Using mouse models overexpressing human TREM2 in microglia, this study shows that WT TREM2 expression reduces amyloid deposition and suppresses disease-associated microglia only during the early amyloid seeding stage, whereas TREM2-R47H exacerbates amyloid burden during the middle amyloid rapid growth stage.
Abstract
TREM2 is exclusively expressed by microglia in the brain and is strongly linked to the risk for Alzheimer’s disease (AD). As microglial responses modulated by TREM2 are central to AD pathogenesis, enhancing TREM2 signaling has been explored as an AD therapeutic strategy. However, the effective therapeutic window targeting TREM2 is unclear. Here, by using microglia-specific inducible mouse models overexpressing human wild-type TREM2 (TREM2-WT) or R47H risk variant (TREM2-R47H), we show that TREM2-WT expression reduces amyloid deposition and neuritic dystrophy only during the early amyloid seeding stage, whereas TREM2-R47H exacerbates amyloid burden during the middle amyloid rapid growth stage. Single-cell RNA sequencing reveals suppressed disease-associated microglia (DAM) signature and reduced DAM population upon TREM2-WT expression in the early stage, whereas upregulated antigen presentation pathway is detected with TREM2-R47H expression in the middle stage. Together, our findings highlight the dynamic effects of TREM2 in modulating AD pathogenesis and emphasize the beneficial effect of enhancing TREM2 function in the early stage of AD development.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia defined by the pathological deposition of amyloid-β (Aβ) plaques and neurofibrillary tangles containing hyperphosphorylated tau (DeTure and Dickson, 2019; Hardy and Selkoe, 2002). Activation of microglia in the brain, concentrated around amyloid plaques, is a prominent pathological feature of AD (Itagaki et al., 1989). Recent work using the single-cell RNA sequencing (scRNA-seq) approach clearly demonstrates the transition of microglia from homeostatic to the activated states in response to amyloid plaques. These subsets of microglia responding to disease are defined as disease-associated microglia (DAM; Keren-Shaul et al., 2017), also known as microglial neurodegenerative phenotype (Krasemann et al., 2017) or activation response microglia (Sala Frigerio et al., 2019), as well as the interferon response microglia (IRM), major histocompatibility complex class II expressing microglia (MHCII+), and cell cycling/proliferating microglia (CPM; Ellwanger et al., 2021; Sala Frigerio et al., 2019). Importantly, two prominent AD risk genes, the triggering receptor expressed on myeloid cells 2 (TREM2) and the apolipoprotein E (APOE), play critical roles in triggering, sustaining, or controlling the acquisition of the disease-associated phenotypes of microglia (Keren-Shaul et al., 2017; Krasemann et al., 2017; Pimenova et al., 2017), raising the intriguing possibility that targeting TREM2 or APOE pathways in microglia could be a potential disease-modifying therapy for AD.
TREM2 variant p.R47H has been linked to an increased risk of AD (Guerreiro et al., 2013; Jonsson et al., 2013) with an effect size comparable to that of the APOE4 gene allele, the strongest genetic risk factor for AD (Corder et al., 1993). TREM2 is a cell surface receptor of the immunoglobulin superfamily that is expressed exclusively in the myeloid lineage cells, particularly in microglia in the central nervous system (Ulrich et al., 2017). TREM2 interacts with a wide array of ligands such as anionic lipids (Wang et al., 2015), high-density and low-density lipoproteins (Song et al., 2017; Yeh et al., 2016), apoptotic cells (Hsieh et al., 2009), APOE (Atagi et al., 2015; Bailey et al., 2015), and Aβ (Zhao et al., 2018; Zhong et al., 2018). Engagement of TREM2 by ligands initiates signaling through its adaptor DAP12 or DAP10 (Xing et al., 2015) to trigger downstream signaling pathways (Konishi and Kiyama, 2018; Ulland et al., 2017). Importantly, TREM2 signaling is required for the sequential microglia activation from homeostatic to disease-associated state (Deczkowska et al., 2018). Deficiency of Trem2 locks microglia in a homeostatic state and leads to attenuated microglial responses to pathological cues (Deczkowska et al., 2020; Keren-Shaul et al., 2017; Krasemann et al., 2017). Compelling experimental evidence suggests that microglia could play either a beneficial or a detrimental role during distinct pathological states (Aguzzi et al., 2013). Supporting this, studies on TREM2 function in AD using Trem2-knockout mice have generated inconsistent findings of either reducing or enhancing amyloid or tau pathologies (Bemiller et al., 2017; Fitz et al., 2020; Jay et al., 2017; Lee et al., 2021; Leyns et al., 2017; Parhizkar et al., 2019; Yuan et al., 2016). On the other hand, elevating TREM2 levels (Lee et al., 2018) or signaling (Schlepckow et al., 2020; Wang et al., 2020) may ameliorate amyloid pathogenesis, but the impacts seem to be affected by the age of the animals. In addition, there is limited information on the molecular pathobiology of TREM2-R47H, with one study reporting that the humanized TREM2-R47H mice showed less microglia activation but comparable Aβ burden with the mice expressing the common variant of TREM2 (Song et al., 2018). Collectively, studies to date suggest that TREM2 and related microglial functions are likely dynamic depending on the pathological stages throughout AD initiation and progression, highlighting the importance of using appropriate model systems to modulate TREM2 or TREM2-R47H expression during different stages of AD pathogenesis to inform therapeutic strategies.
Toward this, we herein generated microglia-specific conditional mouse models allowing induced expression of either the human WT TREM2 (TREM2-WT) or its R47H variant (TREM2-R47H) during different stages of AD development (Fig. 1 A). Upon breeding to the 5xFAD amyloid mouse model, we found that expression of TREM2-WT in the early amyloid seeding stage, but not during later stages of amyloid development, significantly reduced the amyloid deposition. In contrast, the expression of TREM2-R47H during the amyloid rapid growth period exacerbated amyloid burden. Microglia morphological and functional analysis by two-photon imaging demonstrated higher dynamic and increased response to Aβ in TREM2-WT expressing microglia compared with TREM2-R47H microglia. Finally, the scRNA-seq analyses revealed that elevating TREM2-WT expression suppresses the DAM signature and reduces the DAM population in the early stage, whereas the overexpression of TREM2-R47H in the middle stage is associated with upregulated antigen presentation pathway. Together, our study demonstrates the dynamic effects of TREM2 in AD pathogenesis and defines the effective window to target TREM2 in AD therapy.
Figure 1.
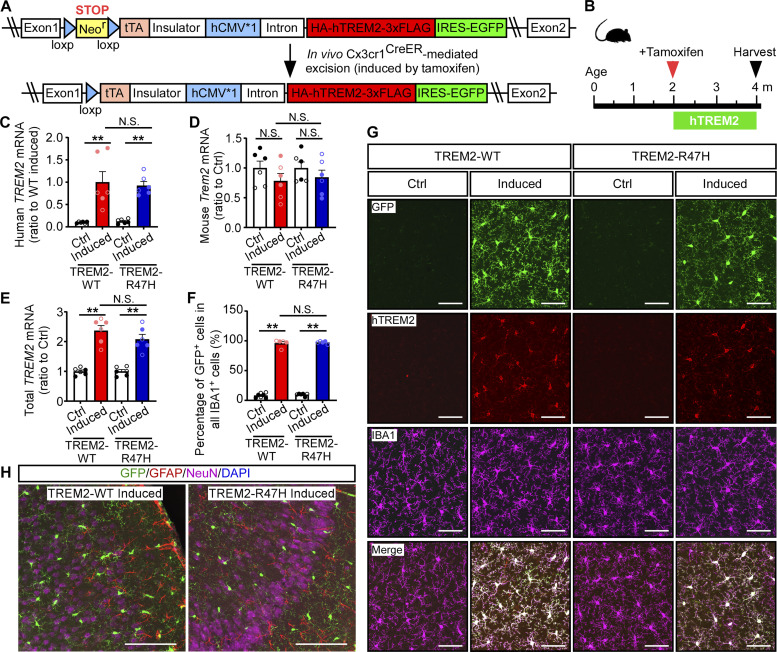
Microglia-specific and inducible mice expressing human TREM2-WT or TREM2-R47H variant. (A) Illustrative structure of the Rosa-targeting vector for Cre-mediated conditional expression of TREM2-WT or TREM2-R47H and EGFP. The TREM2 floxed mice were bred to the Cx3cr1CreER mice to generate TREM2-WT (TREM2-WT+/+; Cx3cr1CreER/+) or TREM2-R47H (TREM2-R47H+/+; Cx3cr1CreER/+) mice. Administration of tamoxifen to these mice led to the removal of the loxP-flanked Neor gene and expression of human TREM2-WT or TREM2-R47H in microglia. (B) Illustration of the induction paradigms for expression of human TREM2-WT or TREM2-R47H. (C–E) The mRNA levels of human TREM2 (C), mouse Trem2 (D), and total TREM2 (human + mouse; E) in the brain were quantified by qPCR. (F and G) Representative images of the GFP, human TREM2, and IBA1 co-staining (G) and the quantification of the percentage of GFP+ cells in all the IBA1+ microglia (F). Scale bar, 50 µm. (H) Immunofluorescence co-staining of GFP, GFAP, NeuN, and DAPI. Scale bar, 100 µm. n = 6 mice/group (mixed sexes). In C–F, the data from male and female mice are labeled as solid and open circles, respectively. Two-tailed unpaired Student’s t tests with Bonferroni correction were used for statistical analysis. P values <0.0167 were considered to be statistically significant. **, P < 0.01; N.S., not significant. Data are mean ± SEM.
Results
Generation of mouse models with inducible expression of human TREM2-WT or TREM2-R47H
To address the dynamic effects of human TREM2 and its AD risk-associated R47H variant in microglia, we generated mouse models with inducible human TREM2 gene expression in a specific cell type using a knock-in strategy targeting the Rosa26 locus (Liu et al., 2017; Miyazaki et al., 2005). The transgene construct contained a loxP-flanked STOP cassette harboring a Neor gene for the selection of integrated clones and for Cre-mediated cell type-specific expression. An enhanced green fluorescent protein (EGFP) cDNA was inserted with its expression driven by an internal ribosomal entry site, which serves as a surrogate of human TREM2 expression (Fig. 1 A). An N-terminal HA tag and a C-terminal 3xFLAG tag were introduced to distinguish the N-terminal soluble TREM2 (sTRMEM2) and the C-terminal fragment (CTF) from the full-length (FL) TREM2, respectively. To establish microglia-specific mouse models, we crossed our TREM2-WT and TREM2-R47H mice with Cx3cr1CreER mice (Parkhurst et al., 2013) to allow tamoxifen-induced, microglia-specific expression of human TREM2-WT or TREM2-R47H in the background of mouse endogenous Trem2 (Fig. 1 A).
At 2 mo of age, the TREM2-WT (TREM2-WT+/+; Cx3cr1CreER/+) and TREM2-R47H (TREM2-R47H+/+; Cx3cr1CreER/+) mice were treated with tamoxifen (referred to as “induced” group), while control groups were given a corn oil vehicle (referred to as “Ctrl” group; Fig. 1 B). Mice were then maintained for 2 mo to allow for turnover/replenishment of peripheral myeloid cells and harvested at 4 mo of age. To confirm the expression of TREM2, we assessed the mRNA levels of both human TREM2 and endogenous mouse Trem2 by quantitative PCR (qPCR). Using primers that specifically recognize human TREM2, we detected significantly higher expression of human TREM2 in the induced groups compared with the Ctrl groups with no significant difference between TREM2-WT– and TREM2-R47H–induced groups (Fig. 1 C). The mouse endogenous Trem2 mRNA levels were comparable between Ctrl and induced groups in both TREM2-WT and TREM2-R47H mice (Fig. 1 D). Using primers that recognize both human TREM2 and murine Trem2 with the same efficiency, we found that the induced groups had approximately twofolds of total TREM2 mRNA relative to Ctrl animals, with no difference detected between TREM2-WT– and TREM2-R47H–induced groups (Fig. 1 E). The Ctrl animals also showed a very low-level expression of human TREM2 mRNA (Fig. 1 C), which was likely due to the tamoxifen-independent Cre activity as previously reported (Parkhurst et al., 2013). To further verify the microglia-specific expression of human TREM2, we performed costaining of human TREM2, the microglia-specific marker IBA1, and GFP (the surrogate of human TREM2). Our results demonstrated that the human TREM2 and GFP were exclusively expressed in the microglia (Fig. 1, F and G), but not in astrocytes or neurons (Fig. 1 H), confirming microglia-specific expression of human TREM2 in our mouse models. In summary, our novel mouse models display tamoxifen-induced, microglia-specific expression of human TREM2-WT or TREM2-R47H as designed.
Characterization of microglia-specific 5xFAD/TREM2 inducible mouse models
To assess the dynamic effect of TREM2 during different stages of amyloid development, we bred our microglia-specific TREM2-WT and TREM2-R47H mice to 5xFAD amyloid mouse model (hereafter referred to as 5xFAD/TREM2-WT and 5xFAD/TREM2-R47H). We designed three paradigms to investigate the specific effects of TREM2-WT and TREM2-R47H on amyloid deposition: (1) during the Aβ seeding (0–3.5 mo, early stage); (2) during the rapid growth period (2–5 mo, middle stage); and (3) during saturation stage (5–8 mo, late stage; Fig. 2 A; Oakley et al., 2006).
Figure 2.
