Abstract
Acyl-homoserine lactones (acyl-HSLs) serve as dedicated cell-to-cell signaling molecules in many species of the class Proteobacteria. We have addressed the question of whether these compounds can be degraded biologically. A motile, rod-shaped bacterium was isolated from soil based upon its ability to utilize N-(3-oxohexanoyl)-l-homoserine lactone as the sole source of energy and nitrogen. The bacterium was classified as a strain of Variovorax paradoxus. The V. paradoxus isolate was capable of growth on all of the acyl-HSLs tested. The molar growth yields correlated with the length of the acyl group. HSL, a product of acyl-HSL metabolism, was used as a nitrogen source, but not as an energy source. Cleavage and partial mineralization of the HSL ring were demonstrated by using radiolabeled substrate. This study indicates that some strains of V. paradoxus degrade and grow on acyl-HSL signals as the sole energy and nitrogen sources. This study provides clues about the metabolic pathway of acyl-HSL degradation by V. paradoxus.
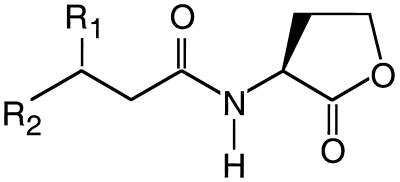
A diversity of bacterial species self-regulate expression of specific sets of genes in response to their own population density, a phenomenon that has become known as quorum sensing (for reviews, see references 9, 27, and 33). Many members of the class Proteobacteria have quorum-sensing systems that rely on acyl-homoserine lactone (acyl-HSL) signals. The nature of the acyl side chain of the signal molecule depends upon the quorum-sensing system. A diversity of acyl-HSL structures have been elucidated. Signal specificity depends on the length of and the substitutions in the acyl side chain (Fig. 1). Acyl-HSLs are dedicated signaling molecules with no other known function, and a specific enzyme is required for their synthesis (13, 20, 22, 30). These signal molecules reach concentrations on the order of 10 μM in laboratory cultures of quorum-sensing bacteria (5, 24, 26).
FIG. 1.
Generalized structure of acyl-HSLs produced by quorum-sensing bacteria: R1, -H, -OH, or ⩵O; R2, -CH3, -(CH2)2–10CH3 or -(CH2)3CH⩵CH(CH2)5CH3.
The available evidence is consistent with the idea that bacteria which synthesize acyl-HSLs do not degrade them, and acyl-HSLs are chemically stable at neutral or acidic pH in aqueous solutions (29). However, the HSL ring is subject to alkaline hydrolysis (32). The potential for biological decomposition of these signals is intriguing for several reasons. Other bacteria sharing the same local environment as quorum-sensing bacteria could conceivably gain a competitive advantage by degrading acyl-HSL signals. Enzymes that degrade acyl-HSLs might have commercial value as modulators of cell-to-cell signaling. Since acyl-HSLs are stable under slightly acidic conditions, biological degradation could play an important role in maintaining these signals at low environmental concentrations.
A recent report shows that acyl-HSL signaling molecules can be biologically inactivated by specific soil bacteria (4). A gene encoding this degradative ability was cloned from a Bacillus isolate. The purified gene product showed acyl-HSL-inactivating ability. It was not clear how the gene product served to inactivate acyl-HSLs or whether the Bacillus could use acyl-HSLs as nutrients for growth. To initiate our investigations into the biological degradation of acyl-HSL molecules, we have used enrichment and isolation techniques to obtain a pure culture of a bacterium capable of utilizing these signals as the sole source of energy and nitrogen. This is our initial description of that bacterium and its acyl-HSL-degrading capabilities.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains used were Variovorax paradoxus VAI-C (isolation described below), V. paradoxus ATCC 17713, and, for biological production of radioactive N-butanoyl-HSL (C4-HSL), Escherichia coli XL1-Blue containing pRHL1 (21). For growth of E. coli, we used Luria-Bertani (28) broth or agar amended as indicated. For enrichment, isolation, and growth experiments with V. paradoxus, we used a defined medium. The composition of the medium (per liter) was 1 g of NaCl, 0.5 g of KCl, 0.4 g of MgCl2 · 6H2O, 0.3 g of NH4Cl (unless otherwise specified), 0.1 g of CaCl2 · 2H2O, 0.2 g of KH2PO4, 0.15 g of Na2SO2, and 1 g of 2-(N-morpholino)-ethanesulfonic acid (MES). Trace elements and a selenate-tungstate mixture (18) were added, and the pH was adjusted to 5.5 with 1 M NaOH. This basal medium was autoclaved, and, after cooling, vitamins were added unless otherwise noted. The final vitamin composition per liter of medium was 100 μg of riboflavin and 1 mg of l-ascorbic acid, biotin, dl-calcium-panthothenate, folic acid, niacinamide, nicotinic acid, p-aminobenzoic acid, pyridoxal-HCl, thiamine-HCl, lipoic acid, and cyanocobalamin. For cultivation on solid media, agarose (Gibco Ultrapure) was incorporated at a final concentration of 1.0%. Growth substrates were added to the autoclaved, vitamin-amended medium as indicated.
