Abstract
Vancomycin is commonly used to treat methicillin‐resistant Staphylococcus aureus infections and is known to cause nephrotoxicity. Previous Vancomycin Consensus Guidelines recommended targeting trough concentrations but the 2020 Guidelines suggest monitoring vancomycin area under the curve (AUC) given the reduced risk of acute kidney injury (AKI) at similar levels of efficacy. This meta‐analysis compares vancomycin‐induced AKI incidence using AUC‐guided dosing strategies versus trough‐based monitoring. Literature was queried from Medline (Ovid), Web of Science, and Google Scholar from database inception through November 5, 2021. Interventional or observational studies reporting the incidence of vancomycin‐induced AKI between AUC‐ and trough‐guided dosing strategies were included. In the primary analysis, the Vancomycin Consensus Guidelines definition for AKI was used if reported; otherwise, the Risk, Injury, and Failure; and Loss, and End‐stage kidney disease (RIFLE) or Kidney Disease Improving Global Outcomes (KDIGO) definitions were used. The incidence of nephrotoxicity was evaluated between the two strategies using a Mantel–Haenszel random‐effects model, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Subgroup analyses for adjusted ORs and AKI definitions were performed. Heterogeneity was identified using Cochrane's Q test and I 2 statistics. A total of 10 studies with 4231 patients were included. AUC‐guided dosing strategies were associated with significantly less vancomycin‐induced AKI than trough‐guided strategies [OR 0.625, 95% CI (0.469–0.834), p = 0.001; I 2 = 25.476]. A subgroup analysis of three studies reporting adjusted ORs yielded similar results [OR 0.475, 95% CI (0.261–0.863), p = 0.015]. Stratification by AKI definition showed a significant reduction in AKI with the Vancomycin Consensus Guidelines definition [OR 0.552, 95% CI (0.341–0.894), p = 0.016] but failed to find significance in the alternative definitions. Area under the curve‐guided dosing strategies are associated with a lower incidence of vancomycin‐induced AKI versus trough‐guided dosing strategies (GRADE, low). Limitations included the variety of AKI definitions and the potential for confounding bias.
Keywords: acute kidney injury, area under the curve, nephrotoxicity, trough, vancomycin
1. INTRODUCTION
Vancomycin, a glycopeptide antibiotic, is the drug of choice to treat serious methicillin‐resistant Staphylococcus aureus (MRSA) infections and may be used for other susceptible gram‐positive infections. 1 Previous studies have reported that approximately 10% of hospitalized patients receive vancomycin annually in the United States. 2 According to the American Hospital Association, 33 million individuals were hospitalized in 2020. 3 This leads to an estimation of 3.3 million patients on vancomycin each year. The 2020 Vancomycin Guidelines state that 5%–43% of patients exposed to vancomycin may experience acute kidney injury (AKI). 4 The daily cost of hospitalization associated with managing vancomycin‐induced AKI ranges from $9379 to $20,467, 5 thus emphasizing the importance of effectively monitoring vancomycin therapy to minimize AKI occurrence.
In vitro data suggests that vancomycin‐induced AKI may result from mitochondrial damage and dose‐dependent proliferation of proximal tubular cells. 6 , 7 This increases oxidative phosphorylation, leading to oxidative stress and tubular cell damage. 7 Data suggest that vancomycin‐induced AKI involves other segments of the kidney, including the medullary region. 8 Concurrent administration of other nephrotoxic agents, such as aminoglycosides and intravenous contrast can increase the risk of vancomycin‐induced AKI. 6 Vancomycin‐induced AKI is thought to be dependent on the intensity and duration of exposure and mostly occurs within 4–5 days of therapy. 9
Vancomycin therapy is guided by therapeutic drug monitoring (TDM) to reduce AKI risk and optimize effectiveness. Previously, TDM of vancomycin for serious infections caused by MRSA was based on targeting trough concentrations of 15–20 mg/L as a surrogate of area under the curve (AUC):minimum inhibitory concentration (MIC) ≥400 mg*h/L, assuming a minimum inhibitory concentration (MIC) of 1 mg/L. The most recent Vancomycin Consensus Guidelines published in 2020 changed this recommendation to a target AUC:MIC ratio of 400–600 mg*h/L. 4 This change was based on data suggesting that trough‐guided dosing often overestimates AUC and results in greater risk of toxicity. 10 , 11 , 12
Area under the curve‐guided dosing can be performed using Bayesian dose optimization software or traditional pharmacokinetic (PK) equations. An advantage of Bayesian dosing is that it accounts for dynamic covariates such as renal function and calculates individualized patient dosing based on Bayesian priors, patients' drug concentrations, and creatinine clearance. 13 , 14 , 15 On the other hand, PK equations provide an estimation of AUC solely based on two vancomycin levels within the same dosing period. 13 , 15 One factor to consider is that these equations rely on serum creatinine (SCr); however, SCr is a delayed marker of renal injury and may take 24–36 h to display a notable change in renal function. 9 Additionally, the Bayesian method concentrations do not need to be drawn at steady‐state and can provide an estimation of AUC prior to reaching steady‐state. 13 , 14 , 15 Disadvantages of the Bayesian dose optimization are the logistics associated with implementation, including cost and education. 9 , 14 Transitioning to AUC‐guided dosing using Bayesian software would require the development and revision of vancomycin dosing and monitoring policies. 9 PK equations also rely on fewer assumptions than Bayesian software programs. 14 The recent shift toward AUC‐guided vancomycin dosing prompts further evaluation to identify the most appropriate method to determine AUC.
