Abstract
Purpose:
Greater articular cartilage T1ρ magnetic resonance imaging relaxation times indicate less proteoglycan density and are linked to posttraumatic osteoarthritis development following anterior cruciate ligament reconstruction (ACLR). While changes in T1ρ relaxation times are associated with gait biomechanics, it is unclear if excessive or insufficient knee joint loading is linked to greater T1ρ relaxation times 12 months post-ACLR. The purpose of this study was to compare external knee adduction (KAM) and flexion (KFM) moments in individuals after ACLR with high vs. low tibiofemoral T1ρ relaxation profiles and uninjured controls.
Methods:
Gait biomechanics were collected in 26 uninjured controls (50% females, age 22±4 yrs., BMI 23.9±2.8 kg/m2) and 26 individuals after ACLR (50% females, age 22±4 yrs., BMI 24.2±3.5 kg/m2) at 6 and 12 months post-ACLR. ACLR-T1ρHigh (n=9) and ACLR-T1ρLow (n=17) groups were created based on 12-month post-ACLR T1ρ relaxation times using a k-means cluster analysis. Functional analyses of variance were used to compare KAM and KFM.
Results:
ACLR-T1ρHigh exhibited lesser KAM than ACLR-T1ρLow and Uninjured Controls 6 months post-ACLR. ACLR-T1ρLow exhibited greater KAM than Uninjured Controls 6 and 12 months post-ACLR. KAM increased in ACLR-T1ρHigh and decreased in ACLR-T1ρLow between 6-12 months, both groups becoming more similar to Uninjured Controls. There were scant differences in KFM between ACLR-T1ρHigh and ACLR-T1ρLow 6 or 12 months post-ACLR, but both groups demonstrated lesser KFM compared to Uninjured Controls.
Conclusions:
Associations between worse T1ρ profiles and increases in KAM may be driven by the normalization of KAM in individuals who initially exhibit insufficient KAM 6-months post-ACLR.
Keywords: POSTTRAUMATIC OSTEOARTHRITIS, T1ρ, MAGNETIC RESONANCE IMAGING, KNEE ADDUCTION MOMENT, GAIT
INTRODUCTION
Between 50-90% of anterior cruciate ligament (ACL) injured patients develop post-traumatic knee osteoarthritis (PTOA) despite undergoing surgical ACL reconstruction (ACLR) and therapeutic exercise (1). Aberrant knee kinetics during gait have been linked to the progression of posttraumatic osteoarthritis (PTOA) in patients with ACLR (2, 3). Specifically, both altered frontal (external knee adduction [KAM]) and sagittal (external knee flexion [KFM]) plane knee moments contribute to joint loading (4) and early changes in tibiofemoral cartilage composition following ACLR measured using T1ρ magnetic resonance imaging (MRI) (5-8). Greater T1ρ MRI relaxation times indicate lesser articular cartilage proteoglycan density which is an early in vivo indicator of joint tissue changes linked to PTOA development (9). As early as 12-months post-ACLR, tibiofemoral articular cartilage on the ACL injured limb exhibit higher T1ρ relaxation times compared to the contralateral uninjured limb (10) and reference limbs of uninjured controls (11). The association between T1ρ MRI and gait kinetics suggests that modifying gait kinetics may be a viable intervention for mitigating early compositional cartilage changes related to PTOA development. Unfortunately, it remains unclear whether the changes in tibiofemoral proteoglycan density are related to excessively high, insufficiently low, or a combination of high and low, peak KAM and KFM magnitudes during gait as both greater and lesser knee gait moments reportedly associate with greater T1ρ MRI relaxation times (2, 3, 16, 17, 5-8, 12-15). A greater understanding of the relationship between peak KAM and KFM magnitudes and articular cartilage health following ACLR would improve the ability to direct the most optimal biomechanical PTOA prevention interventions following ACLR.
Greater initial peak KAM has historically been associated with the progression of idiopathic knee osteoarthritis severity and greater initial peak KAM is hypothesized to hasten tibiofemoral cartilage breakdown by excessively loading the medial compartment during gait (12, 13, 18). Similarly, having controlled for walking speed, individuals with higher initial peak KAM 18 ± 10 months following ACLR exhibit greater T1ρ MRI relaxation times compared to those with lesser initial peak KAM (7), suggesting that excessive kinetics may contribute to PTOA onset. Furthermore, in a longitudinal study adjusting for age, sex, BMI and concomitant injury, greater initial peak KFM at 6 months post-ACLR was associated with greater T1ρ MRI relaxation times between 1- and 2-years post-ACLR (5). Finally, having controlled for walking speed, increased initial peak KAM between pre-operative and 6 months post-ACLR timepoints was associated with increased T1ρ MRI relaxation times between the same timepoints (6). Overall, these studies concluded that greater initial peak knee moments are associated with deleterious changes in cartilage composition and seem to implicate excessive gait kinetics with peak magnitudes that exceed values that would be normal in uninjured controls, as a potential mechanism associated with the development of PTOA following ACLR.
Conversely, others have demonstrated that lesser initial peak KFM and KAM associates with PTOA-related markers, including T1ρ MRI relaxation times. ACLR patients exhibit lesser initial peak KAM and KFM through the first 12-70 months following ACLR compared to uninjured individuals (3, 14-16). Further, a previous cross-sectional study reported that while statistically accounting for walking speed and concomitant injury, lesser initial peak KAM was associated with greater T1ρ MRI relaxation times for both the medial and lateral femoral cartilage 6 months following ACLR (8). A separate longitudinal study of individuals after ACLR with no pain or effusion at the time of enrollment demonstrated that individuals with lesser KAM in the involved limb compared to contralateral limb at 6 months post-ACLR were more likely to develop PTOA 5-years post-ACLR (2). These data suggest that individuals with lesser initial peak KFM and KAM may be insufficiently loading the injured joint in the first 6-12 months following ACLR; thereby exerting lesser than normal loads to the joint and failing to stimulate optimal tissue metabolism (17), thus increasing the likelihood that joint tissues undergo early PTOA related changes.
The current literature evaluating associations between initial peak KFM and KAM and T1ρ MRI relaxation times remains contradictory. Without the contextualization provided by comparisons to a control group, it remains unclear if ACLR patients with greater T1ρ MRI relaxation times demonstrate abnormally greater (i.e., excessive loading) or lesser (i.e., insufficient loading) peak knee moments relative to uninjured controls. Understanding the magnitude of KAM and KEM relative to uninjured controls is critical for determining how best to prescribe changes to gait biomechanics in the future to prevent PTOA following ACLR. Additionally, differences in KFM and KAM during periods of stance other than the initial peaks may exist. Therefore, the purpose of this study was to compare KAM and KFM throughout the entire stance phase at 6 and 12 months post-ACLR between ACLR individuals with high vs. low tibiofemoral cartilage T1ρ MRI relaxation profiles at 12 months post-ACLR and uninjured controls. We hypothesized that individuals with high T1ρ MRI relaxation profiles would demonstrate lesser KAM and KFM throughout stance compared to the individuals with low T1ρ relaxation times and uninjured controls at both 6 and 12 months post-ACLR. Additionally, we hypothesized that KAM and KFM in individuals with low T1ρ MRI relaxation profiles at 12 months post-ACLR would not differ from uninjured controls at both 6 and 12 months post-ACLR.
