Abstract
Background:
Previous studies have shown that inflammation plays an important role in intracranial atherosclerotic stenosis (ICAS). The platelet-to-lymphocyte ratio (PLR) has recently emerged as a potential inflammatory biomarker. This study aimed to explore the association of the PLR with ICAS in a Chinese Han population.
Methods:
A total of 2134 participants (518 with ICAS, 1616 without ICAS) were enrolled in this study. ICAS was defined as atherosclerotic stenosis >50% or the occlusion of several main intracranial arteries. Multivariable logistic regression analyses were used to assess the association of the PLR with ICAS. Additional subgroup analyses were performed according to age (<60 vs. ≥60 years) and acute ischemic stroke.
Results:
Multivariate regression analysis showed that a high PLR was associated with a higher risk of ICAS in all participants (P < 0.001). Compared with the lowest quartile, the fourth PLR quartile was significantly associated with ICAS (OR 1.705, 95% confidence interval 1.278–2.275, P < 0.001). In the subgroups stratified by age, an association between the PLR and ICAS was found in the late-life group (P < 0.001), but not in the mid-life group (P = 0.650). In the subgroups stratified by acute ischemic stroke, the relationship between an elevated PLR and a higher risk of ICAS remained unchanged (stroke group, P < 0.001; non-stroke group, P = 0.027).
Conclusions:
An elevated PLR was associated with a higher risk of ICAS in a Chinese Han population. The PLR might serve as a potential biomarker for ICAS in the elderly population.
Keywords: Platelet-to-lymphocyte ratio, Intracranial atherosclerotic stenosis, Atherosclerosis, Inflammation
Introduction
Intracranial atherosclerotic stenosis (ICAS), a progressive pathological process that induces cerebral hypoperfusion, is recognized as the most common cause of the occurrence of ischemic stroke globally and more easily leads to the recurrence of ischemic stroke compared with other stroke subtypes.[1,2] Asian, Hispanic, and African populations have a higher risk of intracranial atherosclerosis.[3] In Chinese populations, ICAS induces approximately 50% of transient ischemic attacks (TIAs) and 33% to 50% of strokes.[4] In consideration of the heavy burden that stroke places on society, it is crucial to identify ICAS to improve preventive strategies.
Previous studies have demonstrated that inflammation plays an essential role in the initiation and progression of ICAS. Inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6, and matrix metalloproteinases have been identified as reliable markers of ICAS.[2] The platelet-to-lymphocyte ratio (PLR), which is calculated by dividing the platelet count by the lymphocyte count, has recently been identified as a potential inflammatory marker. What is more, a higher PLR is regarded as a better reflection of atherosclerosis and platelet activation.[5] Studies have proven that the PLR is significantly correlated with the severity of coronary artery disease (CAD).[6–8] Moreover, it was found that the PLR might be a prognostic marker for acute coronary syndrome[9] and acute cerebral infarction.[10] Many prospective epidemiological studies have demonstrated that an increased PLR value is associated with stenosis in the carotid artery.[11] It was also shown that the occurrence of critical limb ischemia was significantly increased with the increase in PLR among patients with the peripheral arterial occlusive disease.[12] However, rare studies to date have focused on the association between the PLR and ICAS. This study aimed to investigate the association between the PLR and ICAS in a Chinese Han population, as well as whether the PLR could be used as a potential biomarker for ICAS.
Methods
Ethical approval
This study was approved by the Institutional Ethics Committee of Qingdao Municipal Hospital (No. QDSSLYY-2014-034) and written informed consent was obtained from all participants or their legal representatives.
Study population
The participants were prospectively recruited from Qingdao Municipal Hospital of the patients for suspected stroke in the department of neurology and individuals who underwent comprehensive health screening at the health screening center from January 2014 to June 2018. The inclusion criteria were as follows: (1) aged over 40 years; (2) of Han Chinese ethnicity; (3) underwent systemic investigations, including magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and other essential laboratory tests; (4) had available comprehensive clinical information. The exclusion criteria were as follows: (1) had been taking antiplatelet drugs before admission that may affect the PLR; (2) had a history of stroke; (3) had an intracerebral hemorrhage, extracranial atherosclerotic stenosis, atrial fibrillation, cardiac embolism, valvular heart disease, vascular disease and had undergone replacement; (4) had intracranial and external artery dissection, muscular fiber dysplasia, arteritis, moyamoya disease; (5) had an infection, tumor, hematologic disease, autoimmune diseases, chronic liver disease, and renal disease.
Finally, a total of 2134 participants were included for analysis in this study (a flowchart of the screening process for the included participants is listed in [Supplementary Figure 1)]. The majority of the participants were from the department of neurology (the basic characteristics of participants from the two different clinical departments are presented in [Supplementary Table 1)].
