To the Editor: As of July 4, 2021, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has affected >0.18 billion individuals and caused >3.9 million deaths worldwide.[1] Currently, the only method to radically overcome this pandemic is extensive vaccination. However, the clinical trials assessing most vaccines against SARS-CoV-2 have excluded patients with hematological tumors. Thus, only limited data are available in hematological tumor patients with respect to vaccine safety, tolerability, and effectiveness. The lack of such data in hematologic tumor patients leads to hesitation towards the immunization approach and undermines current policies regarding vaccination. Currently, six vaccines against SARS-CoV-2 are available in China. However, vaccination agencies in most communities in China do not allow or recommend vaccination for hematological tumor patients. Therefore, acquiring the safety data of SARS-CoV-2 vaccines in such patients is imperative for individual choice and public health policies.
Chronic myeloid leukemia (CML) is a chronic hematological tumor. CML patients have a life expectancy close to that of healthy individuals. Hitherto, the safety of SARS-CoV-2 vaccination in CML patients has been reported only in a few cases.[2,3] Therefore, we performed a multicenter cross-sectional survey in CML patients who had undergone SARS-CoV-2 vaccination. Online questionnaires were dispatched to CML patients by physicians in nine medical centers through social media. Each questionnaire included an informed consent form and 17 anonymous questions addressing demographics, comorbidities, CML status and treatments, and SARS-CoV-2 vaccination status and adverse events (AEs), as well as contact information. The questionnaires were filled by CML patients voluntarily. Missed or unclear data were verified by contacting the patients through telephone calls, e-mail, or WeChat. AEs were graded according to the following scale: Grade 1 (mild, not interfering with activity); Grade 2 (moderate, interfering with activity); Grade 3 (severe, preventing daily activity); Grade 4 (potentially life-threatening, emergency department visit, or hospital admission). Logistic regression analysis was performed to examine the associations of clinical parameters (gender, age, disease course, Tyrosine Kinase Inhibitor (TKI) types, comorbidities, and BCR-ABL1 level) with vaccination-related factors (vaccines type and doses) and AEs. Variables with P < 0.05 were considered significant. Analyses were performed with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The study was approved by the Ethics Committee of West China Hospital, Sichuan University.
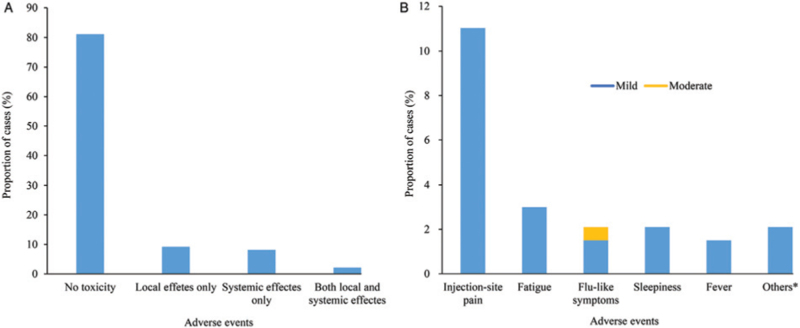
We finally recruited 335 CML patients across 29 provinces in China who were vaccinated against SARS-CoV-2. All these respondents completed the online questionnaire, and 268 also participated in telephone interviews. Fifty-one respondents had interviews though e-mails or social media channels such as WeChat. Sixteen respondents declined interviews besides the online questionnaire. The median time between vaccination and questionnaire submission was seven (range: 1–172) days. The characteristics of the respondents are listed in [Supplementary Table 1]. All respondents were in the chronic phase (CP). Among them, 89.3% (299/335) achieved major molecular response or deeper molecular response, and 80.5% (270/335) of respondents received the inactivated vaccine. Totally 75.5% (253/335) of respondents received one dose of the vaccine, and the remaining individuals received two doses. A total of 64 (19.1%) respondents reported AEs after vaccination. The most common (11.0%, 37/335) AEs were injection-site pain. The most common systemic AEs were fatigue (3.0%, 10/335), sleepiness (2.1%, 7/335), and flu-like symptoms (2.1%, 7/335). Other AEs included fever, headache, diarrhea, dizziness, hypogeusia, knee pain, lumbago, and gout attack. The median duration of AEs was 1 day (ranging from 1 to 7 days). Two respondents presented Grade 2 flu-like symptoms. AEs in all the remaining respondents were Grade 1, and no Grade 3 to 4 AEs were reported. The detected AEs are summarized in [Figure 1]. Interestingly, the AEs of vaccination were not significantly associated with vaccine brand, TKI types, other vaccine characteristics, or patient features, except for younger age (P = 0.001) [Supplementary Table 1], which may indicate a stronger immune response in young people.