Although TDM and AUC dosing strategies are purported to reduce the incidence of vancomycin‐associated AKI, this has been difficult to prove conclusively in part due to heterogeneity in study designs, especially the definition of outcome. There are four definitions most commonly used to classify AKI. The Vancomycin Consensus Guidelines define AKI as an increase in serum creatinine (SCr) of ≥0.5 mg/dl or ≥50% increase in baseline SCr over ≥2 consecutive measurements. 4 In comparison, the Kidney Disease: Improving Global Outcomes (KDIGO) Criteria propose a more sensitive threshold of an increase in SCr of ≥0.3 mg/dl over 48 h. 4 Alternatively, the Acute Kidney Injury Network (AKIN) and the Risk, Injury, Loss of Kidney Function, and End‐Stage Renal Disease (RIFLE) Criteria have defined AKI in various stages based on changes in SCr, glomerular filtration rate (GFR), and urine output. 16
This meta‐analysis examines the incidence of vancomycin‐induced AKI observed with AUC‐guided versus conventional trough‐guided dosing strategies. In addition, subgroup analyses evaluate the influence of AKI definition on estimating the benefits of AUC‐based vancomycin dosing.
2. METHODS
2.1. Search strategy
A literature search identified all studies that reported the outcome of interest: incidence of vancomycin‐induced AKI using trough‐based monitoring versus AUC‐based monitoring. Medline (Ovid), Web of Science, and Google Scholar were queried for relevant journal articles using a combination of the search terms “vancomycin,” “trough,” “AUC,” “nephrotoxicity,” “acute renal failure,” and/or “kidney injury.” Specific search criteria are provided in Table A1. All studies from the inception of each database to November 5, 2021, were considered for inclusion. An independent researcher queried each database or search engine. The search was not restricted by publication date. A summary of the search strategy, including study selection, is provided in Figure 1. This meta‐analysis was registered in the PROSPERO database CRD42022306784.
FIGURE 1.
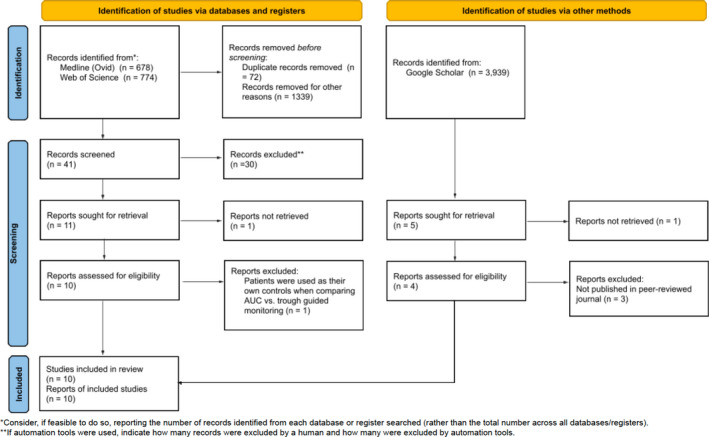
PRISMA diagram. Summary of evidence search and selection. AUC, area under the curve.
2.2. Study selection
All studies that provided data for the outcome of interest (AKI) and included a comparison of AUC versus trough‐based vancomycin dosing were evaluated for inclusion. Studies that included only one dosing strategy or that did not compare the two were excluded. Only studies published in peer‐reviewed journals were considered. Pediatric studies in patients under the age of 18 were excluded. A summary of the study characteristics is provided in Table 1.
TABLE 1.
