Abstract
NTRK-rearranged uterine sarcomas are rare spindle-cell neoplasms that typically arise in the uterine cervix of young women. Some tumors recur or metastasize, but features which predict behavior have not been identified to date. Distinguishing these tumors from morphologic mimics is significant because patients with advanced stage disease may be treated with TRK inhibitors. Herein, we present fifteen cases of NTRK-rearranged uterine sarcomas, the largest series to date. Median patient age was 35 years (range 16–61). The majority arose in the uterine cervix (n=14) and all but two were organ-confined at diagnosis. Tumors were composed of an infiltrative, fascicular proliferation of spindle cells and most showed mild-to-moderate cytologic atypia. All were pan-TRK positive by immunohistochemistry (13/13); S100 (11/13) and CD34 (6/10) were usually positive. RNA or DNA sequencing found NTRK1 (10/13) and NTRK3 (3/13) fusions with partners TPR, TPM3, EML4, TFG, SPECC1L, C16orf72, and IRF2BP2. Unusual morphology was seen in two tumors which were originally diagnosed as unclassifiable uterine sarcomas, one of which also harbored TP53 mutations. Follow up was available for nine patients, of whom three died of disease. By incorporating outcome data of previously reported tumors, adverse prognostic features were identified, including a mitotic index ≥8 per 10 high power fields, lymphovascular invasion, necrosis, and NTRK3 fusion. Patients with tumors which lacked any of these four features had an excellent prognosis. This study expands the morphologic spectrum of NTRK-rearranged uterine sarcomas and identifies features which can be used for risk stratification.
Keywords: NTRK, Uterine sarcoma, Pan-TRK
Introduction
In 2018, Chiang and colleagues described a novel uterine sarcoma predominantly located in the uterine cervix in young women with the potential for aggressive behavior. It is defined by NTRK gene fusions, and characterized by spindle cell/fibrosarcoma-like morphology and S100 expression.1 Since its original description, more than 30 NTRK-rearranged uterine sarcomas have been reported in the literature,1–13 and NTRK1–3 fusions are now recognized to underlie most of the tumors described several years previously as “endocervical fibroblastic malignant peripheral nerve sheath tumors (MPNST)” by Mills et al.3,14 Typically, the histologic features are that of a cellular spindle-cell fascicular proliferation usually without significant pleomorphism (although symplastic/atypical foci have been reported).4 By immunohistochemistry, they are positive for pan-TRK, with variable CD34 and S100 expression. Identification of these tumors has clinical significance as patients with recurrent or metastatic disease may benefit from treatment with TRK inhibitors.15,16 However, the morphologic and immunophenotypic spectrum of this recently reported tumor is ever widening and the clinical behavior remains difficult to predict. Although the number of cases described in the literature has steadily increased since 2018, most reports are small series or single cases. Moreover, even though most tumors behave indolently, some recur or metastasize3,4 and the histologic or molecular features which can predict their behavior have not yet been established. Therefore, herein we describe the morphologic, immunohistochemical, molecular and clinical features of fifteen additional NTRK-rearranged uterine sarcomas and review the literature to identify potential prognostic factors and better understand their morphologic spectrum and the salient features that help distinguish them from morphologic mimics.
Materials and Methods
Case Selection
Cases reviewed at Brigham and Women’s Hospital (BWH, Boston, MA) were retrospectively identified based on either documented NTRK fusion or morphologic and immunohistochemical features consistent with an NTRK-rearranged uterine sarcoma. All tumors included in the series were reviewed by a gynecologic pathologist, and representative slides were re-reviewed for study inclusion. The BWH Institutional Review Board approved this study.
Immunohistochemistry
Immunohistochemistry was performed on 4 μm thick formalin-fixed paraffin-embedded (FFPE) tissue for pan-TRK (clone EPR17341, 1:300, Abcam, Cambridge, MA), S100 (polyclonal, 1:3000, Dako, Carpinteria, CA), SOX10 (clone EP268, 1:2000, Cell Marque, Rocklin, CA), CD34 (clone M7165, 1:150, Dako), desmin (clone DE-U-10, 1:5000, Sigma-Aldrich, Saint Louis, MO), smooth muscle actin (SMA, clone 1A4, 1:20 000, Sigma), h-caldesmon (clone h-CD, 1:300, Dako), estrogen receptor (ER, clone SP1, 1:40, Fisher Scientific, Hampton, NH), progesterone receptor (PR, PgR636, 1:200, Dako), and p53 (DO-7, 1:500, Dako).
RNA-Sequencing (RNA-Seq)
Total RNA was extracted from FFPE tissue using the ExpressArt FFPE Clear RNA Ready kit (Amsbio, Cambridge, MA). Total RNA was quantified using the Qubit RNA HS Assay Kit (ThermoFisher Scientific, Mississauga, ON). RNA-seq libraries were prepared following the manufacturer’s instructions using an input of 20–100 ng RNA and the TruSight RNA Fusion Panel (Illumina, San Diego, CA). The results were analyzed using both STAR and BOWTIE2 aligners, and Manta and JAFFA fusion callers,17,18 respectively.
DNA Sequencing
DNA sequencing was performed as previously described using the Oncopanel assay.19,20 Tumor was macrodissected from FFPE unstained slides, and the QIAamp DNA kit (Qiagen, Germantown, MD) was used to extract DNA. Oncopanel is a targeted solution-phase hybrid-capture technique which uses an Illumina HiSeq2500 to sequence the coding regions of 447 tumor suppressors and oncogenes. Additionally, regions of 60 genes, including NTRK1, NTRK2, and NTRK3, are included specifically for rearrangement detection.
Literature Review
Published cases of NTRK-rearranged uterine sarcoma were identified in the English literature by searching PubMed and Google Scholar using the term “NTRK” in combination with “uterus”, “uterine”, “cervix”, and “cervical”.
Statistical Analyses
Statistical analyses were performed in Python (3.8.5)21 using pandas (1.3.3)22 and SciPy (1.7.1).23 Survival analyses used the lifelines package (0.25.7).24 For regression analyses, missing data were imputed using the multivariable IterativeImputer from the scikit-learn library (0.24.2).25
Results
Clinical Features
The clinical characteristics of the fifteen cases in the study cohort are presented in Table 1. The median age was 35 years (range 16–61). Fourteen tumors arose in the uterine cervix (14/15; 93%), and one in the uterine corpus. The average tumor size was 6.8 cm (range 3.5–12). Of the ten cases with staging information, three were FIGO stage IA, five were stage IB and two were stage IIB. Two tumors extended beyond the uterus at the time of diagnosis: one involved the parametrium, and one the vagina. Eight patients underwent hysterectomy (8/15; 53%), one (1/15; 7%) had a myomectomy, one had a biopsy, and five others had local excision of the cervical mass only (5/15; 33%). However, four of the patients who had initially had uterine-sparing procedures were lost to follow up and may have had subsequent hysterectomies. Four patients received additional treatment: two received radiation alone (one each in adjuvant and neoadjuvant settings) and two received both adjuvant radiation and chemotherapy.
Table 1.
Clinical features, including treatment and follow up, for NTRK-rearranged uterine sarcomas of the cohort.
| Case | Age (years) | Presentation | Site | Size (cm) | FIGO Stage | Surgical Therapy or Procedure | Adjuvant Therapy | Recurrence | Time to Recurrence (months) | Follow up (months) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | Anemia, “fibroid” | Corpus | 5.1 | IB | Myomectomy | NK | NK | NA | NA | LTFU |
| 2 | 35 | Dysmenorrhea, dyspareunia | Cervix | 3.5 | IA | TAH BSO | NK | NK | NA | NA | LTFU |
| 3 | 47 | Mass | Cervix | 7.8 | IB | TAH BSO, bilateral pelvic & para-aortic nodes | Radiation & chemotherapy | NK | NA | 12 | DOD |
| 4 | 30 | Enlarged, vascular, friable cervix | Cervix | 4.0 | IIB | TAH BS & omentectomy | N | N | NA | 35 | NED |
| 5 | 39 | NK | Cervix | NK | NK | Local excision | NK | NK | NA | NA | LTFU |
| 6 | 16 | NK | Cervix | NK | IA | Biopsy, LEEP, CKC | N | N | NA | 30 | NED |
| 7 | 26 | NK | Cervix | 12 | IIB | TAH BS | Radiation | Pelvis | 13 | 82 | NED |
| 8 | 26 | NK | Cervix | 5.5 | ≥IB | TAH | Radiation & chemotherapy | Peritoneum, lymph nodes, bone | 6 | 22 | DOD |
| 9 | 26 | NK | Cervix | NK | NK | Local excision | NK | Lung | 72 | 84 | DOD |
| 10 | 61 | Vaginal bleeding | Cervix | 7 | IB | TAH BSO | N | N | NA | 16 | NED |
| 11 | 24 | NK | Cervix | NK | IA | Local excision | N | N | NA | 16.5 | NED |
| 12 | 42 | NK | Cervix | NK | NK | Local excision | NK | NK | NA | NA | LTFU |
| 13 | 42 | Vaginal bleeding | Cervix | 5.6 | IB | TAH BSO | N | N | NA | 44 | NED |
| 14 | 46 | NK | Cervix | 10.0 | IB | Radical hysterectomy BSO | Radiation* | NK | NA | NA | LTFU |
| 15 | 26 | Vaginal bleeding | Cervix | 8.0 | NK | Cervical biopsy | NK | NK | NA | NA | LTFU |
neoadjuvant radiation therapy; LEEP = loop electrosurgical excision procedure; CKC = cold knife cone biopsy; LTFU = lost to follow up; NED = no evidence of disease; DOD = died of disease; NA = not applicable; NK = not known; TAH = total abdominal hysterectomy; BSO = bilateral salpingo-oophorectomy; BS = bilateral salpingectomy
Follow up was available for nine patients (60%) with a median follow up of 22 months (range 6–84 months). Six (6/9; 67%) were alive with no evidence of disease; one experienced a recurrence at thirteen months (pelvis), with subsequent resection and is currently disease-free at 82 months. A second patient experienced recurrences (peritoneum, lymph nodes, and bone metastases) at six months. She progressed through multiple chemotherapy regimens (doxorubicin and ifosfamide, followed by trabectedin, and finally ifosfamide and etoposide), and died with disseminated disease 22 months after initial diagnosis. Two additional patients (total 3/9; 33%) died of disease at 12 and 84 months.