For routine maintenance of V. paradoxus we used the medium described above supplemented with 5 g (wt/vol) of Difco yeast extract · liter−1 as an energy and nitrogen source. Stock solutions of acyl-HSLs were used at 5 mg · ml−1 (except for N-3-oxohexanoyl-l-HSL [3OC6-HSL], which was used at 50 mg · ml−1) in ethyl acetate acidified with glacial acetic acid (0.1% [vol/vol]). The stock solutions were stored at −20°C. For liquid media, the solutions of acyl-HSLs were dispensed into sterile tubes, the ethyl acetate was removed by evaporation under a stream of nitrogen gas, and sterile medium was added to the remaining acyl-HSL. The acyl-HSL-containing media were used immediately after preparation. Acyl-HSLs with carbon chain lengths of >8 did not fully dissolve in the medium at concentrations of >100 μM. Cells were grown in 3 or 5 ml of medium in 13- or 18-mm-diameter tubes, respectively, with shaking at 30°C unless otherwise noted. For agarose plates, the ethyl acetate solutions were spread on the surface of the agarose medium, and the plates were used shortly after the ethyl acetate evaporated. Acyl-HSL molecules are stable for weeks under the conditions of low pH in our defined medium. At higher pH values (ca. 8.0), the half-life of an acyl-HSL can be <3 h (29; A. Eberhard, personal communication).
Enrichment and isolation procedures.
Turf soil was collected in September 1998 at the University of Iowa. The soil was disrupted with a metal spatula until all particles were finely dispersed, and the remaining large particles were removed. One hundred milligrams of the soil preparation was added to 3 ml of the basal medium containing 3OC6-HSL as the sole source of nitrogen and energy (500 μg · ml−1). Vitamins were not added to the enrichment medium. After 48 h, a 5% (vol/vol) transfer was made to fresh enrichment medium, and after an additional 48 h, a second transfer was made. After a further 48-h incubation, cells in the third transfer tube were streaked on a plate of 3OC6-HSL-containing agarose medium.
Growth studies.
We constructed a 3OC6-HSL consumption curve by analyzing duplicate 10-μl samples of culture fluid collected during growth. Duplicate 1-ml samples were collected after the culture had entered the stationary phase. The analysis was by means of a 3OC6-HSL bioassay as described elsewhere (23).
Molar growth yields with acyl-HSLs as energy sources were determined in NH4Cl-replete medium containing the indicated acyl-HSL at a final concentration of 0.5, 0.75, 1.0, or 1.5 mM. Growth yields with nitrogen sources other than NH4Cl were determined in a medium containing 20 mM sodium succinate as the energy source. The nitrogen sources used in place of NH4Cl were HSL (at concentrations of 0 to 10 mM), homoserine (at concentrations of 0 to 10 mM), or 3OC6-HSL (at concentrations of 0 to 1 mM). A factor for converting optical density to cell dry mass was constructed by using cells grown in a medium containing succinate as the energy source and NH4Cl as the nitrogen source, washed with 50 mM ammonium acetate buffer (pH 5.5), and then dried to a constant weight. Experiments were done at least twice.
Metabolism of radiolabeled C4-HSL.
We prepared C4-l-[1-14C]HSL for radiotracer experiments by modification of a previously described procedure (12). E. coli XL1-Blue cells containing the C4-HSL synthase expression vector pRHLI were grown in 50 ml of Luria-Bertani broth containing ampicillin (100 μg · ml−1). Isopropyl-β-thiogalactoside (1 mM) was added after 2 h at 37°C. Cells were harvested by centrifugation when the culture reached an optical density of 0.7 at 600 nm. The cells were suspended in 2 ml of phosphate-buffered saline (28) containing 10 mM glucose in a 15-ml conical tube. After 10 min at 37°C with shaking, we added 10 μCi of l-[1-14C]methionine (55 mCi · mmol−1; American Radiolabeled Chemicals, Inc., St. Louis, Mo.) and incubated the cell suspension for an additional 4 h. The cells were then removed by centrifugation, and the C4-HSL was extracted from the remaining culture fluid with 2 equal volumes of acidified ethyl acetate. The ethyl acetate evaporated, and the residue was dissolved in 200 μl of 20% methanol in water. The C4-HSL in the methanol-water was purified by reversed-phase high-performance liquid chromatography (29). The purified, radioactive C4-HSL was dried and stored at −20°C. Radioactivity was measured with a liquid scintillation counter and was quench corrected by using an internal standard.