Summary of characteristics of studies included in the analysis
| Study | Design of study | Duration of study | Country | Patient population | Primary outcome | Antibiotic indication | Mode of AUC calculation | Target AUC (mg*h/L) | Target trough (mg/L) | AKI definition | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
D'Amico 2021 25 N = 1024 |
Retrospective, single center cohort study | 2015–2019 | USA | Obese | AKI for entire population and for subgroups by obesity class | N/A | Two‐level Pharmacokinetic equations | 400–600 | 15–20 | KDIGO, RIFLE classification | Multivariable regression analysis |
|
Eads 2021 26 N = 44 |
Retrospective quasi‐experimental | 2018–2019 | USA | Veterans | Safety and efficacy of AUC/MIC monitoring compared to a historical cohort | N/A | Trapezoidal rule | 400–600 | 15–20 | RIFLE and KDIGO | No |
|
Lines 2021 27 N = 156 |
Retrospective cohort | 2013–2017 | USA | Adult inpatients | Treatment failure a | MRSA infections | Institutional nomogram | N/A | 15–20 | Vancomycin consensus guidelines | No |
|
Muklewicz 2021 28 N = 636 |
Retrospective quasi‐experimental | 2019–2020 | USA | Adult inpatients | Incidence of vancomycin‐associated AKI in the total population | N/A | Excel‐based calculator | 400–600 | 15–20 | AKIN classification, RIFLE classification, vancomycin consensus guidelines | No |
|
Wolfe 2021 29 N = 254 |
Retrospective, observational, single center | 2017–2020 | USA | Obese | Comparison of the development of nephrotoxicity after vancomycin initiation | N/A | Excel‐based calculator | 400–600 | 10–20 | KDIGO, RIFLE classification | No |
|
Oda 2020 30 N = 74 |
Retrospective cohort | 2016–2020 | Japan | Adult med‐surg | Incidence of AKI and 30‐day survival rate | N/A | Bayesian | 400–600 | 15–20 | AKIN classification, RIFLE classification | Multivariable regression analysis |
|
Vali 2020 31 N = 243 |
Retrospective quasi‐experimental | 2017–2019 | UK | Vascular surgery | Comparison of AUC24 values for the two groups a | N/A | Bayesian | 350–450 | 10–20 | KDIGO | No |
|
Meng 2019 34 N = 296 |
Prospective cohort | 2017–2018 | USA | Hospitalized adults | Achievement of therapeutic AUC values in the postimplementation group or therapeutic trough levels in the preimplementation group a | N/A | Trapezoidal rule | 400–800 | 10–20 | Vancomycin consensus guidelines | No |
|
Neely 2018 32 N = 252 |
Prospective cohort | 2012–2016 | USA | Adult inpatients | Determination of the proportion of all available trough concentrations that were therapeutic versus the proportion of all corresponding AUCs a | N/A | Bayesian | 400–800 | 10–20 | Vancomycin consensus guidelines | No |
|
Finch 2017 33 N = 1280 |
Retrospective quasi‐experimental | 2014–2015 | USA | Hospitalized patients | Comparative rate of acute kidney injury | N/A | Trapezoidal rule | 400–600 | 15–20 | AKIN classification, RIFLE classification, vancomycin consensus guidelines | Multivariable regression analysis |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; AUC, area under the curve; KDIGO, Kidney disease: Improving global outcomes; MIC, minimum inhibitory concentration; MRSA, methicillin‐resistant Staphylococcus aureus; N/A, not available; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End‐stage kidney disease.
These studies did not report AKI as a primary outcome.
2.3. Data extraction and outcomes
Three independent researchers (N.P., J.V., E.A.) collected data including trough target, AUC target, the incidence of AKI, method of AUC calculation, and AKI definition. The primary outcome assessed vancomycin‐induced AKI between trough‐guided and AUC‐guided dosing. If a study reported results for more than one AKI definition, the primary analysis was performed using the outcomes based on the Vancomycin Consensus Guidelines AKI definition. In the absence of outcomes based on this definition, the RIFLE or KDIGO Criteria were used, as these definitions are similar and would, therefore, increase the standardization of our results. Subgroup analyses were performed stratifying the outcome of interest by AKI definitions, which included AKIN, RIFLE, Vancomycin Consensus Guidelines, and KDIGO, and for studies that adjusted for covariates. All definitions are summarized in Table A2.
2.4. Assessment of the risk of bias and heterogeneity
The methodologic quality and risk of bias of each study were assessed using the Risk of Bias in Non‐Randomized Studies of Interventions (ROBINS‐I) scoring tool by two independent reviewers (N.P. and J.V.). Any disagreements in the study assessment were adjudicated by a third individual (E.A.). Visual inspection of the funnel plot and analysis of Egger's statistic were used to evaluate study heterogeneity.
2.5. GRADE assessment
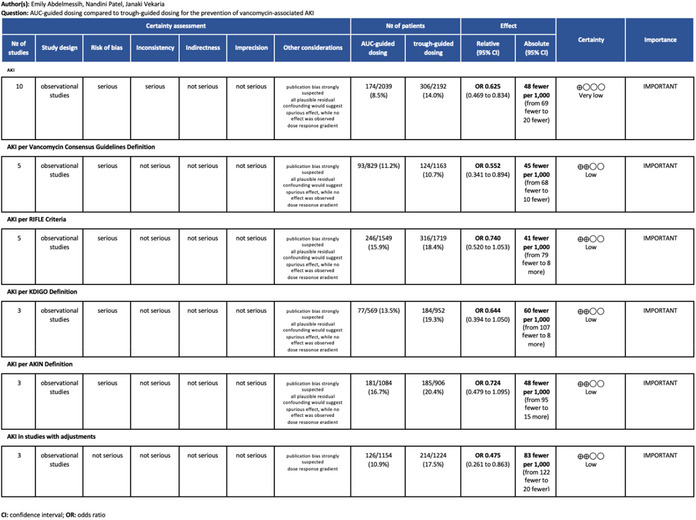
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate each meta‐analysis performed. This method reproducibly provides a quality assessment of meta‐analyses. 17 The scoring of each study within each of the 5 GRADE domains is provided in Table A3.
2.6. Statistical analysis
Comprehensive Meta‐Analysis version 3.0 was used for all statistical analyses. Funnel plots were created in R. 18 , 19 , 20 Odds ratios (ORs) and 95% confidence intervals (CI) were calculated using a Mantel–Haenszel random‐effects model. The heterogeneity between studies was evaluated using Cochrane's Q test and I 2 statistics. The study team considered an I 2 index <25%, 25%–75%, and >75% as low, moderate, and high, respectively. A sensitivity analysis was conducted by excluding studies with a high risk of bias.