METHODS
Study Design
All individuals after ACLR participated in a prospective longitudinal cohort study that consisted of two study related visits (i.e., 6 months post-ACLR and 12 months post-ACLR). As part of the current study, we included an embedded comparison-control design to compare gait biomechanics of uninjured controls from a single timepoint and of individuals after ACLR at both 6 and 12 months post-ACLR retrospectively assigned into subgroups (ACLR-T1ρLow and ACLR-T1ρHigh). ACLR participants were recruited from a local orthopaedic clinic in a consecutive order. Uninjured Controls were recruited from the University and surrounding community to match the ACLR group. All participants provided written informed consent approved by the University’s Institutional Review Board prior to participating in any research related procedures.
Participants
Twenty-six individuals after ACLR and 26 Uninjured Controls between the ages of 16 and 35 years were enrolled in the study. All individuals after ACLR and Uninjured Controls met the following inclusion criteria: (1) a BMI between 18-35 kg/m2, (2) no history of neurological disorder, (3) no lower extremity joint injury in the previous 6 months (other than the initial ACL injury), (4) no previous diagnosis of any diseases that affect joints, (5) no history of osteoarthritis, and (6) not currently pregnant. All ACL injured individuals underwent arthroscopically assisted bone-patellar tendon-bone autograft ACLR from one of three participating orthopeadic surgeons. All individuals after ACLR were prescribed physical therapy and were provided a standardized timeline for rehabilitation goals adapted from a review of best practice guidelines (19). Individuals enrolled in the Uninjured Control group were physically active and had no history of: (1) lower or upper extremity joint surgery, (2) ligamentous knee injury, (3) concussion or head injury in the previous 6 months, (4) chronic ankle instability or balance disorders, and (5) cardiac condition or stroke. Uninjured Controls were sex and BMI (±2 kg/m2) matched to the ACLR participants.
A previous study (20) demonstrated a moderate effect (0.61BWxHeight; d = 0.65) for the largest magnitude differences in KFM waveforms between individuals after ACLR with low and high concentrations of inflammatory cytokines throughout stance using the functional waveform gait analysis. We utilized parameters consistent with previously published literature (20-22) to define the calculation of mean differences between the groups and variability estimates across the waveform using 5 gait trials from each participant. Therefore, we estimated that groups with 8 individuals (with 5 gait trials) would be capable of detecting a statistically significant moderate mean difference between waveforms, assuming similar inter-trial variability as previously reported (two tailed alpha= 0.05; 1-ß = 0.8; G*Power Statistical power Analysis Software v3 (23).
Procedures
Gait biomechanics and Knee Injury and Osteoarthritis Outcome Scores (KOOS) (24) were collected on individuals after ACLR at the 6-month and 12-months post-ACLR sessions. At the 12-month post-ACLR session, MRI T1ρ relaxation times were collected on the involved limb of the individuals after ACLR following a 30-minute period of unloading where participants remained seated to unload the knee cartilage. Gait biomechanics were collected on uninjured controls at a single timepoint.
Walking Gait Biomechanics Collection and Processing
All participants were outfitted with 26 retroflective markers and a rigid cluster of three retroreflective markers placed over the sacrum (25). A static trial was collected to create a segment-linkage model prior to testing. Participants were instructed to “walk at a speed as if you were comfortably walking on a sidewalk” over a 6-meter walkway. Practice trials were conducted until participants felt comfortable walking in the laboratory. Preferred gait speed was then measured and averaged over five walking trials via infrared timing gates (TF100, Trac Tronix, Lenexa, Kansas, United States). Force data from 2 staggered and embedded force-plates (40 × 60 cm, FP406010, Bertec Corporation, Columbus, Ohio, United States) and marker position data from a 10-camera motion capture system (Vicon, Nexus, Denver, Colorado, United States) from five error-free walking trials were then collected at 1200 Hz and 120 Hz, respectively. Errors were considered if participants: (1) failed to individually strike an individual force plate with each foot, (2) exceeded walking speed by ±5% of the pre-determined self-selected walking speed, and (3) underwent any visible alterations to gait during the trial (e.g., trip or stutter step). Kinematic and kinetic data were low-pass filtered at 10 Hz (4th order Butterworth). We evaluated kinematic and kinetic outcomes from the injured limb of the ACLR group. Approximately, 69% of the individuals after ACLR injured their dominant limb; therefore, we assessed the dominant limb for 69% of the Uninjured Controls via random assignment (26). The dominant limb was defined as the limb chosen to kick a ball.
Biomechanical outcomes during the stance phase of walking were analyzed on a global coordinate system using Visual3D software (C-Motion, Germantown, Maryland, United States). Hip joint centers were estimated using the Bell and Brand hip joint CODA coordinate system (27). Knee and ankle joint centers were identified using a radius half the distance between the medial and lateral epicondyles and malleoli, respectively. Knee kinematics were calculated based on the angle of the shank relative to the thigh using Euler angles (sagittal/frontal/transverse sequence) such that knee flexion, adduction, and internal rotation represented positive values. The resulting knee frontal angle during the standing calibration trials was used as a proxy measurement of knee alignment on the individuals after ACLR. Joint moments were calculated using anthropometrics, synchronized kinematics and ground reaction force data, and a standard inverse dynamics approach on Visual3D software (C-Motion, Germantown, MD). Joint moments were reported as external moments and normalized to the product of body weight (N) and height (m). Data during the stance phase of walking were time normalized to 101 data points prior to analysis (20, 22).
