Abstract
Spinocerebellar ataxia type 6 (SCA6) is a neurodegenerative disease resulting in motor coordination deficits and cerebellar pathology. Expression of brain-derived neurotrophic factor (BDNF) is reduced in postmortem tissue from SCA6 patients. Here, we show that levels of cerebellar BDNF and its receptor, tropomyosin receptor kinase B (TrkB), are reduced at an early disease stage in a mouse model of SCA6 (SCA684Q/84Q). One month of exercise elevated cerebellar BDNF expression and improved ataxia and cerebellar Purkinje cell firing rate deficits. A TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF), likewise improved motor coordination and Purkinje cell firing rate and elevated downstream Akt signaling. Prolonged 7,8-DHF administration persistently improved ataxia when treatment commenced near disease onset but was ineffective when treatment was started late. These data suggest that 7,8-DHF, which is orally bioavailable and crosses the blood-brain barrier, is a promising therapeutic for SCA6 and argue for the importance of early intervention for SCA6.
Reduced BDNF-TrkB signaling in SCA6 is restored with exercise or a TrkB agonist that reverses ataxia and cerebellar dysfunction.
INTRODUCTION
Spinocerebellar ataxia type 6 (SCA6) is a rare neurodegenerative disorder characterized by impaired motor coordination and cerebellar pathology. SCA6 is caused by a CAG-repeat expansion mutation in the CACNA1A gene (1). This leads to a progressive loss of motor coordination, which typically onsets in middle age (2). There is presently no treatment for SCA6 (2), making an understanding of its pathophysiology and identification of novel treatment strategies a high priority. We have used a mouse model of SCA6 to characterize disease pathophysiology. The SCA684Q/84Q model has a CAG-repeat insertion (84 repeats) in the CACNA1A gene (3). We have previously shown that SCA684Q/84Q mice develop motor coordination deficits at 7 months (4), consistent with the mid-life onset of motor dysfunction in SCA6 patients. Purkinje cell loss occurs long after disease onset in SCA684Q/84Q mice, at 2 years (4), reminiscent of Purkinje cell loss observed in postmortem tissue from SCA6 patients (2). The long latency between the onset of motor deficits and Purkinje cell loss in mice suggests that early motor coordination deficits in SCA6 patients might arise from altered cerebellar function, rather than from cell death, and thus might be reversible if cerebellar function can be restored.
Neurotrophins are molecules that promote neuronal health and survival in both the developing and the adult brain (5). A reduction in the neurotrophin brain-derived neurotrophic factor (BDNF) has been observed in postmortem SCA6 cerebellum (6), which made us wonder whether BDNF signaling deficits contributed to SCA6 pathology. A reduction in BDNF expression is observed in many neurodegenerative diseases, including Huntington’s disease (7), which, like SCA6, is caused by a triplet-repeat expansion mutation, Parkinson’s disease (8) and Alzheimer’s disease (9). In addition, reduced BDNF levels have been reported in several mouse models of ataxia, including SCA1 (10, 11), Stargazer (12), Lurcher (13), and Purkinje cell degeneration (13). BDNF signals via two receptors: the high-affinity tropomyosin receptor kinase B (TrkB) receptor, which has been implicated as the disrupted signaling pathway in other disease models (14), and the low-affinity p75 receptor.
To explore whether BDNF signaling deficits contribute to SCA6 pathophysiology, we measured expression levels of both BDNF and its high-affinity receptor, TrkB, in SCA684Q/84Q mice. We found that both BDNF and TrkB receptors were reduced in the cerebellum at the age of onset of motor coordination deficits. Exercise has been shown to elevate BDNF levels in other brain regions in healthy animals (15–18) and in mouse models of Alzheimer’s disease (14, 19, 20). We found that chronic voluntary exercise partially restored cerebellar BDNF levels and rescued motor coordination deficits. Purkinje cell firing deficits, which we have previously reported in SCA684Q/84Q mice (21), were also reversed, suggesting that Purkinje cell firing deficits can be used as a readout of cerebellar function in ataxia (22). To test whether cerebellar BDNF signaling mediated the effects of exercise on ataxia in SCA6, we used a TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF), to mimic BDNF signaling. We found that 7,8-DHF recapitulated the therapeutic effects of exercise in SCA684Q/84Q mice and that this improvement in cerebellar function was associated with the reversal of Purkinje cell firing rate deficits and elevation of the Akt signaling pathway downstream of the TrkB receptor. Furthermore, since the TrkB agonist we used is orally bioavailable and is able to cross the blood-brain barrier, its use represents a promising novel therapeutic strategy for SCA6 and other forms of ataxia associated with reduced cerebellar BDNF.
RESULTS
SCA684Q/84Q mice display deficits in cerebellar BDNF-TrkB signaling
Deficits in BDNF signaling have been characterized in multiple neurological diseases (7–11, 14, 19). Reduced BDNF levels have been observed in tissue from postmortem human SCA6 cerebellum (6), representing an advanced stage of disease progression. We wondered whether BDNF is altered at earlier disease stages and if altered BDNF levels contribute to disease pathology. To address this question, we used a mouse model of SCA6 with a hyper-expanded triplet repeat, SCA684Q/84Q mice (3), which allow us to study early disease stages at the time of onset of motor deficits.
We performed an enzyme-linked immunosorbent assay (ELISA) assay to detect BDNF protein in cerebellar vermis from litter-matched male and female wild-type (WT) and SCA684Q/84Q mice at various ages. We tested at several ages, including at 7 months, an age at which we have previously observed the onset of motor deficits (4), at 5 to 6 months, before the onset of detectable motor deficits (4), and at 12 months, an advanced disease stage (Fig. 1A). BDNF levels were indistinguishable between WT and SCA684Q/84Q mice before disease onset (Fig. 1B) with ELISA, consistent with the lack of an overt phenotype at this age. However, at both 7 and 12 months, SCA684Q/84Q mice showed a significant reduction in the levels of BDNF protein in the cerebellar vermis (Fig. 1, C and D). Thus, BDNF levels are reduced in the cerebellar vermis of SCA684Q/84Q mice at and after the onset of motor and cellular deficits.
Fig. 1. SCA684Q/84Q mice have reduced cerebellar BDNF and TrkB at disease onset.
(A) Schematic of sample collection for BDNF ELISA. (B) At 5 to 6 months, the level of BDNF (pg/mg of soluble protein) in the cerebellar vermis of SCA684Q/84Q mice is not significantly different from WT. However, at 7 months (C) and 12 months (D), there is a significant reduction in BDNF levels detected with an ELISA in cerebellar vermis tissue in SCA684Q/84Q mice compared to WT (BDNF pg/mg soluble protein). (E) Representative images show that BDNF is expressed in the cerebellar cortex at 5 to 6 months but is decreased in SCA684Q/84Q mice. Scale bar, 50 μm. (F) Quantification shows that BDNF levels are reduced in the Purkinje cell somata, the molecular layer, and the granule cell layer in SCA684Q/84Q mice. (G) Representative images show that BDNF is expressed in the cerebellar cortex at 7 months, but expression is decreased in SCA684Q/84Q mice. Scale bar, 50 μm. (H) Quantification showing that BDNF levels are reduced in all the three cortical layers: Purkinje cell layer, the molecular layer, and the granule cell layer. (I) Representative images show that TrkB is expressed in the cerebellar cortex at 7 months, but expression is decreased in SCA684Q/84Q mice. Scale bar, 50 μm. (J) Quantification of TrkB expression in the three cortical layers. TrkB is reduced in the Purkinje cell layer but not in the molecular layer or the granule cell layer. All statistical tests and P values are reported in table S2. ***P < 0.005; **P < 0.01; *P < 0.05; not significant (n.s.), P > 0.05. All data points are normalized to the median WT value of the group.
To determine whether BDNF was altered in specific cell types within the cerebellum, we performed immunohistochemistry on sections of cerebellar vermis from litter-matched WT and SCA684Q/84Q mice at 5 to 6 months (pre-onset) and 7 months (disease onset). BDNF immunoreactivity was observed in all layers of the cerebellar cortex (Fig. 1, E and G). To determine whether BDNF was expressed in neurons or glia, we costained for calbindin and glial fibrillary acidic protein (GFAP) to label Purkinje cells and Bergmann glia, respectively (fig. S1). We observed a high degree of colocalization between BDNF and calbindin, but little between BDNF and GFAP, indicating that the BDNF signal in the molecular layer reflects localization predominantly in Purkinje cell dendrites (Fig. 1, E and G, and fig. S1). We compared BDNF levels in SCA684Q/84Q and WT mice at both pre-onset (Fig. 1, E and F) and disease onset stages (Fig. 1, G and H) and found that BDNF immunoreactivity was significantly reduced in cerebellar slices from SCA684Q/84Q mice in all three cortical layers at both pre-onset (Fig. 1F) and disease onset stages (Fig. 1H). The discrepancy between findings from immunohistochemistry (Fig. 1F) and ELISA at pre-onset age (Fig. 1B) may arise because ELISA involved homogenization of the whole vermis and the Purkinje cells, which are particularly affected in SCA6, make up only a small portion of the cells in the vermis. The more numerous granule cells also express BDNF and show deficits in SCA6, but the deficit is more pronounced at 7 months (Fig. 1H) compared to pre-onset age (Fig. 1F). This could explain why the reduction in BDNF is only detectable by ELISA after onset, once the deficit is more pronounced in this more abundant cell type. These findings suggest that immunohistochemistry is a more sensitive assay than ELISA for detecting small changes in BDNF expression in the cerebellar vermis.
BDNF is known to be highly expressed in the hippocampus, and BDNF deficits are observed in the hippocampus in both Alzheimer’s disease and Down syndrome (14, 19, 20), so we wondered whether hippocampal BDNF levels were altered in SCA6. We compared BDNF intensity in hippocampal sections from SCA684Q/84Q and WT mice and observed no significant differences (fig. S1). This suggests that BDNF alterations in SCA684Q/84Q mice occur predominantly in the cerebellum, the primary locus of degeneration in SCA6 (23).