Demographic information and clinical measurements
Demographic information and clinical data were gathered by experienced and certified neurologists. Blood pressure was measured three times consecutively at admission after 15 min of rest in a supine position and the average was taken for analysis. Acute ischemic stroke included TIA and MRI confirmed acute ischemic stroke (within 7 days of onset). An average systolic pressure of at least 140 mmHg and/or an average diastolic pressure of at least 90 mmHg on ≥3 occasions or taking anti-hypertensive drugs was defined as hypertension.[13] The use of anti-diabetic drugs, a random blood glucose level ≥11.1 mmol/L, or a fasting plasma glucose ≥7.0 mmol/L were defined as diabetes mellitus.[14] Coronary heart disease (CHD) was defined as previous or newly diagnosed CHD. Self-reported behaviors of past or present smoking and drinking were defined as smoking and drinking, respectively. Information on medication usage, including lipid-lowering drugs, was obtained through self-report at admission.
Laboratory measurements
Fasting peripheral venous blood samples were collected in the morning within 24 h after admission and all data were obtained from the same blood sample. The lipid profile and fasting blood glucose (FBG) were assayed using an automated analytical platform (Beckman Coulter AU5800: Beckman Coulter Inc., Brea, CA, USA). The platelet count and lymphocyte count were automatically analyzed from EDTA-anticoagulated whole-blood samples using automated particle counters. Following the measurement, the PLR was calculated by dividing the platelet count by the lymphocyte count.
Assessment of ICAS
All enrolled participants had undergone 3D-Time-of-flight magnetic-resonance-angiography (3D-TOF MRA) at 3.0-T. In this study, the Warfarin-Aspirin Symptomatic Intracranial Disease trial criteria were used to evaluate the degree of intracranial stenosis.[15] More than 50% atherosclerotic stenosis or occlusion in single or multiple large intracranial arteries, including the M1/M2 middle cerebral artery, A1/A2 anterior cerebral artery, P1/P2 posterior cerebral artery, vertebral artery, basilar artery, and intracranial section of the internal carotid artery, was considered as ICAS. Two experienced radiologists read all images of the participants in the absence of clinical information and consulted a third neuroimaging physician if there was a dispute.
Statistical analysis
Eleven participants with data values outside 4 standard deviation limits were removed to eliminate the influence of extreme values. The normality of the distribution of continuous variables was tested using the Kolmogorov–Smirnov test. Non-normally distributed continuous variables were expressed as medians and interquartile ranges, and intergroup differences were compared by the Mann-Whitney U test. Categorical variables were expressed as frequencies and percentages, and Chi-squared tests were used to explore the differences. The relationship between the PLR and ICAS was evaluated by multivariate logistic regression models, adjusted by related risk factors (P < 0.05 by univariate logistic regression) including age, systolic blood pressure (SBP), FBG, high-density lipoprotein (HDL), acute ischemic stroke, a medical history of hypertension, a medical history of diabetes, and lipid-lowering drugs. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The trend test was performed by taking the median values of each quartile into the logistic regression model as a continuous variable. The analyses were further conducted in subgroups stratified by age (<60 vs. ≥60 years) and acute ischemic stroke. Moreover, in participants who completed the CRP test, the same analyses described above were conducted by adding CRP in the multivariate-adjusted models. Statistical analyses were performed using R software (version 3.6.3; R Foundation, Vienna, Austria) and SPSS version 21 (IBM Corp., Armonk, NY, United States). The significance level of all analyses was set at P < 0.05.
Results
Participant characteristics and intergroup comparisons
A total of 2134 participants were included in this analysis, among whom there were 518 patients with ICAS and 1616 patients without ICAS. The study population had a median age of 67 years and they were more likely to be male (59.9%). The clinical and demographic characteristics of the included participants are presented in [Table 1]. As the results showed, compared with the non-ICAS group, patients with ICAS were older and had higher levels of SBP and FBG, as well as a higher prevalence of acute ischemic stroke, hypertension, and diabetes mellitus, but a lower level of HDL. The PLR was significantly increased among patients with ICAS compared with patients without ICAS. We also found that patients with 1 to 2 stenotic arteries and ≥3 stenotic arteries had significantly higher PLRs than those without ICAS, but the comparison between the group with 1 to 2 stenotic arteries and the group with ≥3 stenotic arteries revealed no significant differences [Supplementary Figure 2].
Table 1.
