Abstract
Oxidation of malate to oxaloacetate in Escherichia coli can be catalyzed by two enzymes: the well-known NAD-dependent malate dehydrogenase (MDH; EC 1.1.1.37) and the membrane-associated malate:quinone-oxidoreductase (MQO; EC 1.1.99.16), encoded by the gene mqo (previously called yojH). Expression of the mqo gene and, consequently, MQO activity are regulated by carbon and energy source for growth. In batch cultures, MQO activity was highest during exponential growth and decreased sharply after onset of the stationary phase. Experiments with the β-galactosidase reporter fused to the promoter of the mqo gene indicate that its transcription is regulated by the ArcA-ArcB two-component system. In contrast to earlier reports, MDH did not repress mqo expression. On the contrary, MQO and MDH are active at the same time in E. coli. For Corynebacterium glutamicum, it was found that MQO is the principal enzyme catalyzing the oxidation of malate to oxaloacetate. These observations justified a reinvestigation of the roles of MDH and MQO in the citric acid cycle of E. coli. In this organism, a defined deletion of the mdh gene led to severely decreased rates of growth on several substrates. Deletion of the mqo gene did not produce a distinguishable effect on the growth rate, nor did it affect the fitness of the organism in competition with the wild type. To investigate whether in an mqo mutant the conversion of malate to oxaloacetate could have been taken over by a bypass route via malic enzyme, phosphoenolpyruvate synthase, and phosphenolpyruvate carboxylase, deletion mutants of the malic enzyme genes sfcA and b2463 (coding for EC 1.1.1.38 and EC 1.1.1.40, respectively) and of the phosphoenolpyruvate synthase (EC 2.7.9.2) gene pps were created. They were introduced separately or together with the deletion of mqo. These studies did not reveal a significant role for MQO in malate oxidation in wild-type E. coli. However, comparing growth of the mdh single mutant to that of the double mutant containing mdh and mqo deletions did indicate that MQO partly takes over the function of MDH in an mdh mutant.
In Escherichia coli, several enzymes or pathways are able to convert malate to oxaloacetate. The NAD-dependent (cytoplasmic) malate dehydrogenase (MDH; EC 1.1.1.37) has always been considered to be the principal malate-oxidizing enzyme in the citric acid cycle (tricarboxylic acid [TCA] cycle) of this organism (9). Recently, a malate:quinone-oxidoreductase (MQO; EC 1.1.99.16) which is an essential enzyme in the TCA cycle of Corynebacterium glutamicum (reference 23 and accompanying paper) was described. MQO is a flavin adenine dinucleotide (FAD)- and lipid-dependent peripheral membrane protein catalyzing the oxidation of l-malate to oxaloacetate. The electrons are donated to the electron transfer chain at the level of quinones. This reaction is essentially irreversible (17). In C. glutamicum, MQO and the cytoplasmic NAD-dependent malate dehydrogenase, which is capable of reversible oxidation of malate to oxaloacetate, are active at the same time. Due to the different natures of their electron acceptors, the Gibbs standard free energies for the reactions catalyzed by MQO and MDH differ radically. For the MDH-dependent reaction, the standard free energy is equal to +28.6 kJ · mol−1, and for the MQO-dependent reaction, it is −55 or −18.9 kJ · mol−1, depending on whether the acceptor is ubiquinone or menaquinone, respectively (23). These figures suggest that, when both enzymes are active, under standard-state conditions, MQO should catalyze the oxidation of malate whereas MDH should catalyze the reduction of oxaloacetate. In C. glutamicum, it was, indeed, clearly demonstrated that MQO is the principal enzyme responsible for the oxidation of malate to oxaloacetate, whereas there were indications that MDH catalyzes oxaloacetate reduction (accompanying paper). MDH could be forced to function in the direction of malate oxidation only when the mqo gene was deleted and the NAD concentration in the cell was increased by addition of nicotinamide to the growth medium.
In E. coli, an MQO-like enzyme activity (at the time usually referred to as malate oxidase) was described before. This activity was thought to be induced to significant levels only in certain mutants lacking MDH activity (12). Because an MDH-negative mutant which still contained inactive MDH protein exhibited very high MQO activity, it was surmised that the expression of the gene encoding MQO was regulated by substrates or products of the MDH reaction (25). MQO was partially purified from an MDH-negative E. coli strain which overexpressed this enzyme (25). Properties of the partially purified protein were similar to those found for the purified protein of C. glutamicum (23). Initial studies by us, using a more sensitive MQO assay and optimized assay conditions (unpublished results), indicated that in contrast with previous observations MDH and MQO were active at the same time in E. coli. Because of this observation, and regarding the findings in C. glutamicum, a reinvestigation of the role of MDH and more detailed studies of the role of MQO in E. coli were deemed necessary.
The open reading frame (ORF) yojH (EMBL accession no. AE000310) found on the genome of E. coli has a high similarity (43.9% identical residues) to the mqo gene of C. glutamicum (23). This ORF (here called mqo) was used to overexpress MQO activity in E. coli. Furthermore, by using a construct of the mqo promoter region fused with the reporter gene lacZ, the regulation of expression was examined. To investigate the importance of MQO and MDH for the physiology of E. coli, and to investigate the supposed regulation of mqo expression by MDH, deletions of mqo and mdh were constructed on the chromosome. To assess the roles of MDH and MQO in malate oxidation, site-directed deletions of the genes coding for the malic enzymes and for phosphoenolpyruvate (PEP) synthase were constructed to block alternative metabolic routes leading from malate to oxaloacetate.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli DH5α (supE44 ΔlacU169 Φ80lacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA1) (31) was used for standard genetic experiments. Measurement of MQO activity and gene replacements were carried out in E. coli K-12 strains DSM 5698 (ATCC 25404) (F− λ−) and MC4100 [F− λ− araD139 Δ(argF-lac) U169 rpsL150 flb5301 deoC1 ptsF25 rbsR] (4, 30). E. coli PC2 (parent, MC4100; Δfnr2) and PC35 (parent, MC4100; ΔarcA Kanr) were a generous gift from R. P. Gunsalus (7).
