Abstract
Cell-released biological nanoparticles, including extracellular vesicles (EVs), are emerging drug carriers with high complexity. EV-based drug delivery has the potential to efficiently exploit intrinsic mechanisms for molecular transport in the body. Integrating the expanding knowledge of EV biology and manufacturing with clinical insights from synthetic nanoparticles is likely to substantially advance the field of drug delivery.
Synthetic nanoparticles have been widely used for clinical drug delivery since the 1990s.1 Nanodelivery strategies can be designed to improve the spatial and temporal distribution of therapeutic agents in the body, which results in decreased side effects and/or increased therapeutic efficacy. Improved delivery has been achieved through optimization of the size, shape and surface properties of nanocarriers. However, complex molecular targeting strategies have repeatedly failed in clinical trials; for example, BIND-014, a polymeric nanoparticle with surface ligands that binds to the tumour-enriched prostate-specific membrane antigen (PSMA).1 The clinical failure of ligand-targeted nanodelivery approaches is often attributed to complex interactions between nanoparticles and the biological environment, including the formation of a protein corona that can mask surface ligands and trigger immunological recognition.1 Such interactions may not be apparent in preclinical studies, because major components of the protein corona, for example, complement proteins, substantially differ in humans and animal models. In addition, ligand type, orientation, density and surface patterning are crucial for optimal delivery and binding to target molecules. Thus, computational tools are often necessary to understand how design parameters affect nano-bio interactions.
Optimal nanoparticle design may require a level of complexity similar to that of the biological environment to enable successful navigation of numerous molecular, cellular and tissue components. However, complex designs are typically incompatible with cost-effective and time-efficient clinical-grade manufacturing processes. The majority of nanomedicines on the market are simple liposomes, which consist of up to two therapeutic agents and up to four lipid or lipidoid types that form a spherical bilayer. Although liposomes are clinically used for the delivery of a wide variety of therapeutic agents, including small molecules, peptides and RNA,1 realization of the true potential of nanodelivery to improve patient outcomes awaits implementation of more complex, multipronged designs that allow navigation of biological realities without introducing too many variables. The most basic functional features to consider…and display on the particle surface…are those that avoid rapid clearance, enhance local retention by specific cells (“targeting”), and maximize the likelihood of transmission of one or more mission-critical signals or drugs.
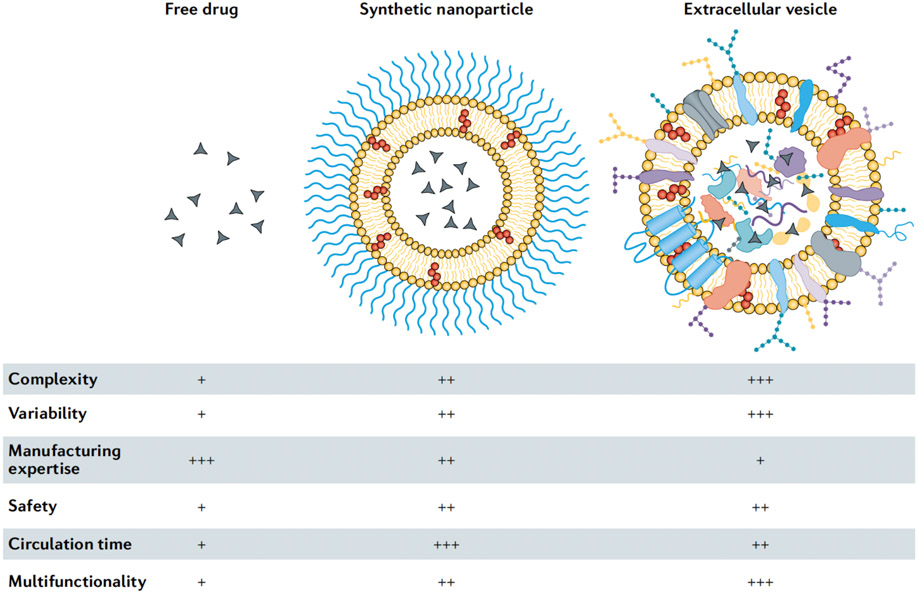
Extracellular vesicles (EVs) are naturally occurring nanoparticles released by prokaryotic and eukaryotic cells.2,3 EVs resemble liposomes in terms of size, shape and structure, but have more complex bilayers, containing up to hundreds of different lipid, protein and carbohydrate types, as well as internal cargo and surface-associated molecules (FIG. 1). EVs play a major role in short and long-distance intercellular communication in various (patho)physiological processes.2 The ability of EVs to transport biomolecules to recipient cells has made them attractive for drug delivery purposes. EVs can be obtained from the conditioned medium of cultured cells or from biological tissues or fluids, and various methods, such as electroporation, extrusion and sonication, have been used for loading therapeutic agents into EVs.3 Multiple design and manufacturing challenges in nanomedicine could potentially be bypassed by exploiting the evolutionary selection of EV structures for transport of molecular cargo in the body. However, the potential of EVs for drug delivery remains uncertain owing to challenges in EV isolation and characterization, which have impeded basic and translational studies. In particular, the heterogeneity among EV types and the existence of other biological nanoparticles with overlapping characteristics make EV isolation difficult.2 In addition, caution should be taken in the premature portrayal of EVs as superior drug carriers to synthetic nanoparticles in terms of biocompatibility and site-specific delivery.