Magnetic Resonance Image Acquisition, Registration, and Segmentation
MRI was collected on a Siemens Magnetom TIM Trio 3-T scanner with a 4-channel Siemens larger flex coil (516 × 224 mm; Siemens Munich Germany) or a Siemens Magnetom Prisma 3-T Powerpack scanner with an XR 80/200 gradient coil (60 × 213 cm; Siemens) using parameters described in Table 1 (10, 28). Excellent inter-scanner reliability of both the medial (ICC2,1 = 0.99) and lateral (ICC2,1 = 0.96) tibiofemoral compartments have been reported (8). Voxel by voxel T1ρ relaxation times were calculated as previously reported(10, 28, 29) using a five-image T1ρ spin-lock sequence and curve-fit with a MATLAB program [MATLAB R2014b (8.4.0) MathWorks, Natick, MA, USA] to Equation 1(30):
| (Equation 1) |
where TSL is the time of the spin-lock, S0 is signal intensity when TSL equals zero, S corresponds to signal intensity, and T1ρ is the T1 relaxation time in the rotating frame. The articular cartilage of the medial and lateral femoral and tibial condyles acquired during the 0 ms spin-lock duration was manually segmented using ITK-SNAP software (version 3.6; http://www.itksnap.org) (31). Specifically, the entire weight-bearing regions of the femoral and tibial condyles were evaluated by identifying the articular cartilage between the posterior edge of the posterior horn of the meniscus and the anterior edge of the anterior horn of the meniscus in the sagittal plane. The femoral and tibial articular cartilage were further sub-sectioned into 4 regions of interest (ROI) including: [1] the medial femoral condyle (Medial Femoral Cartilage; MFC), [2] the lateral femoral condyle (Lateral Femoral Cartilage; LFC), [3] the medial tibial condyle (Medial Tibial Cartilage; MTC), and [4] the lateral tibial condyle (Lateral Tibial Cartilage; LFC). The medial and lateral condyles were separated based on the center of the intercondylar notch of femur in the coronal plane. T1ρ relaxation time means were calculated as an average of each region of interest that extended coronally from the intercondylar notch to the most medial or lateral portion of the MFC or LFC, respectively. This segmentation method has previously been reported to demonstrate strong test–retest reliability (10). Higher T1ρ relaxation times are interpreted as tissue consisting of lower proteoglycan density (30).
Table 1. Participant Demographics.
Table 1 shows the parameters of the Siemens 3T scanner used to determine T1ρ relaxation times.
| T1ρ Parameters | |
|---|---|
| Sequence | Fast Low Angle Shot (FLASH) |
| Spin Lock Frequency | 500 Hz |
| Spin-lock Durations | 40, 30, 20, 10, 0 ms |
| Voxel Size | 0.8 mm × 0.4 mm × 3 mm |
| Field of View | 288 mm |
| Slice Thickness | 3.0 mm |
| Repetition Time | 9.2 ms, 160 × 320 matrix |
| Gap | 0 mm |
| Flip Angle | 10° |
| Echo-Train Duration Time | 443 ms |
| Phase Encode Direction | Anterior/Posterior |
Statistical Analyses: k-means Cluster Analysis
A k-means cluster analysis was used to identify groups of individuals after ACLR with similar tibiofemoral T1ρ MRI relaxation times profiles at 12 months post-ACLR. We were then able to utilize a waveform analysis to KAM and KFM throughout the entire stance phase. Z-scores were calculated for the T1ρ relaxation times of the regions of interest (i.e., LFC, LTC, MFC, and MTC) and used in the k-means cluster analysis to reduce the potential influence of differing scale magnitudes between the regions. To determine the number of clusters used in the analysis, a Silhouette Ranking Measure of mean silhouette coefficients was calculated and compared between two- and three-mean cluster analyses. The silhouette coefficients were similar between the two and three mean cluster silhouette coefficients (coefficient = 0.17); therefore, we chose to proceed with the two-mean cluster analysis in order to retain the maximum number of participants per group. The cluster analysis led to the division of individuals after ACLR into two groups, one group with high average T1ρ relaxation times (ACLR-T1ρHigh) and one group with low T1ρ relaxation times (ACLR-T1ρLow). Independent t-tests were used to compare LFC, LTC, MFC, and MTC T1ρ relaxation times between ACLR-T1ρHigh and ACLR-T1ρLow groups. We compared demographic and patient reported outcomes between ACLR-T1ρHigh and ACLR-T1ρLow using independent t-tests or chi square tests for continuous and dichotomous variables, respectively.
Statistical Analyses: Gait Analyses
We performed functional waveform gait analyses (20, 22, 32) to evaluate planned comparisons between [1] ACLR-T1ρHigh and ACLR-T1ρLow, [2] ACLR-T1ρHigh and Uninjured Controls, and [3] T1ρLow and Uninjured Controls. The functional waveform gait analysis allows for the detection of between group differences throughout stance for biomechanical outcomes of interest. Functional waveform gait analyses were performed using version 0.3.0 of the bayesFDA package within version 2.2.6 of the R statistical computing software. The bayesFDA package is a modified version of the warptk package proposed by Horton et. al. (2020) in which Bayesian P-splines were fit (34) and average waveforms for each group were estimated and used to compute difference curves and 95% confidence intervals. Comparisons between waveforms were considered different at any percentile of the stance phase where mean differences and corresponding 95% confidence intervals did not cross zero. We reported the largest difference between the ensemble curves and corresponding between-group effect sizes (Cohen's d) within the proportions of stance demonstrating differences for each comparison as described above.
RESULTS
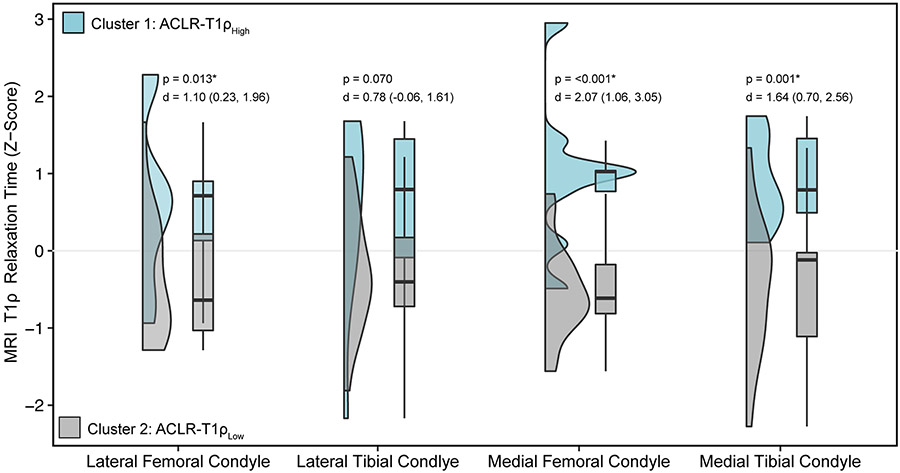
No significant differences were found between ACLR-T1ρHigh and ACLR-T1ρLow demographic and patient reported outcome variables (Table 2). MRI T1ρ relaxation times for the LFC, MFC, and MTC condyles were significantly higher in ACLR-T1ρHigh compared to ACLR-T1ρLow (Figure 1). Percentages of stance phase exhibiting mean differences and corresponding effect sizes for KFM and KAM are presented in Table 3. A figure distinguishing the individual KFM and KAM waveforms for the ACLR-T1ρHigh and ACLR-T1ρLow participants can be found in the supplement (see Supplemental Figure 1, Supplemental Digital Content, SDC 1, Individual knee sagittal and frontal moment waveforms of ACLR-T1ρHigh and ACLR-T1ρLow at 6 months and 12 months post-ACLR).