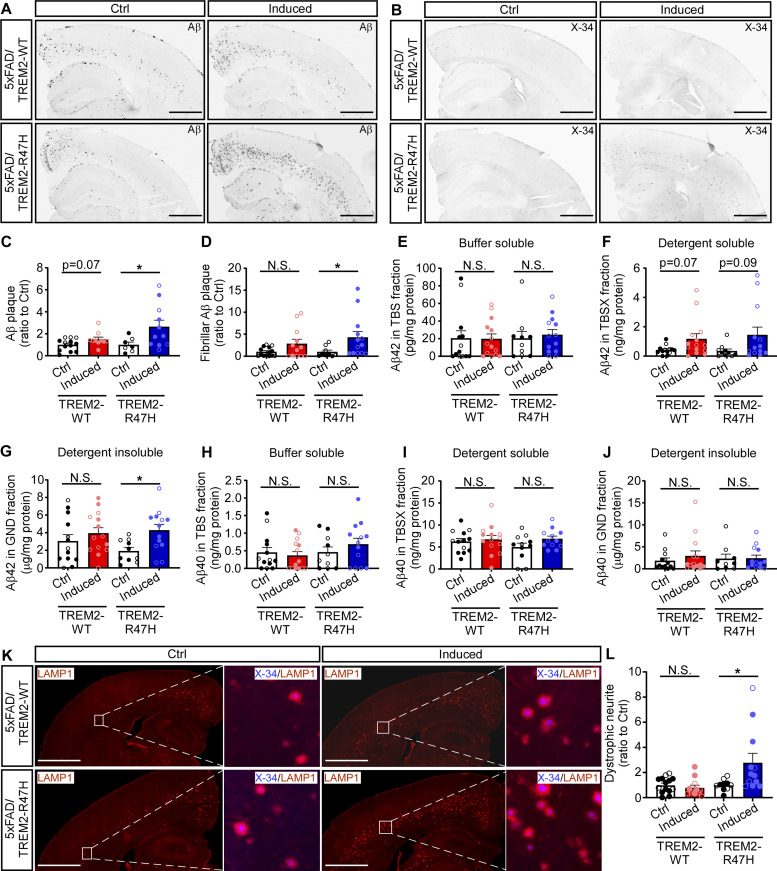
TREM2-mediated suppression of amyloid accumulation and neuritic dystrophy in the early stage of pathological development. (A) Illustration of induction paradigms for the expression of human TREM2-WT or TREM2-R47H during different stages of the amyloid development in the 5xFAD background. (B–E) Representative images (B and C) and quantification (D and E) of Aβ plaque load by immunofluorescence staining with MOAB2 antibody (B and D) and fibrillar Aβ by X-34 labeling (C and E) in the mice induced for TREM2-WT or TREM2-R47H expression during 0–3.5 mo of age. Scale bar, 1 mm. (F–H) The levels of Aβ42 in the detergent soluble (TBSX) and insoluble (GND) fractions and the Aβ40 in the insoluble (GND) fraction were detected by ELISAs in the mice expressing TREM2-WT or TREM2-R47H during 0–3.5 mo of age. (I and J) The representative images (J) and quantification (I) of dystrophic neurites by immunofluorescence staining with LAMP1 antibody in the mice induced for TREM2-WT or TREM2-R47H expression during 0–3.5 mo of age. Scale bar, 1 mm. n = 11–13 mice/group, mixed sexes. Data are shown as mean ± SEM. Mann–Whitney tests with Bonferroni correction were used for statistical analysis. P values <0.025 were considered to be statistically significant. *, P < 0.025; **, P < 0.01; N.S., not significant. (K–P) The ISF from the hippocampus of the 5xFAD mice expressing TREM2-WT (K–M) or TREM2-R47H (N–P) at 0–3.5 mo was collected via in vivo microdialysis. (K, L, N, and O) The levels of Aβ42 at baseline or after γ-secretase inhibitor treatment were quantified by ELISAs. (M and P) The half-life of Aβ42 in ISF was calculated and compared. n = 3–6 mice/group, mixed sexes. Data are shown as mean ± SEM. Two-tailed unpaired Student’s t tests were used for statistical analysis. *, P < 0.05; N.S., not significant. In D–I, L, M, O, and P, the data from male and female mice are labeled as solid and open circles, respectively.
We first confirmed the Cre recombination efficiency in all three 5xFAD/TREM2 animal cohorts by quantifying the percentage of GFP+ cells in all IBA+ cells in the brain (Fig. S1). There were >98% of IBA1+ cells expressing GFP in all three cohorts, including the 5xFAD induced at 0–3.5 (Fig. S1, A and D), 2–5 (Fig. S1, B and E), and 5–8 (Fig. S1, C and F) mo of age by tamoxifen treatment. No differences were found between 5xFAD/TREM2-WT and 5xFAD/TREM2-R47H groups induced at different ages.
Figure S1.
Microglia-specific and inducible expression of human TREM2 in the 5xFAD background. The expression of human TREM2-WT or TREM2-R47H in microglia were detected by immunofluorescence analysis of GFP and IBA1 signals in the 5xFAD/TREM2-WT mice or 5xFAD/TREM2-R47H mice in different stages of AD development. (A–F) Representative images (A–C) of the GFP and IBA1 signals and the percentage of GFP+ cells in all the IBA1+ microglia (D–F) are shown. Scale bar, 50 µm. n = 12–14, 6–9, and 10–15 mice/group (mixed sexes) for the 0–3.5, 2–5, and 5–8 mo cohorts, respectively. Data are mean ± SEM. The data from male and female mice are labeled as solid and open circles, respectively. Mann−Whitney tests followed by Bonferroni correction for multiple comparisons were used for statistical analysis. P values <0.0167 were considered to be statistically significant. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; N.S., not significant.
Next, we confirmed the expression of human TREM2 at the protein level in the cortical brain lysate of these induced mice after sequentially extracting proteins in TBS (buffer soluble proteins) and TBSX (TBS with 1% Triton X-100, membrane-bound detergent soluble proteins) fractions. Using an antibody recognizing the C-terminus of human TREM2, we detected a band above 37 kD corresponding to the FL-TREM2 (plus the HA and 3xFLAG tags) in the TBSX fraction (Fig. S2, A, B, E, F, I, and J). No differences in the amount of FL-TREM2 were found between TREM2-WT– and TREM2-R47H–induced groups. Interestingly, we found significantly lower levels of CTF-TREM2/FL-TREM2 ratio in TREM2-R47H mice compared with TREM2-WT mice in all the three cohorts (Fig. S2, D, H, and L), whereas the ratio of sTREM2 to FL-TREM2 was significantly reduced in TREM2-R47H mice in the middle and late cohorts (Fig. S2, G and K), but not in the early cohort (Fig. S2 C), suggesting that the R47H mutation might affect the proteolytic processing of TREM2 or the metabolism of sTREM2 in the presence of abundant Aβ pathology.
Figure S2.
Characterization of human TREM2 protein in the 5xFAD/TREM2 mice. The protein levels of human TREM2 were detected in the TBS and TBSX fraction of 5xFAD/TREM2 mouse brain lysates by Western blotting (A, E, and I). The soluble TREM2 (sTREM2) in the TBS fraction was blotted by HA antibody. The FL- and CTF-TREM2 in the TBSX fraction were detected by a human-specific TREM2 antibody recognizing the C-terminus of TREM2. The amount of FL-TREM2 (B, F, and J), the ratio of sTREM2 to FL-TREM2 (C, G, and K), and the ratio of CTF- to FL-TREM2 (D, H, and L) were quantified. (A–D) The assessment of human TREM2 in the cohort of 5xFAD/TREM2 animals when TREM2 was expressed from 0 to 3.5 mo of age. n = 11–13 mice/group, mixed sexes. (E–H) The assessment of human TREM2 in the cohort of 5xFAD/TREM2 animals when TREM2 was expressed from 2 to 5 mo of age. n = 11–13 mice/group, mixed sexes. (I–L) The assessment of human TREM2 in the cohort of 5xFAD/TREM2 animals when TREM2 was expressed from 5 to 8 mo of age. n = 9–15 mice/group, mixed sexes. Data are mean ± SEM. The data from male and female mice are labeled as solid and open circles, respectively. Mann–Whitney tests with Bonferroni correction were used for statistical analysis. P values <0.0167 (B, F, and G) or < 0.05 (C, D, G, H, K, and L) were considered to be statistically significant. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001; N.S., not significant. Source data are available for this figure: SourceData FS2.
Elevating human TREM2-WT expression in the early seeding stage reduces amyloid pathology
Using these 5xFAD/TREM2 inducible mice, we first investigated whether TREM2 expression in the early amyloid seeding stage impacts amyloid pathology by inducing human TREM2 expression at birth and harvested the mice at 3.5 mo of age. The amyloid pathology was assessed by immunofluorescence staining of Aβ with a MOAB-2 antibody, labeling Aβ fibrils with X-34, and measuring soluble and insoluble Aβ40 and Aβ42 levels in different fractions of brain lysates by ELISAs. We found that the expression of TREM2-WT in the Aβ seeding stage significantly reduced the total amyloid plaque burden (Fig. 2, B and D), the fibrillar Aβ deposition (Fig. 2, C and E), the detergent soluble and insoluble Aβ42 levels (Fig. 2, F and G), and detergent insoluble Aβ40 levels (Fig. 2 H). To further assess the effects of TREM2 on dystrophic neurites, a key pathological feature of AD (DeTure and Dickson, 2019), we performed immunofluorescence staining with an antibody against the lysosome-associated membrane protein 1 (LAMP1) that typically accumulates in damaged neurites. Consistent with the lower amyloid burden, we found a significant reduction of dystrophic neurites in these mice when TREM2-WT was expressed during the early Aβ seeding stage (Fig. 2, I and J). In contrast, the expression of TREM2-R47H in this stage did not influence the overall amyloid pathologies (Fig. 2, B–H) or dystrophic neurites (Fig. 2, I and J), suggesting a loss-of-function of TREM2-mediated protection against the early amyloid development.
Strong evidence indicates that the Aβ clearance is impaired in AD (Mawuenyega et al., 2010); however, it is not clear whether TREM2-mediated microglia function plays a role in Aβ clearance. To address this, we performed in vivo microdialysis to analyze the elimination kinetics of Aβ42 in the interstitial fluid (ISF) using our 5xFAD/TREM2 mice induced during the early Aβ seeding stage (Liu et al., 2017). We found that the baseline level of Aβ42 in ISF was significantly reduced to 50% upon expression of TREM2-WT (Fig. 2, K and L), consistent with the lower level of amyloid burden in the brain parenchyma. Importantly, after halting Aβ production by injecting a γ-secretase inhibitor, we found that the ISF Aβ42 half-life was reduced by the expression of TREM2-WT (Fig. 2 M), suggesting a potential contributing mechanism of TREM2-mediated microglia function in reducing Aβ seeding. Consistent with the unchanged amyloid burden, TREM2-R47H expression did not affect the ISF Aβ42 clearance, including the baseline level, clearance rate, and half-life of Aβ42 (Fig. 2, N–P).
TREM2-R47H expression in the rapid growth stage exacerbates amyloid pathology
When inducing TREM2 expression during the rapid growth amyloid stage (2–5 mo of age), we found that TREM2-R47H expression led to an increased overall amyloid burden, including the MOAB-2 antibody labeled Aβ plaques (Fig. 3, A and C), X-34 labeled Aβ fibrils (Fig. 3, B and D), and Aβ42 levels in the detergent-insoluble fraction of the brain lysate measured by ELISAs (Fig. 3 G). The soluble Aβ42 levels and the soluble and insoluble Aβ40 levels did not change (Fig. 3, E, F, and H–J). Additionally, TREM2-R47H expression significantly increased dystrophic neurites (Fig. 3, K and L), suggesting a toxic function of TREM2-R47H in this stage. The expression of TREM2-WT, however, did not affect the overall amyloid burden and neuritic dystrophy during this stage of amyloid development (Fig. 3, A–E, and G–L), except for a slight increase in detergent-soluble Aβ42 levels by ELISA (Fig. 3 F).
Figure 3.
TREM2-R47H expression in the rapid growth period of amyloid development exacerbates amyloid accumulation and neuritic dystrophy. (A–D) The representative images (A and B) and quantification (C and D) of Aβ plaque load by immunofluorescence staining with MOAB2 antibody (A and C) and fibrillar Aβ by X-34 labeling (B and D) in the mice induced for TREM2-WT or TREM2-R47H expression during 2–5 mo of age. Scale bar, 1 mm. (E–J) The levels of Aβ42 (E–G) and Aβ40 (H–J) in the buffer soluble (TBS), detergent soluble (TBSX), and insoluble (GND) fractions detected by ELISAs in the mice with TREM2-WT or TREM2-R47H expression during 2–5 mo of age. (K and L) The representative images (K) and quantification (L) of dystrophic neurites by immunofluorescence staining with LAMP1 antibody in the mice expressing TREM2-WT or TREM2-R47H during 2–5 mo of age. Scale bar, 1 mm. n = 11–13 mice/group, mixed sexes. Data are shown as mean ± SEM. Mann–Whitney tests with Bonferroni correction were used for statistical analysis. P values <0.025 were considered to be statistically significant. *, P < 0.025; **, P < 0.01; N.S., not significant. In C–J and L, the data from male and female mice are labeled as solid and open circles, respectively.
To evaluate whether the mouse endogenous Trem2 function is interrupted by human TREM2 overexpression, we measured mouse Trem2 by ELISA and found significantly increased soluble (in TBS fraction) and membrane-associated (in TBSX fraction) mouse Trem2 in TREM2-R47H–induced group compared to the control group, whereas no changes were found with or without TREM2-WT expression (Fig. S3, A and B). The levels of mouse Trem2 in the TBS fraction were significantly associated with those of insoluble Aβ42 (Fig. S3 C), whereas the levels of mouse Trem2 in the TBSX fraction were not significant but showed the same trend of association with those of insoluble Aβ42 (Fig. S3 D; P = 0.08). These data suggest that the level of mouse endogenous Trem2 changes with microglia activation and the function of endogenous Trem2 are unlikely affected by human TREM2 overexpression.
Figure S3.
The protein levels of mouse endogenous Trem2 in 5xFAD/TREM2 mice. The protein levels of mouse Trem2 were detected in the buffer soluble (TBS) and detergent soluble (TBSX) fraction of 5xFAD/TREM2 mouse brain lysates by ELISA. (A–D) The assessment of mouse Trem2 in the middle cohort of 5xFAD/TREM2 animals when TREM2 was expressed from 2 to 5 mo of age (A and B). n = 11–13 mice/group, mixed sexes. The correlation between Trem2 and insoluble Aβ42 was plotted (C and D). (E–H) The assessment of mouse Trem2 in the late cohort of 5xFAD/TREM2 animals when TREM2 was expressed from 5 to 8 mo of age. n = 9–15 mice/group, mixed sexes. The correlation between Trem2 and insoluble Aβ42 were plotted (G and H). Mann–Whitney tests with Bonferroni correction were used for statistical analysis in panels (A, B, E, and F). P values <0.025 were considered to be statistically significant. **, P < 0.01; N.S., not significant. Data are mean ± SEM. Spearman correlation tests were used for statistical analysis in C, D, G, and H. P values <0.05 were considered to be statistically significant. In A, B, E, and F, the data from male and female mice are labeled as solid and open circles, respectively.
TREM2 expression does not affect amyloid pathology in the advanced stage of amyloid development
Finally, we tested the effects of TREM2 expression on amyloid pathology during the plaque saturation stage (5–8 mo of age). We found that TREM2 expression, either WT or R47H, in this stage did not have any impact on total Aβ plaques (Fig. 4, A and C), Aβ fibrils (Fig. 4, B and D), or soluble and insoluble Aβ40 and Aβ42 levels by ELISAs (Fig. 4, E–J). The mouse endogenous Trem2 levels were significantly associated with insoluble Aβ42 levels but were not changed with or without human TREM2 overexpression (Fig. S3, E–H). Additionally, there were no differences in the levels of the dystrophic neurites after TREM2 expression (Fig. 4, K and L). These results are consistent with the previous finding that TREM2 has a limited role in the late or advanced stage of amyloid development in AD (Parhizkar et al., 2019).
Figure 4.
TREM2 expression in the late stage of amyloid development does not affect amyloid accumulation and neuritic dystrophy. (A–D) The representative images (A and B) and quantification (C and D) of Aβ plaque load by immunofluorescence staining with MOAB2 antibody (A and C) and fibrillar Aβ by X-34 labeling (B and D) in the mice induced for TREM2-WT or TREM2-R47H expression during 5–8 mo of age. Scale bar, 1 mm. (E–J) The levels of Aβ42 (E–G) and Aβ40 (H–J) in the buffer soluble (TBS), detergent soluble (TBSX), and insoluble (GND) fractions detected by ELISAs in the mice expressing TREM2-WT or TREM2-R47H during 5–8 mo of age. (K and L). The representative images (K) and quantification (L) of dystrophic neurites by immunofluorescence staining with LAMP1 antibody in the mice expressing TREM2-WT or TREM2-R47H during 5–8 mo of age. Scale bar, 300 µm. n = 9–15 mice/group, mixed sexes. Mann–Whitney tests followed by Bonferroni correction were used for statistical analysis. P values <0.025 were considered to be statistically significant. N.S., not significant. Data are mean ± SEM. In D–I, L, M, O, and P, the data from male and female mice are labeled as solid and open circles, respectively.
