Abstract
Background:
Syphilis can cause neurologic, ocular, or otic manifestations, possibly resulting in permanent disability or death. In 2018, CDC began collecting syphilis clinical manifestation data via the National Notifiable Diseases Surveillance System (NNDSS). We present the first reported U.S. syphilis neurologic, ocular, and otic manifestation prevalence estimates.
Methods:
We reviewed 2019 NNDSS data to identify jurisdictions reporting ≥70% of syphilis cases ≥15 years old with clinical manifestation data (considered “complete reporting”). Among these jurisdictions, we determined reported neurologic, ocular, and otic manifestation prevalence, stratified by demographic, behavioral, and clinical characteristics.
Results:
Among 41,187 syphilis cases in 16 jurisdictions with complete reporting, clinical manifestations were infrequently reported overall: neurologic (n=445, 1.1%), ocular (n=461, 1.1%), otic (n=166, 0.4%), any (n=807, 2.0%). Reported clinical manifestation prevalence was highest among cases ≥65 years old (neurologic: 5.1%; ocular: 3.5%; otic: 1.2%) and those reporting injection drug use (neurologic: 2.8%; ocular: 3.4%; otic: 1.6%). Although reported neurologic and ocular manifestation prevalence was slightly higher among HIV-infected vs. HIV-negative persons, approximately 40% of cases with manifestations were HIV-negative. Reported otic manifestation prevalence was similar regardless of HIV status. When stratifying by HIV status and syphilis stage, reported prevalence was highest among HIV-infected persons with unknown duration/late syphilis (neurologic: 3.0%; ocular: 2.3%; otic: 0.7%).
Conclusions:
Reported neurologic, ocular, and otic manifestation prevalence was low among syphilis cases, but these data are likely an underestimate given potential underreporting. Reported clinical manifestation frequency, including among HIV-negative persons, emphasizes the importance of evaluating all syphilis cases for signs/symptoms of neurosyphilis, ocular syphilis, and otosyphilis.
Keywords: Syphilis, Neurosyphilis, Ocular syphilis, Otosyphilis, Surveillance
Summary
Neurologic, ocular, and otic manifestations were infrequently reported overall, but prevalence increased with age and was higher among HIV-infected persons reporting injection drug use and staged as unknown duration/late syphilis.
INTRODUCTION
Syphilis is an infection caused by the spirochete Treponema pallidum. T. pallidum can invade the central nervous system, visual system, and/or cochleovestibular system at any stage of infection resulting in neurosyphilis, ocular syphilis, and otosyphilis, respectively.[1] Neurologic, ocular, and otic manifestations can occur together or in isolation and have a wide spectrum of presentations ranging from mild to severe. If left untreated, these syphilitic complications may result in permanent disability or death.[1, 2]
U.S. rates of primary and secondary syphilis have increased nearly every year since 2001, with reported total syphilis cases up 74% in 2019 compared to 2015.[3] These increasing syphilis case rates raise concerns about a possible accompanying increase in severe sequelae of syphilitic infections such as neurologic, ocular, and otic manifestations.[4–6] Although some population-based syphilis data indicate that the prevalence of reported neurologic, ocular, and/or otic manifestations is low,[4, 7, 8] other estimates suggest the actual prevalence may be higher.[9] Recent population-based reports on the prevalence of these manifestations among syphilis cases of all stages are lacking.
In 2018, the Council of State and Territorial Epidemiologists (CSTE) updated the case definition for syphilis including classification of neurologic, ocular, and otic manifestations, and CDC subsequently began collecting clinical manifestation data for syphilis cases reported through the National Notifiable Diseases Surveillance System (NNDSS).[10] In this report, we present the first prevalence estimates of reported neurologic, ocular, and otic manifestations among U.S. syphilis cases of all stages that were ≥15 years old and captured through NNDSS using the 2018 CSTE syphilis case definition.
MATERIALS AND METHODS
After excluding cases of congenital syphilis and syphilitic stillbirth and those <15 years old, we reviewed 2019 NNDSS data from 51 jurisdictions (50 states and the District of Columbia) to identify syphilis cases reported with neurologic, ocular, and/or otic manifestations. When present, these manifestations are reported as verified, likely, or possible (Table 1).1 For our prevalence analyses, we combined verified, likely, and possible classifications for each of these manifestations. We separately determined the proportion of cases with each manifestation that were reported as verified, likely, and possible.
Table 1.
Council of State and Territorial Epidemiologists (CSTE) case definitions for neurologic, ocular, and otic manifestations of syphilis
| Classification | Neurologic manifestations | Ocular manifestations | Otic manifestations |
|---|---|---|---|
| Verified | Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
| Likely | Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
Reactive nontreponemal test and a reactive
treponemal test with both of the following:
|
| Possible | Reactive nontreponemal test and a reactive treponemal test and clinical symptoms or signs consistent with neurosyphilis without other known causes for these abnormalities. | Reactive nontreponemal test and a reactive treponemal test and clinical symptoms or signs consistent with ocular syphilis without other known causes for these abnormalities. | Reactive nontreponemal test and a reactive treponemal test and clinical symptoms or signs consistent with otosyphilis without other known causes for these abnormalities. |
To limit bias introduced by missing data, jurisdictions reporting ≥70% of syphilis cases with response values that were not missing or unknown for all of these clinical manifestations were considered to have “complete reporting.” Among jurisdictions with complete reporting, we determined the overall prevalence of reported neurologic, ocular, and otic manifestations across syphilis cases of all stages. We then stratified these results by demographic characteristics, sex of sex partners, HIV status, syphilis surveillance stage, and reported injection drug use during the past 12 months (IDU). For sex of sex partners, we categorized cases as women, men who had sex with women only (MSW) in the past 12 months, and men who had sex with men including men who had sex with men and women (MSM) in the past 12 months.
