Abstract
In recent years, there have been significant innovations in the development of nanoparticle-based vaccines and vaccine delivery systems. For the purposes of both design and evaluation, these nanovaccines are imaged using the wealth of understanding established around medical imaging of nanomaterials. An important insight to the advancement of the field of nanovaccines can be given by an analysis of the design rationale of an imaging platform, as well as the significance of the information provided by imaging. Nanovaccine imaging strategies can be categorized by the imaging modality leveraged, but it is also worth understanding the superiority or convenience of a given modality over others in a given context of a particular nanovaccine. The most important imaging modalities in this endeavor are optical imaging including near-infrared fluorescence imaging (NIRF), emission tomography methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) with or without computed tomography (CT) or magnetic resonance (MR), the emerging magnetic particle imaging (MPI), and finally, multimodal applications of imaging which include molecular imaging with magnetic resonance imaging (MRI) and photoacoustic (PA) imaging. One finds that each of these modalities has strengths and weaknesses, but optical and PET imaging tend, in this context, to be currently the most accessible, convenient, and informative modalities. Nevertheless, an important principle is that there is not a one-size-fits-all solution, and that the specific nanovaccine in question must be compatible with a particular imaging modality.
Keywords: fluorescence imaging, medical imaging, nanomaterials, positron emission tomography, vaccines
1 |. INTRODUCTION
In recent years, the development of vaccines has become an increasingly productive field of research (Lindsay et al., 2019; Pollard & Bijker, 2021; Ruiz-de-Angulo et al., 2016; Zhang et al., 2020). Vaccines have been developed for a variety of purposes, ranging from prevention of infectious diseases such as polio and COVID-19 to the treatment of non-infectious diseases such as cancer, becoming a major player in the area of cancer immunology (Beatty & Gladney, 2015; Binnewies et al., 2018; Pollard & Bijker, 2021). The basis of immunization for every disease is different, but they all follow the same principle - that the vaccine encourages and equips the immune system to respond to a particular disruption in the status quo, whether it’s the presence of a virus or the development of a cancer cell.
1.1 |. Nanovaccines
Two important aspects of vaccine design are the delivery of antigens and vaccine adjuvants, as well as the evaluation of their biodistribution and longevity (Beatty & Gladney, 2015; Binnewies et al., 2018; Pollard & Bijker, 2021). Additionally, evaluation of vaccines is important not only for drug development but also for drug approval, which can be a great obstacle to the translation of research into the clinic. For these reasons, nanovaccines have emerged as an innovative strategy for the delivery of vaccines (Cruz et al., 2011; Gheibi Hayat & Darroudi, 2019; Tian et al., 2020). Nanovaccines utilize nanoparticles as carriers of antigens and vaccine adjuvants and consequently provide a plethora of benefits for the stable and targeted delivery of immunogens and adjuvants.
A contemporary example of the application of nanotechnology to vaccination of an infectious disease is in mRNA-based COVID-19 vaccines. Lipid nanoparticle-based delivery systems were favored by researchers from the development stage to the translational stage (Lasting Impact of Lipid Nanoparticles, 2021; Zhang et al., 2020). As a matter of fact, many of us have received our COVID-19 vaccines in this form. Lipid nanoparticles are a cutting-edge tool for mRNA delivery, which is a rising strategy for vaccination against viruses, due in part to the convenience of their synthesis, their flexibility, and biodegradability.
1.2 |. Cancer vaccines
A perhaps lesser-known application of vaccines is in the treatment of cancer. Yet, from an understanding of cancer immunology, cancer vaccines become a natural application of vaccine research. Due to the need for intricate and smart delivery design in, nanovaccines have been one of the interesting platforms for cancer vaccination (Persano et al., 2021; Petersen et al., 2010; Saxena et al., 2021; Vinay et al., 2015). The rationale for cancer vaccines is derived from the basic principles of the immune system and vaccination. If properly trained, the immune system is capable of eliciting a precise therapeutic effect on a pathogen or native tissue. When it comes to cancer, tumor-associated antigens are typically recognized by cell-killing T cells such as cytotoxic T lymphocytes (CTLs), however, cancer cells are capable of evading immune response (Vinay et al., 2015). In particular, cancer cells can target regulatory T cell function, down-regulate antigen productions and presentation, as well as produce immune suppressive mediators. Tumors which are particularly unlikely to trigger a strong immune response are referred to as “cold” tumors, and they can often arise as a consequence of acquired resistance to conventional immunotherapies (Persano et al., 2021). Cancer immunotherapy aims to overcome these hurdles and create a powerful immune response to cancer. Immunotherapeutic approaches to cancer include chimeric antigen receptor T cell (CAR-T) therapy, immune checkpoint blockade (ICB), and cancer vaccines, which is the focus of this review. Cancer vaccines encourage the immune system to eliminate cancer through exposure to tumor-specific antigens (Persano et al., 2021; Saxena et al., 2021). It is generally successful for tumors with low immune infiltrates, for which it can induce T cell priming and expands tumor specific T cell responses. A common method for cancer vaccination is out-lined in Figure 1. Briefly, it consists of identification of the tumor antigen or antigenic epitope, and then administration to a patient as a major histocompatibility complex class I (MHC I)-restricted peptides or nucleic acids. For the response to be effective, the antigens must be delivered to so-called antigen-presenting cells, in particular to dendritic cells (DCs) at the site of injection, which is usually the dermis, or at lymphoid organs (Cruz et al., 2011). This is because DCs are important messengers between CD4+ T cells and CD8+ cells. Specifically, they are important in the generation of T cell immune response, since they mediate the processing of antigens and their presentation to naive CD8+ and CD4+ T cells in the draining lymph nodes. T cell activation requires, among other things, secretion of pro-inflammatory cytokines by DCs, such as interleukin-12 (IL-12). Major goals of cancer vaccination include the induction of tumor regression, eradicate minimal residual disease, establish lasting antitumor memory, and avoid non-specific or adverse reactions (Saxena et al., 2021). Important principles of an effective cancer vaccine are delivery of large amounts of antigen to DCs or lymphoid organs, induction of strong and sustained CD4+ T cell response and CTL response, infiltration of the tumor microenvironment, and a durable, maintained response. Four important components of a cancer vaccine are the selection of a tumor antigen, the choice of an antigenic platform, the use of immune adjuvants to enhance immune response, and the antigen-delivery system which gives the antigens to the DCs or lymphoid organs (Persano et al., 2021). The second and last components are typically where nanoparticles come into play.
FIGURE 1.
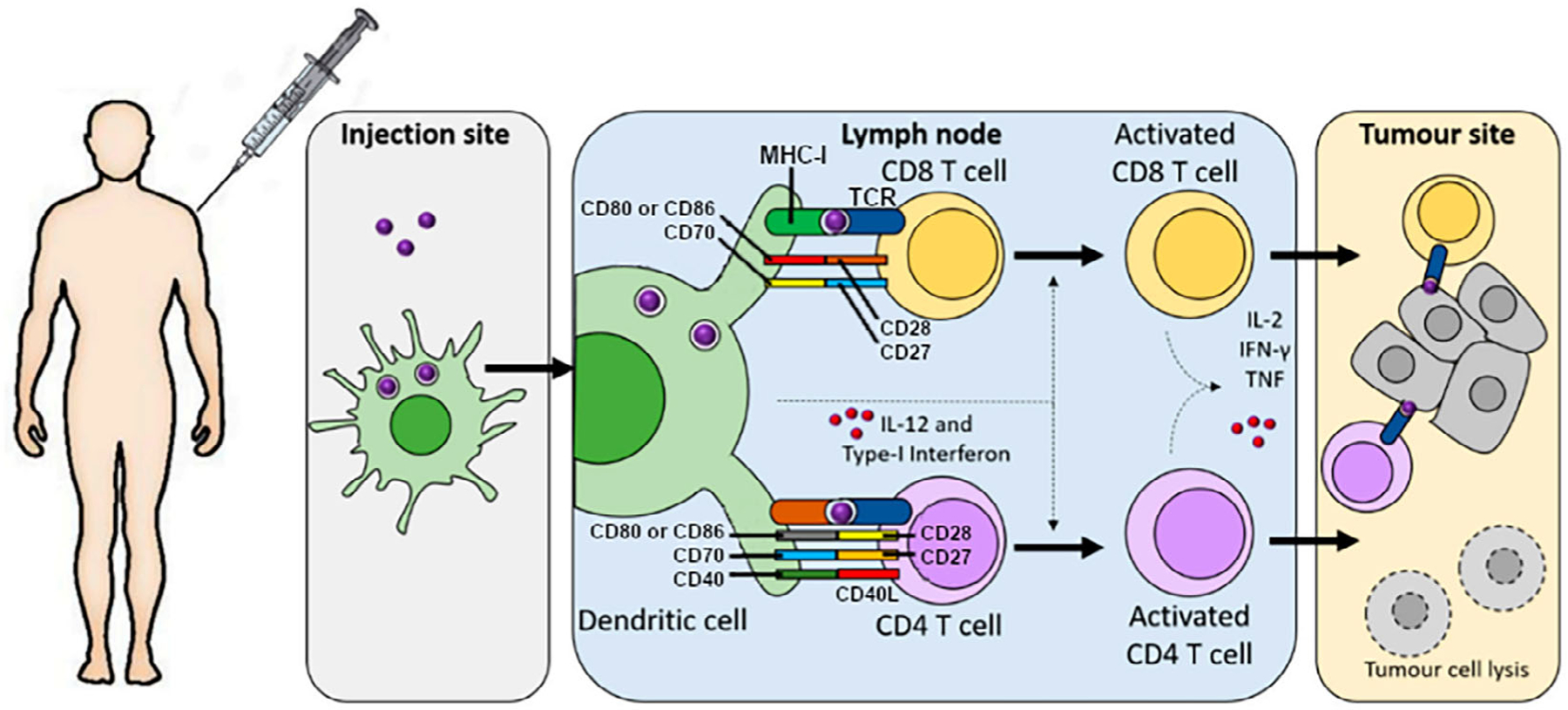
The process of tumor antigen presentation which is caused by cancer vaccination. Injection of an antigen causes uptake by antigen presenting cells (APCs). APCs then travel to draining lymph nodes, wherein mature dendritic cells (DCs) present antigen-derived peptides on MHC class I molecules to CD8+ and those on MHC class II molecules to CD4+ T cells. Several factors are involved in the activation of these T cells (CD28, CD27, CD40, IL-12, and Type-I interferon). With activated, antigen-specific T cells now formed, the cancer cells can now be recognized and killed by the production of effector cytokines such as IFN-γ and tumor necrosis factor (TNF). Adapted with permission from Persano et al. (2021).
1.3 |. The imaging capabilities introduced by nanomaterial-based vaccines
Nanovaccines not only provide benefits for delivery of the immunogen and adjuvants, but they also allow us to apply years of research into nanoparticle imaging to the imaging of vaccines, which will be the foremost focus of this review. The various methods of imaging which can now be applied to vaccines include optical imaging of fluorescent and nearinfrared fluorescent (NIRF) agents, emission tomography methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), magnetic particle imaging (MPI), magnetic resonance imaging (MRI), and photoacoustic (PA) imaging (Beard, 2011; Grover et al., 2015; Vaquero & Kinahan, 2015; Welch et al., 2009; Zhou et al., 2018; Zhu & Sevick-Muraca, 2015). All of these imaging methods provide exquisite sensitivity and quantification of the presence of nanoparticles, noninvasively and in vivo. Additionally, images can be analyzed over intervals of time to learn about the time-dependent distribution of the vaccine, therefore providing an understanding of its pharmacokinetics. These capabilities allow researchers and regulatory bodies to analyze the efficacy of the drug, thereby making them useful for translation as well as development. The aforementioned imaging modalities make the structure of this review, wherein we focus on recent studies demonstrating the design rationale and briefly the methodology to employ and interpret them in vaccine development.
