Abstract
A ubiquitous feature of animal development is the formation of fluid-filled cavities or lumina, which transport gases and fluids across tissues and organs. Among different species, lumina vary drastically in size, scale, and complexity. However, all lumen formation processes share key morphogenetic principles that underly their development. Fundamentally, a lumen simply consists of epithelial cells that encapsulate a continuous internal space, and a common way of building a lumen is via opening and enlarging by filling it with fluid and/or macromolecules. Here, we discuss how polarized targeting of membrane and secreted proteins regulates lumen formation, mainly focusing on ion transporters in vertebrate model systems. We also discuss mechanistic differences observed among invertebrates and vertebrates and describe how the unique properties of the Na+/K+-ATPase and junctional proteins can promote polarization of immature epithelia to build lumina de novo in developing organs.
Keywords: Lumen, epithelial, polarity, fluid secretion, morphogenesis, zebrafish
1. Introduction
During animal development, tube formation allows the transport of gases, fluid, and macromolecules across organs [1]. Tubes range from simple structures made from just a few cells, as in the Caenorhabditis elegans excretory system, to complex branched ductal networks, as in the mammalian lung [2]. Tubes consist of epithelial cells that encapsulate an interior space or lumen. A variety of mechanisms are known to generate lumina from epithelial sheets or unpolarized mesenchyme [2–4]. De novo lumen formation, in which a fluid-filled cavity is generated within clusters of cells or through individual cells, is a common and conserved process, as examples have been described in nearly all invertebrate and vertebrate model systems [5–12]. While distinct cellular mechanisms can facilitate de novo lumen formation, some fundamental principles are prevalent among different systems. For example, luminal contents (fluid or solid) drive the opening and enlargement of lumina and tubes [11–18]. The roles of cytosolic trafficking machinery in lumen formation has been recently reviewed [3], and lumen formation in vitro (e.g. 3D cell culture models) has also been thoroughly characterized and reviewed [19–24]. Here, we focus on how polarized sorting and trafficking of key proteins facilitates lumen opening, expansion, and coalescence during organ development mostly in in vivo models, and we describe some in vitro work that has offered molecular insights. Throughout this review, we consider how epithelial polarization relates to lumen formation.
2. The problem of polarity and trafficking in immature, developing epithelial organs
Cell polarization is a general feature of mature lumina, with distinct domains of the plasma membrane in contact with the lumen and surrounding tissue. The apical membrane generally faces the lumen, and the basolateral membrane is adhered to the basement membrane by cell-extracellular matrix (ECM) interactions. Some tissues, such as the mammalian blastocyst, have a less common organization, with the basolateral membrane facing the lumen [25]. Epithelial polarization is critical for cell and organ physiology, which depend on secretion and absorption of ions and macromolecules to and from the lumen [1]. Epithelial cells accomplish these tasks by expressing membrane proteins such as channels and transporters asymmetrically, at either the apical or basolateral surface. Membrane proteins are generally sorted, or segregated, into different vesicular carriers during biosynthetic transport. Segregation of apical and basolateral proteins can occur in the trans-Golgi network (TGN) and sorting endosomes, or in recycling endosomes after internalization [26–28]. While mature, highly polarized epithelia have robust sorting and trafficking mechanisms delivering cargoes to the cell surface in a highly polarized fashion, in developing organs, lumen morphogenesis can coincide with epithelial polarization [29]. The lack of polarization in immature epithelia presents a problem because channels and transporters must precisely localize to their target membrane to facilitate lumen formation. Randomized sorting of polarized channels and transporters to apical and basolateral membranes would abolish ion gradients that are needed for secondary, coupled transport and fluid secretion. Recent studies have revealed how critical polarized membrane proteins can be correctly targeted in newly polarizing epithelial cells, and how lumina can rapidly form de novo within immature epithelia. In the following sections, we highlight the key roles of the Na+/K+-ATPase in this process and describe how its basolateral sorting can be tightly regulated prior to mature epithelial polarization.