To understand potential differences in prevalence by HIV status and other possible modifying factors, we evaluated the prevalence of neurologic, ocular, and otic manifestations when stratifying HIV status by syphilis stage, race/Hispanic ethnicity, IDU, and sex of sex partners. When stratifying HIV status by syphilis stage, cases were categorized as early or late syphilis with early syphilis defined as those assigned a syphilis surveillance stage of primary, secondary, or early non-primary non-secondary and late syphilis defined as those staged as unknown duration or late.
We compared point prevalence estimates and described differences under the assumptions that the response values for neurologic, ocular, and otic manifestations were accurately reported and that the estimates reflect the prevalence of all reported clinical manifestations for the included jurisdictions; therefore, no statistical testing was performed, and 95% confidence intervals are not provided. We have provided numerators and denominators for those who may be interested in making such calculations.
This study used data that are routinely collected as a part of public health surveillance for the purpose of guiding public health disease control efforts and was therefore not subject to institutional review board approval for human subjects’ protection. Data were collected during 2019–2020 and analyzed in 2021. All analyses were performed using Stata version 16.1.
RESULTS
In 2019, 16 jurisdictions representing all U.S. Census Bureau regions had complete reporting for neurologic, ocular, and otic manifestations (Figure 1). After excluding 29 syphilis cases <15 years old, a total of 41,187 syphilis cases were reported across these jurisdictions, accounting for approximately one-third of the 127,855 total syphilis cases ≥15 years old reported to CDC nationwide for 2019.[3] Approximately 86% of the 41,187 syphilis cases reported in the 16 included jurisdictions in 2019 were reported with response values that were not missing or unknown for all of the neurologic, ocular, and otic clinical manifestations variables. These manifestations were infrequently reported overall: neurologic (n=445, 1.1%), ocular (n=461, 1.1%), otic (n=166, 0.4%), and any (n=807, 2.0%). Of note, none of the excluded 29 syphilis cases <15 years old were reported to have neurologic, ocular, or otic manifestations. The majority of these reported manifestations were classified as “possible” (Table 2).
Figure 1.
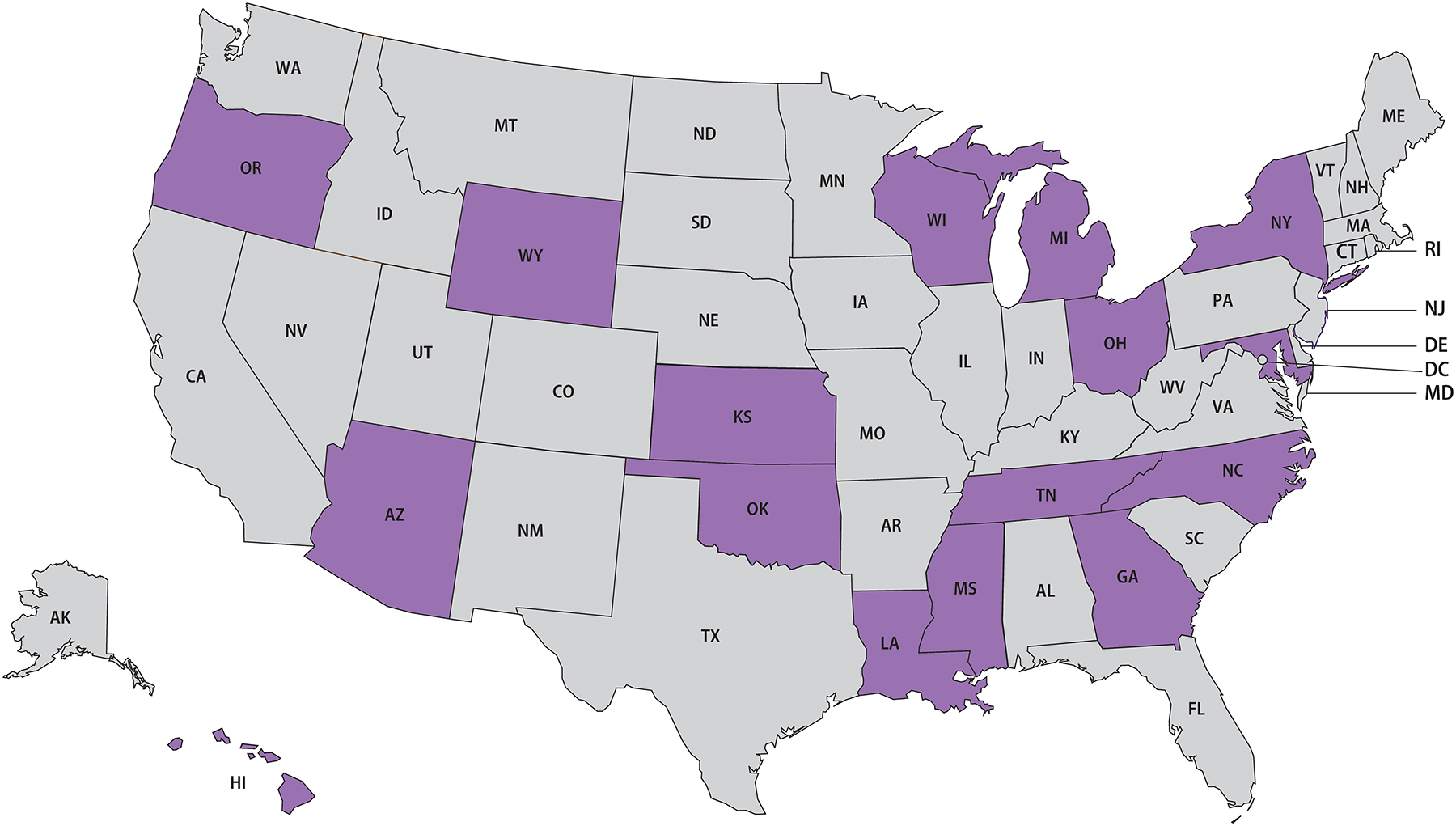
Sixteen included states reporting ≥70% of syphilis cases (all stages) with neurologic, ocular, and otic manifestation response values that were not missing or unknown, 2019.