Table 2. Participant Demographics and Patient Reported Outcome Measures.
Table 2 shows the demographical, injury, and time data associated with the Uninjured Controls and ACLR Individuals.
| Uninjured Control Group |
Entire ACLR Group |
P-value between Uninjured Control and ACLR Groups |
ACLR-T1ρHigh | ACLR-T1ρLow | P-value between ACLR-T1ρHigh and ACLR-T1ρLow |
|
|---|---|---|---|---|---|---|
| n | 26 | 26 | NA | 9 | 17 | NA |
| Sex (Female) | 13 | 13 | 1.000 | 6 | 7 | 0.216 |
| Age (yrs.) | 21.8 ± 3.5 | 22.19 ± 3.95 | 0.684 | 21.22 ± 2.4 | 22.7 ± 4.5 | 0.373 |
| Height (m) | 1.77 ± 0.11 | 1.77 ± 0.11 | 0.973 | 1.81 ± .15 | 1.77 ± 0.78 | 0.238 |
| 6 Month Body Mass Index (kg/m2) | 23.9 ± 2.8 | 24.2 ± 3.5 | 0.998 | 22.9 ± 0.87 | 24.9 ± 4.1 | 0.176 |
| 6 Month Gait Speed (m/s) | 1.27 ± 0.13 | 1.24 ± 0.12 | 0.416 | 1.23± 0.12 | 1.25 ± 0.13 | 0.639 |
| 12 Month Gait Speed (m/s) | NA | 1.20 ± 0.14 | NA | 1.20 ± 0.12 | 1.20 ± 0.15 | 0.960 |
| Chondral Injury | NA | 11 | NA | 5 | 6 | 0.383 |
| Lateral Meniscus Tear | NA | 13 | NA | 7 | 6 | 0.629 |
| Medial Meniscus Tear | NA | 7 | NA | 1 | 6 | 0.158 |
| Lateral Meniscus Repair | NA | 8 | NA | 3 | 5 | 0.915 |
| Medial Meniscus Repair | NA | 7 | NA | 1 | 6 | 0.158 |
| Any Concomitant Injury | NA | 23 | NA | 9 | 14 | 0.180 |
| KOOS Symptoms Score (6 Month) | NA | 76.9 ± 15.8 | NA | 84.9 ± 6.5 | 73.2 ± 17.6 | 0.085 |
| KOOS Pain Score (6 Month) | NA | 83.2 ± 12.9 | NA | 86.8 ± 6.8 | 81.5 ± 14.9 | 0.358 |
| KOOS Activities of Daily Living Score (6 Month) | NA | 93.74 ± 12.2 | NA | 95.6 ± 4.7 | 92.7 ± 14.6 | 0.589 |
| KOOS Sports Score (6 Month) | NA | 64.4 ± 20.2 | NA | 68.8 ± 18.5 | 62.3 ± 21.1 | 0.471 |
| KOOS Quality of Life Score (6 Month) | NA | 54.04 ± 17.3 | NA | 57.9 ± 16.3 | 57.9 ± 16.3 | 0.459 |
| KOOS Symptoms Score (12 Month) | NA | 86.3 ± 10.7 | NA | 85.1 ± 10.5 | 87.0 ± 11.1 | 0.680 |
| KOOS Pain Score (12 Month) | NA | 92.6 ± 6.8 | NA | 90.9 ± 8.5 | 93.5 ± 5.8 | 0.366 |
| KOOS Activities of Daily Living Score (12 Month) | NA | 97.7 ± 3.7 | NA | 96.8 ± 5.4 | 98.2 ± 2.4 | 0.343 |
| KOOS Sports Score (12 Month) | NA | 85.4 ± 15.1 | NA | 83.9 ± 22.5 | 86.2 ± 10.1 | 0.721 |
| KOOS Quality of Life Score (12 Month) | NA | 76.5 ± 17.2 | NA | 72.2 ± 21.3 | 78.8 ± 14.9 | 0.362 |
| Time Between Surgery and 6mo Biomechanics (Days) | NA | 204.3 ± 26.7 | NA | 206.5 ± 27.4 | 203.1 ± 27.2 | 0.780 |
| Time Between Surgery and 12mo Biomechanics (Days) | NA | 372.2 ± 17.8 | NA | 368.1 ± 16.3 | 374.4 ± 18.7 | 0.406 |
| 6 Month Knee Alignment Angle (Degrees) | NA | −0.59 ± 3.89 | NA | −1.21 ± 4.34 | −0.21 ± 4.12 | 0.617 |
| 12 Month Knee Alignment Angle (Degrees) | NA | −0.17 ± 4.13 | NA | −1.28 ± 4.54 | 0.42 ± 4.04 | 0.336 |
ACLR: Anterior Cruciate Ligament Reconstruction; KOOS: Knee Injury and Osteoarthritis Outcome Score
Figure 1. MRI T1ρ Relaxation Times of k-Means Clusters.
Figure 1 shows the distributions of lateral and medial femoral and tibial condyles z-scores for the ACLR-T1ρHigh and ACLR-T1ρLow groups using violin and box and whisker plots. Box and whisker plots display the minimum, first quartile, median, third quartile, and maximum z-scores. Associated p values and Cohen’s d effect sizes and associated 95% confidence intervals are provided above the plots for each comparison.
Table 3. Portions of Stance Demonstrating Relevant Mean Differences and Corresponding Peak Mean Differences.
Table 3 shows the Peak Mean Differences of knee frontal and sagittal moments between both ACLR-T1ρHigh and ACLR-T1ρLow as well as between the Uninjured Control group and each ACLR-T1ρHigh and ACLR-T1ρLow.