Cells were routinely grown on Luria broth (LB) (31) or on Neidhardt minimal medium (NMM) (26) containing carbon source as indicated. When required, the media were supplemented with 100 μg of carbenicillin per ml or 50 μg of kanamycin per ml. Controlled batch fermentations were carried out in a 1.5-liter vessel. The culture was maintained at 37°C, and oxygen was maintained at 5.5 mg of O2 (±10%) liter−1 by regulating the sparge flow.
Construction of pECmqo.
Oligonucleotides used are listed in Table 1. The mqo ORF (EMBL accession no. AE000310) was amplified by PCR from the E. coli MC4100 genome using YojH_01 as forward primer and YojH_02 as reverse primer. PCR conditions were a standard 30 cycles consisting of a temperature profile of 30 s at 94°C, 30 s at 60°C, and 2 min at 72°C. The YojH_01 primer is complementary to a region −101 bp in front of the +1 ATG start codon of the mqo ORF, and YojH_02 is located 92 bp following the TAA stop codon. The resulting PCR fragment was cloned in the vector pET324 (36), resulting in pECmqo for expression in E. coli. For expression in C. glutamicum, the fragment was cloned in pJCBW1, a derivative of pJC1 (8, 20) containing the lacZ complementation region and multiple cloning site from pSK+ (Stratagene), using EcoRV.
TABLE 1.
Oligonucleotides used for PCRs
| Designation | Sequence | bp on E. coli chromosome (2)a |
|---|---|---|
| Y_01 | 5′-GCTGGATGAATGGGCGGCGG-3′ | c2305202–2305221 |
| Y_04 | 5′-CGCGGATCCCCGGTTTCAACGATGATG-3′ | 2302220–2302240 |
| YojH_01 | 5′-GGATCCGTTCATGCCGCGCAAATC-3′ | c2304816–2304829 |
| YojH_02 | 5′-GTTACGCCGCATCCAACATC-3′ | 2303020–2303039 |
| F_01 | 5′-GGGGCCGGAGGGTAAACC-3′ | c2305381–2305398 |
| F_02 | 5′-GAATCCGTAATCATGGTCATCTTGTTAATGCCTTAC-3′ | 365511–365530 and 2304772–2304791 |
| F_03 | 5′-GTAAGGCATTAACAAGATGACCATGATTACGGATTC-3′ | c365511–365530 and c2304772–2304791 |
| F_04 | 5′-GCGGAATTCTTAAACGCCATCAAAAATAATTCG-3′ | 365090–365110 |
| Komdh_01 | 5′-CGCGGATCCGCGAGACTTTAGACT-3′ | c3382466–3382484 |
| Komdh_02 | 5′-CGCGAATTCCAACAGGTGGTGAC-3′ | 3382097–3382114 |
| Komdh_03 | 5′-CGCGAATTCCTGAATGGTGACCTGCAG-3′ | c3380865–3380886 |
| Komdh_04 | 5′-CGCGGATCCGGTGTAATCATGGC-3′ | 3380073–3380091 |
| Mao1_01 | 5′-GCATGGATCCAGTATCAGG-3′ | c1553499–1553517 |
| Mao1_02 | 5′-GCGGATCCGGCAGTACCATACCTTC-3′ | 1552163–1552182 |
| Mao2_01 | 5′-CCGGGATCCTCAACGGCTTGCGC-3′ | c2575862–2575879 |
| Mao2_02 | 5′-CAGGATCCATGTTCGGCATCACC-3′ | 2574297–2574314 |
| Pps_01 | 5′-CGCGGATCCGCCAGTGGATTATC-3′ | c1784850–1784867 |
| Pps_02 | 5′-CGCGGATCCCGACATATTTGCCC-3′ | 1782889–1782906 |
c, complement.
Construction of Φ(mqo-lacZ).
Oligonucleotides used are listed in Table 1. A construct was made in which the lacZ gene was fused to the promoter region of mqo. Since the mqo ORF has at least four possible start sites (ATG), and since it is unclear which one is used by the organism, the most probable start had to be determined by an educated guess. To this end, the hypothetical protein sequence of the mqo ORF was compared to that of MQO from C. glutamicum (23). The amino-terminal amino acid sequence of the purified MQO protein of C. glutamicum was SDSPK (D. Molenaar, unpublished results). This sequence can be recognized in the translated DNA sequence of the C. glutamicum mqo region and is preceded there by a methionine (see Fig. 5 in reference 23), which is apparently posttranslationally removed. The MQO protein from C. glutamicum therefore starts with the methionine immediately in front of a putative FAD binding site referred to in reference 23. The analogous methionine (ATG codon) in the mqo ORF, immediately in front of its putative FAD binding site, was defined as the most probable start of MQO of E. coli, and the corresponding hypothetical protein starts with the amino acid sequence MAAKA (see Fig. 5 in reference 23). The fusion of the mqo promoter region with the lacZ gene was constructed in such a way that the lacZ ORF starts with the ATG codon of the mqo ORF corresponding to this most probable start. A 681-bp region preceding this ATG codon of the mqo ORF was isolated using primer F_01 as forward primer and primer F_02 as reverse primer in reaction A. Since the most probable ATG is also the one furthest downstream in the sequence of possible ATG starts, all regulatory elements of the mqo promoter should be present in the construct, even if this ATG is not the true start codon. The lacZ ORF was isolated using the primer F_03 as forward primer and the primer F_04 as reverse primer in reaction B. The 3′ region of primer F_02 and the 5′ region of primer F_03 are complementary. In a crossover PCR, the products of reactions A and B are combined with the primers F_01 and F_04. The resulting PCR product is the desired fusion between the promoter region of the mqo ORF and the lacZ ORF starting with the most probable ATG start codon. The PCR product was digested with HindIII and EcoRI and inserted into the vector pBR322 (3), resulting in the plasmid pML2.
Construction of E. coli mdh, mqo, sfcA, b2463, and pps deletion strains.