Table 2.
Clinical manifestation classification of reported syphilis cases (all stages) with neurologic, ocular, and otic manifestations — 16 statesa, 2019
| Classification | Neurologic manifestations n = 445 |
Ocular manifestations n = 461 |
Otic manifestations n = 166 |
|---|---|---|---|
| n (%, Col) | n (%, Col) | n (%, Col) | |
| Verified | 104 (23.4) | 53 (11.5) | 12 (7.2) |
| Likely | 79 (17.8) | 127 (27.5) | 27 (16.3) |
| Possible | 262 (58.9) | 281 (61.0) | 127 (76.5) |
States reporting ≥70% of syphilis cases ≥15 years old with clinical manifestation response values that were not missing or unknown in 2019 were included in the analysis.
Table 3 shows the characteristics of reported syphilis cases with neurologic, ocular, and otic manifestations when combining verified, likely, and possible clinical manifestation classifications together. The prevalence of these reported manifestations increased with age and was higher among those reported as White, non-Hispanic compared to other race/Hispanic ethnicity categories. Prevalence of reported neurologic, ocular, and otic manifestations was highest among cases that were ≥65 years old (neurologic: n=44, 5.1%; ocular: n=30, 3.5%; otic: n=10, 1.2%) followed by those with reported IDU (neurologic: n=45, 2.8%; ocular: n=53, 3.4%; otic: n=26, 1.6%). When stratifying by reported HIV status, cases that were reported as HIV-infected had a higher prevalence of reported neurologic (n=179, 1.4%) and ocular (n=168, 1.3%) manifestations, but prevalence of reported otic manifestations was similar regardless of reported HIV status. Approximately 40% of cases with reported neurologic and ocular manifestations and 50% with reported otic manifestations were reported as HIV-negative. Prevalence of these reported clinical manifestations was higher among cases staged as either unknown duration or late syphilis (neurologic: n=230, 1.6%; ocular: n=220, 1.6%; otic: n=68; 0.5%) or secondary syphilis (neurologic: n=110, 1.3%; ocular: n=127, 1.5%, otic: n=53, 0.6%) compared with those assigned primary (neurologic: n=24, 0.5%; ocular: n=21, 0.4%; otic: n=7, 0.2%) or early non-primary non-secondary stages (neurologic: n=81, 0.6%; ocular: n=92, 0.7%; otic: n=38, 0.3%).
Table 3.
Characteristics of reported syphilis cases (all stages) with neurologic, ocular, and otic manifestations — 16 states, 2019a
| Characteristic | Syphilis cases | Neurologic manifestations | Ocular manifestations | Otic manifestations |
|---|---|---|---|---|
| n | n (%, Row) | n (%, Row) | n (%, Row) | |
| Total | 41,187 | 445 (1.1) | 461 (1.1) | 166 (0.4) |
| Age category at diagnosis | ||||
| 15–24 years old | 7,618 | 29 (0.4) | 30 (0.4) | 19 (0.3) |
| 25–34 years old | 16,210 | 116 (0.7) | 124 (0.8) | 54 (0.3) |
| 35–44 years old | 9,012 | 103 (1.1) | 120 (1.3) | 30 (0.3) |
| 45–54 years old | 5,056 | 85 (1.7) | 96 (1.9) | 33 (0.7) |
| 55–64 years old | 2,432 | 68 (2.8) | 61 (2.5) | 20 (0.8) |
| ≥ 65 years old | 854 | 44 (5.1) | 30 (3.5) | 10 (1.2) |
| Missing/unknown | 5 | 0 (0) | 0 (0) | 0 (0) |
| Sex | ||||
| Female | 8,521 | 106 (1.2) | 108 (1.3) | 58 (0.7) |
| Male | 32,653 | 338 (1.0) | 352 (1.1) | 108 (0.3) |
| Missing/unknown | 13 | 1 (7.7) | 1 (7.7) | 0 (0) |
| Race/Hispanic ethnicity | ||||