3. RESULTS
A total of 5391 records were identified through the initial literature search: 678 from Medline (Ovid), 774 from Web of Science, and 3939 from Google Scholar. There were 5273 records removed based on the review of the record title and abstract and 72 duplicate records. After applying the inclusion and exclusion criteria, 32 studies were excluded. The majority of these exclusions were due to a lack of comparison of AUC and trough or lack of one of the dosing strategies altogether. A total of 14 full‐text articles were examined for inclusion. Three of these articles were not published in a peer‐reviewed journal and were excluded. 21 , 22 , 23 One study was excluded as it was descriptive and used each patient as their own control. 24 After these articles were excluded, 10 studies were included in the primary analysis. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 A full description of the study selection process can be found in Figure 1. Publication dates ranged from 2017 to 2021 and a total of 4231 patients were included. All studies included were conducted in noncritically ill adult patients. A summary of the study characteristics is reported in Table 1. Three studies reported significant reductions in AKI with AUC‐guided dosing, 25 , 27 , 32 while six studies 26 , 28 , 29 , 30 , 33 , 34 favored AUC‐guided dosing but with nonsignificant results. One study favored trough‐guided dosing, though the results were not statistically significant. 31 The ROBINS‐I analysis revealed that most studies (n = 8) had an overall low risk of bias, which was defined as high‐risk in less than three categories (Table 2). Two studies scored “high” in more than one category: Oda et al. (two categories) and Lines et al. (five categories). All the included studies scored “high” in the “bias of confounding variables” category due to their retrospective design.
TABLE 2.
Assessment of bias in the included studies using ROBINS‐I
| Study | Bias of confounding variables | Bias of selection of participants into the study | Bias in classification from intended intervention | Bias due to deviation from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of reporting results | Overall assessment of high degree of bias |
|---|---|---|---|---|---|---|---|---|
| D'Amico 2021 25 | High | Low | Low | Low | Low | Low | Low | I |
| Eads 2021 26 | High | Low | Low | Low | Low | Low | Low | I |
| Lines 2021 27 | High | High | High | Low | High | Moderate | High | IIIII a |
| Muklewicz 2021 28 | High | Low | Low | Low | Low | Low | Low | I |
| Wolfe 2021 29 | High | Low | Low | Low | Low | Low | Low | I |
| Oda 2020 30 | High | High | Low | Low | Moderate | Low | Low | II |
| Vali 2020 31 | High | Low | Low | Low | Low | Moderate | Low | I |
| Meng 2019 34 | High | Low | Low | Low | Low | Moderate | Low | I |
| Neely 2018 32 | High | Low | Low | Moderate | Low | Moderate | Low | I |
| Finch 2017 33 | High | Low | Low | Low | Low | Low | Low | I |
Overall high risk of bias, as defined by high risk of bias in 3 or more categories.
Area under the curve‐guided dosing strategies significantly reduced the incidence of vancomycin‐induced AKI versus trough‐guided strategies [OR 0.625, 95% CI (0.469–0.834), p = 0.001; Figure 2; GRADE: Very Low]; moderate heterogeneity was observed among the studies evaluated (I 2 = 25.746%). A sensitivity analysis was performed excluding the Lines et al.’s study and AUC‐guided dosing remained significantly associated with a lower incidence of AKI [OR 0.675, 95% CI (0.539–0.845), p = 0.001; Figure A1]. Although visual inspection of the funnel plot (Figure 3) showed some asymmetry, the Egger's weighted regression statistic did not identify significant publication bias (p = 0.0660). A subgroup analysis was performed with the three studies that reported ORs with adjustments for confounding variables, which showed that AUC‐guided dosing was associated with significantly reduced risk of nephrotoxicity compared to trough‐guided dosing [OR 0.475, 95% CI (0.261–0.863), p = 0.015, I 2 = 65.375%; Figure 4; GRADE: Low].
FIGURE 2.
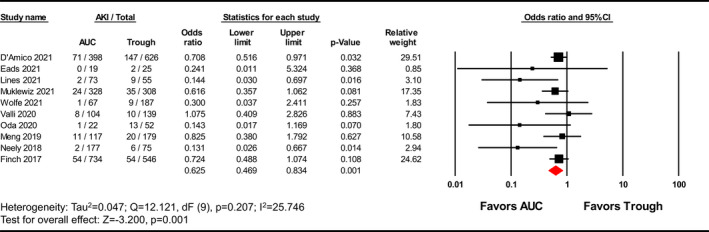
Forest plot examining incidence rate of AKI reported as odds ratio (OR). The overall meta‐analysis compares AKI incidence of AUC‐guided and trough‐guided dosing. AKI, acute kidney injury; AUC, area under the curve; CI, confidence interval.
FIGURE 3.
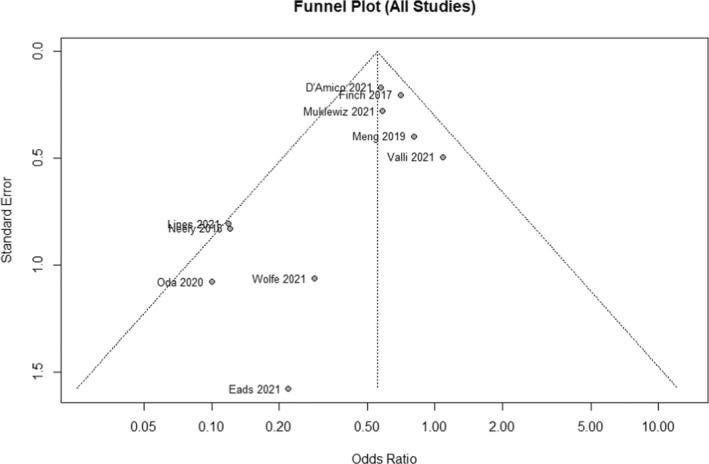
Funnel plot for all included studies. The funnel plot including all studies to visualize risk of bias in the overall analysis.