Clinical and demographic characteristics of participants with ICAS or without ICAS.
| Characteristics | Total participants (n = 2134) | ICAS (n = 518) | Non-ICAS (n = 1616) | Statistics | P values |
| Age, median (IQR), years | 67 (60–77) | 70 (62–79) | 66 (59–76) | −5.325∗ | <0.001 |
| Male, n (%) | 1278 (59.9) | 306 (59.1) | 972 (60.1) | 0.189† | 0.664 |
| SBP, median (IQR), mmHg | 145 (130–160) | 150 (135–170) | 144 (130–160) | −4.703∗ | <0.001 |
| DBP, median (IQR), mmHg | 84 (80–90) | 85 (80–90) | 82 (80–90) | −0.911∗ | 0.362 |
| Lipid profile, median (IQR) | |||||
| TG (mmol/L) | 1.30 (0.95–1.78) | 1.32 (0.98–1.74) | 1.30 (0.94–1.78) | −0.619∗ | 0.536 |
| TC (mmol/L) | 4.95 (4.21–5.77) | 4.98 (4.13–5.90) | 4.93 (4.23–5.73) | −0.232∗ | 0.817 |
| HDL (mmol/L) | 1.14 (0.96–1.35) | 1.12 (0.93–1.32) | 1.14 (0.98–1.36) | −2.376∗ | 0.018 |
| LDL (mmol/L) | 3.04 (2.48–3.62) | 3.08 (2.44–3.64) | 3.03 (2.50–3.61) | −0.534∗ | 0.593 |
| FBG, median (IQR), mmol/L | 5.32 (4.69–6.77) | 5.69 (4.80–7.83) | 5.23 (4.66–6.50) | −5.261∗ | <0.001 |
| Platelet, median (IQR), (109/L) | 205 (173–239) | 207 (172.75–244) | 205 (173–238) | −0.644∗ | 0.520 |
| Lymphocytes, median (IQR), (109/L) | 1.95 (1.55–2.42) | 1.86 (1.45–2.33) | 1.99 (1.57–2.46) | −3.720∗ | <0.001 |
| PLR, median (IQR) | 104.76 (82.20–136.45) | 110.17 (87.49–146.17) | 102.97 (81.77–131.83) | −3.620∗ | <0.001 |
| Medical history | |||||
| Hypertension, n (%) | 1646 (77.1) | 424 (81.9) | 1222 (75.6) | 8.644† | 0.003 |
| Diabetes mellitus, n (%) | 751 (35.2) | 224 (43.2) | 527 (32.6) | 19.441† | <0.001 |
| CHD, n (%) | 791 (37.1) | 208 (40.2) | 583 (36.1) | 2.796† | 0.094 |
| Acute ischemic stroke, n (%) | 1293 (60.6) | 362 (69.9) | 931 (57.6) | 24.743† | <0.001 |
| Smoking, n (%) | 728 (34.1) | 174 (33.6) | 554 (34.3) | 0.083† | 0.773 |
| Drinking, n (%) | 526 (24.6) | 127 (24.5) | 399 (24.7) | 0.006† | 0.937 |
| Lipid-lowering drugs, n (%) | 247 (11.6) | 107 (20.7) | 140 (8.7) | 55.126† | <0.001 |
Mann-Whitney U test.
Chi-squared test.
CHD: Coronary heart disease; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; HDL: High-density lipoprotein; ICAS: Intracranial arterial stenosis; IQR: Interquartile range; LDL: Low-density lipoprotein; PLR: Platelet-to-lymphocyte ratio; SBP: Systolic blood pressure; TC: Total cholesterol; TG: Triglyceride.
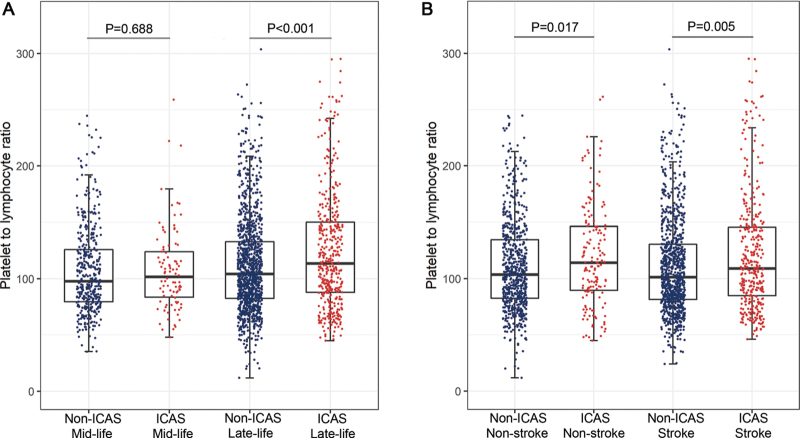
In our subgroup analysis stratified by age, all participants were divided into two subgroups: the <60 years group (mid-life group) and the ≥60 years group (late-life group). We found that PLR levels were higher in patients with ICAS than in those without ICAS in the late-life group (P < 0.001), but no significant difference in PLR level was found while comparing patients with and without ICAS in the mid-life group (P = 0.688) [Figure 1A]. Other characteristics of participants in subgroups stratified by age are also shown in [Table 2].
Figure 1.
The level of PLR and distribution of participants. (A) PLR levels were significantly higher in ICAS groups compared to non-ICAS groups in late-life participants, but no statistical significance in mid-life participants. (B) PLR levels were significantly higher in ICAS groups in both stroke and non-stroke participants. ICAS: Intracranial arterial stenosis; PLR: Platelet-to-lymphocyte ratio.