Oligonucleotides used are listed in Table 1. PCR conditions were chosen as described above. Chromosomal gene disruptions were carried out in E. coli MC4100 according to the method of Link et al. (19).
The region in front of the mdh ORF (EMBL accession no. M24777) was amplified by PCR using the oligonucleotides Komdh_01 and Komdh_02 (reaction 1). The region following the mdh ORF was amplified using oligonucleotides Komdh_03 and Komdh_04 (reaction 2). The 3′ end of Komdh_02 and the 5′ start of Komdh_03 are complementary. Using crossover PCR with the products of reactions 1 and 2 and primers Komdh_01 and Komdh_04, an 1,100-bp PCR fragment was created, joining the regions on the E. coli genome in front of and behind the mdh ORF. The fragment was inserted into the BamHI site of the vector pKO3 (19). The resulting plasmid pMDH was used for gene disruption on the chromosome according to the protocol described by Link et al. (19).
For the disruption of the mqo ORF, a 3,002-bp fragment containing the mqo ORF was amplified using the oligonucleotides Y_01 and Y_04. Both oligonucleotides introduce BamHI sites. The amplified fragment was digested with BamHI and inserted into the BamHI site of pKO3. The resulting plasmid was digested with MluI to remove a 416-bp fragment from the mqo ORF, thereby disrupting the ORF. The disrupted mqo ORF in plasmid pKO3 was used for gene disruption on the chromosome.
The ORF coding for the NAD-dependent malic enzyme A (EMBL accession no. AE000245) was amplified from the E. coli chromosome using the oligonucleotides Mao1_01 and Mao1_02. The Mao1_01 oligonucleotide contains a BamHI restriction site, and the oligonucleotide Mao1_02 introduces a BamHI restriction site following the sfcA ORF. The resulting PCR fragment was digested with BamHI and ligated into the pKO3 vector opened with BamHI. The resulting plasmid was digested using the restriction enzyme PvuI, thereby deleting a 717-bp fragment from the sfcA ORF. The disrupted sfcA ORF in pKO3 was used for gene disruption. The NADP-dependent malic enzyme (EC 1.1.1.40) is not annotated on the E. coli chromosome. The enzyme encoded by b2463 (EMBL accession no. AE000333) was suggested elsewhere to be an NADP-linked malic enzyme (21), but no experimental data were provided. Using the oligonucleotides Mao2_01 and Mao2_02, the b2463 ORF was amplified from the E. coli chromosome. Both oligonucleotides introduce a BamHI site flanking the PCR fragment. These BamHI sites were used to clone the fragment into the pKO3 vector. The resulting plasmid was digested with NdeI, thereby removing a 1,078-bp fragment from the ORF. The disrupted ORF in pKO3 was used for gene disruption on the chromosome.
The ORF pps (EMBL accession no. M69116) coding for the PEP synthase was amplified from the E. coli chromosome using the oligonucleotides Pps_01 and Pps_02. Both oligonucleotides introduce a BamHI restriction site in front of and following the pps ORF. The resulting PCR fragment was digested with BamHI and the restriction enzymes HpaI and DraI to delete a 1,038-bp fragment from the pps ORF. The BamHI-digested fragments were ligated into the pKO3 vector opened with BamHI. The disrupted pps ORF in pKO3 was used for gene disruption on the chromosome.
Preparation of membrane fragments.
A sample of 5 × 1011 cells (calculated by assuming that an optical density at 600 nm equal to 1 corresponds to approximately 5 × 108 cells ml−1) was harvested and washed twice with 25 ml of ice-cold buffer A consisting of 50 mM HEPES, 10 mM K-acetate, 10 mM CaCl2, and 5 mM MgCl2 titrated with NaOH to pH 7.5. The washed cells were resuspended in 10 ml of buffer A and passed once through a French pressure cell at 10,000 lb/in2 (69 MPa). Cell debris was removed by centrifuging it for 10 min at 10,000 × g. The supernatant was centrifuged for 30 min at 75,000 × g, and membranes were resuspended in buffer A to 10 to 15 mg of protein per ml.
Enzyme assays.
Malate dehydrogenase activity was determined in cells grown overnight in LB. The cells were washed twice in 150 mM Tris–15 mM EDTA, titrated to pH 7.4 with acetic acid, and passed once through a French pressure cell at 10,000 lb/in2 (69 MPa). After a low-speed spin at 2,500 × g, the cell extract was used for the assay. Malate dehydrogenase activity was assayed at pH 9.0 in the direction of malate oxidation as described in reference 32.
NADP-dependent malic enzyme activity was measured in cell extract using the method described by Diesterhaft and Freese (11).
MQO activity in membrane fragments was measured using 2,6-dichlorophenolindophenol (DCPIP) as an electron acceptor as described before (23). The reaction mixture additionally contained 30 μM ubiquinone 1.
β-Galactosidase activity was measured according to the method of Sambrook et al. (31).
Competition experiments with wild type and Δmqo.
The strain MC4100 and its derivative Δmqo were precultured overnight on LB, and the optical densities at 600 nm of these cultures were measured. The cultures were then mixed to obtain a 1:1 proportion of MC4100 and Δmqo cells. Of this mixture, 2 μl was used to inoculate 2 ml of NMM (26) with different carbon and energy sources. For each carbon and energy source, five cultures were inoculated and maintained in parallel throughout the experiment. The cultures were incubated in a shaker at 37°C for 24 h, and approximately 2 μl of each culture was transferred to 2 ml of fresh NMM. The optical density at 600 nm was measured, and the rest of the culture volume was kept frozen at −20°C. The transfer to fresh medium was repeated seven times. The number of generations after the last cultures had reached stationary phase was approximately 50 to 60. PCR was carried out as described above on 1 μl of a 50-fold-diluted sample of the culture to estimate the proportions of MC4100 and mqo using oligonucleotides Yojh_01 and Yojh_02. The linear quantitative character of the PCR method was confirmed by testing with defined mixtures of these strains.
Estimation of growth rates.