FIGURE 4.
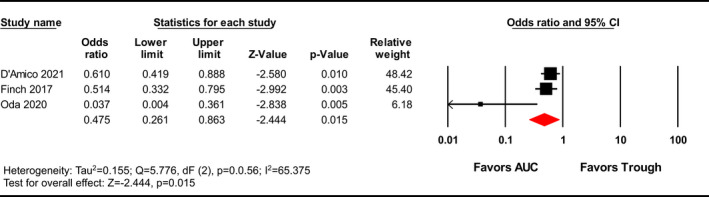
Forest plot examining effects of adjusting for confounders on AKI incidence. Subgroup analysis includes studies reporting adjusted odds ratios. AUC, area under the curve; CI, confidence interval.
Subgroup analysis by AKI definition showed a significant decrease in vancomycin‐induced AKI using AUC‐guided dosing strategies when AKI was classified using the Vancomycin Consensus Guidelines definition [OR 0.552, 95% CI (0.341–0.894), p = 0.016, I 2 = 49.437%; GRADE: Low] as shown in Figure 5. However, there was no significant difference in vancomycin‐induced AKI based on RIFLE, AKIN, and KDIGO definitions [RIFLE OR 0.740, 95% CI (0.520–1.053), p = 0.095, I 2 = 53.805%; GRADE: Low; AKIN OR 0.724, 95% CI (0.479–1.095), p = 0.126, I 2 = 51.206%; GRADE: Low; KDIGO OR 0.644, 95% CI (0.394–1.050), p = 0.078, I 2 = 31.433%; GRADE: Low]. Moderate heterogeneity was observed among each subgroup and was highest in the RIFLE subgroup (I 2 = 53.805%) and lowest in the KDIGO subgroup (I 2 = 31.433%). The Egger's statistic was nonsignificant for the subgroup analysis for the RIFLE, AKIN, and KDIGO definitions, but significant for the Vancomycin Consensus Guidelines definition (RIFLE: p = 0.1650; AKIN: p = 0.3136; Vancomycin Consensus Guidelines: p = 0.038; KDIGO: p = 0.6092).
FIGURE 5.
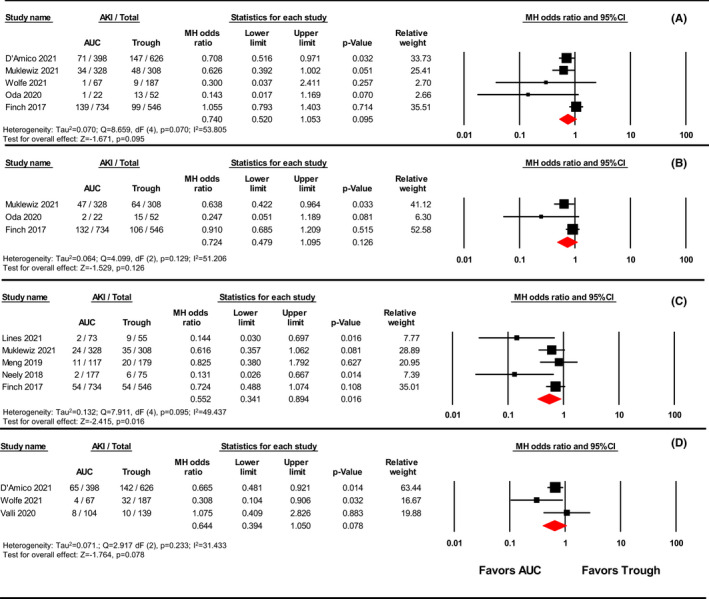
Forest plot examining the effect of different AKI definitions on AKI incidence. The forest plots present the results of the subgroup analyses by AKI definition. Panel A—RIFLE; Panel B—AKIN; Panel C—Vancomycin Consensus Guidelines; Panel D—KDIGO. AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; AUC, area under the curve; CI, confidence interval; KDIGO, Kidney Disease Improving Global Outcomes; MH, Mantel–Haenszel; RIFLE, Risk, Injury, Failure, Loss, End‐stage kidney disease.
4. DISCUSSION
The results of our study indicate that AUC‐guided dosing strategies are less likely to result in vancomycin‐mediated nephrotoxicity than trough‐based dosing strategies. Of the three studies identifying a difference in AKI incidence, two 25 , 27 reported a statistically significant higher trough level in the trough‐guided dosing groups, while the third 32 did not provide this data. This signifies that the observed difference in AKI may be due to the inherently higher trough concentrations in the trough‐guided group. An expected but important ancillary finding is that the definition of AKI influences the significance of the study results. These findings underscore the importance of uniformity in assessing vancomycin‐induced AKI in future studies. The GRADE of each meta‐analysis ranged from very low to low, suggesting that the true effect of AUC‐based dosing is markedly different from the estimate provided in this analysis. Regardless, the magnitude of the estimate warrants consideration.
