ABSTRACT
Cadmium (Cd) causes serious damage to plants. Although calcium (Ca) signal has been found to respond to certain stress, the localization of Ca and molecular mechanisms underlying Ca signal in plants during Cd stress are largely unknown. In this study, Ca2+-sensing fluorescent reporter (GCaMP3) transgenic duckweed showed the Ca2+ signal response in Lemna turionifera 5511 (duckweed) during Cd stress. Subsequently, the subcellular localization of Ca2+ has been studied during Cd stress by transmission electron microscopy, showing the accumulation of Ca2+ in vacuoles. Also, Ca2+ flow during Cd stress has been measured. At the same time, the effects of exogenous glutamic acid (Glu) and γ-aminobutyric (GABA) on duckweed can better clarify the signal operation mechanism of plants to Cd stress. The molecular mechanism of Ca2+ signal responsed during Cd stress showed that Cd treatment promotes the positive response of Ca signaling channels in plant cells, and thus affects the intracellular Ca content. These novel signal studies provided an important Ca2+ signal molecular mechanism during Cd stress.
KEYWORDS: Calcium, Cd stress, GCaMP3 duckweed, ruthenium red, subcellular localization
Introduction
Cadmium (Cd) is a highly biotoxic heavy metal, which causes great harm to plants. Plants usually grow short when affected by Cd,1 then leading to plant cell damage, and plays an inhibitory effect on plant growth. Cd affects growth morphology and physiological levels of plants. From the whole plant, Cd causes delayed growth rate, leaves blade yellowing, suppressed respiration and photosynthesis, and decreased ability to absorb nutrients.2 When the Cd content in plant reached a certain level, it reduces its chlorophyll abundance, thus damaging the photosynthetic structure and changing its respiration rate.3 It also suppresses the absorption of water by plant roots, leading to their water imbalance.4 The biomass of duckweed has been studied in our previous study, including the chlorophyl content,5 photosynthetic rate,6 and declined total sugar content (8.2%, unpublished data). Cd also competes for channels for other metal ions, preventing the plants to absorb essential elements.7 Therefore, it is very necessary to find out the signal path under Cd stress.
Calcium (Ca) signal is a universal second messenger, which occupies a crucial role in plant adversity as a key regulator react to a variety of stimuli, including aspects of heavy metal stress, low temperature, salt, and pathogens.8 First, recent research has shown that Ca signal played a significant role through Ca2+ and calmodulin-mediated signal responsiveness during temperatures drop sharply.9 Secondly, plants respond to salt stress by Ca2+ perception and signaling. Within seconds of exposure to salt stress, the cell membrane Ca2+ level increased rapidly.10 In addition, Ca signaling was coordinated with pathways such as the ubiquitin and proteasome system to keep a balanced and effective defense response against pathogens in plants.11 The addition of exogenous Ca reflects the biological repair function, and relieves effectively the smaller leaves and early bolting caused by Cd stress.12 Ca2+ around the plasma membrane is important in mitigating Cd toxicity by competing for influx of Cd2+ ions.13
Ca2+ channels play a significant role during Ca2+ signal response. Glutamate receptors (GLRs), the Ca2+ channels, take a part during Ca2+ signaling coding,14 which provides the possibility of the crosstalk between Glu and Ca2+ signaling. In addition, Ruthenium Red (RR), a complex of ammoniated ruthenium chloride oxide, is known as a Ca2+ channel blocker vacuole,15 and RR reacts specifically with phospholipids, fatty acids, and mucopolysaccharides.16 Hence, the function of Glu during Ca2+ signal net as well as the influence of RR on Ca2+ signal is still needed to be investigated during Cd stress.
Despite the great progress in the past made by researchers on the response of Ca signal in Cd stress, where the Ca2+ release remains to be discovered. Thus, Ca2+ is regarded as one of the most representative elements when plants are under Cd stress.
The use of aquatic plants such as duckweed for the repair of Cd pollution in water bodies can play an environmental role, so bioremediation methods are widely used.17 Duckweed can grow and reproduce faster, is easy to obtain, has wide distribution and has low cost.18 The growth period of duckweed and the rate of accumulation of biomass longer than most plants,19 many deleteriousness metals such as Cd can be efficiently removed from water by absorption and accumulation.20Therefore, duckweed can be effectively used in the treatment of Cd pollution in water bodies.21
When the content of glutamate increases between cells, the continuous opening of glutamate channels also leads to a large increase in Ca influx and neurotoxicity. 22 Glutamate involved in growth and development of plants,23 seed germination,24 and heavy-metal stress response.21 Not only that the addition of exogenous glutamate can induce resistance of rice and tomatoes to fungal pathogens and Arabidopsis thaliana to pathogens.25
GABA can act as an important inhibitory neurotransmitter in the vertebrate central nervous system.26Experiments have confirmed that with the increase of GABA added to the culture medium, the biomass and mineral element content of duckweed also increased, indicating that GABA can regulate ion transport in plants (Kinnersley and Lin, n.d.).
The aims of this paper are to: 1) study the Ca2+ flux with Cd stress; 2) investigate the responses of Ca signal by GCaMP3 transgenic duckweed under Cd stress; 3) analyze the differentially expressed genes involved in the Ca signaling pathway during Cd stress; 4) the subcellular localization of Ca was accumulated in duckweed.
Materials and methods
Cultivation of duckweed
Lemna turionifera 5511 (Duckweed) was taken from the Fengchan river of Tianjin, which has been cultured with an aseptic condition with Datko that contains 0.4 mM MgSO4 · 7H2O, 1.4 mM Ca (NO3)2 · 4H2O, 1.1 mM KNO3, 0.4 mM KH2PO4, 0.4 mM Mg (NO3)2 · 6H2O, 55 µM CaCl2 · 2H2O, 55 µM KCl, 6.2 µM Na2MoO4 · 2H2O, 71 µM H2BO3, 30 µM K2H2EDTA·2H2O, 56.7 µM FeNH4EDTA, 13.8 µM MnCl2 · 4H2O, 2.8 µM ZnNa2EDTA·4H2O, 4.8 µM CoSO4 · 7H2O, 18.6 µM Na2EDTA·2H2O following our previous research with a temperature of 23°C.27 Duckweed was cultured with the light period set as 16 h light (light intensity 95 ± 5 μmol·m−2 · s−1) and 8 h dark a day. The duckweeds are subcultured every 12 days.
Duckweeds were treated with 50 μM CdCl2 or without for 15 h in GCaMP3 duckweed analysis, and for 24 h in Ca content, localization, and differentially expressed genes study.
Construction of binary vector pCAMBIA-1301-GCaMP3
The structure of GCaMP3 plasmid is shown in Figure 1. The gene of A GFP-based calmodulin protein 3 (GCaMP3) was a gift from Loren Looger (Addgene plasmid # 22692; http://n2t.net/addgene:22692; RRID: Addgene- 22692). The GCaMP3 open reading frame was obtained by PCR with the template from this plasmid, which was added by CaMV-35S promoter (5’ end) the nopaline synthase terminator (3’ end). Then that was linked to pCAMBIA-1301, a binary vector, to construct vector pCAMBIA-1301-GCaMP3. pCAMBIA-1301-GCaMP3 was transformed to the Agrobacterium tumefaciens strain Gv3101, according to the method of freeze-thaw 28
Figure 1.

Construction of pCAMBIA-1301-GCaMP3. T35S, CaMV 35S terminator; HPT: hygromycin B phosphotransferase gene; GCaMP3: GCaMP3-A GFP-based Ca sensor; Poly A: terminator; GUS: β- Glucuronidase.