Table 2.
Characteristics of participants in subgroups stratified by age.
| Mid-life group (n = 519) | Late-life group (n = 1615) | |||||
| Characteristics | ICAS (n = 94) | Non-ICAS (n = 425) | P (sta) | ICAS (n = 424) | Non-ICAS (n = 1191) | P (sta) |
| Age, median (IQR), years | 53 (51–57) | 54 (49–57) | 0.483 (−0.701∗) | 74 (66–81) | 71 (64–79) | <0.001 (−3.631∗) |
| Male, n (%) | 73 (77.7) | 326 (76.7) | 0.843 (0.039†) | 233 (55.0) | 646 (54.2) | 0.800 (0.064†) |
| SBP, median (IQR), mmHg | 147 (130–160) | 140 (130–160) | 0.381 (−0.877∗) | 150 (135–170) | 145 (130–160) | <0.001 (−4.686∗) |
| DBP, median (IQR), mmHg | 90 (80–96) | 90 (80–100) | 0.705 (−0.379∗) | 84 (80–90) | 80 (80–90) | 0.066 (−1.837∗) |
| Lipid profile, median (IQR) | ||||||
| TG (mmol/L) | 1.49 (1.11–2.06) | 1.51 (1.12–2.05) | 0.683 (−0.408∗) | 1.27 (0.96–1.70) | 1.22 (0.89–1.69) | 0.092 (−1.687∗) |
| TC (mmol/L) | 4.87 (4.01–5.72) | 5.01 (4.16–5.75) | 0.395 (−0.850∗) | 5.00 (4.15–5.95) | 4.90 (4.24–5.72) | 0.497 (−0.679∗) |
| HDL (mmol/L) | 1.04 (0.89–1.27) | 1.11 (0.95–1.29) | 0.124 (−1.538∗) | 1.13 (0.94–1.33) | 1.16 (0.99–1.38) | 0.026 (−2.222∗) |
| LDL (mmol/L) | 3.01 (2.40–3.48) | 3.08 (2.46–3.66) | 0.279 (−1.082∗) | 3.09 (2.47–3.70) | 3.01 (2.51–3.58) | 0.247 (−1.157∗) |
| FBG, median (IQR), mmol/L | 5.72 (4.82–8.07) | 5.31 (4.70–6.83) | 0.055 (−1.917∗) | 5.69 (4.80–7.68) | 5.21 (4.65–6.42) | <0.001 (−5.081∗) |
| Platelet, median (IQR), (109/L) | 209 (178–244) | 210 (180–248) | 0.575 (−0.561∗) | 207 (172–244) | 202 (171–234) | 0.210 (−1.254∗) |
| Lymphocytes, median (IQR), (109/L) | 2.05 (1.67–2.59) | 2.14 (1.72–2.55) | 0.502 (−0.671∗) | 1.82 (1.39–2.28) | 1.93 (1.53–2.40) | 0.001 (−3.293∗) |
| PLR, median (IQR) | 101.71 (82.26–124.72) | 97.89 (79.40–126.08) | 0.688 (−0.401∗) | 113.65 (87.67–150.33) | 104.32 (82.33–133.12) | <0.001 (−3.651∗) |
| Medical history | ||||||
| Hypertension, n (%) | 73 (77.7) | 304 (71.5) | 0.228 (1.455†) | 351 (82.8) | 918 (77.1) | 0.014 (6.045†) |
| Diabetes mellitus, n (%) | 41 (43.6) | 141 (33.2) | 0.055 (3.685†) | 183 (43.2) | 386 (32.4) | <0.001 (15.837†) |
| CHD, n (%) | 23 (24.5) | 87 (20.5) | 0.391 (0.736†) | 185 (43.6) | 496 (41.6) | 0.477 (0.506†) |
| Acute ischemic stroke, n (%) | 72 (76.6) | 257 (60.5) | 0.003 (8.625†) | 290 (68.4) | 674 (56.6) | <0.001 (18.111†) |
| Smoking, n (%) | 56 (59.6) | 215 (50.6) | 0.114 (2.491†) | 118 (27.8) | 339 (28.5) | 0.804 (0.062†) |
| Drinking, n (%) | 39 (41.5) | 154 (36.2) | 0.340 (0.910†) | 88 (20.8) | 245 (20.6) | 0.936 (0.006†) |
| Lipid-lowering drugs, n (%) | 8 (8.5) | 19 (4.5) | 0.110 (2.548†) | 99 (23.3) | 121 (10.2) | <0.001 (46.229†) |
Mann-Whitney U test.
Chi-squared test.
CHD: Coronary heart disease; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; HDL: High-density lipoprotein; ICAS: Intracranial arterial stenosis; IQR: Interquartile range; LDL: Low-density lipoprotein; PLR: Platelet-to-lymphocyte ratio; SBP: Systolic blood pressure; sta: Statistics; TC: Total cholesterol; TG: Triglyceride.
