Abstract
Background
HIV preexposure prophylaxis (PrEP) remains underutilized despite its efficacy and potential population impact. Achieving PrEP’s full potential depends upon providers who are knowledgeable and comfortable prescribing it to individuals at risk of acquiring HIV. Previous educational interventions targeting provider-related uptake barriers have had limited success. We designed and tested an electronic medical record (EMR) interpretative comment to improve the delivery of PrEP.
Methods
An EMR comment provided information on PrEP eligibility and referral resources to providers delivering positive chlamydia and gonorrhea results. Positive tests for bacterial sexually transmitted infections pre-intervention (01/01/19 – 08/23/19) and post-intervention (08/24/19 – 12/31/19) were identified. A retrospective chart review was conducted to ascertain provider documentation of PrEP discussions or provision, HIV prevention discussions, and HIV screening. Pretest-posttest analysis was performed to compare the provision of PrEP and HIV prevention services.
Results
We reviewed 856 pre-intervention encounters spanning eight months and 461 post-encounters spanning four months. Patient demographics were comparable. We observed an increase in provider documentation of safe sex and condom counseling (respectively, OR 1.2, 95% CI 1.07–1.18: OR 1.11, 95% CI 1.05–1.17), and the absence of any HIV prevention discussion decreased (OR 0.85, 95% CI 0.80–0.90), but not HIV screening or PrEP documentation.
Conclusions
We demonstrated that an EMR lab comment had a modest effect on increasing risk reduction counseling, though not HIV screening or PrEP prescriptions. Future strategies to encourage provider delivery of sexual health services may benefit from more targeted strategies that combine behavioral and IT approaches.
Keywords: preexposure prophylaxis, HIV prevention, electronic medical record, STI testing
Summary:
An interpretative lab comment added to positive STI results in the EMR may have increased HIV prevention discussions, though not HIV screening or provision of or referral to PrEP.
Introduction
Despite advances in HIV prevention, including treatment as prevention and the expansion of primary prevention services, HIV remains a persistent public health challenge. In the United States (US), approximately 34,800 new HIV infections occur annually, decreasing by less than 10% since 2015.1 Furthermore, progress has not been uniform, exposing inequalities between subgroups and regions with men who have sex with men (MSM) accounting for 66% of new HIV diagnoses by transmission risk category.1 African American and Hispanic individuals, representing a minority of the US population, constituted 41% and 29% of new HIV diagnoses.1 Additionally, the HIV burden falls heaviest in urban environments, like New York City (NYC).1 Without a cure, primary and secondary prevention remain the only viable pathways toward eradication.
Daily oral pre-exposure prophylaxis (PrEP) effectively reduces acquisition of HIV by 99% when taken consistently.2 When taken consistently, oral combination of emtricitabine-tenofovir reduces the risk of transmission by up to 99%.3 Current CDC guidelines recommend a PrEP discussion as part of comprehensive HIV prevention for all sexually active patients and prioritize facilitating PrEP access for patients at risk of HIV acquisition.4 However, despite its efficacy and prioritization, PrEP continues to be vastly underutilized, particularly among people of color. According to the CDC, only 18.1% of Americans with indications for PrEP receive it.4
Furthermore, less than 6% of African American and 10% of Hispanic individuals with HIV risk factors are prescribed PrEP, revealing disparities in coverage that mirror higher rates of new HIV infections among these communities.4 This slow uptake of PrEP represents missed opportunities for HIV prevention. A retrospective study conducted by our group at a large academic medical center in NYC found that from 2015 to 2017, nearly half (42%) of patients newly diagnosed with HIV had at least one missed opportunity for PrEP and that PrEP was rarely discussed (4.7%) with patients testing positive for a sexually transmitted infections (STI).5,6
To improve PrEP provision at our institution, trainees in Internal Medicine, Pediatrics, Emergency Medicine, and Obstetrics and Gynecology (OB/GYN) were queried to understand potential provider-related uptake barriers and enable focused implementation efforts. While most trainees recognized the importance of HIV testing and linkage to prevention services, few felt comfortable prescribing PrEP or knew where to refer a patient.7 Lack of formal PrEP training, belief that PrEP was outside their scope of practice, inadequate knowledge of referral networks, higher perceived patient care priority issues, and time constraints were cited as top barriers to the provision of PrEP across groups.7 This is consistent with previous studies that found numerous barriers to PrEP delivery among providers, including lack of PrEP awareness and knowledge, negative attitudes towards PrEP, concerns about efficacy, safety, risk compensation, patient adherence, and time commitment.8 While, in our experience, educational efforts have modestly increased PrEP knowledge and referral to PrEP services, these tactics are likely to be more sustainable and have greater efficacy when paired with healthcare system changes.