We investigated the localization and expression levels of the BDNF receptor TrkB by staining cerebellar vermis sections for TrkB, calbindin, and GFAP. Similar to BDNF expression, we observed TrkB immunoreactivity in all cerebellar cortical layers (Fig. 1I). TrkB expression in the Purkinje cell and molecular layer colocalized with calbindin but not GFAP (Fig. 1I and fig. S1), suggesting that TrkB is expressed in Purkinje cell dendrites and somata. We observed the strongest signal in Purkinje cell bodies, with relatively weak signal in the dendrites. We found that TrkB staining was reduced in Purkinje cell somata in SCA684Q/84Q mice compared to WT controls but was not significantly altered in the molecular layer or granule cell layer at disease onset (Fig. 1J). However, since signal was relatively low, we may not be able to detect small changes in TrkB expression in these layers. Thus, we found that both BDNF and its receptor TrkB are reduced in the cerebellar vermis of SCA684Q/84Q mice at disease onset, indicating that a deficit in BDNF signaling is observed in the early stages of disease progression in SCA684Q/84Q mice.
Exercise elevates BDNF in the SCA684Q/84Q mouse cerebellum
Physical exercise can up-regulate BDNF expression in several brain regions (15–18), including in other neurodegenerative disorders in which BDNF levels are reduced (14, 19, 20). We wondered whether exercise could increase BDNF levels in the cerebellum of SCA684Q/84Q mice. To address this, randomly chosen WT and SCA684Q/84Q mice were provided with running wheels (or locked wheels as a sedentary control) for 1 month beginning at age 6 months, before the onset of motor deficits has been reported (Fig. 2A) (4). Both WT and pre-onset SCA684Q/84Q mice engaged in running behavior, although we found that SCA684Q/84Q mice ran shorter distances and with lower intensity. We found no significant differences in the number of active hours each day between WT and SCA684Q/84Q mice (fig. S2).
Fig. 2. Exercise increases cerebellar BDNF levels in SCA684Q/84Q mice.
(A) Schematic of exercise protocol. IHC, immunohistochemistry. (B) BDNF immunoreactivity in sedentary (left) and exercise (right) WT (top) and SCA684Q/84Q (SCA6, bottom) mice at 7 months. Scale bars, 50 μm. (C to E) Cerebellar BDNF expression in cortical layers: (C) Purkinje cell somata, (D) the molecular layer, and (E) the granule cell layer. (F) Cerebellar TrkB expression in sedentary (left) and exercised (right) WT (top) and SCA684Q/84Q (bottom) mice at 7 months. Scale bars, 100 μm. (G to I) Quantification of changes in TrkB expression. (G) Purkinje cell, (H) molecular layer, and (I) the granule cell layer. All statistical tests and P values are reported in table S2. *P < 0.05, **P < 0.01, ***P < 0.005, P > 0.05 when comparison is not shown. All data points are normalized to the median WT value of the group.
To determine whether cerebellar BDNF levels were altered by exercise, we measured BDNF staining intensity in cerebellar vermis of WT and SCA684Q/84Q mice that were either sedentary or exercised (Fig. 2B). We found that exercise elevated BDNF levels in SCA684Q/84Q mice in Purkinje cell somata (Fig. 2C), but not in the molecular and granule cell layers (Fig. 2, D and E). Exercise did not alter BDNF levels in the cerebellum of WT mice (Fig. 2, C to E), which is consistent with previous reports (24–26), and argues that chronic exercise increases cerebellar BDNF expression only when it is pathologically reduced and that levels are likely saturated in WT mice. Thus, although SCA684Q/84Q mice run less than WT mice when given free access to a running wheel, they exercise at sufficient levels to alter cerebellar BDNF levels.
Since expression of the BDNF receptor TrkB is also reduced in SCA684Q/84Q mice (Fig. 1J), and because TrkB expression can itself be influenced by its ligand BDNF (27), we wondered whether its expression was also modulated by exercise. We found that TrkB levels were not significantly different in exercised or sedentary SCA684Q/84Q mice (Fig. 2, G to I), although we saw a modest reduction in TrkB receptor expression in Purkinje cell soma in WT mice (Fig. 2G; P = 0.016). This suggests that in SCA684Q/84Q mice, exercise enhances cerebellar BDNF expression without affecting expression of its high-affinity TrkB receptor.
Exercise alleviates motor coordination and Purkinje cell firing deficits in SCA684Q/84Q mice
Exercise has been shown to rescue deficits in an SCA1 mouse model (28, 29) and in ataxic Snf2h-null mice (30), although BDNF has not been identified as the mechanism underlying its therapeutic action in these models. We wondered whether exercise, perhaps via elevation of cerebellar BDNF levels, would rescue motor behavioral deficits in SCA684Q/84Q mice. To test this, we used an accelerating rotarod to assay motor coordination (Fig. 3A). We found that motor coordination in SCA684Q/84Q mice significantly improved after 1 month of exercise compared to sedentary SCA684Q/84Q mice (Fig. 3B), although not to WT levels (Fig. 3B). Consistent with the lack of change in BDNF expression levels, WT mice showed no significant changes with exercise compared to sedentary controls (Fig. 3B). This shows that, in addition to restoring cerebellar BDNF levels, exercise reduces ataxia in SCA684Q/84Q mice.
Fig. 3. Exercise rescues motor coordination and Purkinje cell firing deficits in SCA684Q/84Q mice.
(A) Schematic of exercise protocol. (B) Motor coordination is assessed with an accelerating rotarod assay after 1 month of voluntary wheel running by measuring the latency to fall for 5 days of testing. Latency to fall is significantly different between WT and SCA684Q/84Q mice, SCA684Q/84Q and SCA684Q/84Q exercise mice, and SCA684Q/84Q exercise and WT mice. (C) Sample juxtacellular Purkinje cell recordings from WT (left) and SCA684Q/84Q (right) sedentary (top) or exercise (bottom) mouse cerebellar acute slices. (D) Purkinje cell firing frequency (left) is restored in SCA684Q/84Q exercise mice. However, action potential regularity (as measured by the CV of interspike intervals) is not restored by exercise. All statistical tests and P values are reported in table S2. *P < 0.05, **P < 0.01, ***P < 0.005, P > 0.05 when not shown.
We next wondered whether other cellular deficits in SCA684Q/84Q mice were altered by exercise. We have previously shown that spontaneous Purkinje cell firing frequency and regularity are reduced at 7 months in SCA684Q/84Q mice (21). Similar deficits in intrinsic Purkinje cell firing have been observed in several ataxia models (10, 21, 31–33) [summarized in (22)], suggesting that such firing deficits may be a readout of cerebellar dysfunction in ataxia. Using juxtacellular recordings of spontaneous Purkinje cell firing in acute sagittal slices, we found that exercise rescued Purkinje cell firing frequency deficits in SCA684Q/84Q mice to levels indistinguishable from WT mice (Fig. 3, C and D). However, firing regularity, quantified by the coefficient of variation (CV) of interspike intervals, was unaltered and remained significantly higher in exercise SCA684Q/84Q mice compared to WT controls (Fig. 3D). Exercise did not significantly alter firing properties of Purkinje cells in WT mice (Fig. 3D), consistent with the finding that BDNF levels and motor coordination are unaltered by exercise in WT mice. Thus, exercise restores Purkinje cell firing rate and motor coordination deficits, perhaps by restoring cerebellar BDNF levels.
The TrkB agonist 7,8-DHF improves motor coordination and Purkinje cell firing rate deficits in SCA684Q/84Q mice
If the behavioral rescue we observed in pre-onset mice with exercise was due to enhanced BDNF expression, we wondered whether targeting BDNF-TrkB signaling alone would be sufficient to rescue motor coordination. To test this, we administered a small-molecule TrkB agonist, 7,8-DHF (34, 35), in drinking water (Fig. 4, A and B). 7,8-DHF has been shown to rapidly cross the blood-brain barrier in mice (36), and chronic oral administration has been shown to activate TrkB and downstream signaling (37, 38) and rescue deficits in multiple mouse models of disease (14, 38–40).
Fig. 4. The TrkB agonist 7,8-DHF restores ataxia and Purkinje cell firing deficits in SCA684Q/84Q mice at disease onset.
(A) Proposed model of altered BDNF-TrkB signaling in SCA684Q/84Q mice and its subsequent restoration with 7,8-DHF, a TrkB agonist. (B) Schematic of 7,8-DHF administration protocol. (C) 7,8-DHF improves motor coordination in SCA684Q/84Q mice as measured with rotarod assay. (D) Example traces from juxtacellular recordings of Purkinje cell firing at 7 months after 1 month of 7,8-DHF administration. (E) Purkinje cell firing frequency is elevated after 7,8-DHF administration. However, Purkinje cell firing regularity is unchanged with 7,8-DHF administration (CV). All statistical tests and P values are reported in table S2. ***P < 0.005; n.s., P > 0.05.
SCA684Q/84Q mice were given access to drinking water with sucrose and either 7,8-DHF or dimethyl sulfoxide (DMSO) vehicle alone as a control at 6 months before onset of motor deficits (Fig. 4B), after which we tested motor coordination using the rotarod assay. We found that after 1 month, 7,8-DHF administration alleviates motor coordination deficits in SCA684Q/84Q mice compared to vehicle controls (Fig. 4C). Furthermore, we observed a weak positive correlation between the amount of 7,8-DHF consumed daily and rotarod performance (fig. S3), supporting the hypothesis that 7,8-DHF intake influences motor coordination. If 7,8-DHF acts in the cerebellum of SCA684Q/84Q mice to improve motor coordination, we would predict that it would restore Purkinje cell firing abnormalities (22). To test this, we carried out juxtacellular recordings of intrinsic Purkinje cell action potentials and found that, like the effects of exercise, 7,8-DHF elevated Purkinje cell firing frequency but did not rescue firing regularity deficits in SCA684Q/84Q mice (Fig. 4, D and E). Thus, chronic 7,8-DHF treatment recapitulated the therapeutic effect of exercise on motor coordination and reversed Purkinje cell firing rate deficits that have been previously associated with impaired motor coordination (21, 22). These findings support our hypothesis that exercise and 7,8-DHF act via the same signaling pathway in SCA684Q/84Q mice.