2 |. OPTICAL IMAGING
Optical imaging describes a set of imaging methods which utilizes light in the range of visible to infrared (IR) wavelengths. Of these methods, perhaps the most notable in medical imaging, and certainly in the context of nanovaccine imaging, is fluorescence, which itself can be divided into fluorescence in the visible light range and in the IR to near-IR range. Additionally, bioluminescence, which is closely related to fluorescence, will be noteworthy. Most generally, fluorescence is a physical phenomenon in which an excited system (it could be a molecule, an atom, or even a nucleus) relaxes through the emission of a photon (Hassan & Klaunberg, 2004). Molecules which can undergo fluorescence are called fluorophores. For imaging applications, excitation is typically induced by a beam of incident photons so that the intensity emission photons can be measured as signal. Owing to the intricacies of quantum chemistry, the wavelengths of excitation and emission are not arbitrary. Excitation occurs most often at some wavelength(s) which we will simply refer to as “excitation peaks,” and similarly for emission which we will refer to as “emission peaks,” with particular excitation wavelengths producing particular emission wavelengths within a remarkably narrow window. Generally excitation and emission do not occur at the same wavelength. This is because excitation and emission do not occur between exactly the same physical states. Foregoing an extraneously technical explanation, the energy of an excitation photon is greater than that of its corresponding emission photon, and so the wavelength of the excitation photon is shorter than that of the emission photon. Additionally, the intensity of emission will not match the intensity of excitation. Finally, bioluminescence is the process in which the enzyme luciferase catalyzes the formation of oxyluciferin, which is excited upon production and relaxes through the release of a visible light photon (Pozzo et al., 2018).
2.1 |. Fluorescence and bioluminescence imaging in the visible spectrum
Fluorescence is a very broad phenomenon, and not just any fluorophore or good fluorophore finds convenient application to medical imaging. In fact, in the context of medicine it is broadly perhaps most often applied to in vitro cellular experiments such as cell tracking and cell staining, to name only two (Hassan & Klaunberg, 2004). The primary reason for this is that optical imaging outside of the red to near infrared (IR) range of the visible light spectrum is often less desirable depending on the tissue depth, since tissue penetration of visible light is poorer for shorter wavelengths (Ferreira et al., 2019). Consequently, a fluorophore with both an excitation and emission wavelength in this region is therefore doubly disadvantaged, as both the excitation beam and emission beam will be attenuated by tissue resulting in a decrease in signal and sensitivity. In spite of this, medical imaging with these fluorophores is not impossible and often most convenient. For instance, Zhang et al. (2020) have successfully imaged an mRNA lipid nanoparticle (mRNA-LNP) using firefly luciferase (FLuc). This study is worthy of mention for a few reasons. For contemporary reasons, it sought to develop a basis for a vaccine against COVID-19. In fact, more generally, a current topic of interest in immunology is the use of mRNA vaccines, which have been showcased in the vaccination of COVID-19 (Hou et al., 2021; Zhang et al., 2020). With imaging of biodistribution being an important aspect of vaccine development, especially when it occurs in the midst of a widespread pandemic such as the COVID-19 pandemic, it utilizes FLuc, which is a molecule with fascinating properties. FLuc is an enzyme isolated from fireflies which is responsible for their namesake, that is, their bioluminescence (Pozzo et al., 2018). FLuc is not a fluorophore itself, but it catalyzes a reaction to yield one, oxyluciferin. Interestingly, oxyluciferin produced by FLuc is already excited as-is and relaxes through fluorescence emission of a 560 nm photon, which is in the yellow region of the visible light spectrum. Thus, no excitation wavelength is required. This is one justification for the use of FLuc rather than NIRF, since it is better not to require an excitation beam in a region of the spectrum which is attenuated by biological matter. Finally, FLuc was incorporated in the nanovaccine in an innovative manner, by using a FLuc reporter encoding mRNA in the nanoparticle.
Nevertheless, the basis of immunization is that this lipid nanoparticle carries mRNA which encodes the receptor binding domain (RBD) of SARS-CoV-2 in addition to encoding FLuc (Zhang et al., 2020). The schematic of the study of shown in Figure 2a. Regarding in vivo imaging, the group tested intramuscular and subcutaneous injection as well as intranasal inoculation, and obtained images at 6, 12, 24, and 48 h post injection (p.i.). At 48 h p.i., they sacrificed the mice, harvested organs, and obtained ex vivo images to determine biodistribution in particular organs of interest. Both in vivo and ex vivo imaging results are displayed in Figure 2b and c, respectively. Importantly, images demonstrated that subcutaneous injection led to FLuc expression in the upper abdomen, while the same was not observed following intranasal inoculation. On the other hand, mice in the intramuscular injection group showed a peak in total fluorescence intensity in the upper abdomen 12 h after injection, and undetectable intensity by 48 h. It is also seen in this group at 48 h, uptake remains in glutamine synthetase-positive pericentral hepatocytes, which surround the CD31-positive central vein, as well as CD163-positive liver macrophages. These findings corroborate the immunization effect of this nanovaccine which is observed in mice challenged with a SARS-CoV-2 mouse-adapted strain, where it is found that two doses of this nanovaccine, at 10 μg of nanovaccine formulation each, confer complete protection to mice.
FIGURE 2.
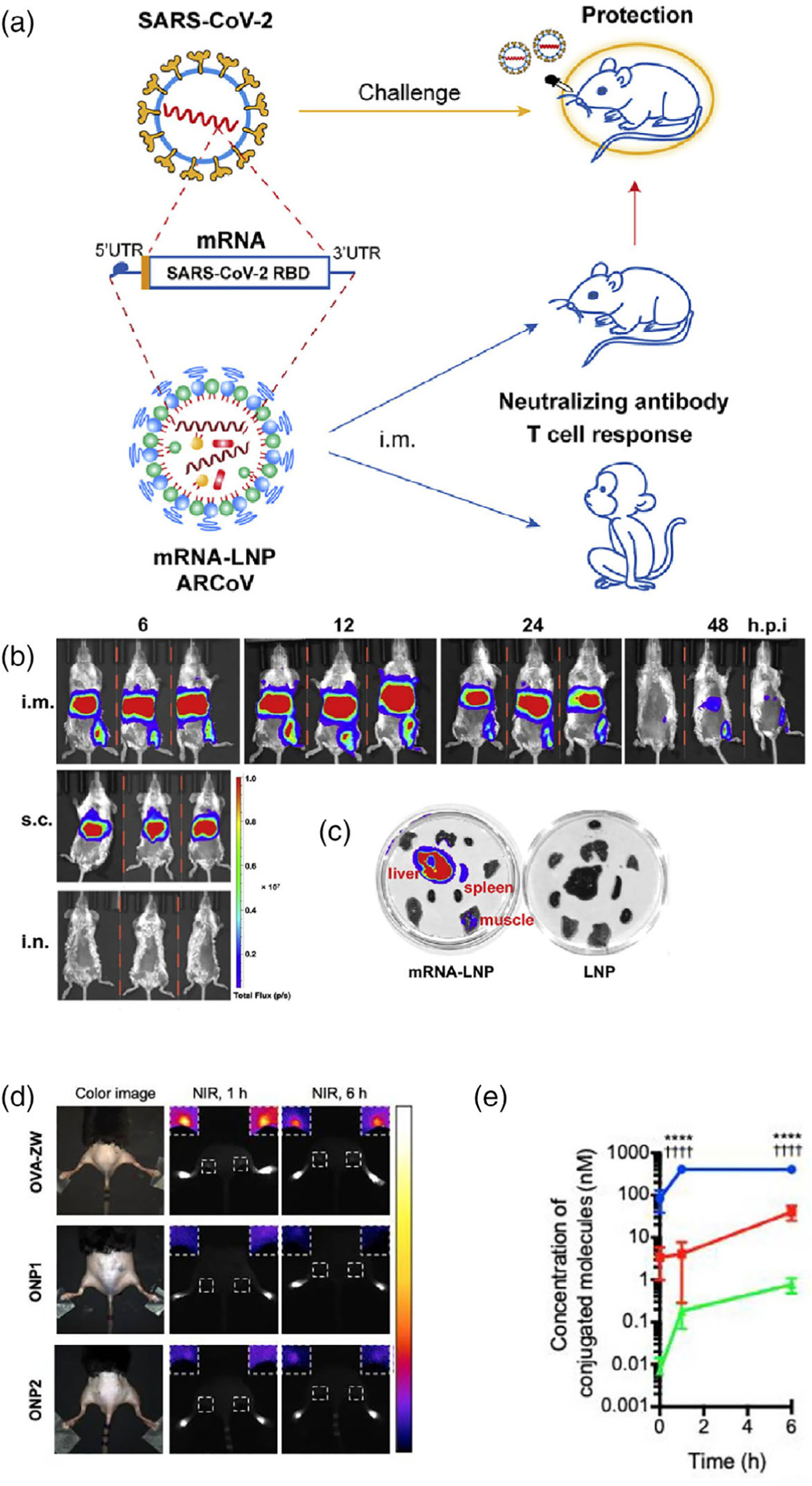
(a) A SARS-CoV-2 vaccine nanoplatform named ARCoV, wherein lipid nanoparticles are designed to carry an mRNA sequence corresponding to the receptor binding domain (RBD) of SARS-CoV-2. Immunization confers protection from SARS-CoV-2 in mice. (b) Fluorescence imaging of FLuc indicating presence of mRNA-LNP in mice, intramuscular (i.m.) and subcutaneous (s.c.) injections as well as intranasal (i.n.) inoculation. (c) Ex vivo imaging of organs from mice in the i.m. injection group (left) and free injection of LNP group (right). Adapted with permission from Zhang et al. (2020). (d) NIRF imaging of mice injected with OVA-ZW, ONP1, and ONP2, at 1 and 6 h. (e) Concentration of conjugated molecules versus time for each group. Both (d) and (e) demonstrated that OVA-ZW had higher uptake in the popliteal lymph nodes for the given time frames. Adapted with permission from Katagiri et al. (2019).
2.2 |. Near infrared fluorescence imaging
Near infrared fluorescence (NIRF) imaging specifically is when fluorophores that have excitation and emission peaks at wavelengths in the far-red to near IR region between 690 and 900 nm are used (Zhu & Sevick-Muraca, 2015). As mentioned in the previous subsection, the reason to distinguish fluorescent agents which have both an excitation and emission peak in the IR or near IR region is that light of these wavelengths has particularly good penetration into biological matter, therefore making it more viable to both excite the fluorophores exogenously and measure the emission signal, when compared with other optical imaging methods. However, despite better tissue penetration it remains a 2D imaging technique rather than a tomographic 3D imaging technique. The tissue penetration can be anywhere between 4 and <2 cm, and consequently 3D tomographic techniques using 2D projection data have not proven to be clinically interesting. In general, however, tissue penetration, sensitivity, and resolution of this technique is dependent on the NIRF device being used. In particular, and unlike the majority of imaging modalities, NIRF does not have phantoms or standards with which to quantify or compare performance.
Molecular imaging being the primary use of NIRF imaging, it is generally performed with a biomolecule that is conjugated with a near IR fluorophore (Zhu & Sevick-Muraca, 2015). In contrast to PET and SPECT, NIRF has the benefit that it does not require the introduction of a radioisotope into the subject, which can be an important consideration in the development of radiotracers, for instance, considering pharmacokinetics and radiolabeling, as well as in the clinical administration of radiotracers, considering the dose of radioactivity given to the patient.
One common fluorophore used for NIRF imaging, and one which certainly sees much use in nanovaccine imaging, is Cyanine (Cy) 5.5. Cy5.5 has an excitation peak at around 675 nm and an emission peak at around 694 nm, owing to its highly conjugated structure and aromatic groups (Umezawa et al., 2009). Additionally, Cy5.5 can be readily tagged to ovalbumin (OVA) and therefore purchased directly, which greatly increases its applicability to the research of nanovaccine biodistribution (Deluco & Wilson, 2021; Han et al., 2021; Katagiri et al., 2019; Zhong et al., 2019). Now, OVA is important because it is a common “model antigen” (Bayne-Jones, 1917; Tiselius & Kabat, 1939). Model antigens are used in the design and evaluation of a vaccine delivery system, and an ideal model antigen is biologically inert and does not introduce any complications to the body when delivered by vaccine, a role which OVA readily fulfills (Hopkins, 1900; Hu & Du, 2000).