We designed an intervention to improve the delivery of comprehensive HIV prevention services, including PrEP, at a large urban medical center. Behavioral science suggests that small contextual cues may “nudge” individuals towards the desired behavior.9,10 One example of this tactic is clinical decision support (CDS) tools, such as alerts and messages to providers, which can be embedded within an electronic medical record (EMR). These have been shown to promote behavior change and reduce medical errors.11,12 Interpretive lab comments have also been shown to change antibiotic prescribing behavior.13 We created an EMR lab comment attached to positive bacterial sexually transmitted infection (STI) results to alert providers to potential PrEP eligibility and referral services. These test results were selected for this intervention because STIs are a reliable marker of HIV risk and can be easily identified through EMR review. 14–17 We hypothesized that reminding providers about patients’ PrEP eligibility and lowering the barriers to PrEP delivery through resource sharing would increase the provision of PrEP and other HIV prevention services.
Methods
Study Population
This study was performed at Columbia University Irving Medical Center (CUIMC), a large urban academic medical center consisting of an academic medical center hospital campus in Northern Manhattan, an affiliated community hospital, and multiple ambulatory care sites throughout New York City. The medical center serves a diverse community, in which 72% self-identify as Latino, 47% were born outside the US, and 37% have limited English proficiency.18 Twenty-nine percent of residents have not completed high school, 12% are unemployed, and 20% live below the federal poverty line.18 Despite the proximity to a major medical center, 14% of adults lack health insurance, and 17% report going without needed medical care in the past year.18 The local HIV burden exceeds that of NYC, with 31.1 new HIV diagnoses per 100,000 people.18
Intervention
An EMR alert, constructed as an interpretive lab comment, accompanying positive gonorrhea (GC) and chlamydia (CT) test results. All GC/CT testing was performed on the Hologic Aptima Combo 2 assay, a nucleic acid amplification test (NAAT), and included both genitourinary and extra-genital sites. The EMR alert was designed to inform providers of patients’ potential PrEP eligibility and provide further resource assistance for PrEP prescription or referral. The comment includes a hotline number, email address, and a website URL for the HIV Prevention Program (Figure 1). The comment appears one line below positive test results for permanent reference and went live institution-wide on August 24, 2019. Screening for syphilis, another bacterial STI closely related to increased HIV acquisition, was chosen to serve as a control, and the comment was not included in positive RPR results. Syphilis screening was done using the traditional algorithm that starts with a rapid plasma reagin result (RPR).
Figure 1. Interpretative Lab Comment Alert –

This is the lab comment that appeared in the EMR below positive gonorrhea and chlamydia tests during the post-intervention period.
Data Collection
An electronic sexual health dashboard was used to identify encounters for all patients 18 years or older with positive gonorrhea, chlamydia, and/or RPR test during the 2019 calendar year. A single patient encounter, for review, was defined as the date of patient presentation for STI testing as well as any visits involving the relaying of test results or treatment. Patients could be included more than once if they had multiple independent encounters during the study period. However, to focus on new STI diagnosis, we excluded repeat encounters, defined as patients testing positive for the same organism at the same site of infection within 14 days of the initial encounter. We excluded patients living with HIV and patients already taking PrEP. We also excluded participants in HIV prevention research and patients treated by an Infectious Disease or sexual health providers in our comprehensive health program (CHP). CHP patients were excluded as our mature HIV prevention program has a team of prevention navigators and extremely high rates of PrEP discussions. Patients whose RPR titers were not consistent with new or current infection based on the treating physician’s documentation and those with positive RPR titers exclusively sent as part of a dementia workup were also excluded. Finally, positive results for which there was no clinical data available in the EMR or positive RPR after a workplace needlestick exposure were omitted (Figure 2).
Figure 2. Study Population Prior to and Following Intervention -.