We hypothesize that WT BDNF levels are near saturation since exercise does not influence motor coordination (Fig. 3B), BDNF levels (Fig. 2, C to E), or Purkinje cell firing properties (Fig. 3D) in these mice. We thus expected that 7,8-DHF would not affect motor coordination in WT mice. To test this, we administered 7,8-DHF to WT mice for 1 month and found that it did not significantly affect motor coordination compared to sucrose-treated WT controls (fig. S4). This reinforces our hypothesis that 7,8-DHF affects motor coordination only when cerebellar BDNF levels are reduced.
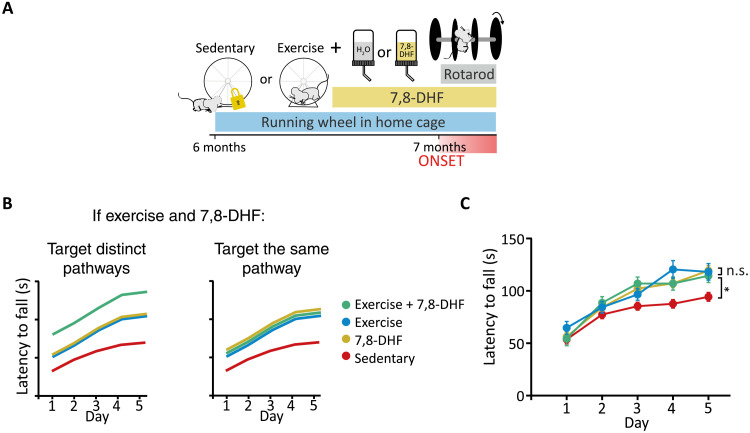
If exercise restores motor coordination in SCA684Q/84Q mice through elevation of cerebellar BDNF, we predict that the combination of exercise and 7,8-DHF treatments should not be additive, whereas if they restore motor coordination through different pathways, combining these treatments should be. To differentiate between these possibilities, we compared exercise, 7,8-DHF, and the combination of these two while testing motor coordination using rotarod (Fig. 5, A and B). We found that exercise alone, exercise + 7,8-DHF, and 7,8-DHF alone all significantly rescued motor coordination in SCA684Q/84Q mice compared to sedentary SCA684Q/84Q mice but that there was no significant difference in performance between these three conditions (Fig. 5C). Notably, SCA684Q/84Q mice administered exercise + 7,8-DHF demonstrated no additional improvement in motor coordination compared to either treatment alone (Fig. 5C). This supports the hypothesis that exercise improves motor coordination in SCA684Q/84Q mice by enhancing BDNF-TrkB signaling in the cerebellum. These data suggest that both exercise and 7,8-DHF improve motor coordination via TrkB signaling and that signaling is near saturation in both conditions.
Fig. 5. Combining exercise with administration of a TrkB agonist provides no further rescue of ataxia in SCA684Q/84Q mice.
(A) Schematic of exercise and 7,8-DHF combination therapy on SCA684Q/84Q mice (SCA6). (B) Schematics of possible outcomes if exercise and 7,8-DHF are additive, suggesting that they act via distinct pathways (left), or if no further improvement is observed in motor coordination when exercise and 7,8-DHF are co-administered, suggesting that they act on the same signaling pathway (right). (C) No additional improvement is observed when exercise and 7,8-DHF administration are combined, and exercise and 7,8-DHF improve motor coordination, similarly suggesting that they act on the same pathway. All statistical tests and P values are reported in table S2. *P < 0.05; n.s., P > 0.05.
7,8-DHF activates Akt signaling pathway
BDNF has been reported to elevate TrkB levels (27, 41), and both BDNF and the TrkB agonist 7,8-DHF have been reported to induce TrkB phosphorylation and activation of downstream signaling pathways (Fig. 6A) (34, 35, 41, 42). To determine whether 7,8-DHF affects TrkB signaling in SCA684Q/84Q mice, we performed immunoblotting for both TrkB and phosphorylated TrkB (pTrkB). We found that 7,8-DHF administration resulted in a significant increase in total TrkB in the cerebellar vermis and a small but not significant increase in pTrkB levels compared to untreated SCA684Q/84Q mice (Fig. 6, B and C). This increase in TrkB upon 7,8-DHF administration is not unexpected given that TrkB activation has been reported to lead to both local increases in TrkB (27, 41), and sustained up-regulation of TrkB via Akt and subsequent hypoxia-inducible factor 1α (HIF1α) activation (43–45). However, it is unclear why TrkB levels were unchanged after exercise in SCA684Q/84Q mice (Fig. 2, G to I), in contrast to the increase after 7,8-DHF administration, although this may be due to differences in the duration and strength of TrkB activation produced by exercise. TrkB receptor signaling leads to the activation of downstream signaling pathways, which have been reported to include the kinases Akt and extracellular signal–regulated kinase (ERK) (Fig. 6A) (46, 47). Both Akt (48) and ERK (49) are dysregulated in cerebellar diseases, including models of neuroinflammation and ataxia. We used a high-throughput reverse phase protein array (RPPA) to determine the levels of total and phosphorylated proteins (50) in 7,8-DHF–treated and sucrose-treated SCA684Q/84Q mice. We found that 1 month of 7,8-DHF administration resulted in no change in the total Akt protein level in 7,8-DHF–treated animals (Fig. 6E), a significant increase in the levels of Akt phosphorylated at threonine-308 (Fig. 6F), and a slight but not significant increase in the amount of Akt phosphorylated at serine-473 (Fig. 6G). This indicates that an increase in the activation of Akt is mediating the effects of 7,8-DHF. In contrast, we did not see significant changes in the ERK signaling pathway, and levels of phosphorylated RafB, ERK, and total ERK protein were all not significantly different between 7,8-DHF–treated and sucrose-treated SCA684Q/84Q mice (Fig. 6, H to K). Since Akt and ERK are parallel downstream signaling pathways, these results suggest that 7,8-DHF acts predominantly via Akt signaling in SCA684Q/84Q mice. In mouse models of traumatic brain injury (37) and intracerebral hemorrhage (51), 7,8-DHF was also shown to lead to phosphorylation of Akt but not ERK, suggesting that 7,8-DHF may preferentially activate Akt signaling pathways in some disorders, despite evidence for ERK activation in other disease models (52, 53). As the RPPA was carried out on lysates prepared from the whole vermis, we next performed immunohistochemistry to determine whether the increase in phosphorylated Akt detected with the RPPA could be localized to cerebellar cell types. We found that pAkt(T308) was detected at high levels in Purkinje cells (Fig. 6L) and that pAkt(T308) levels were elevated in Purkinje cell somas (Fig. 6M), and the molecular layer (Fig. 6M) of SCA684Q/84Q mice treated with 7,8-DHF. Since most of the pAkt(T308) signal in the molecular layer appears in Purkinje cell dendrites, this suggests that most of the downstream signaling by pAkt(T308) occurs in Purkinje cells. Consistent with this, little pAkt(T308) was detected in the granule cell layer, and no statistical differences were observed in the granule cell layer after 7,8-DHF administration (Fig. 6M). This confirms that 7,8-DHF leads to increased phosphorylation of Akt in the SCA684Q/84Q cerebellum, particularly in Purkinje cells. Thus, 7,8-DHF rescues behavioral and cellular deficits in SCA684Q/84Q mice and activates Akt signaling in the cerebellar vermis.
Fig. 6. The TrkB agonist 7,8-DHF activates the Akt signaling pathway in SCA684Q/84Q mice.
(A) Top: Schematic of 7,8-DHF administration followed by tissue collection for Western blots and RPPA. Bottom: TrkB and downstream signaling pathways. (B) Total TrkB levels are significantly increased in the cerebellar vermis of SCA684Q/84Q mice after chronic 7,8-DHF administration, while levels of phosphorylated TrkB are slightly increased by 7,8-DHF (C). (D) Schematic of putative Akt signaling downstream of TrkB activation. (E) Total Akt levels are unchanged in vermis tissue after 7,8-DHF administration in SCA684Q/84Q mice, but phosphorylated Akt levels are increased (F and G), indicating Akt activation. (H) Schematic of putative ERK signaling downstream of TrkB activation. (I to K) 7,8-DHF administration does not lead to activation of the ERK signaling pathway in SCA684Q/84Q mice. (L) Representative images showing pAkt(T308) in the Purkinje cells of the cerebellar vermis. Scale bar, 50 μm. (M) Quantification of pAkt(T308) staining showing that levels are increased in the Purkinje cells and molecular layer of SCA684Q/84Q mice that received 7,8-DHF treatment. All statistical tests and P values are reported in table S2. *P < 0.05, **P < 0.01, ***P < 0.005, P > 0.05 when not shown.
7,8-DHF can restore motor coordination at later disease stages when administration starts early
SCA6 is a progressive disorder, which means that motor coordination worsens over time in both patients and mouse models (2, 4). To determine whether targeting TrkB signaling at later ages in SCA684Q/84Q mice would improve motor coordination, we administered 7,8-DHF at 7 months, after disease onset. SCA684Q/84Q mice were assayed on rotarod for 5 days and then divided into two equally performing groups, which were given either 7,8-DHF or DMSO vehicle in drinking water (Fig. 7A). Rotarod testing continued daily to determine whether and when changes in motor coordination occurred. SCA684Q/84Q mice treated with 7,8-DHF performed significantly better than untreated controls after 14 days, and performance tended to increase for the next 2 weeks in treated mice (Fig. 7B). The relatively slow time course of 7,8-DHF action is consistent with our finding that 4-hour 7,8-DHF treatment on acute cerebellar slices was insufficient to alter Purkinje cell firing properties (fig. S5), supporting our observation of a slow time course to ameliorate motor coordination (Fig. 7B). Our findings that treatment with 7,8-DHF at the onset of ataxia still rescued motor coordination in SCA684Q/84Q mice are particularly promising since mice did not exercise at high levels at this age, and exercise was unable to rescue deficits in SCA684Q/84Q mice when started after onset, even when supplemented with bouts of forced exercise on a treadmill (fig. S6). The difference we observe in the efficacy of exercise and 7,8-DHF treatment post-onset may arise from motor impairment because of disease progression that prevents SCA684Q/84Q mice from exercising at sufficiently high levels.