Two growth curves were recorded by monitoring the optical density at 600 nm of cultures which had been inoculated with overnight cultures on the same medium from two different colonies. The specific growth rates were estimated by simultaneous least-squares fitting of both growth curves to exponential growth equations.
Protein determination.
Protein was determined with bicinchoninic acid in the presence of 0.5% (wt/vol) sodium dodecyl sulfate, according to a protocol adapted from reference 34.
RESULTS
The gene mqo, formerly called yojH, encodes an MQO in E. coli.
The high amino acid sequence similarity of MQO from C. glutamicum and the hypothetical protein encoded by the gene mqo (previously called yojH) of E. coli suggested that this gene encodes an MQO in E. coli (23). To test this possibility, the mqo gene was amplified by PCR. The PCR product containing the mqo ORF was inserted in a high-copy-number vector under the control of the trc (lac) promoter, resulting in plasmid pECmqo. This plasmid was introduced in E. coli DSM 5698 (wild-type K-12 strain). The resulting strain, when grown on LB, had an MQO activity of 32 nmol · min−1 · mg−1 of protein. The detection of activity in E. coli membranes with DCPIP as electron acceptor was absolutely dependent on the addition of a quinone and varied with the particular quinone added. The highest activity is found in the presence of ubiquinone 1 (results not shown). Addition of the inducer isopropyl-β-d-thiogalactopyranoside (IPTG) increased the activity to 176 nmol · min−1 · mg−1 of protein. Using a C. glutamicum-E. coli shuttle plasmid, pJCBW1, the mqo gene could also be expressed in the C. glutamicum DM22 mutant which lacks MQO activity (23). The addition of IPTG to these cells gave rise to MQO activity, albeit with lower specific activities than those found in membranes from C. glutamicum strain ATCC 13032 (wild type).
Effects of carbon substrates on Φ(mqo-lacZ) expression.
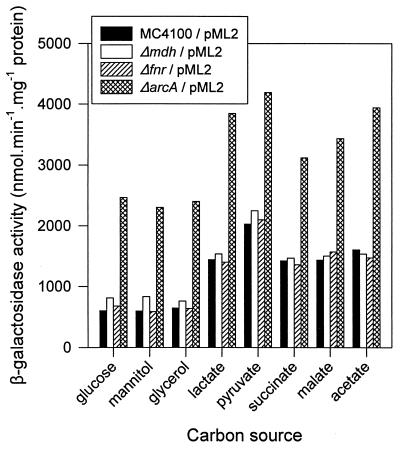
To determine the effect of the carbon source on mqo gene expression, an mqo-lacZ fusion Φ(mqo-lacZ) was constructed on a plasmid (pML2). Strain MC4100/pML2, lacking the lacZ gene on the chromosome, was grown in NMM on different carbon sources to mid-logarithmic phase, and β-galactosidase levels were determined. Expression was low on NMM with glucose and high when nonfermentable substrates were used as the carbon source (Fig. 1). Expression of mqo appears to be under catabolite control, similar to the regulation of mdh and the succinate dehydrogenase gene (sdh) (28, 29). Expression of Φ(mqo-lacZ) in a ΔarcA strain (PC35 [7]) was increased approximately two- to fourfold (Fig. 1). This shows that mqo, like many other genes encoding enzymes from the electron transfer chain, is regulated by the ArcA-ArcB two-component system. Deletion of fnr had no effect on expression of Φ(mqo-lacZ), neither under aerobic conditions (Fig. 1) nor under anaerobic growth on glucose. The β-galactosidase levels were approximately 10-fold lower under anaerobic conditions (data not shown).
FIG. 1.
Expression of Φ(mqo-lacZ) from plasmid pML2 in E. coli MC4100 and in strains lacking ArcA, Fnr, or MDH. Cells were grown in NMM containing the indicated carbon source (0.5% [wt/vol]) to an optical density at 600 nm of 0.4. Hydrolysis of ortho-nitrophenyl-β-d-galactopyranoside (ONPG) was used as a measure of β-galactosidase activity (31).
Earlier investigations suggested that MQO in E. coli was present in only (some) mutants lacking the cytoplasmic malate dehydrogenase (12, 25). These authors interpreted the results as indicating that there existed a repressing effect of MDH on MQO expression, which was dependent on the presence of active MDH. The observed increase of MQO activity in MDH mutants was by at least a factor of 10 to 50 and in one case of 200 to 300 (25). A basis for this regulation was not found, and in this study it is shown that MQO and MDH are active at the same time in E. coli (Fig. 2). The mutants used in the earlier studies were generated by random mutation using nitrosoguanidine and are therefore ill defined. The defined mdh knockout strain described in this study allowed a clear-cut evaluation of the effect of the absence of MDH activity on MQO expression. Expression of Φ(mqo-lacZ) in the Δmdh strain resulted in only slightly increased β-galactosidase levels (Fig. 1). MQO activity was increased by at most a factor of 2 in the mdh strain in the exponential phase during growth on NMM with glucose. Finally, deletion of the mqo gene also had no effect on Φ(mqo-lacZ) expression (data not shown), ruling out the idea that MQO regulates its own synthesis.
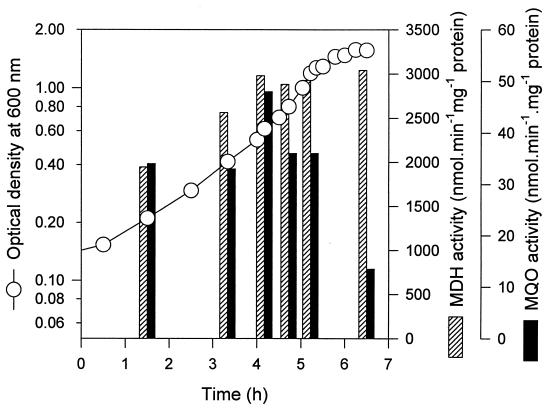
FIG. 2.
Effect of the growth phase on MQO activity. E. coli MC4100 was grown in NMM using 0.5% (wt/vol) pyruvic acid as carbon source in a controlled batch under constant oxygen pressure. Membrane vesicles and cleared extracts were prepared from cells harvested at indicated positions. MQO and MDH were measured as described in the text.
