Abstract
Development is tightly connected to aging, but whether pharmacologically targeting development can extend life remains unknown. Here, we subjected genetically diverse UMHET3 mice to rapamycin for the first 45 days of life. The mice grew slower and remained smaller than controls for their entire lives. Their reproductive age was delayed without affecting offspring numbers. The treatment was sufficient to extend the median life span by 10%, with the strongest effect in males, and helped to preserve health as measured by frailty index scores, gait speed, and glucose and insulin tolerance tests. Mechanistically, the liver transcriptome and epigenome of treated mice were younger at the completion of treatment. Analogous to mice, rapamycin exposure during development robustly extended the life span of Daphnia magna and reduced its body size. Overall, the results demonstrate that short-term rapamycin treatment during development is a novel longevity intervention that acts by slowing down development and aging, suggesting that aging may be targeted already early in life.
Rapamycin given only during early life extends life span and health span of male mice and extends life span of Daphnia magna.
INTRODUCTION
The evolutionary history of mammals is a prominent example of how life span may be changed over 100-fold. Cross-species analyses have revealed a strong positive association of species’ maximum life span with time to maturity and a positive association with body mass (1, 2). On the contrary, smaller animals within species tend to live longer or be protected from age-related diseases as evidenced by studies of dogs and humans (3–6). These associations could be explained by the importance of growth rate for longevity (1, 2). Knockouts of the growth hormone pathway or genes involved in insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) lead to extended life span of organisms from worms to mice (7–13), and this is further associated with decreased body size. Moreover, wild-type mice preselected for slower growth live longer than their fast-growing siblings (14). Likewise, the growth rate of longer-lived species of mammals is lower compared with those with shorter life span (15). These findings suggest a causal link between the organismal pace of growth, body size, and longevity.
Some indirect evidence supports the causal relationship between inhibition of growth signaling and longevity if targeted during development. For example, growth hormone knockout mice and mice lacking GH production live up to 50% longer than their wild-type siblings (10, 12). However, their longevity was diminished if they were treated with growth hormone during early postnatal development (16, 17). At the same time, growth hormone knockout induced at adult age had limited to no effects on longevity (18). However, there have been no experiments where growth pathways are directly inhibited only during development and the longevity outcomes measured.
Rapamycin is a well-characterized mechanistic target of rapamycin (mTOR) inhibitor and is among the most validated and potent pharmaceutical interventions that extend life span in mice. Rapamycin can extend life span if given in adulthood (19, 20) or later in life (21, 22) in various mouse strains, including genetically diverse UMHET3 mice (a cross of four inbred strains). Rapamycin failed to extend the life span of growth hormone receptor knockout mice (20). Furthermore, early life (EL) rapamycin treatment was previously shown to suppress growth of mice (23). Thus, rapamycin is a perfect candidate to test how targeting growth only early in life can affect life span, and we used it in our study, examining its effects on longevity, health span, biological age, and gene expression.
RESULTS
Rapamycin treatment during mouse development attenuates growth and onset of reproduction
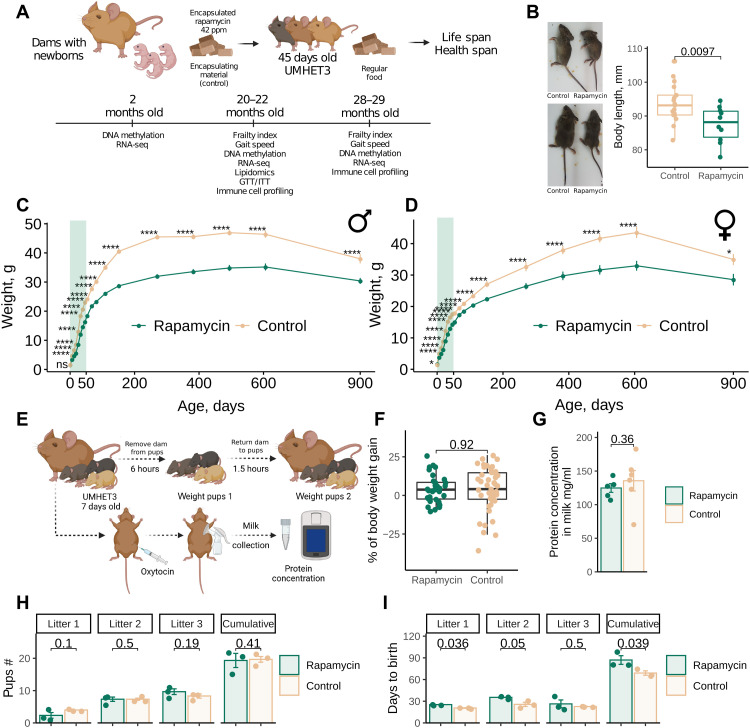
To evaluate the effect of rapamycin on longevity, we set up a cohort of 130 newborn UMHET3 mice for a life span experiment and a separate cohort of 40 mice for health span analyses (Fig. 1A). Dams with newborns were fed chow containing 42 parts per million (ppm) of eudragit-encapsulated rapamycin or the corresponding amount of encapsulating material as a control. Pups were separated from dams at 20 to 22 days of age and continued on the same rapamycin diet until 45 days of age (Fig. 1A). These animals were then transferred to a regular chow diet and followed for the rest of their lives.
Fig. 1. Rapamycin inhibits growth and delays reproduction of genetically heterogeneous mice.
(A) Experimental design for evaluating the effect of EL rapamycin treatment on mouse longevity and health. RNA-seq, RNA sequencing. GTT, glucose tolerance test; ITT, insulin tolerance test. (B) Representative images of 2-month-old UMHET3 mice subjected to eudragit-encapsulated rapamycin or control (eudragit-containing) diets during the first 45 days of their lives. (C and D) Body weight change of male (C) and female (D) mice subjected to rapamycin (n = 30 per sex) or control (n = 34 to 39 per sex) diets from 0 to 45 days of age. The shaded area shows the period of rapamycin treatment. The data are means ± SE. (E) Experimental design for evaluating the effects of EL rapamycin treatment on pups’ nutrition. (F) Percentage of 7-day-old pups’ body weight gain calculated as weight of pup after it consumed milk divided by the mean weight of the group after a 6-hour starving period and exactly before pups were returned to dams and multiplied by 100. Mean weight was used for normalization within each group because of the baseline difference in weight between rapamycin and control pups. (G) Milk protein concentration in dams subjected to rapamycin or control diets starting from the day they give birth. (H) Number of pups born to breeders subjected to rapamycin or control diets during development. (I) Time between pregnancies for rapamycin-treated and control groups of breeders. *P < 0.05; ****P < 0.0001; ns, not significant.
Rapamycin treatment in EL resulted in smaller mice (Fig. 1B), and the reduced weight was preserved across the life span in both males (Fig. 1C) and females (Fig. 1D). Rapamycin showed an effect within days. Small body size was seen upon completion of treatment, with males being affected more than females. Accordingly, the weight of the organs (brain, liver, kidney, and spleen) was also reduced in the treatment group (fig. S1A).
Because rapamycin was initially delivered to the pups through the milk, we examined whether rapamycin directly inhibited the growth of animals or affected nutrient consumption. Thus, we tested whether pups consumed the same amount of milk and whether total protein of the milk was affected in the treated animals (Fig. 1F). We focused on protein levels because rapamycin inhibits protein synthesis (24, 25). We found no difference in milk consumption between treated and control groups (Fig. 1F); each group gained the same percentage of weight after being separated from dams and returned for refeeding. We further constructed a custom milking pump and collected milk from the dams (fig. S2). The total protein content of the milk was not different between treated and control dams (Fig. 1G). We conclude that the observed effect of growth patterns is the direct consequence of rapamycin treatment rather than nutritional deficits.