We also conducted a subgroup analysis stratified by acute ischemic stroke (stroke group and non-stroke group). The characteristics of participants in subgroups stratified by stroke status are summarized in [Supplementary Table 2]. In the non-stroke group, PLR levels were higher in patients with ICAS than in those without ICAS (P = 0.017). Similar results were also found in the stroke group (P = 0.005) [Figure 1B].
Association of the PLR and ICAS in all recruited participants
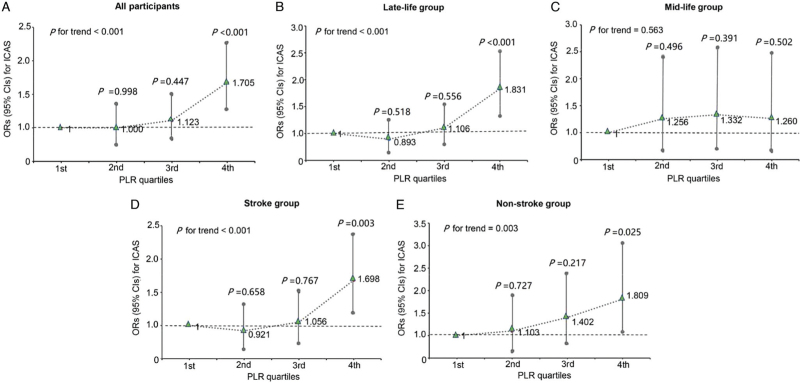
Findings from multivariable logistic regression for the association between the PLR and ICAS are provided in [Table 3]. After adjustment for confounding factors, the PLR was demonstrated to be associated with ICAS in all participants (OR 1.005, 95% CI 1.003–1.007, P < 0.001). Further analyses were performed using quartiles of the PLR. The regression analyses indicated that patients in higher PLR quartiles were more inclined to suffer ICAS than those in the first quartile. Specifically, with the first quartile as the reference category, the ORs and 95% CIs were 1.000 (0.739–1.352) in the second quartile, 1.123 (0.833–1.513) in the third quartile, and 1.705 (1.278–2.275) in the fourth quartile [Figure 2A]. The fourth (P < 0.001) PLR quartile was significantly associated with ICAS. We also conducted the trend test and finally obtained the P value for trend (P < 0.001) which was significant, and the test also indicated a dose–response relationship between the PLR and the risk of suffering ICAS. In addition, repeated analyses were performed separately in participants from the two different clinical departments, and similar results were obtained [Supplementary Table 3].
Table 3.
Multivariate-adjusted OR and 95% CI for ICAS in mid-life and late-life groups.
| Total (n = 2134) | Mid-life group (n = 519) | Late-life group (n = 1615) | ||||||
| Characteristic | Univariate, OR (95% CI) | P value | Multi-OR (95% CI) | P value | Multi-OR (95% CI) | P value | Multi-OR (95% CI) | P value |
| Age | 1.024 (1.015–1.033) | <0.001 | 1.020 (1.011–1.030) | <0.001 | 1.026 (0.979–1.077) | 0.288 | 1.017 (1.003–1.032) | 0.015 |
| Male | 1.046 (0.854–1.278) | 0.664 | – | – | – | – | – | – |
| SBP | 1.010 (1.006–1.015) | <0.001 | 1.006 (1.001–1.011) | 0.021 | 0.998 (0.987–1.008) | 0.666 | 1.008 (1.003–1.013) | 0.004 |
| DBP | 0.998 (0.992–1.001) | 0.300 | – | – | – | – | – | – |
| FBG, mmol/L | 1.094 (1.054–1.134) | <0.001 | 1.070 (1.020–1.123) | 0.006 | 1.015 (0.991–1.126) | 0.787 | 1.093 (1.034–1.156) | 0.002 |
| TG, mmol/L | 0.985 (0.895–1.069) | 0.737 | – | – | – | – | – | – |
| TC, mmol/L | 1.023 (0.947–1.104) | 0.562 | – | – | – | – | – | – |
| HDL, mmol/L | 0.617 (0.445–0.834) | 0.003 | 0.629 (0.452–0.846) | 0.004 | 0.646 (0.273–1.321) | 0.288 | 0.640 (0.447–0.881) | 0.010 |
| LDL, mmol/L | 0.994 (0.926–1.017) | 0.710 | – | – | – | – | – | – |
| Hypertension | 1.454 (1.136–1.877) | 0.003 | 1.201 (0.914–1.588) | 0.193 | 1.395 (0.776–2.579) | 0.276 | 1.154 (0.847–1.585) | 0.369 |
| Diabetes | 1.574 (1.285–1.927) | <0.001 | 1.150 (0.878–1.502) | 0.307 | 1.292 (0.675–2.434) | 0.432 | 1.107 (0.820–1.489) | 0.502 |
| CHD | 1.189 (0.970–1.455) | 0.095 | – | – | – | – | – | – |
| Acute ischemic stroke | 1.707 (1.383–2.115) | <0.001 | 1.601 (1.282–2.006) | <0.001 | 2.119 (1.261–3.678) | 0.006 | 1.503 (1.174–1.930) | 0.001 |
| Smoking | 0.970 (0.785–1.194) | 0.773 | – | – | – | – | – | – |
| Drinking | 0.991 (0.785–1.244) | 0.937 | – | – | – | – | – | – |
| Lipid-lowering drugs | 2.745 (2.083–3.609) | <0.001 | 2.219 (1.652–2.975) | <0.001 | 1.592 (0.618–3.785) | 0.309 | 2.333 (1.702–3.196) | <0.001 |
| PLR | 1.005 (1.003–1.007) | <0.001 | 1.005 (1.003–1.007) | <0.001 | 1.001 (0.995–1.007) | 0.650 | 1.006 (1.003–1.008) | <0.001 |
CHD: Coronary heart disease; CI: Confidence interval; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; Multi-OR: Multivariate OR; OR: Odds ratio; PLR: Platelet-to-lymphocyte ratio; SBP: Systolic blood pressure; TC: Total cholesterol; TG: Triglyceride.