| AI/AN, non-Hispanic | 691 | 9 (1.3) | 9 (1.3) | 2 (0.3) |
| Asian, non-Hispanic | 631 | 5 (0.8) | 8 (1.3) | 1 (0.2) |
| Black, non-Hispanic | 18,715 | 145 (0.8) | 128 (0.7) | 45 (0.2) |
| NH/PI, non-Hispanic | 138 | 2 (1.5) | 2 (1.5) | 0 (0) |
| White, non-Hispanic | 10,770 | 214 (2.0) | 229 (2.1) | 95 (0.9) |
| Other, non-Hispanic | 646 | 6 (0.9) | 10 (1.6) | 1 (0.2) |
| Multirace, non-Hispanic | 1,809 | 25 (1.4) | 24 (1.3) | 10 (0.6) |
| Hispanic | 6,303 | 28 (0.4) | 37 (0.6) | 9 (0.1) |
| Missing/unknown | 1,484 | 11 (0.7) | 14 (0.9) | 3 (0.2) |
| Sex and sex of sex partners | ||||
| Men who have sex with menb | 15,026 | 158 (1.1) | 174 (1.2) | 67 (0.5) |
| Men who have sex with women only | 5,763 | 60 (1.0) | 71 (1.2) | 18 (0.3) |
| Men with unknown sex of sex partners | 11,864 | 120 (1.0) | 107 (0.9) | 23 (0.2) |
| Womenc | 8,521 | 106 (1.2) | 108 (1.3) | 58 (0.7) |
| Missing/unknown sex | 13 | 1 (7.7) | 1 (7.7) | 0 (0) |
| HIV-infected | ||||
| Yes | 12,558 | 179 (1.4) | 168 (1.3) | 51 (0.4) |
| No | 18,489 | 168 (0.9) | 193 (1.0) | 82 (0.4) |
| Missing/unknown | 10,140 | 98 (1.0) | 100 (1.0) | 33 (0.3) |
| Syphilis surveillance stage | ||||
| Primary | 4,859 | 24 (0.5) | 21 (0.4) | 7 (0.1) |
| Secondary | 8,264 | 110 (1.3) | 127 (1.5) | 53 (0.6) |
| Early non-primary non-secondary | 14,047 | 81 (0.6) | 93 (0.7) | 38 (0.3) |
| Unknown duration or late | 14,017 | 230 (1.6) | 220 (1.6) | 68 (0.5) |
| Injection drug use past 12 months | ||||
| Yes | 1,582 | 45 (2.8) | 53 (3.4) | 26 (1.6) |
| No | 21,696 | 235 (1.1) | 267 (1.2) | 97 (0.5) |
| Missing/unknown | 17,909 | 165 (0.9) | 141 (0.8) | 43 (0.2) |
Abbreviations: AI/AN, American Indian/Alaska Native; NH/PI, Native Hawaiian/Other Pacific Islander
States reporting ≥70% of syphilis cases ≥15 years old with clinical manifestation response values that were not missing or unknown in 2019 were included in the analysis. Values shown for prevalence represent the total of verified, likely, and possible neurologic, ocular, and otic clinical manifestations.
The men who have sex with men category included gay, bisexual, and other men who have sex with men.
A total of 200 women were reported to have neurologic, ocular, and/or otic manifestations of syphilis in the 16 included jurisdictions during 2019. Among these women, 137/200 (68.5%) were reported as having only male partners, 9/200 (4.5%) were reported as having male and female partners, and 54/200 (27.0%) were reported with unknown or missing sex of sex partners.
When stratifying by reported HIV status and syphilis stage, prevalence of reported neurologic, ocular, and otic manifestations was higher among cases staged as late syphilis regardless of reported HIV status. However, prevalence was highest among cases reported as HIV-infected with late syphilis (neurologic: n=84, 3.0%; ocular: n=66, 2.3%; otic: n=20, 0.7%) (Figure 2a; Table 1, Supplemental Digital Content 1).
Figure 2.
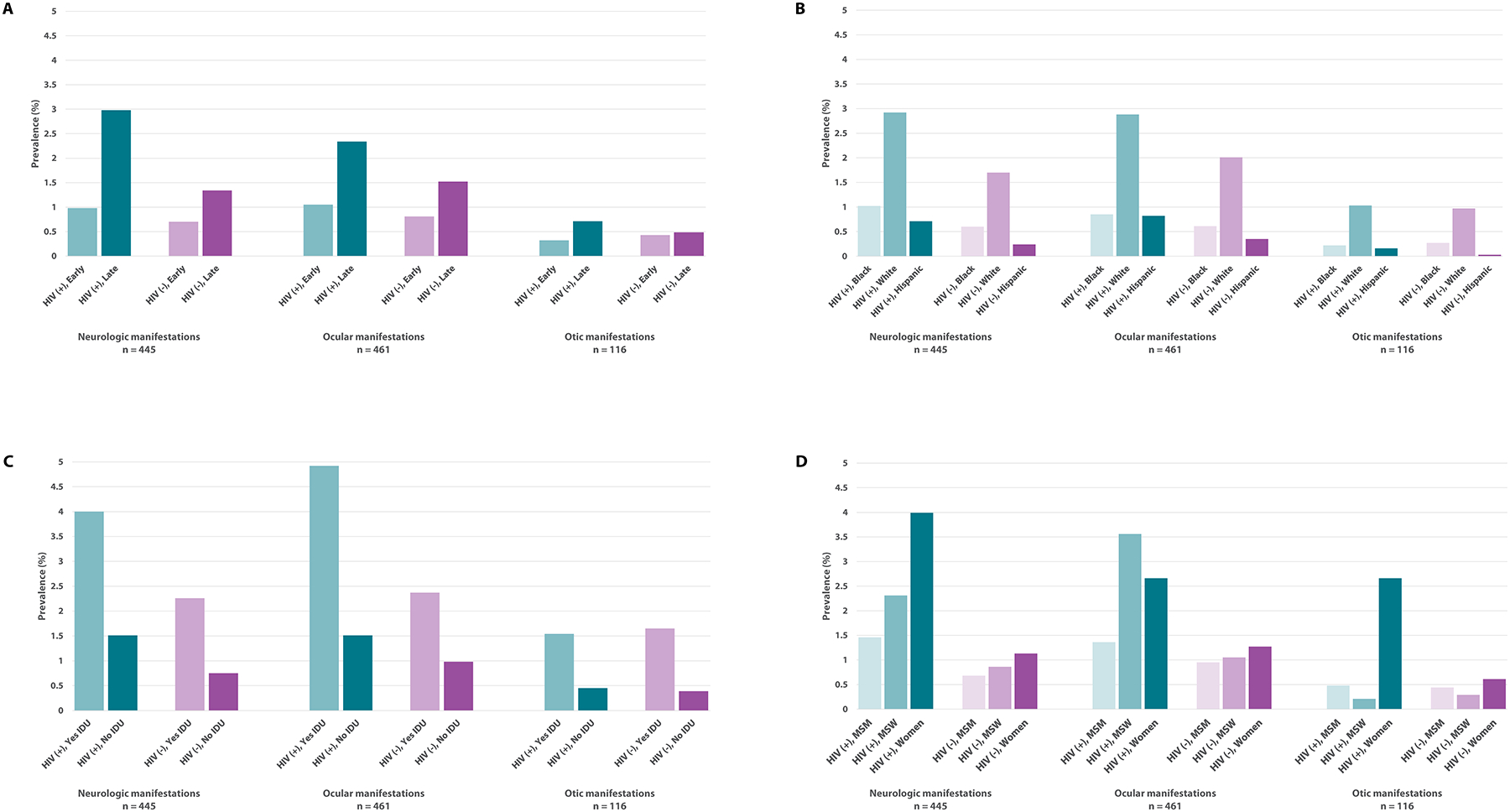