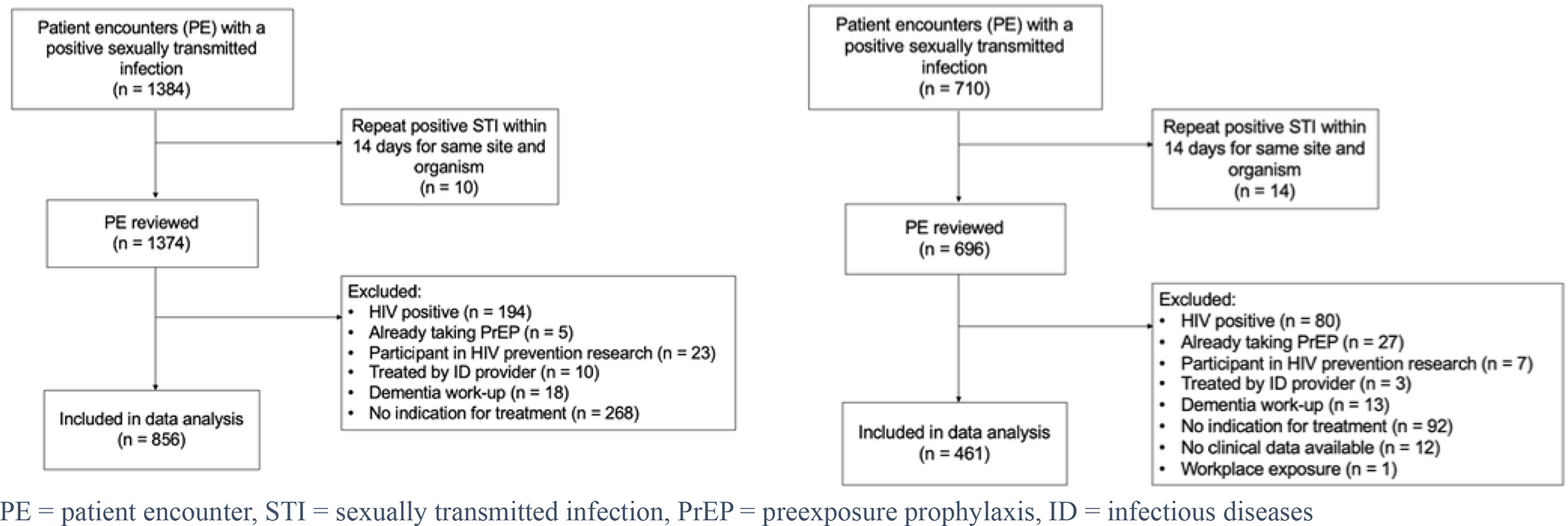
Inclusion and exclusion criteria utilized in this study, 856 pre-intervention and 461 post-intervention encounters were identified to be included in the analysis.
Encounters were divided into two distinct time intervals: January 1, 2019, to August 23, 2019 (pre-intervention) and August 24, 2019, to December 31, 2019 (post-intervention). A retrospective chart review was conducted for each eligible encounter to determine patient demographics and EMR documentation of PrEP prescription, HIV prevention discussion topics, and HIV screening. Two different data abstractors performed chart reviews (one pre-intervention one-post intervention) using a standardized chart abstraction form in REDCap.19 Abstractors were trained and supervised by a single senior team member to ensure consistency and who, as a quality check, reviewed at least 10% of all charts for consistency. All chart reviews were conducted from the electronic medical record; reviews included the entirety of the medical chart, but abstractors were instructed to focus on physician and nursing notes and orders first.
Definition of Outcomes
Our primary outcome was provider documentation of PrEP, including the writing or offer of a PrEP prescription, the placement of a PrEP referral, or documentation of PrEP education during a patient encounter in any other documentation associated with that visit. Secondary outcomes included documentation of additional HIV prevention services, further classified into HIV prevention discussions (primary prevention) and HIV screening (secondary prevention). HIV prevention discussions as a category included safe sex, condoms, or PrEP. Safe sex was defined as any form of STI prevention education, while condoms included recommending or providing condoms and other forms of barrier protection. PrEP included any mention of PrEP, pre-exposure prophylaxis, or medications that prevent HIV. It is important to note that sexual health education is moving away from ambiguous, imprecise, and stigmatizing language such as “unsafe” sex, “risky” sex, and others.20 However, the institutional EMR at this time still had templates that included “safe sex” and many providers used the term; therefore, we chose to include it in our data analysis. In the future, we hope that patient-centered educational interventions will change the way sexual behaviors are conceptualized and documented and lead to the application and use of more neutral and non-stigmatizing language.
Additionally, encounters that did not address PrEP, safe sex, or condoms, were recorded as “no HIV prevention discussion documented.” HIV screening was assessed as the date of the last HIV test before or within one week following a positive STI encounter. Inadequate HIV screening was defined as never having been screened or having been screened greater than 12 months before the positive STI test, based on current CDC guidelines for persons at elevated risk of HIV infection.21
Statistical Analysis
Provision of PrEP and other HIV prevention services were compared pre-and post-intervention using odds ratios with a 95% confidence interval calculation. As a secondary analysis, we divided both the pre-and post-intervention groups into encounters with a positive test result associated with an alert (gonorrhea or chlamydia) and encounters with a positive test result not associated with an alert (control group, RPR for syphilis). SAS University Edition (SAS Institute Inc. 2015. SAS/IML® 14.1) and R version 4.1.2 were used for data cleaning and statistical analyses.