Fig. 7. Chronic administration of 7,8-DHF after disease onset leads to a slow improvement of ataxia in SCA684Q/84Q mice.
(A) Schematic of 7,8-DHF administration protocol. (B) SCA684Q/84Q mice (SCA6) were administered 7,8-DHF for 25 days after 5 days of baseline testing. Improvement in motor coordination was observed after ~2 weeks. (C) Motor performance remained elevated for ~2 weeks after 7,8-DHF withdrawal. All statistical tests and P values are reported in table S2. *P < 0.05, **P < 0.01, ***P < 0.005, P > 0.05 when not shown.
Given the slow time course of 7,8-DHF administration to produce an effect on motor coordination, we wanted to determine the time course of its withdrawal as well. To test this, we withdrew 7,8-DHF after 1 month while testing motor coordination weekly. We found that 7,8-DHF–treated mice maintained elevated motor coordination for 2 weeks after 7,8-DHF was removed but that motor coordination returned to control levels by 3 weeks (Fig. 7C). Thus, 7,8-DHF treatment had an effect over a slow time course on motor coordination for both drug administration and withdrawal.
SCA6 patients often live with the progressively worsening disease for decades. We wondered whether 7,8-DHF could provide long-lasting benefits in SCA684Q/84Q mice. To address this, we continued 7,8-DHF treatment for 4 months in a subset of SCA684Q/84Q mice (Fig. 8A) and assayed motor coordination monthly (Fig. 8A). We found that SCA684Q/84Q mice chronically administered 7,8-DHF showed improved performance at 7, 9, and 10 months (Fig. 8B). This suggests that the continued administration of 7,8-DHF may have a long-lasting therapeutic benefit in SCA6 patients. To determine whether chronic 7,8-DHF administration had similar cellular effects in older mice that it did in younger mice, we recorded Purkinje cell firing rates in acute slices made after 4 months of 7,8-DHF administration and found that they were not significantly different than controls (Fig. 8C), unlike what we observed in younger mice where 7,8-DHF restored Purkinje cell firing rate deficits (Fig. 4E).
Fig. 8. Early, but not late, 7,8-DHF administration rescues deficits at later stages of disease progression in SCA684Q/84Q mice.
(A) Schematic of long-term chronic 7,8-DHF administration in SCA684Q/84Q mice started near disease onset. (B) Mice treated chronically with 7,8-DHF performed better at 7, 9, and 10 months old. (C) Purkinje cell firing rate (left) was no longer restored after 4 months of drug administration, nor was regularity affected. (D) Schematic of 7,8-DHF administration started in late disease progression. (E) Late-administered 7,8-DHF does not restore firing deficits at 10 months when treatment starts after disease onset at 9 months. (F) Purkinje cell firing rate and regularity (CV) are not significantly different after late administration of 7,8-DHF. All statistical tests and P values are reported in table S2. *P < 0.05; ***P < 0.001; n.s., P > 0.05.
Our observation that treatment with the TrkB agonist 7,8-DHF provides long-term therapeutic benefits in the SCA684Q/84Q mouse model (Fig. 8C) is promising for SCA6 patients. However, SCA6 is often diagnosed after disease onset, meaning that early treatment may not always be practical. We thus wondered whether 7,8-DHF would ameliorate ataxia in SCA684Q/84Q mice when drug administration was initiated at a later stage of disease progression. To determine whether this was likely to succeed, we first determined that TrkB receptors were still expressed in 9-month SCA684Q/84Q cerebellum (fig. S7). We then administered 7,8-DHF for 1 month to SCA684Q/84Q mice aged 9 months (Fig. 8D). We observed no significant improvement in motor coordination in late-treated 7,8-DHF mice (Fig. 8E) and similarly observed no changes in Purkinje cell firing properties (Fig. 8F). These data suggest that the lack of a therapeutic effect of late-administered 7,8-DHF is unlikely to arise because of TrkB receptors but rather from changes in downstream signaling mechanisms. Together, our results demonstrate that 7,8-DHF is a promising therapeutic with the potential for long-lasting benefits for SCA6-associated ataxia when administration is started around disease onset but not at later stages.
DISCUSSION
We identified BDNF-TrkB signaling disruptions as a contributor to pathophysiology at early disease stages in SCA684Q/84Q mice. Changes in the level of BDNF in the cerebellar vermis were detectable before onset using immunohistochemistry. Although BDNF-TrkB signaling alterations have been observed in postmortem SCA6 cerebellum (6), it was unclear whether this alteration contributed to early pathophysiology or not. Here, we showed that BDNF did contribute to pathophysiology and successfully targeted this pathway to improve disease phenotype using two approaches. First, we showed that cerebellar BDNF could be rescued by a chronic exercise program, which also rescued deficits in motor coordination and Purkinje cell firing. While exercise has been shown to be beneficial in ataxia (54–57), this is the first study linking therapeutic exercise in ataxia to cerebellar BDNF levels. We then used 7,8-DHF, a TrkB agonist, to rescue deficits in the SCA684Q/84Q mouse. In addition to rescuing behavioral and cellular deficits, 7,8-DHF increased expression of TrkB receptors and phosphorylation of Akt, a signaling pathway downstream of TrkB. Phosphorylated Akt is largely expressed in cerebellar Purkinje cells, suggesting that much of the therapeutic action of 7,8-DHF is localized to these cells. Combining these two therapies provided no additional rescue of motor coordination deficits, suggesting that they act via similar signaling mechanisms in the cerebellum. 7,8-DHF treatment improved motor coordination for months if treatment was initiated around the onset of ataxia but was insufficient if treatment was started later in the disease progression. Our work represents a promising novel therapeutic strategy for SCA6 and sheds light on the mechanisms underlying the therapeutic properties of exercise for SCA6.
BDNF levels were reduced in both the soma and dendrites of Purkinje cells, which are known to function abnormally at disease onset in SCA684Q/84Q mice (21). However, we also observed changes in BDNF in the granule cell layer, which is intriguing because granule cell dysfunction has not previously been described in SCA684Q/84Q mice. Multiple studies have described modest degeneration of granule cells in postmortem tissue from SCA6 patients (58, 59), and while CACNA1A is predominantly expressed in the Purkinje cells, it is also expressed at lower levels in granule cells (60). In addition, it is known that BDNF can be synthesized in one cell type and exert effects on other cell types, such as retrograde BDNF signaling from Purkinje cells to the presynaptic climbing fiber terminals (61), meaning that the actions of BDNF may not be limited to the cell types in which we observe that its expression is reduced. In the future, it will be interesting to determine whether granule cell dysfunction also contributes to disease in SCA6.
Many studies using animal models of human diseases focus on early, peri-onset disease stages [e.g., (21)]. Yet, for progressive, neurodegenerative diseases like SCA6, patients often live with symptoms for years or decades, and diagnosis is sometimes made long after disease onset. For this reason, we chose to focus on multiple stages of disease progression: pre-onset, early progression, and late progression. We found that in our mouse model, both exercise and TrkB agonist treatment mitigated the development of motor coordination and Purkinje cell firing deficits when administered pre-onset. Exercise did not lead to further improvement of motor coordination than treatment with 7,8-DHF alone, suggesting that exercise and 7,8-DHF act via similar pathways. We showed that this was likely via the Akt signaling pathway downstream of the TrkB receptor, although since other signaling pathways also activate Akt (62), it is possible that other signaling molecules and pathways may also contribute to the effects of exercise and/or 7,8-DHF. Later, as disease progressed, exercise ceased to rescue motor function, likely because the mice did not run far enough. Nonetheless, treatment with 7,8-DHF was still able to reverse deficits, demonstrating that restoring TrkB signaling was still possible at early stages of disease progression. However, the deficits in Purkinje cell firing that 7,8-DHF rescued at an earlier disease stage were no longer restored by 7,8-DHF at later stages, perhaps because of other factors that may contribute to Purkinje cell firing alterations as disease progresses and SCA684Q/84Q mice age. Our findings argue that different windows of opportunity may exist for different interventions, although exercise in humans after disease onset may be more successful than it was in our mouse model due to differences in motivation between people and mice. Notably, at a more advanced stage later in disease progression, 7,8-DHF treatment was only able to restore motor deficits when administration began peri-onset and continued for 4 months, but not when administration began later. With increased prevalence of predictive genetic testing in neurodegenerative diseases like SCA6 (63), an awareness of the importance of early treatment may be of benefit to patients, especially given that we observed changes in BDNF before motor coordination deficits have been reported (4).
Exercise is an intervention that is accessible to many patients and has been successfully used to improve outcomes for ataxia; for example, exercise leads to some alleviation of motor symptoms and prevention of cerebellar decline in patients with various types of ataxia, including SCA6 (54–57), although until now there was no evidence that this therapeutic action was mediated by BDNF. Exercise has also proven therapeutic in other animal models of ataxia (28–30), although the mechanism of rescue described in those models appears at least partially different from the mechanism we describe here, perhaps because of differences in the duration and intensity of exercise animals undergo. It is also likely that the difference in the molecular mechanism of therapeutic exercise in different ataxias arises because of differences in the molecular pathophysiology of different disorders. While it is unclear what the equivalent of 30 days of voluntary wheel running by a mouse would be for an SCA6 patient, we know that there are exercise protocols, such as high-intensity interval training, that target BDNF-TrkB signaling in people (64, 65). Thus, although our finding that exercise ameliorates ataxia may be generalizable, it will be nonetheless important to determine how exercise acts in each form of ataxia to determine whether a strategy targeting BDNF signaling will be beneficial.
Exercise has been shown to up-regulate BDNF expression in the hippocampus (66), where even short bouts of exercise lead to up-regulation of BDNF, although a number of different mechanisms may be involved (19, 67–69). In the cerebellum, however, the effects of exercise on BDNF have been less clear. Some studies in rodents have reported no change in BDNF expression in the cerebellum with exercise (24–26), while others report that exercise increases levels of cerebellar BDNF (15, 70). These discrepancies could arise because of differences in the duration and type of exercise in each protocol. We found that although 4 weeks of exercise did not alter cerebellar BDNF protein levels in WT mice, it did increase BDNF protein levels in the SCA684Q/84Q cerebellum. This differential effect on WT and disease model mice is not unprecedented, and other studies similarly report that exercise acts differently in different models and circumstances (71, 72). We surmise that the chronic exercise protocol that we used increased BDNF levels only when they were already pathologically reduced in the SCA684Q/84Q mice.