Growth is usually coupled with time to sexual maturity. To test whether EL rapamycin treatment not only attenuated body growth but also the age at sexual maturity, we set up breeding pairs of treated and untreated age-matched UMHET3 mice (46- to 49-day-old females and 47-day-old males) and recorded the dates when the females gave birth to the first, second, and third litters as well as the number of pups born. Treated and untreated mice had the same litter size (Fig. 1H). However, treated breeders had their first litters on average 4.6 days later than untreated breeders (P = 0.036). The period between the first and second litters was also longer in rapamycin-treated mice (now by 9.6 days, P = 0.05), and the period between the second and third litters was not statistically significantly (P = 0.5) longer in rapamycin-treated breeders (Fig. 1I).
EL rapamycin treatment during development extends life span and health span of mice
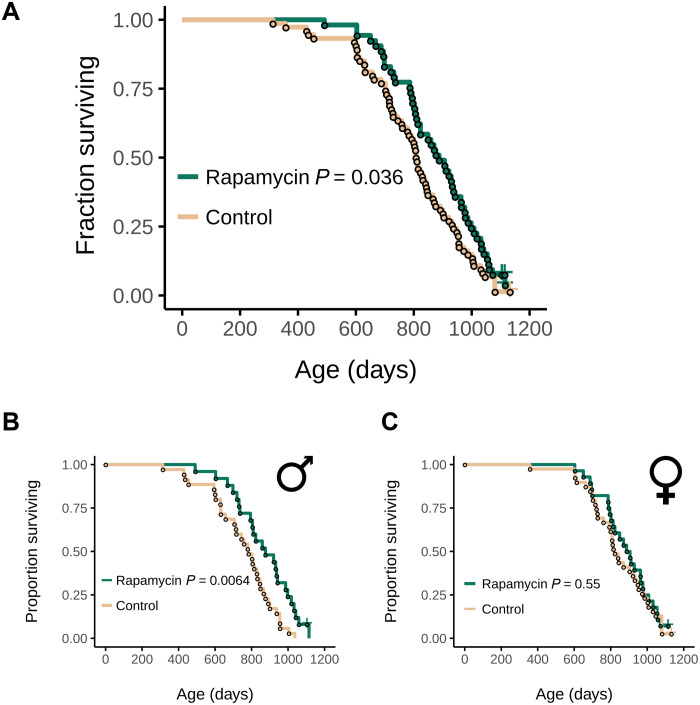
We found that EL rapamycin treatment extended the median life span of UMHET3 mice by 10% (P = 0.036; Fig. 2A). This effect was primarily due to the effect of treatment in males (P = 0.0064, median life span extension by 11.8%; Fig. 2B), whereas females did not live longer (P = 0.55; Fig. 2C).
Fig. 2. Rapamycin treatment during development extends the life span of UMHET3 mice.
(A) Survival curve of treated and untreated UMHET3 mice, sexes combined. (B and C) Survival curves of (B) male and (C) female UMHET3 mice that were subjected to encapsulated rapamycin or encapsulating material as a control from birth until 45 days of age. P values were calculated with the log-rank test.
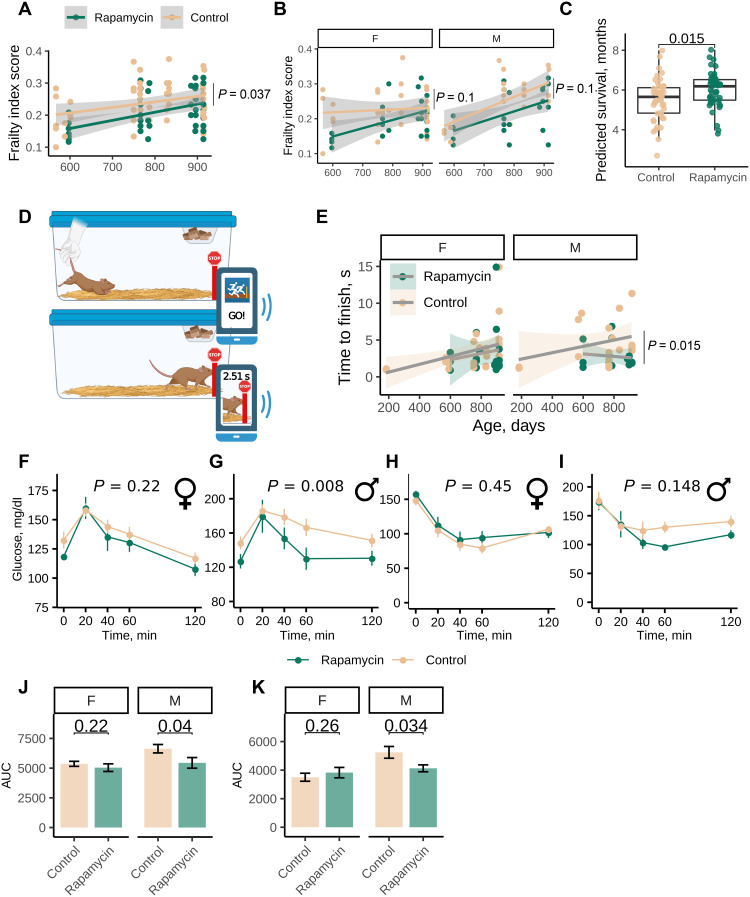
To understand whether health span was also improved in the EL treated mice later in their lives, we measured frailty index (26–28), gait speed, and glucose and insulin tolerance at the age of 20 to 29 months. Frailty index is a 31-parameter estimate of overall frailty of mice based on noninvasive assessment of mouse age–associated features (26–28). EL rapamycin treatment made mice less frail than the control group [repeated-measures analysis of variance (ANOVA), P = 0.037; Fig. 3A], and we observed lower, but not statistically significant, frailty index scores in treated males and females separately (Fig. 3B). We further applied a frailty index clock (26) that predicts the survival of mice based on frailty score features and takes mouse age into account and found that mice treated with EL rapamycin had a longer predicted survival time (analysis of covariance adjusted for sex, P = 0.015; Fig. 3C). We conclude that EL rapamycin treatment delays the onset of frailty phenotypes, including features that are associated with mouse survival. Feature analyses revealed that the forelimb grip strength, gait disorders, body condition score, and distended abdomen were improved in the treatment group (fig. S3A).
Fig. 3. Rapamycin treatment during development extends the health span of mice.
(A) Frailty index score of 20- to 29-month-old UMHET3 mice subjected to diets containing encapsulated rapamycin or encapsulating material (eudragit) from birth until 45 days of age. P value was calculated with repeated-measures analysis of variance (ANOVA) test. (B) Frailty index score of 20- to 29-month-old male (M) and female (F) UMHET3 mice subjected to rapamycin or control diets from birth until 45 days of age. P value was calculated with repeated-measures ANOVA test. (C) Predicted survival (in months) based on frailty index calculated using the AFRAID (analysis of frailty and death) clock. (D) Scheme of gait speed measurement in mice. (E) Median time mice spent in seconds to reach the finish line. P value was calculated with repeated-measures ANOVA test. (F and G) Glucose tolerance test results for (F) females and (G) males (G) (n = 4 to 6 per group per sex). Insulin tolerance test result for (H) females and (I) males (n = 4 to 6 per group per sex). P values were calculated with repeated-measures ANOVA test. (J and K) Area under the curve (AUC) for glucose tolerance and insulin tolerance tests. P values were calculated with a one-tailed Student’s t test.