Our results support the updated guidelines for vancomycin, which aim to minimize vancomycin‐induced AKI and maintain efficacy against invasive MRSA infections through AUC‐guided dosing. 4 However, widespread implementation of these guidelines has been slow and widely debated due to various concerns ranging from logistic to financial. A 2020 survey showed that the majority of institutions continue to use trough‐based monitoring as their primary method of dosing vancomycin. 35 Many institutions are currently implementing or planning to incorporate AUC dosing in the future. 35 , 36 Questions that need to be addressed before transitioning from trough‐based to AUC‐based vancomycin dosing include who will lead the implementation of the new program, which populations AUC dosing should be used in, and which AUC strategy should be used (institutional vs. commercial). The guidelines recommend AUC‐based dosing only for patients with invasive MRSA infections. Nonetheless, many institutions plan to incorporate AUC‐based dosing for all adult patients, which may be due to pragmatic reasons to limit confusion or additional training requirements. 35 A recent survey revealed that the most commonly used AUC calculation method among hospitals with an AUC‐guided dosing strategy was Bayesian software (38.3%), followed closely by in‐house software (35%), typically using Microsoft Excel. 36
As mentioned above, cost is often a barrier to switching vancomycin dosing methods. Lee et al. performed a cost–benefit analysis comparing vancomycin dosing for trough, two‐concentration AUC, and Bayesian AUC. Costs included phlebotomy, Bayesian software, and complications from nephrotoxicity. The cost of Bayesian software ranges from $10,000 to $50,000 per year while that of managing AKI for a single patient on vancomycin therapy is estimated to be $2982 with trough dosing, $2136 with two‐sample AUC, and $917 with Bayesian AUC dosing methods, showing that there may be a favorable cost–benefit ratio. Although many institutions are hesitant to transition, this study supports the use of AUC‐guided dosing and predicts that the overall cost savings associated with the transition may outweigh the potential burden. 37 Importantly, the study provided estimates of AKI based on trough, two‐concentration AUC, and Bayesian dosing using available literature. These data support the use of Bayesian software to generate the greatest cost savings benefit.
The 2020 Vancomycin Consensus Guidelines recommend AUC using Bayesian software programs over pharmacokinetic equations. 4 Out of the 10 studies included in this meta‐analysis, only three utilized Bayesian software for AUC dosing. Bayesian software programs use an established pharmacokinetic model and patient parameters to optimize vancomycin dosing and account for dynamic changes such as renal function. 13 , 14 , 15 Bayesian software programs are the preferred method of AUC monitoring since concentrations do not need to be drawn at steady‐state, and thus, therapeutic drug monitoring can be initiated as early as the first dosing interval. This may quicken the time to effective drug concentrations as trough levels need to be drawn at steady‐state. 14 Additionally, AUC monitoring is beneficial as steady‐state conditions may be difficult to predict in clinical practice since they can be influenced by renal function and other factors such as loading doses and body mass index. 14 Bayesian monitoring can be performed using one‐ or two‐concentrations. Based on the Vancomycin Consensus Guidelines, it is preferred to calculate AUC using two‐concentration Bayesian monitoring due to the lack of data using single concentration estimates. 4
This meta‐analysis has several limitations as it attempts to combine multiple studies with different methodologies. An important consideration is that the small sample size of this analysis reduces the power of our meta‐analysis. However, the detection of a difference despite the small sample size suggests that AUC‐guided dosing strategies are less likely to lead to vancomycin‐induced AKI than trough‐based dosing strategies. Given the multiple definitions of nephrotoxicity, such as RIFLE, AKIN, KDIGO, or Vancomycin Consensus Guidelines, the ascertainment of the AKI end point was not consistent. To increase the standardization of the results, we used the most common definition throughout all 10 studies, which was the Vancomycin Consensus Guidelines definition. For the remaining studies, we used the RIFLE Criteria and KDIGO definition, as they were the second and third most utilized definitions, respectively.
In addition, we performed subgroup analyses based on each definition reported throughout the studies. Although AUC‐based dosing was associated with a reduced incidence of AKI in all subgroups, significance was only reached in the subgroup analyses including studies utilizing the Vancomycin Consensus Guidelines definition of AKI, which is the strictest based on the change in SCr. Statistically significant results may have been more profound with stricter definitions such as Vancomycin Consensus Guidelines, which suggest a cut‐off of 0.5 mg/L or a 50% increase in SCr. More lenient definitions (0.3 mg/L or a 30% increase in SCr) as the lower threshold may be too sensitive and captures more non‐vancomycin‐related AKI. Thus, the results may be biased toward the null hypothesis in studies using the 0.3 mg/dl cutoff. Since the results remain significant in the subgroup using the strictest definition for AKI, AUC‐guided dosing would likely remain beneficial in clinical settings where more lenient criteria may be used to define AKI. Additionally, no randomized studies were available for inclusion and most studies included in this analysis were retrospective, leading to an increased risk of confounding bias. Only three of the 10 studies adjusted for these potential covariates by performing regression analyses and reporting adjusted ORs. A subgroup analysis was performed with these three studies and the results remained significant in favor of AUC‐guided dosing to reduce nephrotoxicity.