Results
In the pre-intervention period, 1384 encounters were identified, and 856 encounters (815 unique patients) were included. 710 encounters were identified in the post-intervention period, and 461 encounters (450 unique patients) were included (Figure 2).
Patient demographics in the two study intervals were similar, although notable there were more MSM (11% vs. 4%) in the pre-intervention arm. In both, patients had a median age of 24 or 25 and were predominately female. Data on race and ethnicity were incomplete; however, of those reporting, the majority were Hispanic, and nearly one-fourth were African American. Among encounters with available data on sexual orientation (84% pre and 93% post), most patients identified as heterosexual (88% pre vs. 92% post), followed by MSM (11% vs. 4%). (Table 1).Patients presented for STI testing at 57 (pre) and 41 (post) unique locations. The most popular location was the Emergency Department (pre 27.2% vs. post 30.1%), followed by family planning clinics (26.4% vs. 25.5%), an ambulatory clinic for at-risk young men (14.5% vs. 10.9%), and OB/GYN clinics (10.3% vs. 10.5%)
Table 1:
Baseline characteristics of study participants
| Pre-intervention (N=856) | Post-intervention (N=461) | P value** | |
|---|---|---|---|
| Age, median (range) | 24 (IQR 21–29) | 25 (IQR 21 – 30) | 0.6 |
| Sex | 0.13 | ||
| Female | 551 (64) | 316 (69) | |
| Male | 305 (36) | 145 (31) | |
| Ethnicity | 0.5 | ||
| Hispanic | 385 (83) | 241 (81) | |
| Non-Hispanic | 78 (17) | 55 (19) | |
| Unknown | 392 | 165 | |
| Race | |||
| White | 131 (36) | 68 (23) | <.001 |
| Black/African American | 88 (24) | 71 (24) | |
| Other | 145 (40) | 154 (53) | |
| Unknown | 491 | 168 | |
| Sexual Preference | <.001 | ||
| Heterosexual | 631 (88) | 393 (92) | |
| Men who have sex with men | 78 (11) | 19 (4) | |
| Women who have sex with women | 0 (0) | 0 (0) | |
| Bisexual | 8 (1) | 17 (4) | |
| Unknown | 138 | 32 |
Presented as n(%) of known unless otherwise specified.
Pearson’s Chi-squared test; Wilcoxon rank sum test
No change in PrEP documentation was observed when comparing GC/CT (with lab comment) positive encounters (6.6% pre vs. 6.8% post, OR 1.01, CI 0.91–1.12) to RPR (no lab comment) positive encounters (20% pre vs. 7.5% post, OR 0.77, 95% CI 0.56–1.06). (Figure 3, Table 2) In a sensitivity analysis these results were effectively unchanged when removing the 14 patients with concurrent positive GC/CT and RPR encounters and examining patients with only a positive GC/CT or RPR. (Not shown).