One study which employs OVA and Cy5.5 was carried out by Zhong et al. (2019), who reported the successful utilization of a zeolitic imidazole framework (ZIF-8) in the delivery of OVA. Zeolitic imidazole frameworks are a subset of metal organic frameworks (MOFs), which have been recognized as desirable drug delivery systems owing to their large surface area to volume ratio, tunable pore size, and high loading capacity. However, synthesis of MOFs typically requires organic solvents, high temperatures, and high pressures, none of which are very easy to achieve in the presence of biomacromolecules such as an antigen. On the other hand, ZIF-8 is more readily formed by the coordination of two Zn2+ ions with 2-methylimidazole (MeIM). ZIF-8 is promising for drug delivery not only due to its high loading capacity, but also due to pH-sensitive degradation, which can be leveraged for the release of their load in a target environment. The novelty of this research is the use of a mild and facile synthesis method to form OVA-loaded ZIF-8 (ZNP) without organic solvents or stabilizing agents. Additionally, aluminum adjuvants, common in many vaccines, were integrated with ZIF-8 utilizing a biomimetic mineralization process. This formed nanoporous shells which could be loaded with OVA (ZANPs). Finally, they employed yet another adjuvant, the toll-like receptor 9 agonist, cytosine-phosphate-guanine oligodeoxynucleotides (CpG), using electrostatic interaction to adsorb the adjuvant onto the surfaces of ZNPs and ZNAPs (CpG/ZNP or CpG/ZNAP, respectively). It was shown that CpG/ZANP induced a strong antigen-specific humoral and cytotoxic T lymphocyte response against EG7-OVA tumors in mice, while showing little toxicity in mice. In order to image this nanoplatform, Cy5.5-labeled OVA was used. C57BL/6 mice were divided into the following groups corresponding to the treatment with which they would be injected: free Cy5.5-Ova, Cy5.5-OVA-ZNPs, and Cy5.5-OVA-ZANPs. Subcutaneous injections in the footpad were given, and NIRF imaging was performed at 1, 6, 12, and 24 h p.i. It was seen that at 6 and 12 h p.i., the ZNP and ZANP groups showed strong NIRF signals in the axillary lymph nodes, while OVA showed a weaker signal. Importantly, these results agreed with flow cytometry that was performed.
Another study which demonstrates the versatility of NIRF imaging as well as the significance of imaging in development and evaluation of a vaccine was carried out by Katagiri et al (2019). This group conjugated a zwitterionic NIR fluorophore, ZW800–1C, on the surface of OVA (OVA-ZW), and subsequently conjugated this to silica nanoparticles of 20 nm (SiNP-20 nm) and 100 nm (SiNP-100 nm) in diameter to form what are referred to as ONP1 and ONP2, respectively. The authors chose ZW800–1C, a fluorophore with an excitation peak at around 753 and emission peak at around 722 nm, since it has minimal interactions with tissue, allowing the nanovaccine to be distributed without interference. Additionally, the authors tested two nanoparticle sizes because, as discussed previously, the size of a nanovaccine is important in determining the kinetics and mechanics of its migration to the draining lymph nodes after injection. Typically, a larger nanoparticle like ONP2 may be expected to be slower, while a smaller nanoparticle like ONP1 may be expected to be quicker.
The nanovaccine was then evaluated for in vivo biodistribution (Katagiri et al., 2019). Mice were divided into three groups depending on the vaccine formulation they received: ONP1, ONP2, and free OVA-ZW. Intradermal injection in the footpad was performed, and NIRF images were taken at 1, 6, 24, 48, and 72 h p.i. Mice injected with OVA-ZW showed rapid increase in signal in the popliteal lymph node after 1 h, and this remained high until 6 h, after which it steadily decreased. On the other hand, ONP1 and ONP2 mice showed steady accumulation in the draining lymph nodes over 72 h p.i. Figure 2d shows a comparison of the three groups imaged at 1 and 6 h with a comparison of the calculated concentration of conjugated molecules across the three groups in Figure 2e. The fluorescence signal due to OVA-ZW from 1 to 6 h was much higher than that of ONP1 and ONP2, although concentration of OVA-ZW in the popliteal lymph node was much higher than ONP1 and ONP2 over the whole 72 h. Notably, ONP2 showed the lowest concentration in the lymph node over the whole 72 h, corresponding to the inverse relationship between nanoparticle size and concentration in the draining lymph nodes.
Another interesting example is a study by Gong et al. (2020), in which two proton-driven nanotransformer-based vaccines (NTVs) were engineered for immunotherapy of cancer. These NTVs are polymer-peptide conjugate-based nanotransformers loaded with an antigen. The remarkable thing about these nanostructures is that in an acidic environment, such as an endosome, they change from a nanosphere (~100 nm diameter) to either a nanofiber or a nanosheet with several μm in length and width. This is interesting to solve a very particular problem in cancer vaccination strategies, which is endosomal trapping of tumor antigens. Such a structure, if trapped in an endosome, can unravel and mechanically destroy the endosomal membrane while also delivering the antigen into the cytoplasm. The method of synthesis for this structure was reversible addition-fragmentation chain transfer polymerization, which is described in depth in the source. Nevertheless, with OVA241–270 as a model antigen, NIRF imaging occurred readily with OVA241–270-Cy5.5.
Specifically, p(DMAEMA22-OGEMA4)-b-p(MAVE)30 was synthesized using reversible addition-fragmentation chain transfer polymerization (Gong et al., 2020). Then, naphthalene-conjugated D-peptide (NDP), which forms a nanofiber in cells, was conjugated to the hydroxyl groups of p(DMAEMA22-OGEMA4)-b-p(MAVE)30 with an acetal bond. The polymer p(DMAEMA22-OGEMA4)-b-p((MAVE)16-(MAVE-NDP)14) forms a spherical nanostructure at pH 7.4 but forms a nanofiber in an acidic environment and is referred to as nanotransformer 1 (NT1), representing a sphere-to-nanofiber transformation. Alternatively, pyrene-conjugated-D-peptide (PDP) was also used instead of NDP, which formed p(DMAEMA22-OGEMA4)-b-p((MAVE)18-(MAVE-PDP)12), which is also a spherical nanostructure at pH 7.4 but transforms into a nanosheet in an acidic environment, representing sphere-to-nanosheet transformation and referred to as nanotransformer 2 (NT2). These nanotransformers, loaded with OVA241–270-Cy5.5 via a double emulsion method, represent NTV1 and NTV2, respectively, and form nanovaccines capable of delivering the OVA antigen without endosomal trapping. In B16F10-OVA melanoma mice, NTV2 was found to inhibit tumor growth and improve survival, while NTV1 only exhibited moderate inhibition of tumor growth. This was justified by imaging. Formulations of free OVA241–270-Cy5.5, OVA241–270-Cy5.5-NTV1, and OVA241–270-Cy5.5-NTV2 were injected into C57BL/6 mice three times at 1 week intervals. It was found that NTV2 was perhaps superior to NTV1, delivering OVA to lymph nodes and inducing a strong and sustained cross-presentation to CD8+ T cells.
3 |. RADIONUCLIDE-BASED IMAGING
Radionuclide-based imaging is most broadly a nuclear medicine technique of forming a tomographic image from nuclear decay signals (Vaquero & Kinahan, 2015; Wernick & Aarsvold, 2004). Generally, nuclear decay can provide useful information if the radioisotope in question is tagged to a molecule. This forms the basis of the so-called tracer principle, by which one can ascertain to a biochemical or metabolic function or analyze biodistribution of a molecule by radiolabeling it, so long as the molecule and radioisotope meet certain criteria such as not disturbing biological function besides being radioactive. It is important to understand that radioisotopes will behave chemically like any isotope of the same atomic number, which is the motivation for many radiolabeling procedures. While not every decay mode finds application to medical imaging, only two decay modes find application to imaging of nanovaccines. They are positron decay and gamma decay.
3.1 |. Positron emission tomography
Positron emission tomography (PET) is a well-established and widely used imaging modality (Vaquero & Kinahan, 2015; Wernick & Aarsvold, 2004). The purpose of PET is to image the location at which a positron emitting radioisotope decays, and as such, it can be used to image the location of a tracer in the body. The decayed positron propagates until it meets an electron and they annihilate, characteristically leaving behind two anti-parallel (or very nearly anti-parallel) photons of energy about 0.511 MeV, which can be detected by a PET scanner and used to determine the location of the annihilation. Due to the range of positrons in biological matter, scatter of annihilation photons, and attenuation despite being generally well-corrected by a computed-tomography (CT) attenuation scan, PET is said to have a fundamental resolution limit of about 2 mm (Persano et al., 2021; Vaquero & Kinahan, 2015). However, in practice the resolution is typically about 2.5 mm in clinical PET scanners. Regardless, the resolution is variable depending on the scanner and subject.
The major novelty in the application of PET to a given situation, such as the imaging of a nanoparticle, is radiolabeling (Ferreira et al., 2019; Ni et al., 2017; Welch et al., 2009). The study of nanomaterials in conjunction with radioisotopes is a highly interdisciplinary topic which holds promise for simultaneous therapy and imaging (i.e., theranostics). Two strategies for their simultaneous use are to have the nanoparticle carry the radioisotope as a tracer itself, or have the radioisotope and nanoparticle both conjugated to the same tracer. Of course, the introduction and stable conjugation of the radioisotope is its own problem which involves an understanding of radiochemistry. But in addition to that, there are always important considerations in the synthesis of such nanoplatforms. For instance, the selected radioisotope should typically have a half-life suitable for the pharmacokinetics of the tracer or nanoparticle. These are some of the challenges faced in the engineering of such nanoplatforms.
Han et al. (2021) reported the development of Poly(D, L-lactic-co-glycolic acid) (PLGA) nanoparticles as antigen delivery systems and adjuvants. One desirable characteristic of these nanoparticles is that they are both biodegradable and biocompatible. These solid lipid nanoparticles were formed using PLGA and dimethyl-dioctadecyl-ammonium bromide (DDAB) and loaded with a model antigen ovalbumin, OVA257–264. The nanoparticles thus formed are referred to as OVA@DDAB/PLGA nanovaccines. As a delivery system, these nanovaccines may be compared with pure OVA or antigen-loaded alumina hydroxide adjuvant (OVA@Al). In this case, it is found that antigen uptake of bone-marrow derived DCs after incubation with OVA@DDAB/PLGA is two times higher than with pure OVA or OVA@Al, and subsequently there is better activation of CD4+ T cells, CD8+ T cells, and B cells, contributing to powerful immune response and memory. Additionally, when delivered by DDAB/PLGA, the antigen remained in lymph nodes for over 7 days. Evaluation of biodistribution was performed using PET imaging with 89Zr labeled OVA.
PET imaging would be performed on mice divided into groups corresponding to their injection with either free 89Zr, 89Zr-OVA, 89Zr-OVA@Al, and 89Zr-OVA@DDAB/PLGA. Specifically, the study used female D57 mice (6–8 weeks), and intramuscular injections of 120–150 μCi per mouse. Images were taken using a micro PET/CT at 5 min, 2, 4, 6, and 9 h, 1, 2, 4, 6, 8, 10, 12, and 14 days (d) p.i., with 20 million coincidence events each scan. Partial results of this imaging are shown in Figure 3a, and Figure 3b depicts a PET/CT image which distinguishes the injection site and various ROIs. It was observed that OVA@Al maintained a long-term antigen storage effect at the injection site, trapping the antigens and preventing their delivery to the draining lymph nodes. Conversely, OVA@DDAB/PLGA delivered the antigen to the draining lymph nodes and maintained a signal in the lymph nodes which remained strong even at 2 days after injection. Afterwards, however, the antigen distributed to the spleen, liver, and kidneys, remaining there even at 14 days after injection. These results, owed to the power of PET imaging of the radiolabeled nanovaccine, demonstrated that OVA@DDAB/PLGA may be a more effective antigen delivery system than OVA@Al.
FIGURE 3.
