Abstract
Background:
Response to immune checkpoint inhibitors (ICIs) is affected by multiple factors. This study aimed to explore whether sites of metastasis are associated with clinical outcomes of ICIs in advanced non-small-cell lung cancer (NSCLC) patients.
Methods:
The data of NSCLC patients with high programmed death-ligand 1 expression and good performance status receiving first-line ICIs monotherapy from Guangdong Provincial People's Hospital between May 2019 and July 2020 were retrospectively analyzed. Metastatic sites included liver, bone, brain, adrenal gland, pleura, and contralateral lung. Progression-free survival (PFS) and overall survival (OS) were compared between different metastatic sites and metastatic burden by the Kaplan-Meier method. Organ-specific disease control rate (OSDCR) of different individual metastatic sites was evaluated.
Results:
Forty NSCLC patients meeting the criteria were identified. The presence of liver metastasis was significantly associated with shorter PFS (3.1 vs. 15.5 months, P = 0.0005) and OS (11.1 months vs. not reached, P = 0.0016). Besides, patients with bone metastasis tend to get shorter PFS (4.2 vs. 15.5 months, P = 0.0532) rather than OS (P = 0.6086). Moreover, the application of local treatment could numerically prolong PFS in patients with brain metastasis (15.5 vs. 4.3 months, P = 0.1894). More metastatic organs involved were associated with inferior PFS (P = 0.0052) but not OS (P = 0.0791). The presence of liver metastasis or bone metastasis was associated with more metastatic organs (Phi[ϕ]: 0.516, P = 0.001). The highest OSDCR was observed in lung (15/17), and the lowest in the liver (1/4).
Conclusions:
Metastases in different anatomical locations may be associated with different clinical outcomes and local tumor response to ICIs in NSCLC. ICIs monotherapy shows limited efficacy in patients with liver and bone metastasis, thus patients with this type of metastasis might require more aggressive combination strategies.
Keywords: Metastatic sites, Immunotherapy, Liver metastases, Bone metastasis, Non-small-cell lung cancer, Tumor response
Introduction
Lung cancer ranks the first in the incidence of malignancy in men and is also the key contributor to cancer-related deaths worldwide regardless of gender. Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer.[1] Distant metastases are characteristic of advanced cancer, and the most frequent metastatic sites in NSCLC include the nervous system, bone, liver, respiratory system, and adrenal gland.[2] It has been demonstrated that different metastatic pattern shows different prognostic value in patients with NSCLC. For example, a Surveillance, Epidemiology, and End Results based study suggested that liver metastasis and multiorgan metastases are associated with high mortality risk.[3]
The emergence of immunotherapy has transformed the systemic treatment strategies for patients with advanced NSCLC. Several phase III trials showed the significant survival benefits of immunotherapy targeting immune checkpoints, including programmed death-1/programmed death-ligand 1 (PD-L1) interaction in patients with advanced NSCLC. The efficacy of immune checkpoint inhibitors (ICIs) is closely related to the tumor microenvironment as it induces antitumor effects by restoring systemic antitumor immunity. Yet, heterogeneous tumor microenvironments (gene profile alteration and infiltrated immune cells located at specific organs) have been seen in advanced NSCLC patients with different metastatic sites, which may potentially lead to a discrepant response to ICIs, also known as organ-specific efficacy.[4–6]
Several trials have suggested liver metastasis as an independent negative predictive and prognostic factor of ICIs efficacy. Besides, a translational study analyzed the data of the NSCLC cohort from KEYNOTE-001, which found that liver metastases were associated with reduced marginal CD8+ T-cell infiltration, providing a potential mechanism for this outcome.[7] Furthermore, patients with brain metastasis or bone metastasis showed dismal results regarding the efficacy of ICIs.[8,9] However, these studies always included patients with various PD-L1 tumor proportion scores (TPS) statuses, disparate Eastern Cooperative Oncology Group (ECOG) performance status (PS), and different lines of therapy, all of which may potentially influence the clinical outcomes of ICIs as reported in the literature.[10–12] The association of different metastatic sites with the efficacy of ICIs needs to be further elucidated in a relatively homogeneous population. Thus, we designed this study by collecting information from NSCLC patients who received first-line ICIs monotherapy and had high PD-L1 expression as well as good performance status, to make sure this group of patients included is relatively homogeneous and comparable.
In the present study, we investigated systemic and local efficacy of ICIs between different metastatic sites in advanced NSCLC patients with high PD-L1 expression and good performance status who received ICIs monotherapy as first-line treatment. The goal was to explore whether sites of metastasis are associated with clinical outcomes to ICIs in advanced NSCLC patients.
Methods
Ethical approval
The study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Committee of Guangdong Provincial People's Hospital (approval number: GDREC2019304H[R1]). The ethics committee waived the individual consent due to the retrospective nature of the study.