3. Na+/K+-ATPase and fluid secretion in lumen formation
Ion transport, which is mediated both by active transport and by secondary transport along electrochemical gradients, can promote lumen formation by controlling fluid flux [30]. The resulting hydrostatic pressure from accumulating fluid drives lumen inflation and can also promote coalescence of many smaller lumina [11]. Active Na+ and K+ transport by the Na+/K+-ATPase provides the driving force for fluid transport in most mature epithelia [1], and it is ultimately responsible for lumen inflation in most developing organs. This was first shown in the Drosophila tracheal system [31] and the zebrafish brain ventricle [15]. Subsequently, work in the zebrafish gut, which develops from an endodermal rod of unpolarized mesenchyme that undergoes cord hollowing [32], established a role for basolateral transporters and junctional pores in lumen morphogenesis [11]. Lumen formation in the zebrafish gut begins as small cavities arise adjacent to newly forming junctional domains marked by ZO1 [11]. Coalescence into a single lumen then proceeds as the cavities enlarge, expand, and remodel membrane contacts [33]. The process is genetically controlled by transcriptional regulation via Tcf2, which is required for expression of the Na+/K+-ATPase and the paracellular pore forming protein Cldn15la in the gut [11]. Perturbation of the pathway results in many small lumina that fail to resolve due to impaired fluid secretion that is needed for lumen expansion. Apical trafficking, on the other hand, is required for later stages of single lumen formation, where Rab11a promotes membrane contact remodeling during the final step of coalescence. Contact remodeling is facilitated by recycling of basolateral membrane proteins, including E-cadherin [33]. A similar sequence of events, but controlled by parasympathetic innervation, was shown for lumen formation in the mouse salivary gland [34], although fluid secretion in that organ relies on the CFTR channel (see section 5).
Similar to the zebrafish gut [11], mammalian salivary glands [34], and avian lung [35], the mouse blastocoel forms as several pressurized microlumina arise throughout the embryo in a process that has been compared to hydraulic fracturing [36]. Formation of microlumina depend both on fluid transport via the activity of the basolateral Na+/K+-ATPase [36] and on secretion of cytoplasmic vesicles [37]. Microlumina begin to coalesce as they are guided to a centralized cavity according to differences in cell adhesion and tension [36]. Cells lining the cavity polarize and rapidly inflate the lumen by additional fluid transport that is driven largely by the basolateral Na+/K+-ATPase [38] and tight junction (TJ)-localized paracellular pores of the Claudin family [39]. Basolateral adhesion molecules are also crucial for morphogenesis during blastocoel inflation. Growth of the embryo occurs cyclically as hydrostatic pressure exceeds the tensile strength of adherens junctions, leading to their collapse and repair, allowing them to withstand yet increased pressure during subsequent growth cycles [5, 38]. Lumen formation within the proamniotic cavity, which forms shortly after the blastocyst stages, follows similar principles [29]. Cells of the epiblast are initially unpolarized, and restriction of the adherens junction protein E-Cadherin to the nascent basolateral domain decreases adhesion at presumptive apical membrane domains. Basolateral membrane protein segregation coincides with the activity of ion transporters that drive fluid secretion, allowing for hydraulic fracturing to initiate lumen formation at polarized membrane domains. Apical polarization commences concurrently and facilitates the delivery of proteins that further inhibit adhesion at apical membrane domains, while the continued activity of transporters enhances fluid secretion to inflate the proamniotic cavity. Much of the fluid used for inflating the lumen of the proamniotic cavity appears to derive from the blastocoel [29], suggesting that one of its functions is to act as a fluid reservoir for other tissues in the embryo. Formation of the blastocoel and proamniotic cavity, while generally similar, also show important mechanistic differences. For example, as described above, disruption of the epithelial barrier of the blastocyst results in fluid leakage and lumen size reduction [36]. By contrast, when epiblast cells surrounding the proamniotic cavity undergo division and disrupt the epithelial barrier, this allows additional fluid flow into the lumen rather than leakage [29]. This finding suggests that, unlike the blastocoel, osmotic gradients are not necessarily limiting for lumen expansion of the proamniotic cavity, and that much of the fluid that enters the lumen is coupled to cell division, which may help to relax tension that builds with rapid lumen growth.
The zebrafish inner ear is another organ highly dependent on basolateral transporters and fluid secretion for morphogenesis. The inner ear develops from the otic vesicle, a simple epithelium enclosing a lumen that rapidly inflates during development, increasing in volume nearly four-fold in just a day [40, 41]. Fluid secretion in the otic vesicle relies on the activity of at least two transporters, the Na+/K+-ATPase and cation-chloride co-transporter Nkcc1 [42]. In vivo studies demonstrated that lumen inflation in the otic vesicle generates hydrostatic pressure and because it is pressurized, puncturing the epithelium induces rapid volume loss [41]. Upon puncture, the otic epithelium quickly repairs and reinflates the lumen, but lumen growth during the repair process is abolished when Na+/K+-ATPase pump activity is inhibited [41]. Thus, the pump activity of Na+/K+-ATPase is a key driver of tubulogenesis across different vertebrate organs.