| External Knee Frontal Moment (BW×Height) | External Knee Sagittal Moment (BW×Height) | ||||||
|---|---|---|---|---|---|---|---|
| Portions of Stance with Differences between Ensemble Curves (%) |
Largest Difference Between Ensemble Curves± (Corresponding % of stance) |
Effect Size (95% Confidence Interval) of Largest Difference Between Ensemble Curves |
Portions of Stance with Differences between Ensemble Curves (%) |
Largest Difference Between Ensemble Curves± (Corresponding % of stance) |
Effect Size (95% Confidence Interval) of Largest Difference Between Ensemble Curves |
||
| 6 Months Post-ACLR | ACLR-T1ρHigh minus ACLR-T1ρLow | 11-47 | −0.0723 (24%) | d=−0.67 (−1.00, −0.34) | 13-20 | 0.0411 (16%) | d=0.37 (0.05, 0.69) |
| 59-95 | −0.0765 (80%) | d=−0.83 (−1.16, −0.49) | |||||
| ACLR-T1ρHigh minus Uninjured Controls | 17-27 | −0.0410 (21%) | d=−0.46 (−0.77, −0.16) | 14-27 | −0.0498 (21%) | d=−0.41 (−0.71, −0.11) | |
| 78-88 | −0.0281 (81%) | d=−0.33 (−0.64, −0.03) | 42-90 | 0.0943 (72%) | d=1.30 (0.97, 1.62) | ||
| ACLR-T1ρLow minus Uninjured Controls | 4-98 | 0.0487 (79%) | d=0.52 (0.28, 0.76) | 9-33 | −0.0843 (19%) | d=−0.68 (−0.92, −0.43) | |
| 48-89 | 0.0807 (71%) | d=1.21 (0.95, 1.47) | |||||
| 12 Months Post-ACLR | ACLR-T1ρHigh minus ACLR-T1ρLow | 18-33 | −0.0443 (24%) | d=−0.50 (−0.81, −0.19) | 13-18 | 0.0334 (16%) | d=0.33 (0.02, 0.33) |
| 59-85 | −0.0300 (61%) | d=−0.47 (−0.78, −0.16) | 53-46 | 0.0357 (55%) | d=0.46 (0.15, 0.78) | ||
| ACLR-T1ρHigh minus Uninjured Controls | NA | NA | NA | 12-31 | −0.0733 (22%) | d=−0.61 (−0.90, −0.32) | |
| 53-83 | 0.0511 (72%) | d=−0.78 (0.46, 1.05) | |||||
| ACLR-T1ρLow minus Uninjured Controls | 4-13 | 0.0222 (8%) | d=0.57 (0.33, 0.81) | 8-31 | −0.0970 (19%) | d=−0.82 (−1.06, −0.57) | |
| 21-92 | 0.0452 (74%) | d=0.50 (0.26, 0.75) | 69-82 | 0.0311 (74%) | d=−0.44 (0.20, 0.68) | ||
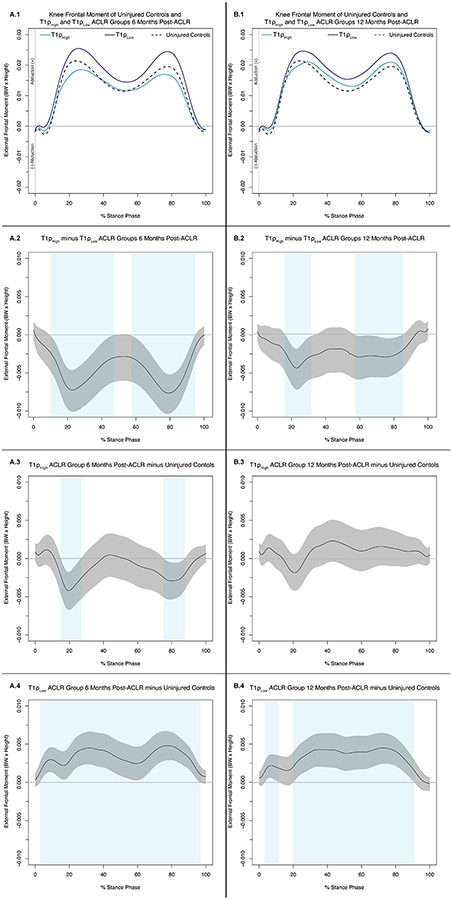
Knee Adduction Moment Differences
6 Months Post-ACLR (Figure 2A.1):
Figure 2: Knee Frontal Moments (.1) and Mean Difference Curves (.2-.4) of Uninjured Controls and ACLR-T1ρHigh and ACLR-T1ρLow Individuals at 6 Months (A) and 12 Months Post-ACLR (B).
Figures 3A.1 and 3B.1 depict mean ensemble waveforms plotted over the stance phase of walking, for mean knee frontal moments normalized to body weight (BW × Height), for the Uninjured Controls and ACLR-T1ρHigh and ACLR-T1ρLow at 6 Months (A) and 12 Months Post-ACLR. Figures 3A.2-4 and 3B.2-4 depict corresponding pairwise comparison functions, and associated 95% confidence intervals (grey bands), indicating the mean differences between ACLR-T1ρHigh and ACLR-T1ρLow (.2), Uninjured Controls and ACLR-T1ρHigh (.3), and Uninjured Controls and ACLR-T1ρLow (.4) at 6 (A) and 12 (B) months post-ACLR. Differences between conditions existed whenever the 95% confidence intervals did not cross zero and are indicated with light blue bands.
KAM was lesser in ACLR-T1ρHigh compared to ACL-T1ρLow between 11-47% and 59-95% of stance (Figure 2A.2) and compared to Uninjured Controls between 17-27% and 78-88% of stance (Figure 2A.3). KAM was higher in ACL-T1ρLow compared to Uninjured Controls between 4-98% of stance (Figure 2A.4).
12 Months Post-ACLR (Figure 2B.1):
KAM was lesser in ACLR-T1ρHigh compared to ACL-T1ρLow between 18-33% and 59-85% of stance (Figure 2B.2). There were no differences between the ACLR-T1ρHigh compared to Uninjured Controls (Figure 2B.3). KAM was higher in ACL-T1ρLow compared to Uninjured Controls between 4-13 and 21-92% of stance (Figure 2B.4).
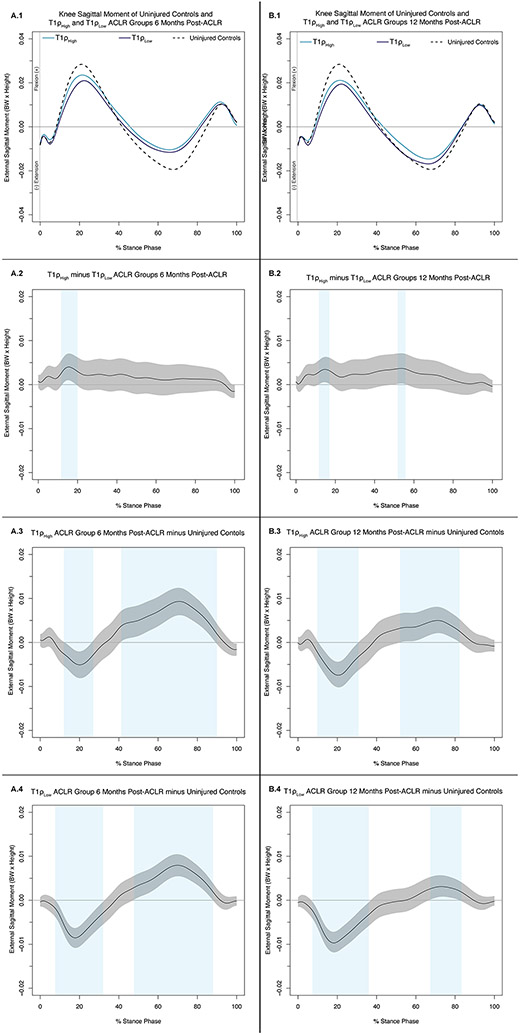
Knee Flexion Moment Differences
6 Months Post-ACLR (Figure 3A.1):
Figure 3: Knee Sagittal Moments (.1) and Mean Difference Curves (.2-.4) of Uninjured Controls and ACLR-T1ρHigh and ACLR-T1ρLow at 6 Months (A) and 12 Months Post-ACLR (B).