Patient population
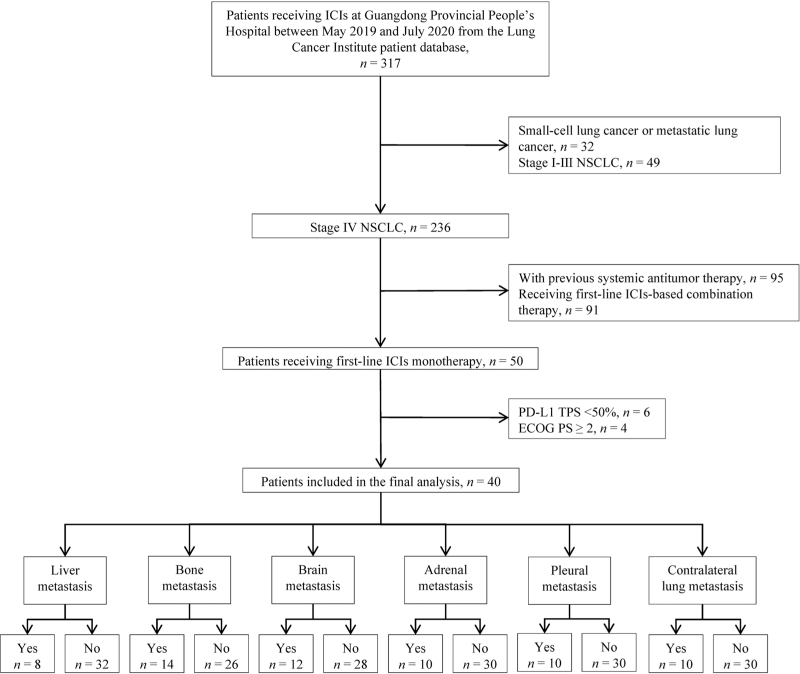
Patients who were diagnosed as advanced NSCLC with different sites of metastasis and treated with ICIs at Guangdong Provincial People's Hospital between May 2019 and July 2020 from the Lung Cancer Institute patient database were selected for this retrospective study. The inclusion criteria were: (1) histologically/cytologically confirmed stage IV NSCLC, (2) receiving first-line ICIs monotherapy, (3) with high PD-L1 expression (TPS ≥ 50%), (4) without sensitizing epidermal growth factor receptor or anaplastic lymphoma kinase genomic aberration, (5) with an ECOG PS of 0 or 1. The exclusion criteria were: (1) small-cell lung cancer or metastatic lung cancer, (2) previous systemic antitumor therapy, (3) receiving first-line ICIs-based combination therapy, (4) ECOG PS ≥ 2. A flowchart of the screening process of cases included in the present study and further study sample details was presented in Figure 1.
Figure 1.
Flowchart describing the selection of advanced non-small cell lung cancer patients treated with first-line single-agent immune checkpoint inhibitors.
PD-L1 assessment by immunohistochemistry (IHC)
PD-L1 IHC was performed with Dako PD-L1 IHC 22C3 pharmDx (clone 22C3; Dako/Agilent Technologies, Carpinteria, CA, USA) on the Dako Autostainer Link 48 autostainer (Dako/Agilent Technologies) according to the manufacturer's protocol. PD-L1 controls were run concurrently with test samples, including positive and negative cell line controls provided in the assay kit, an in-house tonsil control as a positive tissue control, and a negative control without a secondary antibody. PD-L1 expression on tumor cells from patients’ tumor samples obtained by biopsy (sample types are summarized in Supplementary Table 1) was determined by TPS, which was defined as the percentage of tumor cells with complete or partial membranous staining at any intensity. High PD-L1 expression was defined as at least 50% of tumor cells with staining. The stained tissue sections were independently scored by two pathologists. PD-L1 results were obtained from pathology reports.
Data collection and outcome assessment
Clinical data of enrolled patients including age, sex, smoking history, pathology, metastatic sites, and the number of metastatic organs were retrieved from the patients’ medical records. Metastatic burden was defined as the number of metastatic organs involved and a high metastatic burden meant more than one site of distant metastasis. Progression-free survival (PFS) was defined as the time from the first ICIs prescription date to the day of physician assessment of disease progression or death from any cause. Overall survival (OS) was defined as the time from the first ICIs prescription date to either the date of death or the final follow-up date. The data of patients who survived were censored.
Tumor response was assessed in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). In brief, the organ-specific tumor responses, including complete responses (CR; complete disappearance), partial responses (PR; ≥30% reduction, taking the baseline sum of maximal diameters as reference), progressive diseases (PD; ≥20% increase, taking the smallest sum of maximal diameters as reference), or stable diseases (SD; neither CR, PR, nor PD), were evaluated in each organ system by RECIST 1.1. The best response of the lesions in each organ during the study period was recorded. The organ-specific disease control rate (OSDCR) was defined as the ratio of the total number of patients reaching CR, PR, or SD as the best organ-specific tumor response to the total number of patients. All patients were followed up until November 8, 2021.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Science (SPSS) software (version 23, IBM, Armonk, NY, USA) and GraphPad Prism software (Version 8, GraphPad Software; San Diego, CA, USA). Categorical variables were expressed as numbers of cases (%). Continuous variables with normal distribution were presented as mean ± standard deviation; non-normally distributed variables were reported as median (range). The Kaplan-Meier method was used to analyze the survival probability, and the log-rank test was used to calculate the significance of differences. The Cox proportional hazard model was applied for the univariable and multivariable analyses to calculate the hazard ratios and 95% confidence intervals (95%). Only variables with P < 0.20 in univariable analysis were included as candidate variables in the model selection procedure for multivariable analyses. The correlation of liver or bone metastasis and the metastatic burden was evaluated by the Spearman's rank correlation test. Two-sided P values < 0.05 were considered statistically significant.