In vivo two-photon imaging reveals TREM2-mediated dynamic interaction of microglia with amyloid plaques
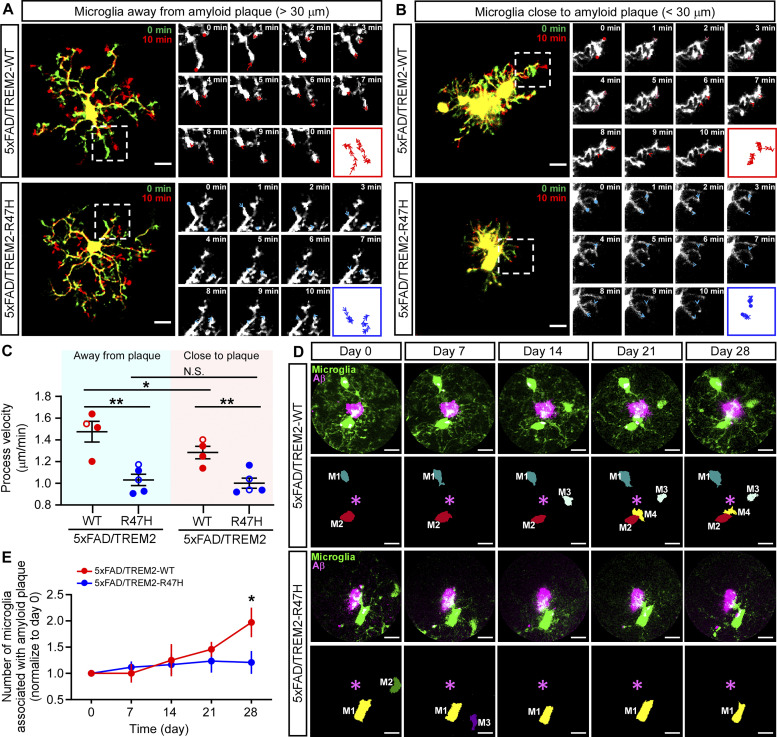
To investigate the TREM2-mediated functional changes of microglia in the environment with amyloid deposition, we monitored the dynamics of microglia processes away from amyloid (>30 µm) and close to amyloid (<30 µm) by two-photon microscopy. Microglial TREM2-GFP expression was induced at birth, and the amyloid plaque was labeled by Methoxy-X04 via intraperitoneal injection prior to imaging (Meyer-Luehmann et al., 2008). We started to evaluate the deposition of amyloid plaques at 3 mo; however, the X04-labeled amyloid plaques were not enough to be visualized in the somatosensory cortical region under two-photon microscopy until the animals reached 5–6 mo of age. As such, we first monitored the dynamics of microglia away from the amyloid plaques at 6 mo of age. Time-lapse imaging in a 10-min period showed that the microglial processes were remarkably motile, undergoing cycles of de novo extensions and retractions as previously reported (Nimmerjahn et al., 2005; Fig. 5 A). On average, the process velocity of TREM2-WT-expressing microglia was around 1.48 µm/min, whereas the TREM2-R47H microglia exhibited significantly reduced dynamics with an average process velocity of around 1.03 µm/min (Fig. 5 C). This TREM2-WT–associated higher resting motility might enable microglia to effectively surveil the microenvironment, which leads to efficient Aβ clearance. Interestingly, the dynamics of TREM2-WT–expressing microglia were reduced when they were close to the amyloid plaques (1.28 µm/min), although still faster than those expressing the TREM2-R47H at around 1.00 µm/min (Fig. 5, B and C).
Figure 5.
Greater microglial surveillance and dynamic responses to amyloid plaque in TREM2-WT mice than TREM2-R47H mice. (A and B) Two-photon in vivo imaging of microglial process velocity in the 5xFAD/TREM2-WT mice or 5xFAD/TREM2-R47H mice at 6 mo of age. Overlaid images taken 10 min apart show process dynamics. Close-up images show process trace per minute of microglia away from amyloid plaque (A) and close to amyloid plaque (B). Scale bar, 10 μm. (C) Quantification of the microglial process velocity in the 5xFAD/TREM2-WT mice and 5xFAD/TREM2-R47H mice. n = 4–5 mice/group, mixed sexes. Two-tailed unpaired Student’s t tests with Bonferroni correction were used for statistical analysis. P values <0.0125 were considered to be statistically significant. *, P < 0.0125; **, P < 0.01; N.S., not significant. (D) Representative time-lapse images of microglia response to amyloid plaque in the 5xFAD/TREM2-WT mice or 5xFAD/TREM2-R47H mice. The same field of views were traced and recorded every 7 d for 28 d. Scale bar, 10 μm. (E) Quantification of the number of microglia within a 30 μm radius of the plaque. Microglia number in each field of view is normalized to day 0. n = 4–5 mice/group, mixed sexes. In C, the data from male and female mice are labeled as solid and open circles, respectively. Two-tailed unpaired Student’s t tests were used for statistical analysis. *, P < 0.05. Data are shown as mean ± SEM.
It has been reported that in amyloid mouse models, Trem2 deficiency impairs microglia clustering around Aβ plaques (Mazaheri et al., 2017; Wang et al., 2015). To evaluate how the expression of TREM2-WT or TREM2-R47H impacts the clustering of microglia toward amyloid plaque, we longitudinally monitored the temporal relationships between microglia and plaques weekly for 4 wk starting from 6 mo of age (Fig. 5 D). Interestingly, the microglia number surrounding amyloid doubled in the TREM2-WT group after 4 wk, whereas no significant changes were observed in the TREM2-R47H group (Fig. 5 E). These results indicate that TREM2-WT enhances microglial interaction with amyloid plaque.
TREM2 expression modifies the microglia gene expression signatures and phenotypes
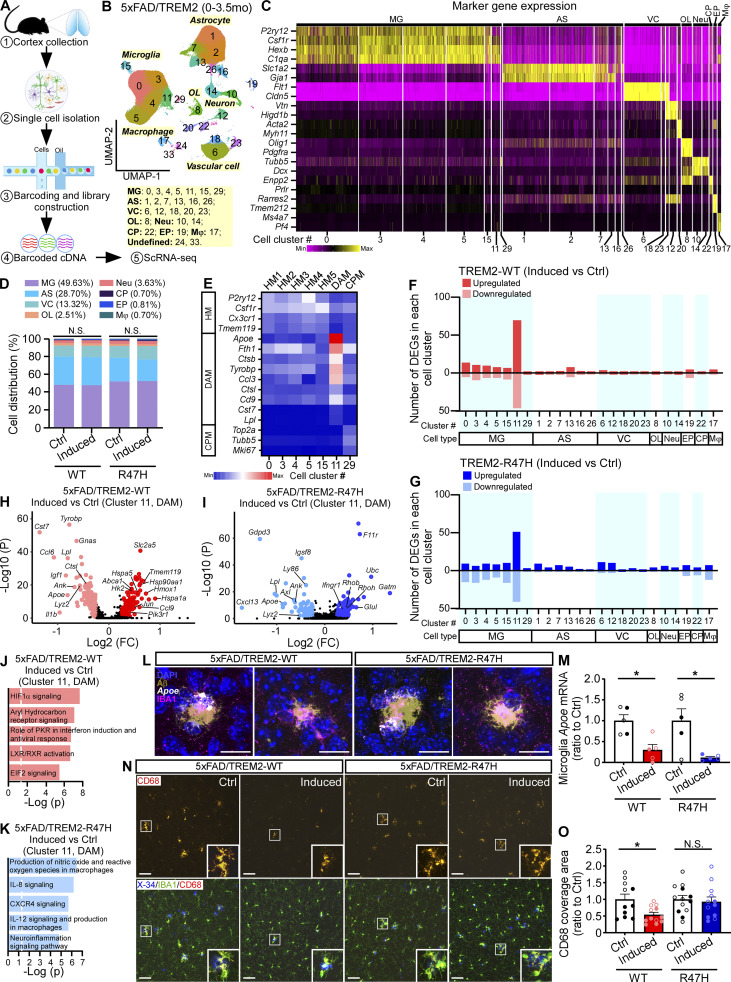
To gain insights into the molecular pathways underlying the microglial responses to amyloid deposition, we isolated cortical single cells and performed scRNA-seq analysis using animals with induced TREM2 expression at 0–3.5 mo of age as aforementioned (Fig. 6 A). We removed myelin after dissociating the cells, thus the cell populations of oligodendrocytes and neurons were largely reduced. A total of 216,436 single cells were recovered after manually removing the doublet cell clusters, which formed 26 distinct cell clusters after unsupervised clustering. The clusters were visualized with uniform manifold approximation and projection (UMAP) dimensions (Fig. 6 B). The cell types of the clusters were annotated based on the expression of known cell type–specific marker genes (Fig. 6 C). The major cell type captured by scRNA-seq was microglia (clusters 0, 3, 4, 5, 11, 15, and 29; 49.63% of total cells). Other cell types included astrocytes (clusters 1, 2, 7, 13, 16, and 26; 28.70% of total cells), vascular cells (clusters 6, 12, 18, 20, and 23; 13.32% of total cells), oligodendrocyte lineage cells (cluster 8; 2.51% of total cells), neurons (clusters 10 and 14; 3.63% of total cells), choroid plexus cells (cluster 22; 0.70% of total cells), ependymal cells (cluster 19; 0.81% of total cells), and macrophages (cluster 17; 0.70% of total cells; Fig. 6, B and D). Two clusters that cannot be defined as specific cell types (clusters 24 and 33) were removed from downstream analyses. The composition of the major cell types (Fig. 6 D) was similarly represented in the TREM2-WT and TREM2-R47H mice compared to their own controls. Based on published subtype-specific microglial marker gene expression data (Keren-Shaul et al., 2017; Krasemann et al., 2017; Sala Frigerio et al., 2019; Wang et al., 2020), we further identified subclusters of microglia, including the homeostatic microglia (HM1-5; clusters 0, 3, 4, 5, and 15; high expression of P2ry12, Csf1r, Cx3cr1, and Tmem119); DAM (cluster 11, high expression of DAM genes identified by Keren-Shaul et al. [2017] such as Apoe, Fth1, Ctsb, Tyrobp, Ccl3, Ctsl, Cd9, Cst7, and Lpl); and CPM (cluster 29; high expression of Top2a, Tubb5, and Mki67 identified by Sala Frigerio et al. [2019]; Fig. 6 E).
Figure 6.
Suppression of DAM signature with TREM2 expression at the early amyloid seeding stage. (A) Schematic representation of experimental procedures for scRNA-seq of brains from 5xFAD/TREM2 mice with TREM2 induction at the early amyloid seeding stage (0–3.5 mo of age). (B) UMAP plot showing 26 distinguished cell clusters and their annotated cell types. n = 6 mice per group (equal male and female). 216,436 total cells were analyzed. (C) Heatmap showing the expression of marker genes in different annotated cell types. (D) Bar graph showing the proportion of cell types in each of the four groups. (E) Heatmap showing the expression of marker genes in the microglia subclusters. (F and G) Number of DEGs for all the cell clusters in the comparison between induced versus Ctrl groups of 5xFAD/TREM2-WT (F) or 5xFAD/TREM2-R47H (G) mice. (H and I) Volcano plots for DEGs of induced versus Ctrl groups in microglia subcluster 11 (DAM) in 5xFAD/TREM2-WT (H) or 5xFAD/TREM2-R47H (I) group. (J and K) Top five canonical pathways enriched by DEGs from cluster 11 in 5xFAD/TREM2-WT (J) or 5xFAD/TREM2-R47H (K) group. The threshold of significant P value is 0.05. MAST tests were used for statistical analysis of DEGs while correcting for sex differences. (L and M) Combined RNAscope and immunofluorescent analyses of Apoe expression by microglia in the presence of Aβ plaques. Expression of Apoe was visualized using RNAscope probe, while amyloid plaques and microglia were visualized by staining with the anti-Aβ (MOAB2) and anti-IBA1 antibodies, respectively. Nuclei were visualized with DAPI. Representative images showing Apoe (white), microglia (violet), amyloid plaque (olive), and DAPI (blue) staining (L). Scale bar, 20 μm. The microglial Apoe expression was quantified and compared (M). n = 5 mice/group. Two-tailed unpaired Student’s t tests were used for statistical analysis. *, P < 0.05. Data are mean ± SEM. (N and O) Representative images showing CD68 (red), IBA1 (green), and amyloid plaque (X-34+, blue) staining (N). Scale bar, 50 μm. The CD68 coverage areas in the IBA1+ microglia were quantified and compared (O). n = 11–13 mice/group, mixed sexes. Data are shown as mean ± SEM. Mann–Whitney tests with Bonferroni correction were used for statistical analysis. P values <0.025 were considered to be statistically significant. *, P < 0.025; **, P < 0.01; N.S., not significant. In M and O, the data from male and female mice are labeled as solid and open circles, respectively. MG, microglia; AS, astrocyte; VC, vascular cell; OL, oligodendrocyte lineage cell; Neu, neuron; CP, choroid plexus cell; EP, ependymal cell; Mφ, macrophage.
We next focused on differentially expressed genes (DEGs), specifically comparing 5xFAD/TREM2-WT– and 5xFAD/TREM2-R47H–induced mice versus their own littermate controls. We found that most DEGs were identified in microglia clusters after TREM2-WT or TREM2-R47H expression, yet very few DEGs in other cell types (Fig. 6, F and G), suggesting a profound cell-autonomous effect of TREM2 on microglia gene expression profile. Importantly, TREM2 overexpression primarily induced DEGs in the DAM microglia subcluster (cluster 11), which is consistent with the notion that TREM2 signaling regulates microglia responding to disease (Keren-Shaul et al., 2017). Specifically, we identified 69 upregulated and 46 downregulated genes in the 5xFAD/TREM2-WT–induced versus Ctrl groups (Fig. 6 F) and 51 upregulated and 41 downregulated genes in the 5xFAD/TREM2-R47H–induced versus Ctrl groups (Fig. 6 G) in this DAM subcluster. Pathway analysis showed that the DEGs in the DAM cluster were enriched in hypoxia-inducible factor 1 subunit alpha (HIF1α) signaling as the top pathway (Hk2, Hmox1, Hsp90aa1, Hspa1a, Hspa5, Jun, Pik3r1, Slc2a5, etc.) in 5xFAD/TREM2-WT–expressing mice (Fig. 6, H and J), whereas production of oxide and reactive oxygen species was the top pathway (Ifngr1, Rhob, Rhoh, etc.) in 5xFAD/TREM2-R47H–expressing mice (Fig. 6, I and K). The HIF1α signaling pathway plays important roles in regulating microglia functions, such as phagocytosis (Grubman et al., 2021), which might underlie the beneficial effect of TREM2-WT in accelerating Aβ clearance and reducing Aβ seeding in this stage. Interestingly, the TREM2 expression, both TREM2-WT and TREM2-R47H, significantly downregulated the genes associated with DAM signature (including Apoe, B2m, Ccl6, Cst7, Lpl, Igf1, Tyrobp, etc.; Fig. 6, H and I). To further confirm the results from scRNA-seq, we performed in situ hybridization (ISH) with RNAscope probes against Apoe, coupled with immunofluorescent detection of Aβ plaques by MOAB-2 antibody and microglia by IBA1 antibody (Fig. S4 A). Consistent with our observations from scRNA-seq analysis in this cohort, the TREM2 expression significantly downregulated the microglial Apoe mRNA in both TREM2-WT and TREM2-R47H groups (Fig. 6, L and M). These data suggest that elevating TREM2 expression in the early stage of amyloid development suppresses the disease-related signature of microglia.