Figures 2A.1 and 2B.1 depict mean ensemble waveforms plotted over the stance phase of walking, for mean knee sagittal moments normalized to body weight (BW × Height), for the Uninjured Controls and ACLR-T1ρHigh and ACLR-T1ρLow at 6 Months (A) and 12 Months Post-ACLR. Figures 2A.2-4 and 2B.2-4 depict corresponding pairwise comparison functions, and associated 95% confidence intervals (grey bands), indicating the mean differences between ACLR-T1ρHigh and ACLR-T1ρLow (.2), Uninjured Controls and ACLR-T1ρHigh (.3), and Uninjured Controls and ACLR-T1ρLow (.4) at 6 (A) and 12 (B) months post-ACLR. Differences between conditions existed whenever the 95% confidence intervals did cross zero and are indicated with light blue bands.
KFM was greater in ACLR-T1ρHigh compared to ACL-T1ρLow between 13-20% (Figure 3A.2). KFM and external knee extension moment (KEM) were lesser in magnitude in ACLR-T1ρHigh compared to Uninjured Controls between 14-27% and 42-90% of stance, respectively (Figure 3A.3). KFM and KEM were lesser in magnitude in ACLR-T1ρLow compared to Uninjured Controls between 9-33% and 48-89% of stance, respectively (Figure 3A.4).
12 Months Post-ACLR (Figure 3B.1):
KFM and KEM were greater in ACLR-T1ρHigh compared to ACL-T1ρLow between 13-18% and 53-56% of stance, respectively (Figure 3B.2). KFM and KEM were lesser in magnitude in ACLR-T1ρHigh compared to Uninjured Controls between 12-31% and 53-83% of stance, respectively (Figure 3B.3). KFM and KEM were lesser in magnitude in ACLR-T1ρLow compared to Uninjured Controls between 8-31% and 69-82% of stance, respectively (Figure 3B.4).
DISCUSSION
Consistent with our hypothesis, individuals after ACLR with higher T1ρ profiles exhibited lesser KAM 6 months post-ACLR compared to both Uninjured Controls and individuals after ACLR with lower T1ρ profiles. While our results demonstrate higher KFM prior to the initial peak KFM in the ACLR-T1ρHigh group compared to the ACLR-T1ρLow group at 6 and 12 months post-ACLR, both ACLR groups demonstrated lesser peak magnitudes of KFM compared to Uninjured Controls 6 and 12 months post-ACLR. Individuals with lower T1ρ profiles exhibited higher KAM 6 months post-ACLR compared to Uninjured Controls. At 12 months post-ACLR, KAM in both the ACLR-T1ρHigh and ACLR-T1ρLow groups became more similar to that of the Uninjured Controls, thus suggesting a normalization of KAM profiles with the ACLR-T1ρHigh increasing KAM from lesser than normal at 6 months and the ACLR-T1ρLow group decreasing KAM from higher than normal at 6 months. Our results are consistent with previous findings (6) reporting that increased KAM associates with worse T1ρ profiles. The inclusion of uninjured controls in our study suggests that individuals with worse T1ρ profiles who increase in KAM between 6 and 12 months do not display excessive loading, but rather are returning to KAM values more similar to the KAM values of uninjured controls. Therefore, comparing subsets of ACLR KAM profiles to uninjured controls seems critical for fully interpreting the nature of aberrant kinetics that associate with deleterious cartilage changes. These findings are important as they may be used to direct future interventions that seek to apply personalized modifications in knee kinetics to normalize gait biomechanics for the purpose of maximizing joint tissue health.
We expected ACLR-T1ρHigh individuals to demonstrate lesser KAM at 6 months post-ACLR compared to ACLR-T1ρLow individuals and Uninjured Controls as associations between lesser KAM and worse T1ρ profiles are supported by previous studies (2, 8). Specifically, lesser KAM has been associated with greater T1ρ relaxation times at 6 months post-ACLR (8) and individuals who developed PTOA 5 years post-ACLR demonstrated lesser KAM at 6 months post-ACLR compared to individuals 5 years post-ACLR without PTOA (2). While the ACLR-T1ρHigh group exhibited lower KAM peaks compared to Uninjured Controls at 6 months post-ACLR, there were no differences between ACLR-T1ρHigh and the Uninjured Controls 12 months post-ACLR. While this difference between time points was contrary to our hypothesis, the magnitudes of KAM in ACLR-T1ρHigh at 12 months post-ACLR did not exceed that of Uninjured Controls. Thus, a potential increase in KAM between 6 and 12 months should not be misinterpreted as excessive KAM magnitudes that exceed those of uninjured controls. These results are consistent with the findings of Kumar et al. who linked an early increase in KAM with deleterious changes in T1ρ relaxation times (6). However, previous studies have not included an uninjured control group to guide the interpretation of the absolute KAM magnitudes and our results caution the interpretation that early increases in KAM magnitudes are representative of excessive kinetics on the ACLR limb. The current study demonstrates that while KAM profiles tend to increase between 6 and 12 months post-ACLR in individuals with T1ρHigh profiles the KAM profiles are not greater than uninjured controls at both timepoints. Further, we speculate that individuals with the most deleterious changes in cartilage composition who demonstrate lower KAM profiles than uninjured controls 6 months following ACLR may benefit from interventions seeking to increase KAM magnitudes to values that match uninjured controls. As a single session of gait retraining in individuals with an ACLR that cues an increase in peak vertical ground reaction force results in biomechanical gait patterns more similar to uninjured controls and decreases serum biomarker concentrations of cartilage turnover (22, 35, 36). In contrast, individuals with ACLR-T1ρLow profiles who decrease KAM between 6 and 12 months post-ACLR start with higher KAM values than the uninjured controls, and the trend to decrease KAM between 6 and 12 months post-ACLR in the ACLR-T1ρLow group may be an attempt to express loading profiles similar to uninjured controls. These data suggest that interventions which seek to decrease KAM may not be appropriate for all individuals after ACLR. Instead, future interventions should utilize a personalized medicine approach that compares ACLR gait profiles to matched uninjured control data to contextualize gait biomechanics in patients prior to implementing cues to either increase or decrease magnitudes of KAM.