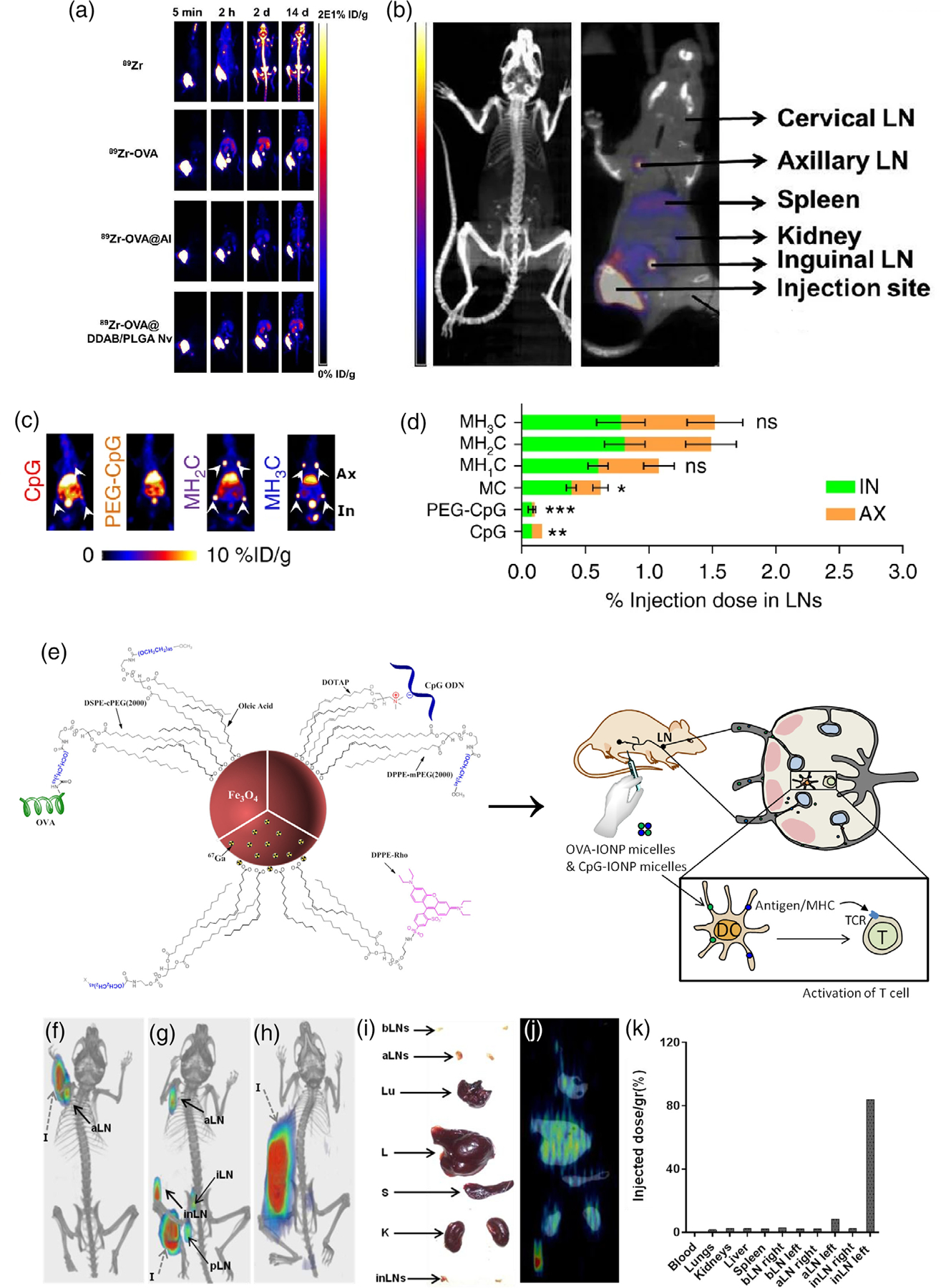
(a) PET imaging of different 89Zr-OVA-loaded formulations in mice. (b) PET/CT image showing the injection site with respect to various ROIs as well as uptake values. Adapted with permission from Han et al. (2021). (c) PET images at 6 h p.i. of CpG, PEG-CpG, MH2C, and MH3C formulations of AlbiVax. Axillary (Ax) and inguinal (In) lymph nodes are indicated in the MH3C image. (d) %ID of all formulations of AlbiVax. Adapted with permission from Zhu et al. (2017). (e) Design of PEGylated magnetite nanoparticles carrying immunogen OVA, adjuvant CpG, as well as gamma emitting radioisotope 67Ga and fluorophore DPPE-Rho. Injection in mice shows that the nanovaccine targets T-cells in lymph nodes to elicit immunogenic effect. (f) 67Ga-SPECT/CT of mice 3 h p.i. with nanovaccine formulation, injection in the forearm, (g) injection in the hock, and (h) injection in the flank. (i) Image of organs harvested from a mouse in the flank injection group 24 h p.i. (j) 67Ga-SPECT/CT of harvested organs. (k) %ID/g in various ROIs. Adapted with permission from Ruiz de-Angulo et al. (2016).
One more interesting and recent study was carried out by Zhu et al. (2017), who developed a nanoplatform for efficient delivery of a molecular vaccine. This nanoplatform is based on the conjugation of molecular vaccines with Evans blue (EB) into an albumin-binding vaccine (AlbiVax) to assist with delivery to the lymph nodes. These vaccines are capable of self-assembly in vivo. In particular, AlbiVax is synthesized by conjugating thiol-modified vaccines with a maleimide-functionalized EB derivative (MEB), forming a complex which naturally binds human serum albumin (HSA). In order to demonstrate the ability of this nanoplatform to transport molecular vaccines, they used the adjuvant CpG. MEB was conjugated to the 3′-end of CpG, forming MEB-CpG. However, the authors had suspicions that this conjugation would interfere with the ability of HSA to bind with CpG, and so they used hexaethylene glycol (HEG) linkers to connect MEB and CpG. Therefore, they formulated four different nanoplatform complexes depending on the number of HEG linkers used. With 0, 1, 2, and 3 units of HEG linkers, they formed MC, MH1C, MH2C, and MH3C, respectively. Imaging was now crucial, not just to evaluate the nanoplatform design in the first place, but also to compare the numerous formulations they developed. Taking advantage of the use of MEB in this nanovaccine, they were able to use NOTA-MEB which is capable of chelating Cu ions, and so they were able to radiolabel their formulations with 64Cu. PET imaging of these formulations as well as control groups, most notably polyethylene glycol (PEG)-CpG and free CpG, was performed 6, 12, 24, 48, and 72 h after subcutaneous tail injection.
PET imaging allowed the authors to conclude that MH2C and MH3C were the most successful formulations, while all formulations were substantially more successful than injection of PEG-CpG and free CpG (Zhu et al., 2017). Only <0.3% of the injected dose (ID) of CpG was delivered to the inguinal and axillary lymph nodes. At 6 h, PEG-CpG delivered to the inguinal lymph nodes about as well as free CpG, while its delivery to the axillary lymph nodes was negligible. Meanwhile, at 6 h, MH2C and MH3C were able to deliver 1.49% and 1.52% of ID, respectively, to the two lymph nodes. Refer to Figure 3c for a comparison of the PET images obtained for MH2C, MH3C, PEG-CpG, and free CpG, 6 h p.i., and to Figure 3d for a comparison of the %ID at 6 h p.i. among all formulations and controls. Further evaluation of this nanoplatform demonstrated that it could potentially contribute significantly to tumor immunotherapy.
3.2 |. Single photon emission computed tomography
Single photon emission computed tomography (SPECT) is a method of imaging isotopes which decay through gamma emission (Wernick & Aarsvold, 2004). Gamma decays are the release of a high-energy photon from the nucleus of a radioisotope as a form of relaxation. Notably, they do not change the atomic number of the isotope. Nevertheless, having a unique physical mechanism means that SPECT imaging systems are quite unlike PET imaging systems. Typically, SPECT systems utilize so-called gamma cameras, which collimate gamma rays and register them as signals. Nevertheless, SPECT usually has worse resolution than PET because scattering of gamma rays is a more significant problem. SPECT’s utility over PET is typically related to the use of a particular radioisotope. For instance, the emergence of radionuclide therapy, in which a radiolabeled molecule is used to target and damage a tumor rather than simply provide an image, has increased interest in the use of many molecules radiolabeled by a β− (electron) emitter, such as the clinically approved 177Lu-DOTATATE (lutathera) (Strosberg et al., 2017). In radionuclide therapy, it is sometimes desirable to be able to image the distribution of radioisotope for a variety of purposes, ranging from tumor tracking to internal dosimetry (Marquis et al., 2021). This approach is termed “theranostics.” While it sometimes involves dual-radiolabeling with both a positron emitter for PET imaging and a β− emitter for therapy, some β− emitters may also emit gamma rays at a rate desirable for SPECT imaging, allowing one to use a single radioisotope for both therapy and imaging. Another, separate reason that one would choose a gamma emitter over a positron emitter may involve the half-life of the emitter in question. The half-life of a radioisotope chosen for a tracer should be short or long dependent on the pharmacokinetics of the molecule which is being radiolabeled.
One recent study opted to use SPECT over PET due to the half-life of a gamma emitting isotope of Ga compared with that of a positron emitting isotope of Ga. Ruiz de-Angulo et al. (2016) reported the use of lipid-coated magnetite micelles to carry and deliver the model antigen OVA and CpGs as an adjuvant. In this study, the authors propose a novel and convenient method of labeling these nanoparticles with isotopes of Ga, of which there are two particularly useful ones for either PET (68Ga) or SPECT (67Ga). The SPECT isotope has a half-life more suited to the timescale of the study and pharmacokinetics of the nanovaccine. Specifically, the half-life of 68Ga (68 min) is quite a bit shorter than that of 67Ga (3.3 days). The schematic of this study is shown in Figure 3e, depicting the magnetite nanoparticle PEGylated, labeled with 67Ga, and carrying both OVA and CpG, in addition to a (non-IR) fluorophore, rhodamine B-modified 1,2-Bis(diphenylphosphino)ethane (DPPE). Rhodamine B has an excitation peak and emission peak at around 556 and 580 nm, respectively. However, in this study, fluorescence was used exclusively for in vitro and ex vivo analysis, and so we will focus on SPECT. Still, it is interesting to note that the conjugation of a NIRF agent could possibly be achieved in a similar manner, and so imaging in vivo with a NIRF agent is likely a possibility.
Nevertheless, one of the benefits of a magnetite nanoparticle is that it is biocompatible (Ruiz-de-Angulo et al., 2016). Additionally, they are viable as MRI contrast agents, although this is not desirable in this context as it has been demonstrated that high nanoparticle concentrations would be required for reasonable diagnostic performance. That said, the novel approach of radiolabeling utilizes the size, about 40 nm diameter, and surface properties of these magnetite micelles. The Ga3+ ions are able to be effectively attached to the nanoparticle without chelators, representing an “organic chemistry-free” radiolabeling method. Additionally, both their size and their ability to present the antigen on their surface facilitates the delivery of cargo to lymph nodes and APCs, forming the basis of their immunization effect.
SPECT/computed tomography (CT) imaging was performed at 3 and 24 h after subcutaneous injection in the left flank, forearms, and hock of the mice (Ruiz-de-Angulo et al., 2016). After 24 h, mice were sacrificed and organs were harvested, then imaged. Results of in vivo imaging at 3 h p.i. are shown in Figure 3f–j. The three methods of injection are able to be compared, where Figure 3f shows injection into the forearm, Figure 3g shows injection into the hock, and Figure 3h shows injection into the flank. 3 h after injection in the forearm, the nanovaccines had migrated to the draining left axillary lymph nodes, while after injection in the hock, they migrated to the left inguinal, popliteal, axillary, and iliac lymph nodes. However, 3 h after injection in the flank, the signal at the site of injection was prevented the detection of uptake in the draining inguinal lymph nodes. Nevertheless, in the ex vivo image corresponding to this injection, shown in Figure 3i,j, they were able to detect accumulation in the left inguinal and axillary lymph nodes. Finally, Figure 3k shows a %ID/gram (g) analysis of various organs. These results inform the conclusion that their nanovaccine is well capable of reaching the lymph nodes near the injection site as well as some that are more distant and being internalized by APCs.
4 |. MAGNETIC PARTICLE IMAGING
Magnetic particle imaging (MPI) is a relatively young tomographic technique in the field of molecular imaging (Graeser et al., 2019; Persano et al., 2021; Wu et al., 2019; Zhou et al., 2018). Like PET and SPECT, MPI is a tracer imaging technique rather than a structural imaging technique like MRI and CT. Like MRI, it does not use ionizing radiation, however it does use a magnetic field albeit one different in structure from those used by MRI, and therefore the purpose is to obtain a very different type of information. MPI specifically images superparamagnetic iron oxide nanoparticles (SPIONs), which are also occasionally used as MRI contrast agents. Importantly, biomatter does not generate or attenuate the gradient fields that are used in MPI, so MPI images have contrast that is independent of source depth and reduced tissue background noise. MPI resolution is about 700 μm full width at half maximum, and point sources of MPI signal can be distinguished at about 600 μm separation. This is good compared with PET, which has a fundamental limit of about 2 mm, due in part to the range of positrons in biological matter, and a practical limit of about 2.5 mm. Additionally, it has been shown that MPI can detect as low as 1.1 ng of SPIONs in one voxel with a gradient field of 5.7 T/m, as well as achieve a native resolution of 800 μm full width at half maximum. MPI also has great temporal resolution, making it viable for real time imaging.
Research on MPI systems is ongoing, and novel MPI systems are being developed. One example is the point-of-care testing MPI system (PoCT-MPI) created by Choi et al. (2020). The PoCT-MPI is a device which uses a frequency mixing magnetic detection method and hybrid FFL generator. As a consequence, it is smaller, lighter, and uses less power than a typical MPI, making it more practical. Another interesting use of MPI is color MPI, which can distinguish between different nanoparticles by considering distinct relaxation dynamics and allow for imaging and distinction of more than one tracer (Zhou et al., 2018). A demonstration of this with two separate tracers, one of which targets the lungs and one of which targets the liver, is shown in Figure 4a.
FIGURE 4.