Results
Baseline characteristics and efficacy of ICIs in the total population
A total of 317 patients received ICIs between May 2019 and July 2020 at Guangdong Provincial People's Hospital. The patients’ demographic information and disease characteristics are summarized in Table 1. A total of 40 patients with metastatic NSCLC as well as good performance status receiving ICIs monotherapy as the first-line treatment strategy were identified. All the enrolled patients had high expression of PD-L1 (assessed by biopsy) as shown in Supplementary Table 1, and all received pembrolizumab 200 mg intravenously every 3 weeks. The median age of the overall population of patients was 66 (range 31–77) years; the majority of patients (83%, 33/40) were male. Among them, 30% (12/40) were diagnosed with lung squamous carcinoma. In our cohort, 68% of patients (27/40) had more than one site of distant metastasis before starting treatment. The most frequent site of metastasis recorded in this retrospective cohort was bone (35%, 14/40), followed by the brain (30%, 12/40), adrenal (25%, 10/40), pleura (25%, 10/40), contralateral lung (25%, 10/40), and the liver (20%, 8/40) was the least frequent site of metastasis. Of note, half of the patients with brain metastasis (6/12) received local treatment targeting brain metastasis, including surgical resection and radiotherapy, before the application of ICIs. The median PFS was 11.7 months in the total population, and the 2-year OS rate was 60%.
Table 1.
Characteristics of advanced non-small-cell lung cancer patients treated with first-line single-agent immune checkpoint inhibitors.
| Characteristics | No. of patients (n = 40) |
| Age (years), median (range) | 66 (31–77) |
| Sex, n (%) | |
| Male | 33 (83) |
| Female | 7 (17) |
| Smoking history, n (%) | |
| Current/former | 24 (60) |
| Never | 16 (40) |
| Pathology, n (%) | |
| Non-squamous | 28 (70) |
| Squamous | 12 (30) |
| PD-L1, n (%) | |
| >50% and <75% | 18 (45) |
| ≥75% | 22 (55) |
| Number of metastatic sites, n (%) | |
| 1 | 13 (33) |
| 2 | 16 (40) |
| ≥3 | 11 (27) |
| Baseline adrenal metastasis, n (%) | |
| Yes | 10 (25) |
| No | 30 (75) |
| Baseline brain metastasis, n (%) | |
| Yes | 12 (30) |
| No | 28 (70) |
| Baseline bone metastasis, n (%) | |
| Yes | 14 (35) |
| No | 26 (65) |
| Baseline liver metastasis, n (%) | |
| Yes | 8 (20) |
| No | 32 (80) |
| Baseline contralateral lung metastasis, n (%) | |
| Yes | 10 (25) |
| No | 30 (75) |
| Baseline pleural metastasis, n (%) | |
| Yes | 10 (25) |
| No | 30 (75) |
Clinical outcomes influenced by different metastatic sites
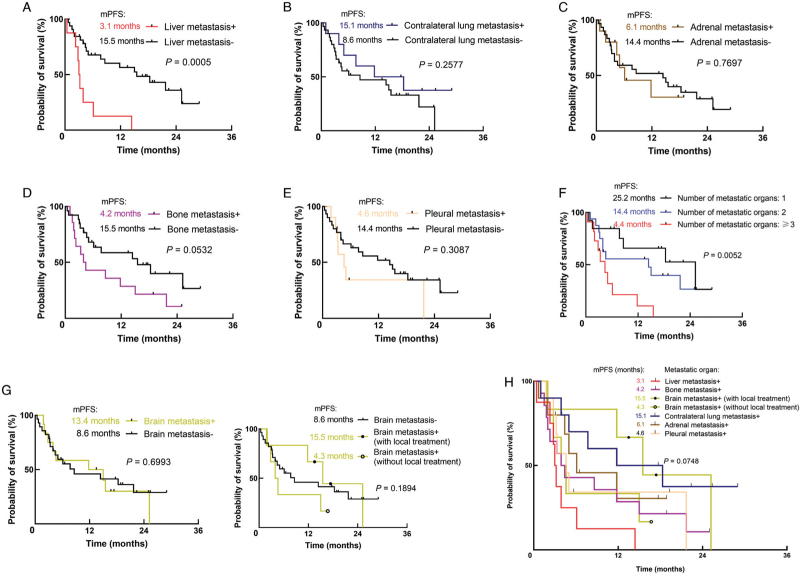
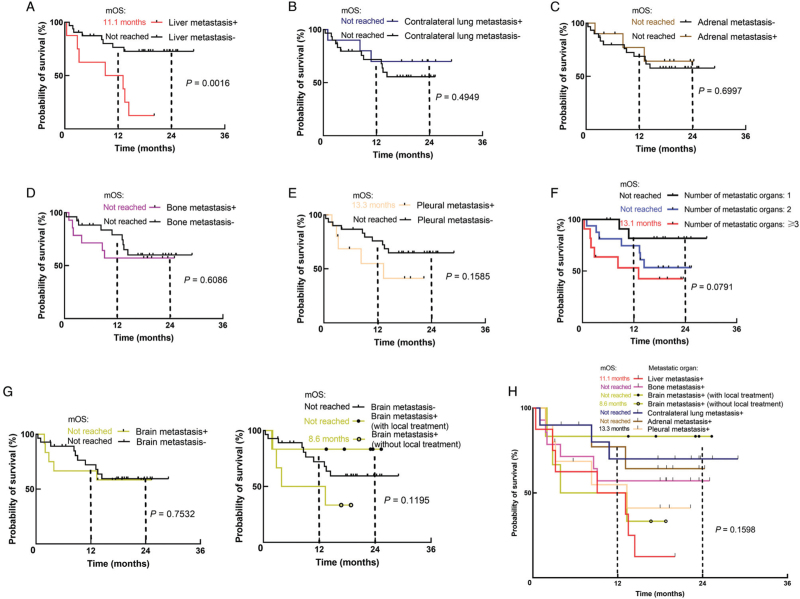