We further developed and applied a new test to assess mobility fitness in mice that does not require specific equipment and can be conducted by using a cell phone and a common app (Fig. 3D). Such a test can be performed at a house facility and at near zero cost. In this test, mice are placed at one end of the cage and allowed to run voluntarily to the other end of the cage (Fig. 3D). The time to finish is determined using a photo-finish app. We found that mice ran significantly slower with age (fig. S3B). We further verified that the results of our test are comparable to widely used tests that assess muscle function. Thus, we performed three tests in the same group of 27-month-old mice: rotarod, treadmill, and gait speed analysis presented here. We found a significant correlation between the measures of our test and treadmill, as well as rotarod (fig. S4, C and D). EL rapamycin attenuated this phenotype in aged males (Fig. 3E), suggesting that mobility fitness is better preserved in these animals as a result of the intervention.
In addition, we analyzed whether our EL intervention affects the metabolic health of old animals and induces glucose intolerance similarly to the acute rapamycin treatment. Glucose and insulin tolerance tests revealed some sex-specific improvements of glucose and insulin tolerance in treated males of older ages (Fig. 3, F to K), consistent with sex specificity of the treatment on life span.
To test whether EL rapamycin might cause hyperlipidemia similarly to the acute rapamycin treatment (29), we performed lipidomics analyses of the liver and serum of 20- to 22-month-old male and female rapamycin groups. There were a total of 3093 lipid species detected in the serum and 3075 in the liver. EL rapamycin had a limited effect on the lipid content of both tissues, and we observed most of the changes in the female liver, where 95 lipids showed increased levels and 73 lipids showed decreased levels (table S5). Overall, the number of lipids with increased levels was comparable to those with decrease levels, without bias toward increased levels of triglycerides, characteristic of hyperlipidemia (fig. S7, A and B). In addition, gene expression of Prox1 remained unchanged in treated animals (fig. S7C); Prox1 was shown to regulate hyperlipidemia caused by acute rapamycin treatment (30).
Unlike rapamycin given in adulthood, EL rapamycin did not up-regulate genes from gluconeogenesis pathways in the liver of young animals (G6Pase encoded by G6pc and G6pc3, PEPCK encoded by Pck1 and Pck2, and PGC-1α encoded by Ppargc1a) (fig. S7D). This finding suggests that the EL rapamycin effects on glucose metabolism are different from those of rapamycin treatment in adulthood.
We further analyzed immune cell composition of the spleen and bone marrow of the treated and control groups of animals 20 to 29 months of age. We previously established new age-related phenotypes of the immune system that are predictive of mouse life span and lymphoma incidence [increased B cell size, clonal B cells, age-associated B cells (ABCs), and myeloid bias] (31). Analysis of control mice revealed that UMHET3 mice accumulate the same age-related phenotypes as C57BL/6 mice (B cell size increase, myeloid skewing, accumulation of age-associated clonal B cells, and ABCs). We found that these phenotypes (fig. S4, A, B, D, and E) as well as percentage (fig. S4C) and cell size (fig. S4F) of hematopoietic stem cells were not affected in the treated mice in comparison to controls.
Last, necropsy analyses of aged mice that died of natural causes revealed no difference in the incidence of common mouse age–related pathologies (table S1), indicating that the longevity effect of the EL rapamycin treatment cannot be attributed to the prevention of a particular age-related disease.
EL rapamycin treatment induces pro-longevity gene expression changes that are preserved with age in a sex-specific manner
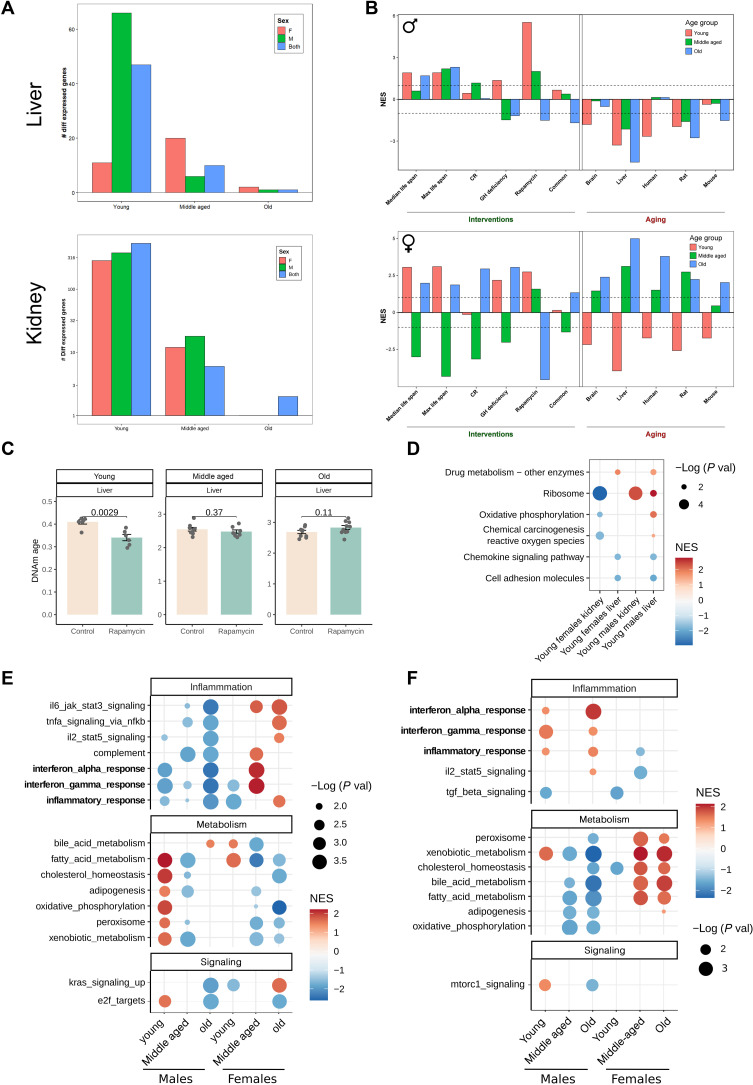
To gain insights into molecular mechanisms of the EL rapamycin regimen, we analyzed gene expression changes in response to the treatment in the livers and kidneys of young (2 months), middle-aged (20 to 22 months), and old (28 to 29 months) mice. We first found that the number of differentially expressed genes (DEGs) triggered by treatment was reduced with age (Fig. 4A). We then compared transcriptome changes in response to EL rapamycin to those induced by known longevity interventions and those induced by aging (i.e., compared gene expression signatures) (32). In both males and females, EL rapamycin triggered transcriptome changes in the young liver that were opposite to age-related changes in this organ and were similar to those induced by longevity interventions (lifelong rapamycin and growth hormone deficiency) (Fig. 4C). In males, a negative association with aging signatures persisted throughout life; however, in females, the effect of treatment vanished with time (Fig. 4C). A positive association of EL rapamycin effect with the effect of lifelong rapamycin treatment was lost with age in both males and females; it was strongest in young animals days after the treatment was stopped (fig. S5C). This indicates that the effect of rapamycin on the liver transcriptome fades away over time once the treatment is terminated. However, other antiaging and pro-longevity effects on the transcriptomes were preserved in treated male mice. This was supported by the negative association with the liver aging signature and positive association with the gene expression biomarkers of mice with higher median and maximum life span (median and max life span signatures) (Fig. 4C). The sex-specific life span and health span effects of the treatment were reflected in hepatic gene expression changes. In particular, males that benefited the most from the treatment retained the negative association with aging signatures across ages. Treated females, on the other hand, acquired a positive association with aging signatures at older ages.