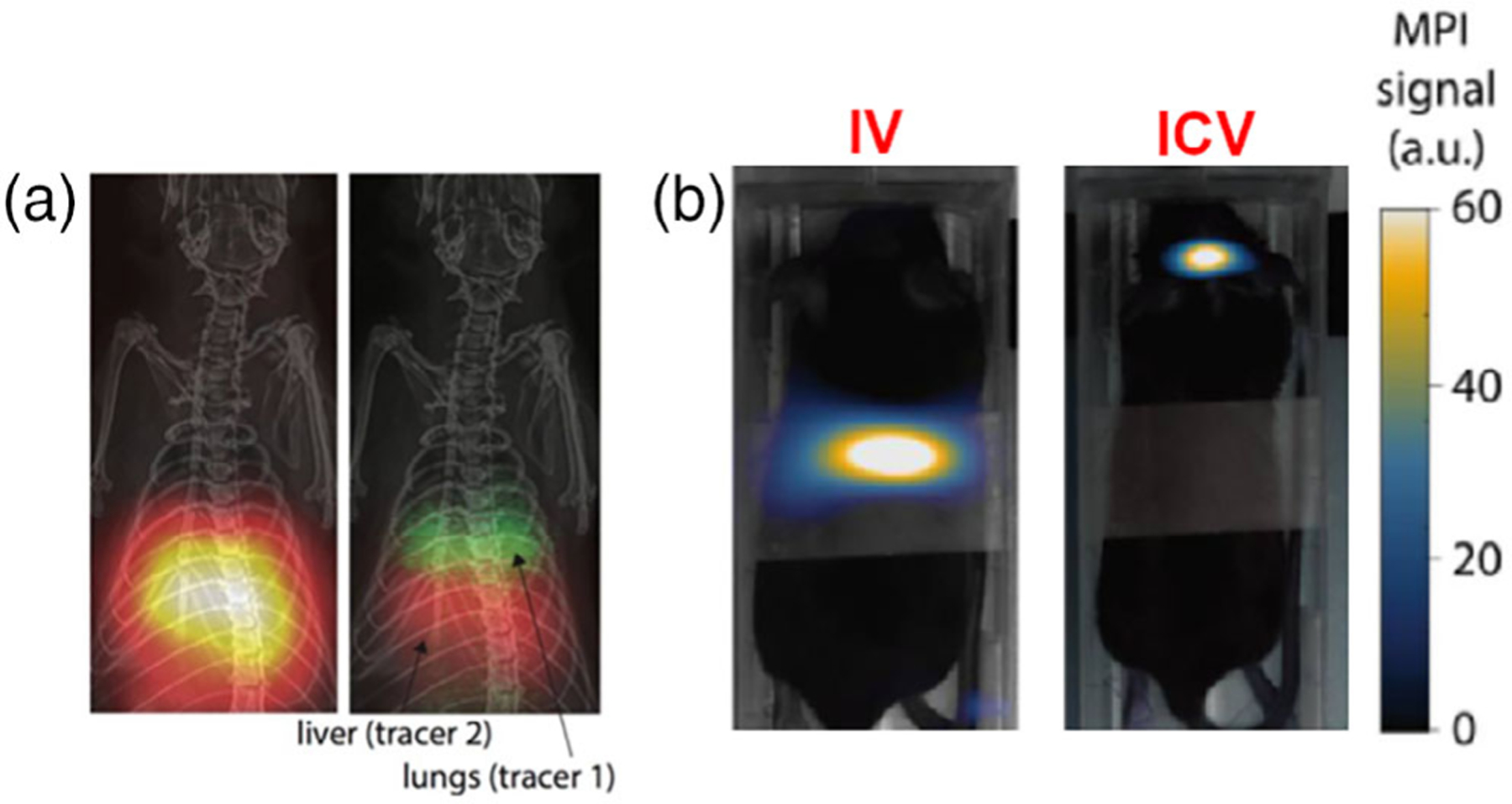
(a) A side-by-side comparison of standard MPI and “dual-color” MPI (with CT for the anatomical background), using both a lung-targeting tracer (green) and a liver-targeting tracer (red). Adapted with permission from Zhou et al. (2018). (b) MPI of mice injected with ferucarbotran-labeled tumor-effective T cells for brain tumor therapy, 24 h p.i. with intravenous (IV) injection, and intracerebroventricular (ICV) injection. Adapted with permission from Rivera-Rodriguez et al. (2021).
Regarding the application of MPI, its spatial and temporal resolution make it promising for applications in vascular and perfusion imaging, oncology, cell tracking, inflammation imaging, trauma imaging, and brain imaging. However, MPI has not yet seen very much clinical use. It is currently confined to research, with small-animal MPI devices being available for research facilities. In fact, only recently has a human-sized MPI device been reported by Graeser et al., (2019) which is specifically designed for imaging of brain perfusion. This device was reported to be able to detect a SPION concentration of 263 pmol/ml with a gradient field of 0.2 T/m.
Being based on molecular imaging of SPIONs, MPI naturally finds application in nanovaccine imaging. One powerful proof-of-concept study performed by Riviera-Rodriguez et al., in which T-cells transferred during adoptive cellular therapy (ACT) were imaged in vivo in a mouse model of brain cancer (Rivera-Rodriguez et al., 2021). ACT is a method of vaccination involving the direct administration of T cells, and in the treatment of cancer it typically involves administration of tumor-effective memory T cells. This group attempted ACT for brain tumors in C57BL/6 mice with intracranial KLuc-gp100 tumors, and opted to image the cells to determine their biodistribution. It was convenient to label the cells with ferucarbotran, a SPION, and subsequently image with MPI. The T cells were delivered in the tail vein or intracerebroventricularly, and the mice were imaged 24 h p.i. A comparison of the T cell distribution under both injection methods is shown in Figure 4b, where it is seen that intracerebroventricular injection causes T cell accumulation in the brain, but intravenous injection appears not to. This study also demonstrates that the in vivo MPI signal accurately and linearly quantifies the ferucarbotran-labeled T cells, a direct consequence of the physical basis of MPI described earlier in this section.
Finally, there are several recent studies which have utilized SPIONs but have not been evaluated or re-evaluated with MPI, (Liu et al., 2020; Mukherjee et al., 2020; Rivera-Rodriguez et al., 2021) but could perhaps benefit from it. One was performed by Kim et al., (Kim et al., 2012) in which SPIONs were investigated in the delivery of mouse melanoma antigen hgp10025–33. While this study utilized the fluorescent agent fluorescein isothiocyanate (FITC, excitation 490 nm, emission 525 nm) and SPIONs were utilized for their ability to carry the antigen, MPI could have introduced an alternative, more minimalistic method of imaging.
5 |. MULTIMODALITY IMAGING
In nanovaccine imaging, it is also common to find the application of more than one modality of imaging. Note that by multimodality we do not mean combinations such as PET/CT, SPECT/CT, PET/magnetic resonance (MR), that is, combinations of two modalities for simultaneous molecular imaging and anatomical imaging. This section describes the reasons for which two imaging modalities have been used for simultaneous or sequential molecular imaging of nanovaccines.
5.1 |. PET and optical imaging
One group recently applied both PET/MR and optical imaging to an HIV vaccine nanoplatform in the rhesus macaque model in part with the intention of quantifying vaccine uptake in specific ROI (Martin et al., 2021). The vaccine designed by this group is intended to build mucosal immunity to HIV, it is a nanoplatform comprised of a separate nanoparticle-based immunogen/antigen and an adjuvant. Specifically, the immunogen was a MD39 trimer, which is an HIV envelope trimer and capable of existing as either a soluble trimer or a self-assembled trimer nanoparticle. It is also capable of entering lymph nodes due to its size. The adjuvant was CpG7909 bound to an albumin-binding lipid moiety (amph-CpG7909), which is capable of targeting lymph nodes through a process referred to as albumin hitchhiking. Now, the authors realized they could target mucosal immunity through an injection into the thigh, which could lead to accumulation of the immunogen and adjuvant in the inguinal and iliac lymph nodes that drain the vaginal tract and rectum, or more generally the genitourinary tract-draining lymph nodes, which are linked to mucosal immunity to HIV. The specific advantage of anatomical imaging in this case is to identify the inguinal and iliac lymph nodes with respect to the injection site, and subsequently to isolate and quantify the signal of the vaccine in these three ROIs.
A schematic of the study design is shown in Figure 5a, which demonstrates the separate role of PET and optical imaging (Martin et al., 2021). That is to say, the use of these imaging modalities was not simultaneous but rather to obtain information which is complementary. To highlight this, we will consider first the approach, rationale, and results given by PET/MR imaging. It is then worth noting that the use of MR is motivated by anatomical information, specifically for the identification of the lymph nodes with respect to the PET image and subsequent quantitative ROI analysis. The authors considered two formulations: one with 64Cu radiolabeled immunogen and one with 64Cu radiolabeled adjuvant. The injection occurred subcutaneously in the thigh, scans were taken in vivo at 24 h and then ex vivo post autopsy at 48 and 72 h. In Figure 5b, one can compare the PET-only images of three mice 24 h p.i. of MD39-NP against the PET/MR image in Figure 5c (a composite of two mice 24 h p.i.), where the lymph nodes and injection site are isolated and exclusively shown in the PET overlaying the MR image. In Figure 5d, SUVmean quantities are provided for these scans. Importantly, the PET-only images demonstrated the general uptake of the vaccine, pointing out the uptake of vaccine in important organs such as the liver. Meanwhile, the PET/MR image is comprised of only the signals in the inguinal lymph node, the iliac lymph node, and the injection site, which was able to be isolated using the MR image. Importantly, this study found that MD39-NPs appeared to accumulate around follicular dendritic cell networks (FDC) in B cell follicles, providing a basis for therapeutic success of this nanoplatform.
FIGURE 5.
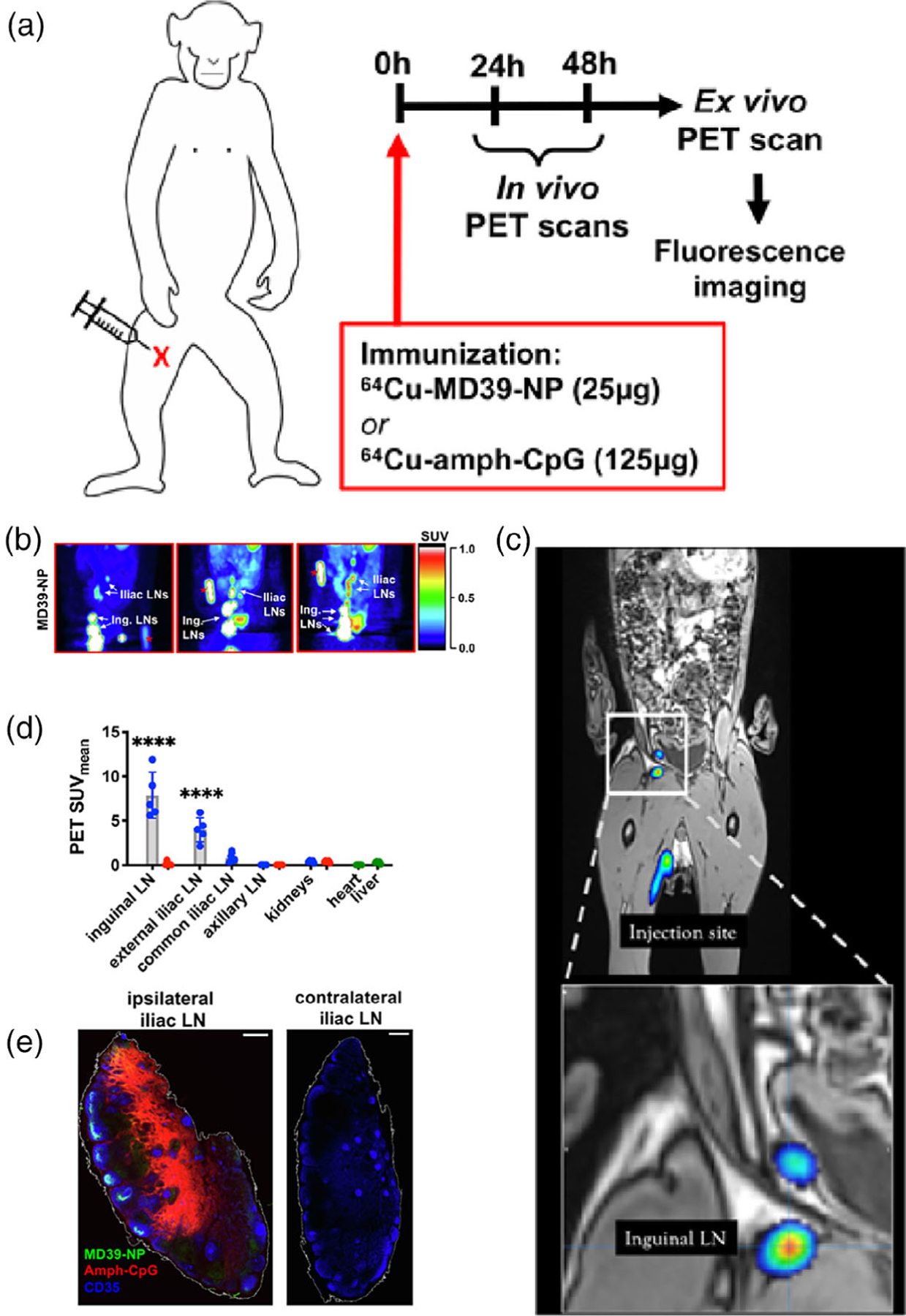
(a) Schematic of the study design wherein a nanovaccine was developed for PET/MR imaging and subsequent fluorescence imaging of a rhesus macaque model. (b) PET-only images of three rhesus macaques 24 h p.i. of 64Cu-MD39-NPs. (c) Combined PET/MR composite of two rhesus macaques 24 h p.i. 64Cu-MD39-NPs, injection site and lymph nodes are isolated in the PET scan while MRI provides anatomical context. (d) SUVmean of all three rhesus macaques, indicating high uptake in the targeted lymph nodes (LNs). (e) Ex vivo (24 h p.i.) fluorescence imaging of ipsilateral and contralateral iliac LNs of rhesus macaques injected with fluorescent MD39-NP (green), Amph-CpG (red), and CD35 (blue). Adapted with permission from Martin et al. (2021).