Figure 2.
Multivariate-adjusted OR and 95% CI for ICAS according to PLR levels. (A) All participants, (B) late-life group, (C) mid-life group, (D) stroke group, and (E) non-stroke group. CI: Confidence interval; ICAS: Intracranial arterial stenosis; OR: Odds ratio; PLR: Platelet-to-lymphocyte ratio.
To remove the potential impact of CRP on the relationship between the PLR and ICAS, sensitivity analysis was further conducted on participants who completed the CRP test. In this analysis, we added CRP to the multivariate-adjusted models and found that the results remained stable [Supplementary Table 4].
Association of the PLR and ICAS in the mid-life and late-life groups
In the late-life group, the PLR was a significant risk factor for ICAS in regression analysis (OR 1.006, 95% CI 1.003–1.008, P < 0.001). However, no association between the PLR and ICAS was observed in the mid-life group (OR 1.001, 95% CI 0.995–1.007, P = 0.650). When referenced to the first quartile, higher PLR levels had a significant association with the risk of ICAS in the late-life group. The OR was 0.893 (95% CI 0.635–1.257; P = 0.518) for the second quartile, 1.106 (95% CI 0.791–1.546; P = 0.556) for the third quartile, and 1.831 (95% CI 1.327–2.527; P < 0.001) for the fourth quartile (P for trend < 0.001) [Figure 2B]. However, no significant association mentioned above was found in the mid-life group [Figure 2C]. The results indicated that the relationship between the PLR and ICAS did differ significantly in different age subgroups.
Association of the PLR and ICAS in the stroke and non-stroke groups
In the multivariate logistic regression model, an elevated PLR was associated with ICAS in the stroke (OR 1.005, 95% CI 1.002–1.008, P < 0.001) and non-stroke groups (OR 1.005, 95% CI 1.001–1.009, P = 0.027) [Supplementary Table 5]. In addition, taking the first quartile as a reference, the fourth PLR quartile had the most significant association with ICAS in both the stroke (OR 1.698, 95% CI 1.198–2.407, P = 0.003) [Figure 2D] and non-stroke groups (OR 1.809, 95% CI 1.075–3.045, P = 0.025) [Figure 2E]. In both groups, the P values for trend were still remarkable. These findings indicated that the relationship between an increased PLR and ICAS remained unchanged, regardless of stroke status.
Discussion
Our results indicated high levels of PLR were associated with the occurrence of ICAS. Moreover, an association between the PLR and ICAS was found in the late-life group, but not in the mid-life group. In addition, regardless of stroke status, the risk of ICAS increased with an increase in the PLR. We are the first to explore the association between the PLR and ICAS.
The PLR, which has been recognized as a systemic inflammatory marker, is easily obtained by measuring the whole blood count. Moreover, the PLR is an integrated reflection of inflammatory and thrombotic pathways.[16] Atherosclerosis is a chronic inflammatory disease, and multiple inflammatory cells and inflammatory factors play a significant role in its pathogenesis.[17] Previous research has rarely focused on both PLR and ICAS. However, an association of the PLR with atherosclerotic stenosis was demonstrated in the coronary artery and carotid artery. In a large-scale study,[18] the results revealed that the PLR was positively correlated with the severity of CAD and CRP levels. Furthermore, a greater PLR was demonstrated to be an independent predictor for more severe CAD. Varim et al's[19] study showed that the PLR of patients in the critical carotid stenosis group was higher than that in the non-critical carotid stenosis group. Moreover, they found that PLR had an important predictive value for critical stenosis in the carotid arteries. These results were consistent with our finding of the association of high levels of PLR with the presence of ICAS.