The fate of radioactive C4-HSL during growth of V. paradoxus VAI-C was assessed in the following manner: V. paradoxus was cultured in 5 ml of medium in 25-ml butyl rubber-stoppered tubes containing sufficient concentrations of oxygen for aerobic growth. Radioactive C4-HSL (10 μmol, 32 μCi · mmol−1) was added to each reaction vessel (as described above). Immediately after inoculation, the amount of radioactivity in the culture broth was measured. After cultures had reached the stationary phase, the headspace above each culture was flushed with N2 for 60 min into two CO2 traps connected in tandem (1). The radioactivity in the second vial ranged from 0.1 to 1.0% of that trapped in the first. Culture fluid was then removed, and after centrifugation, the clarified culture fluid was extracted with 8 equal volumes of acidified ethyl acetate. Radioactivity in both the extract and the culture fluid after extraction was measured. The cell pellet was washed three times with 10 mM MES (pH 5.5). The radioactivity present in the washed cells was determined after boiling for 2 min in 100 μl of 1 M NaOH.
Nucleotide sequence analysis of the 16S rDNA.
The nucleotide sequence of a PCR-amplified fragment of the 16S ribosomal DNA (rDNA) of our bacterial isolate was determined by previously described procedures (18). Genomic DNA was isolated with a QIAamp tissue kit (QIAGEN, Inc., Valencia, Calif.). We used the Expand Long Template PCR system (Boehringer-Mannheim) to amplify 16S rDNA with about 50 ng of bacterial DNA as the template with previously described 27-forward and 1494-reverse primers (17). The PCR product was purified and ligated into pCRII by using a TOPO TA cloning kit (Invitrogen, San Diego, Calif.). DNA sequencing was by routine automated methods at the University of Iowa DNA Core Facility. The sequencing primers were the standard M13 forward and M13 reverse primers and primers previously designed to target internal regions of the 16S rRNA genes of most bacteria (17). For sequence analysis, we used ARB software (www.mikro.biologie.tu-muenchen.de/pub/ARB/linux/).
Other analyses.
Phase-contrast microscopy and epifluorescence microscopy were performed with an Olympus BH2 microscope. Acridine orange staining and confocal-epifluorescence microscopy were performed as described elsewhere (2, 6). To demonstrate that HSL was released as a product of acyl-HSL degradation during culture growth, growth supernatants were analyzed at the University of Iowa College of Medicine Molecular Analysis Facility with a Beckman 6300 high-performance ion-exchange analyzer operated according to the manufacturer's specifications. The bioassays used to screen for production of 3OC6-HSL, C4-HSL, and related molecules by V. paradoxus have been described previously (23, 25).
Nucleotide sequence accession number.
The 16S rDNA sequence has been assigned GenBank accession no. AF250030. All other rDNA sequences were from the ARB database or from GenBank.
RESULTS
Enrichment and isolation of acyl-HSL-degrading bacteria.
Enrichment was in vitamin-free basal medium containing 3OC6-HSL. Enrichment tubes were inoculated with soil, and growth was evident as turbidity within 48 h. No obvious turbidity was observed in the absence of 3OC6-HSL. After two transfers in the 3OC6-HSL-containing medium, a complex microbial community including a variety of bacterial and eukaryotic microbes was observed by phase-contrast microscopy. There was an obvious biofilm near the air-liquid interface. The biofilm was disrupted by vortexing the culture for 30 s, and then a sample of the culture was streaked on a plate of vitamin-free agarose medium containing 3OC6-HSL. Three colony types arose over a period of a week. Pure cultures of each colony type were obtained by repeated streaking. The three isolates obtained were designated VAI-A (a dimorphic rod-coccus), VAI-B (a highly motile spirillum), and VAI-C (a weakly motile rod). These isolates were screened for growth on 3OC6-HSL in the liquid medium used for the enrichments. The pure VAI-A and VAI-C cultures grew on 3OC6-HSL, and VAI-C was chosen for further study.
Phylogenetic analysis of VAI-C.