Figure 3. Comparison of Outcomes Prior to and Following the Intervention: Discussion and Provision of Primary and Secondary HIV Prevention -.
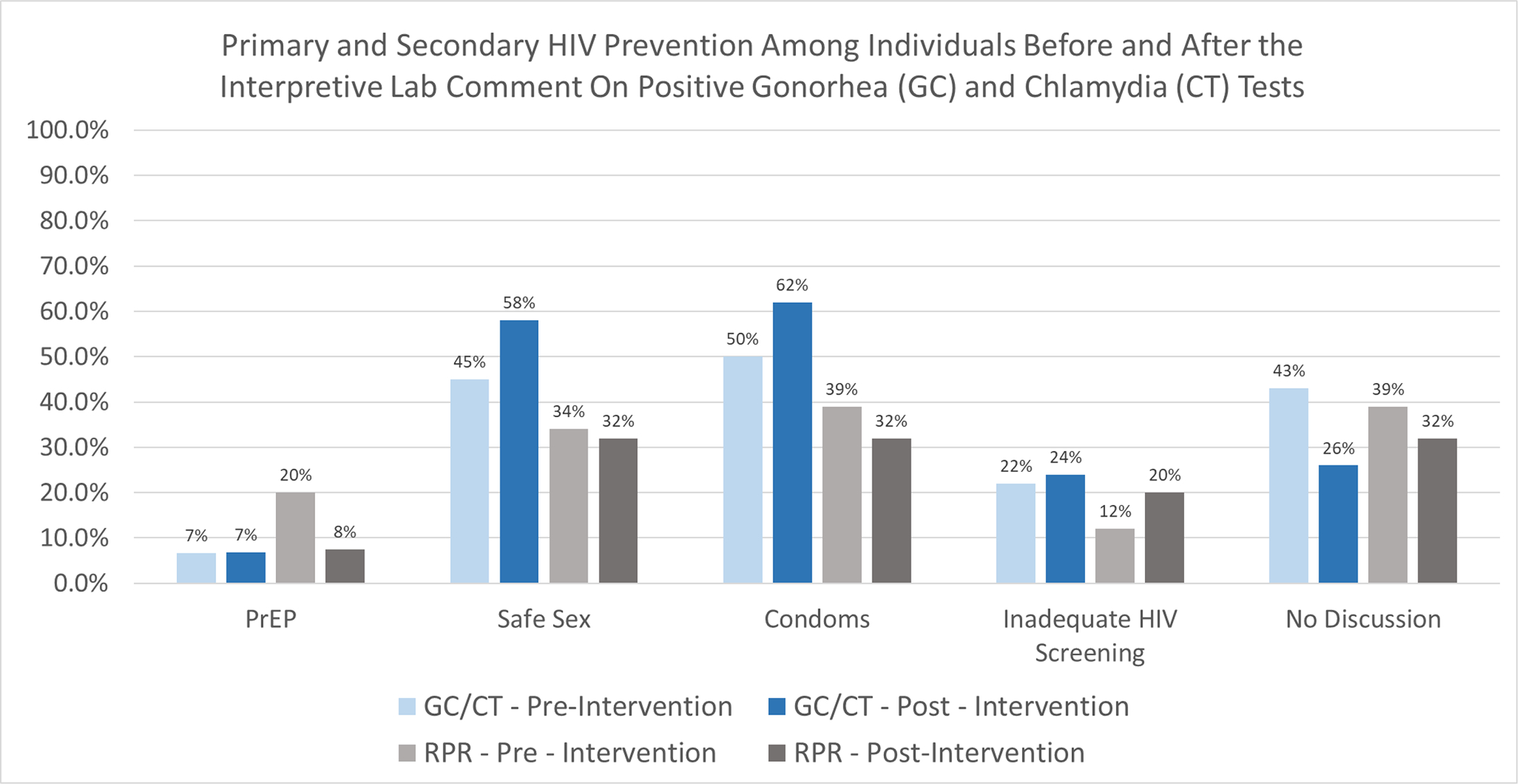
Figure 3 represents the results of the primary analysis, comparing HIV primary and secondary prevention pre- and post-intervention. Percentages represent the percentage of encounters that included discussion or provision of each prevention method. Inadequate HIV screening was defined as never having been screened or having been screened greater than 12 months before the positive STI test.
Table 2:
HIV prevention outcomes
| Pre-intervention (%,n) | Post-intervention (%, n) | OR (95% CI) | |
|---|---|---|---|
| Chlamydia or Gonorrhea Diagnosis | |||
| HIV prevention discussions | N = 822 | N = 427 | |
| PrEP | 6.6% (54) | 6.8% (29) | 1.01 (0.91 – 1.12) |
| Safe sex | 45% (372) | 58% (248) | 1.12 (1.07 – 1.18) |
| Condoms | 50% (415) | 62% (263) | 1.11 (1.05 – 1.17) |
| None | 43% (351) | 26% (110) | 0.85 (0.80 – 0.90) |
| Inadequate HIV screening | 22% (184) | 24% (104) | 1.03 (0.96 – 1.09) |
| Syphilis Diagnosis | |||
| HIV prevention discussions | N = 41 | N = 40 | |
| PrEP | 20% (8) | 7.5% (2) | 0.77 (0.56 – 1.06) |
| Safe sex | 34% (14) | 32% (13) | 0.98 (0.78 – 1.24) |
| Condoms | 39% (16) | 32% (13) | 0.93 (0.74 – 1.17) |
| None | 54% (22) | 60% (24) | 1.07 (0.85 – 1.33) |
| Inadequate HIV screening | 12% (5) | 20% (8) | 1.16 (0.86 – 1.56) |
14 Patients had both a positive GC/CT and RPR at the same visit
Regarding the HIV prevention discussion outcomes, amongst those with a positive GC/CT test provider documentation of safe sex counseling increased from 45% pre- to 58% post-intervention (OR 1.12, 95% CI 1.07–1.18). Likewise, condoms and other barrier protection recommendations or provision increased from 50% to 62% (OR 1.11, 95% CI 1.05–1.17). Absence of any HIV prevention discussion decreased from 43% to 26% (OR 0.85, 95% CI 0.80–0.90). No change in HIV prevention outcomes was noted among the RPR positive encounters group. (Figure 3, Table 2).