Prevalence of reported neurologic, ocular, and otic manifestations by HIV status and syphilis stage (Panel A), race/Hispanic ethnicity (Panel B), injection drug use in the past 12 months (IDU) (Panel C), and sex and sex of sex partners (Panel D) among syphilis cases (all stages) reported by jurisdictions with complete reporting, 2019. Values shown for prevalence represent the total of verified, likely, and possible neurologic, ocular, and otic clinical manifestations. A total of 16 states reporting ≥70% of syphilis cases with clinical manifestation response values that were not missing or unknown were considered to have complete reporting. Syphilis cases ≥15 years of age were included in this analysis. Approximately 25% of cases were missing HIV status. Early syphilis was defined as those cases assigned a syphilis surveillance stage of primary, secondary, or early non-primary non-secondary, and late syphilis was defined as those cases staged as unknown duration or late. Of note, >40% of cases were missing IDU status. The men who have sex with men (MSM) category included gay, bisexual, and other MSM while the men who have sex with women (MSW) category included men who reported only having sex with women. Complete supporting data for this figure are available in Table 1, Supplemental Digital Content 1.
The prevalence of reported neurologic and ocular manifestations was higher among cases that were reported to be HIV-infected when compared to those that were reported to be HIV-negative within all race/Hispanic ethnicity categories, but the prevalence of reported otic manifestations was similar regardless of reported HIV status (Figure 2b; Table 1, Supplemental Digital Content 1). For example, White, non-Hispanic cases had approximately three times the prevalence of reported neurologic manifestations compared to Black, non-Hispanic cases regardless of reported HIV status (HIV-infected: Black [n=70, 1.0%] vs. White [n=71, 2.9%]; HIV-negative: Black [n=46, 0.6%] vs. White [n=95, 1.7%]). This difference in prevalence was even more pronounced when comparing White, non-Hispanics to Hispanics, with the greatest difference in prevalence across all of these manifestations noted between White, non-Hispanic and Hispanic cases that were reported to be HIV-negative.
Cases reported with IDU had more than twice the prevalence of reported neurologic, ocular, and otic manifestations compared to those reported with no IDU when stratifying by reported HIV status (Figure 2c; Table 1, Supplemental Digital Content 1). The prevalence of reported neurologic and ocular manifestations among cases that were reported as HIV-infected and reported with IDU was also approximately twice that of those that were reported as HIV-negative and reported with IDU (neurologic, HIV-infected [n=13, 4.0%] vs. HIV-negative [n=22, 2.3%]; ocular, HIV-infected [n=16, 4.9%] vs. HIV-negative [n=23, 2.4%]). Prevalence of reported otic manifestations was similar by IDU category regardless of reported HIV status.
When stratifying by reported HIV status, sex, and sex of sex partners, women had the highest prevalence of reported neurologic and otic manifestations (neurologic: n=12, 4.0%; otic: n=8, 2.7%) (Figure 2d; Table 1, Supplemental Digital Content 1). For ocular manifestations, MSW who were reported as HIV-infected had the highest prevalence (n=17, 3.6%). MSM had a lower prevalence of reported neurologic and ocular manifestations than MSW and women when stratifying by reported HIV status while MSW had a lower prevalence of reported otic manifestations than MSM and women.