4. Polarization of the Na+/K+-ATPase in mature and immature epithelia
Given the important roles of fluid transport highlighted above, polarized sorting and trafficking of ion pumps and channels emerged as crucial for lumen formation in vivo. However, mechanisms underlying the asymmetric distribution of transporters during the onset of lumen formation in developing organs in animals are poorly described. In particular, how the Na+/K+-ATPase is sorted to the basolateral membrane in most epithelia during cell polarization is a major open question. In this section, we discuss mechanisms of Na+/K+-ATPase basolateral transport in mature versus immature epithelia, and we consider how in vitro studies of Na+/K+-ATPase trafficking may help us to understand the fundamental requirements of lumen opening in newly polarizing organs.
Basolateral sorting is generally mediated by receptor-like mechanisms in which cytoplasmic adaptor proteins bind to short peptide motifs on cargo proteins. An example is the di-leucine motif on the cytosolic tail of E-Cadherin, which partly relies on the AP (adaptor protein)-1A and AP-1B clathrin adaptors [43, 44]. Many basolateral membrane proteins are segregated in this way and become polarized by sorting and trafficking [45], whereas others are initially transported to the plasma membrane in a less polarized fashion and become asymmetrically enriched by selective stabilization through interactions with scaffolds and cytoskeletal adaptors [46, 47]. Basolateral targeting of the Na+/K+-ATPase in mammalian cells is complex and may be mediated by a combination of direct sorting and plasma membrane stabilization [48]. The minimal Na+/K+-ATPase holoenzyme consists of a catalytic α-subunit and an obligate ß-subunit, which is essential for biosynthetic trafficking of the pump and modulates its activity. In the absence of the ß-subunit, newly synthesized α-subunit is trapped in the endoplasmic reticulum (ER) lumen by the Coat Protein 1 (COP-1) factor ß-COP and subsequently degraded [49, 50]. ER export of the Na+/K+-ATPase requires assembly of the α- and ß-subunits [50] and proper folding for release from chaperones, which occurs coincidently with binding of the α-subunit cytosolic tail to Ankyrin [51], an adaptor protein to the Spectrin cytoskeleton. These prerequisites seem to make ER export rate-limiting for biosynthetic transport of the Na+/K+-ATPase [52] and ensure that only functional pumps are trafficked to the plasma membrane [53]. After ER export, the Na+/K+-ATPase is transported to the plasma membrane rapidly [52, 54], segregating from other basolateral membrane proteins at the TGN and reaching the basolateral membrane directly without traversing intermediate endosomes [54]. Na+/K+-ATPase polarization has also been attributed to its stabilization at the basolateral membrane through its linkage to Spectrin [55, 56] by the adaptor Ankyrin [57], although this has not been shown directly and instead may relate to Ankyrin’s role in the early biosynthetic pathway rather than at the plasma membrane [51].
The mechanism by which the Na+/K+-ATPase is segregated into basolateral carriers is unknown, and no specific sorting signals have been found on either the α- or ß-subunits. Both subunits lack common peptide sorting motifs, and their basolateral localization does not require AP-1A or AP-1B clathrin adaptors in mammalian cells [43, 45]. By contrast, in Drosophila photoreceptor cells, basolateral localization of the Na+/K+-ATPase does depend on Clathrin/AP-1 [58, 59]. Additionally, the GTPase Rab10, its GEF (Guanine Exchange Factor) Crag, its effector Ehbp1, and an unidentified GPI (Glycosylphosphatidylinositol)-anchored protein also regulate basolateral localization of the Na+/K+-ATPase [58, 59]. Because these factors localize to the TGN and their loss-of-function causes apical mistargeting [58], these results strongly suggest that this pathway regulates biosynthetic sorting of the Na+/K+-ATPase in Drosophila photoreceptors. Given the mechanistic differences observed for Na+/K+-ATPase targeting in Drosophila photoreceptor cells versus mammalian cells [43, 45, 58, 59], it is unclear whether Clathrin/AP-1/Rab10 is generally involved in Na+/K+-ATPase sorting in other epithelial organs.