Eight cases (53%) were initially diagnosed as NTRK-rearranged sarcoma (including one “endocervical MPNST”). The others were diagnosed as unclassifiable sarcoma (n=3, 2 of which were “high grade” or pleomorphic), melanoma (n=2), adenosarcoma with sarcomatous overgrowth (n=1), and atypical myxoid and spindle cell tumor of uncertain malignant potential (n=1).
Gross Features
A gross description was available for 6 cases. Tumors were tan-white or white and cut surfaces ranged from solid to fleshy and friable (Figure 1). Three contained focal hemorrhage within the tumor. Two were exophytic or polypoid. Two cases were ill-defined on gross examination including one tumor that was not identified on an initial gross examination.
Figure 1.
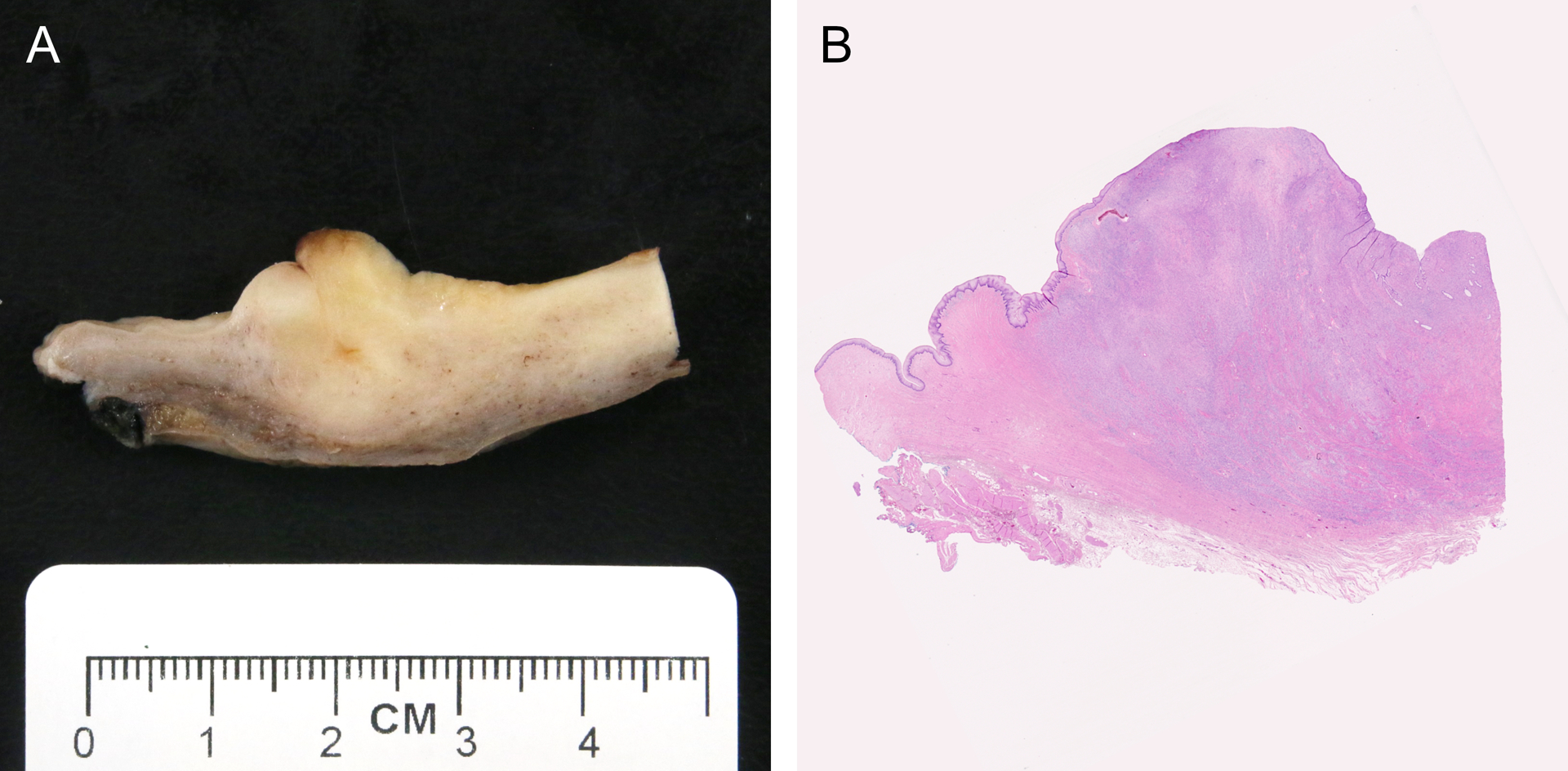
(A) Grossly ill-defined tan-white cervical mass and (B) corresponding microscopic image of tumor harboring a TPR-NTRK1 fusion in a 30-year-old female (perpendicular section of endocervical canal, case 4).
Microscopic Features
The microscopic features are summarized in Table 2. Most tumors (14/15; 93%) were comprised predominantly of a fascicular proliferation of spindle cells, frequently entrapping benign endocervical glands (Figure 2). All cases with an evaluable interface (14/14) exhibited an infiltrative growth pattern. In two cases, stromal expansion imparted leaf-like architecture reminiscent of a Mullerian adenosarcoma, but these areas lacked periglandular cuffing. Most cases (11/15; 73%) showed mild or moderate cytologic atypia, with the remainder showing either moderate-to-severe (2/15; 13%) or diffuse, severe (2/15; 13%) atypia (Figure 3A–C). All tumors showed regions of at least moderate cellularity, but cellularity was frequently variable within a tumor (Figure 3D–F). Intra-tumoral hyalinized vessels were noted in 40% of tumors (6/15; Figure 4A), and nuclear pseudoinclusions were seen in most tumors (9/15; 60%) (Figure 4B). Myxoid stroma was focally present in four cases (27%; Figure 4C), and whorling in one (Figure 4D). Mitotic counts were variable, with a mean count of 11 per 10 high power fields (HPFs; range 1–43). Atypical mitotic figures were identified in two tumors (13%) and were only present in those with high-grade atypia. Lymphovascular invasion and necrosis were seen in two cases each (13%). A lymphocytic infiltrate was noted in almost all cases (14/15; 93%) and was mostly mild, although some tumors demonstrated prominent intratumoral or peritumoral lymphoid aggregates (Figure 5). Two tumors exhibited unusual morphology. Case 13 lacked a spindle cell component and was instead composed entirely of diffuse sheets of pleomorphic cells (Figure 2C). A scant pre-treatment biopsy in case 14 showed epithelioid cells with prominent hyalinization, and a post-radiation resection specimen demonstrated high-grade cytologic atypia with areas of rhabdoid cells (Figure 6).
Table 2.
Morphologic features of NTRK-rearranged uterine sarcomas of the cohort.
| Case | Atypia | Cellularity | Mitoses/10 HPF | Atypical mitoses | LVI | Necrosis | Infiltrative edge | Hyalinized vessels | Lymphocytic inflammation | Myxoid stroma | Nuclear pseudoinclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1–2 | 2 | 1 | N | N | N | Y | N | + | Focal | Y |
| 2 | 2 | 2 | 5 | N | N | N | Y | Y | + | N | N |
| 3 | 1 | 2 | 8 | N | Y | N | Y | N | + | N | N |
| 4 | 1–2 | 1–2 | 4 | N | N | N | Y | Y | + | N | Y |
| 5 | 2–3 (focal) | 2–3 | 16 | N | N | Y | Y | Y | ++ | N | Y |
| 6 | 2 | 2 | 6 | N | N | N | Y | Y | ++ | N | N |
| 7 | 1 | 3 | 4 | N | N | N | Y | N | + | N | N |
| 8 | 1 | 3 | 43 | N | N | N | Y | N | + | N | N |
| 9 | 1–2 | 2 | 23 | N | N | N | Y | N | 0 | Focal | N |
| 10 | 2 | 2–3 | 4 | N | N | Y | Y | Y | + | Focal | Y |
| 11 | 2–3 (focal) | 2 | 2 | N | N | N | Y | N | +++ | N | Y |
| 12 | 1–2 | 1–2 | 7 | N | N | N | Y | N | +++ | Focal | Y |
| 13 | 3, diffuse | 2 | 26 | Y | N | N | Y | N | + | N | Y |
| 14 | 3, diffuse | 1–3 | 3 | Y | Y | Y | Y | Y | ++ | N | Y |
| 15 | 2 | 2 | 1 | N | N | N | NA | N | + | N | Y |
LVI = lymphovascular invasion; HPF = high power fields; NA = not assessable
Figure 2.