DISCUSSION
Although the prevalence of reported neurologic, ocular, and otic manifestations was low overall among syphilis cases from the 16 jurisdictions included in this analysis, our findings almost certainly underestimate the true burden of these clinical presentations due to underascertainment and underreporting related to variability in assessment and documentation of these manifestations among syphilis cases. Despite these limitations, our findings are useful for describing the minimum burden of these manifestations in the included jurisdictions. While our results demonstrate a low overall reported prevalence of these syphilitic manifestations, prevalence was higher among certain groups (e.g., ocular manifestations among cases reported as HIV-infected with IDU: 4.9%) and over 800 syphilis cases were reported with neurologic, ocular, and/or otic manifestations in 2019 from the 16 included jurisdictions alone, representing a large number of cases with severe, potentially irreversible sequelae of syphilis that were entirely preventable. These data emphasize that syphilis is not a benign condition that can necessarily be treated easily. The variability of presentation and serious nature of these syphilitic complications; reports of neurologic, ocular, and otic manifestations among cases of all demographic, behavioral, and clinical characteristics assessed; and the ongoing increases in syphilis morbidity noted in recent years [3] emphasize the importance of comprehensively evaluating all persons with syphilis for signs and symptoms of neurosyphilis, ocular syphilis, and otosyphilis.
Furthermore, these estimates should only be interpreted as the prevalence of reported neurologic, ocular, and otic manifestations. For a syphilis case with neurologic, ocular, or otic manifestations to be captured by NNDSS, proper diagnosis of syphilis, evaluation for these clinical manifestations, documentation of relevant findings, and reporting to the local/state health department are required. The health department must then verify that the case meets the CSTE case definition, stage the case, classify the manifestations, and report the case and manifestations to CDC via NNDSS. Issues at any step in this process could result in failure to capture a syphilis case with neurologic, ocular, and/or otic manifestations or impact data quality. Also, because these manifestations may present with nonspecific symptoms such as headache or tinnitus, they can easily be missed or mistakenly attributed to other etiologies, further contributing to underascertainment and underreporting. The introduction of the 2018 CSTE case definition updates for these clinical manifestations relatively close in time to the data included in our analyses likely also impacted ascertainment, reporting, and/or data quality for these conditions given the time and expense of staff training and system upgrades in reporting jurisdictions. These case notification challenges further emphasize the importance of interpreting our results as minimum estimates of the prevalence of reported neurologic, ocular, and otic manifestations of syphilis. The true burden of these complications is undoubtedly greater.
Although several reports suggest that the prevalence of otic manifestations among syphilis cases is similar to the prevalence of neurologic and ocular manifestations,[4, 9] the overall prevalence of otic manifestations reported in our analysis was less than half that of reported neurologic and ocular manifestations. The lower reported prevalence of otic manifestations may be related to the nonspecific presentation of otosyphilis (e.g., tinnitus, hearing loss),[1] or a greater focus on neurologic and ocular manifestations of syphilis in previous national sexually transmitted diseases treatment guidelines, surveillance case definitions, and peer-reviewed literature.[8, 10–12] By contrast, updated treatment guidelines place more equal emphasis on clinical assessment of all of these manifestations among syphilis cases, and the 2018 CSTE case definition allows for reporting of isolated or any combination of neurologic, ocular, or otic manifestations.[1, 10] Continued monitoring of trends in the prevalence of these clinical manifestations will improve understanding of the impact of these updates on reporting.
Our finding of a higher prevalence of reported neurologic and ocular manifestations among cases that were HIV-infected compared to those that were HIV-negative is consistent with other studies.[4, 7, 13] Although a higher proportion of cases with otosyphilis were noted to be HIV-infected in a recent case series,[6] our data demonstrated no differences in reported prevalence of otic manifestations when stratifying by HIV status. Our finding that the overall prevalence of neurologic, ocular, and otic manifestations was similarly highest among syphilis cases staged as secondary and those staged as unknown duration or late is consistent with findings from other studies.[14] With the exception of cases reported as HIV-negative with otic manifestations, reported prevalence of these manifestations was generally much higher among late stage compared to early stage syphilis cases when stratifying by HIV status. Although few past population-based studies report prevalence of neurologic, ocular, and otic manifestations stratified by HIV status and syphilis stage, Dombrowski et al noted nearly twice the prevalence of these manifestations among syphilis cases staged as late or unknown duration compared to those staged as early.[9] Consistent with our findings, Dombrowski et al also found that prevalence of these manifestations among syphilis cases assigned an early stage was highest in secondary syphilis cases.[9] While our analysis found that prevalence of neurologic, ocular, and otic manifestations was highest among cases that were HIV-infected and staged as unknown duration or late, the prevalence among HIV-negative cases and those assigned an early stage emphasizes the importance of screening all individuals with syphilis for these clinical manifestations regardless of HIV status or syphilis stage.
The results of our analyses stratified by HIV status and race/Hispanic ethnicity (Figure 2b; Table 1, Supplemental Digital Content 1) suggest that the differences noted in prevalence of neurologic, ocular, and otic manifestations by race and Hispanic ethnicity are not related to HIV status differences. While we cannot discern from available NNDSS data what factors may be contributing to these differences by race/Hispanic ethnicity, we cannot rule out the possibility that differences in sexual networks among racial and/or ethnic groups may be playing a role. Past studies have demonstrated that sexual networks often differ by racial and/or ethnic group,[15, 16] which may result in different strains of T. pallidum circulating in different networks. However, evidence of specific strains of T. pallidum that are more likely to invade the central nervous system, visual system, and/or cochleovestibular system is mixed with some studies suggesting increased neuroinvasive potential for certain strains and other studies failing to find an association between T. pallidum strain and neurologic, ocular, and/or otic manifestations of syphilis.[16, 17] A more likely explanation is that differential ascertainment bias is playing a role. Recent evidence suggests that despite improvements in some measures of healthcare disparities in recent years, racial and ethnic disparities persist.[18] Factors such as implicit bias, structural racism, and reduced access to healthcare negatively impact outcomes among those who identify as persons of Black or African American race and/or persons of Hispanic ethnicity.[19–22] These systemic issues may also be contributing to what appears to be underascertainment of neurologic, ocular, and otic manifestations among cases reported as Black or African American race or Hispanic ethnicity, but further research is needed to understand the reasons for these differences.