We first evaluated the association between included metastatic sites and clinical outcomes in NSCLC patients treated with first-line ICIs. As shown in Figures 2 and 3, the presence of liver metastasis instead of other metastatic organs was significantly associated with shorter PFS (3.1 vs. 15.5 months, P = 0.0005) and OS (11.1 months vs. not reached, P = 0.0016). Additionally, patients with liver metastasis had marginally significant shortest PFS compared with those with other metastatic patterns (P = 0.0748) and numerically shortest OS; though, the statistical significance was not reached (P = 0.1598). In addition, patients with bone metastasis tend to get shorter PFS (4.2 vs. 15.5 months, P = 0.0532) rather than OS (P = 0.6086). Moreover, for patients with brain metastasis, the application of local treatment such as radiotherapy and surgical resection before immunotherapy could numerically improve clinical efficacy. Compared to patients with brain metastasis who did not receive local treatment, the median PFS of those receiving local treatment was 15.5 vs. 4.3 months (P = 0.1894). The 2-year OS rate was 83% vs. 33% (P = 0.1195). With regard to patients without brain metastasis, the median PFS was 8.6 months (P = 0.3045 compared with patients with brain metastatis and not receiving local treatment), and the 2-year OS was 59% (P = 0.1422 compared with patients with brain metastatis and not receiving local treatment). No significant difference in PFS and OS was found in those with contralateral lung metastasis, pleural metastasis, or adrenal metastasis.
Figure 2.
PFS in NSCLC patients with (A) liver metastasis; (B) contralateral lung metastasis; (C) adrenal metastasis; (D) bone metastasis; (E) pleural metastasis; (F) different metastatic burden; (G) brain metastasis; (H) different metastatic sites treated with first-line ICIs monotherapy. ICIs: Immune checkpoint inhibitors; NSCLC: Non-small-cell lung cancer; PFS: Progression-free survival; mPFS: Median PFS.
Figure 3.
OS in NSCLC patients with (A) liver metastasis; (B) contralateral lung metastasis; (C) adrenal metastasis; (D) bone metastasis; (E) pleural metastasis; (F) different metastatic burden; (G) brain metastasis; (H) different metastatic sites treated with first-line ICIs monotherapy. ICIs: Immune checkpoint inhibitors; NSCLC: Non-small-cell lung cancer; OS: Overall survival; mOS: Median OS.
Association between metastatic burden, metastatic sites, and efficacy of ICIs
We further investigated the influence of metastatic burden on the efficacy of ICIs. As shown in Figures 2F and 3F, we divided patients into three subgroups according to the number of metastatic organs: 1, 2, and ≥3 organs involved. More metastatic organs involved were associated with inferior PFS (4.4 vs. 14.4 vs. 25.2 months, P = 0.0052) and lower 2-year OS rate (42% vs. 53% vs. 82%, P = 0.0791).