5. CONCLUSIONS
Compared to trough‐based dosing strategies, the use of AUC‐guided dosing strategies is associated with a reduced incidence of vancomycin‐induced AKI, which supports the 2020 Vancomycin Consensus Guidelines. However, the certainty of the findings remains low due to the overall low quality of many of the studies included in the meta‐analysis. Ultimately, randomized controlled studies are necessary to affirm the safety benefits of AUC‐guided vancomycin therapy.
AUTHOR CONTRIBUTION
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVAL
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
ACKNOWLEDGEMENTS
Funding: LB reports that research in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Number R01DK131214. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
APPENDIX 1.
FIGURE A1.
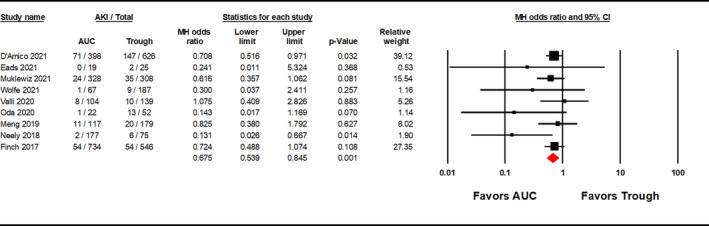
This figure shows the results of a sensitivity analysis performed excluding Lines et al., due to its high risk of bias. AUC, area under the curve; CI, confidence interval; MH, Mantel–Haenszel.
TABLE A1.
Search strategy
| Database | Search terms |
| Google Scholar | “vancomycin” and (“AUC” or “area under the curve”) and “trough” |
| “vancomycin” and “nephrotoxicity or acute renal failure or kidney injury” and “AUC or area under the curve” | |
| “vancomycin” and “acute renal failure or nephrotoxicity or kidney injury” and “trough” | |
| Web of Science | “vancomycin” and "AUC or area under the curve" and “trough” |
| “vancomycin” and “AUC/area under the curve” and “nephrotoxicity/acute renal failure/kidney injury” | |
| “vancomycin” and “trough” and “nephrotoxicity/acute renal failure/kidney injury” | |
| Medline | “vancomycin” and “AUC or area under the curve” and “trough” |
| “vancomycin” and “nephrotoxicity or acute renal failure or kidney injury” and “AUC or area under the curve” | |
| “vancomycin” and “acute renal failure or nephrotoxicity or kidney injury” and “trough” |
TABLE A2.
AKI definitions
| Criteria | Definition |
|---|---|
| AKIN | |
| Stage 1 |
|
| Stage 2 |
|
| Stage 3 |
|
| KDIGO | |
| Stage 1 |
|
| Stage 2 |
|
| Stage 3 |
|
| RIFLE | |
| Risk |
|
| Injury |
|
| Failure |
|
| Loss |
|
| ESRD |
|
| 2020 Vancomycin consensus guidelines |
|
Note: Summary of the AKI Definitions for AKIN, KDIGO, RIFLE, and 2009 Vancomycin Consensus Guidelines.
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ESRD, end‐stage renal disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; kg, kilograms; ml, milliliters; RIFLE, Risk, Injury, Failure, Loss, End‐stage kidney disease; SCr, serum creatinine.
TABLE A3.
Grading of recommendations, assessment, development, and evaluation (GRADE)
|
Abdelmessih E, Patel N, Vekaria J, et al. Vancomycin area under the curve versus trough only guided dosing and the risk of acute kidney injury: Systematic review and meta‐analysis. Pharmacotherapy. 2022;42:741‐753. doi: 10.1002/phar.2722
Registration: CRD42022306784.
REFERENCES
- 1. Patel S, Preuss CV, Bernice F. Vancomycin. In: StatPearls. StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC; 2022. [Google Scholar]
- 2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May–September 2011. Jama. 2014;14:1438‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Association AH . Fast facts on U.S. hospitals; 2022. Accessed July 1, 2022. https://www.aha.org/statistics/fast‐facts‐us‐hospitals
- 4. Rybak MJ, Le J, Lodise TP, et al. Executive summary: therapeutic monitoring of vancomycin for serious methicillin‐resistant Staphylococcus aureus infections: a revised consensus guideline and review of the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2020;4:363‐367. [DOI] [PubMed] [Google Scholar]
- 5. Patel N, Huang D, Lodise T. Potential for cost saving with iclaprim owing to avoidance of vancomycin‐associated acute kidney injury in hospitalized patients with acute bacterial skin and skin structure infections. Clin Drug Investig. 2018;10:935‐943. [DOI] [PubMed] [Google Scholar]
- 6. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;3:459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. King DW, Smith MA. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro. 2004;6:797‐803. [DOI] [PubMed] [Google Scholar]
- 8. Le Moyec L, Racine S, Le Toumelin P, et al. Aminoglycoside and glycopeptide renal toxicity in intensive care patients studied by proton magnetic resonance spectroscopy of urine. Crit Care Med. 2002;6:1242‐1245. [DOI] [PubMed] [Google Scholar]
- 9. Lodise TP, Hall RG 2nd, Scheetz MH. Vancomycin area under the curve‐guided dosing and monitoring: "is the juice worth the squeeze"? Pharmacotherapy. 2020;12:1176‐1179. [DOI] [PubMed] [Google Scholar]
- 10. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta‐analysis of vancomycin‐induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;2:734‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can't get there from here. Clin Infect Dis. 2011;8:969‐974. [DOI] [PubMed] [Google Scholar]
- 12. Neely MN, Youn G, Jones B, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;1:309‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner RB, Kojiro K, Shephard EA, et al. Review and validation of Bayesian dose‐optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy. 2018;12:1174‐1183. [DOI] [PubMed] [Google Scholar]
- 14. Aljutayli A, Thirion DJG, Bonnefois G, Nekka F. Pharmacokinetic equations versus Bayesian guided vancomycin monitoring: pharmacokinetic model and model‐informed precision dosing trial simulations. Clin Transl Sci. 2022;15:942‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olney KB, Wallace KL, Mynatt RP, et al. Comparison of Bayesian‐derived and first‐order analytic equations for calculation of vancomycin area under the curve. Pharmacotherapy. 2022;42:284‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mustafa RA, Santesso N, Brozek J, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J Clin Epidemiol. 2013;7:736‐742; quiz 42.e1‐5. [DOI] [PubMed] [Google Scholar]
- 18. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;4:153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;3:1‐48. [Google Scholar]
- 20. Team RC . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. https://www.R‐project.org/ [Google Scholar]
- 21. McQueen J, Nadeau L, Hussein AA. Determination of the relationship between vancomycin trough concentrations and the AUC/MIC dosing; 2020. "Unpublished Work".