Our results suggest that the KFM peaks, in both ACLR-T1ρHigh and ACLR-T1ρLow groups, is lesser than that of Uninjured Controls at 6 and 12 months post-ACLR. These findings are consistent with gait outcomes linked to lower quadriceps strength, a clinical impairment commonly identified in individuals after ACLR which hinders the ability to produce adequate internal extension moments required to counteract external knee flexion moments during stance (37, 38). Between ACLR groups, KFM was greater in ACLR-T1ρHigh compared to ACLR-T1ρLow prior to the initial peak KFM between 13-20% and 13-18% of stance at 6 and 12 months post-ACLR respectively. In addition to decreased quadriceps strength, individuals after ACLR exhibit decreased quadriceps activation and increased hamstring activation during heel strike in the ACLR limb compared uninjured controls (39). As increases in hamstring activation are believed to increase tibiofemoral joint contact forces (40), we speculate that the higher KFM shortly after heel-strike in the T1ρHigh group may be a contributing factor to the higher T1ρ MRI profiles. However, without musculoskeletal simulations we were unable to draw conclusions regarding the overall magnitudes of tibiofemoral contact forces between ACLR groups. The regions around the KFM peaks were not different between the ACLR groups and subsequently, may not contribute to the differentiation of 12-month post-ACLR T1ρ relaxation times. It remains unknown how increasing KFM peaks in all individuals after ACLR would improve T1ρ relaxation profiles in for all individuals.
It is possible that different biomechanical phenotypes develop following ACLR and may be most accurately identified by comparing ACLR gait biomechanics to uninjured controls. Specifically, data from the current study suggests that a portion of individuals after ACLR exhibit biomechanical underloading KAM profiles while others exhibit biomechanical overloading KAM profiles in the first 6 months post-ACLR that are linked to tibiofemoral cartilage composition status. We did not conduct musculoskeletal simulations and subsequently were unable to measure tibiofemoral contact forces. Instead, measurements of KAM have been used as surrogate measures for medial tibiofemoral knee loading during gait (41, 42). Further, there is some evidence to suggest that individuals who demonstrate clinically relevant knee symptoms post-ACLR may similarly exhibit biomechanical patterns of vertical ground reaction force underloading < 12 months post ACLR but may overload vertical ground reaction forces 24 months post-ACLR (43). Thus, prolonged underloading of KAM and vertical ground reaction forces in the first 6-12 months post-ACLR may lead to deconditioning joint tissues, which may contribute to further joint tissue damage if the initially unloaded cartilage is excessively loaded at later timepoints post-ACLR. Therefore, it may be important to screen individuals after ACLR at multiple timepoints post-ACLR and apply individually directed gait retraining to normalize specific gait biomechanics.
Our study was the first to demonstrate differences in knee moments of individuals after ACLR with high and low T1ρ MRI profiles relative to uninjured controls; yet, there are limitations that should be addressed in future research. We used a k-means cluster analysis to identify those that may be at higher risk of developing PTOA due to 12-month T1ρ MRI profiles (44), yet it remains uncertain if there would be a higher prevalence of PTOA in the ACLR-T1ρHigh group compared to the ACLR-T1ρLow group. T1ρ MRI relaxation times are correlated with articular cartilage proteoglycan concentrations (44) and commonly used to assess early changes in cartilage composition (44, 45). Future studies may also include T2 MRI relaxation times to evaluate type-II collagen orientation or anatomical MRI sequences to assess changes in tissue morphology. Further, we did not assess changes in T1ρ MRI profiles overtime nor in Uninjured Controls. Not including 6-month T1ρ measurements on out participants prevents the ability to interpret whether T1ρ MRI profiles increased or decreased from 6-12 months and infer if 6-month biomechanics may have influenced changes in T1ρ MRI profiles between 6 and 12 months. Moreover, while the Uninjured Controls and entire ACLR group included 50% females, the ACLR-T1ρHigh group included 67% (6/9) females while the ACLR-T1ρLow included 41% (7/17) females. Sex differences in KAM following ACLR have been reported, where females demonstrate higher magnitudes of initial peak KAM following ACLR compared to males (46). While not statistically different between groups, the prevalence and location of concomitant injury trended to be different between ACLR groups where ACLR-T1ρHigh group had 78% (7/9) and 11% (1/9) with lateral and medial meniscal injuries respectively, while the ACLR-T1ρLow had 35% (6/17) and 35% (6/17) with lateral and medial meniscal tears respectively. Presence of concomitant injury are thought to increase subsequent risk of PTOA (47). Future studies with larger samples sizes should be conducted to determine the influence of sex and concomitant injury on the association between gait biomechanics and changes in cartilage composition. Additionally, this study did not evaluate walking gait biomechanics or T1ρ MRI profiles during time points beyond 12 months post-ACLR, thus it is unclear if the findings are applicable to individuals at different periods of time post-ACLR.
CONCLUSIONS
In conclusion, lesser KAM at 6 months post-ACLR is linked to worse T1ρ MRI profiles 12 months post-ACLR. Moreover, KAM profiles in both the ACLR-T1ρHigh and ACLR-T1ρLow became more similar to Uninjured Controls between 6 and 12 months post-ACLR by increasing and decreasing KAM, respectively. While there were scant differences in KFM peaks between ACLR-T1ρHigh and ACLR-T1ρLow at either 6 or 12 months post-ACLR, KFM peaks of both ACL groups were less than the peaks of Uninjured Controls at both 6 and 12 months post-ACLR.
Supplementary Material
Supplemental Figure 1: Individual Knee Sagittal and Frontal Moment Waveforms of ACLR-T1ρHigh and ACLR-T1ρLow at 6 Months and 12 Months Post-ACLR
Conflict of Interest and Sources of Funding
There are no conflicts of interest. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health [1R03AR066840-01A1], North Carolina Translational and Clinical Sciences (TraCS) Institute and National Athletic Trainers Association Research and Education Foundation [14NewInv001]. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. There are no professional relationships with companies or manufacturers who will benefit from the results of the present study to disclose.