Reduced BDNF expression is a hallmark of many neurological diseases. BDNF is known to be up-regulated by neuronal activity, and so the reduction in SCA6 and other ataxias could be a secondary effect caused by the decreased firing frequency of Purkinje cells that has been reported for SCA6 (21) and many other ataxic mouse models (22). However, other mechanisms leading to reduced BDNF in disease cannot be excluded, and BDNF disruption may arise from a variety of insults in different diseases, including transcriptional and posttranscriptional mechanisms [e.g., see (5)]. It will be interesting to determine by which mechanism(s) exercise influences BDNF in the SCA684Q/84Q cerebellum, as this may provide other possible therapeutic avenues for SCA6 treatment.
The restoration of BDNF-TrkB signaling we describe here for SCA6 is a promising potential therapeutic approach to multiple ataxias. Our results are consistent with previous findings that viral reintroduction of the BDNF gene rescued ataxia in Stargazer mice (12) and acute cerebellar delivery of BDNF to SCA1 ATXN1(82Q) mouse model had a similar therapeutic effect (11, 73). However, overexpression of BDNF has been associated with negative outcomes in some cases (74–76), likely due to the complex signaling pathways that are known to be activated by BDNF. Thus, instead of virally reintroducing BDNF in the SCA684Q/84Q mouse, we chose a different approach to administering a putative BDNF mimetic that acts as a TrkB agonist, as this signals exclusively through the neuroprotective TrkB pathway and may be more translatable to the clinic (5). Although 7,8-DHF can be detected within minutes of systemic delivery in mice (36), its ability to restore motor coordination is incremental and requires a few weeks to be detected. This suggests that the effects of 7,8-DHF may not be entirely related to its acute effects on TrkB signaling but may involve changes in network activation or other slower processes in the brain.
In summary, we have found that both BDNF and its high-affinity TrkB receptor are down-regulated at the age of onset in a mouse model of SCA6. We found that exercise elevated BDNF in the cerebellum of SCA6 mice, and that this was associated with an improvement of motor coordination deficits and cellular firing deficits in cerebellar Purkinje cells. We then used 7,8-DHF to activate TrkB receptors directly and found that this restored motor coordination and Purkinje cell firing deficits in a manner similar to exercise. When administered together, exercise and 7,8-DHF were not additive, suggesting that they were acting via similar pathways, likely involving Akt signaling in cerebellar Purkinje cells. Chronic administration of the BDNF mimetic that was initiated around the age of disease onset led to a sustained improvement in motor coordination, but this was not true when administration started later during disease progression. Together, our data suggest that exercise modulates ataxia in SCA6 through the restoration of BDNF-TrkB signaling in the cerebellum and that a TrkB agonist can mimic this therapeutically. Given that the TrkB agonist we studied, 7,8-DHF, has rescued behavior in mouse models of several other neurodegenerative diseases (14, 38–40), we believe that 7,8-DHF or a related molecule (53) is a highly promising therapeutic approach to treating both SCA6 and other neurodegenerative diseases.
MATERIALS AND METHODS
Experimental design
The objective of this study was to explore the role of BDNF-TrkB signaling at disease onset and during disease progression using a mouse model of SCA6. Mice were compared to litter-matched WT mice as controls, or in some cases, SCA6 mice with treatment were compared to those without. Outliers were not removed. Experimental design had to be altered during the pandemic because of McGill University regulations. All immunohistochemistry was done with controlled studies with at least three or up to six replicates. Representative data are included in figure image panels.
Animals
We used a knock-in mouse model of SCA6 with a humanized 84-CAG-repeat expansion mutation at the CACNA1A locus (SCA684Q/84Q). Mice that were heterozygous at this locus were obtained from The Jackson Laboratory (Bar Harbor, ME) (strain B6.129S7-Cacna1atm3Hzo/J, stock number 008683) and were bred to obtain SCA684Q/84Q and litter-matched WT control mice. Generally, animal husbandry was carried out by moving a heterozygous male between cages of two heterozygous sister mice each week. Genotyping was performed at weaning using primer sequences published by The Jackson Laboratory. Bedding materials were provided in all cages, but no additional environmental enrichment was added except in cages described as having running wheels. Male and female mice were used in all experiments, and no animals were excluded from analysis. Experimental groups were determined so that they were litter- and sex-matched. Once that was achieved, mice were randomly assigned to groups by an experimenter who had not previously worked with the mice and who could not see the mice, only their ID numbers. All animal procedures were carried out with the approval of the McGill Animal Care Committee in accordance with the Canadian Council on Animal Care guidelines.
Immunohistochemistry
Tissue fixation was carried out as previously described (31). Mice were deeply anesthetized with intraperitoneal injection of 2,2,2-tribromoethanol (Avertin), and an intracardiac perfusion was performed with a flush of ice-cold phosphate-buffered saline (PBS; 0.1 M, pH 7.4) with heparin salt (5.6 μg/ml), followed by 40 ml of 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4). Extracted tissue was stored in 4% PFA at 4°C for a further 24 hours of fixation before being transferred to PBS with 0.5% sodium azide for storage at 4°C. The cerebellar vermis was dissected and sliced into 100-μm sagittal slices with a Vibratome 3000 sectioning system (Concord, ON, Canada). Immunohistochemistry on free-floating slices was carried out with antibodies for proteins of interest along with anti-calbindin, anti-parvalbumin, or anti-GFAP as markers for Purkinje cells, Purkinje cells and molecular layer interneurons, and Bergmann glia, respectively. For immunohistochemistry involving anti-mouse secondary antibodies, additional 30-min incubation with Fab fragments was carried out before incubation with the secondary antibodies. Antibodies are listed in table S1. The use of anti-BDNF included an additional heat-induced epitope retrieval step comprising 10 min of heating to 95°C in PBS, after which slices were allowed to cool before proceeding with the primary antibody staining. After staining, slices were immediately mounted in ProLong Gold Antifade mounting medium (Thermo Fisher Scientific, Waltham, USA) and stored in the dark at 4°C.
Image acquisition and analysis
Imaging was performed using an LSM800 confocal microscope (Zeiss) and using Zeiss Zen software for image acquisition. The raw fluorescence intensity was recorded in arbitrary units and was normalized to the mean or median WT value within a batch, depending on whether the data were normally distributed. Because of limitations of litter size, immunohistochemistry was typically carried out in several batches. To minimize batch-specific effects on the data, all data are shown normalized to the mean WT value of that batch (defining WT intensity = 1). Image analysis was performed in FIJI (ImageJ, U.S. National Institutes of Health) (77, 78). All image analysis was carried out by an experimenter who was blind to the genotype and treatment group of the animal. Raw images of lobule 3 of cerebellar vermis were used, and manual tracing around Purkinje cell bodies in the calbindin channel allowed the delineation of regions of interest (ROIs) around the Purkinje cell bodies. Missing or out of the plane of focus cells were skipped. These ROIs were then exported to the protein of interest channel, and fluorescence intensity was measured (integrated density, arbitrary units). For measurements of fluorescence intensity in the molecular layer and granule cell layer, we used four ROIs of consistent size (5193 μm2) to determine the total intensity at randomly chosen locations in the molecular and granule cell layers (fig. S8).
For representative images in figure panels only, linear adjustments of the brightness and contrast were applied to improve the readability of the figures. The same adjustments were applied to images from all conditions, and this occurred after image analysis.
Voluntary running
Mice were individually housed in low-profile rat cages with running wheels (Columbus Instruments, OH, USA). Sedentary control mice received wheels that were locked in position to control for environmental enrichment provided by the presence of the wheel and the larger cages. Each cage was also furnished with bedding materials. The number of wheel rotations was recorded hourly using Multi-Device software (Columbus Instruments, OH, USA).
Treadmill exercise
Mice were moved to the behavior room for acclimatization 1 hour before the start of exercise. Mice were placed individually on an enclosed single-lane treadmill (Treadmill Simplex II, Columbus Instruments). The treadmill speed was set to 6 m/min for the first 5 min, increased to 10 m/min for 5 min, and then increased in 1 m/min increments for each subsequent 5-min period of the 20-min exercise period. The treadmill was equipped with a shock plate, which distributed 2-mA shocks twice per minute when mice stepped off the treadmill. If a mouse remained on the shock plate for more than 30 s, the treadmill was stopped for an additional 30 s, allowing the mouse to return to the treadmill. Mice in the control condition were placed on the stationary treadmill and allowed to explore it for 20 s with the shock platform on, encouraging the mice to remain on the treadmill. This exercise protocol was repeated daily for 6 weeks.
Accelerating rotarod
We used an accelerating protocol on a rotarod (Stoelting, IITC) as previously described (4, 21, 31). We have previously shown that this assay is able to detect differences in motor coordination in WT and SCA684Q/84Q mice in both control and treatment conditions (4, 21). Mice were moved to the testing room and left to acclimatize for 1 hour before testing. Mice underwent four trials per day with a 10- to 15-min rest period between trials. Testing was carried out at the same time each day for five consecutive days or, in some cases, for up to 30 days. The protocol started with a speed of 4 rpm and then accelerated to 40 rpm over 5 min, after which the assay continued at 40 rpm for up to five further minutes. Latency to fall off the rotarod was recorded in each trial. All behavioral tests were carried out by individuals who were blind to the genotype of the animals, and the placement order of mice on the rotarod was rotated to minimize the effects of confounders such as position on the apparatus.