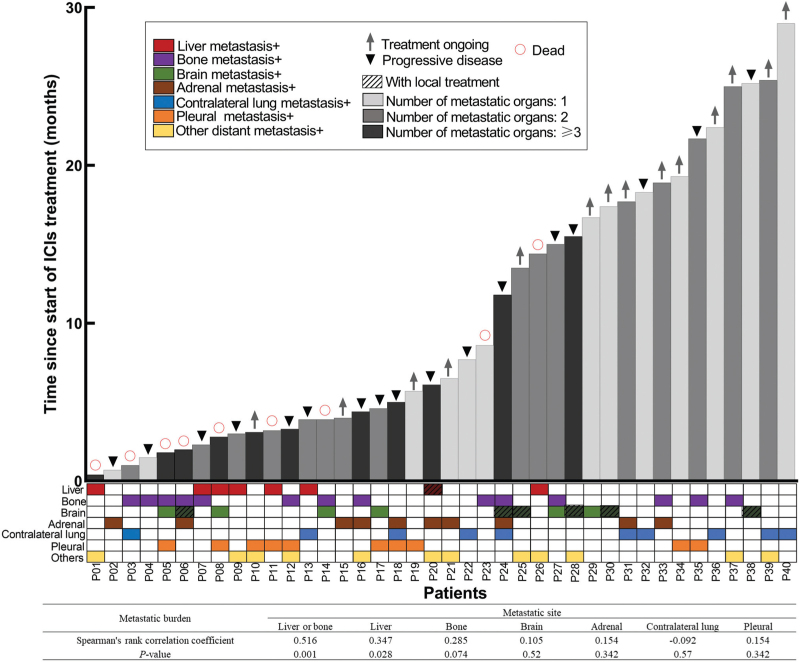
Next, we examined whether there was a correlation between the presence of specific organ metastases and the number of metastatic organs. As shown in Figure 4, patients with liver metastasis or bone metastasis tend to suffer from multiple metastases (Phi(ϕ): 0.516, P = 0.001), while no significant relationship was found between brain, adrenal, contralateral lung, and pleural metastasis with the metastatic burden.
Figure 4.
Relevance of metastatic burden, metastatic sites with PFS. PFS: progression-free survival.
Potential factors including metastatic sites associated with the efficacy of ICIs in metastatic NSCLC patients
Univariable and multivariable analyses were performed to explore how metastatic site, along with other characteristics (sex, age, smoking history, and pathological type) affected patients’ survival (PFS and OS). In order to improve the efficiency of analysis and the stability and reliability of the results, only possible metastatic sites (including liver, bone, and brain metastases) that may affect survival analyzed by the Kaplan-Meier method were included as variables in the Cox proportional hazard model. As shown in Tables 2 and 3, the presence of liver metastases was significantly associated with shorter PFS (univariable: HR 4.485, 95% CI: 1.807–11.131, P = 0.001; multivariable: HR 7.411, 95% CI: 2.668–20.585, P = 0.001) and OS (univariable: HR 4.473, 95% CI: 1.612–12.409, P = 0.004; multivariable: HR 4.473, 95% CI: 1.612–12.409, P = 0.004). In addition, patients with bone metastasis had less PFS benefit (univariable: HR 2.129, 95% CI: 0.969–4.678, P = 0.060; multivariable: HR 3.475, 95% CI: 1.438–8.395, P = 0.006) rather than OS benefit in our analysis. In terms of brain metastasis, patients with brain metastasis who received local treatment were excluded in order to eliminate the effect of local treatment on ICIs; however, although those with brain metastasis showed a higher hazard ratio for disease progression or death, the P-value did not show a significant difference (PFS: HR 1.765, 95% CI: 0.639–4.874, P = 0.273; OS: HR 2.310, 95% CI: 0.722–7.390, P = 0.158). In addition, no significant difference was observed concerning other clinical parameters, including age, sex, smoking history, or pathological type in our cohort.
Table 2.
Univariable and multivariable analyses of the influence of clinical parameters on PFS in NSCLC patients with different sites of metastases receiving first-line ICIs monotherapy.
| Univariable analysis | Multivariable analysis† | |||||
| Factors | HR | 95% CI | P | HR | 95% CI | P |
| Age (≥65 years/<65 years) | 0.477 | 0.220–1.036 | 0.062 | |||
| Sex (male/female) | 0.544 | 0.216–1.369 | 0.196 | |||
| Smoking (ever/never) | 0.815 | 0.368–1.807 | 0.614 | |||
| Pathology (squamous/non-squamous) | 0.732 | 0.320–1.676 | 0.460 | |||
| Liver metastasis (yes/no) | 4.485 | 1.807–11.131 | 0.001 | 7.411 | 2.668–20.585 | 0.001 |
| Bone metastasis (yes/no) | 2.129 | 0.969–4.678 | 0.060 | 3.475 | 1.438–8.395 | 0.006 |
| Brain metastasis (yes/no)∗ | 1.765 | 0.639–4.874 | 0.273 | |||
Patients with brain metastasis who previously received local treatment were excluded.
Variables with P < 0.20 in univariable analysis were taken forward for consideration in the multivariable analysis, and multivariable analysis was conducted using the forward stepwise regression method. CI: Confidence interval; HR: Hazard ratio; ICIs: Immune checkpoint inhibitors; NSCLC: Non-small-cell lung cancer; PFS: Progression-free survival.
Table 3.