REFERENCES
- 1.Friel NA, Chu CR. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin Sports Med. 2013;32(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44(1):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50(10):597–612. [DOI] [PubMed] [Google Scholar]
- 4.Saxby DJ, Bryant AL, Van Ginckel A, et al. Greater magnitude tibiofemoral contact forces are associated with reduced prevalence of osteochondral pathologies 2-3 years following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):707–15. [DOI] [PubMed] [Google Scholar]
- 5.Teng H-LL, Wu D, Su F, et al. Gait characteristics associated with a greater increase in medial knee cartilage T 1ρ and T 2 relaxation times in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(14):3262–71. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Su F, Wu D, et al. Frontal plane knee mechanics and early cartilage degeneration in people with anterior cruciate ligament reconstruction: a longitudinal study. Am J Sports Med. 2018;46(2):378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar D, Kothari A, Souza RB, Wu S, Benjamin Ma C, Li X. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: a pilot study. Knee. 2014;21(5):881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeiffer SJ, Spang J, Nissman D, et al. Gait mechanics and T1ρ MRI of tibiofemoral cartilage 6 months after ACL reconstruction. Med Sci Sports Exerc. 2019;51(4):630–9. [DOI] [PubMed] [Google Scholar]
- 9.Rautiainen J, Nissi MJ, Salo EN, et al. Multiparametric MRI assessment of human articular cartilage degeneration: correlation with quantitative histology and mechanical properties. Magn Reson Med. 2015;74(1):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrosimone B, Nissman D, Padua DA, et al. Associations between cartilage proteoglycan density and patient outcomes 12 months following anterior cruciate ligament reconstruction. Knee. 2018;25(1):118–29. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1ρand T2 - Initial experience with 1-year follow-up. Radiology. 2011;258(2):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maly MR, Acker SM, Totterman S, et al. Knee adduction moment relates to medial femoral and tibial cartilage morphology in clinical knee osteoarthritis. J Biomech. 2015;48(12):3495–501. [DOI] [PubMed] [Google Scholar]
- 14.Kaur M, Ribeiro DC, Theis J-C, Webster KE, Sole G. Movement patterns of the knee during gait following ACL reconstruction: a systematic review and meta-analysis. Sport Med. 2016;46(12):1869–95. [DOI] [PubMed] [Google Scholar]
- 15.Davis-Wilson HC, Pfeiffer SJ, Johnston CD, et al. Bilateral gait 6 and 12 months post-anterior cruciate ligament reconstruction compared with controls. Med Sci Sports Exerc. 2020;52(4):785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater L V, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-Analysis. J Athl Train. 2017;52(9):847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics six-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erhart-Hledik JC, Favre J, Andriacchi TP. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. J Biomech. 2015;48(14):3868–75. [DOI] [PubMed] [Google Scholar]
- 19.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sport Phys Ther. 2012;42(7):601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans-Pickett A, Longobardi L, Spang JT, et al. Synovial fluid concentrations of matrix Metalloproteinase-3 and Interluekin-6 following anterior cruciate ligament injury associate with gait biomechanics 6 months following reconstruction. Osteoarthr Cartil. 2021;29(7):1006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Seeley MK, Francom D, Reese CS, Hopkins JT. Functional vs. traditional analysis in biomechanical gait data: an alternative statistical approach. J Hum Kinet. 2017;60:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans-Pickett A, Davis-Wilson HC, Luc-Harkey BA, et al. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6–12 months after anterior cruciate ligament reconstruction. Clin Biomech. 2020;76:105014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 24.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA, Pietrosimone B. Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2016;39:9–13. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski E, Catelli DS, Lamontagne M. Side does not matter in healthy young and older individuals – Examining the importance of how we match limbs during gait studies. Gait Posture. 2019;67:133–6. [DOI] [PubMed] [Google Scholar]
- 27.Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Hum Mov Sci. 1989;8(1):3–16. [Google Scholar]
- 28.Pfeiffer S, Harkey MS, Stanley LE, et al. Associations between slower walking speed and T1ρ magnetic resonance imaging of femoral cartilage following anterior cruciate ligament reconstruction. Arthritis Care Res (Hoboken). 2018;70(8):1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrosimone B, Pfeiffer SJ, Harkey MS, et al. Quadriceps weakness associates with greater T1ρ relaxation time in the medial femoral articular cartilage 6 months following anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2019;27(8):2632–42. [DOI] [PubMed] [Google Scholar]
- 30.Theologis AA, Haughom B, Liang F, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. [DOI] [PubMed] [Google Scholar]
- 32.Davis HC, Pfeiffer SJ, Johnston CD, et al. Walking biomechanics six and twelve months following anterior cruciate ligament reconstruction compared to healthy controls. Med Sci Sport Exerc. 2019;51(6S):265. [Google Scholar]
- 33.Horton WZ, Page GL, Reese CS, Lepley LK, White M. Template priors in bayesian curve registration. Technometrics. 2021;63(4):487–99. [Google Scholar]
- 34.Lang S, Brezger A. Bayesian P-splines. J Comput Graph Stat. 2004;13(1):183–212. [Google Scholar]
- 35.Luc-Harkey BA, Franz J, Hackney AC, et al. Immediate biochemical changes after gait biofeedback in individuals with anterior cruciate ligament reconstruction. J Athl Train. 2020;55(10):1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luc-Harkey BA, Franz JR, Hackney AC, Blackburn JT, Padua DA, Pietrosimone B. Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2018;60:13–9. [DOI] [PubMed] [Google Scholar]
- 37.Pietrosimone B, Davis-Wilson HC, Seeley MK, et al. Gait biomechanics in individuals meeting sufficient quadriceps strength cutoffs after anterior cruciate ligament reconstruction. J Athl Train. 2021;56(9):960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002;17(1):56–63. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn T, Pietrosimone B, Goodwin JS, Johnston C, Spang JT. Co-activation during gait following anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2019;67:153–9. [DOI] [PubMed] [Google Scholar]
- 40.Catalfamo PF, Aguiar G, Curi J, Braidot A. Anterior cruciate ligament injury: compensation during gait using hamstring muscle activity. Open Biomed Eng J. 2010;4(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlrich SD, Silder A, Beaupre GS, Shull PB, Delp SL. Subject-specific toe-in or toe-out gait modifications reduce the larger knee adduction moment peak more than a non-personalized approach. J Biomech. 2018;66:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutzner I, Trepczynski A, Heller MO, Bergmann G. Knee adduction moment and medial contact force--facts about their correlation during gait. PLoS One. 2013;8(12):e81036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietrosimone B, Seeley MK, Johnston CD, Pfeiffer SJ, Spang JT, Blackburn JT. Walking ground reaction force post-ACL reconstruction: analysis of time and symptoms. Med Sci Sport Exerc. 2019;51(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson HF, Birmingham TB, Moyer RF, et al. MRI T2 and T1ρ relaxation in patients at risk for knee osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutcliffe HC, Kottamasu PK, McNulty AL, Goode AP, Spritzer CE, DeFrate LE. Mechanical metrics may show improved ability to predict osteoarthritis compared to T1rho mapping. J Biomech. 2021;129:110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2012;46(5):355–9. [DOI] [PubMed] [Google Scholar]
- 47.MOON Knee Group, Jones MH, Oak SR, et al. Predictors of radiographic osteoarthritis 2 to 3 years after anterior cruciate ligament reconstruction: data from the MOON On-site Nested Cohort. Orthop J Sport Med. 2019;7(8):2325967119867085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Individual Knee Sagittal and Frontal Moment Waveforms of ACLR-T1ρHigh and ACLR-T1ρLow at 6 Months and 12 Months Post-ACLR