Figure 1. Characteristics of free drugs, clinically approved synthetic nanoparticles and extracellular vesicles.
Low (+), medium (++), and high (+++).
Pharmacokinetics
A challenge in nanomedicine is rapid macrophage-mediated hepatic clearance of synthetic nanoparticles from the circulation.1 Sequestration of nanocarriers in the liver impedes site-specific delivery mechanisms, such as size- and shape-based targeting of nanocarriers to tumours.1 Many clinically approved synthetic nanoparticles have been functionalized with polyethylene glycol (PEG), which reduces macrophage uptake and substantially prolongs the circulation half-life from hours to days.1 The use of PEG also has several disadvantages, and EV-based drug delivery has been proposed as an alternative strategy to avoid immunological clearance owing to the intrinsic nature of the carrier.1 However, multiple studies have demonstrated that exogenous EVs also undergo rapid hepatic clearance and that they have circulation half-lives of only a few minutes.3 It is possible that EV isolation, drug loading and labelling procedures make EVs more susceptible to rapid uptake by macrophages. Additionally, it remains largely unknow how EV biogenesis, cellular origin and biomolecular composition affect pharmacokinetics. Certain EV types may have superior circulation half-lives and/or site-specific targeting mechanisms that outcompete hepatic clearance. Experimental design in terms of appropriate EV isolation, authentication and characterization is crucial for evaluating the potential of EVs as drug carriers. A direct comparison with synthetic nanoparticles is often omitted; however, this would allow the identification of benefits of EV delivery in terms of drug toxicity and efficacy.
In addition to biodistribution profiles that may outperform synthetic carriers, EV-based drug delivery has another benefit; the possibility to exploit cellular processes for drug loading and surface modifications.3 Cells can be genetically engineered to express and package protein- and RNA-based therapeutic agents and/or targeting ligands in EVs.3 The use of the cellular machinery for drug loading and EV surface modifications can be advantageous, because RNA and proteins may degrade or become damaged during nanoparticle synthesis. Additionally, EV endocytosis pathways or fusion events with recipient cell membranes may facilitate intracellular delivery, that is, targeting therapeutic agents to specific intracellular compartments or organelles. In a recent study, cells were genetically engineered to enrich specific small interfering RNAs (siRNAs) in EVs, which led to a more than tenfold improvement in functional siRNA delivery to mice compared to synthetic lipid nanocarriers.4 The superiority of EV carriers was attributed to the ability of siRNA to escape the lysosome and localize in the cytoplasm.4 Notably, siRNA-mediated knockdown efficiency was dependent on both the EV source and the recipient cell type. Some cell types, such as resident macrophages, accumulated high levels of EV-delivered siRNA, but displayed minimal target knockdown.4 These results suggest that EV heterogeneity in terms of both biodistribution and intracellular delivery have to be considered.
Biocompatibility
Nanomedicines often cause fewer side effects than free drugs owing to less exposure of healthy tissue to therapeutic agents and by obviating the need for toxic excipients to solubilize non-water-soluble small molecules.1 In fact, several nanomedicines have received clinical approval based on equivalent efficacy but improved safety compared with freely administered small molecules.1 Nevertheless, clinically approved nanoparticles that contain PEG can activate the complement system, and in certain cases, lead to life-threatening hypersensitivity reactions, which can usually be mitigated by lowering the infusion rate.1
EV-based therapeutics have yet to receive clinical approval, but early phase clinical trials have assessed the effects of autologous dendritic cell-derived EVs for cancer immunotherapy and of allogeneic mesenchymal stem cell-derived EVs for regenerative and anti-inflammatory applications.5 The majority of these trials reported mild to moderate side effects, generally concluding that EV administration was safe.5 Allogeneic EVs have been proposed to pose a risk because they may elicit immune reactions in recipients. However, blood and plasma also contain high concentrations of EVs (with estimates as high as 1010 EVs per mL),6 which are released into circulation from all cell types in the body. Indeed, plasma and blood transfusions seldom cause adverse immune reactions, indicating that allogeneic EVs are unlikely to present a safety risk.3 However, a disadvantage of allogeneic EVs may be accelerated hepatic clearance compared with autologous EVs. In addition, although initial safety results from clinical trials are promising, endogenous cargo, which is challenging to remove without damaging the EV structure, could trigger unwanted effects. On the contrary, endogenous cargo could contribute to additive or synergistic effects, especially if EVs are obtained from cells that display therapeutic properties, such as mesenchymal stem cells.