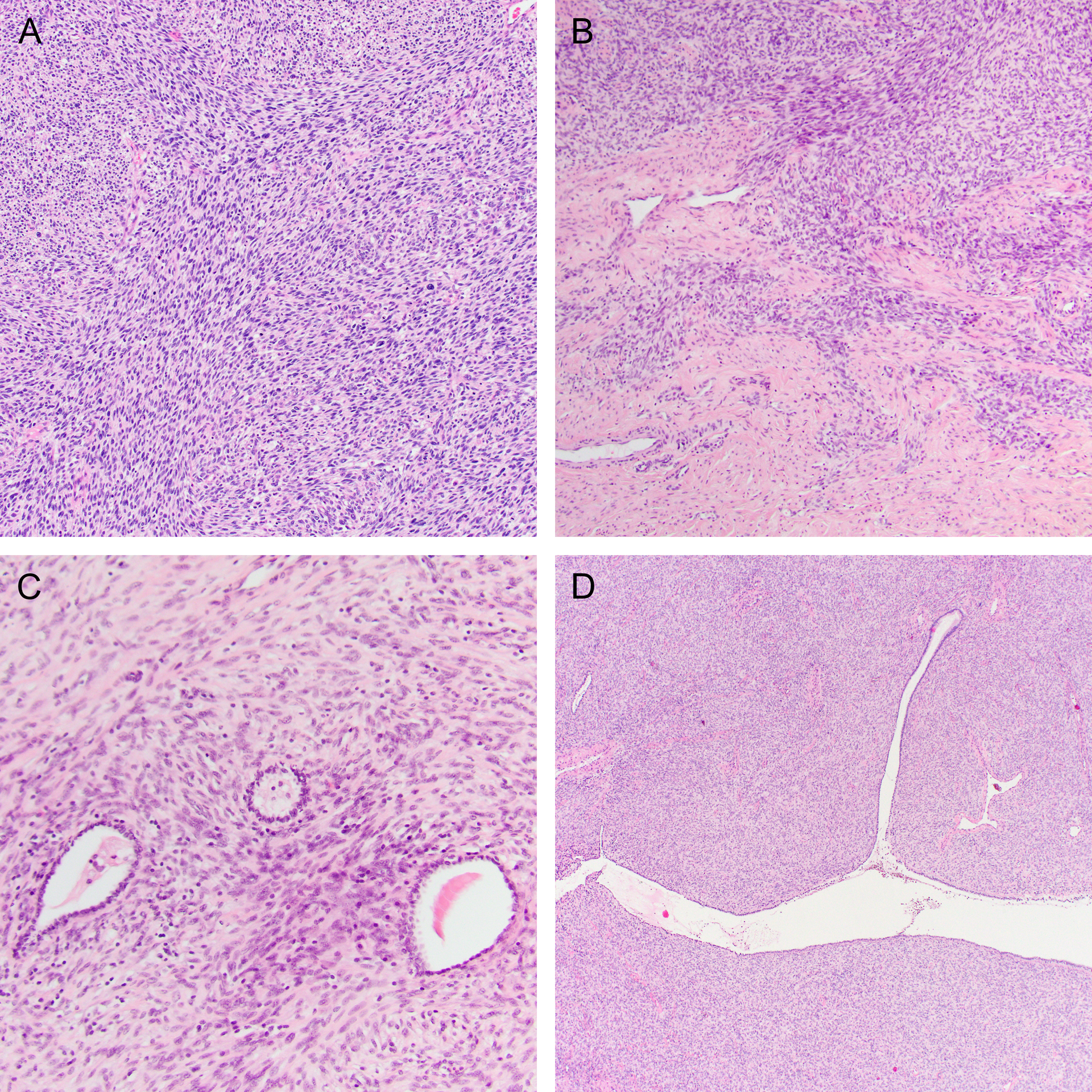
(A) Most NTRK-rearranged sarcomas were composed of spindle cells arranged in short fascicles. (B) All tumors demonstrated an infiltrative growth pattern and (C) frequently entrapped benign endocervical glands. (D) Stromal expansion resulting in a leaf-like architecture, reminiscent of a Mullerian adenosarcoma, was present in two cases.
Figure 3.
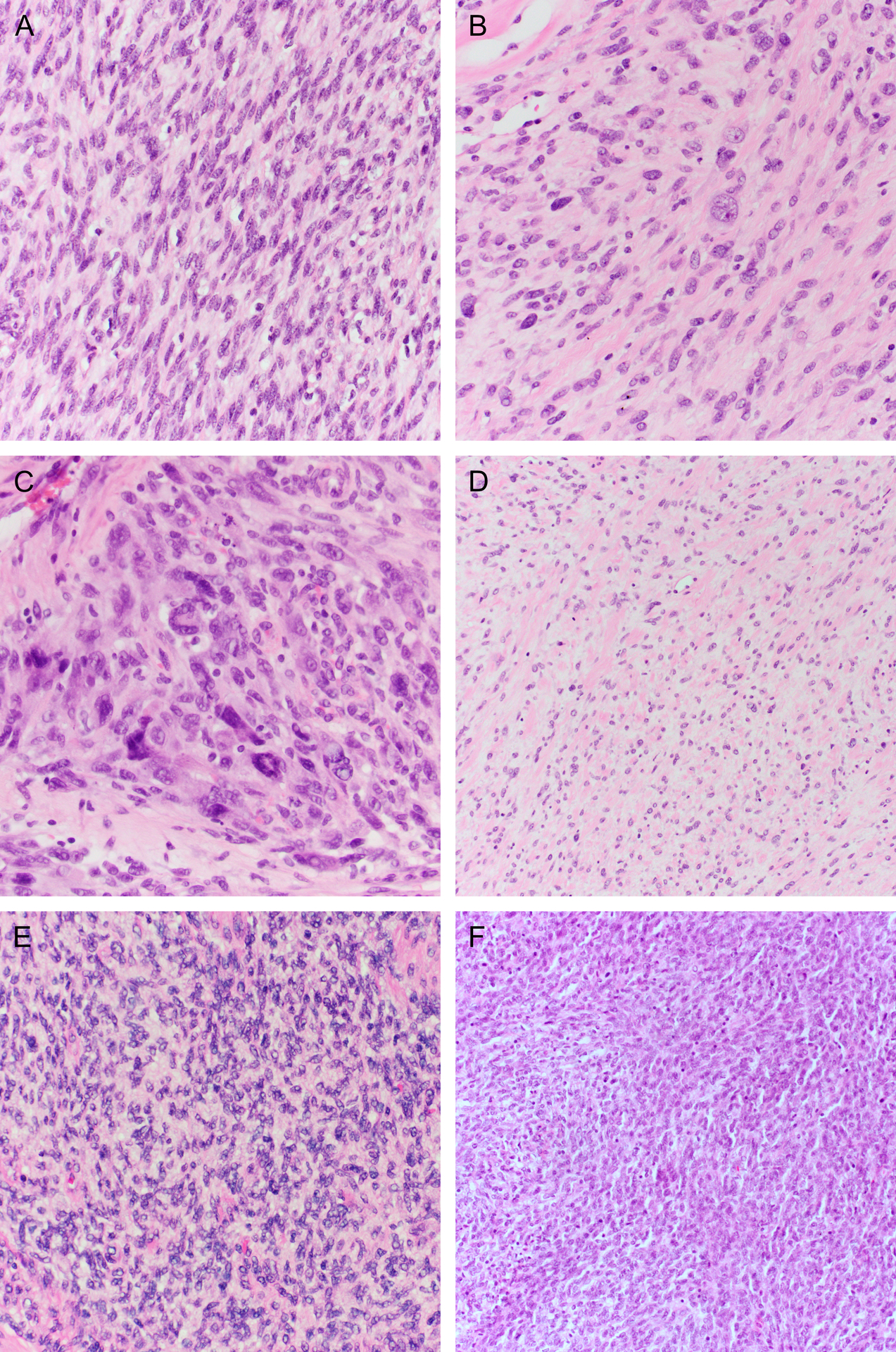
Cytologic atypia was usually (A) mild to (B) moderate. (C) High-grade atypia was seen in a subset of cases, including occasional prominent symplastic tumor giant cells. Tumor cellularity was variable, ranging from (D) low to (E) moderate to (F) high.
Figure 4.
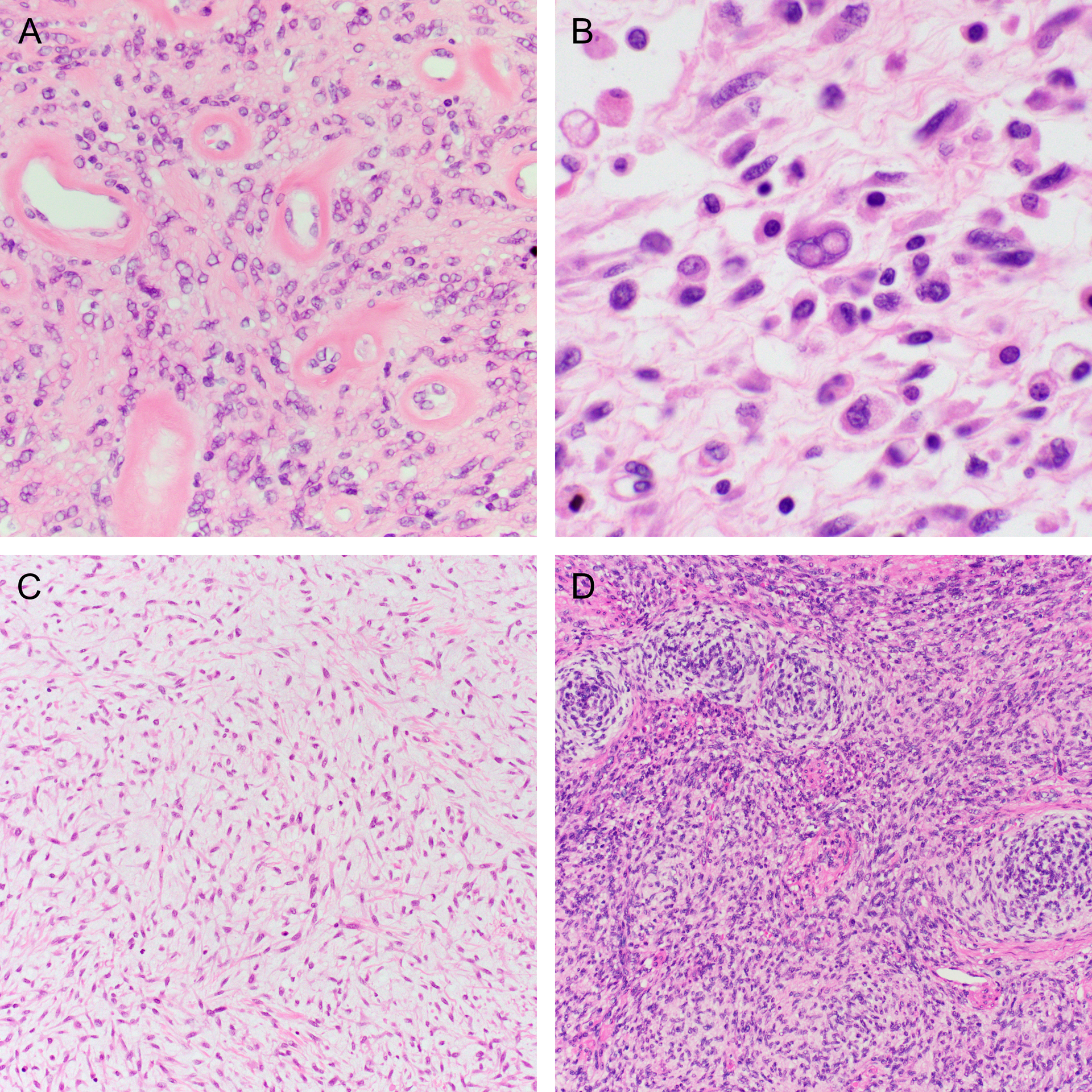
Histologic features seen in some NTRK-fusion uterine sarcomas include (A) hyalinized vessels, (B) nuclear pseudoinclusions, (C) tissue culture-like growth in a myxoid matrix, and (D) whorling.