Fig. 4. Rapamycin treatment during development attenuates aging of the transcriptome and DNA methylation (DNAm) of young animals, with males preserving these younger transcriptomes across the life span.
(A) Number of differentially expressed genes (DEGs) [false discovery rate (FDR) < 0.1] in the liver and kidney of young, middle-aged, and old animals treated with EL rapamycin and compared to controls. (B) Association between gene expression changes induced by EL rapamycin and measured in young (red), middle-aged (green), and old (blue) animals, and signatures of aging and life span extension in males (top plot) and females (bottom plot). The intervention signatures include gene signatures of individual interventions [caloric restriction (CR), growth hormone (GH) deficiency, and rapamycin), common gene expression signatures across different interventions (common), and signatures associated with the effect of interventions on maximum or median life span (max and median life span). Aging signatures include age-related gene expression changes across different tissues of humans, mice, and rats (human, mouse, and rat) as well as liver- and brain-specific changes (liver, brain). (C) DNAm age of livers estimated by the Liver epigenetic aging clock in young, middle-aged, and old treated and untreated animals. P values are calculated with a two-tailed Student’s t test. (D) GSEA for pathways from the KEGG database using ranked DEGs in livers and kidneys from young treated animals. Only significant enrichments (FDR < 0.1) for at least two conditions are shown. Dots are colored according to normalized enrichment score (NES) and sized according to −log10 of P value. (E) GSEA for hallmark pathways from the MSigDB using ranked differentially expressed and (F) differentially methylated genes in treated animals. Only significant enrichments (FDR < 0.1) for at least two conditions are shown. Dots are colored according to NES and sized according to −log10 of P value.
Pathway gene set enrichment analysis (GSEA) based on the Molecular Signatures Database (MSigDB) revealed that young animals up-regulated metabolic pathways and down-regulated inflammation pathways in response to the treatment in both sexes (Fig. 5D). These changes were consistent with other longevity interventions, including rapamycin at adult age, caloric restriction, growth hormone deficiency, and heterochronic parabiosis (fig. S5, A and B) and were opposite to age-related changes. Consistent with the life span and health span benefits, we observed sex-specific effects on the transcriptomes. First, unlike females, treated males preserved the down-regulation of inflammation pathways with age (Fig. 4E). Second, young males up-regulated metabolic pathways more robustly than young females (Fig. 4E).
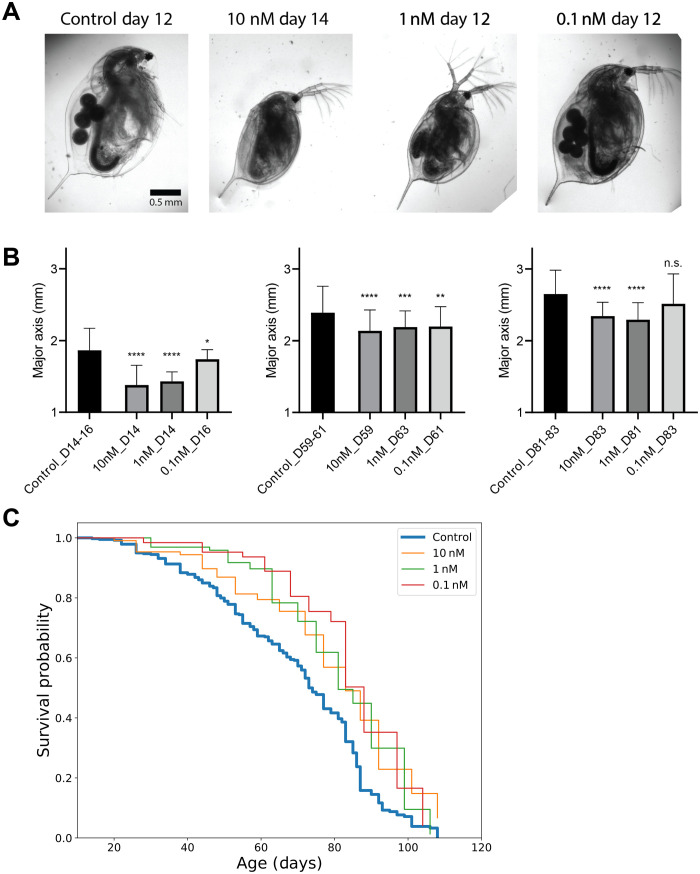
Fig. 5. Rapamycin treatment during development extends life span and reduces the body size of Daphnia magna.
(A) Representative images of D. magna at 12 to 14 days following rapamycin exposure. Also shown is a control animal at the same chronological age. (B) Daphnia body size measurement at days 14 to 16, days 59 to 63, and days 81 to 83. P values are calculated using two-sided Student’s t test. The data are means ± SD. Kruskal-Wallis test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). n.s., not significant. (C) Survival curves for control and three different concentrations of rapamycin (control, n = 379; 0.01 nM, n = 107; 1 nM, n = 97; and 0.1 nM, n = 63).
We further compared pathways enriched in gene expression changes caused by the treatment in the liver and kidney. Both liver and kidney in males up-regulated ribosomal genes in response to EL rapamycin, whereas those in females down-regulated these genes (Fig. 4D and tables S3 and S4). Interferon-γ and inflammatory response pathways were consistently down-regulated in response to the treatment in both tissues in males and in kidneys in females, suggesting that some of the effects of EL rapamycin might be mediated by systemic effects on inflammation (fig. S5D).
Overall, we found that the EL rapamycin treatment initially triggered gene expression changes that were similar to potent longevity interventions and were opposite to age-related changes, with males better preserving these effects across the life span. However, females lost negative association with aging signatures at older ages, consistent with the sex-specific effects of the EL rapamycin treatment on life span and health span. Moreover, there was no correlation between gene expression ranks of old males and females (Spearman correlation coefficient, r = −0.02), further underlying the sex specificity of the impact of EL rapamycin on gene expression into older ages of animals (fig. S5, E and F).
EL rapamycin perturbs global DNA methylation patterns in mouse liver
To test a potential role of the epigenome on the long-lasting effects of EL rapamycin, we performed analyses of DNA methylation (DNAm) of the livers that were analyzed with RNA sequencing. The treatment significantly affected 83 CpG sites [false discovery rate (FDR) < 0.1] independent of age and sex of the animals (table S2). We further performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of genes that carry differentially methylated CpG sites and are differentially expressed. These genes in young animals were significantly enriched in steroid, thyroid, and hormone biosynthesis (fig. S6). This finding suggests that EL rapamycin regulates the expression of genes involved in hormone biosynthesis by altering their DNAm patterns. This is consistent with the changes in body size and sex-specific effects of the treatment.