However, by 72 h post injection, 64Cu signal was difficult to quantify, due to the half-life of 64Cu of about 12.7 h (Anderson & Ferdani, 2009; Martin et al., 2021). For this reason, in both of their vaccine formulations (radiolabeled immunogen vs. radiolabeled adjuvant), they included both fluorescently labeled immunogen and adjuvant, specifically rhodamine-amph-CpG7909 and AF647-MD39-NP, where rhodamine and AF647 are fluorophores. The latter has an excitation peak and emission peak at around 633 and 667 nm, respectively. Due to their distinct excitation and emission wavelengths, they can be distinguished when imaged simultaneously, providing a similar tracer distinction as does multi-color MPI. Fluorescence imaging enabled them to image ex vivo as late as 7 days p.i., at which point in time the 64Cu would have decayed to about 0.01% of its original amount. This way they were able to obtain important ex vivo imaging information without having to compromise on their choice of radioisotope by picking a longer-lived but potentially less convenient isotope than 64Cu. Nevertheless, using fluorescence they were able to confirm results from PET which suggested that vaccine uptake in the inguinal and iliac lymph nodes. For instance, Figure 5e shows a fluorescence image of the ipsilateral and contralateral iliac lymph nodes at 7 days p.i. They were also then able to obtain other quantities of significance not provided by PET or MR, such as the total antigen accumulation, for which they used a flatbed laser fluorescence scanner.
A similar study was carried out by Lindsay et al. (2019), wherein they employed PET/CT and NIRF to track messenger ribonucleic acid (mRNA) vaccine delivery in primates, cynomolgus macaques. Once again, PET/CT was used in vivo while NIRF was used ex vivo. As a model mRNA, they used yellow fever prME mRNA. Importantly, they labeled the mRNA directly with a dual imaging probe, 64Cu-DyLight 680, where DyLight 680 is a NIR fluorophore (excitation 692 nm, emission 712 nm). Note that method of labeling is employed without constraining the design by choosing a particular nanoplatform, thus demonstrating the flexibility of imaging mRNA vaccines.
Nevertheless, the mRNA was complexed with a lipid derivative of natural amino sugars that has been designed in the past for the purpose of preserving and delivering mRNA (Colombani et al., 2017; Desigaux et al., 2007; Habrant et al., 2016; Lindsay et al., 2019). The labeled mRNA was complexed with aminoglycoside lipidic derivative CholK which contained a kanamycin polar head group and a cholesterol moiety as a hydrophobic group. At low mRNA-to-nanoparticle charge ratios, it has previously been demonstrated by this group that these nanoparticles are capable of entering cells via clathrin-mediated endocytosis, after which they are released in the cell and efficiently undergo translation.
Following right quadricep injection of the nanovaccine into the primates, imaging occurred with whole-body PET/CT after 4 and 28 h (Lindsay et al., 2019). It was found that at 4 h, the vaccine was able to reach inguinal, iliac, and para-aortic lymph nodes, with the distance between the injection site and the farthest lymph node being 9.2 cm away. With the 4 h PET/CT images they obtained, they were able to conclude that the vaccine travels from the injection site to the inguinal, iliac, and then para-aortic lymph nodes in that order, while remaining on the ipsilateral side of injection. Additionally, images at 28 h enabled longitudinal monitoring, which showed that while mRNA reaches the lymph nodes quickly, it continues to accumulate at least up to 28 h.
Fluorescence imaging then occurred ex vivo 28 h after injection (Lindsay et al., 2019). A handheld NIR imaging device was used to identify tissue with mRNA uptake during autopsy. In general, they were able to find one lymph node with mRNA signal in each anatomical region they imaged. However, they compared the PET signal and NIRF in the analyzed regions, and after verifying that PET signal was not due to dissociation of the radioisotope from the nanovaccine, determined that PET was able to identify mRNA in particular lymph nodes that were not captured by NIRF, indicating the potential superiority of PET imaging over fluorescence imaging when compared head-to-head with their methodology.
5.2 |. MRI and optical imaging
Magnetic resonance imaging (MRI) is a primarily (but not exclusively) anatomical imaging modality based on nuclear magnetic resonance (NMR) (Geethanath & Vaughan, 2019; Grover et al., 2015). Its application to molecular imaging is not nearly as prominent as its application to anatomical imaging. But to understand what kinds of molecules and nanoparticles can be imaged by MRI, one should understand how MRI images are obtained in the first place. Very briefly, a strong external magnetic field applied to a material will align nuclear spins either along or against the direction of the field while making them “wobble” or precess about this axis, at a frequency proportional to the strength of the magnetic field referred to as the Larmor frequency. Spins aligned in different directions in a magnetic field obtain an energy difference with respect to one another, which can be exploited to provide information about the density of nuclear spins in a region and therefore provide imaging data. If a radiofrequency magnetic field is now applied perpendicular to the original one, then nuclei can be excited and jump between energy levels, either absorbing radiofrequency radiation or emitting it, which can induce a voltage in a nearby coil and be used to record a signal. The radiofrequency magnetic field is usually pulsed, and its frequency is varied providing a pulsing signal intensity as a function of the frequency of the magnetic field, which is reconstructed to form an image that provides the density of nuclear spins in a region of interest (ROI).
Unlike popular molecular imaging modalities such as PET and SPECT, MRI does not utilize tracers. The closest analogue of a tracer in MRI is a contrast agent (Cruz et al., 2011; Hengerer & Grimm, 2006). These contrast agents are typically paramagnetic (they should not be magnetic, because MRIs produce intense magnetic fields). While this means that there are more MRI contrast agents than MPI tracers (of which are only SPIONs), molecular imaging is not as readily performed with MRI as tracer-based modalities. It is for this reason that MRI is not generally employed alone in the imaging of nanovaccines.
Nevertheless, MRI can contribute to imaging of nanovaccines. For instance, Cruz et al (Cruz et al., 2011). reported an antibody-guided nanovaccine system with the intention of vaccine delivery directly to the dendritic cells, the significance of which was covered earlier in the review, and also carries SPIONs for the sole purpose of MRI contrast imaging. However, in addition to this, they also employed fluorescently labeled antigens. This nanovaccine system was tested with in vitro and ex vivo, but not in animal models. Nevertheless, the justification to use MRI rather than other forms of molecular imaging in this context is not trivial, as MRI does not involve ionizing radiation and is therefore a safer imaging modality than PET. Additionally, SPIONs are already clinically approved and used as MRI contrast agents. Specifically, the nanovaccine consists of poly(D, L-lactide-co-glycolide) (PLG) which carried SPIONS as well as a peptide antigen, the TT epitope which was used in the tetanus vaccine, labeled by FITC to make SPIO-TT-FITC. A crucial aspect of this design, the fluorescent label did not prevent the antigens from being presented to antigen-specific T-cells. The antibody is humanized anti-DC-SIGN, which recognizes the human dendritic cell-specific antigen receptor DC-SIGN. In an uptake and binding assay of this nanovaccine in dendritic cells, SPIONs were identified by MRI at the subcellular level within endolysosomal compartments within 24 h. This was confirmed by FITC-TT presence using confocal microscopy. Additionally, MRI imaging allowed real time monitoring of dendritic cell migration via lymphatic drainage into lymph nodes in an ex vivo collagen scaffold model. MRI therefore played a crucial role in evaluating a nanovaccine completely absent of animal models, and its findings were confirmed by fluorescence imaging.
Another study by Zhao et al. (2019) utilizes NIRF and MRI to image a nanovaccine based upon a nanoscale coordination polymer (NCP) of Mn2+ and nucleotide oligomerization binding domain 1 (Nod1) agonist, with Mn2+ being a natural MRI contrast agent. As is common practice in the evaluation of developmental vaccine platforms, they employ this vaccine to deliver OVA with the Nod1 agonist as the adjuvant. A nanovaccine delivery system for Nod1 is particularly interesting. It has been shown that activation of the Nod1 signal pathway improves innate and adaptive immune responses. Despite this, delivery of the Nod1 agonist has been a challenge since the Nod1 receptor is not located on the cell surface but rather within the cell. These nanoparticles are thus designed to deliver both OVA and Nod1 to DCs. The schematic of this study is shown in Figure 6a. The nanoparticle consists of this NCP and meso-2,6-diaminopimelic acid (DAP), which, at blood pH levels, forms a nanoparticle which can encapsulate OVA. The full nanoparticle loaded with OVA is referred to as OVA@Mn-DAP. Now, MRI imaging occurred readily with the employment of Mn2+, and NIRF imaging also occurred readily with the simple adjustment of the use of OVA-Cy5.5 rather than plain OVA. Importantly, following footpad injection in mice, both NIRF and MRI imaging allowed quantification of OVA-Cy5.5 presence in the popliteal lymph nodes at various time points. The images collected are shown in Figure 6b. They were thus able to see that OVA@Mn-DAP nanoparticles accumulated satisfactorily in popliteal lymph nodes, which justified their findings in the use of OVA@Mn-DAP for treatment of mice with B16-OVA tumors. Specifically, they found that combination of anti-programmed cell death protein 1 antibody (a-PD-1) immune checkpoint blockage therapy and OVA@Mn-DAP effectively inhibited growth of already-established tumors.
FIGURE 6.