Importantly, the above-mentioned studies all described basolateral transport of the Na+/K+-ATPase in fully polarized cells. By contrast, de novo lumen formation in developing organs often proceeds before or together with epithelial polarization [11, 38]. This raises the question of how immature epithelial cells in vivo target the Na+/K+-ATPase to the basolateral membrane before membrane identity is specified. A possible answer may stem from the surprising discovery that the Na+/K+-ATPase, in addition to being an ion pump, also functions as a cell adhesion molecule [60, 61]. Co-culture studies of cells expressing ß-subunits from different species demonstrated that the Na+/K+-ATPase accumulates at the basolateral membrane only when homotypic interactions occur between neighboring cells, despite the fact that heterotypic cell borders still form adherens junctions [62]. Moreover, expression of the canine ß-subunit in Chinese Hamster Ovary (CHO) cells mediates localization of the Na+/K+-ATPase at heterotypic cell borders between canine MDCK and hamster CHO cells [62]. These experiments indicate that trans interaction of the ß-subunit across epithelial cells is both necessary and sufficient for basolateral localization of the Na+/K+-ATPase [31, 63].
Overall, these studies raise the intriguing possibility is that the sorting adaptor for the Na+/K+-ATPase catalytic α-subunit is simply its obligate ß-subunit. This mode of polarized targeting would be unique from that of any other known membrane protein and could conceivably mediate proper localization of the Na+/K+-ATPase prior to establishment of epithelial polarization, provided that cells have established adherens junctions. This idea is supported by studies showing that expression of E-cadherin in unpolarized fibroblasts alone can mediate basolateral localization of the Na+/K+-ATPase [64], presumably by bringing cells close enough in proximity to facilitate trans interaction of ß-subunits [65]. In this system, basolateral targeting of the Na+/K+-ATPase occurs even before formation of TJ [64], suggesting that the Na+/K+-ATPase can be properly sorted in immature epithelial cells simply due to its intrinsic ability to adhere with pumps on neighboring cells. Further support for this model comes from studies showing that co-expression of E-cadherin and the Na+/K+-ATPase ß-subunit is sufficient to suppress a mesenchymal phenotype and induce epithelial polarization of a sarcoma-transformed MDCK cell line [58]. Moreover, the only know mechanism for apical targeting of the Na+/K+-ATPase, which supports the unique physiology of some organs [1], is expression of a separate, more highly glycosylated ß-subunit isoform [66–68], indicating that ß-subunit switching can mediate differential sorting of the Na+/K+-ATPase. Finally, of the many P-type ATPase subfamilies, a ß-subunit is unique to the Na+/K+-ATPase and H+/K+-ATPase pumps that share common ancestry [69]; some P4-ATPases also have an accessory subunit that is distinct from the Na+/H+K+-ATPase ß-subunit [70]. These findings suggest that the ß-subunit may have evolved separately as an adhesion molecule before its integration with ion pumps [71].
Basolateral polarization of E-cadherin, although mechanistically distinct from that of the Na+/K+-ATPase, follows similar principles. In mature epithelia, the steady state localization of E-Cadherin relies on both biosynthetic sorting and recycling [43, 44]. E-Cadherin, like the Na+/K+-ATPase, is stabilized by homotypic interactions across neighboring cells to inhibit its turnover at the lateral membrane, and this is mediated in part by binding to Ankyrin [47]. Studies of newly polarizing cells showed that E-Cadherin localization to forming cell contacts immediately preceded delivery of subsequent basolateral membrane proteins, including the Na+/K+-ATPase and water channel forming protein Aquaporin-3 (AQP3). This finding led to the conclusion that Cadherin-mediated adhesion establishes a major positional domain to trigger epithelial polarization [72]. AQP3 also appears to reciprocally enhance cell adhesion complexes through expression of E-Cadherin [73] and β-catenin [74], suggesting that adhesion complexes, water channels, and ion pumps may exert a positive feedback effect to promote epithelial polarization and stabilize the basolateral machinery needed for lumen formation. Moreover, the stability of E-Cadherin at the plasma membrane and paracellular barrier function both depend on trans interactions of the Na+/K+-ATPase ß-subunits [75]. Because E-Cadherin and the Na+/K+-ATPase do not directly interact, their positive reinforcement may stem from binding to Ankyrin, which restricts their mobility at the basolateral membrane [57, 76]. This mode of positive feedback and basolateral stabilization may be particularly crucial for maintaining adhesion and barrier function during periods of mechanical stress caused by lumen inflation.