We then estimated the DNAm age of the livers using pan-tissue and liver-specific epigenetic aging mouse clocks. The EL rapamycin treatment initially reduced epigenetic age of the livers of young animals in both sexes, but this effect was lost with age (Fig. 4C). Enrichment of differentially methylated regions of young, middle-aged, and old treated males and females revealed a limited number of enriched pathways that changed following the treatment and were also preserved across the life span in both sexes (Fig. 4F). Moreover, correlation of CpG ranks among ages and sexes was relatively high only between young males and young females (Spearman correlation coefficient, r = 0.12) and between old males and middle-aged males (r = 0.15) (fig. S5F).
Rapamycin treatment during development extends the life span of Daphnia magna
To understand whether the EL rapamycin treatment operates through evolutionarily conserved longevity mechanisms, we subjected the model invertebrate D. magna to rapamycin only during postnatal development. We chose a window from days 3 and 4 to days 11 and 12 as most of the animals begin to produce offspring by day 12 (33).
Like mice, EL rapamycin-treated Daphnia were smaller in size, and the effect was dose dependent (Fig. 5, A and B). Whereas animals treated with 1 and 10 nM rapamycin stayed smaller throughout their life span, Daphnia treated with a 0.1 nM rapamycin caught up in size as they aged, and by the age of 83 days were insignificantly different from controls (Fig. 5B, right). Despite catching up in size, these animals still lived longer, suggesting that staying small throughout the life span is not necessary for the longevity effect of the treatment.
Concentrations above 10 μM were found to be toxic and were not studied further. We did not observe a significant effect of 0.1 μM rapamycin on life span (χ2 = 1.505 and P = 0.220). We then examined the effect of three lower doses of rapamycin (10, 1, and 0.1 nM) on the life span and found a significant increase in life span for all three conditions (control versus 10 nM: χ2 = 22.72 and P < 0.0001; control versus 1 nM: χ2 = 16.23 and P < 0.0001; and control versus 0.1 nM: χ2 = 18.67 and P < 0.0001 in log-rank test) (Fig. 5C).
DISCUSSION
In this study, we demonstrated that longevity can be achieved by rapamycin treatment during postnatal development, which is associated with the delay of growth and development in evolutionarily distant model organisms: mice and the invertebrate Daphnia. Because many longevity interventions that target growth pathways and body size are inversely associated with longevity within species, our findings provide the first evidence that slowing mTOR-mediated growth results in longer life span.
There is much evidence that EL exposures may affect aging and life span in evolutionarily distant organisms. For example, mouse pups that get less nutrition in the crowded litter model are longer lived, although the mechanisms involved are unknown (34). There are also some extreme cases of longevity achieved by changing the diet during development of insects. Bees fed royal jelly during development become queens and live more than twice as long as untreated worker bees (35, 36). It was also reported that natural increase in reactive oxygen species during early development extends the life span of Caenorhabditis elegans by acting on H3K4me3 marks (37). These findings were further confirmed in mice, where conditional knockout of Sod2 during development in mice improved resistance to a subsequent oxidant challenge in adult animals (38). However, none of the previous studies involved pharmacological interventions, which makes interpretation of underlying mechanisms difficult. The fact that EL exposure to rapamycin, an mTOR inhibitor, during postnatal development increases the life span and inhibits the growth of two evolutionarily distant organisms supports the idea that mTOR is a crucial gene for organismal growth and that it mediates the relationship between pace of growth and longevity. However, rapamycin also has off-targets that affect growth and cell division, for example, STAT3 (signal transducer and activator of transcription 3) and c-MYC in human cells (39). Future studies may test whether mTOR-deficient mice respond to the EL rapamycin regimen similarly to wild-type animals.
The ways EL exposures translate into longevity phenotypes are unknown. One possibility is that the epigenetic memory of the treated tissues is supported by altered chromatin structure and DNAm. In our analysis, few changes in DNAm in the liver were retained by the treated animals all the way into old age. However, it is possible that the EL rapamycin treatment has a long-lasting impact on chromatin structure rather than DNAm as was recently shown in the mouse intestine in vivo (40). Alternatively, tissues other than the liver (analyzed here) may be involved in the epigenetic memory of the EL rapamycin treatment.
It is also possible that a boost of longevity mechanisms during EL is sufficient to attenuate aging phenotypes later in life. Both gene expression signatures and DNAm clocks revealed the strongest longevity effects of EL rapamycin in young animals, whereas these effects mostly were lost later in life. Driver somatic mutations may occur at a young age and evolve into a full-blown cancer only later in life (41). EL treatments can prevent or delay the appearance of somatic mutations, leading to delayed age-related clonal expansion and cancer. Another explanation may involve an improved proteostasis. A nuclear pore complex and histones are examples of proteins that are synthesized early in life and preserved throughout organismal life span in nonreplicative tissues (42, 43). At the same time, there is a recent finding that rapamycin improves translation precision in vitro (44). Rapamycin treatment may reduce the number of translation errors in long-lived proteins and improve their cumulative function throughout life span, even if given briefly at a young age. Future studies may test whether EL rapamycin does so in vivo. Another explanation for the longevity benefits of EL rapamycin might be a transient activation of pathways involved in stress and damage response. Transient increase in reactive oxygen species and mitohormesis induced during development were previously linked to an improved response to oxidative challenge in mice and to longevity in C. elegans (37, 38). In our gene expression data, xenobiotic metabolism and peroxisome were up-regulated in young males by EL rapamycin, which further suggests that activation of stress response during development by rapamycin could carry longevity benefits later in life.
Although we observed a significant decrease in body size following EL rapamycin, the effect was smaller than that usually observed in dwarf mouse models with deficiency in the growth hormone pathway. If longevity is linearly dependent on the effect size on body size, then it might partly explain why life span effects in our study were smaller than in dwarf mice (10% versus 40% in dwarf mice) (10, 11, 16). Future studies may test whether higher doses of rapamycin retard development more strongly, thereby extending life span even further. In addition, our study used genetically heterogeneous mice, whereas longevity studies of dwarf mice were done in the inbred background, which may also explain some difference in the effect sizes.
The longevity effect of EL rapamycin was also smaller than that of crowded litter experiments (median life span increased by 16%) (34), which tested EL exposures on mouse life span too. Females from crowded litters benefited more than males with regard to life span extension. This finding is different from our work where only males showed an extended life span. This difference could be due to different mechanisms of action between crowded litter, where pups get less nutrition, and EL rapamycin, where pups consumed the same amount of milk in treatment and control groups.
The longevity effect of EL rapamycin was biased toward males, whereas rapamycin given in adulthood and old age extended female life span more than that of males (19, 21). Longevity interventions may have different effects at different time periods, for example, calorie restriction fails to extend life span if started at old ages (45), and conditional knockout of the growth hormone receptor in adult animals extended maximum life span of females only (18). Further studies are needed to clarify this phenomenon.
The sex-specific effects of EL rapamycin are consistent with previous studies using growth manipulation where life span was measured. Unlike males, female mice benefited from conditional knockout of the growth hormone receptor during adulthood (18). This demonstrates that the benefits of growth hormone receptor knockout for female life span are a combination of EL and late-life effects, but only EL exposure matters for the male life span. Similarly, growth hormone–deficient males respond more strongly to EL growth hormone therapy than females (16). One explanation for these effects could be that females are naturally smaller, and thus, growth retardation will have a smaller impact on their phenotypes. We found that EL rapamycin affected growth of male mice more than females. Another explanation could be a nontrivial relationship between molecular mechanisms of growth control, sex, and longevity. In this study, we found that treated females reduce inflammation pathways at first but later in life activate them. This indicates an active gain of detrimental functions at the old age in females alongside the loss of pro-longevity effects following treatment. Last, in the mouse experiment, only one dosage of rapamycin was tested. However, our Daphnia experiments revealed that lower dosages can be as beneficial as the higher ones, with the highest rapamycin concentrations being detrimental. Future studies may explore the dosage effect of EL rapamycin on mouse life span.