Transient transformation of transgenic GCaMP3-duckweed
Activate the Agrobacterium carrying the pCAMBIA-1301-GCaMP3 plasmid three times on the LB solid plate containing antibiotics (Kanamycin 50 mg·L−1, Rifampin 25 mg·L−1, Streptomycin 50 mg·L−1) at 28°C. Then, single colonies were selected and inoculated in LB liquid medium (5 mL) containing antibiotics for 28 hours. After that, 1 ml of the resultant bacterial solution was transferred to the new liquid LB (20 mL) for 4 hours. The solution was cultured at 28°C until OD600 was 0.6, then set the centrifuge for 10 min at 25°C and 3000 g. And the sediment was resoluted by 0.04% Silwet-77 (v/v) and 5 g·L−1 sucrose. 8 ml solution was added to the centrifuged thallus, after which it was gently suspended with a pipette gun. The dissolved thallus was poured into a sterile six-well plate after it was fully mixed with the lysate, with the duckweed picked out on the filter paper. After pricking a small hole (without penetrating the duckweed) at the bag on the back of the frond with a sterile needle, place it in the lysate for 20 mins, after which pick the duckweed out with tweezers. Subsequently, we transferred it to a fluid nutrient medium and cultured it in darkness for 24 h after sucking up the surface bacterial solution on the filter paper, then the transient transformation of transgenic GCaMP3 duckweed was observed by the fluorescence microscope. The plant sample was observed with a fluorescence microscope (Leica DFC450C, DM5000, Berlin, Germany) and the excitation wavelength is 488 nm, the fluorescence intensity on the point 300 μm from the root tip was measured by Image J software (NIH, Bethesda, MD, USA).
RNA extraction and purification
10 days old duckweeds were used for study. When duckweed was exposed to 50 μM Cd for 24 h, the samples for sequencing analysis were collected. The extraction and purification of RNA in duckweed treated with Cd (Cd group) or without Cd (CK) were carried out in strict accordance with the operating instructions of the RNA prep pure plant Kit (DP441). The purity and integrity of RNA have been detected by Nano Photometer® spectrophotometer (IMPLEN, CA, USA) and by the RNA Nano 6000 Assay Kit using Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Library construction was followed by the Illumina’s NEBNext ® Ultra TM RNA Library Prep Kit instruction. To obtain the library, PCR amplification was applied and PCR product purification was performed by AMPure XP beads. Then, initial quantification was studied by Qubit2.0 Fluorometer, and then the library size was analyzed by Agilent 2100 Bio Analyzer. Finally, qRT-PCR quantified the concentration of the library (the library concentration was higher than 2 nM).
Data filtering and sequence analysis
Firstly, FASTP software was used for quality control and pretreatment to obtain clean readings, including deleting readings with an adapter, and removing the proportion of N with a reading number greater than 10%. The clean reading number has been obtained according to Grabherr et al.29 And the reference sequence has been obtained for subsequent analysis. Cluster profiler was used to analyze the potential functions of RNA molecules by Kyoto gene, Gene Ontology (GO), and genome Encyclopedia (KEGG). The Clue GO application of Cytoscape was used for KEGG pathway enrichment analysis of differential expression.30 In addition, the GO function and KEGG signaling of differential genes were analyzed by GSEA software, in which the screening threshold was set to P < 0.05.
The determination of Ca2+ Net flux
The Ca2+ flow was measured at 0.1 mm from the root tip of duckweed using a non-invasive micromeasurement system (NMT, 100 Series, YoungerUSA LLC, Amherst, MA 01003, USA; Xuyue, Beijing, China and im Fluxes V2.0 (Younger USA LLC, Amherst, MA 01002, USA). Then, the Ca2+ flow was measured by dropping 100 mM (stocking concentration) CdCl2 with a working concentration of 50 μM. After that, 5 mmol L−1 ruthenium red, or 1 mmol L−1 Glu, or 10 μM GABA were added under the Cd stress treatment to determine the effect of the treatment on the Ca ion current. The Ca2+ ion-selective microelectrode achieved selectivity by adding a liquid ion exchanger (Liquid Ion Exchanger LIX) at the front end.
Treatment of duckweed roots Ruthenium red under Cd stress
Ruthenium red, is a Ca release inhibitor of the vacuolar membrane,16 which can inhibit the transfer of Ca ions from the vacuole to the cytoplasm.31
Ca signal responded to duckweed under Cd stress
50 μM CdCl2 was added or not to transgenic GCaMP3 duckweed for 15 h. The roots of duckweed were dissected and observed. The plant sample was examined with a fluorescence microscope (Leica DFC450C, DM5000, Berlin, Germany) and the excitation wavelength is 488 nm, the fluorescence intensity of 300 μm from the root tip was analyzed by Image J (NIH, Bethesda, MD, USA).
Subcellular localization of Ca signaling
Duckweed treated with and without Cd were fixed in PBS (0.1 mol/L) solution (pH = 7.4) containing 2% potassium pyroantimonate and 3% glutaraldehyde for 24 h. The cells were washed with 0.1 mol/L PBS (pH = 7.4) containing 2% potassium pyroantimonate, dehydrated with acetone gradient, embedded with Epon812 resin, ultrathin sections, stained with uranium and lead, and observed by 120kV transmission electron microscopy (TF20, Jeol 2100 F, USA).
Ca element level analysis
Ca content was observed by scanning electronic microscopy (SEM) and energy-dispersive X-ray spectrometer (EDX) analysis followed by our previous study.5 The duckweeds with 24 h CdCl2 treatment were desiccated and sprayed gold. Then the roots and frond were detected by SEM (Nova Nano SEM 230). The Ca element was measured by EDX (Genesis APEX, Genesis Apollo 10).
Statistical analysis
At least triplicate was repeated for all experiments. And also 6 parallel groups of the sets were contained which included about 80–150 samples of duckweed. Variables were analyzed by independent sample T-test and one-way ANOVA by SPSS (IBM SPSS Statistics, Version 22). Significantly difference was referred as asterisks (**P < 0.01, *P < 0.05).
Result
Responses of Ca signal in GCaMP3-duckweed under Cd stress
Transgenic duckweed expressing GCaMP3 was obtained as described in methods. PCR was applied to the target GCaMP3 gene in the GCaMP3 duckweed (Figure 2b). After 50 μM CdCl2 was added to the root of transgenic GCaMP3 duckweed for 15 h, the root cap began to fall off (Figure S1), the change of root fluorescence intensity was observed under 488 nm of an upright fluorescence microscope (Figure 2a). It was found that the green fluorescence intensity of duckweed root increased and obvious green fluorescence spots (Ca signal) appeared after 50 μM CdCl2 was added to transgenic GCaMP3 duckweed. As shown in Figure 2a,c, the fluorescence images of GCaMP3-duckweed with 50 μM Cd or without were different. On the root tips, the result in Figure 2c showed that the fluorescence intensity of in WT was 29.6, a very low intensity. And GCaMP3-duckweed without Cd stress was 111.12, while the fluorescence intensity of GCaMP3-duckweed under Cd stress was 136.17, significantly higher than that without Cd. These results indicate that Cd stress can stimulate Ca signal response in duckweed.
Figure 2.
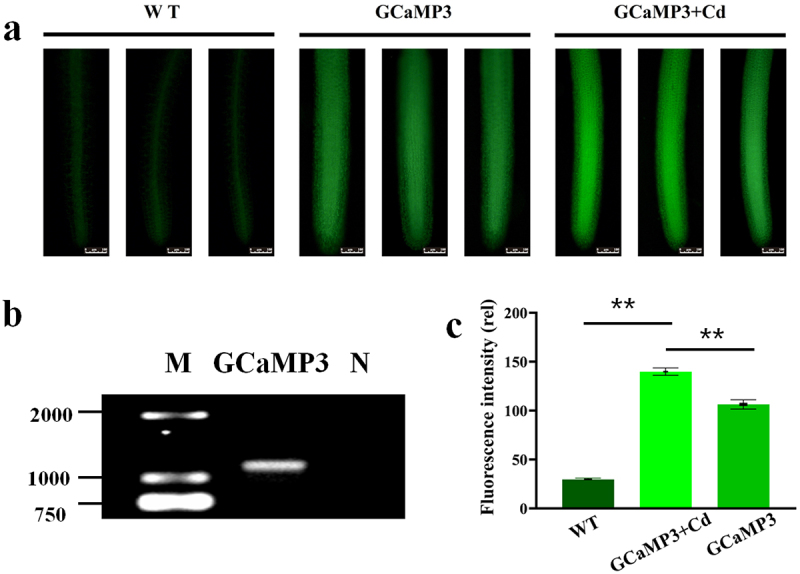
(a). The localization of Ca2+ in the roots of WT-duckweed, GCaMP3-duckweed, and GCaMP3-duckweed was treated with 50 μM CdCl2 for 15 h. Scale bar = 100 μm. (b). PCR results of plasmid validation of GCaMP3 in transgenic GCaMP3-duckweed, M: maker, N: negative control. (c). The different Ca2+ fluorescence intensity was analyzed by image J at 300 μm from the rhizoid tip.