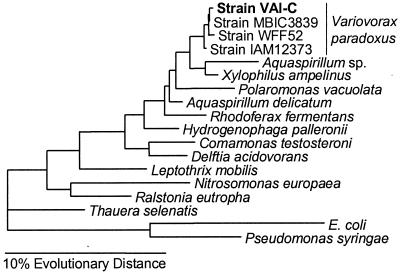
A nearly complete sequence for the 16S rDNA was obtained. The sequence corresponds to E. coli 16S rRNA nucleotide positions 28 to 1489. Web-based similarity searches against the GenBank and Ribosomal Database Project databases suggested that VAI-C belonged to the subclass β-Proteobacteria, clustering with the family Comamonadaceae. The 16S rDNA of VAI-C shared 99.3 to 99.8% sequence identity with the 16S rDNA of three strains of V. (formerly Alcaligenes) paradoxus. A further phylogenetic analysis supported the conclusion that VAI-C is a strain of V. paradoxus. By any of the FastDNAML maximum-likelihood, maximum-parsimony, and Desoete distance treeing algorithms, VAI-C clustered tightly with the other V. paradoxus strains (Fig. 2). Thus, we consider our isolate to be a strain of V. paradoxus. An additional confirmation of this assignment was obtained by demonstrating that the type strain of V. paradoxus (ATCC 17713) was capable of growth in 3OC6-HSL broth. Growth of this strain was not as rapid as growth of VAI-C in the test medium, irrespective of the substrate tested (data not shown).
FIG. 2.
16S rRNA-based evolutionary tree showing the phylogenetic position of strain VAI-C. The horizontal bar at the bottom represents a 10% difference in evolutionary distance as determined by measuring the lengths of the horizontal lines connecting the species. Pseudomonas syringae and E. coli were used as outgroups.
General properties of V. paradoxus VAI-C.
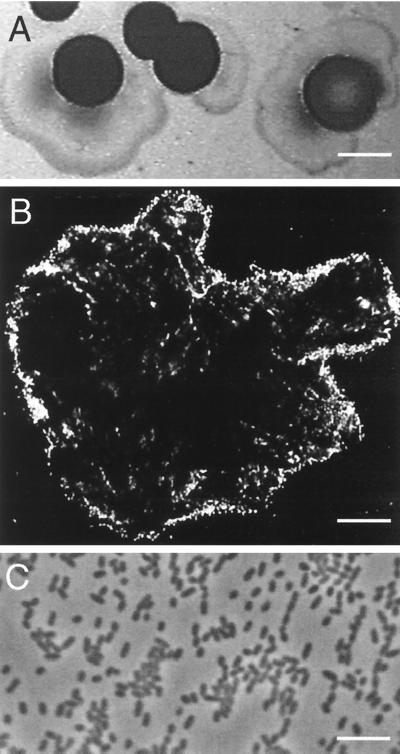
Growth of V. paradoxus VAI-C on 3OC6-HSL occurred at 30°C, but not at 37°C. The isolate formed spreading, yellow colonies on plates of 3OC6-HSL defined agarose medium and raised, yellow colonies with an occasional spreading edge on yeast extract agarose plates (Fig. 3A). Cells from late-logarithmic-phase 3OC6-HSL broth cultures were 1.1 by 0.7 μm in dimension (Fig. 3C). Despite the spreading of colonies on agarose plates, cells in broth were sporadically motile. A putrid aroma was produced during growth, especially on yeast extract agarose plates. As is the case for other strains of V. paradoxus, our isolate was capable of growth with pantothenic acid or folic acid as the sole source of energy and nitrogen (10, 11, 19).
FIG. 3.
Colonial and cell morphology of V. paradoxus VAI-C. (A) Photograph of colonies grown on yeast extract-agarose plates. (B) Confocal fluorescence micrograph of acridine orange-stained cells attached to the surface of a particle of C12-HSL. (C) Phase-contrast micrograph of 3OC6-HSL-grown cells. Bars are 4 mm (A), 17 μm (B), and 5 μm (C).
To examine whether V. paradoxus VAI-C produced an acyl-HSL, we extracted culture fluid with ethyl acetate and used bioassays for C4-HSL, 3OC6-HSL, and related acyl-HSLs to screen the concentrated extracts (see Materials and Methods). We did not detect acyl-HSLs in extracts obtained from low-pH defined medium amended with 0.5% yeast extract or from medium with succinate as the carbon and energy source.
Acyl-HSLs as energy sources for growth.