MATERIALS AND METHODS
Animals
CByB6F1/J females and C3D2F1/J male mice were purchased from the Jackson Laboratory. Breeder pairs were set up and monitored daily. On the day of birth, males were removed from the cages, and the dams were given chow containing encapsulated rapamycin (42 ppm of active compound) or encapsulating material Eudragit at the same concentration (Rapamycin Holdings) in 5053 diet (TestDiet) and were fed ad libitum until 45 days of age. Then, all mice were subjected to a regular 5053 diet (TestDiet) and followed until death or moribund state. Mice that died as a result of fighting were excluded from survival analyses. Animals for cross-sectional studies were euthanized with CO2. Spleens were harvested and stored in cold phosphate-buffered saline until analysis, and liver samples were immediately frozen in liquid nitrogen and stored at −80°C. All experiments using mice were performed in accordance with the institutional guidelines for the use of laboratory animals and were approved by the Brigham and Women’s Hospital and Harvard Medical School Institutional Animal Care and Use Committees.
Food consumption and milk collection
To calculate food consumption by pups, dams were separated from 7-day-old pups for 6 hours; pups were weighted and returned to the dams for 1.5 hours and weighed again. Body weight change of pups post refeeding was considered as a proxy for food consumption. Milk was collected from the dams when the pups were 9 and 14 days old. A custom mouse milking machine was constructed on the basis of the Electric Breast Pump (Toogel). Dams were anesthetized with 3.5% isoflurane, placed on the heating pad, and injected intraperitoneally with 100 μl of 2 IU of oxytocin (Sigma-Aldrich) in sterile saline to stimulate milk production. The milk (50 to 100 μl) was then collected into microtubes and frozen at −80°C. Protein concentration was measured by Qubit with a Qubit Protein Assay Kit.
RNA sequencing
Total RNA was extracted from snap-frozen livers using the Direct-zol RNA Miniprep Kit (Zimo) following the manufacturer’s instructions. RNA was eluted with 50 μl of RNAse-free water. RNA concentration was measured with Qubit using an RNA HS Assay kit. Libraries were prepared with the TruSeq Stranded mRNA LT Sample Prep Kit according to the TruSeq Stranded mRNA Sample Preparation Guide. Libraries were quantified using the Bioanalyzer (Agilent) and sequenced with Illumina NovaSeq6000 S4 (2 × 150 base pairs) to get 20 M read depth coverage per sample. The BCL (binary base calls) files were converted into FASTQ using the Illumina package bcl2fastq. Fastq files were mapped to the mm10 (GRCm38.p6) mouse genome, and gene counts were obtained with STAR v2.7.2b (46). Statistical analyses for gene expression were performed with custom models from DEseq2 3.13 and edgeR 3.34.1 in R (resulting lists of DEGs are in table S3) (47). For GSEA, ranks for each gene were calculated as −log10(P value) multiplied by 1 if log fold change is positive and by −1 if negative. GSEA was performed with the clusterprofiler package in R (48). For signature association analysis and identification of longevity-associated genes perturbed by EL rapamycin, we filtered out genes with low number of reads, keeping only the genes with at least 10 reads in at least 50% of the samples, which resulted in 12,374 detected genes according to Entrez annotation.
Frailty index
Frailty index was measured as described in (26); frailty index score was calculated per the original protocol with small modifications. Because rapamycin-treated mice were significantly smaller than controls, the sd_scores for body weight and temperature were calculated in relation to the mean value of younger animals for each group independently. As such, we avoided bias in these parameters because of body size differences between the groups.
Gait speed
Mice were positioned in the regular mouse cage that had free space on one end and a food container on the other end. Recording started with the SprinTimer app (App Store), and upon the “GO” signal from the app, mice were let run voluntarily to the other end of the cage. In the vast majority of cases, mice run toward the food container, presumably to hide. The finish line was marked by the colored tape at the end of the cage. When mice reached the finish line, recording was stopped, and the time to finish was quantified on the basis of the pictures from the app. The time of finish was determined as the time when the mouse nose crossed the finish line. Each mouse was let run four times, and the median value was recorded. An attempt was considered successful if the mouse crossed the finish line without standing or turning 90° or more during the run. Fifteen seconds were recorded if the mice failed to finish the run in 15 s.
Glucose and insulin tolerance tests
For the glucose tolerance test, mice were placed into food-free cages for 16 hours (usually from 7:00 p.m. until 11:00 a.m. the next day). The next day, mice were weighted, marked, and bled from the tail, and the fasting blood glucose level was measured with a glucometer. Filtered 10% glucose in sterile saline solution (Sigma-Aldrich) was injected intraperitoneally at a final dose of 1 mg/kg of body weight. Injections were done at 30-s intervals between the mice. Glucose level was measured again at 20, 40, 60, and 120 min after the injection with a 30-s interval between the mice. Insulin tolerance test was done similarly, but mice fasted for 6 hours (usually from 10:00 a.m. to 4:00 p.m.) and were injected with insulin (0.75 U/kg; Eli Lilly) in sterile saline solution (Sigma-Aldrich).
DNA methylation
We analyzed DNA samples isolated from the livers with the recently developed mammalian methylation array (HorvathMammalMethylChip40) (49). Two clocks were applied in our analysis, including one clock based on the mouse liver (liver) and the other being a pan-tissue clock (pan) (50). Student’s two-tailed t test was used for statistical testing. For GSEA, first ranks for each CpG site were calculated as −log10(P value) multiplied by 1 if log fold change is positive and by −1 if negative. Then, ranks were aggregated by gene name, and the mean rank value for each gene was calculated. Only genes that were included in differential expression analysis were included in DNAm GSEA for comparative purposes. For GO and KEGG enrichment, genes with the sites significantly changed by treatment (P < 0.05) were overlapped with DEGs (P < 0.05), and an enrichment analysis of overlapped genes was carried out using all genes detected with the microarray as a background using enrichGO and enrichKEGG functions of clusterprofiler package.
Daphnia survival
D. magna animals were used in the Daphnia life span assay. An IL-MI-8 heat-tolerant clone was obtained from the Ebert Lab at the University of Basel, Switzerland, stock collection originating from a pond in Jerusalem, Israel. To collect synchronized cohorts, neonates born within 1 to 2 days were separated from the mothers, and their sex was determined at days 8 to 10. All mothers were also well fed and cultured under the same conditions (25°C). We used females for all experiments in this study. After drug treatment, 40 to 50 animals were randomly assigned to one of our developed culture tanks (51). All cultures (e.g., mother, neonates, and tank cultures) were maintained in ADaM water (52) in the 25°C incubator, exposed to a light cycle of 16 light hours followed by 8 dark hours, and fed every day the suspension of the green alga, Scenedesmus obliquus, at a concentration of 105 cells/ml (for one animal/20-ml density, amount of food prorated by population). Every sixth day, the water was changed, and the offspring were removed manually until animals were transferred to the culture platform. Details of the culture platform operation protocols are similar to those in the previously published protocols (51).