Figure 5.

Inflammation associated with NTRK fusion uterine sarcomas was common, in the form of (A) intratumoral lymphocytic infiltrates. (B) Peritumoral lymphocytes were prominent in a minority of cases.
Figure 6.
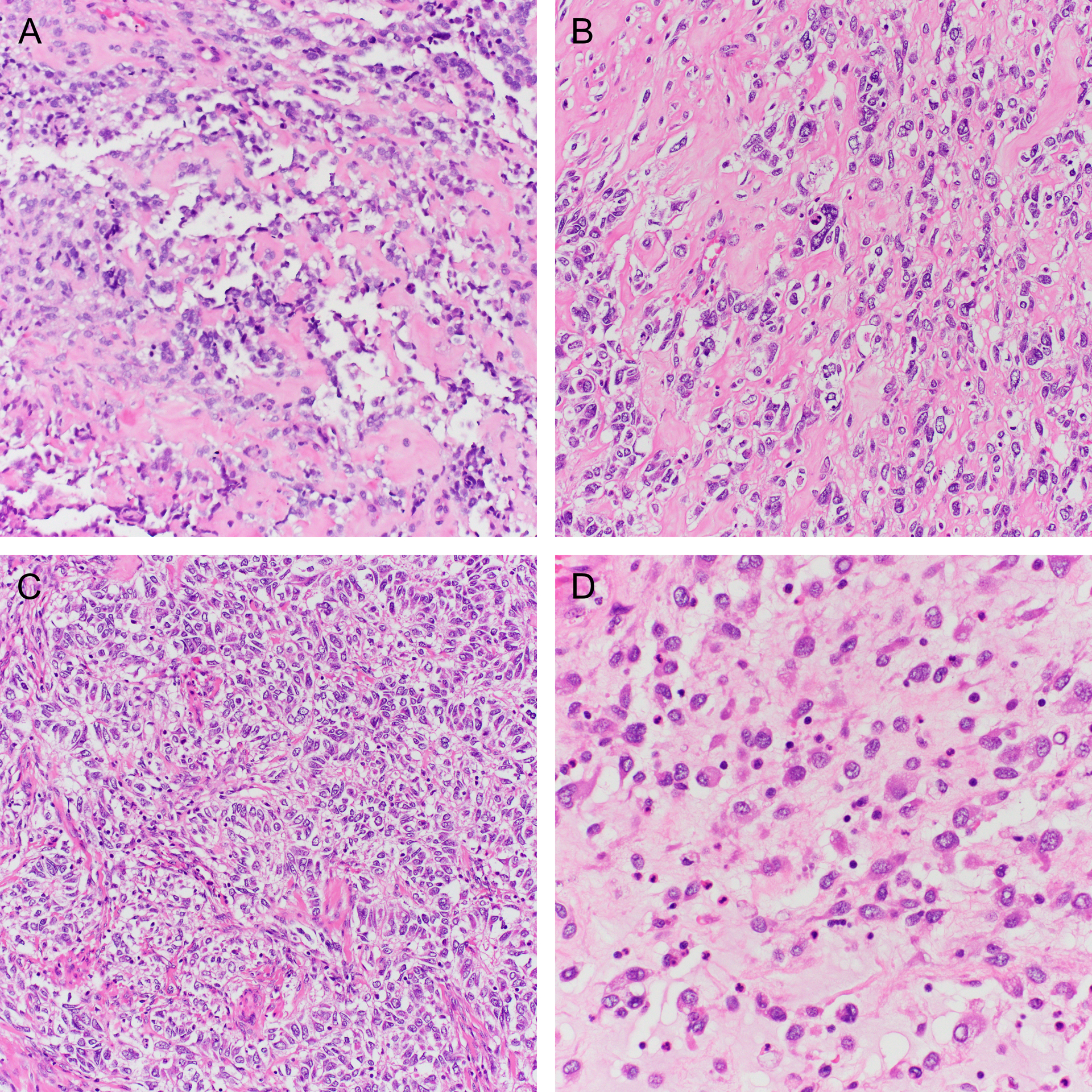
(A) A scant pretreatment biopsy of a tumor with IRF2BP2-NTRK1 fusion (case 14) with prominent stromal hyalinization. The post-radiation resection specimen showed unusual morphology including (B) atypical epithelioid cells embedded in a hyaline matrix, (C) trabecular architecture, and (D) discohesive rhabdoid cells.
Immunohistochemistry
The immunohistochemical (IHC) results are presented in Table 3. Pan-TRK immunohistochemistry was performed in all cases with available tissue. All tested cases (13/13) expressed pan-TRK, with most demonstrating cytoplasmic expression (12/13; 92%) and a single case with nuclear expression. Pan-TRK staining was diffuse in most cases (11/13; 85%) with either strong (6/13; 46%) or moderate (5/13; 38%) staining (Figure 7). Two cases showed only patchy or focal, weak staining (15%). Most tumors expressed S100 (11/13; 85%) with multi-focal or diffuse staining of moderate or strong intensity. CD34 was also commonly expressed (6/10; 60%), with moderate or strong multi-focal staining. Desmin (0/14) and SOX10 (0/6) were negative in all tested cases. SMA showed expression in only a minority of cases (4/12; 33%), typically with focal or multi-focal expression. H-caldesmon was performed in two cases, both negative. Hormone receptors were negative in 3 cases with only rare positive ER and PR cells in one case. Case 14, one of the two cases with diffuse, high-grade atypia, demonstrated wild-type p53 staining.
Table 3.
Immunohistochemical staining and NTRK fusions of the cohort.
| Case | Immunohistochemical stains | NTRK fusion | |||
|---|---|---|---|---|---|
| Pan-TRK | S100 | CD34 | Desmin | ||
| 1 | ND | ND | ND | 0 | C16orf72∷NTRK1 |
| 2 | 3 (D, C) | 1 (F) | POS | 0 | TPM3∷NTRK1 |
| 3 | 3 (D, C) | ND | ND | 0 | TPR∷NTRK1 |
| 4 | 2 (D, C) | 0 | ND | 0 | TPR∷NTRK1 |
| 5 | ND | POS | POS | 0 | TPM3∷NTRK1 |
| 6 | 3 (D, C) | 2 (MF) | 3 (MF) | 0 | TPR∷NTRK1 |
| 7 | 1 (F, C) | 2 (D) | 2 (MF) | 0 | EML4∷NTRK3 |
| 8 | 2 (D, C) | 0 | 0 | 0 | TFG∷NTRK3 |
| 9 | 1 (P, N) | 2 (MF) | 0 | 0 | Failed QC |
| 10 | 2 (D, C) | 3 (MF) | 2 (MF) | 0 | SPECC1L∷NTRK3 |
| 11 | 3 (D, C) | 1 (MF) | 3 (MF) | 0 | TPM3∷NTRK1 |
| 12 | 2 (D, C) | 3 (MF) | 0 | 0 | Insufficient tissue |
| 13 | 2 (D, C) | 3 (MF) | 0 | 0 | TPR∷NTRK1 |
| 14 | 3 (D, C) | 2 (MF) | ND | 0 | IRF2BP2∷NTRK1 |
| 15 | 3 (D, C) | 2 (MF) | ND | ND | TPM3∷NTRK1 |
0 = negative; 1 = weak; 2= moderate; 3= strong; F = focal; MF = multifocal; P = patchy; D = diffuse; C = cytoplasmic; N = nuclear; ND = not done; POS = positive by report (slides not available for review)
Figure 7.

Pan-TRK immunohistochemistry usually demonstrated cytoplasmic staining (A-C) except for one case with patchy nuclear staining (D, case 9) and ranged from (A) weak to (B) moderate to (C) strong intensity.
Molecular Features
Confirmatory RNA (n=12) or DNA (n=2) sequencing was performed on all cases with sufficient tissue (14/15), and the results are presented in Table 3. RNA sequencing failed in one case due to poor quality control metrics. The most common fusions were TPR∷NTRK1 (n=4) and TPM3∷NTRK1 (n=4). There was a predominance of NTRK1 fusions (77%; 10/13) compared to NTRK3 (23%; 3/13). Fusion partners included TPR (n=4), TPM3 (n=4), and one each of EML4, TFG, SPECC1L, C16orf72, and IRF2BP2. Case 13 was subjected to DNA sequencing which, in addition to a TPR∷NTRK1 fusion, identified single nucleotide variants in TP53 (c.843C>A [p.D281E] and c.1009C>G [p.R337G]) and RB1 (c.2206C>T, p.Q736*). The tumor also showed concurrent single copy deletion of RB1, which together with the nonsense mutation, is suggestive of biallelic inactivation of RB1.