Platelets play a major role in the initiation, progression, and destabilization of atherosclerotic plaques.[20,21] An expanding body of evidence indicates that platelets bind to the surface of endothelial cells and secrete pro-inflammatory chemokines to recruit circulating monocytes. The recruitment of monocytes into subendothelial spatium and their differentiation into macrophages initiate the process of atherosclerosis.[22] Moreover, activated platelets release inflammatory mediators that promote inflammation at the site of a vascular lesion.[23] What is more, plaque disruption exposes extracellular matrix proteins, thereby triggering a rapid accumulation of circulating platelets. This process can promote the progression and instability of atherosclerotic plaques, and the transition to the thrombotic phase.[24] Previous studies have suggested that ongoing inflammatory conditions promote the proliferation in megakaryocytic series.[25] Platelet indices including platelet count, mean platelet volume, and soluble mediators released by activated platelets are associated with atherosclerosis, which has been well documented.[26] Moreover, a high platelet count has been proposed to have prognostic value for cardiovascular risk.[27] Low lymphocyte count (LLC) is a general feature of the inflammatory process. Clinical and animal studies have demonstrated that LLC can accelerate the progression of atherosclerosis.[28] An LLC has been identified as a predictor for worse outcomes in patients with heart failure and CAD.[29,30] Various mechanisms underlying lymphopenia have been proposed, including a direct effect of elevated serum levels of cortisol and catecholamines that occur during a systemic stress response.[28] In addition, in atherosclerotic lesions, lymphocyte apoptosis occurs and plays an increasingly important role in the formation of atherosclerotic plaques, eventually promoting plaque growth, lipid core development, plaque rupture, and thrombosis.[31] PLR integrates the predictive risk of both thrombosis and inflammation, and it is more stable than lymphocyte or platelet counts alone.
Since age is the main risk factor for ICAS,[32] we performed age subgroup analyses to remove the potential impact of age on ICAS. The results showed that age affected the association between the PLR and ICAS. With increasing age, arterial walls show complex structural changes, including an inversion of the elastin/collagen ratio and the non-enzymatic glycosylation of some proteins present in the arterial wall, which increases mechanical vessel rigidity and stiffness.[33] Furthermore, due to increased oxidative stress, age-accelerated vascular injury results in inflammation and endothelial dysfunction, but no definite mechanisms have been identified.[34] All situations mentioned above may contribute to the predisposition of ICAS in the elderly population. An age-related effect on the correlation between the PLR and CAD was found in a study by Trakarnwijitr et al[35]. The result of a positive correlation in older (age >55 years) patients was consistent with our finding in the late-life group. However, a negative association found in younger (age <55 years) patients was not in line with our finding in mid-life participants. The reasons for the conflicting results in younger patients were unclear but may be due to the differences in age and racial composition. Another crucial reason might be the differences in the structure and function of arteries.
In this study, we investigated whether the relationship between the PLR and ICAS was affected by an acute ischemic stroke. In the subgroup analyses stratified by acute ischemic stroke, we found that a higher PLR was associated with ICAS, regardless of whether the participants had an acute ischemic stroke. The PLR appeared to be a better discriminator for identifying ICAS risk in stroke patients and non-stroke patients. Therefore, the results from our study may be beneficial to improving preventive strategies for the occurrence or recurrence of ischemic events.
Several limitations exist in our study. First, we adopted a cross-sectional design. The specific causal association between the PLR and ICAS was difficult to establish. Therefore, our findings need to be confirmed by further prospective studies. Second, only MRA, which is not as accurate as catheter angiography, was used to evaluate arterial stenosis. Third, only Chinese Han adults were included in our analysis. Additional research in diverse populations to confirm our findings is essential.
Conclusions
The results indicated that an elevated PLR was associated with a higher risk of ICAS in a Chinese Han population. The association of PLR and ICAS was observed in the patients 60 years old and older but not in the patients under 60 years old. In addition, regardless of stroke status, the risk of ICAS increased with an increase in the PLR. Therefore, the findings from our study suggest that PLR might serve as a potential biomarker for ICAS in the elderly population.
Funding
This study was supported by grants from the Taishan Scholars Program of Shandong Province (Nos. ts201511109 and tsqn20161079) and the Qingdao Key Health Discipline Development Fund.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Huang Y, Wang Z, Zhao B, Ma Y, Ou Y, Hu H, Hou X, Yu J, Tan L. An elevated platelet-to-lymphocyte ratio is associated with a higher risk of intracranial atherosclerotic stenosis. Chin Med J 2022;135:1425–1431. doi: 10.1097/CM9.0000000000002228
Supplemental digital content is available for this article.