Treadmill
Mice were placed in one lane of treadmill and were required to run until exhaustion (no more than 15 min); this time was recorded. Mice were compelled to run by the use of a shock coil (set at frequency of 4 Hz and intensity of 1 mA) at one end of the treadmill, which the animals could feel if they let themselves be carried to the treadmill end. An early endpoint was when mice become so tired that they opt to be shocked rather than run on the treadmill. This was determined when a mouse was idle for 5 s, after which the mouse was removed from the treadmill and allowed to rest. The treadmill speed was set to 7.5 m/min.
Rotarod
Mice were placed on a rotarod apparatus for a maximum of 15 min. During the test, mice walk and maintain balance on the rotarod. Before testing, mice were acclimated for 5 min at rotation speed of 8 rpm, given the rest for an hour, and tested at starting rotation speed of 2 rpm with acceleration of 2 rpm/s. Each mouse was tested once. The time of fall was recorded.
Lipidomics
Analyses of polar and nonpolar lipids were conducted using an liquid chromatography–mass spectrometry (MS) system composed of a Shimadzu Nexera X2 U-HPLC (Shimadzu Corp.) coupled to an Exactive Plus orbitrap mass spectrometer (Thermo Fisher Scientific). Livers were homogenized using a Qiagen Tissuelyser at a 1:20 mass-to-volume (mg/μl) ratio in a 1:20 (v/v) solution of water:isopropyl alcohol. After homogenization for 4 min at 20 Hz, homogenates were incubated for 1 hour before centrifugation for 10 min at 10,000 rcf (relative centrifugal force). The supernatant (2 μl) was injected for analysis. Serum samples (10 μl) were extracted for lipid analyses using 190 μl of isopropanol containing 1,2-didodecanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) as an internal standard. After centrifugation, 2 μl of supernatants was injected directly onto a 100 mm–by–2.1 mm, 1.7-μm ACQUITY BEH C8 column (Waters). The column was eluted isocratically with 80% mobile phase A (95:5:0.1 v/v/v 10 mM ammonium acetate/methanol/formic acid) for 1 min followed by a linear gradient to 80% mobile phase B (99.9:0.1 v/v methanol/formic acid) over 2 min, a linear gradient to 100% mobile phase B over 7 min, and then 3 min at 100% mobile phase B. MS analyses were carried out using electrospray ionization in the positive ion mode using full scan analysis over 200 to 1100 mass/charge ratio at 70,000 resolution and 3-Hz data acquisition rate. Other MS settings were as follows: sheath gas, 50; in-source collision-induced dissociation, 5 eV; sweep gas, 5; spray voltage, 3 kV; capillary temperature, 300°C; S-lens radio frequency, 60; heater temperature, 300°C; microscans, 1; automatic gain control target, 16; and maximum ion time, 100 ms. Raw data were processed using TraceFinder software (Thermo Fisher Scientific) for targeted peak integration and manual review of a subset of identified lipids and using Progenesis QI (Nonlinear Dynamics) for peak detection and integration of both lipids of known identity and unknowns. Lipid identities were determined on the basis of comparison to reference plasma extracts and are denoted by total number of carbons in the lipid acyl chain(s) and total number of double bonds in the lipid acyl chain(s).
Acknowledgments
We thank members of the Gladyshev laboratory for their discussion.
Funding: This work was supported by NIH grants AG065403 and AG067782 to V.N.G. L.P. and Y.C. were supported by Paul G. Allen Frontiers Group and McKenzie Family Charitable Trust.
Author contributions: A.V.S. designed and performed the mouse experiments, interpreted the data, analyzed the data, and drafted the manuscript. Y.C. performed the Daphnia experiments supervised by L.P. A.V.S. and A.K. conceived the project. A.D. and C.B.C. performed lipidomics experiments. A.K., J.P.C., A.T., J.R.P., J.G., L.P., and S.H. performed the experiments, analyzed the data, and revised the manuscript. V.N.G. supervised the overall project, designed the experiments, provided research materials, and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Raw sequencing and processed data are available through GEO under accession number GSE207865. Raw and processed DNA methylation data are available through GEO under accession number GSE210308.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S5
REFERENCES AND NOTES
- 1.Ma S., Gladyshev V. N., Molecular signatures of longevity: Insights from cross-species comparative studies. Semin. Cell Dev. Biol. 70, 190–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fushan A. A., Turanov A. A., Lee S.-G., Kim E. B., Lobanov A. V., Yim S. H., Buffenstein R., Lee S.-R., Chang K.-T., Rhee H., Kim J.-S., Yang K.-S., Gladyshev V. N., Gene expression defines natural changes in mammalian lifespan. Aging Cell 14, 352–365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selman C., Nussey D. H., Monaghan P., Ageing: It’s a dog’s life. Curr. Biol. 23, R451–R453 (2013). [DOI] [PubMed] [Google Scholar]
- 4.He Q., Morris B. J., Grove J. S., Petrovitch H., Ross W., Masaki K. H., Rodriguez B., Chen R., Donlon T. A., Willcox D. C., Willcox B. J., Shorter men live longer: Association of height with longevity and FOXO3 genotype in american men of Japanese ancestry. PLOS ONE 9, e94385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaras T. T., Shorter height is related to lower cardiovascular disease risk – A narrative review. Indian Heart J. 65, 66–71 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C.-W., Hwang D., Martin-Montalvo A., Saavedra J., Ingles S., de Cabo R., Cohen P., Longo V. D., Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 3, 70ra13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R., A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Tatar M., Kopelman A., Epstein D., Tu M. P., Yin C. M., Garofalo R. S., A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Clancy D. J., Gems D., Harshman L. G., Oldham S., Stocker H., Hafen E., Leevers S. J., Partridge L., Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Brown-Borg H. M., Borg K. E., Meliska C. J., Bartke A., Dwarf mice and the ageing process. Nature 384, 33 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Sun L. Y., Spong A., Swindell W. R., Fang Y., Hill C., Huber J. A., Boehm J. D., Westbrook R., Salvatori R., Bartke A., Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2, e01098 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coschigano K. T., Holland A. N., Riders M. E., List E. O., Flyvbjerg A., Kopchick J. J., Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144, 3799–3810 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Flurkey K., Papaconstantinou J., Miller R. A., Harrison D. E., Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U.S.A. 98, 6736–6741 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller R. A., Chrisp C., Atchley W., Differential longevity in mouse stocks selected for early life growth trajectory. J. Gerontol. A Biol. Sci. Med. Sci. 55, B455–B461 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Ricklefs R. E., Embryo growth rates in birds and mammals. Funct. Ecol. 24, 588–596 (2010). [Google Scholar]