Outlook
Currently, there are more than 50 clinically approved nanomedicines, all of which are based on simple designs encompassing a small number of components. The synthesis of more complex nanoparticles that resemble biological structures with hundreds of functional components is unlikely to be compatible with large-scale clinical-grade manufacturing. EVs are a promising alternative to realize the potential of multipronged and multifunctional drug carriers. In addition to identifying EVs with favourable delivery properties, manufacturing and scale-up challenges need to be overcome, including the variability of cell culture, which is the predominant source of EVs. The use of immortalized cell lines can minimize variability, but may introduce safety concerns, because immortalization agents may be loaded into EVs. Large-scale culture is further needed, for example, stirred-tank or fixed-bed bioreactors, as well as chemically defined culture medium or medium with xeno-free supplements, for example, human platelet lysate,7,8 for which contributions of lysate materials to (or against) therapeutic effects should be considered. EV mimetics can also be formed by destructive disruption of cells by extrusion or sonication; however, this process impacts membrane topology. Platelets and red blood cells may be particularly suitable sources for EV mimetics, because they lack nuclear material, which could act as a danger signal for the immune system. Instead of cell culture, human tissues and fluids can be used as EV sources. Indeed, large-scale clinical manufacturing of biological nanoparticles from human plasma has been demonstrated, for example, for lipoprotein therapy trials with thousands of participants.9 However, separation of EVs from biological fluids and tissues is challenging owing to their more complex composition compared to conditioned cell culture media. For certain applications, highly pure EV preparations may not be needed, and efficacy may even decline if co-factors are removed. For example, it was demonstrated that the entire secretome obtained from gamma-irradiated peripheral blood mononuclear cells displayed superior therapeutic efficacy to the EV fraction alone.10 In other cases, it may be necessary to separate EVs from components that interfere with EV function and drug loading or even exert deleterious effects. Regulatory agencies are still in the process of determining release-criteria for EV-based products, and the presence of other biological components is not necessarily a regulatory hurdle as long as physicochemical and potency tests verify batch-to-batch consistency and efficacy.10 Finally, non-human EV sources, such as milk, fruits and algae, have been investigated, but may have restricted applications owing to possible immune reactions if administered intravenously.
Therapeutic EVs in clinical trials are usually separated by ultracentrifugation (including differential and density) and tangential flow filtration.5 However, the majority of EV-based clinical trials have involved only a small number of participants, and ultracentrifugation may not be scalable for larger studies and commercial manufacturing. Additionally, ultracentrifugation-based protocols can result in EV damage and aggregation.3 Tangential flow filtration is compatible with large-scale manufacturing, and achieves both volume reduction and partial purification, while preserving EV structures.3 Post-purification, EVs can be further engineered by loading therapeutic agents (clinical-grade protocols have been reported5) or by fusion with synthetic nanoparticles in a ‘best-of-both-worlds’ approach. Most clinical trials have used saline or sucrose cryoprotectant buffers for EV storage at −80 °C.5 Alternatively, lyophilization may be suitable for some EV cargos and functions and allows simplified storage, distribution and point-of-care reconstitution.
Increased dialogue and collaboration between the synthetic nanomedicine and EV fields is likely to fuel the successful integration of emerging EV biology with decades of expertise in the clinical failures and successes of synthetic drug delivery.
Acknowledgements
This work is partially supported by the Mayo Clinic Center for Regenerative Medicine in Florida (JW), the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), United States under award numbers R21AI152318 (JW) and R01AI144997 (KWW), and the NIH Common Fund through the Office of Strategic Coordination/Office of the NIH Director under award number UG3CA241694 (KWW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Competing interests
The authors declare no competing interests
References
- 1.Wolfram J & Ferrari M Clinical cancer nanomedicine. Nano Today 25, 85–89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Niel G, D'Angelo G & Raposo G Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 19, 213–228, doi: 10.1038/nrm.2017.125 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Walker S et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9, 8001–8017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reshke R et al. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat Biomed Eng 4, 52–68, doi: 10.1038/s41551-019-0502-4 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Elsharkasy OM et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev, doi: 10.1016/j.addr.2020.04.004 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Johnsen KB, Gudbergsson JM, Andresen TL & Simonsen JB What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim Biophys Acta Rev Cancer 1871, 109–116, doi: 10.1016/j.bbcan.2018.11.006 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Witwer KW et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles 8, 1609206, doi: 10.1080/20013078.2019.1609206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K & Rohde E Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int J Mol Sci 18, doi: 10.3390/ijms18061190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busatto S et al. Lipoprotein-based drug delivery. Adv Drug Deliv Rev, doi: 10.1016/j.addr.2020.08.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laggner M et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell. Res. Ther 11, 9, doi: 10.1186/s13287-019-1524-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]