Univariable and multivariable analyses of the influence of clinical parameters on OS in NSCLC patients with different sites of metastases receiving first-line ICIs monotherapy.
| Univariable analysis | Multivariable analysis† | |||||
| Factor | HR | 95% CI | P | HR | 95% CI | P |
| Age (≥60 years/<60 years) | 1.055 | 0.360–3.090 | 0.922 | |||
| Sex (male/female) | 0.579 | 0.184–1.820 | 0.350 | |||
| Smoking (ever/never) | 0.506 | 0.183–1.397 | 0.188 | |||
| Pathology (squamous/non-squamous) | 0.945 | 0.323–2.768 | 0.918 | |||
| Liver metastasis (yes/no) | 4.473 | 1.612–12.409 | 0.004 | 4.473 | 1.612–12.409 | 0.004 |
| Bone metastasis (yes/no) | 1.303 | 0.463–3.664 | 0.616 | |||
| Brain metastasis (yes/no)∗ | 2.310 | 0.722–7.390 | 0.158 | |||
Patients with brain metastasis who previously received local treatment were excluded.
Variables with P < 0.20 in univariable analysis were taken forward for consideration in the multivariable analysis, and multivariable analysis was conducted using the forward stepwise regression method. CI: Confidence interval; HR: Hazard ratio; ICIs: Immune checkpoint inhibitors; NSCLC: Non-small-cell lung cancer; OS: Overall survival.
Organ-specific disease response patterns
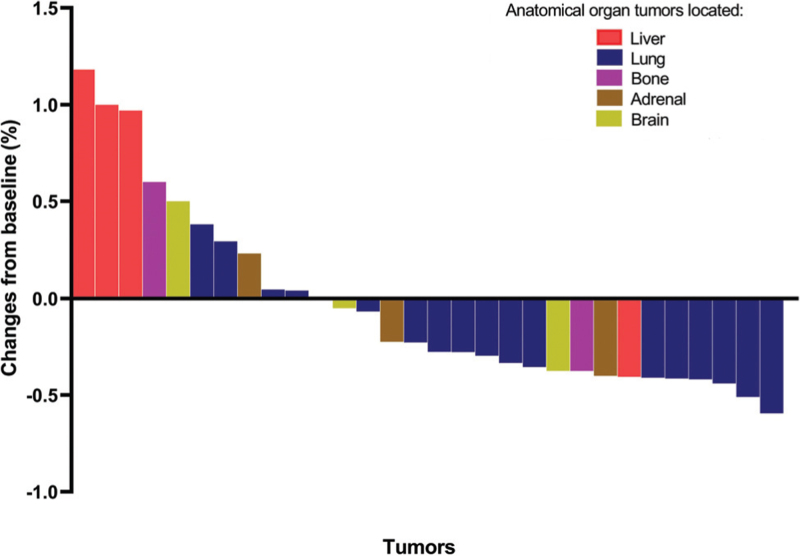
Given the different values of metastatic organs on systemic efficacy of ICIs, we investigated the association of local disease response patterns and the site metastases located. Eighteen patients with detailed data about change in tumor size were included in the analysis of organ-specific disease response patterns. Each patient had at least one metastasis and each metastatic organ had at least one measurable target lesion. According to RECIST1.1, the objective response rate of the enrolled population was 44% (8/18), and the disease control rate was 83% (15/18). The maximum change in tumor size from baseline ranged from −50.98% to 188.00% when all lesions were included as targets. On an individual organ level, the maximum change in tumor size from baseline varied according to the site of disease; as shown in Figure 5, the best tumor response was observed in lung (median of tumor changes from baseline −29.69%; range −59.46% to 38.10%), adrenal (median of tumor changes from baseline −11.22%; range −40.00% to 23.08%), and brain (median of tumor changes from baseline −5.00%; range −37.50% to 50.00%). Liver metastases had the lowest tumor response (median of tumor changes from baseline 98.53%; range −40.54% to 118.18%). Similarly, the likelihood of a disease control also differed according to the site of metastasis; the highest OSDCR was observed in lung (15/17), adrenal (3/4), and brain (2/3) sites, and the lowest was observed in the liver (1/4).
Figure 5.
Best tumor response of each site shows organ-specific disease response patterns (18 patients with 30 tumors).
Discussion
Previous studies on the relationship between metastatic organs and the efficacy of immunotherapy always included patients with various PD-L1 TPS statuses, disparate ECOG performance status, and different lines of therapy, all of which may potentially influence the clinical outcomes of ICIs as reported in the literature.[10–12] In this study, we collected and analyzed the data from a relatively homogeneous group of advanced NSCLC patients who received single-agent ICIs as first-line treatment with high PD-L1 expression and good performance status, to determine the association between different metastatic sites and efficacy to ICIs. We found that metastases in different anatomical locations, especially the liver, bone, and brain metastasis, may be associated with different clinical outcomes and local tumor response to ICIs, which is consistent with some previous studies.[13–17] The efficacy of ICIs is closely related to the tumor microenvironment. ICIs induce antitumor effects by reactivating exhausted T cells and thus restoring systemic antitumor immunity, in which the tumor microenvironment plays a vital role. Advanced NSCLC patients with different metastatic sites represented high heterogeneity of tumor microenvironment according to the gene profile alteration and infiltrated immune cells located at specific organs.[4–6] A case of a patient with high-grade serous ovarian cancer suggested that different tumor immune microenvironments may be seen in different metastatic sites, which may partly explain the different response to immunotherapy.[6] As the efficacy of ICIs is closely related to the tumor microenvironment, heterogeneous tumor microenvironments of various organs may potentially lead to a discrepant response to ICIs.