Figure S4.
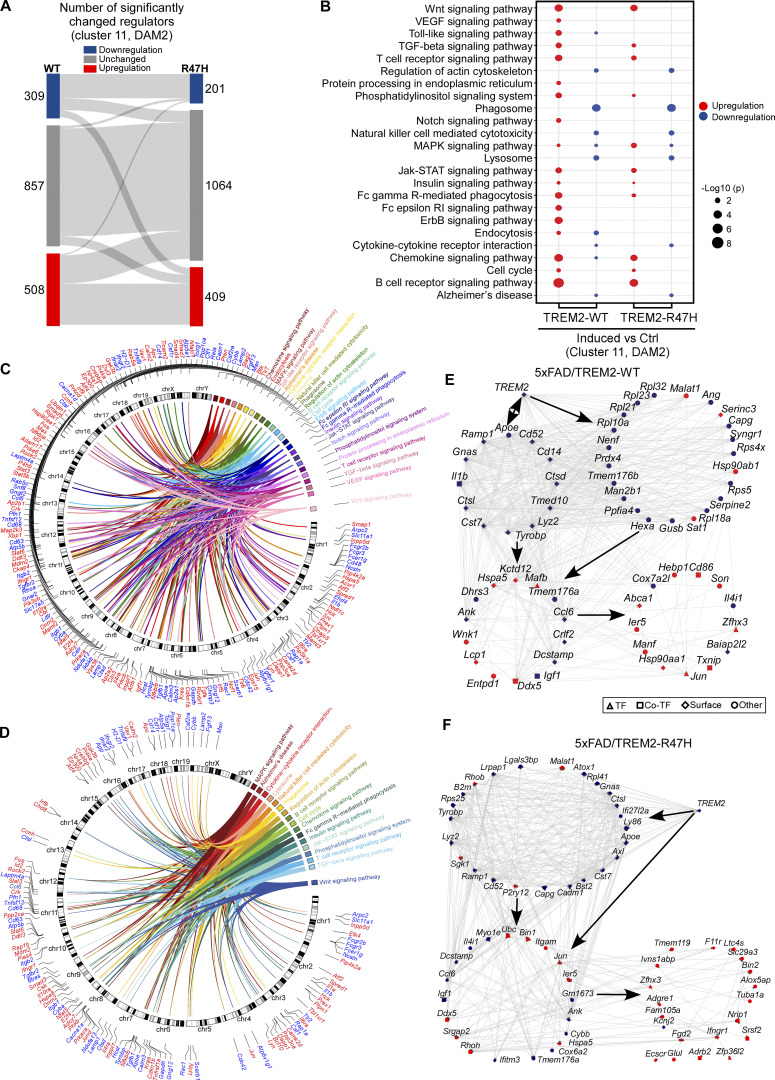
TREM2-mediated regulatory network in the early amyloid seeding stage. (A) Sankey plot showing comparison of activity of the regulators between induced and control groups in 5xFAD/TREM2-WT (left) and 5xFAD/TREM2-R47H (right) mice. n = 6 mice per group (equal male and female). (B) Dot plot for KEGG pathway enrichment analysis. KEGG enrichment analysis of significantly upregulated and downregulated regulators (FDR < 0.05). A total of 24 enriched pathways are shown (red dots: upregulated regulators; blue dots: downregulated regulators). The size of circle represents significance level (−Log10 P values). (C and D) Circos plot summarizing the 24 enriched pathways and associated regulators in the 5xFAD/TREM2-WT (C), and 17 enriched pathways and associated regulators in the 5xFAD/TREM2-R47H (D) groups. (E) TREM2 regulatory networks in 5xFAD/TREM2-WT mice. TREM2 (regulator) and its downstream genes are shown. The network includes 31 regulators (3 TFs, 5 co-TFs, and 23 surface proteins) with the significant activity (Benjamini–Hochberg adjusted P value <0.05). Each TF, co-TF, surface protein, and DEG is represented by a diamond, square, hexagon, and circle, respectively. The regulators with positive activity and DEGs with upregulation are shown in red color, and the regulators with negative activity and DEGs with downregulation are indicated in blue color. The arrow shows the direction of regulation. (F) TREM2 regulatory networks in 5xFAD/TREM2-R47H mice. The network includes 40 regulators (7 TFs, 3 co-TFs, and 30 surface proteins) with the significant activity (Benjamini–Hochberg adjusted P value <0.05).
To further define how TREM2 regulates downstream genes in DAM, we constructed transcriptional regulatory networks with pseudo-bulk transcriptomics data through in silico aggregation of the scRNA-seq data covering microglia subcluster 11 using the ARACNe algorithm (Fig. S4). The ARACNe-inferred regulatory networks included 1,674 regulators (transcriptional factors [TFs], transcription cofactors [co-TFs], and surface proteins). By comparing the microglia in induced versus Ctrl groups in both TREM2-WT and TREM2-R47H mice, we inferred the master regulators, which were candidate drivers in the networks by metaVIPER algorithm. We found 508 upregulated and 309 downregulated regulators in the TREM2-WT group and 409 upregulated and 201 downregulated regulators in the TREM2-R47H group after TREM2 expression was induced (Fig. S4 A). The KEGG pathway analyses revealed that these upregulated regulators were enriched in the pathways related to Wnt signaling, Toll-like signaling, and TGF-β signaling, whereas the downregulated regulators were enriched in the pathways such as phagosome, lysosome, and AD (Fig. S4, B–D). To further investigate how downstream genes are triggered by TREM2, we projected all the identified DEGs to the regulatory networks to generate TREM2-WT–specific and TREM2-R47H–specific networks, respectively. Consistent with the DEG analyses, the regulatory networks showed direct regulation of TREM2 on multiple DAM genes such as Apoe, Tyrobp, Cst7, Ctsd, etc., in both TREM2-WT (Fig. S4 E) and TREM2-R47H groups (Fig. S4 F).
Next, to understand the molecular mechanism underlying the different effects of TREM2-WT and TREM2-R47H during amyloid development, we also isolated the cortical single cells and performed RNA-seq using animals induced at 2–5 mo of age. We used an unsupervised mapping strategy to identify the cell types from this cohort by mapping the scRNA-seq data and its cell type labels from the early cohort (Fig. 7 A). A total of 92,689 microglia were used for downstream analysis (Fig. 7 B). To compare the microglia gene changes in different stages, we reclustered all the microglia from both early and middle cohorts and identified 10 unique subclusters (Fig. 7 C), including HM (HM1-4), stage 1 DAM (DAM1), stage 2 DAM (DAM2), IRM, MHCII+, CPM, and ribosomal gene enriched microglia (REM) based on the expression of marker genes (Fig. 7 D; Ellwanger et al., 2021; Keren-Shaul et al., 2017; Wang et al., 2020). Of note, in addition to homeostatic microglia gene markers, the HM1 subcluster also expressed gene markers such as Fos, Jun, and Junb (Fig. 7 D), which are recently shown to be the markers of ex vivo microglia activation (Marsh et al., 2022). This suggests the potential ex vivo activation of microglia during single-cell preparation. Additionally, HM3 also expressed Ccl3 and Ccl4, and HM4 had a high expression of Ccl2 (Fig. 7 D).
Figure 7.
Reduction of DAM with TREM2-WT expression in the amyloid seeding stage but not the amyloid rapid growth period. (A) scRNA-seq data from the early cohort of 5xFAD/TREM2 mice (0–3.5 mo) were mapped to the middle amyloid cohort (2–5 mo). UMAP visualization of the middle cohort identifies clusters of the major cell types from predicted labels. (B) The prediction scores of cell types mapped from the early to middle cohort are shown. (C) Microglia subclusters from the early cohort were transferred to microglial cells from the middle cohort. The resulting de novo UMAP shows microglial subtypes of the early cohort and the label-transferred subtypes of the middle cohort. (D) Heatmap showing the expression of specific maker genes in different annotated microglia subtypes. (E and F) Prediction scores of early cohort microglia (E) and middle cohort microglia (F) mapped from cluster 2 (XO4+, Aβ-phagocytosing) microglia from Grubman et al. (2021). (G and H) The changes in cell numbers in each microglia subcluster were compared between induced versus Ctrl groups in 5xFAD/TREM2-WT and 5xFAD/TREM2-R47H mice from the early (G) and middle cohort (H). Differential abundance was assessed with Milo (miloR version 0.1.0). Subclusters with differentially abundant neighborhoods are highlighted. **, spatial FDR < 0.01.
To further understand the function of these microglia subpopulations, in particular their association with Aβ, we mapped our microglia to the published transcriptional signature of microglia that are associated with Aβ plaque phagocytosis (Grubman et al., 2021). In the report from Grubman et al. (2021), methoxy-XO4+ microglia were identified as amyloid plaque–containing microglia. By utilizing the label transfer methods from the Seurat pipeline (Stuart et al., 2019), we sought to identify microglia isolated at the single-cell level in our data that correspond to the XO4+ microglia from Grubman et al. (2021). As the median prediction scores for mapping to Grubman et al. (2021) for early and middle cohorts were respectively, 0.91 and 0.92, we chose a prediction score of 0.9 to distinguish microglia as being more or less likely to be XO4+, and therefore to phagocytose plaque.
The microglia of the early cohort were mapped to clusters of Grubman et al. (2021) as follows: cluster 1 (5xFAD XO4−), 117 cells; cluster 2 (5xFAD XO4+), 6,396 cells; cluster 3 (6-mo-old WT), 97,687 cells; and cluster 4 (24-mo-old WT and 5xFAD XO4−), 1,987 cells. Thus, only 8.0% of early cohort microglia were mapped to XO4+ microglia. The high prediction scores of microglia mapping to XO4+ microglia were predominantly DAM2 microglia: 64.9% of microglia with prediction scores >0.9 were DAM2 (Fig. 7 E). DAM1 comprised another 16.2% of such microglia. Thus, among the subpopulations of microglia we identified, we have the highest level of confidence for DAM2 microglia to be the microglia that phagocytose plaque in the early amyloid seeding stage.
Microglia of the middle cohort were also mapped to clusters of Grubman et al. (2021) with the distribution of cluster predictions as follows: cluster 1: 0 cells; cluster 2: 39,168 cells; cluster 3: 53,521 cells; cluster 4: 0 cells. Thus, 42.2% of middle cohort microglia mapped to XO4+ microglia, which likely reflects an increase of phagocytosis of Aβ plaque by microglia, caused by the increase of Aβ pathology between early and middle cohorts. In contrast to the early cohort, the middle cohort microglia mapping to XO4+ microglia with a high prediction score (>0.9) were more diverse and included predominantly DAM2 (21%), HM2 (21%), HM3 (20%), MHCII+ (15%), and HM1 (10%; Fig. 7 F). This observation suggests that the DAM2 is the major responder that phagocytoses Aβ in the early stage, and perhaps, the other subtypes of microglia become involved in this process upon amyloid plaque growth during the disease development.
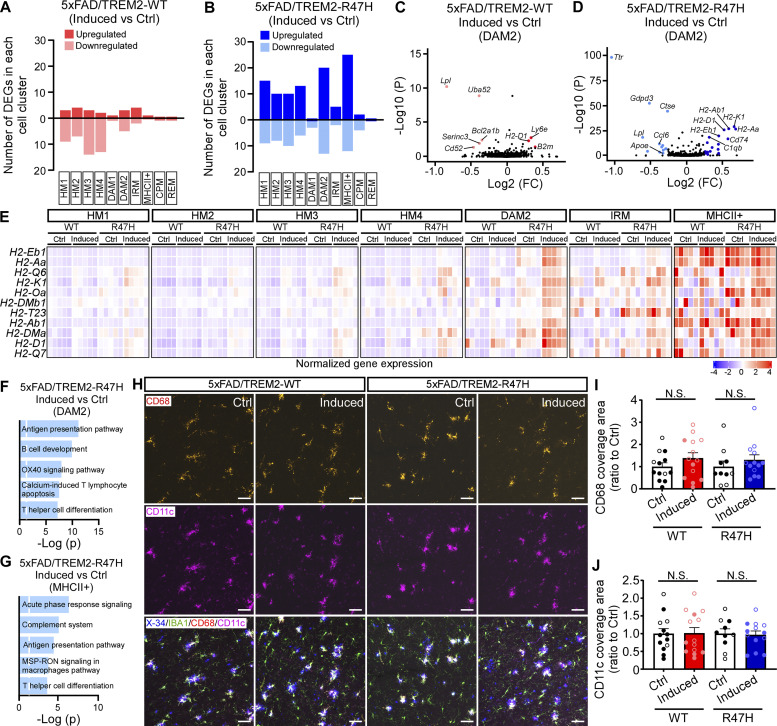
When evaluating the effects of TREM2 expression on cell distribution changes, we found that TREM2-WT expression in the early stage significantly reduced DAM2 cell population and increased HM1 subcluster, whereas TREM2-R47H did not change the overall cell distribution (Fig. 7 G). This result is consistent with the reduced microglia CD68 signal in the TREM2-WT expressing mice at this stage (Fig. 6, N and O), together suggesting that the phagocytic DAM2 microglia are reduced in overall abundance.
In the middle stage cohort, the cell distributions of microglia subclusters were similarly represented in mice expressing TREM2-WT or TREM-R47H compared with their respective controls (Fig. 7 H). DEG analyses in the middle stage cohort revealed that TREM2 expression, in particular TREM2-R47H, affected DAM2, MHCII+, and HM1-4 microglia subclusters (Fig. 8, A and B). For example, in most of the subclusters, especially in DAM2, TREM2-R47H expressing mice showed upregulated MHCII genes, such as H2-Aa, H2-Ab1, H2-D1, etc., whereas such changes were subtle or absent in the TREM2-WT–expressing mice compared to their own controls (Fig. 8, C–E). Pathway analyses showed upregulated antigen presentation pathways in both DAM2 and MHCII+ microglia subclusters in TREM2-R47H–expressing mice (Fig. 8, F and G). The upregulation of MHCII genes and related antigen presentation pathway might be related to the increased Aβ burden in these mice (Fig. 3). Several DAM genes, such as Lpl, were downregulated in both TREM2-WT– and TREM2-R47H–expressing mice in the DAM2 subcluster (Fig. 8, C and D). However, this effect of regulating DAM gene changes was much milder compared with the early cohort (Fig. 6, H and I). To further assess the role of TREM2 expression in regulating the microglia phagocytic phenotypes at this stage, we performed CD68 and CD11c staining and found no significant changes with or without TREM2-WT or TREM2-R47H expression (Fig. 8, H–J). Overall, these findings suggest that overexpression of TREM2 in the middle stage did not strongly modify microglia response to the amyloid plaque, although the MHCII genes were upregulated in TREM2-R47H–expressing mice.