The studies highlighted above provide a framework for understanding, in a minimalist fashion, how a lumen can rapidly form de novo within an unpolarized group of cells (Fig. 1), as often occurs during animal development. Cell contacts mediated by homotypic interactions of Cadherins across cells positionally establish a lateral domain. Formation of adherens junctions promotes cohesion and compaction of epithelial cells, which then facilitates trans interactions of the Na+/K+-ATPase, stabilizing it at the lateral domain. Selective stabilization of Cadherin and the Na+/K+-ATPase, in essence, provides a means for asymmetric transport prior to the expression of a robust polarity network that is present in more mature epithelia. The formation of TJ adjacent to the lateral domain then allows for selective ion transport to establish osmotic gradients. Paracellular fluid transport can then generate hydrostatic pressure to separate the newly forming apical domains of cells lined by microlumina. This model of lumen formation contrasts with others describing apical membrane polarization as a prerequisite that is inextricably linked to lumen formation [2, 77]. However, as explained in later sections, apical secretion has many important roles in lumen growth, particularly in invertebrates. The basic principles described here may represent the minimal requirements for lumen opening in unpolarized organ precursors or those that polarize slowly, such as those that give rise to the mammalian blastocoel and zebrafish gut.
Fig. 1. Proposed mechanisms of lumen initiation and growth in vertebrate unpolarized epithelial organs.
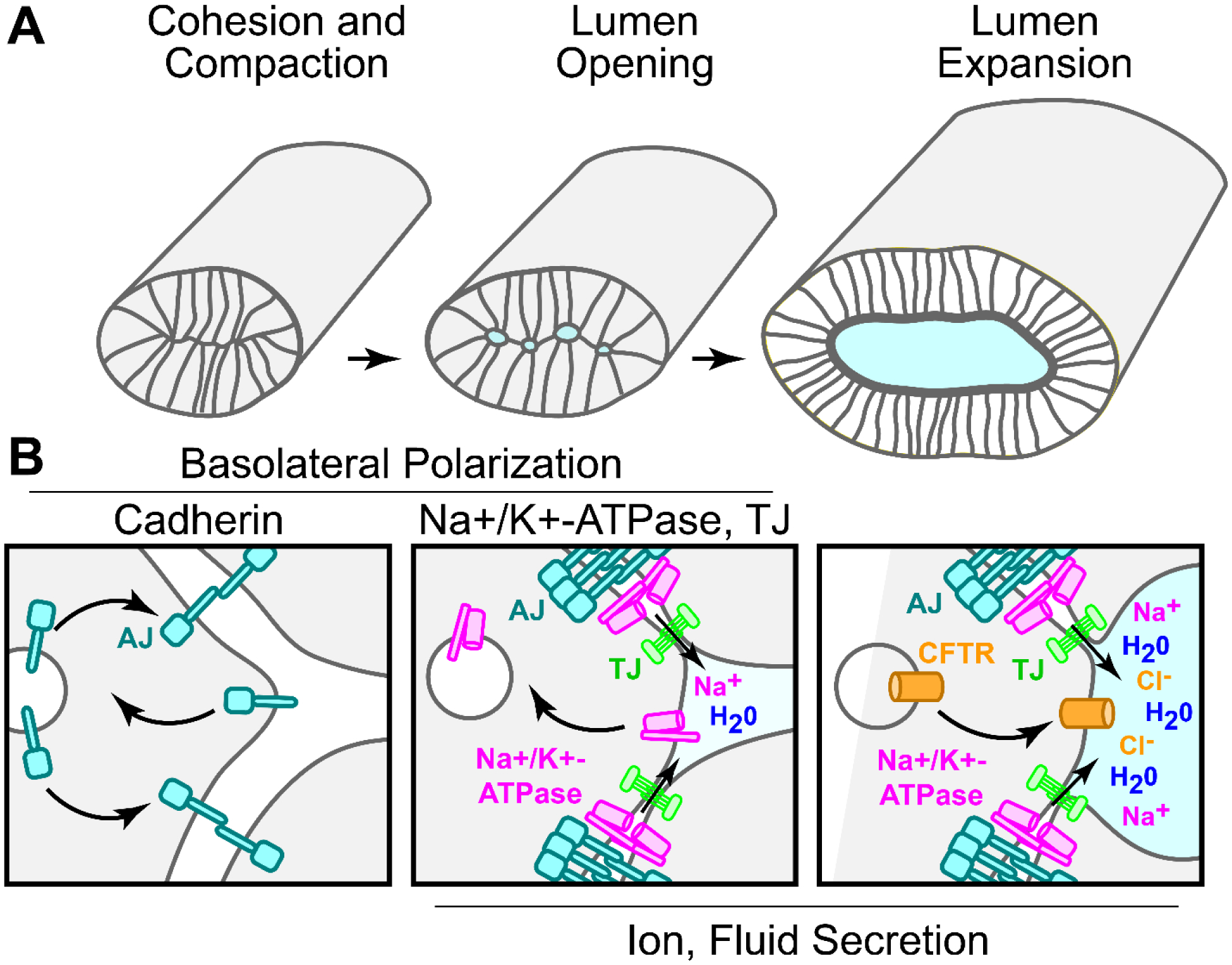
(A) Basic morphogenetic events occurring during de novo lumen formation in newly polarizing organ precursors. Cell adhesion is a fundamental requirement that establishes positional domains required for proper development of other critical adhesion complexes. (B) Minimal molecular events that may facilitate lumen morphogenesis. Left panel: After differentiation, epithelial cells express adherens junction (AJ, cyan) proteins such as E-Cadherin that mediate cohesion. Transcellular interactions of cadherin extracellular domains stabilize the AJ at the newly forming basolateral domain. Cadherins lacking trans-interactions undergo internalization (inward arrow) and recycling (outward arrow). Middle panel: Cohesion and basolateral polarization facilitates transcellular interaction of Na+/K+-ATPase (magenta) ß-subunits, thereby stabilizing the complex. Like cadherins, Na+/K+-ATPase lacking trans-interactions are subject to internalization (inward arrow). Basolateral polarization also promotes the formation of tight junctions (TJ, green), allowing the epithelium to establish selectively permeable paracellular barriers to mediate ion and fluid transport. Hydrostatic pressure commences lumen opening. Right panel: Continued activity of ion channels such as basolateral Na+/K+-ATPase and apical CFTR in vertebrates drives enhanced fluid transport needed for lumen expansion.