The prevalence of neurologic, ocular, and otic manifestations among syphilis cases reported with IDU was nearly three times that of those reported with no IDU, and these prevalence differences remained when stratifying by HIV status. While evidence indicates a recent intersection between the heterosexual syphilis and drug use epidemics,[23, 24] at least one past report noted no association between drug use and ocular syphilis.[12] However, few other recent studies have evaluated for associations between drug use and neurologic, ocular, and otic manifestations of syphilis. Factors such as delayed care-seeking, lack of access to healthcare, comorbid mental health disorders, and IDU-associated co-infections could complicate timely diagnosis and treatment of these clinical manifestations and exacerbate case notification challenges discussed earlier,[25, 26] but interpretation of prevalence differences by IDU category is challenging because more than 40% of cases had missing or unknown IDU status. Future studies of syphilis clinical manifestations should consider evaluating for possible associations between behaviors such as drug use, especially IDU, to determine whether certain behaviors may increase risk of these syphilitic complications.
Consistent with our findings, several past studies have demonstrated increased risk of neurosyphilis among individuals infected with T. pallidum in older age categories,[14, 27] and at least one study has demonstrated increased prevalence of ocular manifestations among older syphilis cases.[12] Although the overall rates of primary and secondary syphilis are higher among younger age categories, rates appear to be increasing faster among the oldest segment of the population.[3] National surveillance data demonstrate that rates of primary and secondary syphilis have more than doubled for individuals in the 55–64 years old and ≥65 years old age groups over the period of 2015–2019.[3] Barriers such as provider discomfort with asking older adults sexual health questions and limited sexual health knowledge among older adults may reduce screening and early detection of sexually transmitted infections in these individuals.[28–30] Among syphilis cases included in our analysis, the increasing reported prevalence of neurologic, ocular, and otic manifestations with age could be explained by factors such as longer duration of unrecognized infection with T. pallidum, more frequent healthcare visits for other underlying comorbidities that increase opportunities for detection, or better access to healthcare among those eligible for Medicare.
Our findings are subject to several additional limitations. Reported prevalence estimates were restricted to a subset of jurisdictions considered to have complete reporting and may not be generalizable to other jurisdictions. This is further complicated by variations in case investigation and reporting practices that may have biased results. Although many syphilis cases reported with ocular and/or otic manifestations likely also had central nervous system involvement, these cases were not all reported as also having neurologic manifestations, further highlighting issues with clinical manifestation identification and reporting. The proportion of missing data for certain variables of interest such as HIV status, sex of sex partners, and IDU limit the interpretability of our findings for these characteristics. Furthermore, the higher prevalence of neurologic and ocular manifestations among HIV-infected persons may be subject to ascertainment bias if this finding reflects a higher likelihood of screening for these clinical manifestations. Additionally, given the limitations of case-based surveillance data, no statistical testing was performed, preventing us from disentangling the relative contributions of various factors to the reported clinical manifestation prevalence estimates.
Finally, while it is reasonable to expect that neurologic, ocular, and otic manifestations among syphilis cases will often be classified as “possible” given the lower threshold of criteria for this surveillance classification relative to “verified” and “likely” classifications, the reduced specificity of the “possible” classification category could have resulted in counting syphilis cases with neurologic, ocular, or otic signs or symptoms caused by something other than syphilis.
CONCLUSIONS
To our knowledge, these analyses provide the first national prevalence estimates of reported neurologic, ocular, and otic manifestations among syphilis cases ≥15 years old of all stages in the United States. Despite the limitations we describe, these estimates are an important step towards understanding the burden of these severe syphilitic complications nationwide. Although prevalence was low overall, our findings are almost certainly an underestimate. As U.S. syphilis prevalence continues to increase, we expect the frequency of reported neurologic, ocular, and otic manifestations will also increase. These findings are a reminder of the significant yet preventable consequences of syphilis, emphasizing the importance of evaluating all individuals with syphilis for clinical signs or symptoms of neurosyphilis, ocular syphilis, and otosyphilis to facilitate early detection and treatment to limit long-term sequelae of these severe syphilitic complications.
Supplementary Material
Footnotes
Case definitions are also available for review at: https://ndc.services.cdc.gov/case-definitions/syphilis-2018/.
Supplemental Digital Content 1.docx
Conflict of interest statements
David A. Jackson has no conflicts of interest.
Robert McDonald has no conflicts of interest.
Laura A. S. Quilter has no conflicts of interest.
Hillard Weinstock has no conflicts of interest.
Elizabeth A. Torrone has no conflicts of interest.
Financial disclosure statements
David A. Jackson has no financial disclosures.
Robert McDonald has no financial disclosures.
Laura A. S. Quilter has no financial disclosures.
Hillard Weinstock has no financial disclosures.