References
- 1.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12:1106–1114. doi: 10.1016/s1474-4422(13)70195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Meng R, Liu G, Cao C, Chen F, Jin K, et al. Intracranial atherosclerotic disease. Neurobiol Dis 2019; 124:118–132. doi: 10.1016/j.nbd.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008; 39:2396–2399. doi: 10.1161/strokeaha.107.505776. [DOI] [PubMed] [Google Scholar]
- 4.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 5.Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015; 26:680–681. doi: 10.3109/09537104.2014.979340. [DOI] [PubMed] [Google Scholar]
- 6.Yüksel M, Yildiz A, Oylumlu M, Akyüz A, Aydin M, Kaya H, et al. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol J Cardiol 2015; 15:640–647. doi: 10.5152/akd.2014.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sari I, Sunbul M, Mammadov C, Durmus E, Bozbay M, Kivrak T, et al. Relation of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio with coronary artery disease severity in patients undergoing coronary angiography. Kardiol Pol 2015; 73:1310–1316. doi: 10.5603/KP.a2015.0098. [DOI] [PubMed] [Google Scholar]
- 8.Li XT, Fang H, Li D, Xu FQ, Yang B, Zhang R, et al. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J (Engl) 2020; 133:415–423. doi: 10.1097/cm9.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep 2017; 7:40426.doi: 10.1038/srep40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Yang P, Wang J. Peripheral blood platelet to lymphocyte ratio as potential diagnostic and prognostic markers of acute cerebral infarction and its clinical significance. Clin Lab 2019; 65.doi: 10.7754/Clin.Lab.2018.180912. [DOI] [PubMed] [Google Scholar]
- 11.Massiot N, Lareyre F, Voury-Pons A, Pelletier Y, Chikande J, Carboni J, et al. High neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with symptomatic internal carotid artery stenosis. J Stroke Cerebrovasc Dis 2019; 28:76–83. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One 2013; 8:e67688.doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LS. 2010 Chinese guidelines for the management of hypertension (in Chinese). Chin J Cardiol 2011; 39:579–615. [PubMed] [Google Scholar]
- 14.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 2019; 42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 15.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology 2019; 70:802–818. doi: 10.1177/0003319719845186. [DOI] [PubMed] [Google Scholar]
- 17.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res 2019; 124:315–327. doi: 10.1161/circresaha.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology 2016; 67:89–95. doi: 10.1177/0003319715583186. [DOI] [PubMed] [Google Scholar]
- 19.Varim C, Varim P, Acar BA, Vatan MB, Uyanik MS, Kaya T, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting the severity of carotid artery stenosis in patients undergoing carotid angiography. Kaohsiung J Med Sci 2016; 32:86–90. doi: 10.1016/j.kjms.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes QE, Fuentes QF, Andrés V, Pello OM, Font de Mora J, Palomo GI. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets 2013; 24:255–262. doi: 10.3109/09537104.2012.690113. [DOI] [PubMed] [Google Scholar]
- 21.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol 2006; 47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 22.Lindemann S, Krämer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost 2007; 5: (Suppl 1): 203–211. doi: 10.1111/j.1538-7836.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- 23.Bakogiannis C, Sachse M, Stamatelopoulos K, Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019; 122:154157.doi: 10.1016/j.cyto.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007; 357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 25.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011; 17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 26.von Hundelshausen P, Schmitt MM. Platelets and their chemokines in atherosclerosis — clinical applications. Front Physiol 2014; 5:294.doi: 10.3389/fphys.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patti G, Di Martino G, Ricci F, Renda G, Gallina S, Hamrefors V, et al. Platelet indices and risk of death and cardiovascular events: results from a large population-based cohort study. Thromb Haemost 2019; 119:1773–1784. doi: 10.1055/s-0039-1694969. [DOI] [PubMed] [Google Scholar]
- 28.Núñez J, Miñana G, Bodí V, Núñez E, Sanchis J, Husser O, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem 2011; 18:3226–3233. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 29.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005; 45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 30.Marçula M, de Souza Buto MF, Madaloso BA, Nunes RA, Cuoco MA, de Paula RS, et al. Lymphocyte count and prognosis in patients with heart failure. Int J Cardiol 2015; 188:60–62. doi: 10.1016/j.ijcard.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004; 107:343–354. doi: 10.1042/cs20040086. [DOI] [PubMed] [Google Scholar]
- 32.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 2012; 111:245–259. doi: 10.1161/circresaha.111.261388. [DOI] [PubMed] [Google Scholar]
- 33.Tesauro M, Mauriello A, Rovella V, Annicchiarico-Petruzzelli M, Cardillo C, Melino G, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med 2017; 281:471–482. doi: 10.1111/joim.12605. [DOI] [PubMed] [Google Scholar]
- 34.McEwen JE, Zimniak P, Mehta JL, Shmookler Reis RJ. Molecular pathology of aging and its implications for senescent coronary atherosclerosis. Curr Opin Cardiol 2005; 20:399–406. doi: 10.1097/01.hco.0000175517.50181.89. [DOI] [PubMed] [Google Scholar]
- 35.Trakarnwijitr I, Li B, Adams H, Layland J, Garlick J, Wilson A. Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. Int J Cardiol 2017; 248:349–354. doi: 10.1016/j.ijcard.2017.06.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.