5. Roles and regulation of CFTR and Cl− transport in lumen formation
While the Na+/K+-ATPase generally provides the driving force for fluid transport in most organs [1], other channels and transporters are also required for lumen formation. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) is a vertebrate apical Cl− and HCO3− channel that functions in concert with the Na+ gradient established by the Na+/K+-ATPase to drive fluid secretion and regulate mucus properties [78]. CFTR function is of great interest biomedically because inactivating mutations are the cause of cystic fibrosis, a life-threatening disease that affects respiratory, gastrointestinal, and urogenital organs [79].
In addition to its roles in human disease, CFTR-dependent fluid transport is also critical for regulating lumen size during development. In the zebrafish embryo, Kupffer’s vesicle (KV), the organ of laterality that controls left-right asymmetry, is a transient fluid-filled cyst-like structure lined by a ciliated epithelium. Lumen formation in KV is critical for establishing asymmetric calcium signaling [80], which depends on cilia motility in the lumen to drive fluid flow [81]. Mutations in cftr impair lumen expansion in KV, but not cilia formation or motility, and results in near randomization of organ laterality [82]. Lumen growth in KV relies on coupled secretion of Na+ and Cl− because chemical inhibition of the Na+/K+-ATPase mimics the effect of cftr mutations, while chemical activation of CFTR causes hyper-inflation [82].
Apical localization of CFTR is essential for its function in Cl− and fluid secretion. In KV, lumen growth is associated not only with CFTR-dependent apical fluid secretion but also proliferation. Interestingly, these cell divisions have an important role in targeting Cftr apically. Prior to KV formation, precursor cells arrange into a rosette formation and undergo cell division. During cytokinesis, daughter cells sever midbody remnants at what will form the apical poles underlying the lumen. These midbody fragments seem to establish a polarized domain by recruitment of Rab11, a GTPase critical for apical trafficking. In fact, optogenetic clustering of Rab11 in KV is sufficient to sequester Cftr and inhibit lumen formation [83]. Thus, KV illustrates another way of how newly polarizing cells target membrane proteins asymmetrically during lumen morphogenesis.