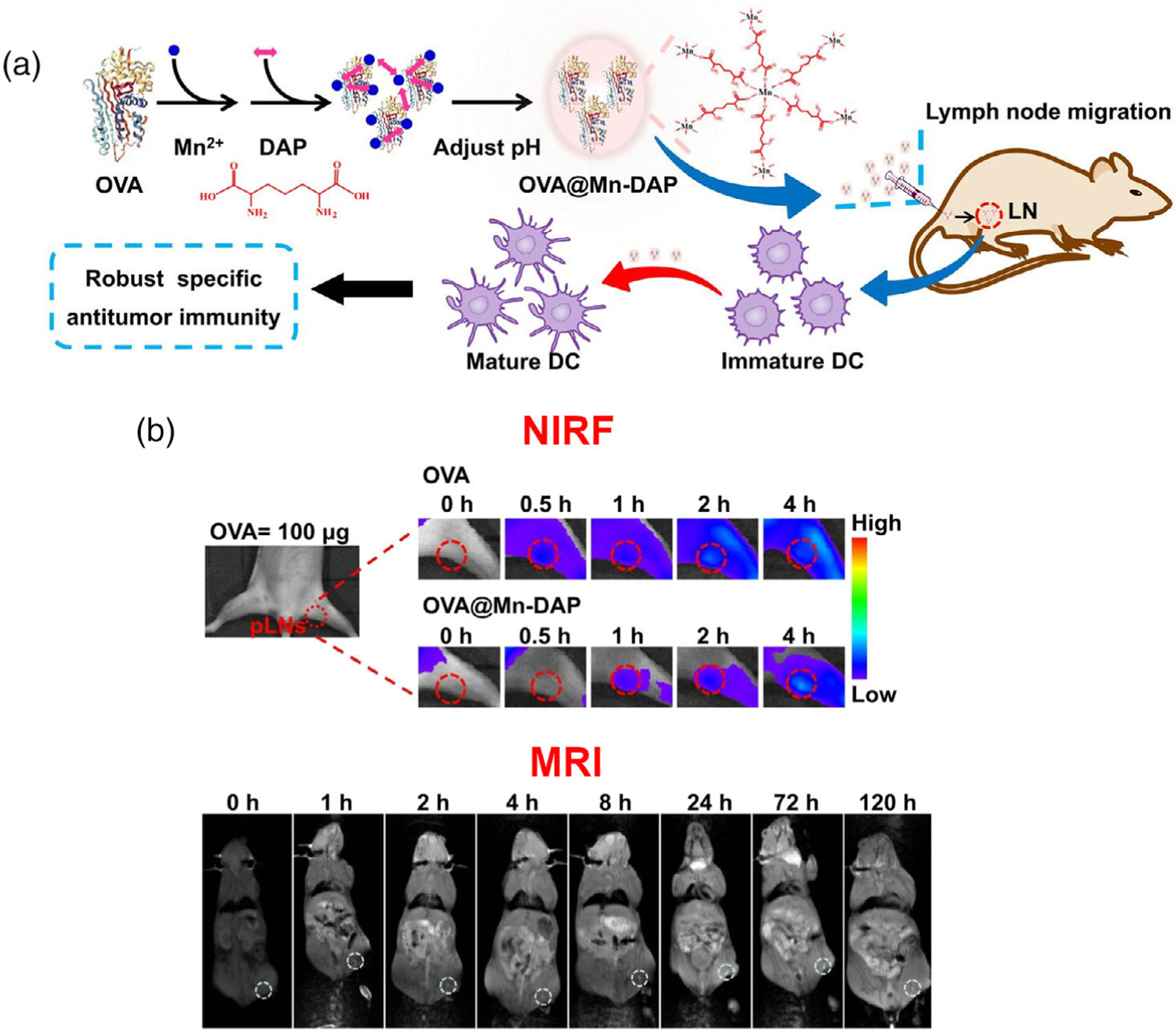
(a) Schematic of the study design by Zhao et al. OVA@Mn-DAP nanoparticles are formed, carrying the model antigen OVA and act as natural MRI contrast agents due to Mn2+ incorporation or fluorophores if OVA-Cy5.5 is used. Following synthesis, this nanovaccine is tested in vivo to analyze antitumor immunity. (b) Side-by-side comparison of NIRF and MRI of OVA@Mn-DAP nanovaccine in mice, time points p.i. in the footpad are above the images. Both imaging modalities demonstrate accumulation of nanovaccine in popliteal lymph nodes. Adapted from Zhao et al. (2019).
5.3 |. Optical imaging and photoacoustic imaging
Photoacoustic (PA) imaging is a young and lesser-known modality. To our knowledge, as of writing this there is only one study involving nanovaccine imaging with PA imaging, so this section focuses on that one study. However, the study, by Liang et al. (2017) also utilizes NIRF imaging because it can conveniently be achieved. Nevertheless, as an imaging modality PA draws upon principles from both optical imaging and ultrasound (Beard, 2011). Briefly, the basis of PA is the internal production and subsequent measurement of ultrasound waves by tissue and other structures irradiated with pulsing NIR light. Again, this frequency of light is favorable due to high tissue penetration. Additionally, the resolution of this modality is highly variable depending on the tissue or structure being imaged as well as the depth, but typically for depths on the order of 1 cm, resolutions of <1 mm are possible. Less depth then corresponds to better resolution. It is also important to note that PA utilizes contrast agents, of which metallic nanostructures of various geometries are some of the most useful as opposed to other contrast agents such as organic dyes. The reason is that these nanoparticles exhibit a great absorption cross section for surface plasmon resonance when exposed to incident NIR light, which can result in ultrasound wave emission and thus a higher signal.
Liang et al. (2017) designed a cancer vaccine for melanoma. A schematic of the study design is shown in Figure 7. The vaccine is designed to carry an immunogen, melanoma antigen peptide TRP2, and an adjuvant, MPLA, which are carried by liposome-coated gold nanocages (Lipos-AuNCs). Using the DC specific antibody aCD11c, this nanovaccine is meant to deliver the immunogen and adjuvant directly to the DCs. Gold nanocages (AuNCs) are attractive nanostructures for this application since their structure allows drugs to be loaded, and their exterior can be modified with polymers or targeting ligands, such as in this study with liposomes and antibodies. The benefit of using liposomes is that they can protect TRP2 from degradation and leakage. Regarding imaging, AuNCs are excellent agents for PA imaging, meaning that it is a natural way to image this vaccine. However, the authors also conjugated NIRF agent FITC to the surface of these nanocages, a well-known capability of AuNCs (Pang et al., 2016), enabling this vaccine platform for convenient NIRF imaging.
FIGURE 7.
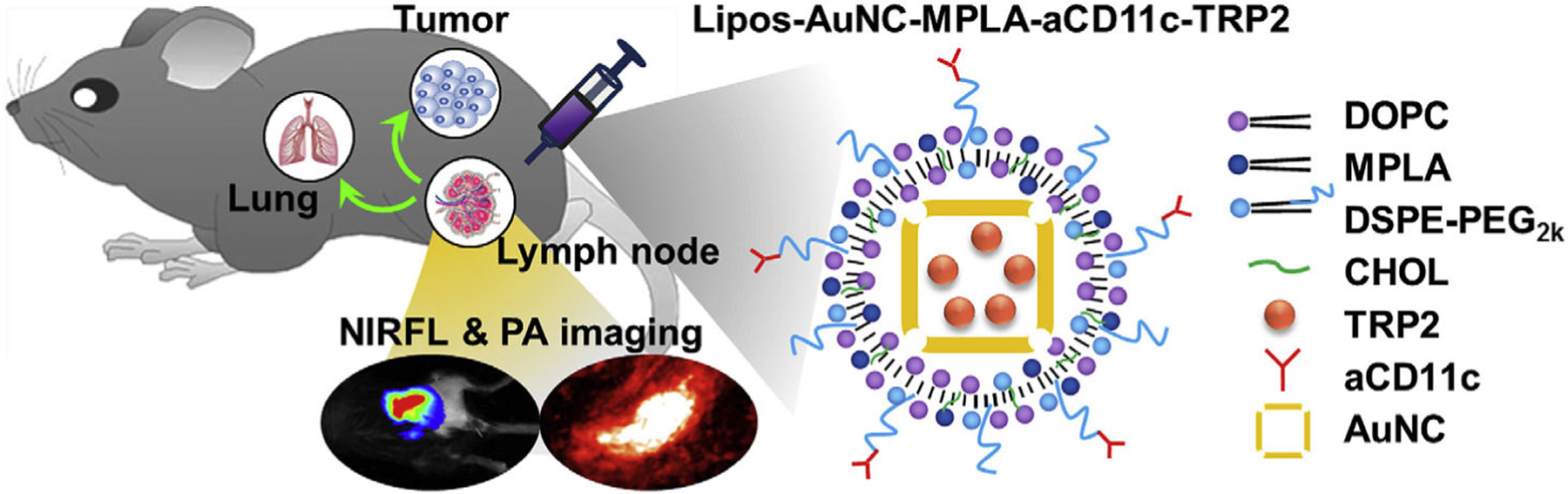
Schematic of the study by Liang et al., in which a fluorescently labeled gold nanocage-based nanovaccine (capable of both fluorescence and PA imaging) is injected in mice to deliver a melanoma antigen and adjuvant. Adapted with permission from Liang et al. (2017).
6 |. CONCLUSION
We have reviewed various unique approaches to the use of nanoparticles in the delivery of immunogenic and adjuvant compounds, highlighting the capability and importance of these nanoparticles being imaged for biodistribution and evaluation of the nanovaccine. In particular, we focused on optical imaging, PET, SPECT, the much lesser known but nonetheless promising MPI modality, and the contributions of MRI and PA to multi-modality imaging. In the literature, it is seen that NIRF and PET are far more common than other modalities. The reason for this is straightforward: the former is a powerful approach when working with biomolecules such as antigens, while the latter is powerful when working with nanoparticles which can be synthesized with a chelator or radiolabeled directly. Both imaging modalities are accessible, informative, and have enough resolution to suit the needs of nanovaccine imaging. The downfall of MPI is not only that it is currently very uncommon in most research facilities, nor that it is clinically unexplored, but also that it is somewhat limited to one very specific type of nanoparticle, the SPION. Nevertheless, it is an elegantly designed modality with potentially unparalleled spatial resolution for molecular imaging, which may certainly find its niche in the near future. It is hoped that, by getting an overview of what kinds of nanovaccines have been developed, and subsequently how imaging was pursued, the reader is able to understand the design process and is therefore now more equipped to delve more deeply into the literature.
In the future, vaccines will likely play an increasingly important role in modern medicine, and as such, nanoparticle-based design and imaging of nanovaccines will be useful for development, characterization, and validation of vaccines (Hou et al., 2021; Luo et al., 2017). As we have seen with the COVID-19 pandemic, development of mRNA-based vaccines is becoming a booming field, and methods of nanoplatform design and imaging will play a key role. Importantly, due to the fragility of mRNA against degradation in vivo, delivery is of utmost importance, creating an opportunity for nanotechnology most generally to take a pivotal role in future research. Many lipid-based nanoparticles facilitate intrinsic radiolabeling with PET and SPECT radioisotopes, depending on the phospholipid being used. Besides this, techniques in cancer vaccines are becoming more popular, as advanced techniques in biology are building a stronger understanding of basic immunology, particularly surrounding cancer immunology. With a greater understanding of cancer immunology, as well as new synthesis methods as part of ongoing research in nanomaterials, cancer nanovaccines can be expected to become increasingly popular methods of targeted molecular therapy, therefore creating the space for imaging of these new nanovaccines. Otherwise, there are plenty of clever methods which have and will be developed to image with a variety of other modalities, such as those mentioned in this review.
This article is categorized under:
Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Therapeutic Approaches and Drug Discovery > Nanomedicine for Infectious Disease
Funding information
The authors are grateful for financial support from Focus-X Therapeutics, Inc., the University of Wisconsin—Madison, and the National Institutes of Health (P30CA014520).