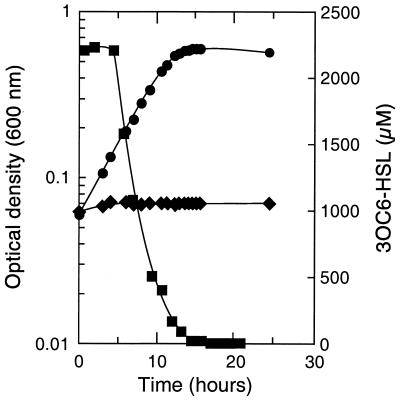
Pure cultures of V. paradoxus VAI-C grew slowly (24- to 48-h doubling times) on 3OC6-HSL in the absence of vitamins. In vitamin-supplemented medium, the doubling time improved to 18 h, with a molar growth yield of 94 g (dry weight) of cell · mol of 3OC6-HSL−1. With NH4Cl added to the medium, the doubling time improved to 3.5 h (Fig. 4); the molar growth yield with NH4Cl was about the same as without it (as was the final pH in the culture medium [5.7 to 6.4]). The concentration of 3OC6-HSL decreased during logarithmic growth, and it was below 100 nM just after the onset of the stationary phase (Fig. 4).
FIG. 4.
Growth of V. paradoxus VAI-C with 3OC6-HSL as the sole energy source. Culture density in medium with (●) and without (♦) 3OC6-HSL is shown, as is the concentration of 3OC6-HSL in the 3OC6-HSL-containing culture (■).
Growth of V. paradoxus VAI-C occurred with a diversity of acyl-HSLs (Table 1). In fact, there was growth with the complete series of saturated acyl-HSLs we tested. Acyl-HSLs with acyl chains ≥8 carbons did not completely dissolve in the medium at the concentrations we used (>100 μM), so accurate growth rates on these substrates were not obtained. However, upon incubation, the insoluble acyl-HSL particles disappeared, and final culture densities could be measured. Epifluorescence microscopy of acridine orange-stained cells during early growth on C12-HSL showed acyl-HSL particles covered with V. paradoxus cells (Fig. 3B). Few cells were found unattached in the growth medium until the visible C12-HSL particles had disappeared.
TABLE 1.
Growth of V. paradoxus VAI-C on acyl-HSLs and other compoundsa
| Growth substrate | Yield (g · mol−1) | Doubling time (h) |
|---|---|---|
| 3OC6-HSL | 95 | 3.5 |
| C4-HSL | 70 | 25 |
| C6-HSL | 98 | 32 |
| C8-HSL | 114 | NDb |
| C10-HSL | 170 | ND |
| C12-HSL | 197 | ND |
| 3-Hydroxybutyrate | 53 | 3.5 |
| Succinate | 39 | 3.5 |
| Isoleucine | 68 | 4.5 |
| Homoserine | 57 | 6 |
| Threonine | 35 | 7 |
| dl-Pantothenate | 52 | 4 |
| Glucose | 71 | 14 |
The data presented are the average of three or more separate determinations. Other substrates utilized were C14-HSL, 4-hydroxybutyrate, polyoxyethylene-20-sorbitan monooleate (i.e., Tween 80), poly[(R)-3-hydroxybutyrate], glycerol, formate, methionine, propanol, folate, proline, and α-ketobutyrate. Substrates not utilized were l-HSL, γ-butyrolactone, dl-pantolactone, dl-homocysteine thiolactone, N-acetyl-l-homocysteine thiolactone, N-acetyl-l-aspartate, hexanoate, and H2 + CO2 + O2.
ND, not determined (see Materials and Methods).
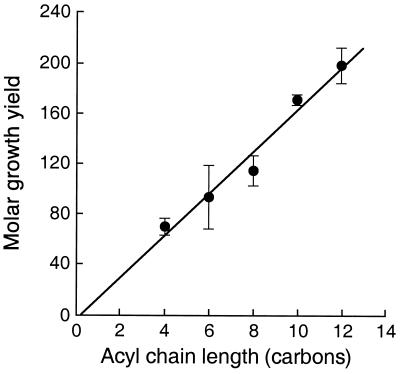
The molar growth yields on different acyl-HSLs showed a direct correlation with the lengths of their acyl side chains (Fig. 5). This is consistent with the conclusion that the acyl group but not the HSL is used as an energy source. Growth was quite slow on C14-HSL, the longest and least soluble of the acyl-HSLs tested.
FIG. 5.
Molar growth yields of V. paradoxus VAI-C on C4-HSL, C6-HSL, C8-HSL, C10-HSL, and C12-HSL. The data points represent the means of four or more separate determinations, and the bars indicate the standard errors.
For the purposes of comparison, we have determined the molar yields and doubling times exhibited by V. paradoxus VAI-C growing on a number of other substrates (Table 1). Of note: growth occurred with homoserine as the sole energy source, but not with l-HSL, γ-butyrolactone, dl-homocysteine thiolactone, or N-acetyl-l-homocysteine thiolactone. Growth on acyl-HSLs was comparable to that with many other energy sources in terms of rate and yield on a per carbon basis.