The content and localization of Ca in duckweed with Cd stress
The Ca content level in the frond and root tissues of duckweed with 24 h Cd treatment have been analyzed. Shown as in Figure 3, the Ca2+ content on the fronds was 0.87 Wt %, and the Ca2+ content of the surface of the roots was 0.66 Wt %.
Figure 3.
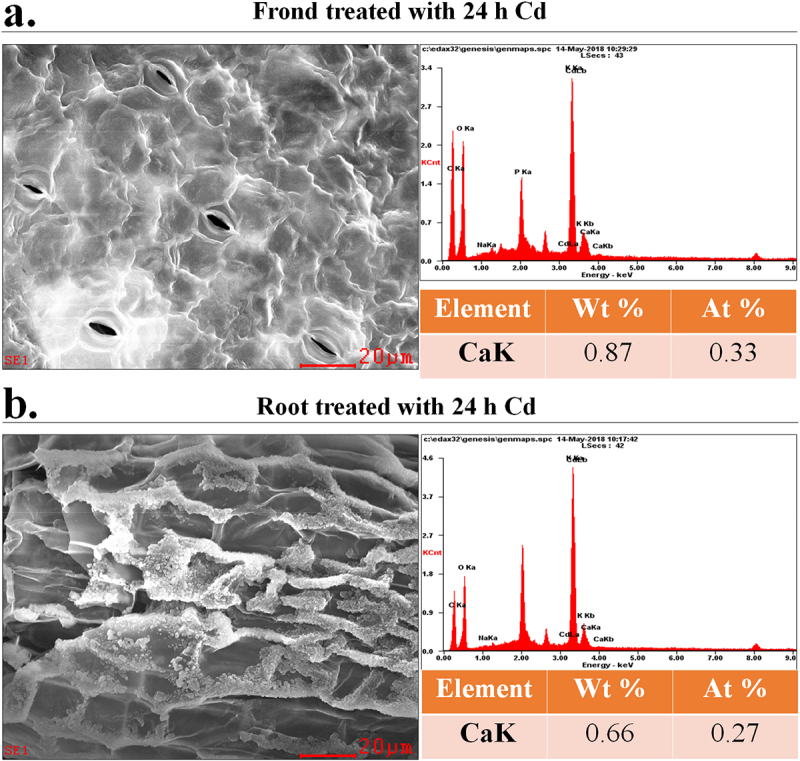
SEM images of the fronds and roots of (a) and OE (b) duckweed treated with Cd (50 μM) for 24 h.The CK represents wide-type duckweed.
The subcellular location of Ca has been studied. As shown in Figure 4a,b, in the normal state, duckweed vacuoles were full and chloroplast morphology was complete. As shown in Figure 4c,d, cytoplasm wall separation occurred in duckweed cells under 50 μM Cd stress for 24 h. Ca stained by potassium pyroantimonate particles increases significantly and accumulates in vacuoles. As shown in Figure 4e,f, vacuoles in local cells with severe Cd stress burst, releasing Ca stored in vacuoles and flowing outwards through cell membranes.
Figure 4.
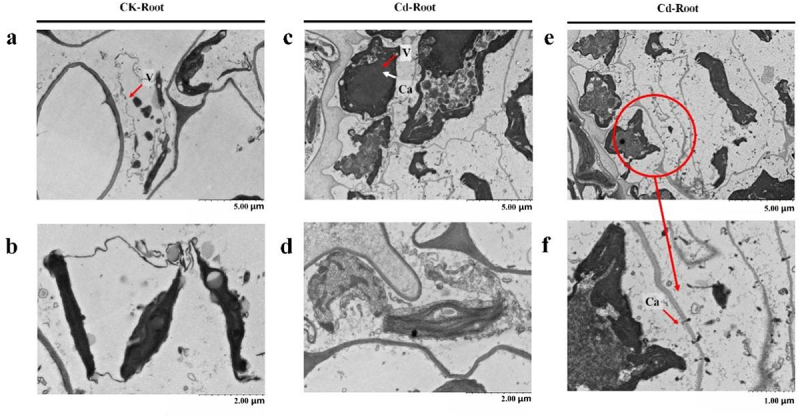
Ca distribution subcellular localization by means of 120 kV transmission electron microscopy (TF20, Jeol 2100 F, USA) of wide-type duckweed with or without 50 μM Cd stress. a&b. The root of wide-type duckweed without 50 μM Cd stress. c&d. The root of wide-type duckweed with 50 μM Cd stress for 24 h. e&f. The root of wide-type duckweed without 50 μM Cd stress for 24 h, and f is the enlarged picture of e.
Investigation of Ca ion flow during Cd stress
As shown in Figure 5a, under normal conditions, Ca ion flow in the root tip was stable at low speed (20 pmol·cm−1·s−1), while under Cd treatment, Ca ion flow in the root tip of duckweed was changed to high-speed outflow (150–250 pmol·cm−1·s−1).
Figure 5.
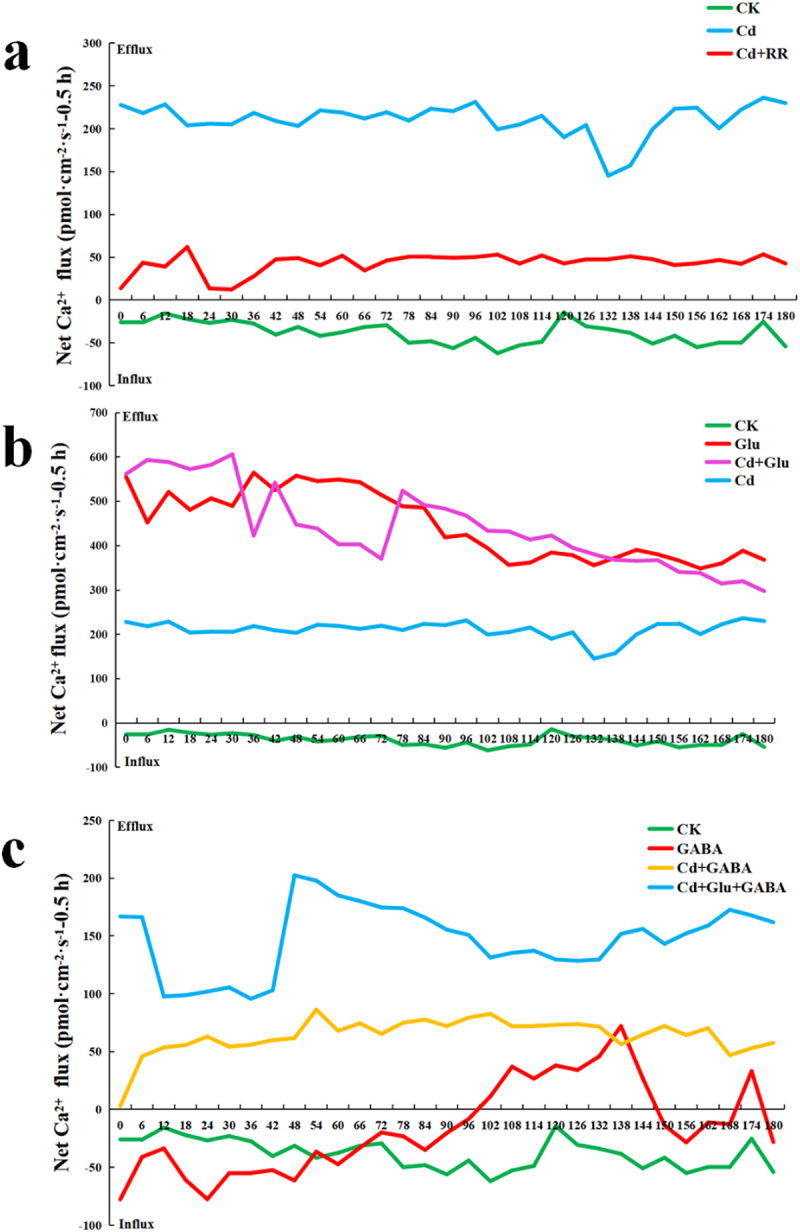
Cd2+ flux was determined by NMT at 100 μm from rhizoid tip, Cd absorption imager (GD-100-CAD) was used to detect duckweed under Cd stress in vivo. a. Duckweed treated with Cd, or with RR in 0.5 h. b. Duckweed was treated with Cd, and with Glu in 0.5 h. c. Duckweed treated with CdCl2, and with GABA or Glu in 0.5 h.