Figure 8.
TREM2-R47H expression in the amyloid rapid growth period upregulates the MHCII genes but does not change the phagocytic phenotype of microglia. (A and B) Numbers of DEGs in all the microglia subclusters between induced versus Ctrl groups of 5xFAD/TREM2-WT (A) or 5xFAD/TREM2-R47H (B) mice in the middle cohort are shown. MAST tests were used for statistical analysis of DEGs while correcting for sex differences. (C and D) Volcano plots showing DEGs of induced versus Ctrl groups in DAM2 microglia subcluster in 5xFAD/TREM2-WT (C) or 5xFAD/TREM2-R47H (D) group. (E) Heatmap showing the upregulation of a group of MHCII genes. (F and G) Top five canonical pathways enriched by DEGs from DAM2 (F) and MHCII+ (G) subclusters in 5xFAD/TREM2-R47H mice. The threshold of significant P value is 0.05. (H–J) CD68 and CD11c staining showing the phagocytic microglia in 5xFAD/TREM2 mice at middle stage. Representative images showing the CD68 (red), CD11c (violet), IBA1 (green), and amyloid plaque (X-34+, blue) staining (N). Scale bar, 50 μm. The CD68 (I) and CD11c (J) coverage areas in the IBA1+ microglia were quantified and compared. n = 11–13 mice/group, mixed sexes (the data from male and female mice are labeled as solid and open circles, respectively). Data are shown as mean ± SEM. Mann–Whitney tests with Bonferroni correction were used for statistical analysis. P values <0.025 were considered to be statistically significant. N.S., not significant.
Taken together, these data suggest that elevating the expression of TREM2-WT and TREM2-R47H affects DAM microglia signature mainly in the early amyloid seeding stage. They both inhibit the disease-associated signature of microglia, with TREM2-WT having much stronger effects than TREM2-R47H.
TREM2 pathway activation inhibits the expression of DAM gene networks
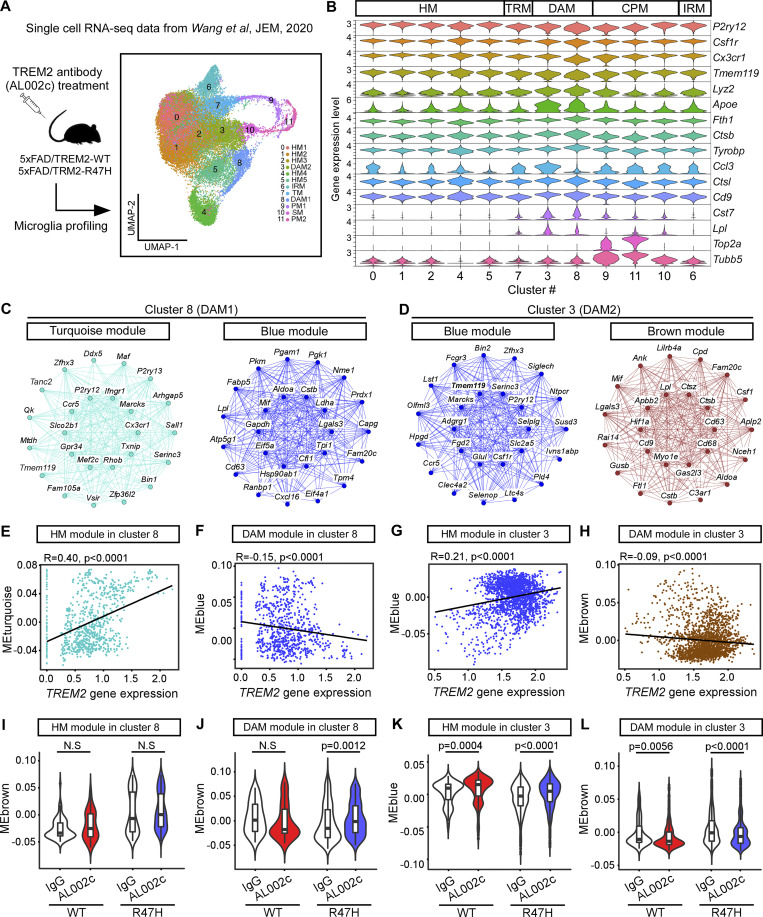
To further understand the effect of TREM2 on the DAM gene signatures in amyloid mouse models, we reanalyzed the microglia scRNA-seq data published by Wang et al. (2020) (Fig. 9 A), where they examined the impact of an anti-human TREM2 agonistic antibody (AL002c) in 5-mo-old 5xFAD mice expressing human TREM2 common variant (5xFAD/CV, referred to as “5xFAD/TREM2-WT”) or TREM2-R47H (5xFAD/R47H, referred to as “5xFAD/TREM2-R47H”). Using a list of marker genes, we confirmed that cluster 3 (identified as DAM2 in Wang et al. [2020]) and cluster 8 (identified as DAM1 in Wang et al. [2020]) were DAM microglia, and these clusters of microglia were similar to the DAM microglia identified in our study (Fig. 9 B). To evaluate the potential effect of TREM2 on DAM signatures, we performed weighted gene coexpression network analysis (WGCNA) for clusters 8 (DAM1) and 3 (DAM2). In both clusters, we identified one gene module enriched with microglia homeostatic genes (HM, including P2ry12, Tmem119, Cx3cr1, Csf1r, etc.). These modules were the turquoise module in cluster 8 (Fig. 9 C) tand the blue module in cluster 3 (Fig. 9 D). We also identified gene modules enriched with DAM genes, including Lpl, Lgals3, Ctsb, Cd9, Ank, Csf1, and Cd68. These modules were the blue modules in cluster 8 (Fig. 9 C) and the brown module in cluster 3 (Fig. 9 D). Interestingly, correlation analyses of TREM2 gene expression and module eigen genes (ME) showed that TREM2 expression level correlated positively with the HM gene modules (Fig. 9, E and G) but negatively with DAM gene modules (Fig. 9, F and H) in both cluster 8 and cluster 3. It was shown that the AL002c antibody treatment activated TREM2 signaling pathway in these mice; we therefore further compared the effects of TREM2 signaling activation on the regulation of these HM and DAM gene modules. We found that the AL002c antibody treatment significantly upregulated the HM gene module (Fig. 9 K) and downregulated the DAM gene module (Fig. 9 L) in cluster 3 (DAM2) of both 5xFAD/TREM2-WT and 5xFAD/TREM2-R47H groups. Such effect was minimal for the DAM1 microglia (cluster 8), with only 5xFAD/TREM2-R47H showing changed expression of DAM gene module (blue) after the antibody treatment (Fig. 9, I and J). These results suggest that the expression of TREM2 and activating TREM2 pathways suppress DAM and promote HM signatures in DAM2 microglia.
Figure 9.
The effects of TREM2 on DAM gene profiles in Wang et al. (2020). The scRNA-seq data of microglia after TREM2 agonistic antibody (AL002c) treatment in amyloid mouse models expressing the TREM2 common variant (5xFAD/CV, referred to as 5xFAD/TREM2-WT) or TREM2-R47H (5xFAD/R47H, referred to as 5xFAD/TREM2-R47H) from Wang et al. (2020) were reanalyzed. (A) Illustration of the scRNA-seq experimental design and the UMAP of microglia subclustered adapted from Wang et al. (2020). HM1-5, homeostatic microglia; TM, transitioning microglia; IRM, interferon-response microglia; PM1-2, proliferating microglia; SM, S-phase microglia. (B) The violin plot showing the microglia-specific marker gene expression. (C and D) WGCNA was performed in the DAM clusters 8 and 3. Network plots of the top 25 genes with the highest intramodular connectivity (hub genes) in turquoise and blue modules from cluster 8 (C), and blue and brown modules from cluster 3 (D) are shown. (E–H) Pearson correlation between TREM2 gene expression level and the eigengene (ME) of turquoise (E) and blue (F) modules in cluster 8, and blue (G) and brown (H) modules in cluster #3 (n = 776 meta cells for cluster 8, 2,054 meta cells for cluster 3). (I–L) The effects of TREM2 antibody AL002c treatment on the MEs of turquoise (I) and blue (J) modules in cluster 8, and the blue (K) and brown (L) modules in cluster 3 were analyzed (n = 75, 81, 369, and 251 meta cells for WT-IgG, WT-AL002c, R47H-IgG, and R47H-AL002c groups in cluster 8, respectively; n = 162, 370, 686, and 836 meta cells for WT-IgG, WT-AL002c, R47H-IgG, and R47H-AL002c groups in cluster 3, respectively). Bonferroni corrected P values were from Kruskal−Wallis tests followed by Dunn’s test.
Altogether, our study and the data from Wang et al. (2020) support the conclusion that TREM2 overexpression or pathway activation does not promote DAM signature of microglia, but rather suppresses it.
Discussion
In this study, we identified a beneficial effect of TREM2 in accelerating amyloid clearance and attenuating amyloid seeding in the early stage of AD pathogenesis. Molecular profiling by scRNA-seq revealed that elevating TREM2 expression strongly suppresses DAM microglial signature in the early stages of amyloid development in the 5xFAD mice. This TREM2-related DAM inhibition was crossvalidated using a publicly available dataset with TREM2 agonistic antibody treatment in 5XFAD mice. In contrast, the pathogenic TREM2-R47H exacerbated amyloid burden and related toxicity during the amyloid rapid growth period with the upregulated antigen presentation pathway in the microglia. Altogether, our study supports a beneficial effect of enhancing TREM2-mediated microglial functions in intervening AD pathogenesis in the early stage.
Most in vivo studies investigating TREM2 and AD use different amyloid transgenic mouse models with total or partial deletions of the murine Trem2 gene. Unfortunately, the effects of Trem2 deficiency on amyloid load have yielded inconsistent results (Jay et al., 2017; Jay et al., 2015; Parhizkar et al., 2019; Ulrich et al., 2017; Wang et al., 2015; Wang et al., 2016). On the other hand, overexpressing TREM2 through a BAC transgenic approach showed beneficial effects on modifying AD pathogenesis (Lee et al., 2018), implying that boosting TREM2 levels or signaling might be a therapeutic approach for AD. In our study, using the microglia-specific, inducible, and humanized TREM2 overexpression mouse models, we demonstrate the need of elevating TREM2 in microglia during the early seeding stage of amyloid development likely by facilitating Aβ clearance. This complements the study that Trem2 deficiency led to increased Aβ plaque seeding likely due to reduced phagocytic clearance of amyloid seeds (Parhizkar et al., 2019). Interestingly, we showed that boosting TREM2 levels after the seeding stage does not reduce Aβ load. Supporting this finding, treatment of 5xFAD mice with TREM2 agonistic antibody (AL002c) from 2 to 5 mo of age (amyloid rapid growth period) has a negligible impact on Aβ burden albeit that the treatment did ameliorate neuronal damages (Wang et al., 2020).
Previous findings have demonstrated that APOE and TREM2 are key components of activated microglia (DAM/microglial neurodegenerative phenotype/activation response microglia), and deletion of either Apoe or Trem2 substantially reduces the number of microglia displaying the DAM signature (Keren-Shaul et al., 2017; Krasemann et al., 2017; Sala Frigerio et al., 2019), suggesting that APOE and TREM2 are required for microglia transitioning from homeostatic to DAM phenotype. However, it is not clear whether exacerbated DAM signature is beneficial or detrimental to AD pathogenesis (Pimenova et al., 2017; Shi et al., 2021), and whether activating the TREM2 pathway will further promote DAM phenotype or do so in an opposite direction, i.e., restricting the over-activation of DAM. In our models, because of the presence of endogenous mouse Trem2 and Apoe, the microglia transitioning from homeostatic to DAM is unaffected during amyloid development. Consistent with the previous report using Trem2-deficient mice (Keren-Shaul et al., 2017), TREM2 overexpression primarily influences the gene expression of DAM microglia subcluster, in particular the stage 2 DAM. However, we found that TREM2 overexpression suppresses the DAM gene expression signature rather than promoting it in the 5xFAD mice. Multiple disease-associated genes in addition to Apoe were downregulated, whereas several homeostatic genes were upregulated. This observation is partially consistent with the results from a study using their TREM2 overexpression model (Lee et al., 2018). By bulk RNA-seq, this study found accelerated expression of microglial homeostatic genes but delayed damage-associated genes (such as Cst7, Ctsd, Tyrobp, etc.) in the 5xFAD/TREM2 mice compared to 5xFAD mice (Lee et al., 2018). However, Lee et al. (2018) also reported several other upregulated neurodegenerative disease–related genes by TREM2 overexpression (such as Spp1, Lgals3, etc.), which were not observed in our study. Moreover, the microglia gene expression changes induced by the dose increase of TREM2 from our study are well validated by a recent report of TREM2 agonistic antibody AL002c treatment (Wang et al., 2020), in particular, the downregulation of DAM-related gene networks in DAM2 microglia after activating TREM2 signaling pathway. Altogether, the evidence from our study supports that elevating TREM2 expression may restrict the disease-associated phenotype of microglia, which might be beneficial in delaying disease development. Further, supporting an underlying protective mechanism of reducing DAM microglia in disease development, a most recent study reported that overexpressing an APOE receptor, the low-density lipoprotein receptor, in a tauopathy mouse model tamed microglial APOE level and reduced DAM signature leading to slowed tau pathology and neurodegeneration (Shi et al., 2021). However, given that amyloid load may also strongly affect DAM cell population and gene signature, it is possible that the reduced DAM signature we observed in our mice may be due to the overall reduction of amyloid load, not reflecting the direct effect of TREM2. Therefore, further studies are needed to clarify the roles and gene signatures of microglial activation during different disease stages, in particular the specific effects of APOE and TREM2.
Interestingly, we observed an increased amyloid deposition and exacerbated neurotoxicity upon TREM2-R47H expression in the rapid amyloid growth period. This might be related to the upregulated antigen presentation pathways in microglia we observed from scRNA-seq. Although the phagocytic microglia phenotype was not changed upon TREM2-R47H expression, the compromised microglial dynamics as shown by in vivo two-photon imaging may contribute to the increased pathology in these mice. Additionally, a recent publication reported that AD brains from TREM2-R47H carriers have increased expression of inflammatory molecules and enhanced AKT signaling in microglia, suggesting a potential gain-of-toxic function of TREM2-R47H, although this needs to be further validated, perhaps in preclinical model systems (Sayed et al., 2021). In addition to the cell-autonomous effect, TREM2-R47H expression could also potentially cause the noncell autonomous effect, such as its influence on neurons or other brain cell types, which leads to increased pathology and neuronal toxicity. Furthermore, it is possible that TREM2-R47H could compete with the mouse endogenous Trem2 for binding of DAP12 and related signaling molecules (Deczkowska et al., 2020), creating a dominant-negative effect on overall TREM2 function. Lastly, it was reported that the TREM2-R47H shows reduced shedding to produce sTREM2 (Kleinberger et al., 2014; Song et al., 2018). Consistently, we observed significantly lower sTREM2 in TREM2-R47H-expressing mice compared to TREM2-WT mice in both middle- and late-stage cohorts. Additionally, we also detected lower CTF of TREM2-R47H compared to TREM2-WT, although this effect has not been observed in the in vitro cellular models (Kleinberger et al., 2014). As sTREM2 is linked to a protective effect against cognitive decline (Ewers et al., 2019) and AD-related pathology and behavior (Zhong et al., 2019), the reduced sTREM2 levels associated with TREM2-R47H in our mouse models may also potentially contribute to the overall increased amyloid pathology. Altogether, the mechanism of TREM2-R47H overexpression leading to increased amyloid burden after amyloid seeding needs to be further investigated.