Elizabeth A. Torrone has no financial disclosures.
CDC Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
David A. Jackson, Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Robert McDonald, Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Laura A. S. Quilter, Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Hillard Weinstock, Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Elizabeth A. Torrone, Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
References
- 1.Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep 2021; 70(4): 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marra CM, Deutsch R, Collier AC, et al. Neurocognitive impairment in HIV-infected individuals with previous syphilis. Int J STD AIDS 2013; 24(5): 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. Atlanta, GA: U.S. Department of Health and Human Services, 2021. [Google Scholar]
- 4.Quilter LAS, de Voux A, Amiya RM, et al. Prevalence of Self-reported Neurologic and Ocular Symptoms in Early Syphilis Cases. Clin Infect Dis 2021; 72(6): 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drago F, Merlo G, Ciccarese G, et al. Changes in neurosyphilis presentation: a survey on 286 patients. J Eur Acad Dermatol Venereol 2016; 30(11): 1886–1900. [DOI] [PubMed] [Google Scholar]
- 6.Theeuwen H, Whipple M, Litvack JR. Otosyphilis: Resurgence of an Old Disease. Laryngoscope 2019; 129(7): 1680–1684. [DOI] [PubMed] [Google Scholar]
- 7.de Voux A, Kidd S, Torrone EA. Reported Cases of Neurosyphilis Among Early Syphilis Cases-United States, 2009 to 2015. Sex Transm Dis 2018; 45(1): 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver SE, Aubin M, Atwell L, et al. Ocular Syphilis — Eight Jurisdictions, United States, 2014–2015. MMWR Recomm Rep 2016; 65(43): 1185–1188. [DOI] [PubMed] [Google Scholar]
- 9.Dombrowski JC, Pedersen R, Marra CM, et al. Prevalence Estimates of Complicated Syphilis. Sex Transm Dis 2015; 42(12): 702–704. [DOI] [PubMed] [Google Scholar]
- 10.Council of State and Territorial Epidemiologists. Syphilis (Treponema pallidum): 2018 Case Definition. Available at: https://ndc.services.cdc.gov/case-definitions/syphilis-2018/.
- 11.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(Rr-03): 1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SE, Cope AB, Rinsky JL, et al. Increases in Ocular Syphilis-North Carolina, 2014–2015. Clin Infect Dis 2017; 65(10): 1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope AB, Mobley VL, Oliver SE, et al. Ocular Syphilis and Human Immunodeficiency Virus Coinfection Among Syphilis Patients in North Carolina, 2014–2016. Sex Transm Dis 2019; 46(2): 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, Peng RR, Gao Z, et al. Risk profiles of neurosyphilis in HIV-negative patients with primary, secondary and latent syphilis: implications for clinical intervention. J Eur Acad Dermatol Venereol 2016; 30(4): 659–666. [DOI] [PubMed] [Google Scholar]
- 15.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis 2005; 191 Suppl 1: S115–S122. [DOI] [PubMed] [Google Scholar]
- 16.Marra C, Sahi S, Tantalo L, et al. Enhanced molecular typing of treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis 2010; 202(9): 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver S, Sahi SK, Tantalo LC, et al. Molecular Typing of Treponema pallidum in Ocular Syphilis. Sex Transm Dis 2016; 43(8): 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. 2019 National Healthcare Quality and Disparities Report. Rockville: U.S. Department of Health and Human Services, 2020. [Google Scholar]
- 19.Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works - Racist Policies as a Root Cause of U.S. Racial Health Inequities. N Engl J Med 2021; 384(8): 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017; 18(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall WJ, Chapman MV, Lee KM, et al. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am J Public Health 2015; 105(12): e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville: U.S. Department of Health and Human Services, 2016. [PubMed] [Google Scholar]
- 23.Kidd SE, Grey JA, Torrone EA, et al. Increased Methamphetamine, Injection Drug, and Heroin Use Among Women and Heterosexual Men with Primary and Secondary Syphilis - United States, 2013–2017. MMWR Recomm Rep 2019; 68(6): 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reno H, Fox B, Highfill C, et al. The Emerging Intersection Between Injection Drug Use and Early Syphilis in Nonurban Areas of Missouri, 2012–2018. J Infect Dis 2020; 222(Suppl 5): S465–S470. [DOI] [PubMed] [Google Scholar]
- 25.Serota DP, Chueng TA, Schechter MC. Applying the infectious diseases literature to people who inject drugs. Infect Dis Clin North Am 2020; 34(3): 539–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug–related infections are missed: A population-based cohort study of inpatient and emergency department settings. Clin Infect Dis 2019; 68(7): 1166–1175. [DOI] [PubMed] [Google Scholar]
- 27.Cai SN, Long J, Chen C, et al. Incidence of asymptomatic neurosyphilis in serofast Chinese syphilis patients. Sci Rep 2017; 7(1): 15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan-Ing M, Seidel L, Ansell P, et al. Addressing sexual health in geriatrics education. Gerontol Geriatr Educ 2018; 39(2): 249–263. [DOI] [PubMed] [Google Scholar]
- 29.Ports KA, Barnack-Tavlaris JL, Syme ML, et al. Sexual health discussions with older adult patients during periodic health exams. J Sex Med 2014; 11(4): 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith ML, Bergeron CD, Goltz HH, et al. Sexually Transmitted Infection Knowledge among Older Adults: Psychometrics and Test-Retest Reliability. Int J Environ Res Public Health 2020; 17(7): 2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