To investigate whether Ca ions came from vacuoles or extracellular, ruthenium red (RR), a vacuolar Ca ion release inhibitor 32 was applied. The results showed that the application of ruthenium red can sharply reduce the Ca2+ efflux induced by Cd stress (22 pmol·cm−1·s−1), suggesting that blocking the vacuolar Ca channel affected the Ca2+ efflux stimulated by Cd, suggesting that the vacuole was a potential Ca reservoir in the plants.33 The rapid block of the Ca2+ channel by RR, applied to the vacuolar membrane, steeply lowed when duckweed under Cd stress.34
Exogenous Glu triggered the Ca2+ signal in duckweed, which has been studied by NMT.35 To investigate the influence of Glu on Ca2+ signal, the net Ca2+ flux was measured by (NMT). As shown in Figure 5b, the net Ca2+ flux of Glu addition in duckweed with or without Cd stress were 422.63 pmol·cm−2·s−1 and 564.56 pmol·cm−2·s−1 at 36s, respectively. The net Ca2+ flux of Glu addition in duckweed with or without Cd stress was 541.66 pmol·cm−2·s−1 and 525.39 pmol·cm−2·s−1 at 42s, respectively. The net Ca2+ flux in duckweed under Cd stress with the addition of Glu was 447.55 pmol·cm−2·s−1 and the net Ca2+ flux of Glu addition in duckweed was 557.66 pmol·cm−2·s−1 at 48s. The net Ca2+ flux of Glu addition in duckweed treated with or without Cd was 523.67 pmol·cm−2·s−1 and 488.76 pmol·cm−2·s−1 at 78s, respectively. The net Ca2+ flux of Glu addition in duckweed without Cd stress was 372.33 pmol·cm−2·s−1, which was higher than the net Ca2+ flux of Glu addition (367.75 pmol·cm−2·s−1) in duckweed with Cd stress at 138 s. In normal conditions, the range of net influx of Ca2+ from the root tip of duckweed was 14.30–62.03 pmol·cm−1·s−1. However, the exogenous addition of Glu resulted in the significant efflux of Ca2+ (348.51–564.56 pmol·cm−1·s−1). At 36s, Ca efflux reached the maximum value of 564.56 pmol·cm−1·s−1, while the minimum efflux was 348.51 pmol·cm−1·s−1 at 162 s. The Ca2+ efflux was also detected in the root tip of duckweed treated with Glu under Cd stress. Under this condition, the maximum net Ca2+ flux was 605.88 pmol·cm−1·s−1 at 30s and the minimum net flux of 297.47 pmol·cm−1·s−1 occurred at 180 s. Compared to the root tip treated only with Glu, Ca efflux trends were approximately the same over a short period.
GABA is synthesized from Glu. GABA can act as an inhibitory neurotransmitter.36 In order to study the immediate influence of GABA on Ca2+ signal, the net Ca2+ flux was also measured by NMT. As shown in Figure 5c, the net Ca2+ flux of GABA addition in duckweed with or without Cd stress was 56.39 pmol·cm−2·s−1 and 72.04 pmol·cm−2·s−1 at 138 s, respectively. the net Ca2+ flux of GABA addition in duckweed with or without Cd stress was 64.62 pmol·cm−2·s−1 and 27.05 pmol·cm−2·s−1 at 144 s, respectively. Moreover, the net Ca2+ flux in duckweed was −41.77 pmol·cm−2·s−1 under normal conditions, while the net Ca2+ flux in duckweed was −36.48 pmol·cm−2·s−1 under GABA treatment at 54s. The net Ca2+ flux of duckweed with or without GABA was −19.85 pmol·cm−2·s−1 and −29.29 pmol·cm−2·s−1 at 72s, respectively. Furthermore, the Ca2+ flow in the root tip of duckweed changed from influx to efflux at 102 s, while it changed from efflux to influx at 150 s under GABA treatment. The direction of Ca2+ flow was transformed in a short period after the root tip of the duckweed was treated with GABA. In this case, the maximum net efflux of Ca2+ was 72.04 pmol·cm−1·s−1 at 138s, and the maximum net influx of Ca2+ was 77.69 pmol·cm−1·s−1 at 0 s. The net Ca2+ flux of Cd and GABA addition in the root tip of duckweed was significantly higher than duckweed treated with GABA. The data suggested that a minimum Ca2+ net flux was 2.81 pmol·cm−2·s−1 at 0s and a maximum Ca2+ net flux of 86.25 pmol·cm−1·s−1 at 54s. When root tips were treated with Glu and GABA under Cd stress, the net Ca2+ flux was significantly higher than duckweed treated with GABA and Cd. Moreover, the maximum net flux of Ca2+ was 202.34 pmol·cm−1·s−1 occurred at 48s, and the minimum net flux was 95.67 pmol·cm−1·s−1 occurred at 36s.
Differently expressed genes involved in Ca signaling pathway during Cd stress
As shown in Figure 6 and Table 1, the differences in genes involved in Ca signaling pathway were explored in the duckweed treated with 50 μM Cd or without 50 μM Cd for 24 h. In cells, the receptors and expression mechanisms involved in Ca signaling are demonstrated.3 Plants have many defense mechanisms to combat Cd. The main strategy is to chelate Cd ions at different sites in plant cells.37 Another approach to improving Cd resistance is to store Cd in vacuole.38
Figure 6.
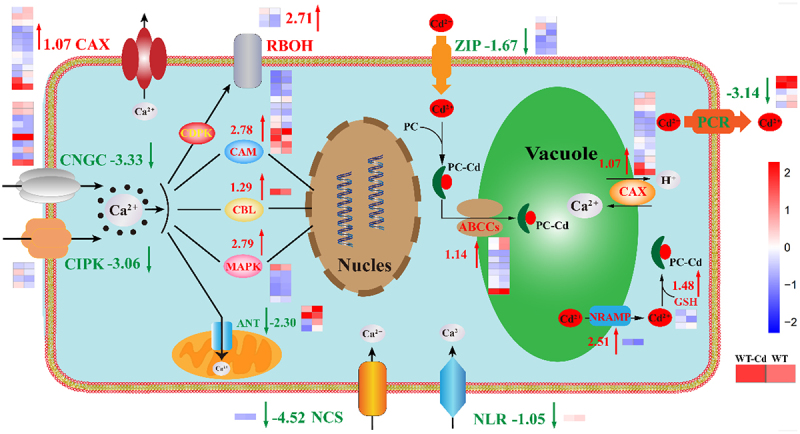
Differences in Ca signaling pathway of duckweed under 50 μM Cd stress. Red arrow is up-regulated, and green arrow is down-regulated. WT group represents wild-type (WT) duckweed, and WT Cd group represents wild-type (WT) duckweed under 50 μM Cd stress. The color in this figure legend is from red to blue, which means log10 (FPKM+1) from high to low.
Table 1.