Overall, 63.6% of pre-intervention patients encounters had an HIV test compared to 63.8% in the post-intervention period. There was no statistically significant change in the rate of inadequate HIV screening pre- to post-intervention in either the GC/CT or RPR groups (Figure 3, Table 2).
Discussion
Interventions similar to ours, with interpretive lab comments, have been demonstrated to reduce inappropriate antibiotic prescribing.22 However other electronic CDS tools, such as best practice alerts, have previously demonstrated mixed results. CDS tools have been shown to improve compliance with screening guidelines by increasing testing rates for hepatitis B and C and decreasing inappropriate testing for Clostridium difficile.23–25 However, CDS tools have been less effective in reducing inappropriate antibiotic use in several cases.26,27 Given that STI testing is a potent biomarker for HIV risk, we hypothesized that including information about PrEP in the laboratory comment with positive STI results would increase PrEP offers and referrals.
In this retrospective evaluation of an EMR lab comment in patients with a positive bacterial STI result, the inclusion of PrEP information in the lab results did not change the provision of PrEP services. This finding was surprising given the comment’s direct reference to PrEP over the other HIV prevention discussion outcomes. One possible explanation is the use of templates for streamlined documentation in healthcare settings. Many providers note templates include checkboxes for discussion of safe sex and/or condoms but not PrEP. Given this bias in documentation, PrEP education, prescriptions, and referrals may have increased in practice but may not have been adequately captured by our study. Future efforts to enhance PrEP uptake among providers should consider adding PrEP as a standard option to relevant note templates, further encouraging providers to employ this prevention strategy.
Another explanation for these findings is that providers did not see or recognize this comment as relevant. This intervention utilized a lab comment and not a “pop-up” that required recognition. Because of that, it is possible that physicians may not have recognized this as an alert or may not have recognized it as being specific to their patients. While future iterations of this intervention could utilize a “best practice alert,” many of these alerts are still dismissed by physicians. Some reasons that alerts are disregarded in clinical practice are alert fatigue, alerts that are long and challenging to interpret, and alerts without clear clinical consequences.28 While we cannot address alert fatigue in this study, the alert used in this intervention was long, five lines, and the clinical consequences of HIV prevention may be harder to conceptualize. Trying a different clinical decision support configuration and finding ways to shorten the information and provide additional saliency may improve uptake of PrEP associated with this type of intervention.
A more extensive potential explanation for our finding is that providers saw the comment associated with their patient’s positive STI result and were still limited by workflow constraints and the need to address higher priority issues. In the previously referenced survey of residents at our institution, a lack of formal training remained the number one barrier to the provision of pre-exposure prophylaxis.7 While condoms and some other STI prevention strategies are commonly known among the general population, PrEP is a novel concept for many and therefore a more complicated, time-intensive topic to introduce. Given that approximately two-thirds of individuals in this study were women, it is also possible that providers incorrectly perceived them as lower risk for HIV and hence did not prescribe PrEP even with prompting. Anecdotally there may also have been hesitancy to discuss PrEP with pregnant women, although pregnancy data was not captured in this study.
Following our intervention, we appreciated an increase in provider documentation of safe sex counseling (OR 1.58 CI 1.26 – 1.98) and condoms (OR 1.48, CI 1.18–1.86). Similarly, the absence of any kind of HIV prevention discussion between providers and patients significantly decreased, indicating an increase in the number of patients receiving some HIV prevention counseling. In our subgroup analysis, we found that these changes were driven by GC/CT positive patient encounters (i.e., those with an attached alert), with no statistically significant contribution from the RPR positive only group.
Similar to the provision of PrEP services, no change in HIV testing was observed, with similar rates of inadequate HIV screening in the pre-and post-intervention periods. One likely reason for this is timing. In contrast to previous studies that examined the effectiveness of electronic provider alerts on same-day HIV testing, our alert accompanied STI test results that returned several days after the patient encounter.29 HIV testing in this scenario requires an additional visit. Similarly, referral-based models result in higher rates of PrEP-eligible individuals lost to follow-up than same-day start approaches.13,30 Phone calls to relay positive STI results also encourage engagement in HIV prevention discussions but do not allow for HIV testing without an in-person visit.31,32
The ED was the most frequent location for an STI diagnosis but the competing priorities of ED staff often do not allow time for counseling and/or documentation.7,33 Provider interventions are most successful when they are timely and, in this case, recommendations were not provided until after a patient had left the ED and results returned. Point of care (POC) STI testing, with results and immediate recommendations, may allow for improved provider-patient discussions. However, even with POC testing the challenge remains that providing PrEP counseling is time consuming and interventions using less time intensive tasks, like HIV testing, have shown greater success in the ED.34 Finally, at our institution follow-ups are frequently done by separate staff that are unlikely to have any relationship to the patient and may be uncomfortable offering them sexual health services during their first conversation. The ED may require specifically designed interventions and dedicated sexual health counselors if we hope to improve the provision of HIV prevention services in the ED.