Literature Review and Risk Stratification
A literature review identified 31 previously reported NTRK-rearranged uterine sarcomas (Table 4),1–14,26 bringing the total number of cases to 46. Considering all cases, the average patient age was 37.7 years (range 13–69). Average tumor size was 6.9 cm (range 1.3–23). Tumors usually arose in the cervix (85%), and occasionally in the corpus (11%). Most were confined to the uterus at diagnosis (91% stage IA or IB). Mitotic counts were highly variable, ranging from 0–43 (mean 9) per 10 high power fields (HPF). Necrosis was present in 36%, and lymphovascular invasion was identified in 14%. NTRK1 fusions were identified in 73% (32/44), NTRK3 in 25% (11/44), and a single case (2%) contained a WWOX∷NTRK2 fusion. NTRK1 fusion partners included TPM3 (20/32; 63%), TPR (7/32; 22%), C16orf72 (2/32; 6%), IRF2BP2 (2/32; 6%), and LMNA (1/32; 3%). NTRK3 partners were SPECC1L (5/10; 50%), EML4 (2/10; 20%), TFG (1/10; 10%), RBPMS (1/10; 10%) and STRN (1/10; 10%). Among 35 patients with follow up, 23 (66%) were with no evidence of disease (NED), seven (20%) were alive with disease (AWD), and five (14%) died of disease (DOD). Patients with stage IA tumors had excellent outcomes: 90% (9/10) were NED, and one patient had a pelvic recurrence. Most patients with stage IB disease were NED (10/17; 59%), but AWD (4/17; 24%) and DOD (2/17; 12%) were more common than in stage IA. Only three cases had stage IIB disease: two patients are NED and one DOD.
Table 4.
Previously reported cases of NTRK-rearranged uterine sarcomas.
| Case | Series | Age (y) | Site | Stage | Time to recurrence (mo) | FU (mo) | FU status | Size (cm) | Atypia | Necrosis | LVI | Mitoses/10 HPF | Fusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chiang1 | 46 | Cervix | IB | 7 | 7 | AWD | 9.3 | 2 | Y | N | 15 | TPM3∷NTRK1 |
| 2 | Chiang1 | 27 | Corpus | IB | NA | 11 | NED | 16.3 | 2 | N | N | 7 | LMNA∷NTRK1 |
| 3 | Chiang1 | 47 | Cervix | IB | 12 | 78 | DOD | 14 | 2 | Y | N | 12 | RBPMS∷NTRK3 |
| 4 | Chiang1 | 42 | Cervix | IB | NA | 2 | NED | 2.6 | 3 | N | N | 30 | TPR∷NTRK1 |
| 5 | Goulding26 | 13 | Cervix | IB | NA | 4 | NED | 9.2 | 1 | NA | NA | NA | TPM3∷NTRK1 |
| 6 | Boyle7 | 42 | Cervix | IB | NA | 11 | NED | 5.2 | 1 | N | NA | 8 | TPM3∷NTRK1 |
| 7 | Croce4 | 39 | Cervix | NA | NA | 0 | LTFU | NA | 1 | Y | N | 3 | TPM3∷NTRK1 |
| 8 | Croce4 | 44 | Cervix | IA | NA | 2 | NED | 4.5 | 2 | N | N | 3 | TPM3∷NTRK1 |
| 9 | Croce4 | 26 | Cervix | IB | NA | 52 | AWD | 12 | 2 | Y | N | 3 | EML4∷NTRK3 |
| 10 | Croce4 | 23 | Cervix | IA | NA | 33 | NED | 3 | 2 | Y | N | 5 | TPM3∷NTRK1 |
| 11† | Croce4, Mills14, Devereaux3 | 32 | Cervix | IA | NA | 156 | NED | 5 | 1 | N | N | 2 | TPM3∷NTRK1 |
| 12† | Croce4, Mills14, Devereaux3 | 21 | Cervix | IIB | 7 | 16 | DOD | 8.0 | 1–2 | Y | Y | 4 | TPM3∷NTRK1 |
| 13† | Croce4, Rabban5, Devereaux3, | 30 | Cervix | IA | 35 | 37 | AWD | 2.5 | 1 | N | N | 18 | TPM3∷NTRK1 |
| 14† | Rabban5, Devereaux3 | 24 | Cervix | IB | 16 | 52 | AWD | 15 | 1–2 | N | Y | 12 | SPECC1L∷NTRK3 |
| 15 | Rabban5 | 49 | Cervix | NA | NA | ≥6 | NED | 1.8 and 1.4 | 1–2 | N | N | 0 | TPR∷NTRK1 |
| 16 | Hodgson8 | 50s‡ | Cervix | IA | NA | 8 | NED | 1.6 | 1–2 | N | N | 0 | SPECC1L∷NTRK3 |
| 17 | Wong10 | 31 | Cervix | NA | NA | 0 | LTFU | 9 | NA | N | NA | 15 | NTRK3 * |
| 18 | Michal9 | 26 | Uterus | ≥IB | NA | 36 | NED | 23 | 1 | N | NA | 0 | STRN∷NTRK3 |
| 19 | Wells6 | 30 | Corpus | IB | NA | 4 | NED | 2.5 | 1–2 | N | N | 2 | TPM3∷NTRK1 |
| 20 | Gatalica2 | NA | Cervix | NA | NA | NA | NA | NA | NA | NA | NA | NA | TPM3∷NTRK1 |
| 21 | Gatalica2 | NA | Uterus | NA | NA | NA | NA | NA | NA | NA | NA | NA | SPECC1L∷NTRK3 |
| 22 | Devereaux3 | 39 | Cervix | IB | NA | 9 | NED | 5.8 | 1–2 | Y | N | 12 | TPM3∷NTRK1 |
| 23 | Devereaux3 | 66 | Cervix | IA | NA | 2 | NED | 1.5 | 1–2 | N | N | 1 | TPM3∷NTRK1 |
| 24 | Devereaux3 | 40 | Cervix | IA | NA | 32 | NED | 2 | 2 | N | N | 1 | TPR∷NTRK1 |
| 25 | Devereaux3 | 37 | Cervix | ≥IB | NA | 0 | LTFU | 6.3 | 1–2 | Y | N | 2 | IRF2BP2∷NTRK1 |
| 26 | Devereaux3 | 35 | Corpus | IB | NA | 31 | NED | 9.4 | 2–3 | N | N | 5 | C16orf72∷NTRK1 |
| 27 | Nilforoushan11 | 54 | Cervix | IB | 8 | 8 | AWD | 5.4 | 2 | Y | NA | 40 | SPECC1L∷NTRK3 |
| 28 | Nilforoushan11 | 52 | Cervix | IA | NA | 6 | NED | 1.3 | 1 | NA | NA | 1 | TPM3∷NTRK1 |
| 29 | Moh13 | 69 | Corpus | IB | NA | 12 | NED | 7 | 3 | Y | Y | 15 | WWOX∷NTRK2 |
| 30 | Tsai12 | 47 | Cervix | NA | 12 | 21 | AWD | 2.7 | 2–3 | Y | NA | 3 | TPM3∷NTRK1 |
| 31 | Tsai12 | 53 | Cervix | NA | 9 | 9 | AWD | 6.8 | 2–3 | Y | NA | 26 | TPM3∷NTRK1 |
Fusion partner not identified.
These cases have been reported in more than one series.
The original report gave the patient age as “6th decade” and for multivariable analysis, the age was estimated as 55. LTFU = lost to follow up; NED = no evidence of disease; DOD = died of disease; NA = not applicable; HPF = high power fields
By univariable and multivariable analysis, both necrosis and increased mitotic activity (as a continuous variable) were associated with disease recurrence (Table 5). However, only lymphovascular invasion was associated with worse overall survival. A mitotic count of ≥8 per 10 HPF was most effective at stratifying cases for disease recurrence.
Table 5.
Cox proportional hazard analysis for disease free survival (DFS) and overall survival (OS) of NTRK-rearranged uterine sarcomas. HR: hazard ratio, CI: confidence interval. For continuous risk factors, the hazard ratio is for a 1-unit increase in the risk factor. For categorical variables, the comparison and reference groups are included in parentheses.
| Univariate | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | p | HR | CI (95%) | p | |
| Age (years) | 1.02 | 0.98–1.06 | 0.42 | 1.02 | 0.94–1.11 | 0.654 |
| Stage (≥1B vs 1A) | 5.9 | 0.81–42 | 0.076 | Not estimable* | ||
| Size (cm) | 1.02 | 0.91–1.13 | 0.78 | 1.02 | 0.85–1.24 | 0.82 |
| Mitoses/10 HPFs | 1.07 | 1.02–1.12 | 0.009 | 1.05 | 0.99–1.12 | 0.12 |
| Lymphovascular invasion (present vs absent) | 3.6 | 0.91–13.9 | 0.068 | 16.9 | 1.4–203 | 0.026 |
| Necrosis (present vs absent) | 3.3 | 1.04–10.8 | 0.043 | 2.7 | 0.4–19 | 0.33 |
| NTRK fusion (NTRK3 vs NTRK1/2) | 2.2 | 0.68–7.1 | 0.19 | 1.6 | 0.2–12 | 0.65 |
| Multivariable | DFS | OS | ||||
| HR | CI (95%) | p | HR | CI (95%) | p | |
| Mitoses/10 HPFs | 1.07 | 1.02–1.13 | 0.008 | 1.13 | 0.997–1.28 | 0.055 |
| Lymphovascular invasion (present vs absent) | 3.3 | 0.8–14 | 0.11 | 51 | 1.3–2041 | 0.036 |
| Necrosis (present vs absent) | 3.7 | 1.03–13.3 | 0.045 | 6.1 | 0.4–88 | 0.19 |
No stage 1A patients died from disease, precluding calculation of HR.
Tumors with NTRK3 fusions were larger than those with NTRK1 fusions (mean 10.5 vs 5.6 cm; p=0.004), and patients with NTRK3 fusion tumors were more likely to recur than those with NTRK1 tumors (p=0.044, Table 6). Tumor stages and mean mitotic activity were higher in NTRK3 tumors, but these differences were not statistically significant. There were no differences in age, lymphovascular invasion, and necrosis between NTRK1 and NTRK3 tumors.
Table 6.
Comparison of clinicopathologic features of NTRK-rearranged uterine sarcomas with NTRK1 and NTRK3 fusions.
| NTRK1 (n=31) | NTRK3 (n=10) | Univariate p | |
|---|---|---|---|
| Mean age (years) (SD) | 37.0 (11.6) | 37.6 (14.8) | 0.89 |
| IIB | 2 (8%) | 1 (11%) | |
| Mean size (cm) (SD) | 5.6 (3.4) | 10.5 (6.1) | 0.004 |
| Mean mitotic activity per 10 HPFs (SD) | 7.3 (8.2) | 13.3 (15.8) | 0.13 |
| Absent | 23 (88%) | 6 (86%) | |
| Absent | 19 (66%) | 6 (60%) | |
| No | 18 (75%) | 3 (33%) | |
| No | 22 (92%) | 7 (22%) |
To investigate the ability of adverse prognostic features to predict behavior, tumors were classified as high risk if one or more of the following were present: lymphovascular invasion, necrosis, mitotic count ≥8 per 10 HPF, or NTRK3 fusion. Tumors without any of these characteristics were classified as low risk. Low risk tumors were associated with a significantly better disease-free survival compared to high-risk tumors (Figure 8; log rank p=0.009), but there was no difference in overall survival (p=0.10).