The difference of GLR expression under Cd stress in duckweed (Cd vs CK).
| Description | Gene ID | Cd | CK | log2Fold | pval | padj |
|---|---|---|---|---|---|---|
| Readcount | Readcount | Change | ||||
| Glutamate receptor 2.8 | Cluster-7365.10892 | 4.62 | 0 | 4.54 | 0.005413 | 0.028602 |
| Glutamate receptor 2.8 | Cluster-7365.97185 | 1695.88 | 22.7 | 6.23 | 3.88E-15 | 8.07E-14 |
| Glutamate receptor 2.8 | Cluster-7365.90108 | 1059.3 | 299.8 | 1.82 | 1.47E-10 | 2.23E-09 |
| Glutamate receptor 2.9 | Cluster-7365.34181 | 50.28 | 15.37 | 1.72 | 0.002368 | 0.013808 |
| Glutamate receptor 2.9 | Cluster-7365.34175 | 356.03 | 123.06 | 1.53 | 0.00000051 | 0.00000588 |
| Glutamate receptor 3.5 | Cluster-7365.29668 | 7.22 | 30.54 | −2.09 | 0.002275 | 0.013334 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.105537 | 0.00 | 14.31 | −6.47 | 0.0052139 | 0.027692 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.78008 | 28.13 | 8.11 | 1.80 | 5.78E-05 | 0.0004685 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.78016 | 17.51 | 0.00 | 6.47 | 0.003248 | 0.018236 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.12653 | 123.58 | 33.41 | 1.89 | 0.0053037 | 0.028115 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.45394 | 518.71 | 205.51 | 1.34 | 0.00084428 | 0.0054782 |
| Ca2+/H+ exchanger (CAX) | Cluster-4392.0 | 9.70 | 2.14 | 2.18 | 0.0056086 | 0.029476 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.40553 | 56.11 | 167.70 | −1.57 | 2.35E-10 | 3.49E-09 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.77997 | 3776.94 | 514.28 | 2.88 | 2.36E-28 | 8.74E-27 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.45137 | 23.86 | 54.89 | −1.20 | 0.00017737 | 0.0013195 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.45385 | 242.87 | 111.42 | 1.13 | 7.43E-06 | 6.89E-05 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.23524 | 11.65 | 71.87 | −2.63 | 0.00011877 | 0.0009116 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.8858 | 41.18 | 7.67 | 2.41 | 0.0067329 | 0.034502 |
| Ca2+/H+ exchanger (CAX) | Cluster-7365.12645 | 1544.28 | 28.92 | 5.73 | 5.51E-192 | 3.84E-189 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.56126 | 183.26 | 687.39 | −1.91 | 7.37E-13 | 1.32E-11 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.53071 | 135.18 | 447.63 | −1.73 | 4.59E-10 | 6.67E-09 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.74926 | 0.00 | 11.80 | −6.19 | 0.0079256 | 0.039709 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.55503 | 0.00 | 167.51 | −10.02 | 4.18E-16 | 9.23E-15 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.57026 | 1.54 | 31.04 | −4.34 | 0.0004331 | 0.0029885 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.56960 | 2336.47 | 8471.62 | −1.86 | 6.15E-48 | 4.09E-46 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.42205 | 7.22 | 22.57 | −1.65 | 0.0089849 | 0.044282 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.19934 | 301.61 | 104.93 | 1.52 | 1.28E-16 | 2.91E-15 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.64466 | 0.00 | 8.69 | −5.75 | 0.00046292 | 0.0031755 |
| cyclic nucleotide-gated channel (CNGC) | Cluster-7365.58747 | 1465.76 | 3735.91 | −1.35 | 0.00029622 | 0.0021097 |
| CBL-interacting protein kinase(CIPK) | Cluster-7365.43393 | 29.43 | 78.02 | −1.41 | 0.00011346 | 0.00087425 |
| CBL-interacting protein kinase(CIPK) | Cluster-7365.57220 | 45.69 | 443.07 | −3.28 | 2.96E-12 | 5.07E-11 |
| CBL-interacting protein kinase(CIPK) | Cluster-7365.83742 | 1.28 | 21.69 | −4.07 | 0.00185 | 0.01108 |
| CBL-interacting protein kinase(CIPK) | Cluster-7365.45428 | 28.57 | 316.92 | −3.47 | 1.51E-08 | 1.92E-07 |
| Respiratory burst oxidase (RBOH) | Cluster-7365.30386 | 269.45 | 106.01 | 1.35 | 1.17E-12 | 2.06E-11 |
| Respiratory burst oxidase (RBOH) | Cluster-7365.107885 | 71.41 | 4.42 | 3.99 | 1.87E-16 | 4.23E-15 |
| Respiratory burst oxidase (RBOH) | Cluster-7365.107886 | 85.66 | 12.48 | 2.79 | 1.50E-11 | 2.44E-10 |
| Calmodulin (CAM) | Cluster-7365.88487 | 100.40 | 49.92 | 1.00 | 0.0057016 | 0.029891 |
| Calmodulin (CAM) | Cluster-7365.42628 | 76.66 | 31.51 | 1.29 | 2.91E-06 | 2.85E-05 |
| Calmodulin (CAM) | Cluster-7365.12951 | 262.31 | 21.96 | 3.59 | 7.46E-29 | 2.81E-27 |
| Calmodulin (CAM) | Cluster-7365.49153 | 479.49 | 152.61 | 1.65 | 1.05E-11 | 1.74E-10 |
| Calmodulin (CAM) | Cluster-7365.34860 | 546.04 | 12.53 | 5.43 | 2.55E-17 | 6.04E-16 |
| Calmodulin (CAM) | Cluster-7365.12947 | 13.72 | 1.39 | 3.33 | 0.0019775 | 0.011757 |
| Calmodulin (CAM) | Cluster-7365.33087 | 153.04 | 23.44 | 2.70 | 1.69E-09 | 2.35E-08 |
| Calmodulin (CAM) | Cluster-7365.72596 | 5586.73 | 62.54 | 6.49 | 1.67E-47 | 1.10E-45 |
| Calmodulin (CAM) | Cluster-7365.58737 | 2019.88 | 712.75 | 1.50 | 2.50E-40 | 1.37E-38 |
| Calmodulin (CAM) | Cluster-7365.68719 | 188.53 | 28.48 | 2.74 | 8.89E-10 | 1.26E-08 |
| Calmodulin (CAM) | Cluster-7365.32431 | 131.65 | 10.88 | 3.61 | 5.15E-06 | 4.89E-05 |
| Calmodulin (CAM) | Cluster-7365.56939 | 3283.27 | 1073.51 | 1.61 | 5.84E-46 | 3.70E-44 |
| Calmodulin (CAM) | Cluster-7365.41749 | 2350.45 | 1033.00 | 1.19 | 1.26E-26 | 4.39E-25 |
| Calcineurin B-like protein (CBL) | Cluster-7365.58129 | 709.36 | 288.74 | 1.29 | 8.96E-42 | 5.10E-40 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.66827 | 68.70 | 10.12 | 2.76 | 0.0022084 | 0.012979 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.97492 | 38.59 | 5.37 | 2.85 | 0.0025563 | 0.01479 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.22659 | 29.99 | 5.87 | 2.35 | 0.0046777 | 0.025198 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.48921 | 1211.82 | 512.35 | 1.24 | 3.31E-07 | 3.63E-06 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.78768 | 13.13 | 0.00 | 6.05 | 0.00011479 | 0.00088337 |
| Mitogen-activated protein kinase (MAPK) | Cluster-7365.43280 | 52.42 | 18.53 | 1.49 | 0.0046022 | 0.024843 |
| Adenine nucleotide translocator (ANT) | Cluster-7365.66271 | 190.38 | 1330.97 | −2.81 | 2.90E-96 | 5.32E-94 |
| Adenine nucleotide translocator (ANT) | Cluster-7365.66270 | 3.54 | 76.12 | −4.42 | 0.002466 | 0.014311 |
| Adenine nucleotide translocator (ANT) | Cluster-7365.92518 | 1394.52 | 564.65 | 1.30 | 5.62E-25 | 1.83E-23 |
| Adenine nucleotide translocator (ANT) | Cluster-7365.68973 | 27.72 | 272.20 | −3.29 | 3.82E-34 | 1.72E-32 |
| neuronal calcium sensors(NCS) | Cluster-7365.1390 | 1.71 | 39.31 | −4.52 | 0.0084973 | 0.042156 |
| nucleotide-binding leucine-rich repeat(NLR) | Cluster-7365.52902 | 159.38 | 331.12 | −1.05 | 2.56E-06 | 2.53E-05 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.73089 | 7.07 | 22.73 | −1.70 | 0.00017456 | 0.0013005 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.16931 | 17.33 | 2.43 | 2.84 | 3.38E-05 | 0.00028439 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.64903 | 0.00 | 113.86 | −9.47 | 9.74E-18 | 2.35E-16 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.38774 | 9.72 | 36.65 | −1.91 | 0.0050219 | 0.026797 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.38773 | 28.70 | 116.06 | −2.01 | 0.0021235 | 0.012527 |
| Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) | Cluster-7365.82922 | 107.73 | 23.10 | 2.22 | 6.94E-15 | 1.41E-13 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.59893 | 367.34 | 117.10 | 1.64 | 0.000082917 | 0.00065505 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.59885 | 221.44 | 24.43 | 3.19 | 5.36E-08 | 6.40E-07 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.59887 | 20,083.94 | 7170.44 | 1.49 | 6.17E-77 | 7.80E-75 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.59883 | 240.97 | 99.72 | 1.28 | 1.99E-08 | 2.49E-07 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.24768 | 124.73 | 41.70 | 1.58 | 0.0008097 | 0.0052743 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.24767 | 36.37 | 8.79 | 2.07 | 0.0030224 | 0.017132 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.57363 | 227.65 | 671.52 | −1.56 | 6.65E-23 | 2.01E-21 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.57364 | 93.08 | 269.73 | −1.53 | 2.71E-14 | 5.30E-13 |
| ATP-binding cassette transporter C proteins (ABCCs) | Cluster-7365.24771 | 422.94 | 96.00 | 2.14 | 6.08E-09 | 8.02E-08 |
| Natural resistance associated macrophage protein (NRAMP) | Cluster-7365.62817 | 12.38 | 2.19 | 2.51 | 0.005133 | 0.027323 |
| glutathione (GSH) | Cluster-7365.56002 | 95.96 | 0.27 | 8.20 | 2.80E-07 | 3.10E-06 |
| glutathione (GSH) | Cluster-7365.35938 | 5.81 | 27.31 | −2.24 | 0.0091648 | 0.04505 |
| glutathione (GSH) | Cluster-7365.64729 | 173.60 | 494.24 | −1.51 | 3.07E-12 | 5.24E-11 |
| Protochlorophyllide reductase (PCR) | Cluster-7365.57067 | 1169.50 | 13,755.89 | −3.56 | 1.56E-16 | 3.54E-15 |
| Protochlorophyllide reductase (PCR) | Cluster-7365.56608 | 1885.58 | 9756.31 | −2.37 | 5.11E-48 | 3.40E-46 |
| Protochlorophyllide reductase (PCR) | Cluster-7365.53631 | 83.81 | 452.05 | −2.43 | 1.78E-15 | 3.78E-14 |
| Protochlorophyllide reductase (PCR) | Cluster-7365.52279 | 28.06 | 167.78 | −2.58 | 3.08E-09 | 4.18E-08 |
| Protochlorophyllide reductase (PCR) | Cluster-7365.54463 | 27.84 | 744.25 | −4.74 | 2.27E-19 | 5.94E-18 |
Vacuoles can transport Cd through the strategy of PC-CD.39 ATP-binding cassette transporter C proteins (ABCCs) participate in PC-CD and tranship Cd into vacuole,33 which has risen by 1.14 log2 Fold Change. Then with the help of glutathione (GSH) participation in the PCCD pathway, which has risen by 1.48 log2 Fold Change. Natural resistance associated with macrophage protein (NRAMP) has been widely studied in Arabidopsis,40 which can transport Cd out of the vacuole,41 has risen by 2.51 log2 Fold Change. In the vacuole, Ca2+/H+ exchanger (CAX) mediates the transport of Cd and Ca ions,42 which has risen by 1.07 log2 Fold Change. Zinc-regulated, Iron-regulated transporter-like Protein (ZIP) has been validated to be involved in the transport of Cd across the cell membrane into the cell,43 which has reduced by 1.67 log2 Fold Change. Protochlorophyllide reductase (PCR) is also involved in the transport of Cd ions from the cell membrane,44,45 which has reduced by 3.14 log2 Fold Change. These results indicated that the main reason for the higher Cd resistance of duckweed was that Cd chelates are transported to vacuoles.46
Adenine nucleotide translocator (ANT) is a Ca ion regulatory channel located in the mitochondrial membrane,47 which was down by 2.30 log2 Fold Change. GLRs, can transport Ca ions from outside the cell into the cell, which has risen by 1.97 log2 Fold Change, and Table 1 for details. Respiratory burst oxidase (RBOH) is related to plant response to wound stress,48 which has risen by 2.71 log2 Fold Change. Calmodulin (CAM) can enhance plant resistance and help transport Ca ions into the nucleus,28 which has risen by 2.78 log2 Fold Change. Activation of Mitogen-activated protein kinase (MAPK) cascade confers resistance to fungal pathogens,49 which has risen by 2.79 log2 Fold Change. Calcineurin B-like protein (CBL) is involved in Ca signaling by interacting with its specific kinase protein,50 which has risen by 1.29 log2 Fold Change.
These results suggest that the addition of Cd promotes the positive response of Ca signaling channels in plant cells, and thus affects the intracellular Ca content.
Discussion
Transient increases of Ca2+ concentration in the plant cell are key signals that initiate many cellular signaling pathways that respond to adversity stress.51 The observation that Cd stress can trigger Ca2+ transient discharging in plant cells raises the intriguing possibility that the Ca signaling not only responds to heavy metal stress but also has a reservoir in the cell that can accumulate and burst in a short time. Here, we investigated the Ca2+ response to Cd stress.
Some studies have produced plants transformed with a construct for constitutive expression of the Ca sensor protein GCaMP, which showed that fluorescence was both increased in leaves subjected to wound stress and freezing stress.52 In our research, we obtained the GCaMP3 duckweed, which could show Ca signal by GFP fluorescence. The result in Figure 2 showed that it showed a higher fluorescence intensity during Cd stress. The result revealed the involvement of the Ca2+ signal in GCaMP3-duckweed during Cd processing. A similar result has been reported that the Ca2+ content was accumulated in duckweed rhizoid under Cd stress.53 Furthermore, Researchers found that Cold stress and mechanical damage can also stimulate Ca2+ signals,11,46 which are mediated by GLR triggered by Glu. Notably, Glu as a crucial signaling molecule in plants,54 which is an activator of glutamate receptors.55 Our results showed that the exogenous addition of Glu resulted in the significant efflux of Ca2+ in duckweed, which is similar to the results of our research on the fluorescence response. In addition, GABA has been extensively studied in animals, and it acts as an inhibitory neuro transmitter,36 while Glu is a key excitatory neurotransmitter in the central nervous system.56Thus, Glu and GABA exert antagonistic effects in animals. In this research, we found that the exogenous addition of GABA stabilized the Ca2+ signal in duckweed. Therefore, they played an important regulatory role on Ca2+ signal under Cd stress in duckweed.
Ruthenium Red, a common Ca2+ channel inhibitor. The inhibitory effect of ruthenium red on Ca2+-permeable vacuolar channels has been demonstrated in a study by Pottosin et al.16 The addition of ruthenium red to Cd-stimulated duckweed showed that ruthenium red inhibited Ca2+ release from the vacuolar membrane (Figure 4a) indicating that blocking vacuolar Ca2+ channels affected Cd-stimulated Ca2+ efflux. This suggested that the vacuole is a potential Ca reservoir. On the other hand, the electron microscopic results showed that the vacuoles of plants without Cd were very full. Nevertheless, after the addition of Cd the vacuoles of the plants were crinkled and aggregation of Ca signals occurred, along with the release of Ca2+ from the vacuole membrane (Electron micrograph). This was consistent with that Ca was stored in the vesicles and that Ca2+ was excreted when the vesicles were crinkled. This has great potential for the study of plant signaling.