In addition to Rab11, many other factors are known to regulate CFTR’s apical targeting. Unlike most apical membrane proteins, during biosynthetic transport, CFTR is randomly sorted for apical and basolateral membrane transport [84]. Apical enrichment of the channel derives from its more rapid removal from the basolateral membrane, much of which is redirected apically by transcytosis [85]. The apical CFTR population is also subject to internalization but undergoes recycling [84]. The relative stability and recycling of the apical CFTR pool depends on its cytosolic PDZ (PSD-95, disks large, zona occludens 1)-interacting domain to bind to scaffolding proteins such as NHERF1/2 [86, 87] and PDZK1 [88]. Apical recycling of CFTR also depends on its phosphorylation by Protein Kinase A (PKA) [89, 90], which is traditionally better known for its influence on CFTR channel gating and activity [91]. In fact, commonly used approaches for CFTR activation, such as Forskolin treatment, act indirectly through the production of cAMP, which stimulates PKA. Thus, while CFTR’s sorting is not robust, its apical localization is reinforced by interactions with scaffolding networks, and the same signaling network that activates its channel activity (cAMP and PKA) also regulates its trafficking by stimulating recycling after apical internalization. CFTR’s steady state polarization, which relies heavily on transcytosis and recycling of existing intracellular pools, contrasts with that of the Na+/K+-ATPase, whose basolateral localization is tightly regulated and reinforced by transcellular interactions and cytoskeletal stabilization (refer to section 4). These differences may reflect the more common and ancient role of the Na+/K+-ATPase in lumen formation compared to CFTR, whose function in lumenogenesis is unique to vertebrates.
Although regulation of CFTR activity and trafficking has been extensively characterized, few in vivo studies have addressed the relevance of these mechanisms to lumen formation. One surprising discovery is that CFTR can be regulated non-cell autonomously to promote lumen formation [34]. During morphogenesis of the mouse salivary glands, the submandibular gland (SMG) becomes innervated by parasympathetic nerves. This innervation is required for lumen resolution in the SMG, which is ultimately dependent on CFTR-dependent fluid secretion. The mechanism by which parasympathetic ganglia regulate CFTR is through the release of vasoactive intestinal peptide (VIP), which signals through the VIPR1 receptor on SMGs. VIPR1 is a G protein-coupled receptor subtype whose activation causes cAMP production, thereby activating CFTR through PKA. Exogenous VIP treatment to isolated SMGs in culture is sufficient to rapidly induce lumen formation, and the effect is blocked by inhibition of PKA or CFTR [34].
Additional insight into CFTR regulation in vivo came from an unbiased forward genetic screen in zebrafish for mutants exhibiting impaired intestinal morphogenesis [92]. Prior studies had established that while the main driving force of fluid accumulation in the zebrafish gut is active Na+ secretion by the Na+/K+-ATPase, Cl− transport also promotes lumen growth in the intestine by further drawing Na+ and water into the lumen [11]. From the genetic screen, mutants for cse1l were found to exhibit hyper-expansion of the gut lumen that phenocopies CFTR activation. Loss of Cse1l causes the gut lumen to increase in volume several fold within a period of hours, and this effect is suppressed by inhibiting CFTR activity. Physical interaction of Cse1l and CFTR in mammalian cells support a model in which direct binding causes negative regulation of channel activity [92], although the exact mechanism remains unknown. Interestingly, cse1l mutants undergo early gut morphogenesis normally and do not show aberrant lumen expansion until nearly a day after lumen formation is complete. These findings indicate that CFTR’s channel activity, which is present only after gut lumen formation [92], must be tightly regulated to limit organ growth, suggesting that the channel is otherwise poised in an active state without Cse1l in the zebrafish intestine. Additional regulation of CFTR comes from its subcellular localization, being mostly in subapical vesicles in CFTR high expresser cells (CHEs) in the absence of PKA-mediated stimulation, which promotes cell surface localization [93]. Interestingly, CHEs have been found in the rat [93] and zebrafish gut (our unpublished results), the mammalian lung [94, 95], fish gills [96] and skin [97] and may be major drivers of fluid secretion in those tissues.
Finally, although CFTR has traditionally been described as a vertebrate-specific gene, recent analysis indicates that Drosophila has a distant ABCC4 ortholog, CG5789, that is functionally related to CFTR. Mutants for CG5789 exhibit ionic and osmotic phenotypes in the intestine, and these can be suppressed by the expression of human CFTR [98]. It remains unknown whether CG5789 regulates lumen morphogenesis in Drosophila similarly to vertebrate CFTR.