There are several limitations of this study. The conditional mouse models we used were generated by employing a targeting vector that was designed and inserted into the mouse Rosa26 locus within ES cells. As the homologous recombination approach was used, the chance of random or nonspecific insertion should be quite low. However, we have not yet performed the whole genome sequencing with these mice to completely rule out any off-target integration. Additionally, we treated the animals with tamoxifen to induce the human TREM2 expression, whereas the control mice received the corn oil injection. Therefore, there is a potential confounding effect of tamoxifen treatment when evaluating the role of human TREM2 that we overexpressed. Furthermore, the human TREM2 expression was controlled by a CMV promoter such that the expression level did not change with the amyloid development. Given that the mouse endogenous Trem2 protein level was increased during the amyloid development, it is possible that in the late cohort, the human TREM2 expression was relatively low compared to the high mouse endogenous Trem2 level, limiting the potential effects on amyloid load during later stages of amyloid development. Lastly, we used an enzymatic digestion method at 37°C to dissociate brain tissues for single-cell isolation and RNA-sequencing. Although this protocol provides us with the best cell yield and viability, which has been widely used (Bordt et al., 2020; Cardona et al., 2006; Grabert and McColl, 2018), it can cause microglia ex vivo activation during the isolation step (Marsh et al., 2022) thereby potentially confounding the TREM2 genotype effects we observed. The modified single cell isolation protocol supplemented with transcriptional and translation inhibitors and under controlled temperature conditions should be applied in future studies to further validate TREM2’s function in our animal models by scRNA-seq.
In summary, by taking advantage of our unique inducible mouse models, we demonstrate a beneficial effect of elevating TREM2 levels in taming DAM microglia signatures and against amyloid pathology only during the initial Aβ seeding stage. Together, our findings support a therapeutic approach by enhancing TREM2-mediated signaling, but caution that such treatment needs to be applied early in the disease process.
Materials and methods
Mouse models
The cell type–specific and inducible TREM2 mouse models were generated by a knock-in strategy targeting the Rosa26 locus as previously described (Liu et al., 2017; Miyazaki et al., 2005). The transgene construct for TREM2-WT or TREM2-R47H contains: (1) Rosa short and long arms at either end for targeted integration of Rosa26 locus; (2) a loxP-flanked STOP cassette that harbors a Neor gene for selection of integrated clones and for Cre-mediated cell type–specific expression; (3) a Tet-off regulatory element to allow for doxycycline-regulated TREM2 expression and a CMV promoter with a tetracycline-responsive element; (4) human TREM2-WT or TREM2-R47H cDNA infused with N-terminal HA and C-terminal 3xFLAG tags; and (5) an EGFP cDNA driven by an internal ribosomal entry site (Fig. 1 A). The vector constructs were confirmed by restriction digestion, sequencing, and electroporation into mouse ES cells. The ES clones were individually screened for homologous recombination, and the positive clones were confirmed by Southern blotting. Three ES cell lines were chosen, verified by karyotyping, and used to generate TREM2 knock-in mice by blastocyst injection into C57BL/6J mice. The generation of inducible TREM2-WT and TREM2-R47H constructs, production, the screening of the ES clones, and the generation of chimeric mice was carried out by Transgenic Vectors Core, Mouse ES Cell Core, and Mouse Genetics Core at the Washington University, respectively. The resulting chimeras were bred with C57BL/6J mice, and one founder mouse bearing the TREM2-WT or TREM2-R47H construct was identified and used for further breeding.
To establish microglial-specific human TREM2-WT– or TREM2-R47H–expressing animal models, we crossed our inducible TREM2-WT and TREM2-R47H mice with Cx3cr1CreER mice (stock # 021160; The Jackson Laboratory) with the Cre recombinase fused to a mutant estrogen ligand-binding domain. Therefore, the TREM2-WT+/+; Cx3cr1CreER/+ (referred to as TREM2-WT) and TREM2-R47H+/+; Cx3cr1CreER/+ (referred to as TREM2-R47H) mice were generated.
To further understand how the expression of human TREM2-WT or TREM2-R47H impacts the function of microglia in the context of amyloid, we crossed TREM2-WT and TREM2-R47H mice with 5xFAD amyloid model mice (stock # 34848; The Jackson Laboratory) and established 5xFAD; TREM2-WT+/+; Cx3cr1CreER/+ mice (referred to as 5xFAD/TREM2-WT) and 5xFAD; TREM2-R47H+/+; Cx3cr1CreER/+ mice (referred to as 5xFAD/TREM2-R47H). To induce the expression of TREM2-WT or TREM2-R47H in microglia, the adult mice were given two doses of 250 mg/kg tamoxifen by gavage with a separation of 48 h between doses (Parkhurst et al., 2013) unless otherwise specified, and the neonatal animals were given 50 mg tamoxifen per day for 3 consecutive days via intragastric administration as described (Pitulescu et al., 2010). The control groups were given a vehicle (corn oil) with the same volume as tamoxifen.
All mice used for experiments were backcrossed to the C57BL/6J background for at least five generations. Unless otherwise specified, male and female mice were used for all experiments. Genotyping was performed by tail biopsy and qPCR for every mouse used in this study. Mice were housed in a temperature-controlled environment with a 12-h light–dark cycle and free access to food and water. All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
qPCR analysis
Reverse transcription of RNA was performed using iScript Reverse Transcription Supermix (Biorad). cDNA was added to a reaction mix (10 µl final volume) containing gene-specific primers and SYBR green supermix (Biorad). All samples were run in duplicates and were analyzed with QuantStudio 7 Flex Real-Time PCR System (Thermo Fisher Scientific). The relative gene expression was normalized to Gapdh controls and assessed using the 2−ΔΔCT method. Primer sequences and information are as follows (5′–3′): murine Gapdh: 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward) and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse); human TREM2: 5′-GCTGCTCATCTTACTCTTTGTC-3′ (forward) and 5′-TCATAGGGGCAAGACACCT-3′ (reverse); murine Trem2: 5′-GCACCTCCAGGAATCAAGAG-3′ (forward) and 5′-GGGTCCAGTGAGGATCTGAA-3′ (reverse); total TREM2/Trem2: 5′-GCCCATGCCAGCGTGTGGT-3′ (forward) and 5′-CACTGGTAGAGGCCCGC-3′ (reverse).
Tissue preparation for immunohistological or biochemical analyses
Mice were deeply anesthetized with isoflurane prior to transcardial perfusion with 0.01 M PBS. The brains were removed and bisected along the midline. For immunohistological analyses, one-half of the brain was drop-fixed in 4% paraformaldehyde (PFA, Fisher Scientific) overnight and then moved to 30% sucrose (Sigma-Aldrich) for 48 h. The tissues were then embedded in O.C.T. compound (Fisher) and snap-frozen in liquid nitrogen before cryostat sectioning. The cortical and hippocampal regions from the other half of the brain were dissected, snap-frozen in liquid nitrogen, and stored at −80°C for biochemical analyses. Three-step sequential protein extraction was performed. The brain tissues were first homogenized and lysed in TBS buffer supplemented by protease inhibitor and phosphatase inhibitor. The samples were ultracentrifuged at 100,000 g for 60 min at 4°C and the supernatants were collected as TBS soluble fractions. The pellets were then resuspended in TBSX buffer (TBS plus 1% Triton X-100) supplemented by protease inhibitor and phosphatase inhibitor. The samples were again ultracentrifuged at 100,000 g for 60 min at 4°C, and the supernatants were collected as TBSX soluble fractions. The residual pellets were then resuspended and sonicated in 5 M guanidine hydrochloride (GND; Sigma-Aldrich). After centrifugation as above, the resultant supernatant was collected as GND insoluble fractions. All fractions were stored in −80°C until they were used for Western blotting or ELISA analyses.
Immunofluorescence staining
Free-floating brain sections (40 μm thickness; coronal) were collected from the same brain region for all the animals, and brain regions were confirmed under the microscope by comparing sections to images on the Allen Mouse Brain Atlas (http://mouse.brain-map.org). The tissue was blocked in 5% goat serum for 1 h and incubated with the primary antibodies overnight. Sections were then incubated with Alexa Fluor-conjugated secondary antibodies for 1 h at room temperature (1:1,000; Invitrogen). The following primary antibodies were used: anti-human TREM2 (16E1, produced in-house at Mayo Clinic, 1:500); anti-IBA1 (Cat# 019-19741, 1:1,000; Wako); anti-GFAP (Cat# Pu020-UP, 1:1,000; BioGenex); anti-NeuN (Cat# MAB377, clone A60, 1:1,000; Millipore); anti-Aβ (MOAB-2, Cat# ab126649, 1:1,000; Abcam); anti-LAMP1 (Cat# ab25245, 1:500; Abcam); anti-CD68 (Cat# MCA1957, 1:300; VWR); and anti-CD11c (Cat# 14011482, 1:200; Thermo Fisher Scientific). To identify fibrillar Aβ plaques, free-floating sections from 5xFAD/TREM2 mice were permeabilized with 0.25% Triton X-100 in PBS and stained with 10 µM X-34 (Cat# SML1953; Sigma-Aldrich) in 40% ethanol + 0.02 M NaOH in PBS as described (Ulrich et al., 2018). Researchers were blinded to genotypes and groups when performing and quantifying the immunofluorescence staining.
Western blotting
Equal amounts of protein from the TBS or TBSX fractions of homogenized tissue lysates or isolated microglia were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. After the membranes were blocked, proteins of interest were detected with proper primary antibodies. The membrane was then probed with HRP-conjugated or LI-COR secondary antibodies and visualized using the films or Odyssey infrared imaging system (LI-COR). The following primary antibodies were used: anti-HA-HRP (Cat# 12013819001, 1:500; Sigma-Aldrich), anti-human TREM2 (Cat# 91068, 1:1,000; Cell Signaling Technology), and anti–β-actin (Cat# A2228, 1:2,000; Sigma-Aldrich) antibodies.
ELISA
The levels of Aβ40 and Aβ42 in ISF samples and TBS, TBSX, and GND fractions of brain lysates were determined by ELISA as previously described (Shinohara et al., 2013) using an end-specific Aβ monoclonal antibody (13.1.1 for Aβ40 and 2.1.3 for Aβ42) and a HRP-conjugated detection antibody (Ab5, all antibodies were produced in-house by Mayo Clinic).
In vivo microdialysis
To assess the concentration of ISF Aβ in the hippocampus of awake, freely moving mice, in vivo microdialysis was performed as previously described (Liu et al., 2017). Briefly, the animals were placed in an animal stereotaxic device equipped with dual manipulator arms and an isoflurane anesthetic mask (David Kopf Instruments). Under isoflurane volatile anesthetic, guide cannula (BR style; Bioanalytical Systems) were cemented into the hippocampus (3.1 mm behind bregma, 2.5 mm lateral to the midline, and 1.2 mm below dura at a 12° angle). 4–6 h after surgery, a microdialysis probe (30-kD MWCO membrane; Bioanalytical Systems) was inserted through the guide cannula into the brain. Artificial cerebrospinal fluid (mM: 1.3 CaCl2, 1.2 MgSO4, 3 KCl, 0.4 KH2PO4, 25 NaHCO3, and 122 NaCl, pH 7.4) containing 4% BSA (Sigma-Aldrich) filtered through a 0.1 mm membrane was used as the microdialysis perfusion buffer. The flow rate was constant at 1.0 ml/min. Samples were collected every 60–90 min overnight which gets through the 5–6 h recovery period into a refrigerated fraction collector. The mean concentration of Aβ over the 6 h preceding treatment was defined as basal levels of ISF Aβ. To assess Aβ42 half-life, the mice were treated subcutaneously with a γ-secretase inhibitor, LY411575 (5 mg/kg) to rapidly block the production of Aβ, and the hippocampal ISF Aβ42 levels were monitored and measured by ELISA.
Two-photon imaging of microglia dynamics and response to Aβ
5xFAD/TREM2 mice were implanted with a chronic cranial window as previously described and mentioned above (Eyo et al., 2018; Liu et al., 2019). After recovery from cranial window surgery (>4 wk), 5xFAD/TREM2 mice were intraperitoneally injected with methoxy-X04 (5 mg/kg) 24 h before in vivo two-photon imaging. GFP and methoxy-X04 were excited by a tunable Ti:Sapphire Mai Tai DeepSee laser (Spectra Physics) at 920 and 700 nm, and the emission was collected at 520/15 and 480/40 nm, respectively (U-MF2; Olympus). An Olympus LUMPLFLN 40× water-immersion objective was used to acquire image stacks. For microglial velocity study, mice were kept under anesthesia (isoflurane, 3% for initiation, 1.5% for maintenance) for at least 20 min before two-photon imaging to avoid anesthetic effects (Liu et al., 2019). A 15-μm stack with 2 μm z-resolution and 1,024 × 1,024 pixels was acquired every minute for a period of 10–20 min. For tracing microglial response to amyloid plaques, mice were repeatedly intraperitoneally injected with methoxy-X04 (5 mg/kg) 24 h before imaging every 7 d. Blood vessels were used as gross landmarks to find the same field of views. A 60-μm stack with 2 μm z-resolution and 1,024 × 1,024 pixels was acquired.
Images were analyzed by Fiji ImageJ. To analyze microglial process velocity, each 15-μm stack was first aligned along the z axis using the StackReg plugin (Rigid Body) and Z-project into one image. Then images of every minute were stacked and aligned using StackReg plugin (translation). Microglial process velocity was measured using the Manual Tracking plugin. For monitoring microglial response to amyloid plaques, methoxy-X04–labeled amyloid plaque was set as the sphere center and the number of microglia within radius of 30 μm was counted using a 3D Object Counter.
Cortical single-cell isolation
Mice were transcardially perfused with PBS and the cortical tissues were quickly dissected and enzymatically digested using the Neural Tissue Dissociation kit (P; Cat# 130-092-628; Miltenyi Biotec) at 37°C in the gentleMACS Octo Dissociator with Heaters (Miltenyi Biotec). The myelin was then removed using magnetic bead separation (Myelin Removal Beads II, Cat# 130-096-733; Miltenyi Biotec) followed by the red blood cell lysis (Cat# 130-094-183; Miltenyi Biotec) and dead cell removal (Cat# 130-090-101; Miltenyi Biotec) according to the manufacturer’s instructions. The final cell pellet was suspended in Ca/Mg-free PBS supplemented with 0.5% FBS for downstream analysis. In all samples, more than 90% of the cells were viable based on trypan blue examination.