The Ca signal during stress has been investigated. Some studies have shown that auxin channel inhibitors block Ca signaling.57 Also, the overexpression of CAX on vacuolar membrane can improve the sodium tolerance of plants,58 and is involved in the exportation of Ca ions from plant cells.59 Ca compete with Cd for binding to the NRAMP site, NRAMP can provide binding sites for Cd ions and uptake and transport of Cd in plant.60 ATP-binding cassette subfamily C member (ABCC) protein can combine the energy generated by ATP hydrolysis with the transfer of toxic substances and help toxic substances out of plant cells.61 ABCC family genes involved in plant growth and development are overexpressed under Cd exposure, which may be related to promoting Cd absorption and transport.33 In this study, we found that genes related to Ca transport were significantly upregulated on the vacuole membrane, indicating that Ca is stored in vacuoles and released by plants under Cd stress. Other Ca transporters on the cell membrane of duckweed were also upregulated, and proteins related to Ca metabolism in cells were also significantly upregulated, which indicated that Ca responded positively to Cd stress.
Conclusion
Conclusively, in this study, Ca2+-sensing fluorescent reporter (GCaMP3) transgenic duckweeds were analyzed to demonstrate the Ca signal response to Cd stress. Also, the subcellular localization of Ca2+ has been studied during Cd stress by transmission electron microscopy, showing the accumulation of Ca2+ in vacuoles. Ca2+ inflow was stable at low speed, while under Cd treatment, it changed to high-speed efflux, which has been influenced by ruthenium red, Glu and GABA. Gene expression analysis results indicated that Ca2+ transport was significantly upregulated on the vacuole membrane, indicating that Ca is stored in vacuoles and released by plants under Cd stress. Thus, these novel signal studies provided important molecular mechanism of Ca2+ signal during Cd stress.
Supplementary Material
Acknowledgments
The present research has been supported by the National Natural Science Foundation of China (No. 32071620), Tianjin Natural Science Foundation of Tianjin (S20QNK618).
Funding Statement
This work was supported by the National Natural Science Foundation of China [No. 32071620]; Tianjin Natural Science Foundation [S20QNK618].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592324.2022.2119340
References
- 1.Holubek R, Deckert J, Zinicovscaia I, Yushin N, Vergel K, Frontasyeva M, Sirotkin AV, Bajia DS, Chmielowska-Bąk J.. The recovery of soybean plants after short-term cadmium stress. Plants. 2020;9(6):782. doi: 10.3390/plants9060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raza A, Habib M, Kakavand SN, Zahid Z, Zahra N, Sharif R, Hasanuzzaman M. Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biology. 2020;9(7):177. doi: 10.3390/biology9070177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naciri R, Lahrir M, Benadis C, Chtouki M, Oukarroum A. Interactive effect of potassium and cadmium on growth, root morphology and chlorophyll a fluorescence in tomato plant. Sci Rep. 2021;11(1):5384. doi: 10.1038/s41598-021-84990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegler JL, Oultram JMJ, Nguyen DQ, Grof CPL, Eamens AL. MicroRNA-mediated responses to cadmium stress in Arabidopsis thaliana. Plants. 2021;10(1):130. doi: 10.3390/plants10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Wei Y, Li N, Zeng J, Han Y, Zuo Z, Wang S, Zhu Y, Zhang Y, Sun J, et al. Declined cadmium accumulation in Na+/H+ antiporter (NHX1) transgenic duckweed under cadmium stress. Ecotoxicol Environ Saf. 2019;182:109397. doi: 10.1016/j.ecoenv.2019.109397. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Han Y, Wu D, Yong W, Liu M, Wang S, Liu W, Lu M, Wei Y, Sun J. Salt and cadmium stress tolerance caused by overexpression of the Glycine Max Na+/H+ Antiporter (GmNHX1) gene in duckweed (Lemna turionifera 5511). Aquat Toxicol. 2017;192:127–11. doi: 10.1016/j.aquatox.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C. Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics. 2019;11(2):255–277. doi: 10.1039/C8MT00247A. [DOI] [PubMed] [Google Scholar]
- 8.Allen GJ, Schroeder JI . Combining genetics and cell biology to crack the code of plant cell calcium signaling. Sci STKE. 2001;(102):re13 . doi: 10.1126/stke.2001.102.re13. [DOI] [PubMed] [Google Scholar]
- 9.Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22(3):541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seifikalhor M, Aliniaeifard S, Shomali A, Azad N, Hassani B, Lastochkina O, Li T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal Behav. 2019;14(11):1665455. doi: 10.1080/15592324.2019.1665455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Du L, Poovaiah BW. Calcium signaling and biotic defense responses in plants. Plant Signal Behav. 2014;9(11):e973818. doi: 10.4161/15592324.2014.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng L, Zhu T, Gao Y, Wang Y, Ning C, Björn LO, Chen D, Li S. Effects of Ca addition on the uptake, translocation, and distribution of Cd in Arabidopsis thaliana. Ecotoxicol Environ Saf. 2017;139:228–237. doi: 10.1016/j.ecoenv.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Wan G, Najeeb U, Jilani G, Naeem MS, Zhou W. Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res. 2011;18(9):1478–1486. doi: 10.1007/s11356-011-0509-1. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, Chang I-F. Pharmacological studies with specific agonist and antagonist of animal iGluR on root growth in Arabidopsis thaliana. GABA Glutamate. 2018. doi: 10.5772/intechopen.72121. [DOI] [Google Scholar]
- 15.Cook NP, Archer CM, Fawver JN, Schall HE, Rodriguez-Rivera J, Dineley KT, Martí AA, Murray IVJ. Ruthenium red colorimetric and birefringent staining of amyloid-β aggregates in vitro and in Tg2576 mice. ACS Chem Neurosci. 2013;4(3):379–384. doi: 10.1021/cn300219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pottosin II, Dobrovinskaya OR, Muñiz J. Cooperative block of the plant endomembrane ion channel by ruthenium red. Biophys J. 1999;77(4):1973–1979. doi: 10.1016/S0006-3495(99)77038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Zhang H, Wang Q, Shao M, Li X, Chen D, Zeng R, Song Y. Intraspecific variations in cadmium tolerance and phytoaccumulation in giant duckweed (Spirodela polyrhiza). J Hazard Mater. 2020;395:122672. doi: 10.1016/j.jhazmat.2020.122672. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhang B, Wu D, Hu L, Huang T, Gao G, Huang S, Wu S. Chemical forms governing Cd tolerance and detoxification in duckweed (Landoltia punctata). Ecotoxicol Environ Saf. 2021;207:111553. doi: 10.1016/j.ecoenv.2020.111553. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Jin Y, Zhang G, Fang Y, Xiao Y, Zhao H. Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies. 2012;5(8):3019–3032. doi: 10.3390/en5083019. [DOI] [Google Scholar]
- 20.Miretzky P, Saralegui A, Cirelli AF. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere. 2004;57(8):997–1005. doi: 10.1016/j.chemosphere.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Ren Q, Ma X, Wang M, Sun J, Wang S, Wu X, Chen X, Wang C, Li Q, et al. New insight into the effect of riluzole on cadmium tolerance and accumulation in duckweed (Lemna turionifera). Ecotoxicol Environ Saf. 2022;241:113783. doi: 10.1016/j.ecoenv.2022.113783. [DOI] [PubMed] [Google Scholar]
- 22.Morizane C, Adachi K, Furutani I, Fujita Y, Akaike A, Kashii S, Honda Y. N v-Nitro-L-arginine methyl ester protects retinal neurons against N-methyl-D-aspartate-induced neurotoxicity in vivo 5. Eur J Pharmacol. 1997;328(1):45–49. doi: 10.1016/s0014-2999(97)83026-9. [DOI] [PubMed] [Google Scholar]
- 23.Qiu X-M, Sun -Y-Y, Ye X-Y, Li Z-G. Signaling role of glutamate in plants. Front Plant Sci. 2020;10:1743. doi: 10.3389/fpls.2019.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong D, Ju C, Parihar A, Kim S, Cho D, Kwak JM. Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol. 2015;167(4):1630–1642. doi: 10.1104/pp.114.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto Y, Maki N, Ichihashi Y, Kitazawa D, Igarashi D, Kadota Y, Shirasu K. Exogenous treatment with glutamate induces immune responses in Arabidopsis. MPMI. 2020;33(3):474–487. doi: 10.1094/MPMI-09-19-0262-R. [DOI] [PubMed] [Google Scholar]