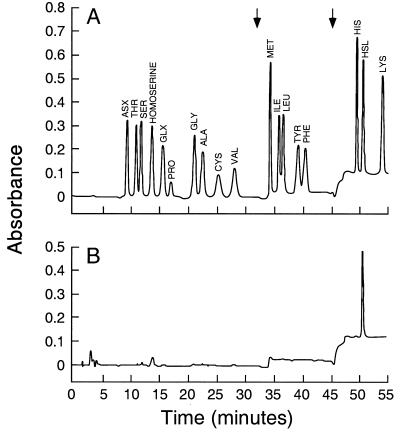
The molar growth yield studies (Fig. 5) indicate that the HSL ring moiety of an acyl-HSL does not serve as an energy source. Nevertheless, the ring may be degraded. To gain insights into the fate of the HSL moiety, we grew cultures with C4-HSL radiolabeled in ring carbon 1. With C4-HSL as the sole source of energy and NH4Cl as a source of nitrogen, nearly half of the radiolabel was recovered as 14CO2. The production of 14CO2 demonstrates the cleavage of a significant fraction of the homoserine lactone ring. Of the radioactive label that was not recovered as 14CO2, most was accounted for in the culture fluid, even after ethyl acetate extraction. We suspected that this might be HSL. To test this hypothesis, we grew a culture on unlabeled C4-HSL. HSL was the only compound detected by quantitative amino acid analysis (Fig. 6). When taken together with the radiolabling experiments, this indicates that about 25% of the HSL in the C4-HSL was recovered as HSL in the culture fluid. These experiments indicate that HSL is an intermediate in the degradation of C4-HSL. Because HSL accumulates in the culture medium, we suggest that acyl-peptide bond cleavage occurs outside of cells or that there is an export system for intracellular HSL.
FIG. 6.
Amino acid analysis of V. paradoxus VAI-C culture fluid. (A) Profile of a mixture of 18 common amino acids, plus homoserine and HSL. (B) Profile of culture fluid analyzed after growth of V. paradoxus VAI-C on C4-HSL as the sole source of energy and NH4Cl as an added nitrogen source. The arrows indicate step changes in the solvent.
Acyl-HSLs as nitrogen sources for growth.
V. paradoxus VAI-C was capable of growth with acyl-HSLs as the sole source of organic carbon, energy, and combined nitrogen. The acyl group serves as an energy source, and HSL, which is a product of acyl-HSL metabolism, can serve as a sole source of combined nitrogen for growth. Furthermore, the HSL ring is metabolized even though it does not appear to be utilized as an energy source (Table 2). Thus, we wanted to study growth of the isolate with succinate as an energy source and acyl-HSLs as the only source of nitrogen. Both C4-HSL and 3OC6-HSL served as a nitrogen source. Homoserine and HSL also served as nitrogen sources, but only the acyl-HSLs and homoserine would serve as the sole energy and nitrogen substrate for growth. The doubling times with homoserine, HSL, or an acyl-HSL as the nitrogen source were slow compared to doubling times with NH4Cl (about 30 h compared to 4 h). However, final cell yields with excess succinate were comparable to the cell yield with NH4Cl (data not shown). When 14C-labeled C4-HSL was used as a nitrogen source, nearly all of the radioactivity was recovered as CO2 (Table 2). We do not know whether CO2 release is an early or late step in the ring cleavage process, but we conclude that ring cleavage and CO2 release occur prior to utilization of the nitrogen. Whether any HSL carbon, or only its amino group, is incorporated into cellular proteins remains unanswered.
TABLE 2.
Metabolism of C4-l-[1-14C]HSL by V. paradoxus VAI-C
| Nitrogen source | Radioactivity (103 dpm)a
|
% Recovery | |||
|---|---|---|---|---|---|
| Consumed | CO2 | Culture fluid | Cells | ||
| NH4+ + C4-HSL | 625 | 285 (46) | 201 (32) | 24 (4) | 82 |
| C4-HSL | 618 | 466 (76) | 8 (1) | 19 (3) | 80 |
Values in parentheses are the amount recovered as a percentage of the amount consumed.