Regulation of MQO activity in E. coli by ArcA, carbon source, and growth phase.
Since expression of MQO in E. coli appears to be highly regulated by carbon source, membrane vesicles were prepared from cells grown on different carbon sources. The cells were harvested from cultures in the exponential growth phase, in the end-exponential phase, and in the stationary phase and from overnight cultures. High MQO activity could be measured in vesicles prepared from exponentially growing cells using pyruvate and acetate as carbon source (Table 2). Cells grown on glucose contained low MQO activities. The difference between MQO activity of cells grown on glucose and activity of cells grown on pyruvate is approximately fourfold and is in accordance with the results obtained for the expression of Φ(mqo-lacZ) (Fig. 1). In cells grown on glucose, the MQO activity was fourfold higher in the ΔarcA strain, in accordance with the Φ(mqo-lacZ) promoter studies. Also, succinate dehydrogenase and MDH were derepressed in the ΔarcA strain (results not shown and references 28 and 29). In cells growing on LB supplemented with 0.1% glucose, MQO activity can also be measured, in contrast to earlier observations (25).
TABLE 2.
MQO activity in E. coli vesicles prepared from cells grown on different media and different carbon sources and harvested at different phases of the growth curve
| Growth phase | MQO activity (nmol · min−1 · mg of protein−1) on medium:
|
|||
|---|---|---|---|---|
| NMM + glucose | NMM + pyruvate | NMM + acetate | LB + glucose | |
| Exponential | 12.0 | 41.3 | 47 | 16.1 |
| End exponential | 13.7 | 23.4 | 4.9 | NDa |
| Stationary | 9.2 | 5.7 | 4.9 | 5.1 |
| Overnight | 4.1 | 1.9 | 0.0 | 0.0 |
ND, not determined.
The growth-phase-dependent MQO activity (Table 2) could be a result of oxygen limitation which occurs at the high cell densities during stationary phase and in overnight cultures. In order to avoid oxygen limitations, MQO activity was measured in vesicles prepared from cells grown on pyruvate in a batch culture under controlled high oxygen pressure. The results shown in Fig. 2 indicate that the MQO activity, in contrast to the MDH activity, is highly dependent on the growth phase and that the activity decreases rapidly when the cells reach the stationary phase. The same observation was made for another strain (K-12 strain DSM 5698) and was also described earlier (12).
Properties of mdh and mqo deletion strains.
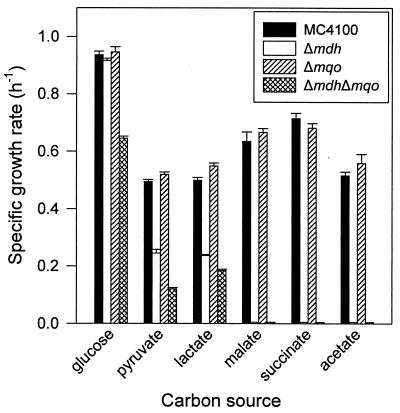
To investigate the role of MQO and MDH in E. coli, deletions of the mqo and mdh genes were generated. The deletion of mdh causes a severe decrease in growth rates on all substrates except glucose (Fig. 3). Strain Δmdh does not grow on acetate or malate. Strain Δmqo appears to have no growth defect on all substrates tested. Nevertheless, the double mutant Δmdh Δmqo grows more slowly on glucose, pyruvate, and lactate than does the Δmdh strain. This effect was confirmed in several independent growth experiments (data not shown). These results show that deletion of the mqo gene has a phenotype although this phenotype is seen only in combination with a deletion of the mdh gene. Overexpression of mqo from a plasmid in the Δmdh strain was deleterious for growth (Table 3). It should be mentioned that the mqo gene is also expressed from the plasmid pECmqo in the absence of the inducer IPTG, which in the Δmqo strain leads to a twofold-higher MQO activity than that present in the wild type. Also, a deleterious effect of both the empty vector pET324 and pECmqo on the growth rate of E. coli MC4100 can be observed, which is relieved by the addition of IPTG. For this reason, the rate of growth with the mqo gene relative to that without the mqo gene is displayed. Increasing overexpression of mqo by increasing inducer concentration led to a decrease of the growth rate.
FIG. 3.
Effect of mqo and mdh deletions on the specific growth rate of E. coli on various carbon sources. Cells were grown on NMM containing carbon sources (0.5% [wt/vol]) as indicated. Error bars represent estimated standard deviations.
TABLE 3.
Growth rates of various strains with the vector pET324 or plasmid pECmqo, containing the mqo gene behind an IPTG-inducible promotera
| Strain | IPTG concn (mM) | Growth rate (h−1)
|
Growth with mqo relative to growth without mqo (%) | |
|---|---|---|---|---|
| pET324 | pECmqo | |||
| MC4100 | 0 | 0.518 ± 0.003 | 0.512 ± 0.003 | 99 ± 1 |
| 0.1 | 0.816 ± 0.010 | 0.284 ± 0.006 | 35 ± 4 | |
| 0.5 | 0.818 ± 0.012 | 0.194 ± 0.017 | 24 ± 10 | |
| Δmdh | 0 | 0.563 ± 0.010 | 0.498 ± 0.009 | 89 ± 4 |
| 0.1 | 0.817 ± 0.013 | 0.252 ± 0.013 | 31 ± 7 | |
| 0.5 | 0.807 ± 0.008 | 0.093 ± 0.014 | 12 ± 16 | |
| Δmdh Δmqo | 0 | 0.513 ± 0.025 | 0.520 ± 0.008 | 101 ± 6 |
| 0.1 | 0.634 ± 0.022 | 0.285 ± 0.017 | 45 ± 9 | |
| 0.5 | 0.622 ± 0.030 | 0.164 ± 0.015 | 26 ± 14 | |
Growth rates and percentages are reported ± estimated standard deviations. The growth was measured on NMM with 0.5% glucose as carbon and energy source.