Acute slice preparation
Slice preparation was carried out as previously described (21). Mice were deeply anesthetized with intraperitoneal injection of 2,2,2-tribromoethanol (Avertin), and an intracardiac perfusion was performed with ice-cold partial sucrose replacement slicing solution (111 mM sucrose, 50 mM NaCl, 2.5 mM KCl, 0.65 mM CaCl2, 10 mM MgCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 25 mM glucose, bubbled with 95% O2 and 5% CO2 to maintain pH at 7.3; osmolality ~320 mOsm). The cerebellar vermis was dissected and sliced into 200-μm sagittal slices with a VT 1200S vibratome (Leica Microsystems, Wetzlar, Germany). Slices were incubated in artificial cerebrospinal fluid (ACSF: 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 20 mM glucose, bubbled with 95% O2 and 5% CO2 to maintain pH at 7.3; osmolality ~320 mOsm) bubbled with carbogen at 37°C for 45 min and then transferred to room temperature for the duration of experiments. All reagents were obtained from Sigma-Aldrich (Oakville, ON, Canada), apart from MgCl2 and CaCl2, which were obtained from Fisher Scientific (Toronto, ON, Canada).
Electrophysiology
Recordings of spontaneous Purkinje cell firing in lobule 3 of cerebellar vermis were carried out as previously described (21, 31). Slices were kept in a bath of ACSF at approximately 33°C during recording. Purkinje cells were visually identified, and juxtacellular recordings were made at the soma with borosilicate pipettes pulled using a P-1000 puller with tip sizes of 1 to 2 μm (Sutter Instruments, Novato, CA, USA). Data acquisition and analysis were performed in IGOR Pro version 6.0 and 7.0 (Wavemetrics, Portland, OR, USA) using custom routines.
Reverse phase protein array
Mice were anesthetized with isoflurane, and once mice were no longer responsive to a toe pinch, they were decapitated, and the brain was removed and placed into ice-cold ACSF prepared as described above. The vermis was quickly dissected, dried, and weighed before snap freezing on dry ice. Tissue samples were stored at −80°C until sample preparation.
RPPA assays were carried out as described previously (79, 80). Specifically, protein lysates were prepared from dissected cerebellar vermis samples with modified Tissue Protein Extraction Reagent (TPER) (Life Technologies Corporation, Carlsbad, CA) and a cocktail of protease and phosphatase inhibitors (Roche, Pleasanton, CA) (80). Lysates were diluted to 0.5 mg/ml in SDS sample buffer and denatured on the same day. The Quanterix 2470 Arrayer (Quanterix, Billerica, MA) with a 40-pin (185-μm) configuration was used to spot samples onto nitrocellulose-coated slides (Grace Bio-labs, Bend, OR) using an array format of 960 lysates per slide (2880 spots per slide). The slides were processed as described and probed with a set of 233 antibodies against total proteins and phosphorylated proteins using an automated slide stainer (Autolink 48, Dako, Santa Clara, CA). Each slide was incubated with one specific primary antibody, and a negative control slide was incubated with antibody diluent without any primary antibody. Primary antibody binding was detected using a biotinylated secondary antibody followed by streptavidin-conjugated IRDye680 fluorophore (LI-COR Biosciences, Lincoln, NE). Total protein content of each spotted lysate was assessed by fluorescent staining with Sypro Ruby Protein Blot Stain according to the manufacturer’s instructions (Molecular Probes, Eugene, OR).
Fluorescence-labeled slides were scanned on a GenePix 4400 AL scanner, along with negative control slides, at an appropriate photomultiplier tube to obtain optimal signal for this specific set of samples. The images were analyzed with GenePix Pro 7.0 (Molecular Devices, Silicon Valley, CA). Total fluorescence signal intensities of each spot were obtained after subtraction of the local background signal for each slide and were then normalized for variation in total protein, background, and nonspecific labeling using a group-based normalization method as described (81). For each spot on the array, the background-subtracted foreground signal intensity was calculated by subtracting the corresponding signal intensity of the negative control slide (omission of primary antibody) and then normalized to the corresponding signal intensity of total protein for that spot. Each image, along with its normalized data, was evaluated for quality through manual inspection and control samples. Antibody slides that failed the quality inspection were either repeated at the end of the staining runs or removed before data reporting. For analysis, the median value of normalized signal intensity of technical triplicates was used for each mouse. All values were normalized to the median value of the control SCA6 group to give values relative to the untreated baseline. Pairwise statistical comparisons were used to detect significant differences.
Enzyme-linked immunosorbent assay
Mice were anesthetized with isoflurane and decapitated, and the vermis was dissected in the same way as previously described for RPPA sample preparation. However, in this case, brains were removed and immediately placed in ice-cold PBS (0.1 M, pH 7.4) for dissection. Tissue samples were dried, snap-frozen on dry ice, and stored at −80°C until sample preparation.
To maximize detection of BDNF from brain tissue, we used an acid extraction protocol for tissue lysis to release BDNF that is bound to receptors or other proteins. This was carried out as described by Kolbeck et al. (81) and the protocol provided by Biosensis. Briefly, tissues were sonicated in an acid extraction buffer at pH 4 with cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail (Roche, Basel, Switzerland) and passed through a fine gauge needle to break up tissue. Sample extracts were centrifuged at 15,000 rcf at 4°C for 30 min, and the pellet was discarded. Protein concentration in the tissue lysate was quantified using a bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, MA, USA) according to the kit’s directions. Following incubation at 37°C, the plate was visualized using a plate reader (Tecan, Männedorf, Switzerland) at 532 nm. Samples were tested in triplicate, and the mean intensity value was taken for each sample. A standard curve was generated and used to calculate the protein concentration in each sample. Samples were stored at −80°C and centrifuged again at 15,000 rcf at 4°C for 30 min after thawing.
We used a mature BDNF Rapid ELISA kit (Biosensis, Thebarton, Australia) to detect BDNF protein in the prepared tissue lysates. To determine the optimal lysate concentration, an initial validation with serial dilution of tissue lysates was carried out. Once determined, samples from litter-matched WT and SCA6 mice at 6, 7, and 12 months of age were tested at 1:8 dilution, and the plate was visualized at 450 nm. 4 parameter analysis was used to generate a standard curve and determine the BDNF concentration in each sample. This was divided by the previously determined protein concentration to give the BDNF concentration per milligram of soluble protein. Tissue preparation and the ELISA protocol were carried out blindly to experimental group.
Western blots
Mice were anesthetized with isoflurane and decapitated. Brains were immediately extracted, and the cerebellar vermis was isolated before being rapidly frozen in a microcentrifuge tube submerged in dry ice. Vermis samples were stored at −80°C until lysate preparation.
Lysates were prepared from cerebellar vermis of DMSO vehicle control and 7,8-DHF–treated SCA6 mice by blending brains submerged in radioimmunoprecipitation assay buffer [150 mM NaCl, 1% NP-40, 50 mM tris-Cl (pH 8.0), 0.5% Na-deoxycholate, 0.1% SDS, and 2 mM EDTA] supplemented with cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail and PhosSTOP phosphatase inhibitor cocktail tablets (Roche, Basel, Switzerland). Lysates were centrifuged at 13,000 rpm for 5 min at 4°C. The resulting supernatants were collected, and protein concentration was measured using a BCA assay (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, MA, USA). Lysates were stored at −80°C until use.
Samples (30 μg/μl) were separated using 8% SDS–polyacrylamide gel electrophoresis resolving gel before being transferred overnight to a nitrocellulose membrane. Membranes were blocked for 2 hours in 5% bovine serum albumin (BSA) in tris-buffered saline–Tween 20 (TBS-T) at room temperature before being incubated overnight in the appropriate antibody (table S1). Membranes were then washed for 15 min with TBS-T before being incubated for 1.5 hours with a horseradish peroxidase–linked secondary antibody (table S1). Last, membranes were washed three times in TBS-T and the bands were visualized using a Western Lightning Plus-ECL chemiluminescence kit (PerkinElmer, NEL103001EA) and an Amersham 600 imager (GE Life Sciences).
Membranes were first probed for phospho-TrkB and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the above methods, and then primary antibodies were stripped following visualization by washing for 7.5 min in 0.5 M NaOH. Following stripping, membranes were washed three times in double-distilled H2O and then three times in TBS-T for 5 min at room temperature before being blocked again in 5% BSA and probed for TrkB overnight. Band densities were measured using ImageJ software and were normalized to GAPDH intensity from the same blot as a loading control. Data from four separate blots were pooled for analysis (four technical replicates). Tissue preparation, blots, and blot analysis were carried out blindly to experimental group.
7,8-DHF administration
7,8-DHF is orally bioavailable (36), and previous studies have demonstrated that oral administration via drinking water is both well tolerated and able to deliver therapeutically relevant dosages of the drug in mice (14, 34, 38–40). Single-housed mice were given free access to solutions containing either 157.3 μM 7,8-DHF (TCI America) dissolved in 0.04% DMSO or control solutions with 0.04% DMSO only, dissolved in autoclaved water. Sucrose (10%) was added to both solutions to make them palatable, as previously described (21). Solutions were made fresh twice weekly and refreshed daily, with solutions stored at 4°C in between refills. Consumption was monitored daily to determine the administered dose of 7,8-DHF. Mice consumed on average 21 ml of the sucrose-sweetened drug solution daily, giving a daily dose of 0.84 mg of 7,8-DHF. The control group with 0.04% DMSO and 10% sucrose but no 7,8-DHF consumed on average 19 ml of the solution each day. The drug treatment period ranged from 25 days to 4 months, and mice underwent behavioral testing on the rotarod assay during this time. Mice were monitored daily when solutions were administered, and no adverse health effects were observed. Mice were weighed at least every second day during behavioral testing, meaning that some mice receiving 7,8-DHF were weighed throughout their drug treatment and did not gain or lose weight during the treatment (fig. S3). Reagents were obtained from Sigma-Aldrich (Oakville, ON, Canada) unless otherwise specified.
Statistical analysis
Comparisons were made using either one-way analysis of variance (ANOVA) followed by post hoc tests using JMP software (SAS, Cary, NC) or two-tailed Student’s t tests or Mann-Whitney U tests using Igor Pro software for parametric or nonparametric testing, respectively. Data are reported as mean ± SEM when normally distributed, or as median when the data are not normally distributed. All statistical tests and P values are reported in table S2.