DISCUSSION
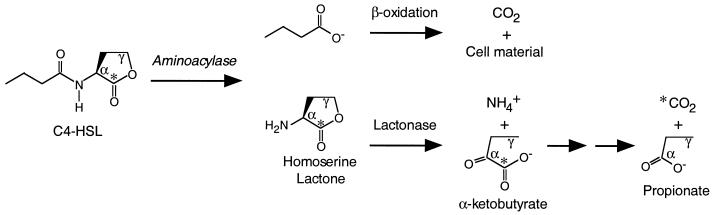
We have isolated a bacterium capable of degrading and growing on a number of different acyl-HSLs. This organism appears to be a strain of V. paradoxus, and it was able to utilize acyl-HSLs as both energy and nitrogen sources. Apparently only the acyl group is used in energy generation (Table 1 and Fig. 5). In fact, HSL is produced by cultures growing on C4-HSL (Fig. 6). Thus, we believe that the first step in the metabolism of acyl-HSLs involves a carboxypeptidase or aminoacylase that releases the fatty acid from HSL (Fig. 7). By using C4-l-[1-14C]HSL, we showed that V. paradoxus cleaves the HSL ring (Table 2). Because the other carbons in the C4-HSL were not labeled, we do not know their metabolic fate.
FIG. 7.
Hypothetical C4-HSL degradation pathway. The asterisks indicate the position of the 14C-labeled carbon.
Although further studies are required to elucidate the pathway of acyl-HSL metabolism, we can speculate that after hydrolysis of the acyl-amide bond, the next step in the degradative pathway might involve the action of a novel α, γ-eliminating deaminase-lyase yielding α-ketobutyrate and NH4+ (Fig. 7). Alternatively, a lactone hydrolase (14, 16) could convert HSL to homoserine. However, the latter hypothesis seems inconsistent with the observation that V. paradoxus VAI-C utilized homoserine, but not HSL (added exogenously or derived from acyl-HSLs), as an energy source.
The acyl-HSL-degrading activity of V. paradoxus VAI-C expands the list of diverse metabolic traits exhibited by members of this bacterial species. Many strains of the species can grow via H2 + CO2 + O2 chemolithoautotrophy (3). Other capabilities of strains of V. paradoxus include degradation of bioplastics (31), involvement in the dechlorination of xenobiotic compounds (7), and the ability to accumulate the rare metal yttrium (15). Of particular interest, strain VAI-C shares with other Variovorax isolates the ability to grow on acyl-amide bond-containing vitamins such as pantothenate (pantoyl-N-3-alanine) and folate (pteroyl-N-glutamate). The degradation of these vitamins requires two different amino acid-specific carboxypeptidases (10, 11). We believe it is likely that V. paradoxus contains an additional acyl-HSL-specific carboxypeptidase.
Acyl-HSLs have received considerable attention as quorum-sensing signals and key regulators of the community behavior of a number of genera of Proteobacteria (9, 27, 33). Many acyl-HSLs are quite stable in mildly acidic or neutral pH environments. However, there is no evidence that they accumulate in such environments. If they accumulated over long periods of time, their function as quorum-sensing signals would be disarmed. The signal concentration would not reflect cell number after fluctuations in population density. An intriguing question arises from the identification of bacteria with acyl-HSL-degrading capabilities. Can acyl-HSL-degrading bacteria influence the gene expression of quorum-sensing bacteria when both groups intermingle with or reside in close proximity to each other? This report, along with the report by Dong et al. (4) on a Bacillus isolate exhibiting acyl-HSL-inactivating activity, should open up investigations of the metabolic pathway for acyl-HSL degradation, the genetics of acyl-HSL degradation, and the ecological significance of acyl-HSL degradation. As pointed out by Dong et al.(4), the application of acyl-HSL degradation could have value in control of specific plant and animal diseases that are caused by bacteria that employ these quorum-sensing signals to control virulence or biofilm formation. Conversely, there has been interest in exploiting beneficial quorum-regulated activities such as the inhibition of pathogenic fungi around the roots of plants (8, 34). It may be useful to consider biological signal degradation as one factor decreasing the potential effectiveness of acyl-HSL-mediated biocontrol regimes.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (GM59026), the National Science Foundation (MCB 9808308), and the Cystic Fibrosis Foundation. J.R.L. was supported in part by a traineeship from the National Institutes of Health (T32-AI07343) and in part by a National Science Foundation Postdoctoral Fellowship in the Biosciences Related to the Environment (DBI-9804278).
We thank M. Parsek and A. Schaefer for many intellectual and technical discussions and E. Rus for performing quantitative amino acid chromatography.