Figure 8.
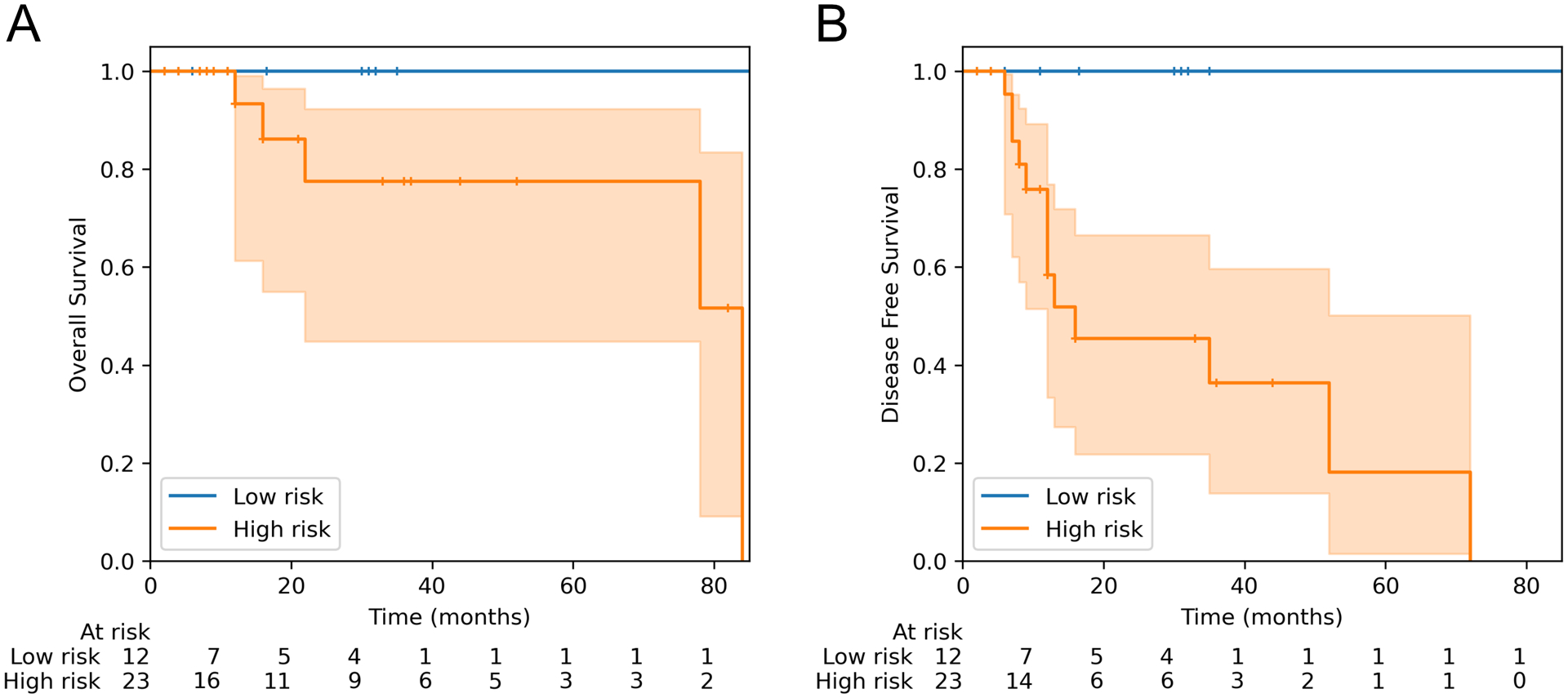
Kaplan-Meier survival curves for NTRK-rearranged uterine sarcomas, stratified based on the presence of at least one high-risk feature: mitotic index ≥8, lymphovascular invasion, necrosis, or NTRK3 fusion. (A) Overall survival, log-rank test p = 0.10. (B) Disease free survival, log-rank test p = 0.009. Shaded regions indicate 95% confidence intervals, and + denotes censored observations.
Discussion
In this study, we present the largest cohort of NTRK-rearranged uterine sarcomas to date, adding significantly to the published literature (Table 4).1–14,26 The total number of reported cases of this rare entity is now 46 and a clearer picture of their clinicopathologic features has emerged, allowing us to identify prognostic factors which may help to predict their behavior.
Morphology
Morphologically, most tumors in our cohort exhibited a similar spectrum of appearances as has been previously described. Conspicuous hyalinized vessels were a readily identifiable feature in almost half of the cases in our cohort and entrapment of benign glands were also frequently seen; both features have been reported previously both within3,5,8 and outside the gynecologic tract.27,28 Mild-to-moderate cytologic atypia was the predominant finding, however four of our cases had severe atypia, which in two cases was diffuse. This finding contrasts with cases that have been described to date, among which very few were described as having severe atypia.1,4 Interestingly, case 13 which displayed diffuse, severe atypia also harbored TP53 mutations; this is consistent with three prior reports of uterine and soft tissue NTRK-fusion sarcomas with TP53 mutations and pleomorphic/anaplastic histology.12,28,29 Case 14 also had diffuse, marked nuclear atypia. However, this tumor demonstrated wild-type p53 staining, suggesting an alternate mechanism underlying the atypia. The clinical significance of increased nuclear atypia in NTRK sarcomas is unclear; of four cases in our series with high grade nuclear atypia, two are with no evidence of disease, and two were lost to follow-up.
Immunohistochemistry
Our study confirms the sensitivity of pan-TRK IHC in the diagnosis of NTRK fusion sarcomas. Pan-TRK IHC was positive in all cases tested and was usually diffusely positive with moderate-to-strong staining intensity. Brčić et al. described a series of 494 soft tissue sarcomas stained with pan-TRK and found that diffuse expression in 4 tumors was associated with an underlying NTRK1/3 fusion, in contrast to 11 tumors with focal weak, moderate, or even strong staining which did not harbor NTRK fusions by RNA sequencing.30 However, case 7 in our series had only focal, weak pan-TRK staining, yet was still found to harbor a EML4∷NTRK3 fusion. Overall, pan-TRK immunohistochemistry is a useful screen in the appropriate clinical and morphologic context. Early studies suggested that pan-TRK immunohistochemistry was 95–97% sensitive and 98–100% specific for tumors harboring NTRK fusions.31,32 However, later studies demonstrated pan-TRK staining is non-specific can be seen in other spindle cell tumors which lack NTRK fusions, such as high grade endometrial stromal sarcoma,33 leiomyosarcoma, and synovial sarcoma.30 Pan-TRK also appears to be less sensitive for NTRK3 fusions: Gatalica et al. reported positive staining in 88% (15/17) of tumors with NTRK1/2 fusions, but only 55% (6/11) tumors with NTRK3 fusions.2 In our series, tumors with NTRK1 fusions showed stronger, more diffuse, expression than those with NTRK3 fusions. Consequently, we suggest confirmatory sequencing or FISH studies even for tumors with only focal and weak pan-TRK staining, if the tumor is otherwise morphologically consistent with an NTRK-rearranged uterine sarcoma, especially if the patient is being considered for targeted therapy.
Nuclear pan-TRK staining was seen in one case with insufficient tissue for sequencing (case 9). This nuclear staining pattern has been reported to be associated with ETV6∷NTRK3.30,31,34 However, FISH failed to identify an ETV6 rearrangement in this case.
While most NTRK-fusion sarcomas co-express S100 and CD34,3,4 it is not uniform and this IHC profile should not be required to screen with pan-TRK immunohistochemistry or to suggest molecular studies. In our series, S100 staining was seen in all but two cases, with co-expression of CD34 seen in just over half of tumors. In reported cases S100 is usually positive, albeit showing a range of staining patterns with only rare cells staining in all cases in one series,1 and a single case report of an S100-negative NTRK-fusion tumor.7 Cases 4 and 8 in our series were S100 negative, highlighting that lack of S100 expression should not exclude the diagnosis.
NTRK Fusions and Related Tumors
NTRK-rearranged uterine sarcomas are a recently described group of rare gynecologic tumors characterized by fascicular spindle cell morphology, expression of S100 and pan-TRK, and a predilection for the cervix of young women. NTRK fusions have previously been described in a wide variety of other tumor types and sites.2,27,28,30 NTRK1, NTRK2 and NTRK3 encode the three corresponding tropomyosin receptor kinases TRKA, TRKB and TRKC that activate a cell signaling cascade involved in nervous system development.35,36 NTRK gene fusions lead to ligand-independent constitutive activation of TRK and have been implicated as oncogenic drivers in a wide variety of adult and pediatric sarcomas, gliomas, and carcinomas.2,30 NTRK fusions promote oncogenesis by constitutive ligand-independent activation of TRK leading to cell proliferation. Recognition of NTRK-rearranged malignancies are of particular clinical importance because of the FDA-approved TRK inhibitors larotrectinib and entrectinib that have demonstrated utility in patients with recurrent, progressive, or metastatic disease.15,16,35,37
A subset of NTRK-rearranged soft tissue tumors show some overlapping histologic characteristics with NTRK sarcomas of the uterus (fascicular spindle cell proliferations with stromal and perivascular hyalinization and S100/CD34 expression),27 however the relationship between the two remains unclear. Similarly, some spindle cell uterine tumors with fibrosarcoma-like morphology harbor targetable non-NTRK fusions, including COL1A1∷PDGFB, FGFR1∷TACC1, and SPECC1L∷RET.3,4,38 The relationship between these tumors and those that are driven by NTRK fusions remains to be fully elucidated, but all of these tumor types may ultimately fall under the same broad diagnostic category of “fibrosarcoma-like uterine sarcomas.” Additional studies examining their morphology, clinical behavior, and molecular characteristics (e.g., methylation profiles) will be helpful to classify them and better understand how they are related.