Acknowledgments
We thank A. McKinney, A. Suvrathan, W.-H. Huang, and J. Sjöström for helpful advice and thoughtful feedback on the project. Custom Igor software routines for electrophysiology data acquisition and analysis were written by J. Sjöström. We thank S. Hekimi, K. Nader, and A. Khoutorsky for the use of equipment and A. Noë and A. Gao for technical guidance. We thank all current and former members of the Watt laboratory for technical and intellectual input and advice, particularly K. Vieira-Lomasney, M. Bhade, and G. Maloney who provided technical support, and B. Toscano-Márquez, A. Smith-Dijak, D. Lang-Ouellette, K. Gruver, and L. Shen for helpful feedback. Imaging was performed in the McGill University Advanced BioImaging Facility (ABIF), and we thank ABIF staff members for technical support. We are grateful for the excellent animal care and training we received from the McGill Animal Resources Centre (CMARC), particularly T. Koch and J. Canale who facilitated the research. We thank X. Wang and Z. Shi from the Antibody-based Proteomics Core/Shared Resource at Baylor College of Medicine for excellent technical assistance in performing RPPA experiments. We thank C. Coarfa and S. L. Grimm for RPPA data processing and normalization.
Funding: This study was supported by the following: CIHR operating grant (MOP-130570) (A.J.W.), CIHR project grant (PJT-153150) (A.J.W.), McGill Biology Doctoral Excellence Award (A.A.C.), Graduate Student Fellowship from the Canada First Research Excellence Fund awarded to McGill University for the Healthy Brains for Healthy Lives initiative (A.A.C. and E.F.), Fonds de recherche du Québec – Santé (FRQS) Doctoral Scholarship (A.A.C.), Returning Student award from the McGill Integrated Program in Neuroscience (S.J. x 2), Natural Sciences and Engineering Research Council (NSERC) Undergraduate Student Research Award (J.S.), Canada Graduate Scholarship (MSc) from the Canadian Institutes of Health Research (CIHR) (E.F.), Cancer Prevention and Research Institute of Texas Proteomics and Metabolomics Core Facility Support Award (RP210227) (S.H.; principal investigator, D. P. Edwards), NCI Cancer Center Support Grant (P30CA125123) (S.H.; principal investigator, H. Heslop), and NIH S10 award (S10OD028648) (S.H.) to Antibody-based Proteomics Core/Shared Resource (S.H.).
Author contributions: A.A.C. designed and ran experiments and analyzed data for all figures except Fig. 3 and wrote the manuscript; S.J. designed and ran experiments and analyzed data for Fig. 3 and helped write the manuscript; J.S. designed and ran experiments and analyzed data for Figs. 1 and 2; E.F. ran experiments for Fig. 6 and analyzed data for Fig. 2; T.C.S.L. analyzed data for fig. S7; S.Q. ran experiments and analyzed data for Fig. 3; E.M. ran experiments and analyzed data for fig. S4; L.L. analyzed data for figs. S1 to S3; S.H. designed and ran experiments and analyzed data for Fig. 6; A.J.W. conceived the project, designed experiments, analyzed data, supervised the project, and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Tables S1 and S2
REFERENCES AND NOTES
- 1.Zhuchenko O., Bailey J., Bonnen P., Ashizawa T., Stockton D. W., Amos C., Dobyns W. B., Subramony S. H., Zoghbi H. Y., Lee C. C., Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat. Genet. 15, 62–69 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Solodkin A., Gomez C. M., Spinocerebellar ataxia type 6. Handb. Clin. Neurol. 103, 461–473 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Watase K., Barrett C. F., Miyazaki T., Ishiguro T., Ishikawa K., Hu Y., Unno T., Sun Y., Kasai S., Watanabe M., Gomez C. M., Mizusawa H., Tsien R. W., Zoghbi H. Y., Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc. Natl. Acad. Sci. U.S.A. 105, 11987–11992 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayabal S., Ljungberg L., Erwes T., Cormier A., Quilez S., El Jaouhari S., Watt A. J., Rapid onset of motor deficits in a mouse model of spinocerebellar ataxia type 6 precedes late cerebellar degeneration. eNeuro 2, ENEURO.0094-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuccato C., Cattaneo E., Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 5, 311–322 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M., Ishikawa K., Sato N., Obayashi M., Niimi Y., Ishiguro T., Yamada M., Toyoshima Y., Takahashi H., Kato T., Takao M., Murayama S., Mori O., Eishi Y., Mizusawa H., Reduced brain-derived neurotrophic factor (BDNF) mRNA expression and presence of BDNF-immunoreactive granules in the spinocerebellar ataxia type 6 (SCA6) cerebellum. Neuropathology 32, 595–603 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Orr H. T., Zoghbi H. Y., Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Murer M. G., Yan Q., Raisman-Vozari R., Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 63, 71–124 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Phillips H. S., Hains J. M., Armanini M., Laramee G. R., Johnson S. A., Winslow J. W., BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7, 695–702 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Hourez R., Servais L., Orduz D., Gall D., Millard I., de Kerchove d’Exaerde A., Cheron G., Orr H. T., Pandolfo M., Schiffmann S. N., Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J. Neurosci. 31, 11795–11807 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellesmoen A., Sheeler C., Ferro A., Rainwater O., Cvetanovic M., Brain derived neurotrophic factor (BDNF) delays onset of pathogenesis in transgenic mouse model of spinocerebellar ataxia Type 1 (SCA1). Front. Cell. Neurosci. 12, 509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H., Larson S. K., Gao R., Qiao X., BDNF transgene improves ataxic and motor behaviors in stargazer mice. Brain Res. 1160, 47–57 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Salomova M., Tichanek F., Jelinkova D., Cendelin J., Abnormalities in the cerebellar levels of trophic factors BDNF and GDNF in pcd and lurcher cerebellar mutant mice. Neurosci. Lett. 725, 134870 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Parrini M., Ghezzi D., Deidda G., Medrihan L., Castroflorio E., Alberti M., Baldelli P., Cancedda L., Contestabile A., Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci. Rep. 7, 16825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neeper S. A., Gómez-Pinilla F., Choi J., Cotman C. W., Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726, 49–56 (1996). [PubMed] [Google Scholar]
- 16.Neeper S. A., Gómez-Pinilla F., Choi J., Cotman C., Exercise and brain neurotrophins. Nature 373, 109 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Gold S. M., Schulz K.-H., Hartmann S., Mladek M., Lang U. E., Hellweg R., Reer R., Braumann K.-M., Heesen C., Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J. Neuroimmunol. 138, 99–105 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Rojas Vega S., Strüder H. K., Vera Wahrmann B., Schmidt A., Bloch W., Hollmann W., Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 1121, 59–65 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Lourenco M. V., Frozza R. L., de Freitas G. B., Zhang H., Kincheski G. C., Ribeiro F. C., Gonçalves R. A., Clarke J. R., Beckman D., Staniszewski A., Berman H., Guerra L. A., Forny-Germano L., Meier S., Wilcock D. M., de Souza J. M., Alves-Leon S., Prado V. F., Prado M. A. M., Abisambra J. F., Tovar-Moll F., Mattos P., Arancio O., Ferreira S. T., De Felice F. G., Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi S. H., Bylykbashi E., Chatila Z. K., Lee S. W., Pulli B., Clemenson G. D., Kim E., Rompala A., Oram M. K., Asselin C., Aronson J., Zhang C., Miller S. J., Lesinski A., Chen J. W., Kim D. Y., van Praag H., Spiegelman B. M., Gage F. H., Tanzi R. E., Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayabal S., Chang H. H. V., Cullen K. E., Watt A. J., 4-Aminopyridine reverses ataxia and cerebellar firing deficiency in a mouse model of spinocerebellar ataxia type 6. Sci. Rep. 6, 29489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook A. A., Fields E., Watt A. J., Losing the beat: Contribution of Purkinje cell firing dysfunction to disease, and its reversal. Neuroscience 462, 247–261 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Kordasiewicz H. B., Gomez C. M., Molecular pathogenesis of spinocerebellar ataxia Type 6. Neurotherapeutics 4, 285–294 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Cechetti F., Fochesatto C., Scopel D., Nardin P., Gonçalves C. A., Netto C. A., Siqueira I. R., Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Res. 1188, 182–188 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Inoue T., Ninuma S., Hayashi M., Okuda A., Asaka T., Maejima H., Effects of long-term exercise and low-level inhibition of GABAergic synapses on motor control and the expression of BDNF in the motor related cortex. Neurol. Res. 40, 18–25 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Johnson R. A., Rhodes J. S., Jeffrey S. L., Garland T., Mitchell G. S., Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience 21, 1–7 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Cheng P.-L., Song A.-H., Wong Y.-H., Wang S., Zhang X., Poo M.-M., Self-amplifying autocrine actions of BDNF in axon development. Proc. Natl. Acad. Sci. U.S.A. 108, 18430–18435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fryer J. D., Yu P., Kang H., Mandel-Brehm C., Carter A. N., Crespo-Barreto J., Gao Y., Flora A., Shaw C., Orr H. T., Zoghbi H. Y., Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science 334, 690–693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang C.-S., Chang J.-C., Soong B.-W., Chuang S.-F., Lin T.-T., Cheng W.-L., Orr H. T., Liu C.-S., Treadmill training increases the motor activity and neuron survival of the cerebellum in a mouse model of spinocerebellar ataxia type 1. Kaohsiung J. Med. Sci. 35, 679–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Saavedra M., De Repentigny Y., Yang D., O’Meara R. W., Yan K., Hashem L. E., Racacho L., Ioshikhes I., Bulman D. E., Parks R. J., Kothary R., Picketts D. J., Voluntary running triggers VGF-mediated oligodendrogenesis to prolong the lifespan of Snf2h-null ataxic mice. Cell Rep. 17, 862–875 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Ady V., Toscano-Márquez B., Nath M., Chang P. K., Hui J., Cook A., Charron F., Larivière R., Brais B., McKinney R. A., Watt A. J., Altered synaptic and firing properties of cerebellar Purkinje cells in a mouse model of ARSACS. J. Physiol. 596, 4253–4267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shakkottai V. G., do Carmo Costa M., Dell’Orco J. M., Sankaranarayanan A., Wulff H., Paulson H. L., Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J. Neurosci. 31, 13002–13014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoyas C. A., Bushart D. D., Switonski P. M., Ward J. M., Alaghatta A., Tang M., Niu C., Wadhwa M., Huang H., Savchenko A., Gariani K., Xie F., Delaney J. R., Gaasterland T., Auwerx J., Shakkottai V. G., La Spada A. R., Nicotinamide pathway-dependent sirt1 activation restores calcium homeostasis to achieve neuroprotection in spinocerebellar ataxia type 7. Neuron 105, 630–644.