Single-cell RNA library preparation and sequencing
scRNA-seq was performed on isolated cortical samples using the chromium platform (10x Genomics) with the 3′ gene expression V3 kit, using a targeted input of ∼6,000–9,000 cells per sample. In brief, gel-beads in emulsions (GEMs) were generated on the sample chip in the Chromium controller. Barcoded cDNA was extracted from the GEMs using Post-GEM RT-cleanup and amplified for 12 cycles. Amplified cDNA was fragmented and subjected to end-repair, poly-A-tailing, adaptor ligation, and 10x-specific sample indexing following the manufacturer’s protocol. Libraries were quantified using the TapeStation (Agilent) analysis and then sequenced on a HiSeq 4000 instrument (Illumina) targeting a depth of 25,000–50,000 reads per cell.
scRNA-seq quality control, integration, clustering, and differential expression analysis
The single cell sequence preprocessing was performed using the standard 10x Genomics Cell Ranger Single Cell Software Suite v.3.0.0. The detailed sample information is shown in Table S1. Briefly, raw sequencing data were demultiplexed, aligned to the mouse genome mm10, and the reads aligned to each gene were counted. Cell filtration, normalization, clustering, and differential expression analyses were performed using Seurat v.3.1 with default parameter settings unless specified otherwise. For quality control, cells that had unique gene counts >6,000 or <200 and >20 mitochondrial content were removed. SCTransform was used to normalize and scale data and to remove the confounding sources of variation of sequencing depth, mitochondria gene percentage, and ribosomal protein gene percentage. All samples of the early cohort were integrated, and canonical correlation analysis was performed to identify common sources of variation among samples and to remove batch effects. The first 30 canonical correlation vectors were used for subsequent clustering analysis and visualization using UMAP. Clusters were determined with “FindClusters,” with a resolution set to 0.5. The middle cohort was integrated by treating the integrated early cohort as the reference using the “MapQuery” function with reference.dims set to 1:50 and reference reduction to “pca.” The middle cohort was then projected into the UMAP coordinates of the early cohort. Finally, a de novo UMAP was constructed by recomputing the UMAP using the first 30 principal components of the combined cohort. Function “FindAllMarkers” was used to identify cluster-specific genes. Cell clusters were manually annotated as specific cell types based on literature and the datasets for murine cell taxonomy. Cell doublets that expressed more than one cell type marker were excluded from downstream analyses. Differential expression in each pairwise comparison within cell clusters (TREM2-WT–induced versus Ctrl or TREM2-R47H–induced versus Ctrl) were calculated using the MAST model while correcting for sex, cellular detection rate, mitochondrial gene percentage, ribosomal gene percentage, and batch (Finak et al., 2015). Cluster-specific genes with Benjamin-Hochberg corrected false discovery rate (FDR) <0.05 and |log2fold change| >0.25 were determined as significant DEGs. Pathway analyses of DEGs from each cluster were performed using ingenuity pathway analysis.
The microglia cell clusters were further classified into HM, DAM, IRM, REM, MHCII+, and CPM based on the expression patterns of marker genes (Keren-Shaul et al., 2017; Krasemann et al., 2017; Sala Frigerio et al., 2019). Microglia subpopulations of the early cohort microglia were determined by reintegration of the microglial cells using the standard Seurat workflow as previously described. The microglia subpopulations were then mapped to middle cohort microglia, as in the previous cell-type mapping step. Again, the reference PCA space from early cohort microglia was used to compute a de novo UMAP for the combined visualization of the microglia subpopulations from both early and middle cohorts. Differential expression for pairwise comparisons within subtypes of microglia cells (TREM2-WT–induced versus Ctrl or TREM2-R47H–induced versus Ctrl) were calculated by using the MAST model, again correcting for sex, cellular detection rate, mitochondrial gene percentage, ribosomal gene percentage, and batch. Log2 fold change values from differential expression tests were used to compare gene change patterns among cohorts.
Data from Grubman et al. (2021) were downloaded from GSE165306. Processing and analysis were performed as described in Grubman et al. (2021) to compute PCA embeddings and cell clusters. Microglial cells from early and middle cohorts were separately mapped to Grubman et al. (2021) cluster labels with the “MapQuery” function as above, with reference.dims set to the first 30 principal components of the PCA cell embeddings. Prediction scores were also computed via “MapQuery” function, as described (Stuart et al., 2019). Briefly, the prediction score is a weighted average of the anchor scores of the 50 (by default) nearest integration anchors (pairs of cells, one from each dataset that is contained in each other’s neighborhoods). Per query cell, each predicted label’s prediction score is computed using only the anchors whose reference label corresponds to the predicted label. Differential abundance was performed using the miloR package (version 0.1.0; Dann et al., 2022), using a k value of 100 and the first seven principal components of the combined PCA space, as determined using the elbow plot of the first 30 principal components. Default parameter settings were used otherwise to compute differentially abundant neighborhoods, performing a comparison of tamoxifen-induced versus control for each genotype (WT and R47H) and each cohort (early and middle). A neighborhood was considered differentially abundant if the spatial FDR was <0.10.
The microglia gene count matrix from Wang et al. (2020) was downloaded from GSE150358. The count matrix was converted to a Seurat object using standard Seurat workflow while maintaining the original microglia cluster identities. Differential gene expression between AL002c and IgG in CV and R47H groups were calculated by the MAST test. Log2 fold change values from differential expression tests were used to compare gene change patterns among cohorts.
Regulatory network construction
Regulatory networks were constructed by ARACNe (Margolin et al., 2006) using in silico bulk microglia RNA-seq data. To generate in silico bulk RNA-seq data, we first extracted microglia scRNA-seq data, performed preprocessing (e.g., filtered genes with no expression are removed), and counts-per-million normalized using Seurat R package (Butler et al., 2018). The normalized data were summed and Log10 transformed to generate in silico bulk RNA-seq data covering microglia subcluster 11 in the early cohort. ARACNe was run with 100 bootstrap iterations using in silico bulk RNA-seq data. We used a set of regulators provided by ARACNe, including 1,465 TFs, 840 co-TFs, and 3,233 surface proteins. Parameters were set to zero data processing inequality tolerance and (mutual information P value threshold of 10−8.
Regulator activity inference
The VIPER algorithm has proven to be a useful tool for estimating regulator activity from gene expression data using enriched regulon analysis (Alvarez et al., 2016). Recently, an extension of the VIPER, metaVIPER, was developed for inferring regulator activity at the single-cell level (Ding et al., 2018). With the constructed regulatory networks, we computed the activity of each regulator (i.e., TFs, co-TFs, and surface proteins) using the metaVIPER program and compared the activity between induced and control samples. A set of regulators with a significant activity difference were defined at Benjamini–Hochberg adjusted P value <0.05. These significant regulators and DEGs were used to construct TREM2 regulatory network. Cytoscape software (version 3.8.0) was used for visualizing the regulatory network (Czerwinska et al., 2015).
Enrichment analysis of regulatory pathways
Identification of enriched pathways for regulators with significant activity was performed using the hypergeometric test. KEGG pathways were used for the enrichment analysis, and significantly enriched pathways were defined at Benjamini–Hochberg adjusted P value <0.05.
Single-cell WGCNA
WGCNA was performed for the scRNA-seq data from Wang et al. (2020) (accessible under accession code GSE150358; Wang et al., 2020), in which 12 cell clusters were identified, including two subsets of DAM, i.e., cluster 8 (DAM1) and cluster 3 (DAM2). The expression of selected marker genes in each cluster was visualized with the VlnPlot function from the Seurat package using the normalized and scaled data from SCT transformation, which removes the confounding effect of the gene detection rate and mitochondria mapping percentage (Hafemeister and Satija, 2019). To overcome the sparsity of the scRNA-seq data, we constructed metacells by aggregating neighboring cells in the k-nearest neighbors space through a bootstrapping-based approach (Morabito et al., 2021). The metacell construction was done separately for each condition, i.e., AL002c-treated TREM2 WT (TREM2-WT) or TREM2 R47H- and IgG-treated TREM2-WT or TREM2 R47H for each DAM cluster using the raw UMI count. The aggregated gene counts were normalized through log transformation for the downstream analysis. The current study included TREM2 and the top 3,000 highly variable genes identified using the FindVaribleFeatures function for WGCNA analysis using the WGCNA packages (v1.70-3). For WGCNA analysis, we computed the correlation matrix with pairwise Pearson correlation for all included genes. A signed co-expression network was obtained through the power transformation |0.5 + 0.5 × coefficient|β with β = 12 for both cluster 8 and cluster 3. To detect gene modules, we performed a topological overlap matrix–based gene dissimilarity measurement for hierarchical clustering. We set the minModuleSize = 50, detectCut Height = 0.995, deepSplit = 4, and mergeCutHeight = 0.2 for module detection. Modules with a correlation coefficient ⩾ 0.8 were merged. Hub genes shown in the network plots for each module were defined based on the intramodular connectivity, and the eigengene of each module used for module-trait analysis was defined as the first principal component of the module.
RNAscope fluorescent ISH combined with immunofluorescence
Fluorescent ISH was performed on cryosections using RNAscope Multiplex Fluorescent Reagent Manual Assay Kit v2 (Cat# 323100; Advanced Cell Diagnostics) according to the manufacturer’s protocol with some modifications. Briefly, 40 µm free-floating sections were removed from cryoprotectant, washed five times in PBS for 10 min each, and mounted onto Superfrost Plus slides (Cat# 12-550-15; Thermo Fisher Scientific). Sections were dried for 30 min at 60°C, then gradually dehydrated in 50, 70, and 100% ethanol for 5 min each, and air-dried for 5 min at room temperature. Next, the slides were incubated in RNAscope hydrogen peroxide at room temperature for 15 min, washed two times in water 1 min each, boiled in 1× Target Retrieval Reagent for 10 min, and washed in water for 15 s. The slides were then dehydrated in 100% ethanol for 3 min, air-dried before creating a hydrophobic barrier around the tissue using an ImmEdge Pen, and dried overnight at room temperature in a desiccator. The following day, the slides were incubated with Protease III for 30 min at 40°C in a HybEZ humidity control tray and oven, then washed in water four times, 1 min each. Next, tissue sections were incubated for 2 h at 40°C with ACD probes for mouse Apoe (Cat# 313271; target NM_009696.3, region 83-1245bp), DapB (negative control, Cat# 310043; target EF191515, region 414-862bp), or mouse Ppib (positive control, Cat# 313911; target NM_011149.2, region 98-856bp), then washed in 1× RNAscope wash buffer. The amplification and detection steps were performed as recommended. The probes were detected using AKOYA Biosciences fluorophore Opal 620 and, diluted 1:750 in TSA buffer (ACD). To combine ISH with immunofluorescence staining, the tissue sections were then blocked with 10% normal goat serum in TBS with 0.1% BSA and 0.3% Triton X-100 and incubated overnight at 4°C with anti-IBA1 (Cat# 019-19741, 1:500; Wako) and anti-Aβ (MOAB2, Cat# ab126649, 1:1,000; Abcam) primary antibodies. Sections were then incubated with the Alexa Fluor-conjugated secondary antibodies for 2 h at room temperature and counterstained with DAPI (ACD) before mounting. Imaging was carried out on a confocal laser scanning fluorescent microscopy (model LSM510 Invert; Carl Zeiss) using a 20× objective. Images (8-bit) were quantified using MATLAB r2020b.
Statistical analyses
All data were reported as mean values ± SEM unless otherwise indicated. Generally, to ensure that results were valid in the presence of non-normal distributions, or differing variances between groups, nonparametric Mann–Whitney tests between-group comparisons with Bonferroni correction for multiple comparisons, and Kruskal–Wallis tests with Dunn’s multiple comparison tests were used to compare outcomes when sample size is larger than 8. With the sample size ≤8, since nonparametric tests would have very low power, a Student’s t test or one-way ANOVA was used to compare outcomes among groups. Statistics were analyzed using GraphPad Prism v8.4.3. All statistical tests were two-sided. The statistical tests used for each analysis, the sample size, and the significance levels were reported in the caption of each figure.
Online supplementary material
Fig. S1 shows the expression of human TREM2-WT or TREM2-R47H in microglia by immunofluorescence analysis of GFP and IBA1 signals in the 5xFAD/TREM2-WT and 5xFAD/TREM2-R47H mice in different stages of AD development. Fig. S2 shows the protein levels of human TREM2 detected in the TBS and TBSX fractions of 5xFAD/TREM2 mouse brain lysates by Western blotting. The uncropped Western blotting gel images from Fig. S2 are shown in SourceDataFS2. Fig. S3 shows the protein levels of mouse endogenous Trem2 detected in the TBS and TBSX fractions of 5xFAD/TREM2 mouse brain lysates by ELISA. Fig. S4 shows the TREM2-mediated regulatory network in the early amyloid seeding stage. Table S1 contains the detailed sample information for scRNA-seq.
Supplementary Material
mouse sample information for scRNA-seq.
contains original blots for Fig. S2.
Acknowledgments
We are grateful to M. Heckman for providing guidance on the statistical analyses for this manuscript.
This work was supported by National Institutes of Health/National Institute on Aging grants R01AG066395 (to G. Bu and N. Zhao), RF1AG056130, R37AG027924, and RF1AG046205 (to G. Bu), R21AG064159 (to L.-J. Wu) and a grant from the Florida Department of Health Ed and Ethel Moore Alzheimer’s Disease Research Program (8AZ07 to C.-C. Liu).
Author contributions: N. Zhao and G. Bu developed the research concept and designed the experiments; N. Zhao, W. Qiao, and F. Li prepared the animals and tissues and performed the majority of experiments including immunofluorescence staining, Western blotting, and ELISA; J. Zheng, K. Chen, and Y. Chen helped with animal tissue collection; N. Zhao, W. Qiao, Y. Chen, and Y.A. Martens performed the single-cell isolation and library preparation; Y. Ren, W. Qiao, X. Wang, L. Li, T.C. Ikezu, Z. Li, N. Zhao, Z.S. Quicksall, and Y.W. Asmann performed the statistical and bioinformatics analysis for scRNA-seq and RNAscope data; J. Zheng, M. Xie, Y.U. Liu, A.D. Umpierre, and K. Haruwaka did the two-photon imaging and quantification; K. Chen, Y. Zhu, A.D. Meneses, L. Job, B. Sonustun, M.L. Smith, and Y. Jin helped with sample preparation, qPCR, image scanning, and quantification; L. Bastea performed RNAscope fluorescent ISH combined with immunofluorescent staining; Y. Chen and C. Linares maintained the animal colonies and performed genotyping; J.A. Knight and Y. Chen did the tamoxifen treatment; J.A. Knight and C-C. Liu performed the in vivo microdialysis; C-C. Liu coordinated the generation of TREM2 mouse lines and participated in the discussion of experimental design; P. Storz supervised the RNAscope experiments; L.J. Wu supervised the two-photon experiments; N. Zhao and G. Bu wrote the manuscript with critical inputs and edits by all the co-authors.
Data availability
All scRNA-seq data were deposited in National Center for Biotechnology Information’s Gene Expression Omnibus under accession numbers GSE161224 and GSE161227.