- 16.Sun L. Y., Fang Y., Patki A., Koopman J. J., Allison D. B., Hill C. M., Masternak M. M., Darcy J., Wang J., McFadden S., Bartke A., Longevity is impacted by growth hormone action during early postnatal period. eLife 6, e24059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panici J. A., Harper J. M., Miller R. A., Bartke A., Spong A., Masternak M. M., Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 24, 5073–5079 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junnila R. K., Duran-Ortiz S., Suer O., Sustarsic E. G., Berryman D. E., List E. O., Kopchick J. J., Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology 157, 4502–4513 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Miller R. A., Harrison D. E., Astle C. M., Fernandez E., Flurkey K., Han M., Javors M. A., Li X., Nadon N. L., Nelson J. F., Pletcher S., Salmon A. B., Sharp Z. D., Van Roekel S., Winkleman L., Strong R., Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y., Hill C. M., Darcy J., Reyes-Ordoñez A., Arauz E., McFadden S., Zhang C., Osland J., Gao J., Zhang T., Frank S. J., Javors M. A., Yuan R., Kopchick J. J., Sun L. Y., Chen J., Bartke A., Effects of rapamycin on growth hormone receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 115, E1495–E1503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A., Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitto A., Ito T. K., Pineda V. V., LeTexier N. J., Huang H. Z., Sutlief E., Tung H., Vizzini N., Chen B., Smith K., Meza D., Yajima M., Beyer R. P., Kerr K. F., Davis D. J., Gillespie C. H., Snyder J. M., Treuting P. M., Kaeberlein M., Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegmund S. E., Yang H., Sharma R., Javors M., Skinner O., Mootha V., Hirano M., Schon E. A., Low-dose rapamycin extends lifespan in a mouse model of mtDNA depletion syndrome. Hum. Mol. Genet. 26, 4588–4605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choo A. Y., Yoon S.-O., Kim S. G., Roux P. P., Blenis J., Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U.S.A. 105, 17414–17419 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux P. P., Topisirovic I., Signaling pathways involved in the regulation of mRNA translation. Mol. Cell. Biol. 38, e00070-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz M. B., Kane A. E., Mitchell S. J., MacArthur M. R., Warner E., Vogel D. S., Mitchell J. R., Howlett S. E., Bonkowski M. S., Sinclair D. A., Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat. Commun. 11, 4618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K., Blodgett J. M., Theou O., Sun M. H., Feridooni H. A., Mitnitski A., Rose R. A., Godin J., Gregson E., Howlett S. E., A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci. Rep. 7, 43068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane A., Schultz M. B., Mitchell S., MacArthur M., Mitchell J., Howlett S. E., Bonkowski M. S., Sinclair D., Machine learning analysis of mouse frailty for prediction of biological age and life expectancy. Innov. Aging 3, S903 (2019). [Google Scholar]
- 29.Houde V. P., Brûlé S., Festuccia W. T., Blanchard P.-G., Bellmann K., Deshaies Y., Marette A., Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 59, 1338–1348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon S., Jeon J.-S., Kim S. B., Hong Y.-K., Ahn C., Sung J.-S., Choi I., Rapamycin up-regulates triglycerides in hepatocytes by down-regulating Prox1. Lipids Health Dis. 15, 41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindyapina A. V., Castro J. P., Barbieri A., Strelkova O. S., Paulo J. A., Kerepesi C., Petrashen A. P., Mariotti M., Meer M., Hu Y., Losyev G., Indzhykulian A. A., Gygi S. P., Sedivy J. M., Manis J. P., Gladyshev V. N., Integrative analysis reveals aged clonal B cells, microenvironment and c-Myc activation in the origin of age-related lymphoma. Immune Aging Cancer 10.1101/2021.02.23.432500 (2021). [Google Scholar]
- 32.Tyshkovskiy A., Bozaykut P., Borodinova A. A., Gerashchenko M. V., Ables G. P., Garratt M., Khaitovich P., Clish C. B., Miller R. A., Gladyshev V. N., Identification and application of gene expression signatures associated with lifespan extension. Cell Metab. 30, 573–593.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D. Ebert, National Center for Biotechnology Information (U.S.), Ecology, epidemiology, and evolution of parasitism in Daphnia (National Library of Medicine (US), National Center for Biotechnology Information, Bethesda, MD, 2005;www.ncbi.nlm.nih.gov/books/NBK2036).
- 34.Sun L., Sadighi Akha A. A., Miller R. A., Harper J. M., Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci. 64, 711–722 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunugi H., Mohammed Ali A., Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 20, 4662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W., Tian Y., Han M., Miao X., Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. PeerJ. 5, e3118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazopoulou D., Knoefler D., Zheng Y., Ulrich K., Oleson B. J., Xie L., Kim M., Kaufmann A., Lee Y.-T., Dou Y., Chen Y., Quan S., Jakob U., Developmental ROS individualizes organismal stress resistance and lifespan. Nature 576, 301–305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox C. S., McKay S. E., Holmbeck M. A., Christian B. E., Scortea A. C., Tsay A. J., Newman L. E., Shadel G. S., Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab. 28, 776–786.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L., Yan Y., Lv H., Li J., Wang Z., Wang K., Wang L., Li Y., Jiang H., Zhang Y., Rapamycin targets STAT3 and impacts c-Myc to suppress tumor growth. Cell Chem. Biol. 29, 373–385.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Lu Y.-X., Regan J. C., Eßer J., Drews L. F., Weinseis T., Stinn J., Hahn O., Miller R. A., Grönke S., Partridge L., A TORC1-histone axis regulates chromatin organisation and non-canonical induction of autophagy to ameliorate ageing. eLife 10, e62233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams N., Lee J., Mitchell E., Moore L., Baxter E. J., Hewinson J., Dawson K. J., Menzies A., Godfrey A. L., Green A. R., Campbell P. J., Nangalia J., Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 602, 162–168 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Fornasiero E. F., Mandad S., Wildhagen H., Alevra M., Rammner B., Keihani S., Opazo F., Urban I., Ischebeck T., Sakib M. S., Fard M. K., Kirli K., Centeno T. P., Vidal R. O., Rahman R.-U., Benito E., Fischer A., Dennerlein S., Rehling P., Feussner I., Bonn S., Simons M., Urlaub H., Rizzoli S. O., Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 9, 4230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyama B. H., Savas J. N., Park S. K., Harris M. S., Ingolia N. T., Yates J. R., Hetzer M. W., Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Miguel V. E., Lujan C., Espie--Caullet T., Martinez-Martinez D., Moore S., Backes C., Gonzalez S., Galimov E. R., Brown A. E. X., Halic M., Tomita K., Rallis C., von der Haar T., Cabreiro F., Bjedov I., Increased fidelity of protein synthesis extends lifespan. Cell Metab. 33, 2288–2300.e12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipman R. D., Smith D. E., Bronson R. T., Blumberg J., Is late-life caloric restriction beneficial? Aging Milan Italy 7, 136–139 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R., STAR: Ultrafast universal RNA-seq aligner. Bioinforma. Oxf. Engl. 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy D. J., Chen Y., Smyth G. K., Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu G., Wang L.-G., Han Y., He Q.-Y., clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arneson A., Haghani A., Thompson M. J., Pellegrini M., Kwon S. B., Vu H., Yao M., Li C. Z., Lu A. T., Barnes B., Hansen K. D., Zhou W., Breeze C. E., Ernst J., Horvath S., A mammalian methylation array for profiling methylation levels at conserved sequences. Nat. Commun. 13, 783 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mozhui K., Lu A. T., Li C. Z., Haghani A., Sandoval-Sierra J. V., Williams R. W., Horvath S., Genetic analyses of epigenetic predictors that estimate aging, metabolic traits, and lifespan. eLife 11, e75244 (2021). [Google Scholar]
- 51.Cho Y., Jonas-Closs R. A., Yampolsky L. Y., Kirschner M. W., Peshkin L., Smart Tanks: An intelligent high-throughput intervention testing platform in Daphnia. Aging Cell 21, e13571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klüttgen B., Dülmer U., Engels M., Ratte H. T., ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743–746 (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Tables S1 to S5