This study has a few notable limitations. First, our pre-intervention period ran twice as long as our post-intervention period. Despite this, the two study groups were demographically similar and proportionately distributed between testing sites, reflecting similar sampling from the same population. Additionally, primary data collection in the two study periods was performed by two different researchers. However, outcomes were discussed and agreed upon before chart review, and discrepancies were reviewed by a senior team member supervising both studies. Reporting bias is also possible as providers may have discussed with patients but failed to document PrEP and other comprehensive HIV prevention services in their notes. Conversely, it is possible that this intervention improved documentation of counseling that was already being provided without impacting the amount of counseling being performed. Furthermore, we used patient encounters with only a positive RPR as a control group. They represent encounters with a positive bacterial STI coming from the same population over the same study period but without an EMR alert. While CHP patients were excluded due to our mature HIV prevention program, this may limit the generalizability of this study to infectious diseases and sexual health clinic settings. However, it is also possible that this intervention may be more effective in settings where people are comfortable counseling for and providing HIV prevention services. Additionally, we did not control at the provider level, and it’s possible that providers could be exposed to both a positive GC/CT or RPR from different patients. Controlling at the provider, and ideally the clinic, level and using a control group in which positive GC/CT patient encounters did not receive an alert over the same period would further control for this. Finally, this study only captured HIV testing that occurred within the CUIMC system. Patients who received recent HIV testing elsewhere may have opted out of testing offered by CUIMC providers.
While previous efforts to improve the delivery of PrEP at our institution have focused on provider education to increase confidence in prescribing PrEP, we aimed to influence provider decision-making through a lab comment. We demonstrated that using a lab comment had a modest effect on increasing some HIV prevention services, though not HIV screening or PrEP. Future strategies to further encourage providers towards positive behavior change in the form of PrEP provision may benefit from different clinical decision support configurations and more targeted, resource-intensive behavioral tactics. These include direct provider performance feedback, education on HIV risk for women, especially women of color, and the highlighting of peer or institution-wide norms.
Supplementary Material
Source of Funding
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1AI069470, K23AI150378 and L30AI133789. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
None
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021;26(No. 1). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2021. Acc. [Google Scholar]
- 2.Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019.321(22):2214–30. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012.4(151):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 Update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guideline.
- 5.Zucker J, Patterson B, Ellman T, Slowikowski J, Olender S, Gordon P, et al. Missed Opportunities for Engagement in the Prevention Continuum in a Predominantly Black and Latino Community in New York City. AIDS Patient Care STDS. 2018.32(11):432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yumori C, Zucker J, Theodore D, Chang M, Carnevale C, Slowikowski J, et al. Women Are Less Likely to Be Tested for HIV or Offered Preexposure Prophylaxis at the Time of Sexually Transmitted Infection Diagnosis. Sex Transm Dis. 2021.48(1):32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zucker J, Carnevale C, Theodore D, Castor D, Meyers K, Gold J, et al. Attitudes and Perceived Barriers to Routine HIV Screening and Provision and Linkage of Postexposure Prophylaxis and Pre-Exposure Prophylaxis Among Graduate Medical Trainees. https://home.liebertpub.com/apc. 2021.35(5):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleuhs B, Quinn KG, Walsh JL, Petroll AE, John SA. Health Care Provider Barriers to HIV Pre-Exposure Prophylaxis in the United States: A Systematic Review. AIDS Patient Care STDS. 2020.34(3):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gigerenzer G, Todd PM. Simple Heuristics That Make Us Smart. Oxford University Press; 1999. [Google Scholar]
- 10.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Yale University Press; 2008. [Google Scholar]