A recent study found that liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination, affecting both systemic and local efficacy of ICIs.[18] In our cohort, patients with liver metastasis had the shortest median PFS (3.1 months) and numerically shortest median OS (11.1 months) compared to those with other organ metastases, and the presence of liver metastasis was significantly associated with shorter PFS and OS, which is in line with some other previous studies.[2,19] In the NSCLC cohort from KEYNOTE-001, a phase I clinical trial, liver metastases were associated with inferior response and PFS to ICIs monotherapy in patients with advanced NSCLC. Its translational research indicated that liver metastases are associated with reduced marginal CD8+ T-cell infiltration, which might provide a potential mechanism for this outcome.[7] Metastases located at the liver also exhibit an inadequate local response to ICIs. Individual lesions from 37 patients with melanoma from KEYNOTE-001 were analyzed; lung lesions were found to have the highest rate of CR (42.3%), followed by peritoneal (37.3%) and liver (24.4%) metastatic lesions.[20] A similar phenomenon was observed in our cohort, further implying that liver metastases might be less responsive to ICI than pulmonary lesions in NSCLC. Thus, ICIs-based combination therapy might be an effective strategy to enhance the benefit of immunotherapy in NSCLC patients with liver metastasis. It might help alter the microenvironment in the liver from the tolerogenic to the immunologic status, causing systemic inflammation influence.[21,22] Data from the subgroup analysis of IMPOWER150 also showed a numerical improvement in terms of OS in the ABCP group (atezolizumab + bevacizumab + carboplatin + paclitaxel; 13.2 months), emphasizing the importance of the combination of anti-angiogenesis and chemotherapy in such a population receiving ICIs as first-line treatment.[23]
Bone is defined as a hematopoietic and immune regulatory organ. Bone marrow has active functions in regulating the immune system and trafficking of immune cells,[24] influencing the response to immunotherapy. A large population-based trial that included 1959 pretreated NSCLC patients evaluated the efficacy of nivolumab according to bone metastases, and found that patients with bone metastases had significantly lower overall response rates, and shorter PFS and OS.[8] Our data are partly consistent with these findings; in this study, patients with bone metastasis tend to have shorter PFS rather than OS compared to those without bone metastasis in first-line immunotherapy. Yet, a longer follow-up time is needed to further prove these findings.
In terms of brain metastasis, subgroup data from KEYNOTE-024 and OAK trials have reported the limited efficacy of ICIs monotherapy in patients with central nervous system metastasis compared with chemotherapy[9,25]; on the contrary, promising results of ICIs plus chemotherapy were seen in KEYNOTE-189 trial.[26] In addition, a recent review suggested that combining immunotherapy with radiotherapy is safe and effective in providing a significant improvement in relevant clinical and radiological outcomes in melanoma and NSCLC patients with brain metastasis.[27] Similarly, we found that applying local treatment such as radiotherapy and surgical resection before immunotherapy could numerically prolong PFS and increase the 2-year OS rate in patients with brain metastasis, emphasizing the importance of systemic therapy in combination with local therapy in brain metastasis.
Multiple metastatic sites are usually viewed as poor prognostic factor. Also, patients harboring multiorgan metastases are often accompanied by worse performance status and cancer-associated cachexia.[3] Immunotherapy is an alternative systemic treatment strategy, providing a more favorable safety even in patients with serious cancer-associated cachexia.[28] Our study suggested that more metastatic organs involved were correlated with worse PFS and OS. Besides, patients with liver metastasis or bone metastasis tend to suffer from multiple metastatic sites, which aggravate the patient's condition.
The study has several limitations. First, since it was difficult to identify such a relatively homogenous group of patients, the sample size of patients with high expression of PD-L1 and good performance status who received ICIs monotherapy as first-line treatment was small, and no conclusive conclusion can be drawn. Thus, larger sample size studies are needed to further confirm these findings. Second, given the nature of a single-center retrospective study, the results need to be interpreted with caution. Also, validation studies need to be performed in prospective and large cohorts. Finally, our study did not explore the mechanisms of differential responses to ICIs in various metastatic organs. Collecting matched tumor samples from multiple organs of the same individual, especially simultaneously, may be challenging. Potential cellular and molecular mechanisms underlying this differential response to ICIs between different metastatic sites warrant further discovery.
In conclusion, we found that metastases in different anatomical locations may be associated with different clinical outcomes and local tumor response of ICIs in NSCLC. The dismal results regarding the efficacy to ICIs monotherapy in patients with liver, bone, or brain metastasis were reported in our cohort, thus more aggressive ICIs-based combination strategies might be recommended for this population. Besides, there showed different local disease response patterns between various sites where metastases are located, among which liver metastasis might be less responsive to ICIs. However, the mechanisms underlying these different responses to ICIs between metastatic sites warrant further investigation.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82072562 to QZ), the High-Level Hospital Construction Project (No. DFJH201810 to QZ), and the GDPH Scientific Research Funds for Leading Medical Talents in Guangdong Province (No. KJ012019428 to QZ).