Differential Diagnosis
In practice, a desmin-negative spindle cell neoplasm in the cervix should trigger consideration of an NTRK-rearranged uterine tumor. Although they occur most frequently in women in their 3rd and 4th decade, they should also be considered in cervical spindle cell tumors in adolescents and post-menopausal patients. S100 and CD34 positivity, while not required, are also supportive of the diagnosis. If pan-TRK immunohistochemistry is available, it is helpful as a sensitive screening test to select cases for further genetic confirmation (e.g., DNA/RNA sequencing or fluorescence in situ hybridization). While RNA-sequencing is the most sensitive technique to detect fusions, many DNA-based gene panels can detect some NTRK1–3 fusions. The rarity of NTRK fusion tumors, however, makes it difficult to rigorously evaluate the sensitivity of these DNA sequencing panels.
The main differential diagnoses include other spindle cell tumors, such as leiomyosarcoma, melanoma, spindled squamous cell carcinoma, COL1A1∷PDGFB sarcoma, solitary fibrous tumor, malignant peripheral nerve sheath tumor (MPNST), inflammatory myofibroblastic tumor, and adenosarcoma with sarcomatous overgrowth. NTRK-rearranged tumors may show focal positivity for SMA,1 but they are negative for other markers frequently positive in leiomyosarcoma, such as desmin, caldesmon, ER and PR. Recently described COL1A1∷PDGFB uterine sarcomas are negative for pan-TRK.4 Solitary fibrous tumors may occur in the gynecologic tract and are CD34 positive, but they are also positive for STAT6.39 Like NTRK-rearranged sarcomas, melanoma and MPNST may show S100 staining. However, in contrast to NTRK-rearranged sarcomas, melanoma is usually diffusely positive for S100, and is also positive for SOX10. MPNST may show S100 reactivity, but about half demonstrate loss of H3K27Me3,40 and they do not harbor diagnostic fusions. Inflammatory myofibroblastic tumors may share some morphologic features with NTRK-rearranged sarcomas, including spindle cells, myxoid stroma, and an inflammatory infiltrate. However, uterine inflammatory myofibroblastic tumors are usually positive for ALK by immunohistochemistry and harbor ALK fusions.41 In some cases, this differential can be especially challenging: there is a single case report of a S100 and CD34 negative myxoid uterine tumor which was diagnosed as an inflammatory myofibroblastic tumor and found to harbor a ETV6∷NTRK3 fusion.42 Adenosarcoma typically shows entrapped glands similar to many NTRK sarcomas; however, adenosarcoma should show well-developed phyllodiform growth and periglandular cuffing. Adenosarcoma-like morphology can occasionally be seen in NTRK sarcomas, and in these cases immunohistochemistry can be useful to indicate molecular testing. In a series of 14 adenosarcomas stained with pan-TRK, all were negative.5
Cotzia and colleagues described a series of 10 uterine tumors initially diagnosed as undifferentiated uterine sarcoma, but were subsequently found, by RNA-Seq or FISH, to harbor genetic alterations characteristic of uterine sarcomas, such as ZC3H7B∷BCOR, YWHAE∷NUTM2, and BRD8∷PHF1.43 Cases 13 and 14 in our series demonstrate that NTRK fusion sarcomas may also initially appear to be unclassifiable/undifferentiated uterine sarcomas, and highlight the utility of RNA or DNA sequencing in such cases to identify a genomic alteration with both diagnostic and predictive significance.
Risk Stratification
Given the relatively small number of cases in the literature, it has been challenging to identify prognostic factors for NTRK-rearranged uterine sarcomas. Devereaux et al. suggested that tumor stage was the most helpful prognostic feature of NTRK uterine sarcomas3 and stage appears to continue to remain prognostically significant as no stage IA patients died of disease. By pooling the clinicopathologic factors and outcomes of published cases, we were able to identify the additional possible prognostic factors of lymphovascular invasion, mitotic index, necrosis, and fusion status (NTRK3 vs NTRK1). While lymphovascular invasion was only seen in four cases with follow up, three had adverse outcomes (two died from disease, one developed distant metastases). Recurrences were more common in tumors with NTRK3 fusions compared to NTRK1 fusions, suggesting that fusion status may have value as a prognostic factor, as has been suggested previously in soft tissue tumors.27 It should be emphasized that these potential prognostic factors were identified using a relatively small number of cases with limited follow up, and therefore future, larger studies should reassess these variables when more cases have been reported.
Cox proportional hazard analysis identified number of mitotic figures (as a continuous variable) as a significant predictor of disease recurrence. For some tumors, such as uterine leiomyosarcoma, mitotic activity is dichotomized (e.g., ≥10 per 10 HPFs for spindle cell leiomyosarcoma) and tumors with counts above that threshold “meet” the diagnostic criteria. However, there was not such binary behavior in this series or a biological basis to dichotomize this variable, as increases in mitotic activity appear to confer a gradual increase in risk. For example, case 9 had a mitotic rate of 23 per 10 HPFs and recurred in 72 months, while case 8 had a mitotic rate of 43 per 10 HPFs and recurred in only 6 months. However, to construct a risk stratification that was easy to apply in practice, a mitotic count of ≥8 per 10 HPFs was selected as a criterion for high-risk tumors. We then demonstrated that NTRK-rearranged uterine sarcomas can be risk stratified by classifying tumors as high risk based on the presence of one of: increased mitotic activity (≥8/10 HPFs), lymphovascular invasion, necrosis, or NTRK3 fusion. While the precise criteria used for prognostication will inevitably be further refined as additional cases are reported, we believe this provides an early framework for predicting behavior based on clinicopathologic features.
Our literature review highlights the variable behavior of these tumors: two thirds of patients were NED at last follow up, one in five were AWD, and 14% DOD. However, the tumors presented herein and those reported in the literature are potentially biased for recurrent, metastatic, and advanced stage tumors as most were seen in consultation at large academic centers. Of note, we and other groups studying NTRK-rearranged tumors in the uterus have used the label “sarcoma” to describe them although the entity appears in the most recent “WHO Classification of Tumors of Female Genital Tract”44 and sometimes in the non-gynecologic literature as “NTRK-rearranged spindle cell neoplasms.” Based on our current knowledge of their potentially aggressive behavior, we believe the uterine tumors should be designated as “sarcomas.” While our proposed risk stratification appears to identify tumors at high risk of recurrence, even the so-called low risk tumors should be considered to have malignant potential and patients should still receive follow up.
Treatment
Surgical resection is the mainstay of treatment for most patients. Just over half of the patients in this series underwent hysterectomy, though this may be an underestimate due to limited clinical follow up. Given the desire to maintain fertility in young patients, initial management may be conservative (polypectomy or cone biopsy). However, several cases in this series highlight challenges associated with local excision. While one patient (case 11) remained disease free 16.5 months after local excision, a second (case 6) required three procedures (polypectomy, cold knife conization and loop electrosurgical excision) to completely remove the tumor, and a third (case 7) initially treated conservatively had a pelvic recurrence which was subsequently successfully salvaged. Unfortunately, one other patient (case 9) who underwent local excision subsequently recurred with distant metastases and died of disease. Because these tumors are usually ER and PR negative and spread to the ovaries is not common, consideration of ovarian conservation is reasonable if these tumors are diagnosed as NTRK-rearranged sarcomas prior to surgery. Three patients received adjuvant treatment, two of whom died of disease; its benefit is difficult to discern with these limited data.
Trials of TRK inhibitors entrectinib and larotrectinib have demonstrated benefit in solid tumors with NTRK fusions, with partial responses in 50–62% of patients and complete responses in 7–13%.15,16 There are two reports in the literature of patients with NTRK-rearranged uterine tumors who were treated with targeted therapy: one patient with a 9 cm cervical sarcoma had a complete response to 27 weeks of neoadjuvant treatment with entrectinib,26 and a second patient with biopsy-proven pleural metastases who was with no evidence of disease following treatment with larotrectinib.5 While no patients in our series received TRK inhibition, the two patients who received chemotherapy died of disease, suggesting that targeted therapy may be more effective than chemotherapy.
Limitations
Our study has some notable limitations. Most significantly, six patients (40%) in our study were lost to follow up. Also, there was insufficient tumor or poor-quality nucleic acid in two cases which precluded molecular confirmation. There is morphologic overlap that can be seen with other uterine spindle cell tumors such as COL1A1∷PDGFB sarcomas.3,4,45 However, given both cases were CD34-negative, in contrast to typically CD34-positive PDGFB-fusion sarcomas, and showed pan-TRK positivity in the context of appropriate morphology we feel that these are best classified as NTRK-rearranged sarcomas. Also, only select slides were available for review in most cases. While pooling outcome data from many different series allowed us to identify potential prognostic features, this approach also introduced several inherent limitations. Specifically, the type and extent of tumor necrosis was not incorporated in the model, the field diameter used for mitotic counts was not standardized, and the recording of atypia was subjective and therefore not included in the analysis. Furthermore, there is significant interobserver variability in the counting of mitotic figures, and because mitotic counts were abstracted from different studies, the values used in our analysis are inherently inaccurate.
Conclusion
We present the clinical, morphologic, immunohistochemical, and molecular features of fifteen cases of NTRK-rearranged uterine sarcomas. We described two tumors with variant morphology not typically associated with NTRK-rearranged uterine sarcomas. One, with a IRF2BP2∷NTRK1 fusion showed high grade cytology and prominent stromal hyalinization. The second demonstrated diffuse pleomorphism and harbored TP53 mutations in addition to a TPR∷NTRK1 fusion. An analysis of previously published cases identified several possible adverse prognostic factors, including increased mitotic index, lymphovascular invasion, necrosis, and NTRK3 fusions. We present a risk stratification model based on these parameters which can predict which tumors may recur. As the number of reported cases increases, this preliminary model can be further revised. NTRK-rearranged uterine sarcomas represent a potentially aggressive neoplasm predominantly seen in the uterine cervix of young women and for which an accurate diagnosis is important because of the utility of TRK inhibitors in the recurrent/metastatic setting. This study further broadens our morphologic, immunohistochemical and clinical understanding of this rare tumor type and has identified clinicopathologic features which may predict tumor behavior. However, larger series are required to better risk stratify these rare tumors.