- 11.Zsenits B, Polashanski WA, Sterns RH, Brown IV DR, Moheet A. Systematically improving physician assignment during in-hospital transitions of care by enhancing a preexisting hospital electronic health record. J Hosp Med. 2009.4(5):308–12. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead NS, Williams L, Meleth S, Kennedy S, Ubaka-Blackmoore N, Kanter M, et al. The Effect of Laboratory Test-Based Clinical Decision Support Tools on Medication Errors and Adverse Drug Events: A Laboratory Medicine Best Practices Systematic Review. J Appl Lab Med. 2019.3(6):1035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamis KF, Marx GE, Scott KA, Gardner EM, Wendel KA, Scott ML, et al. Same-Day HIV Pre-Exposure Prophylaxis (PrEP) Initiation during Drop-in Sexually Transmitted Diseases Clinic Appointments Is a Highly Acceptable, Feasible, and Safe Model that Engages Individuals at Risk for HIV into PrEP Care. Open Forum Infect Dis. 2019.6(7):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathela P, Braunstein SL, Blank S, Shepard C, Schillinger JA. The High Risk of an HIV Diagnosis Following a Diagnosis of Syphilis: A Population-level Analysis of New York City Men. 2015. [DOI] [PubMed]
- 15.Pathela P, Braunstein SL, Blank S, Schillinger JA. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis. 2013.57(8):1203–9. [DOI] [PubMed] [Google Scholar]
- 16.Peterman TA, Newman DR, Maddox L, Schmitt K, Shiver S. Risk for HIV following a diagnosis of syphilis, gonorrhoea or chlamydia: 328,456 women in Florida, 2000–2011. Int J STD AIDS. 2015.26(2):113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz DA, Dombrowski JC, Bell TR, Kerani RP, Golden MR. HIV incidence among men who have sex with men after diagnosis with sexually transmitted infections. Sex Transm Dis. 2016.43(4):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COMMUNITY HEALTH PROFILES 2018. Washington Heights and Inwood 12.
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009.42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus JL, Snowden JM. Words Matter: Putting an End to “Unsafe” and “Risky” Sex. Sex Transm Dis. 2020.47(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006.55(RR-14):1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 22.Musgrove MA, Kenney RM, Kendall RE, Peters M, Tibbetts R, Samuel L, et al. Microbiology Comment Nudge Improves Pneumonia Prescribing. Open forum Infect Dis. 2018.5(7):ofy162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson MR, Freswick PN, Di Pentima MC, Wang L, Edwards KM, Wilson GJ, et al. The use of a computerized provider order entry alert to decrease rates of clostridium difficile testing in young pediatric patients. Infect Control Hosp Epidemiol. 2017.38(5):542–6. [DOI] [PubMed] [Google Scholar]
- 24.Konerman MA, Thomson M, Gray K, Moore M, Choxi H, Seif E, et al. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology. 2017.66(6):1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSilva MB, Kodet A, Walker PF. A best practice alert for identifying hepatitis B-infected patients. Am J Trop Med Hyg. 2020.103(2):884–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen MJ, Carson PJ, Leedahl DD, Leedahl ND. Failure of a best practice alert to reduce antibiotic prescribing rates for acute sinusitis across an integrated health system in the midwest. J Manag Care Spec Pharm. 2018.24(2):154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrest GN, Van Schooneveld TC, Kullar R, Schulz LT, Duong P, Postelnick M. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin Infect Dis. 2014.59:S122–33. [DOI] [PubMed] [Google Scholar]
- 28.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 13(2):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hechter RC, Bider-Canfield Z, Towner W. Effect of an Electronic Alert on Targeted HIV Testing Among High-Risk Populations. Perm J. 2018.22:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatia R, Modali L, Lowther M, Glick N, Bell M, Rowan S, et al. Outcomes of Preexposure Prophylaxis Referrals from Public STI Clinics and Implications for the Preexposure Prophylaxis Continuum. Sex Transm Dis. 2018.45(1):50–5. [DOI] [PubMed] [Google Scholar]
- 31.Palmer MJ, Henschke N, Villanueva G, Maayan N, Bergman H, Glenton C, et al. Targeted client communication via mobile devices for improving sexual and reproductive health. Cochrane database Syst Rev. 2020.8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacio-Vieira J, Reyes-Urueña JM, Imaz A, Bruguera A, Force L, Llaveria AO, et al. Strategies to reengage patients lost to follow up in HIV care in high income countries, a scoping review. BMC Public Health. 2021.21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucker J, Carnevale C, Theodore DA, Castor D, Meyers K, Gold JAW, et al. Attitudes and Perceived Barriers to Sexually Transmitted Infection Screening Among Graduate Medical Trainees. Sex Transm Dis. 2021.48(10):E149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zucker J, Purpura L, Sani F, Huang S, Schluger A, Ruperto K, et al. Individualized Provider Feedback Increased HIV and HCV Screening and Identification in a New York City Emergency Department. AIDS Patient Care STDS. 2022.36(3):106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