In accordance with the results concerning the growth rate, the deletion of mqo also did not affect the fitness in a competition experiment where MC4100 and Δmqo were cocultured. The fitness was tested by successive transfer of inoculum on minimal medium with glucose, acetate, or pyruvate, starting with a 1:1 proportion of wild-type and Δmqo cells (see Materials and Methods for details). The cultures were transferred every 24 h, so that each culture went through the lag, exponential, and stationary phases. After at least 50 generations, the wild-type and Δmqo strains were still present in a 1:1 proportion on all three substrates.
Effects of sfcA, b2463, and pps deletions on growth of E. coli wild type and Δmqo.
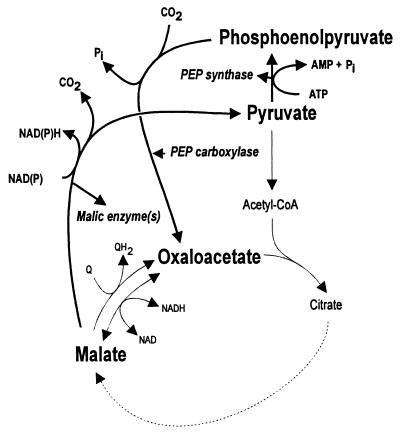
In view of the standard free energies of the MDH and MQO reactions, it would be likely that under physiological conditions (pH 7.0) MDH catalyzes the reduction of oxaloacetate and MQO oxidizes malate to oxaloacetate. The results presented above show that, in contrast to the deletion of the mdh gene, the deletion of the mqo gene in E. coli has no discernible effect on growth. This observation does not rule out the possibility that MQO catalyzes malate oxidation, since an alternative pathway for the conversion of malate to oxaloacetate, formed by the collective action of malic enzyme, PEP synthase, and PEP carboxylase (15) (see Fig. 4), might take over this function from MQO in a Δmqo strain. Furthermore, assuming that MDH catalyzes oxaloacetate reduction, we present an alternative explanation for the deleterious effect of an mdh deletion in the Discussion. Clearly, the alternative route for malate oxidation depicted in Fig. 4 had to be blocked to further investigate the function of MQO.
FIG. 4.
Alternative metabolic pathway (PEP shunt) for the conversion of malate to oxaloacetate in E. coli.
E. coli contains an NAD-dependent and an NADP-dependent malic enzyme (24). It was suggested elsewhere that the NAD-dependent enzyme is involved in gluconeogenesis and that the NADP-dependent enzyme supplies the cell with NADPH when growing on C4 carbon sources (14). The gene for the NAD-dependent malic enzyme has been cloned and characterized and is called sfcA (35). The gene b2463 (EMBL accession no. AE000333) was suggested previously to encode an NADP-dependent malic enzyme (21), but no experimental data were provided. Deletion of b2463 from the E. coli genome abolishes NADP-dependent malic enzyme activity (results not shown), thereby justifying the EC 1.1.1.40 designation for the gene product of b2463. It is unclear whether the b2463 gene product also decarboxylates oxaloacetate. In Table 4, the effects of the various deletions are presented. The mqo deletion had no effect on growth on any of the carbon sources used in agreement with the observations described above. The combined inactivation of sfcA and b2463 resulted in a severe growth defect on the C4 carbon sources tested, whereas no effect on growth was detected on the other carbon sources. Apparently, the conversion of malate to pyruvate is imperative for growth on C4 carbon sources. Introducing the mqo deletion in the strain lacking malic enzyme activity did not result in a distinguishable phenotype. The deletion of the PEP synthase gene pps was deleterious for growth on lactate and pyruvate, but the cells grew normally on C4 sources. Subsequent deletion of mqo had no effect on growth. Surprisingly, during growth on succinate or acetate mutants with normal colony size appeared with high frequency. Since site-directed deletions were introduced, the possibility that these mutants were revertants of the target genes can be ruled out. The appearance of such mutants unfortunately prevented a reliable determination of growth rates.
TABLE 4.
Growth of E. coli strains with deletions of genes encoding MQO, PEP synthase (pps), and malic enzymes (sfcA and b2463) on various carbon sources as judged by growth on platesa
| C source | Growth of strain:
|
|||||
|---|---|---|---|---|---|---|
| MC4100 | Δmqo | Δpps | Δmqo Δpps | ΔsfcA Δb2463 | Δmqo ΔsfcA Δb2463 | |
| Glucose | +++ | +++ | +++ | +++ | +++ | +++ |
| Fructose | +++ | +++ | +++ | +++ | +++ | +++ |
| Mannitol | +++ | +++ | +++ | +++ | +++ | +++ |
| Glycerol | +++ | +++ | +++ | +++ | +++ | +++ |
| Lactate | +++ | +++ | +/− | +/− | +++ | +++ |
| Pyruvate | ++ | ++ | − | − | ++ | ++ |
| Succinate | +++ | +++ | +++ | +++ | +/−⇒+++ | +/−⇒+++ |
| Fumarate | +++ | +++ | +++ | +++ | ++ | ++ |
| Malate | +++ | +++ | +++ | +++ | ++ | ++ |
| Acetate | ++⇒+++ | ++⇒+++ | ++⇒+++ | ++⇒+++ | ++⇒+++ | ++⇒+++ |
Strains were grown on NMM containing 0.5% carbon source. Symbols: +++, large colonies; ++, small colonies; ++⇒+++, small colonies and mutants which grow normally; +/−, growth visible only in regions of dense inoculation; +/−⇒+++, growth visible only in regions of dense inoculation and mutants which grow normally; −, no growth.
Since the introduction of pps or sfcA and b2463 deletions in the wild-type and Δmqo strains did not lead to differential effects, it may be concluded that the alternative pathway does not take over the function of MQO in a Δmqo strain.