REFERENCES
- 1.Brune A, Miambi E, Breznak J A. Roles of oxygen and the intestinal microflora in the metabolism of lignin-derived phenylpropanoids and other monoaromatic compounds by termites. Appl Environ Microbiol. 1995;61:2688–2695. doi: 10.1128/aem.61.7.2688-2695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 3.Davis D H, Doudoroff M, Stanier R, Mandel M. Proposal to reject the genus Hydrogenomonas: taxonomic implications. Int J Syst Bacteriol. 1969;19:375–390. [Google Scholar]
- 4.Dong Y H, Xu J L, Li X Z, Zhang L H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci USA. 2000;97:3526–3531. doi: 10.1073/pnas.060023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 6.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl Environ Microbiol. 1994;60:4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher P R, Appleton J, Pemberton J M. Isolation and characterization of the pesticide-degrading plasmid pJP1 from Alcaligenes paradoxus. J Bacteriol. 1978;135:798–804. doi: 10.1128/jb.135.3.798-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fray R G, Throup J P, Daykin M, Wallace A, Williams P, Stewart G S, Grierson D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat Biotechnol. 1999;17:1017–1020. doi: 10.1038/13717. [DOI] [PubMed] [Google Scholar]
- 9.Fuqua C, Greenberg E P. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 10.Goldman P, Levy C C. Carboxypeptidase G: purification and properties. Proc Natl Acad Sci USA. 1967;58:1299–1306. doi: 10.1073/pnas.58.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodhue C, Snell E. The bacterial degradation of pantothenic acid. I. Overall nature of the reaction. Biochemistry. 1966;5:393–398. doi: 10.1021/bi00866a001. [DOI] [PubMed] [Google Scholar]
- 12.Gray K M, Greenberg E P. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J Bacteriol. 1992;174:4384–4390. doi: 10.1128/jb.174.13.4384-4390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanzelka B L, Parsek M R, Val D L, Dunlap P V, Cronan J E, Jr, Greenberg E P. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J Bacteriol. 1999;181:5766–5770. doi: 10.1128/jb.181.18.5766-5770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakubowski H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J Biol Chem. 2000;275:3957–3962. doi: 10.1074/jbc.275.6.3957. [DOI] [PubMed] [Google Scholar]
- 15.Kamijo M, Suzuki T, Kawai K, Murase H. Accumulation of yttrium by Variovorax paradoxus. J Ferment Bioeng. 1998;86:564–568. [Google Scholar]
- 16.Kobayashi M, Shinohara M, Sakoh C, Kataoka M, Shimizu S. Lactone-ring-cleaving enzyme: genetic analysis, novel RNA editing, and evolutionary implications. Proc Natl Acad Sci USA. 1998;95:12787–12792. doi: 10.1073/pnas.95.22.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, editor. Nucleic acid techniques in bacterial systematics. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 115–175. [Google Scholar]
- 18.Leadbetter J R, Breznak J A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy C, Goldman P. The enzymatic hydrolysis of methotrexate and folic acid. J Biol Chem. 1967;242:2933–2938. [PubMed] [Google Scholar]
- 20.Moré M I, Finger D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through the use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 21.Parsek M R, Schaefer A L, Greenberg E P. Analysis of random and site-directed mutations in rhlI, a Pseudomonas aeruginosa gene encoding an acylhomoserine lactone synthase. Mol Microbiol. 1997;26:301–310. doi: 10.1046/j.1365-2958.1997.5741935.x. [DOI] [PubMed] [Google Scholar]
- 22.Parsek M R, Val D L, Hanzelka B L, Cronan J E, Greenberg E P. Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puskas A, Greenberg E P, Kaplan S, Schaefer A L. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J Bacteriol. 1997;179:7530–7537. doi: 10.1128/jb.179.23.7530-7537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmond G P, Bycroft B W, Stewart G S, Williams P. The bacterial “enigma”: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schaefer A L, Hanzelka B L, Parsek M R, Greenberg E P. Detection, purification and structural elucidation of acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 2000;305:288–301. doi: 10.1016/s0076-6879(00)05495-1. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suyama T, Hosoya H, Tokiwa Y. Bacterial isolates degrading aliphatic polycarbonates. FEMS Microbiol Lett. 1998;161:255–261. doi: 10.1111/j.1574-6968.1998.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 32.Voelkert E, Grant D R. Determination of homoserine as the lactone. Anal Biochem. 1970;34:131–137. doi: 10.1016/0003-2697(70)90093-x. [DOI] [PubMed] [Google Scholar]
- 33.Winans S C. Command, control and communication in bacterial pathogenesis. Trends Microbiol. 1998;6:382–383. doi: 10.1016/s0966-842x(98)01338-9. [DOI] [PubMed] [Google Scholar]
- 34.Wood D W, Gong F, Daykin M M, Williams P, Pierson L S., III N-Acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J Bacteriol. 1997;179:7663–7670. doi: 10.1128/jb.179.24.7663-7670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]