Acknowledgments
We thank the referring pathologists who kindly submitted cases and provided follow up: Dr. B. Gazic (Ljubljana, Slovenia), Dr. D. Payton (Brisbane, Australia), Dr. M. Kieckbusch (Boise, ID), Dr. N. de Saint Aubain (Brussels, Belgium), Dr. A. Dei Tos (Treviso, Italy), Dr. A. Watson (Seattle, WA), and Drs. C. Pauli and M. Buhler (Zurich, Switzerland).
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to declare.
References
- 1.Chiang S, Cotzia P, Hyman DM, et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype with Features of Fibrosarcoma. Am. J. Surg. Pathol 2018;42:791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatalica Z, Xiu J, Swensen J, et al. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol 2019;32:147–153. doi: 10.1038/s41379-018-0118-3. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux KA, Weiel JJ, Mills AM, et al. Neurofibrosarcoma Revisited : An Institutional Case Series of Uterine Sarcomas Harboring Kinase-related Fusions With Report of a Novel FGFR1-TACC1 Fusion. Am. J. Surg. Pathol 2021;45:638–652. doi: 10.1097/PAS.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 4.Croce S, Hostein I, Longacre TA, et al. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol 2019;32:1008–1022. doi: 10.1038/s41379-018-0184-6. [DOI] [PubMed] [Google Scholar]
- 5.Rabban JT, Devine WP, Sangoi AR, et al. NTRK fusion cervical sarcoma: a report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology 2020;77:100–111. doi: 10.1111/his.14069. [DOI] [PubMed] [Google Scholar]
- 6.Wells AE, Mallen AM, Bui MM, et al. NTRK-1 fusion in endocervical fibroblastic malignant peripheral nerve sheath tumor marking eligibility for larotrectinib therapy: A case report. Gynecol. Oncol. Rep 2019;28:141–144. doi: 10.1016/j.gore.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle W, Williams A, Sundar S, et al. TMP3-NTRK1 rearranged uterine sarcoma: A case report. Case Rep. Womens Health 2020;28:e00246. doi: 10.1016/j.crwh.2020.e00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgson A, Pun C, Djordjevic B, et al. NTRK-rearranged Cervical Sarcoma: Expanding the Clinicopathologic Spectrum. Int. J. Gynecol. Pathol 2021;40:73–77. doi: 10.1097/PGP.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 9.Michal M, Hájková V, Skálová A, et al. STRN-NTRK3-rearranged Mesenchymal Tumor of the Uterus: Expanding the Morphologic Spectrum of Tumors With: NTRK: Fusions. Am. J. Surg. Pathol 2019;43:1152–1154. doi: 10.1097/PAS.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 10.Wong DD, Vargas AC, Bonar F, et al. NTRK-rearranged mesenchymal tumours: diagnostic challenges, morphological patterns and proposed testing algorithm. Pathology (Phila.) 2020;52:401–409. doi: 10.1016/j.pathol.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Nilforoushan N, Wethington SL, Nonogaki H, et al. NTRK-Fusion Sarcoma of the Uterine Cervix: Report of 2 Cases With Comparative Clinicopathologic Features. Int. J. Gynecol. Pathol 2021. doi: 10.1097/PGP.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai J-W, Lee J-C, Hsieh T-H, et al. Adult NTRK-rearranged spindle cell neoplasms of the viscera: with an emphasis on rare locations and heterologous elements. Mod. Pathol 2022. doi: 10.1038/s41379-021-01005-3. [DOI] [PubMed] [Google Scholar]
- 13.Moh M, Johnson CM, Geurts J, et al. Uterine Sarcoma With a Novel WWOX-NTRK2 Fusion in a Postmenopausal Woman With Li-Fraumeni–Like Syndrome: A Case That Expands the Spectrum of NTRK-Rearranged Uterine Tumors. AJSP Rev. Rep 2021;26:304–306. doi: 10.1097/PCR.0000000000000476. [DOI] [Google Scholar]
- 14.Mills AM, Karamchandani JR, Vogel H, et al. Endocervical Fibroblastic Malignant Peripheral Nerve Sheath Tumor (Neurofibrosarcoma): Report of a Novel Entity Possibly Related to Endocervical CD34 Fibrocytes. Am. J. Surg. Pathol 2011;35:404–412. doi: 10.1097/PAS.0b013e318208f72e. [DOI] [PubMed] [Google Scholar]
- 15.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Tsai W-H, Ding Y, et al. Comprehensive evaluation of fusion transcript detection algorithms and a meta-caller to combine top performing methods in paired-end RNA-seq data. Nucleic Acids Res. 2016;44:e47. doi: 10.1093/nar/gkv1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Schulz-Trieglaff O, Shaw R, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinforma. Oxf. Engl 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 19.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch. Pathol. Lab. Med 2017;141:751–758. [DOI] [PubMed] [Google Scholar]
- 21.Van Rossum G, Drake FL. Python 3 Reference Manual. Scotts Valley, CA: CreateSpace. 2009. [Google Scholar]
- 22.McKinney W, et al. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference (Vol. 445, pp. 51–56). 2010. [Google Scholar]
- 23.Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson-Pilon C lifelines: survival analysis in Python. J. Open Source Softw 2019;4:1317. doi: 10.21105/joss.01317. [DOI] [Google Scholar]
- 25.Pedregosa F, Varoquaux G, Gramfort A. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res 2011;12:2825–2830. [Google Scholar]
- 26.Goulding EA, Morreau P, De Silva M, et al. Case report: NTRK1-rearranged cervical sarcoma with fibrosarcoma like morphology presenting in a 13-year-old managed with a neo-adjuvant TRK-inhibitor and surgical excision. Gynecol. Oncol. Rep 2021;37:100845. doi: 10.1016/j.gore.2021.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suurmeijer AJ, Dickson BC, Swanson D, et al. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes. Chromosomes Cancer 2019;58:739–746. doi: 10.1002/gcc.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suurmeijer AJH, Dickson BC, Swanson D, et al. A novel group of spindle cell tumors defined by S100 and CD34 co-expression shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes. Genes. Chromosomes Cancer 2018;57:611–621. doi: 10.1002/gcc.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad N, Stewart CJR, Chiang S, et al. p53 immunohistochemical analysis of fusion-positive uterine sarcomas. Histopathology 2021;78:805–813. doi: 10.1111/his.14292. [DOI] [PubMed] [Google Scholar]
- 30.Brčić I, Godschachner TM, Bergovec M, et al. Broadening the spectrum of NTRK rearranged mesenchymal tumors and usefulness of pan-TRK immunohistochemistry for identification of NTRK fusions. Mod. Pathol 2021;34:396–407. doi: 10.1038/s41379-020-00657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hechtman JF, Benayed R, Hyman DM, et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol 2017;41:1547–1551. doi: 10.1097/PAS.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudzinski ER, Lockwood CM, Stohr BA, et al. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol 2018;42:927–935. doi: 10.1097/PAS.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 33.Momeni-Boroujeni A, Mohammad N, Wolber R, et al. Targeted RNA expression profiling identifies high-grade endometrial stromal sarcoma as a clinically relevant molecular subtype of uterine sarcoma. Mod. Pathol 2021;34:1008–1016. doi: 10.1038/s41379-020-00705-6. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto H, Nozaki Y, Kohashi K, et al. Diagnostic utility of pan-Trk immunohistochemistry for inflammatory myofibroblastic tumours. Histopathology 2020;76:774–778. doi: 10.1111/his.14010. [DOI] [PubMed] [Google Scholar]
- 35.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khotskaya YB, Holla VR, Farago AF, et al. Targeting TRK family proteins in cancer. Pharmacol. Ther 2017;173:58–66. doi: 10.1016/j.pharmthera.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Rolfo C, Ruiz R, Giovannetti E, et al. Entrectinib: a potent new TRK, ROS1, and ALK inhibitor. Expert Opin. Investig. Drugs 2015;24:1493–1500. doi: 10.1517/13543784.2015.1096344. [DOI] [PubMed] [Google Scholar]
- 38.Weisman PS, Altinok M, Carballo EV, et al. Uterine Cervical Sarcoma With a Novel RET-SPECC1L Fusion in an Adult: A Case Which Expands the Homology Between RET-rearranged and NTRK-rearranged Tumors. Am. J. Surg. Pathol 2020;44:567–570. doi: 10.1097/PAS.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 39.Yang EJ, Howitt BE, Fletcher CDM, et al. Solitary fibrous tumour of the female genital tract: a clinicopathological analysis of 25 cases. Histopathology 2018;72:749–759. doi: 10.1111/his.13430. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod. Pathol 2016;29:4–13. doi: 10.1038/modpathol.2015.134. [DOI] [PubMed] [Google Scholar]
- 41.Parra-Herran C, Quick CM, Howitt BE, et al. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am. J. Surg. Pathol 2015;39:157–168. doi: 10.1097/PAS.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi A, Kurosawa M, Uemura M, et al. Anaplastic lymphoma kinase-negative uterine inflammatory myofibroblastic tumor containing the ETV6-NTRK3 fusion gene: a case report. J. Int. Med. Res 2018;46:3498–3503. doi: 10.1177/0300060518780873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotzia P, Benayed R, Mullaney K, et al. Undifferentiated Uterine Sarcomas Represent Under-Recognized High-grade Endometrial Stromal Sarcomas. Am. J. Surg. Pathol 2019;43:662–669. doi: 10.1097/PAS.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO Classification of Tumours Editorial Board. Female genital tumours. Lyon (France): International Agency for Research on Cancer; 2020. (WHO classification of tumour series, 5th ed.; vol 4). https://publications.iarc.fr/592. [Google Scholar]
- 45.Grindstaff SL, DiSilvestro J, Hansen K, et al. COL1A1-PDGFB fusion uterine fibrosarcoma: A case report with treatment implication. Gynecol. Oncol. Rep 2020;31:100523. doi: 10.1016/j.gore.2019.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]