- 22. Keats KR. Impact of area‐under‐the‐curve monitoring for vancomycin on incidence of acute kidney injury in orthopedic patients; 2019. "Unpublished Work".
- 23. Giazzon A. Implementation of a pharmacist‐driven two‐level AUC‐based vancomycin dosing strategy at a VA medical center; 2021. "Unpublished Work".
- 24. Johnston MM, Huang V, Hall ST, Buckley MS, Bikin D, Barletta JF. Optimizing outcomes using vancomycin therapeutic drug monitoring in patients with MRSA bacteremia: trough concentrations or area under the curve? Diagn Microbiol Infect Dis. 2021;2:115442. [DOI] [PubMed] [Google Scholar]
- 25. D'Amico H, Wallace KL, Burgess D, et al. Acute kidney injury associated with area under the curve versus trough monitoring of vancomycin in obese patients. Antimicrob Agents Chemother. 2022;66:e0088621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eads AV, Cole JL. Efficacy and safety of vancomycin therapy after the transition to AUC/MIC monitoring in a primary facility. J Pharm Pract. 2021;8971900211003439. doi: 10.1177/08971900211003439. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27. Lines J, Burchette J, Kullab SM, Lewis P. Evaluation of a trough‐only extrapolated area under the curve vancomycin dosing method on clinical outcomes. Int J Clin Pharmacol. 2021;1:263‐269. [DOI] [PubMed] [Google Scholar]
- 28. Muklewicz JD, Steuber TD, Edwards JD. Evaluation of area under the concentration‐time curve‐guided vancomycin dosing with or without piperacillin‐tazobactam on the incidence of acute kidney injury. Int J Antimicrob Agents. 2021;1:106234. [DOI] [PubMed] [Google Scholar]
- 29. Wolfe A, Bowling J, Short MR, Mateyoke G, Berger SC. Assessing nephrotoxicity associated with different vancomycin dosing modalities in obese patients at a community hospital. Hosp Pharm. 2021;57:532‐539. doi: 10.1177/00185787211055791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oda K, Jono H, Nosaka K, Saito H. Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration‐time curve against a trough 15–20 μg/ml concentration. Int J Antimicrob Agents. 2020;4:106109. [DOI] [PubMed] [Google Scholar]
- 31. Vali L, Jenkins DR, Vaja R, Mulla H. Personalised dosing of vancomycin: a prospective and retrospective comparative quasi‐experimental study. Br J Clin Pharmacol. 2021;2:506‐515. [DOI] [PubMed] [Google Scholar]
- 32. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62:e02042‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finch NA, Zasowski EJ, Murray KP, et al. A quasi‐experiment to study the impact of vancomycin area under the concentration‐time curve‐guided dosing on vancomycin‐associated nephrotoxicity. Antimicrob Agents Chemother. 2017;12:e01293‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meng L, Wong T, Huang S, et al. Conversion from Vancomycin trough concentration‐guided dosing to area under the curve‐guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;4:433‐442. [DOI] [PubMed] [Google Scholar]
- 35. Bland CM, Crosby CM, Orvin DL, Smith SE, Jones BM. Transitioning from guideline approval to practical implementation of AUC‐based monitoring of vancomycin. Am J Health Syst Pharm. 2021;14:1270‐1272. [DOI] [PubMed] [Google Scholar]
- 36. Bradley N, Lee Y, Sadeia M. Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J Pharm Pract. 2021;8971900211012395. doi: 10.1177/08971900211012395. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37. Lee BV, Fong G, Bolaris M, et al. Cost‐benefit analysis comparing trough, two‐level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect. 2021;9:1346.e1‐7. [DOI] [PubMed] [Google Scholar]
- 38. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2017;11:R31‐R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204‐R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1‐138. [Google Scholar]