References
- Aguzzi, A., Barres B.A., and Bennett M.L.. 2013. Microglia: Scapegoat, saboteur, or something else?. Science. 339:156–161. 10.1126/science.1227901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, M.J., Shen Y., Giorgi F.M., Lachmann A., Ding B.B., Ye B.H., and Califano A.. 2016. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet. 48:838–847. 10.1038/ng.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atagi, Y., Liu C.C., Painter M.M., Chen X.F., Verbeeck C., Zheng H., Li X., Rademakers R., Kang S.S., Xu H., et al. 2015. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem. 290:26043–26050. 10.1074/jbc.M115.679043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, C.C., DeVaux L.B., and Farzan M.. 2015. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem. 290:26033–26042. 10.1074/jbc.M115.677286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemiller, S.M., McCray T.J., Allan K., Formica S.V., Xu G., Wilson G., Kokiko-Cochran O.N., Crish S.D., Lasagna-Reeves C.A., Ransohoff R.M., et al. 2017. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener. 12:74. 10.1186/s13024-017-0216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordt, E.A., Block C.L., Petrozziello T., Sadri-Vakili G., Smith C.J., Edlow A.G., and Bilbo S.D.. 2020. Isolation of microglia from mouse or human tissue. STAR Protoc. 1:100035. 10.1016/j.xpro.2020.100035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, A., Hoffman P., Smibert P., Papalexi E., and Satija R.. 2018. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36:411–420. 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona, A.E., Huang D., Sasse M.E., and Ransohoff R.M.. 2006. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 1:1947–1951. 10.1038/nprot.2006.327 [DOI] [PubMed] [Google Scholar]
- Corder, E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., and Pericak-Vance M.A.. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 261:921–923. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- Czerwinska, U., Calzone L., Barillot E., and Zinovyev A.. 2015. DeDaL: Cytoscape 3 app for producing and morphing data-driven and structure-driven network layouts. BMC Syst. Biol. 9:46. 10.1186/s12918-015-0189-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann, E., Henderson N.C., Teichmann S.A., Morgan M.D., and Marioni J.C.. 2022. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 40:245–253. 10.1038/s41587-021-01033-z [DOI] [PubMed] [Google Scholar]
- Deczkowska, A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., and Amit I.. 2018. Disease-associated microglia: A universal immune sensor of neurodegeneration. Cell. 173:1073–1081. 10.1016/j.cell.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Deczkowska, A., Weiner A., and Amit I.. 2020. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell. 181:1207–1217. 10.1016/j.cell.2020.05.003 [DOI] [PubMed] [Google Scholar]
- DeTure, M.A., and Dickson D.W.. 2019. The neuropathological diagnosis of Alzheimer's disease. Mol. Neurodegener. 14:32. 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, H., Douglass E.F. Jr., Sonabend A.M., Mela A., Bose S., Gonzalez C., Canoll P.D., Sims P.A., Alvarez M.J., and Califano A.. 2018. Quantitative assessment of protein activity in orphan tissues and single cells using the metaVIPER algorithm. Nat. Commun. 9:1471. 10.1038/s41467-018-03843-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger, D.C., Wang S., Brioschi S., Shao Z., Green L., Case R., Yoo D., Weishuhn D., Rathanaswami P., Bradley J., et al. 2021. Prior activation state shapes the microglia response to antihuman TREM2 in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 118:e2017742118. 10.1073/pnas.2017742118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers, M., Franzmeier N., Suarez-Calvet M., Morenas-Rodriguez E., Caballero M.A.A., Kleinberger G., Piccio L., Cruchaga C., Deming Y., Dichgans M., et al. 2019. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 11:eaav6221. 10.1126/scitranslmed.aav6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo, U.B., Mo M., Yi M.H., Murugan M., Liu J., Yarlagadda R., Margolis D.J., Xu P., and Wu L.J.. 2018. P2Y12R-Dependent translocation mechanisms gate the changing microglial landscape. Cell Rep. 23:959–966. 10.1016/j.celrep.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak, G., McDavid A., Yajima M., Deng J., Gersuk V., Shalek A.K., Slichter C.K., Miller H.W., McElrath M.J., Prlic M., et al. 2015. Mast: A flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16:278. 10.1186/s13059-015-0844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N.F., Wolfe C.M., Playso B.E., Biedrzycki R.J., Lu Y., Nam K.N., Lefterov I., and Koldamova R.. 2020. Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol. Neurodegener. 15:41. 10.1186/s13024-020-00394-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert, K., and McColl B.W.. 2018. Isolation and phenotyping of adult mouse microglial cells. Methods Mol. Biol. 1784:77–86. 10.1007/978-1-4939-7837-3_7 [DOI] [PubMed] [Google Scholar]
- Grubman, A., Choo X.Y., Chew G., Ouyang J.F., Sun G., Croft N.P., Rossello F.J., Simmons R., Buckberry S., Landin D.V., et al. 2021. Transcriptional signature in microglia associated with Aβ plaque phagocytosis.. Nat. Commun. 12:3015. 10.1038/s41467-021-23111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro, R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S., Younkin S., et al. 2013. Genetic analysisTREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368:117–127. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemeister, C., and Satija R.. 2019. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20:296. 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J., and Selkoe D.J.. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 297:353–356. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Hsieh, C.L., Koike M., Spusta S.C., Niemi E.C., Yenari M., Nakamura M.C., and Seaman W.E.. 2009. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J. Neurochem. 109:1144–1156. 10.1111/j.1471-4159.2009.06042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki, S., McGeer P.L., Akiyama H., Zhu S., and Selkoe D.. 1989. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 24:173–182. 10.1016/0165-5728(89)90115-x [DOI] [PubMed] [Google Scholar]
- Jay, T.R., Hirsch A.M., Broihier M.L., Miller C.M., Neilson L.E., Ransohoff R.M., Lamb B.T., and Landreth G.E.. 2017. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer's disease. J. Neurosci. 37:637–647. 10.1523/JNEUROSCI.2110-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay, T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L., Xu G., Margevicius D., Karlo J.C., Sousa G.L., et al. 2015. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 212:287–295. 10.1084/jem.20142322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson, T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., et al. 2013. Variant of TREM2 associated with the risk of Alzheimer's disease. N. Engl. J. Med. 368:107–116. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul, H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. 2017. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 169:1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Kleinberger, G., Yamanishi Y., Suarez-Calvet M., Czirr E., Lohmann E., Cuyvers E., Struyfs H., Pettkus N., Wenninger-Weinzierl A., Mazaheri F., et al. 2014. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6:243ra86. 10.1126/scitranslmed.3009093 [DOI] [PubMed] [Google Scholar]
- Konishi, H., and Kiyama H.. 2018. Microglial TREM2/DAP12 signaling: A double-edged sword in neural diseases. Front. Cell. Neurosci. 12:206. 10.3389/fncel.2018.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann, S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., et al. 2017. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 47:566–581.e9. 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.Y.D., Daggett A., Gu X., Jiang L.L., Langfelder P., Li X., Wang N., Zhao Y., Park C.S., Cooper Y., et al. 2018. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron. 97:1032–1048.e5. 10.1016/j.neuron.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.H., Meilandt W.J., Xie L., Gandham V.D., Ngu H., Barck K.H., Rezzonico M.G., Imperio J., Lalehzadeh G., Huntley M.A., et al. 2021. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by beta-amyloid pathology. Neuron. 109:1283–1301.e6. 10.1016/j.neuron.2021.02.010 [DOI] [PubMed] [Google Scholar]
- Leyns, C.E.G., Ulrich J.D., Finn M.B., Stewart F.R., Koscal L.J., Remolina Serrano J., Robinson G.O., Anderson E., Colonna M., and Holtzman D.M.. 2017. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA. 114:11524–11529. 10.1073/pnas.1710311114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C.C., Zhao N., Fu Y., Wang N., Linares C., Tsai C.W., and Bu G.. 2017. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 96:1024–1032.e3. 10.1016/j.neuron.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.U., Ying Y., Li Y., Eyo U.B., Chen T., Zheng J., Umpierre A.D., Zhu J., Bosco D.B., Dong H., and Wu L.J.. 2019. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22:1771–1781. 10.1038/s41593-019-0511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin, A.A., Nemenman I., Basso K., Wiggins C., Stolovitzky G., Dalla Favera R., and Califano A.. 2006. ARACNE: An algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinf. 7:S7. 10.1186/1471-2105-7-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, S.E., Walker A.J., Kamath T., Dissing-Olesen L., Hammond T.R., de Soysa T.Y., Young A.M.H., Murphy S., Abdulraouf A., Nadaf N., et al. 2022. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 25:306–316. 10.1038/s41593-022-01022-8 [DOI] [PubMed] [Google Scholar]
- Mawuenyega, K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., and Bateman R.J.. 2010. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 330:1774. 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri, F., Snaidero N., Kleinberger G., Madore C., Daria A., Werner G., Krasemann S., Capell A., Trumbach D., Wurst W., et al. 2017. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 18:1186–1198. 10.15252/embr.201743922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann, M., Spires-Jones T.L., Prada C., Garcia-Alloza M., de Calignon A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D.M., Bacskai B.J., and Hyman B.T.. 2008. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 451:720–724. 10.1038/nature06616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, S., Miyazaki T., Tashiro F., Yamato E., and Miyazaki J.. 2005. Development of a single-cassette system for spatiotemporal gene regulation in mice. Biochem. Biophys. Res. Commun. 338:1083–1088. 10.1016/j.bbrc.2005.10.054 [DOI] [PubMed] [Google Scholar]
- Morabito, S., Miyoshi E., Michael N., Shahin S., Martini A.C., Head E., Silva J., Leavy K., Perez-Rosendahl M., and Swarup V.. 2021. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer's disease. Nat. Genet. 53:1143–1155. 10.1038/s41588-021-00894-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn, A., Kirchhoff F., and Helmchen F.. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308:1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Oakley, H., Cole S.L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., et al. 2006. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 26:10129–10140. 10.1523/JNEUROSCI.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhizkar, S., Arzberger T., Brendel M., Kleinberger G., Deussing M., Focke C., Nuscher B., Xiong M., Ghasemigharagoz A., Katzmarski N., et al. 2019. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 22:191–204. 10.1038/s41593-018-0296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst, C.N., Yang G., Ninan I., Savas J.N., Yates J.R. 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., and Gan W.B.. 2013. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 155:1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenova, A.A., Marcora E., and Goate A.M.. 2017. A tale of two genes: Microglial Apoe and Trem2. Immunity. 47:398–400. 10.1016/j.immuni.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Pitulescu, M.E., Schmidt I., Benedito R., and Adams R.H.. 2010. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 5:1518–1534. 10.1038/nprot.2010.113 [DOI] [PubMed] [Google Scholar]
- Sala Frigerio, C., Wolfs L., Fattorelli N., Thrupp N., Voytyuk I., Schmidt I., Mancuso R., Chen W.T., Woodbury M.E., Srivastava G., et al. 2019. The major risk factors for Alzheimer’s disease: Age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 27:1293–1306.e6. 10.1016/j.celrep.2019.03.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed, F.A., Kodama L., Fan L., Carling G.K., Udeochu J.C., Le D., Li Q., Zhou L., Wong M.Y., Horowitz R., et al. 2021. AD-linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation. Sci. Transl. Med. 13:eabe3947. 10.1126/scitranslmed.abe3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlepckow, K., Monroe K.M., Kleinberger G., Cantuti-Castelvetri L., Parhizkar S., Xia D., Willem M., Werner G., Pettkus N., Brunner B., et al. 2020. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol. Med. 12:e11227. 10.15252/emmm.201911227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Andhey P.S., Ising C., Wang K., Snipes L.L., Boyer K., Lawson S., Yamada K., Qin W., Manis M., et al. 2021. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron. 109:2413–2426.e7. 10.1016/j.neuron.2021.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara, M., Petersen R.C., Dickson D.W., and Bu G.. 2013. Brain regional correlation of amyloid-beta with synapses and apolipoprotein E in non-demented individuals: Potential mechanisms underlying regional vulnerability to amyloid-beta accumulation. Acta Neuropathol. 125:535–547. 10.1007/s00401-013-1086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W., Hooli B., Mullin K., Jin S.C., Cella M., Ulland T.K., Wang Y., Tanzi R.E., and Colonna M.. 2017. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer's Dementia. 13:381–387. 10.1016/j.jalz.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.M., Joshita S., Zhou Y., Ulland T.K., Gilfillan S., and Colonna M.. 2018. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 215:745–760. 10.1084/jem.20171529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M. 3rd, Hao Y., Stoeckius M., Smibert P., and Satija R.. 2019. Comprehensive integration of single-cell data. Cell. 177:1888–1902.e21. 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland, T.K., Song W.M., Huang S.C., Ulrich J.D., Sergushichev A., Beatty W.L., Loboda A.A., Zhou Y., Cairns N.J., Kambal A., et al. 2017. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 170:649–663.e13. 10.1016/j.cell.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, J.D., Ulland T.K., Colonna M., and Holtzman D.M.. 2017. Elucidating the role of TREM2 in Alzheimer’s disease. Neuron. 94:237–248. 10.1016/j.neuron.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Ulrich, J.D., Ulland T.K., Mahan T.E., Nystrom S., Nilsson K.P., Song W.M., Zhou Y., Reinartz M., Choi S., Jiang H., et al. 2018. ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 215:1047–1058. 10.1084/jem.20171265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Mustafa M., Yuede C.M., Salazar S.V., Kong P., Long H., Ward M., Siddiqui O., Paul R., Gilfillan S., et al. 2020. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 217:e20200785. 10.1084/jem.20200785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L., Gilfillan S., Krishnan G.M., Sudhakar S., Zinselmeyer B.H., et al. 2015. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 160:1061–1071. 10.1016/j.cell.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Ulland T.K., Ulrich J.D., Song W., Tzaferis J.A., Hole J.T., Yuan P., Mahan T.E., Shi Y., Gilfillan S., et al. 2016. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213:667–675. 10.1084/jem.20151948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, J., Titus A.R., and Humphrey M.B.. 2015. The TREM2-DAP12 signaling pathway in nasu-hakola disease: A molecular genetics perspective. Res. Rep. Biochem. 5:89–100. 10.2147/RRBC.S58057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.L., Wang Y., Tom I., Gonzalez L.C., and Sheng M.. 2016. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 91:328–340. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Yuan, P., Condello C., Keene C.D., Wang Y., Bird T.D., Paul S.M., Luo W., Colonna M., Baddeley D., and Grutzendler J.. 2016. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron. 92:252–264. 10.1016/j.neuron.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Wu X., Li X., Jiang L.L., Gui X., Liu Y., Sun Y., Zhu B., Pina-Crespo J.C., Zhang M., et al. 2018. TREM2 is a receptor for beta-amyloid that mediates microglial function. Neuron. 97:1023–1031.e7. 10.1016/j.neuron.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L., Wang Z., Wang D., Wang Z., Martens Y.A., Wu L., Xu Y., Wang K., Li J., Huang R., et al. 2018. Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol. Neurodegener. 13:15. 10.1186/s13024-018-0247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L., Xu Y., Zhuo R., Wang T., Wang K., Huang R., Wang D., Gao Y., Zhu Y., Sheng X., et al. 2019. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer's disease model. Nat. Commun. 10:1365. 10.1038/s41467-019-09118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mouse sample information for scRNA-seq.
contains original blots for Fig. S2.
Data Availability Statement
All scRNA-seq data were deposited in National Center for Biotechnology Information’s Gene Expression Omnibus under accession numbers GSE161224 and GSE161227.