Footnotes
CONFLICT OF INTEREST
Weibo Cai is a scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc. All other authors declare that they have no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Anderson CJ, & Ferdani R (2009). Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biotherapy & Radiopharmaceuticals, 24(4), 379–393. 10.1089/cbr.2009.0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne-Jones S (1917). Equilibria in precipitin reactions: The coexistence of a single free antigen and its antibody in the same serum. The Journal of Experimental Medicine, 25(6), 837–853. 10.1084/jem.25.6.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard P (2011). Biomedical photoacoustic imaging. Interface Focus, 1(4), 602–631. 10.1098/rsfs.2011.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, & Gladney WL (2015). Immune escape mechanisms as a guide for cancer immunotherapy. Clinical Cancer Research, 21(4), 687–692. 10.1158/1078-0432.CCR-14-1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, & Krummel MF (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine, 24(5), 541–550. 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Jeong JC, Kim J, Lim EG, Kim CB, Park SJ, Song DY, Krause HJ, Hong H, & Kweon IS (2020). A novel three-dimensional magnetic particle imaging system based on the frequency mixing for the point-of-care diagnostics. Scientific Reports, 10(1), 11833. 10.1038/s41598-020-68864-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani T, Peuziat P, Dallet L, Haudebourg T, Mével M, Berchel M, Lambert O, Habrant D, & Pitard B (2017). Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery. Journal of Controlled Release, 249, 131–142. 10.1016/j.jconrel.2017.01.041 [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Tacken PJ, Bonetto F, Buschow SI, Croes HJ, Wijers M, de Vries IJ, & Figdor CG (2011). Multimodal imaging of nanovaccine carriers targeted to human dendritic cells. Molecular Pharmaceutics, 8(2), 520–531. 10.1021/mp100356k [DOI] [PubMed] [Google Scholar]
- Deluco B, & Wilson HL (2021). Assessment of intestinal macromolecular absorption in young piglets to pave the way to oral vaccination: Preliminary results. Veterinary Research Communications, 46, 79–91. 10.1007/s11259-021-09831-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desigaux L, Sainlos M, Lambert O, Chevre R, Letrou-Bonneval E, Vigneron JP, Lehn P, Lehn JM, & Pitard B (2007). Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proceedings of the National Academy of Sciences of the United States of America, 104(42), 16534–16539. 10.1073/pnas.0707431104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CA, Ni D, Rosenkrans ZT, & Cai W (2019). Radionuclide-activated nanomaterials and their biomedical applications. Angewandte Chemie (International Ed. in English), 58(38), 13232–13252. 10.1002/anie.201900594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geethanath S, & Vaughan JT (2019). Accessible magnetic resonance imaging: A review. Journal of Magnetic Resonance Imaging, 49(7), e65–e77. 10.1002/jmri.26638 [DOI] [PubMed] [Google Scholar]
- Gheibi Hayat SM, & Darroudi M (2019). Nanovaccine: A novel approach in immunization. Journal of Cellular Physiology, 234(8), 12530–12536. 10.1002/jcp.28120 [DOI] [PubMed] [Google Scholar]
- Gong N, Zhang Y, Teng X, Wang Y, Huo S, Qing G, Ni Q, Li X, Wang J, Ye X, Zhang T, Chen S, Wang Y, Yu J, Wang PC, Gan Y, Zhang J, Mitchell MJ, Li J, & Liang XJ (2020). Proton-driven transformable nanovaccine for cancer immunotherapy. Nature Nanotechnology, 15(12), 1053–1064. 10.1038/s41565-020-00782-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeser M, Thieben F, Szwargulski P, Werner F, Gdaniec N, Boberg M, Griese F, Möddel M, Ludewig P, van de Ven D, Weber OM, Woywode O, Gleich B, & Knopp T (2019). Human-sized magnetic particle imaging for brain applications. Nature Communications, 10(1), 1936. 10.1038/s41467-019-09704-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover VP, Tognarelli JM, Crossey MM, Cox IJ, Taylor-Robinson SD, & McPhail MJ (2015). Magnetic resonance imaging: Principles and techniques—Lessons for clinicians. Journal of Clinical and Experimental Hepatology, 5(3), 246–255. 10.1016/j.jceh.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habrant D, Peuziat P, Colombani T, Dallet L, Gehin J, Goudeau E, Evrard B, Lambert O, Haudebourg T, & Pitard B (2016). Design of ionizable lipids to overcome the limiting step of endosomal escape: Application in the intracellular delivery of mRNA, DNA, and siRNA. Journal of Medicinal Chemistry, 59(7), 3046–3062. 10.1021/acs.jmedchem.5b01679 [DOI] [PubMed] [Google Scholar]
- Han S, Ma W, Jiang D, Sutherlin L, Zhang J, Lu Y, Huo N, Chen Z, Engle JW, Wang Y, Xu X, Kang L, Cai W, & Wang L (2021). Intracellular signaling pathway in dendritic cells and antigen transport pathway in vivo mediated by an OVA@DDAB/PLGA nano-vaccine. Journal of Nanobiotechnology, 19(1), 394. 10.1186/s12951-021-01116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M, & Klaunberg BA (2004). Biomedical applications of fluorescence imaging in vivo. Comparative Medicine, 54(6), 635–644. [PubMed] [Google Scholar]
- Hengerer A, & Grimm J (2006). Molecular magnetic resonance imaging. Biomedical Imaging and Intervention Journal, 2(2), e8. 10.2349/biij.2.2.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins FG (1900). On the separation of a pure albumin from egg-white. The Journal of Physiology, 25(4), 306–330. 10.1113/jphysiol.1900.sp000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Zaks T, Langer R, & Dong Y (2021). Lipid nanoparticles for mRNA delivery. Nature Reviews Materials, 1–17, 1078–1094. 10.1038/s41578-021-00358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HY, & Du HN (2000). Alpha-to-beta structural transformation of ovalbumin: Heat and pH effects. Journal of Protein Chemistry, 19(3), 177–183. 10.1023/a:1007099502179 [DOI] [PubMed] [Google Scholar]
- Katagiri W, Lee JH, Tétrault MA, Kang H, Jeong S, Evans CL, Yokomizo S, Santos S, Jones C, Hu S, El Fakhri G, Kosuke T, Choi HS, & Kashiwagi S (2019). Real-time imaging of vaccine biodistribution using zwitterionic NIR nanoparticles. Advanced Healthcare Materials, 8(15), e1900035. 10.1002/adhm.201900035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Chang JH, & Kang YJ (2012). Efficient internalization of peptide-conjugated SPIONs in dendritic cells for tumor targeting. Journal of Nanoscience and Nanotechnology, 12(7), 5191–5198. 10.1166/jnn.2012.6379 [DOI] [PubMed] [Google Scholar]
- Liang R, Xie J, Li J, Wang K, Liu L, Gao Y, Hussain M, Shen G, Zhu J, & Tao J (2017). Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials, 149, 41–50. 10.1016/j.biomaterials.2017.09.029 [DOI] [PubMed] [Google Scholar]
- Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, Xiao P, Araínga M, Shirreff LM, Pitard B, Baumhof P, Villinger F, & Santangelo PJ (2019). Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nature Biomedical Engineering, 3(5), 371–380. 10.1038/s41551-019-0378-3 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, Zhang Y, Chen S, Tiwari S, Shi K, Zhang S, Fan HM, Zhao YX, & Liang XJ (2020). Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics, 10(8), 3793–3815. 10.7150/thno.40805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Goel S, Liu HJ, Carter KA, Jiang D, Geng J, Kutyreff CJ, Engle JW, Huang WC, Shao S, Fang C, Cai W, & Lovell JF (2017). Intrabilayer 64Cu labeling of photoactivatable, doxorubicin-loaded stealth liposomes. ACS Nano, 11(12), 12482–12491. 10.1021/acsnano.7b06578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis H, Deidda D, Gillman A, Willowson KP, Gholami Y, Hioki T, Eslick E, Thielemans K, & Bailey DL (2021). Theranostic SPECT reconstruction for improved resolution: Application to radionuclide therapy dosimetry. EJNMMI Physics, 8(1), 16. 10.1186/s40658-021-00362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JT, Hartwell BL, Kumarapperuma SC, Melo MB, Carnathan DG, Cossette BJ, Adams J, Gong S, Zhang W, Tokatlian T, Menis S, Schiffner T, Franklin CG, Goins B, Fox PT, Silvestri G, Schief WR, Ruprecht RM, & Irvine DJ (2021). Combined PET and whole-tissue imaging of lymphatic-targeting vaccines in non-human primates. Biomaterials, 275, 120868. 10.1016/j.biomaterials.2021.120868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Liang L, & Veiseh O (2020). Recent advancements of magnetic nanomaterials in cancer therapy. Pharmaceutics, 12(2), 147–167. 10.3390/pharmaceutics12020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D, Bu W, Ehlerding EB, Cai W, & Shi J (2017). Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chemical Society Reviews, 46(23), 7438–7468. 10.1039/c7cs00316a [DOI] [PMC free article] [PubMed] [Google Scholar]
- No Author. (2021). Lasting impact of lipid nanoparticles. Nature Reviews Materials, 6(1071). 10.1038/s41578-021-00398-6 [DOI] [Google Scholar]
- Pang B, Yang X, & Xia Y (2016). Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomedicine (London, England), 11(13), 1715–1728. 10.2217/nnm-2016-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persano S, Das P, & Pellegrino T (2021). Magnetic nanostructures as emerging therapeutic tools to boost anti-tumour immunity. Cancers (Basel), 13(11), 2735–2766. 10.3390/cancers13112735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TR, Dickgreber N, & Hermans IF (2010). Tumor antigen presentation by dendritic cells. Critical Reviews in Immunology, 30(4), 345–386. 10.1615/critrevimmunol.v30.i4.30 [DOI] [PubMed] [Google Scholar]
- Pollard AJ, & Bijker EM (2021). A guide to vaccinology: From basic principles to new developments. Nature Reviews. Immunology, 21(2), 83–100. 10.1038/s41577-020-00479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo T, Akter F, Nomura Y, Louie AY, & Yokobayashi Y (2018). Firefly luciferase mutant with enhanced activity and thermostability. ACS Omega, 3(3), 2628–2633. 10.1021/acsomega.7b02068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Rodriguez A, Hoang-Minh LB, Chiu-Lam A, Sarna N, Marrero-Morales L, Mitchell DA, & Rinaldi-Ramos CM (2021). Tracking adoptive T cell immunotherapy using magnetic particle imaging. Nano, 5(4), 431–444. 10.7150/ntno.55165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-de-Angulo A, Zabaleta A, Gómez-Vallejo V, Llop J, & Mareque-Rivas JC (2016). Microdosed lipid-coated (67)Ga-magnetite enhances antigen-specific immunity by image tracked delivery of antigen and CpG to lymph nodes. ACS Nano, 10(1), 1602–1618. 10.1021/acsnano.5b07253 [DOI] [PubMed] [Google Scholar]
- Saxena M, van der Burg SH, Melief CJM, & Bhardwaj N (2021). Therapeutic cancer vaccines. Nature Reviews. Cancer, 21(6), 360–378. 10.1038/s41568-021-00346-0 [DOI] [PubMed] [Google Scholar]
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz P, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, van Cutsem E, … Krenning E (2017). Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. The New England Journal of Medicine, 376(2), 125–135. 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Ke C, Rao L, Lau J, & Chen X (2020). Multimodal stratified imaging of nanovaccines in lymph nodes for improving cancer immunotherapy. Advanced Drug Delivery Reviews, 161–162, 145–160. 10.1016/j.addr.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Tiselius A, & Kabat EA (1939). An electrophoretic study of immune sera and purified antibody preparations. The Journal of Experimental Medicine, 69(1), 119–131. 10.1084/jem.69.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa K, Matsui A, Nakamura Y, Citterio D, & Suzuki K (2009). Bright, color-tunable fluorescent dyes in the Vis/NIR region: Establishment of new “tailor-made” multicolor fluorophores based on borondipyrromethene. Chemistry, 15(5), 1096–1106. 10.1002/chem.200801906 [DOI] [PubMed] [Google Scholar]
- Vaquero JJ, & Kinahan P (2015). Positron emission tomography: Current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annual Review of Biomedical Engineering, 17, 385–414. 10.1146/annurev-bioeng-071114-040723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, … Kwon BS (2015). Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in Cancer Biology, 35, S185–S198. 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Welch MJ, Hawker CJ, & Wooley KL (2009). The advantages of nanoparticles for PET. Journal of Nuclear Medicine, 50(11), 1743–1746. 10.2967/jnumed.109.061846 [DOI] [PubMed] [Google Scholar]
- Wernick MN, & Aarsvold JN (2004). Emission tomography: The fundamentals of PET and SPECT Amsterdam, Boston, Elsevier Academic Press. [Google Scholar]
- Wu LC, Zhang Y, Steinberg G, Qu H, Huang S, Cheng M, Bliss T, Du F, Rao J, Song G, Pisani L, Doyle T, Conolly S, Krishnan K, Grant G, & Wintermark M (2019). A review of magnetic particle imaging and perspectives on neuroimaging. AJNR. American Journal of Neuroradiology, 40(2), 206–212. 10.3174/ajnr.A5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NN, Li XF, Deng YQ, Zhao H, Huang YJ, Yang G, Huang WJ, Gao P, Zhou C, Zhang RR, Guo Y, Sun SH, Fan H, Zu SL, Chen Q, He Q, Cao TS, Huang XY, Qiu HY, … Qin CF (2020). A thermostable mRNA vaccine against COVID-19. Cell, 182(5), 1271–1283.e1216. 10.1016/j.cell.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y, Zhou H, Peng R, Wang J, & Liu Z (2019). Nanoscale coordination polymer based nanovaccine for tumor immunotherapy. ACS Nano, 13(11), 13127–13135. 10.1021/acsnano.9b05974 [DOI] [PubMed] [Google Scholar]
- Zhong X, Zhang Y, Tan L, Zheng T, Hou Y, Hong X, Du G, Chen X, Zhang Y, & Sun X (2019). An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. Journal of Controlled Release, 300, 81–92. 10.1016/j.jconrel.2019.02.035 [DOI] [PubMed] [Google Scholar]
- Zhou XY, Tay ZW, Chandrasekharan P, Yu EY, Hensley DW, Orendorff R, Jeffris KE, Mai D, Zheng B, Goodwill PW, & Conolly SM (2018). Magnetic particle imaging for radiation-free, sensitive and high-contrast vascular imaging and cell tracking. Current Opinion in Chemical Biology, 45, 131–138. 10.1016/j.cbpa.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, & Sevick-Muraca EM (2015). A review of performance of near-infrared fluorescence imaging devices used in clinical studies. The British Journal of Radiology, 88(1045), 20140547. 10.1259/bjr.20140547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, Ma Y, Zhang F, Tian R, Ni Q, Cheng S, Wang Z, Lu N, Yung BC, Wang Z, Lang L, Fu X, Jin A, Weiss ID, … Chen X (2017). Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nature Communications, 8(1), 1954. 10.1038/s41467-017-02191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