DISCUSSION
Earlier reports suggested that the level of MQO activity was down-regulated to very low levels by the presence of active MDH (12, 25). The increase in MQO activity in mutants lacking MDH activity could amount to a factor of 200 to 300 over the MQO activity found in the wild type. In the present study, it was shown that E. coli possesses significant MQO activity, even when MDH activity is present. Moreover, a defined deletion of the mdh gene did not lead to an increased MQO activity to the extent reported earlier. Also, the β-galactosidase reporter levels in the Δmdh strain containing the mqo-lacZ fusion were only slightly increased compared to the levels in the wild type. These observations do not suggest a regulation of the mqo gene by active MDH.
The expression levels of the fusion protein in the ΔarcA mutant clearly show that MQO activity is regulated by the ArcA-ArcB regulatory system. MDH expression is regulated by the same system (28). The activity of MQO also seems to be regulated both by carbon catabolite control and by the growth phase. The protein is active only during exponential growth, and when the cells reach stationary phase, it appears to be broken down very rapidly. In view of this intricate regulation, it is surprising that an mqo deletion in the wild type did not lead to an observable phenotype, and hence that no physiological function could be assigned to MQO.
In contrast, an mdh deletion induced severe growth defects. This observation per se does not imply that MDH oxidizes malate in the TCA cycle. The Gibbs standard free energy difference of the MDH reaction (+28.6 kJ · mol−1) is very unfavorable for malate oxidation. The NADH/NAD ratio in E. coli varies from 0.75 under conditions of low oxygen pressure to 0.075 under high oxygen pressure (10). Under low oxygen pressure, the equilibrium oxaloacetate/malate ratio of the MDH reaction would then be 2 × 10−5. In practice, this would mean that the concentration of malate in the cell should be at least 5 to 50 mM, assuming that the concentration of oxaloacetate should be at least 0.1 to 1 μM to meet the requirements of oxaloacetate-consuming enzymes. Such malate concentrations may be difficult to attain.
An alternative route for the oxidation of malate to oxaloacetate, involving malic enzyme and pyruvate decarboxylase, has been suggested previously to exist in Bacillus subtilis and has been named pyruvate shunt (11). This organism possesses an MDH and lacks an MQO. No gene similar to mqo was found in its genome, nor could MQO activity be demonstrated in isolated membrane fragments (D. Molenaar, unpublished results). It was suggested that in B. subtilis malate dehydrogenase reduces oxaloacetate and that, therefore, the pyruvate shunt is necessary for the production of oxaloacetate (11).
Since E. coli does not possess a pyruvate carboxylase and assuming, as seems to be the case in C. glutamicum, that MQO catalyzes malate oxidation while MDH reduces oxaloacetate, the absence of a phenotype for the mqo deletion mutant in E. coli might be due to a PEP shunt taking over its function (Fig. 4). MDH in this case would function as a (down-)regulator of the oxaloacetate concentration, and the effect of an mdh deletion might then be due to increased intracellular oxaloacetate concentrations. Oxaloacetate is known to be a strong inhibitor of key metabolic enzymes, e.g., succinate dehydrogenase (1), malic enzyme (Ki, 10 μM) (33), or PEP synthase (400 μM) (5). This might also be the reason why overexpression of mqo from a plasmid causes growth inhibition (Table 3), because it would disturb the balance between MDH and MQO activity and lead to an increased oxaloacetate concentration. The results in Table 4, however, indicate that the reason for the absence of a phenotype in a strain lacking MQO is not that a PEP shunt takes over the (hypothetical) role of MQO in the conversion of malate to oxaloacetate. Furthermore, since the Δmqo Δpps and Δmqo ΔsfcA Δb2463 strains lack the known alternative routes for conversion of malate to oxaloacetate, it has to be concluded that MDH oxidizes malate in E. coli. However, since the double mutant Δmqo Δmdh still grows on lactate, pyruvate, and glucose, an alternative route has to exist to complete the TCA cycle.
An interesting phenomenon still to discuss is that the additional deletion of the mqo gene has a negative effect on the growth of the Δmdh strain when growing on glucose, pyruvate, or lactate (Fig. 3). The simplest interpretation of these results is that, in the absence of MDH, MQO is capable of sustaining a low TCA cycle activity. This activity is just enough to support a growth rate comparable to that of the wild type during growth on glucose, and it sustains somewhat lower growth rates on lactate or pyruvate. The capacity of MQO is not sufficient for growth on acetate or C4 compounds, when a very high TCA cycle activity is required for the generation of metabolic energy. The fact that MQO completely takes over the function of MDH in the Δmdh strain during growth on glucose is contradictory to the assertion by others that the TCA cycle is split during aerobic growth on glucose (13). Since MQO catalyzes irreversible oxidation of malate (17), it could not take over the function of an MDH which would reduce oxaloacetate in the reductive branch.
We are currently investigating possible reasons for the contrasting functions of MQO and MDH in E. coli and C. glutamicum. One of the obvious reasons to think of is the fact that there are significant differences in the specific activities of MQO and MDH and in the ratios of these activities between the two species. Compare, for example, the activities in Fig. 2 of this paper with those in Fig. 2 of the accompanying paper. Since the presence of both an MQO and MDH or their corresponding genes was observed in different eubacterial genera (6, 12, 16–18, 22, 23, 27), the results of these studies are of general importance for the physiology of the TCA cycle in bacteria.
ACKNOWLEDGMENTS
We thank R. P. Gunsalus, University of California at Los Angeles, for the gift of strains PC2 and PC35 and G. M. Church, Harvard Medical School, Boston, Mass., for the gift of plasmid pKO3.
This research was funded by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie in Germany (project 0316712).