- 26.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Han H, Liu M, Zuo Z, Zhou K, Lü J, Zhu Y, Bai Y, Wang Y. Overexpression of the Arabidopsis photorespiratory pathway gene, serine: glyoxylate aminotransferase (AtAGT1), leads to salt stress tolerance in transgenic duckweed (Lemna minor). Plant Cell Tiss Organ Cult. 2013;113(3):407–416. doi: 10.1007/s11240-012-0280-0. [DOI] [Google Scholar]
- 28.Virdi AS, Singh S, Singh P, Bones AM. Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front Plant Sci. 2015;6:6. doi: 10.3389/fpls.2015.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadi M, Pashangzadeh S, Moraghebi M, Sabetian S, Shekari M, Eini F, Salehi E, Mousavi P. Construction of circRNA‐miRNA‐mRNA network in the pathogenesis of recurrent implantation failure using integrated bioinformatics study. J Cellular Molecular Medi. 2022;26(6):1853–1864. doi: 10.1111/jcmm.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumaker KS, Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986;261(26):12172–12178. doi: 10.1016/S0021-9258(18)67219-9. [DOI] [PubMed] [Google Scholar]
- 32.Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, Philosoph-Hadas S. Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol. 1998;118(2):483–492. doi: 10.1104/pp.118.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng T, He X, Zhuo R, Qiao G, Han X, Qiu W, Chi L, Zhang D, Liu M. Identification and functional characterization of ABCC transporters for Cd tolerance and accumulation in Sedum alfredii Hance. Sci Rep. 2020;10(1):20928. doi: 10.1038/s41598-020-78018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma M-T, Zhang J, Farooqui AA, Chen P, Ong W-Y. Effects of cholesterol oxidation products on exocytosis. Neurosci Lett. 2010;476(1):36–41. doi: 10.1016/j.neulet.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 35.Yao J, Sun J, Chen Y, Shi L, Yang L, Wang Y. The molecular mechanism underlying cadmium resistance in NHX1 transgenic Lemna turonifera was studied by comparative transcriptome analysis. Plant Cell Tiss Organ Cult. 2020;143(1):189–200. doi: 10.1007/s11240-020-01909-z. [DOI] [Google Scholar]
- 36.Hijaz F, Killiny N. The use of deuterium-labeled gamma-aminobutyric (D6-GABA) to study uptake, translocation, and metabolism of exogenous GABA in plants. Plant Methods. 2020;16(1):24. doi: 10.1186/s13007-020-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peñalosa JM, Carpena RO, Vázquez S, Agha R, Granado A, Sarro MJ, Esteban E. Chelate-assisted phytoextraction of heavy metals in a soil contaminated with a pyritic sludge. Science Total Environ. 2007;378(1–2):199–204. doi: 10.1016/j.scitotenv.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 38.Xu Q, Wang C, Li S, Li B, Li Q, Chen G, Chen W, Wang F. Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (Oryza sativa L.). Environ Sci Pollut Res. 2017;24(12):11319–11330. doi: 10.1007/s11356-017-8775-1. [DOI] [PubMed] [Google Scholar]
- 39.Loscos J, Naya L, Ramos J, Clemente MR, Matamoros MA, Becana M. A reassessment of substrate specificity and activation of phytochelatin synthases from model plants by physiologically relevant metals. Plant Physiol. 2006;140(4):1213–1221. doi: 10.1104/pp.105.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cailliatte R, Schikora A, Briat J-F, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for arabidopsis growth in low manganese conditions. Plant Cell. 2010;22(3):904–917. doi: 10.1105/tpc.109.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue X, Song J, Fang B, Wang L, Zou J, Su N, Cui J. BcNRAMP1 promotes the absorption of cadmium and manganese in Arabidopsis. Chemosphere. 2021;283:131113. doi: 10.1016/j.chemosphere.2021.131113. [DOI] [PubMed] [Google Scholar]
- 42.Shigaki T, Sreevidya C, Hirschi KD . Analysis of the Ca2+ domain in the Arabidopsis H+/Ca2+ antiporters CAX1 and CAX3. Plant Mol Biol. 2002;50(3):475–83 . doi: 10.1023/A:1019880006606. [DOI] [PubMed] [Google Scholar]
- 43.Ajeesh Krishna TP, Maharajan T, Victor Roch G, Ignacimuthu S, Antony Ceasar S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front Plant Sci. 2020;11:662. doi: 10.3389/fpls.2020.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim D-Y, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance: role of AtPDR8 in cadmium resistance. Plant J. 2007;50(2):207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- 45.Kinnersley AM, Lin F. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. 2000;32(1): 65-76. doi: 10.1023/A:1006305120202. [DOI] [Google Scholar]
- 46.Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018;361(6407):1112–1115. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 47.Vergun O, Reynolds IJ. Fluctuations in mitochondrial membrane potential in single isolated brain mitochondria: modulation by adenine nucleotides and Ca2+. Biophys J. 2004;87(5):3585–3593. doi: 10.1529/biophysj.104.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum [W]. Plant Cell. 2004;16(3):616–628. doi: 10.1105/tpc.019398. W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415(6875):977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 50.Deng J, Yang X, Sun W, Miao Y, He L, Zhang X. The calcium sensor CBL2 and its interacting kinase CIPK6 are involved in plant sugar homeostasis via interacting with tonoplast sugar transporter TST2. Plant Physiol. 2020;183(1):236–249. doi: 10.1104/pp.19.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haruta M, Monshausen G, Gilroy S, Sussman MR. A cytoplasmic Ca 2+ functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: identification of AtRALF1 peptide. Biochemistry. 2008;47(24):6311–6321. doi: 10.1021/bi8001488. [DOI] [PubMed] [Google Scholar]
- 52.Suda H . Calcium dynamics during trap closure visualized in transgenic Venus flytrap. Nature Plants. 2020;6(10):1219–1224:. doi: 10.1038/s41477-020-00773-1. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Yao J, Sun J, Shi L, Chen Y, Sun J. The Ca2+ signaling, Glu, and GABA responds to Cd stress in duckweed. Aquat Toxicol. 2020;218:105352. doi: 10.1016/j.aquatox.2019.105352. [DOI] [PubMed] [Google Scholar]
- 54.Forde BG. Glutamate signaling in roots. J Exp Bot. 2014;65(3):779–787. doi: 10.1093/jxb/ert335. [DOI] [PubMed] [Google Scholar]
- 55.Li Z-G, Ye X-Y, Qiu X-M. Glutamate signaling enhances the heat tolerance of maize seedlings by plant glutamate receptor-like channels-mediated calcium signaling. Protoplasma. 2019;256(4):1165–1169. doi: 10.1007/s00709-019-01351-9. [DOI] [PubMed] [Google Scholar]
- 56.Tabassum S, Ahmad S, Madiha S, Shahzad S, Batool Z, Sadir S, Haider S. Free l-glutamate-induced modulation in oxidative and neurochemical profile contributes to enhancement in locomotor and memory performance in male rats. Sci Rep. 2020;10(1):11206. doi: 10.1038/s41598-020-68041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006;29(8):1532–1544. doi: 10.1111/j.1365-3040.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J, Shigaki T, Mei H, Guo Y, Cheng N-H, Hirschi KD. Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J Biol Chem. 2009;284:4605–4615. doi: 10.1074/jbc.M804462200. [DOI] [PubMed] [Google Scholar]
- 59.Shigaki T, Hirschi K. Characterization of CAX-like genes in plants: implications for functional diversity. Gene. 2000;257(2):291–298. doi: 10.1016/S0378-1119(00)00390-5. [DOI] [PubMed] [Google Scholar]
- 60.Bozzi AT, Gaudet R. Molecular mechanism of nramp-family transition metal transport. J Mol Biol. 2021;433(16):166991. doi: 10.1016/j.jmb.2021.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kathawala RJ, Wang Y-J, Shukla S, Zhang Y-K, Alqahtani S, Kaddoumi A, Ambudkar SV, Ashby CR, Chen Z-S. ATP-binding cassette subfamily B member 1 (ABCB1) and subfamily C member 10 (ABCC10) are not primary resistance factors for cabazitaxel. Chin J Cancer. 2015;34(3):5. doi: 10.1186/s40880-015-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