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C., Chan C. B., Ye K., 7,8-Dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 5, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang S.-W., Liu X., Yepes M., Shepherd K. R., Miller G. W., Liu Y., Wilson W. D., Xiao G., Blanchi B., Sun Y. E., Ye K., A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. U.S.A. 107, 2687–2692 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X., Qi Q., Xiao G., Li J., Luo H. R., Ye K., O-Methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology 91, 185–200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C.-H., Hung T.-H., Chen C.-C., Ke C.-H., Lee C.-Y., Wang P.-Y., Chen S.-F., Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLOS One 9, e113397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., Liu X., Schroeder J. P., Chan C. B., Song M., Yu S. P., Weinshenker D., Ye K., 7,8-Dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacol. 39, 638–650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson R. A., Lam M., Punzo A. M., Li H., Lin B. R., Ye K., Mitchell G. S., Chang Q., 7,8-Dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J. Appl. Physiol. 112, 704–710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L., Tian M., Zhao H.-Y., Xu Q.-Q., Huang Y.-M., Si Q.-C., Tian Q., Wu Q.-M., Hu X.-M., Sun L.-B., McClintock S. M., Zeng Y., TrkB activation by 7, 8-dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 136, 620–636 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Haapasalo A., Sipola I., Larsson K., Åkerman K. E. O., Stoilov P., Stamm S., Wong G., Castrén E., Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J. Biol. Chem. 277, 43160–43167 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Soppet D., Escandon E., Maragos J., Middlemas D. S., Raid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T., Nikolics K., Parade L. F., The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 65, 895–903 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Martens L. K., Kirschner K. M., Warnecke C., Scholz H., Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J. Biol. Chem. 282, 14379–14388 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z., Yao L., Yang J., Wang Z., Du G., PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol. Med. Rep. 18, 3547–3554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng L., Liu B., Ji R., Jiang X., Yan X., Xin Y., Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 17, 2031–2039 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang E. J., Reichardt L. F., Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Rai S. N., Dilnashin H., Birla H., Sen Singh S., Zahra W., Rathore A. S., Singh B. K., Singh S. P., The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox. Res. 35, 775–795 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Xu J., Hu C., Chen S., Shen H., Jiang Q., Huang P., Zhao W., Neuregulin-1 protects mouse cerebellum against oxidative stress and neuroinflammation. Brain Res. 1670, 32–43 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Lin C.-W., Fan C.-H., Chang Y.-C., Hsieh-Li H. M., ERK activation precedes Purkinje cell loss in mice with Spinocerebellar ataxia type 17. Neurosci. Lett. 738, 135337 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Creighton C. J., Huang S., Reverse phase protein arrays in signaling pathways: A data integration perspective. Drug Des. Devel. Ther. 9, 3519, 3527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C.-H., Chen C.-C., Hung T.-H., Chuang Y.-C., Chao M., Shyue S.-K., Chen S.-F., Activation of TrkB/Akt signaling by a TrkB receptor agonist improves long-term histological and functional outcomes in experimental intracerebral hemorrhage. J. Biomed. Sci. 26, 53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C., Wang Z., Zhang Z., Liu X., Kang S. S., Zhang Y., Ye K., The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, 578–583 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H.-M., Huang C.-C., Tsai M.-H., Poon Y.-C., Chang Y.-C., Systemic 7,8-dihydroxyflavone treatment protects immature retinas against hypoxic-ischemic injury via Müller Glia regeneration and MAPK/ERK activation. Invest. Ophthalmol. Vis. Sci. 59, 3124–3135 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Miyai I., Ito M., Hattori N., Mihara M., Hatakenaka M., Yagura H., Sobue G., Nishizawa M.; Cerebellar Ataxia Rehabilitation Trialists Collaboration , Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil. Neural Repair 26, 515–522 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Ilg W., Synofzik M., Brötz D., Burkard S., Giese M. A., Schöls L., Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 73, 1823–1830 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Chang Y.-J., Chou C.-C., Huang W.-T., Lu C.-S., Wong A. M., Hsu M.-J., Cycling regimen induces spinal circuitry plasticity and improves leg muscle coordination in individuals with spinocerebellar ataxia. Arch. Phys. Med. Rehabil. 96, 1006–1013 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Barbuto S., Martelli D., Omofuma I. B., Lee N., Kuo S. H., Agrawal S., Lee S., O’Dell M., Stein J., Phase I randomized single-blinded controlled study investigating the potential benefit of aerobic exercise in degenerative cerebellar disease. Clin. Rehabil. 34, 584–594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasaki H., Kojima H., Yabe I., Tashiro K., Hamada T., Sawa H., Hiraga H., Nagashima K., Neuropathological and molecular studies of spinocerebellar ataxia type 6 (SCA6). Acta Neuropathol. 95, 199–204 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Gomez C. M., Thompson R. M., Gammack J. T., Perlman S. L., Dobyns W. B., Truwit C. L., Zee D. S., Clark H. B., Anderson J. H., Spinocerebellar ataxia type 6: Gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann. Neurol. 42, 933–950 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Kulik Á., Nakadate K., Hagiwara A., Fukazawa Y., Luján R., Saito H., Suzuki N., Futatsugi A., Mikoshiba K., Frotscher M., Shigemoto R., Immunocytochemical localization of the alpha1A subunit of the P/Q-type calcium channel in the rat cerebellum. Eur. J. Neurosci. 19, 2169–2178 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Choo M., Miyazaki T., Yamazaki M., Kawamura M., Nakazawa T., Zhang J., Tanimura A., Uesaka N., Watanabe M., Sakimura K., Kano M., Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 8, 195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martini M., De Santis M. C., Braccini L., Gulluni F., Hirsch E., PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 46, 372–383 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Paulsen J. S., Nance M., Kim J. I., Carlozzi N. E., Panegyres P. K., Erwin C., Goh A., McCusker E., Williams J. K., A review of quality of life after predictive testing for and earlier identification of neurodegenerative diseases. Prog. Neurobiol. 110, 2–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reycraft J. T., Islam H., Townsend L. K., Hayward G. C., Hazell T. J., MacPherson R. E. K., Exercise intensity and recovery on circulating brain-derived neurotrophic factor. Med. Sci. Sports Exerc. 52, 1210–1217 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Jiménez-Maldonado A., Rentería I., García-Suárez P. C., Moncada-Jiménez J., Freire-Royes L. F., The impact of high-intensity interval training on brain derived neurotrophic factor in brain: A mini-review. Front. Neurosci. 12, 839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliff H. S., Berchtold N. C., Isackson P., Cotman C. W., Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol. Brain Res. 61, 147–153 (1998). [DOI] [PubMed] [Google Scholar]
- 67.El Hayek L., Khalifeh M., Zibara V., Abi Assaad R., Emmanuel N., Karnib N., El-Ghandour R., Nasrallah P., Bilen M., Ibrahim P., Younes J., Abou Haidar E., Barmo N., Jabre V., Stephan J. S., Sleiman S. F., Lactate mediates the effects of exercise on learning and memory through sirt1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sleiman S. F., Henry J., Al-Haddad R., El Hayek L., Haidar E. A., Stringer T., Ulja D., Karuppagounder S. S., Holson E. B., Ratan R. R., Ninan I., Chao M. V., Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β- hydroxybutyrate. Elife 5, e15092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wrann C. D., White J. P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J. D., Greenberg M. E., Spiegelman B. M., Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klintsova A. Y., Dickson E., Yoshida R., Greenough W. T., Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 1028, 92–104 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Rogers J., Chen F., Stanic D., Farzana F., Li S., Zeleznikow-Johnston A. M., Nithianantharajah J., Churilov L., Adlard P. A., Lanfumey L., Hannan A. J., Renoir T., Paradoxical effects of exercise on hippocampal plasticity and cognition in mice with a heterozygous null mutation in the serotonin transporter gene. Br. J. Pharmacol. 176, 3279–3296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adlard P. A., Perreau V. M., Cotman C. W., The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging 26, 511–520 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Sheeler C., Rosa J. G., Borgenheimer E., Mellesmoen A., Rainwater O., Cvetanovic M., Post-symptomatic delivery of brain-derived neurotrophic factor (BDNF) ameliorates spinocerebellar ataxia type 1 (SCA1) pathogenesis. Cerebellum 20, 420–429 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cunha C., Angelucci A., D’Antoni A., Dobrossy M. D., Dunnett S. B., Berardi N., Brambilla R., Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiol. Dis. 33, 358–368 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Croll S. D., Suri C., Compton D. L., Simmons M. V., Yancopoulos G. D., Lindsay R. M., Wiegand S. J., Rudge J. S., Scharfman H. E., Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience 93, 1491–1506 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papaleo F., Silverman J. L., Aney J., Tian Q., Barkan C. L., Chadman K. K., Crawley J. N., Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn. Mem. 18, 534–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rueden C. T., Schindelin J., Hiner M. C., DeZonia B. E., Walter A. E., Arena E. T., Eliceiri K. W., ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coarfa C., Grimm S. L., Rajapakshe K., Perera D., Lu H.-Y., Wang X., Christensen K. R., Mo Q., Edwards D. P., Huang S., Reverse-phase protein array: Technology, application, data processing, and integration. J. Biomol. Tech. 32, 15–29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu H.-Y., Xiong J., Perera D. N., Rajapakshe K., Wang X., Costello M., Holloway K. R., Ramos C., Grimm S. L., Wulfkuhle J., Coarfa C., Edwards D. P., Zhu M. X., Huang S., High-throughput evaluation of metabolic activities using reverse phase protein array technology. Neuron Signal. Metab. Regul. , 293–317 (2021). [Google Scholar]
- 81.Kolbeck R., Bartke I., Eberle W., Barde Y. A., Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J. Neurochem. 72, 1930–1938 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Tables S1 and S2