REFERENCES
- 1.Ackrell B A. Metabolic regulatory functions of oxalacetate. Horiz Biochem Biophys. 1974;1:175–219. [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 4.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 5.Chulavatnatol M, Atkinson D E. Phosphoenolpyruvate synthase from Escherichia coli. J Biol Chem. 1973;248:2712–2715. [PubMed] [Google Scholar]
- 6.Cohn D V. The enzymatic formation of oxalacetic acid by nonpyridine nucleotide malic dehydrogenase of Micrococcus lysodeikticus. J Biol Chem. 1958;233:299–304. [PubMed] [Google Scholar]
- 7.Cotter P A, Gunsalus R P. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol Lett. 1992;91:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- 8.Cremer J, Eggeling L, Sahm H. Cloning the dapA dapB cluster of the lysine-secreting bacterium Corynebacterium glutamicum. Mol Gen Genet. 1990;220:478–480. [Google Scholar]
- 9.Cronan J E, LaPorte D C. Tricarboxylic acid cycle and glyoxylate bypass. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 206–216. [Google Scholar]
- 10.De Graef M R, Alexeeva S, Snoep J L, Teixeira de Mattos M J. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol. 1999;181:2351–2357. doi: 10.1128/jb.181.8.2351-2357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diesterhaft M D, Freese E. Role of pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and malic enzyme during growth and sporulation of Bacillus subtilis. J Biol Chem. 1973;248:6062–6070. [PubMed] [Google Scholar]
- 12.Goldie A H, Narindrasorasak S, Sanwal B D. An unusual type of regulation of malate oxidase synthesis in Escherichia coli. Biochem Biophys Res Commun. 1978;83:421–426. doi: 10.1016/0006-291x(78)91007-0. [DOI] [PubMed] [Google Scholar]
- 13.Guest J R, Russell G C. Complexes and complexities of the citric acid cycle in Escherichia coli. Curr Top Cell Regul. 1992;33:231–247. doi: 10.1016/b978-0-12-152833-1.50018-6. [DOI] [PubMed] [Google Scholar]
- 14.Hansen E J, Juni E. Two routes for synthesis of phosphoenolpyruvate from C4-dicarboxylic acids in Escherichia coli. Biochem Biophys Res Commun. 1974;59:1204–1210. doi: 10.1016/0006-291x(74)90442-2. [DOI] [PubMed] [Google Scholar]
- 15.Hansen E J, Juni E. Properties of mutants of Escherichia coli lacking malic dehydrogenase and their revertants. J Biol Chem. 1979;254:3570–3575. [PubMed] [Google Scholar]
- 16.Jurtschuk P, Bednarz A J, Zey P, Denton C H. l-Malate oxidation by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1969;98:1120–1127. doi: 10.1128/jb.98.3.1120-1127.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kather B, Stingl K, van der Rest M E, Altendorf K, Molenaar D. Another unusual type of citric acid cycle enzyme in Helicobacter pylori: the malate:quinone oxidoreductase. J Bacteriol. 2000;182:3204–3209. doi: 10.1128/jb.182.11.3204-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Tobari J. Participation of flavin-adenine dinucleotide in the activity of malate dehydrogenase from Mycobacterium avium. Biochim Biophys Acta. 1963;73:399–405. doi: 10.1016/0006-3002(63)90441-4. [DOI] [PubMed] [Google Scholar]
- 19.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menkel E, Thierbach G, Eggeling L, Sahm H. Influence of increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl Environ Microbiol. 1989;55:684–688. doi: 10.1128/aem.55.3.684-688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsch M J, Voegele R T, Cowie A, Osteras M, Finan T M. Chimeric structure of the NAD(P)+- and NADP+-dependent malic enzymes of Rhizobium (Sinorhizobium) meliloti. J Biol Chem. 1998;273:9330–9336. doi: 10.1074/jbc.273.15.9330. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno T, Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem (Tokyo) 1978;84:179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- 23.Molenaar D, van der Rest M E, Petrovic S. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) (EC 1.1.99.16) from Corynebacterium glutamicum. Eur J Biochem. 1998;254:395–403. doi: 10.1046/j.1432-1327.1998.2540395.x. [DOI] [PubMed] [Google Scholar]
- 24.Murai T, Tokushige M, Nagai J, Katsuki H. Physiological functions of NAD- and NADP-linked malic enzymes in Escherichia coli. Biochem Biophys Res Commun. 1971;43:875–881. doi: 10.1016/0006-291x(71)90698-x. [DOI] [PubMed] [Google Scholar]
- 25.Narindrasorasak S, Goldie A H, Sanwal B D. Characteristics and regulation of a phospholipid-activated malate oxidase from Escherichia coli. J Biol Chem. 1979;254:1540–1545. [PubMed] [Google Scholar]
- 26.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohshima T, Tanaka S. Dye-linked L-malate dehydrogenase from thermophilic Bacillus species DSM 465. Purification and characterization. Eur J Biochem. 1993;214:37–42. doi: 10.1111/j.1432-1033.1993.tb17893.x. [DOI] [PubMed] [Google Scholar]
- 28.Park S-J, Cotter P A, Gunsalus R P. Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J Bacteriol. 1995;177:6652–6656. doi: 10.1128/jb.177.22.6652-6656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S-J, Tseng C-P, Gunsalus R P. Regulation of succinate dehydrogenase (sdhCDAB) operon expression in Escherichia coli in response to carbon supply and anaerobiosis: role of ArcA and Fnr. Mol Microbiol. 1995;15:473–482. doi: 10.1111/j.1365-2958.1995.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 30.Perkins J D, Heath J D, Sharma B R, Weinstock G M. XbaI and BlnI genomic cleavage maps of Escherichia coli K-12 strain MG1655 and comparative analysis of other strains. J Mol Biol. 1993;232:419–445. doi: 10.1006/jmbi.1993.1401. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sanwal B D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. I. Control characteristics of malate dehydrogenase. J Biol Chem. 1969;244:1831–1837. [PubMed] [Google Scholar]
- 33.Sanwal B D. Allosteric controls of amphibolic pathways in bacteria. Bacteriol Rev. 1970;34:20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olsen B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 35.Stols L, Donnelly M I. Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol. 1997;63:2695–2701. doi: 10.1128/aem.63.7.2695-2701.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Does C, den Blaauwen T, de Wit J G, Manting E H, Groot N A, Fekkes P, Driessen A J. SecA is an intrinsic subunit of the Escherichia coli preprotein translocase and exposes its carboxyl terminus to the periplasm. Mol Microbiol. 1996;22:619–629. doi: 10.1046/j.1365-2958.1996.d01-1712.x. [DOI] [PubMed] [Google Scholar]