Figure 1.
Local and systemic effects reported after SARS-CoV-2 vaccination in patients with CML: (A) Proportions of participants reporting no toxicity or toxicity. (B) Breakdown of specific local and systemic side effects. Symptoms were graded according to the following scale: Grade 1 (mild, not interfering with activity); Grade 2 (moderate, interfering with activity); Grade 3 (severe, preventing daily activity); Grade 4 (potentially life-threatening, emergency department visit or hospital admission). Others include headache, diarrhea, dizziness, hypogeusia, knee pain, lumbago, and gout attack. CML: Chronic myeloid leukemia; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2.
Pimpinelli et al[2] assessed the safety and immunogenicity of the BNT162b2 vaccine (Pfizer–BioNTech) against SARS-CoV-2 in 20 CML patients. The findings showed that the vaccine was safe in CML patients, and no vaccine-related Grade 3 to 4 AEs occurred. Harrington et al[3] drew a similar conclusion in a small cohort of 16 CML patients administered a single dose of the BNT162b2 vaccine. However, both studies had the limitation of a small sample size. The current study is a large multicenter study assessing the safety of SARS-CoV-2 vaccines in CML patients. Moreover, this is a rare report examining five available SARS-CoV-2 vaccines in CML patients. The incidence of AEs in CML patients in this study was not higher than that of healthy individuals reported in previous phase 2 or 3 trials of the inactivated vaccine in China (range: 19.0–48.3%).[4,5] More importantly, no serious AEs were reported. Nevertheless, the present study had several limitations. First, the “Survivorship bias” might characterize this questionnaire survey; in other words, deceased patients would be excluded from the survey by default. However, none of the previous trials of these vaccines[4,5] have reported vaccination-related death, which indicates a minimal possibility of “Survivorship bias”. Second, the number of CML patients vaccinated with recombinant adenovirus vaccine or recombinant protein subunit vaccine was small. Third, CML-related data, including the levels of the fusion gene BCR-ABL1 before and after vaccination, were not collected in this study.
In summary, the current findings suggested that the SARS-CoV-2 vaccines described here are safe for chronic phase-chronic myeloid leukemia (CP-CML) patients. Additional studies with data about CML-related AEs and the levels of protective antibodies are needed to further evaluate the safety and effectiveness of SARS-CoV-2 vaccination in CML patients.
Acknowledgements
Authors would like to thank the patients who volunteered to participate in this study.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yang Y, Zhang Y, Jiang Q, Meng L, Li W, Liu B, Liu X, Zhou L, Liang R, Zhu X, NaXu, Kuang P, Lin T, Zhu H. Safety of SARS-CoV-2 vaccines in patients with chronic myeloid leukemia: a multicenter survey in China. Chin Med J 2022;135:1498–1499. doi: 10.1097/CM9.0000000000001899
Supplemental digital content is available for this article.
References
- 1.Worldometer. COVID-19 coronavirus pandemic, 2021. Available from: https://www.worldometers.info/coronavirus. [Accessed July 4. 2021]. [Google Scholar]
- 2.Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: Preliminary data from a single institution. J Hematol Oncol 2021; 14:81.doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington P, Doores KJ, Radia D, O’Reilly A, Lam HPJ, Seow J, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol 2021; 194:999–1006. doi: 10.1111/bjh.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a Randomized Clinical Trial. JAMA 2021; 326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.