Conflicts of interest
Q. Zhou reports honoraria from AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Roche, and Sanofi, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
Footnotes
How to cite this article: Deng J, Gao M, Gou Q, Xu C, Yan H, Yang M, Li J, Yang X, Wei X, Zhou Q. Organ-specific efficacy in advanced non-small cell lung cancer patients treated with first-line single-agent immune checkpoint inhibitors. Chin Med J 2022;135:1404–1413. doi: 10.1097/CM9.0000000000002217
Supplemental digital content is available for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global CANCER STATISTICS 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014; 86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Zhang Y, Sun X, Gusdon AM, Song N, Chen L, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol 2018; 144:1835–1842. doi: 10.1007/s00432-018-2702-9. [DOI] [PubMed] [Google Scholar]
- 4.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020; 52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Baine MK, Turcu G, Zito CR, Adeniran AJ, Camp RL, Chen L, et al. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 2015; 6:24990–25002. doi: 10.18632/oncotarget.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 2017; 170:927–938. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landi L, D’Inca F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer 2019; 7:316.doi: 10.1186/s40425-019-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C, et al. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017; 112:200–215. doi: 10.1016/j.lungcan.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Facchinetti F, Di Maio M, Perrone F, Tiseo M. First-line immunotherapy in non-small cell lung cancer patients with poor performance status: a systematic review and meta-analysis. Transl Lung Cancer Res 2021; 10:2917–2936. doi: 10.21037/tlcr-21-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanovic M, Knez L, Herzog A, Kovacevic M, Cufer T. Immunotherapy for metastatic non-small cell lung cancer: real-world data from an academic central and eastern european center. Oncologist 2021; 26:e2143–e2150. doi: 10.1002/onco.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol 2017; 12:e140–e141. doi: 10.1016/j.jtho.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S, et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother 2018; 67:1825–1832. doi: 10.1007/s00262-018-2239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang DH, Chung C, Kim JO, Jung SS, Park HS, Park DI, et al. Pleural or pericardial metastasis: a significant factor affecting efficacy and adverse events in lung cancer patients treated with PD-1/PD-L1 inhibitors. Thorac Cancer 2018; 9:1500–1508. doi: 10.1111/1759-7714.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilen MA, Shabto JM, Martini DJ, Liu Y, Lewis C, Collins H, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 2019; 19:857.doi: 10.1186/s12885-019-6073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma SC, Tang XR, Long LL, Bai X, Zhou JG, Duan ZJ, et al. Integrative evaluation of primary and metastatic lesion spectrum to guide anti-PD-L1 therapy of non-small cell lung cancer: results from two randomized studies. Oncoimmunology 2021; 10:1909296.doi: 10.1080/2162402X.2021.1909296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021; 27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin BD, Jiao XD, Liu J, Liu K, He X, Wu Y, et al. The effect of liver metastasis on efficacy of immunotherapy plus chemotherapy in advanced lung cancer. Crit Rev Oncol Hematol 2020; 147:102893.doi: 10.1016/j.critrevonc.2020.102893. [DOI] [PubMed] [Google Scholar]
- 20.Khoja L, Kibiro M, Metser U, Gedye C, Hogg D, Butler MO, et al. Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer 2016; 115:1186–1192. doi: 10.1038/bjc.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat 2019; 46:100644.doi: 10.1016/j.drup.2019.100644. [DOI] [PubMed] [Google Scholar]
- 22.Chambers A, Kundranda M, Rao S, Mahmoud F, Niu J. Anti-angiogenesis revisited: combination with immunotherapy in solid tumors. Curr Oncol Rep 2021; 23:100.doi: 10.1007/s11912-021-01099-7. [DOI] [PubMed] [Google Scholar]
- 23.Socinski MA, Mok TS, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Abstract CT216: IMpower150 final analysis: efficacy of atezolizumab (atezo) + bevacizumab (bev) and chemotherapy in first-line (1L) metastatic nonsquamous (nsq) non-small cell lung cancer (NSCLC) across key subgroups. Cancer Res 2020; 80:CT216–CT1216. doi: 10.1158/1538-7445.Am2020-ct216. [Google Scholar]
- 24.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, et al. Bone marrow and the control of immunity. Cell Mol Immunol 2012; 9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: exploratory analyses of the phase III OAK study. Lung Cancer 2019; 128:105–112. doi: 10.1016/j.lungcan.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 27.Gagliardi F, De Domenico P, Snider S, Roncelli F, Pompeo E, Barzaghi LR, et al. Role of stereotactic radiosurgery for the treatment of brain metastasis in the era of immunotherapy: a systematic review on current evidences and predicting factors. Crit Rev Oncol Hematol 2021; 165:103431.doi: 10.1016/j.critrevonc.2021.103431. [DOI] [PubMed] [Google Scholar]
- 28.Spigel DR, McCleod M, Jotte RM, Einhorn L, Horn L, Waterhouse DM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J Thorac Oncol 2019; 14:1628–1639. doi: 10.1016/j.jtho.2019.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.