6. Secreted and tethered luminal cargos implicated in lumen growth
In contrast to vertebrates, in the Drosophila trachea, the Na+/K+-ATPase functions in lumen formation by regulating the apical secretion of cargoes. This was established by forward genetic screens for mutations affecting morphogenesis of tracheal tubes, which undergo lumen expansion and elongation to reach proper size [3, 99]. These genetic screens uncovered surprising interactions of seemingly disparate pathways - septate junctions (SJ; which are analogous to vertebrate TJ), the Na+/K+-ATPase, and secreted polysaccharides [99].
Early studies found that the megatrachea mutation [10] affects a member of the Claudin family of membrane proteins [100], which are traditionally known for regulating paracellular barriers in vertebrate TJ. This led to the conclusion that SJ function analogously to TJ in lumen morphogenesis by controlling paracellular flux [100]. However, as the name suggests, megatrachea mutants have a phenotype contrary to what would be expected if its relevant role in lumen formation was paracellular barrier formation. Rather than exhibiting smaller lumina, megatrachea mutants and mutations affecting other drosophila Claudins have enlarged and elongated tracheal tubes [101]. Other studies found that mutants of the Na+/K+-ATPase phenocopy those of SJ [31], that the Na+/K+-ATPase is required for proper organization of SJ [31, 102], and that the role of the Na+/K+-ATPase in tracheal growth is independent of its catalytic pump activity [103]. Thus, although Drosophila requires conserved machinery to regulate lumen growth, they paradoxically utilize a different mechanism than is found in vertebrate models.
Subsequent work established that the Na+/K+-ATPase and SJ regulate tracheal lumen growth by controlling the apical secretion of enzymes that modify luminal chitins [104], which are invertebrate oligosaccharide polymers required for proper tracheal tube size and shape [13, 14, 105, 106]. These enzymes, which are called Chitin-binding, LDLRa-containing deacetylases (Ch-LD) are thought to modulate the physical properties of chitins to control lumen expansion [104, 107]. In addition to SJ, the biosynthetic and endocytic pathways, in general, contributes to apical secretion, resolution of luminal chitin, and tracheal lumen growth [108]. While it was postulated that apical secretion regulates tracheal lumen expansion by promoting swelling of the luminal chitin matrix [108], mosaic analysis of mutants deficient in ER-to-Golgi trafficking showed that apical secretion promotes lumen secretion cell-autonomously, even though secreted proteins readily diffuse in the lumen [109]. It was therefore concluded that secretion promotes tracheal lumen growth by promoting apical membrane expansion rather than through fluid accumulation and matrix swelling, and that the luminal chitin matrix acts as a physical cue to shape the tracheal lumen [109, 110]. Recent analysis supports this conclusion and indicates that the mechanical properties of chitinous ECM influence the composition of the apical cytoskeleton to shape lumen growth through an active feedback mechanism [111, 112]. Collectively, these studies suggest that adaptation of phyla-specific genes may influence the different mechanisms regulating lumen morphogenesis among different animals (e.g. mechanical properties of chitin in invertebrates versus CFTR-dependent fluid secretion in vertebrates).
Although a chitin-based luminal matrix is mostly limited to invertebrates, apical secretion of other factors has important roles in lumen formation in vertebrates and invertebrates alike. In particular, proteoglycan secretion regulates lumen formation in many species. Secreted and tethered proteoglycans are thought to promote lumen opening and expansion by their anti-adhesive and water-adsorbing properties. In Drosophila, apical secretion of ECM proteoglycans proteins is required for lumen morphogenesis of the developing eye [113] and salivary gland [114]. Similarly, in the Drosophila hindgut, apical secretion of a large, O-glycosylated mucin-like protein, Tenectin, is required lumen expansion, and exogenous expression of Tenectin in other tubular epithelia is sufficient to increase lumen size [115]. As gel-forming mucins draw fluid, Tenectin is thought to promote lumen expansion by increasing hydrostatic pressure [115]. This notion is supported by experiments showing that Tenectin secretion can promote localized lumen expansion and epithelial cell flattening in multiple tubular organs, and it does so in a dose-dependent manner [115]. It is also formally possible that Tenectin could promote lumen expansion through biochemical signaling cues, such as by binding to ECM receptors or sequestering extracellular ligands [17, 116]. However, the more common and conserved roles of fluid transport in lumen expansion highlighted in this review suggests hydrostatic pressure as a simpler explanation. Similar roles for secreted proteoglycans and mucin-like proteins in lumen morphogenesis have been found in C. elegans [117, 118]. During lumen formation in the C. elegans vulva, expression of several secreted and tethered matrix proteins is dynamically regulated, and mutations in various apical matrix proteins lead to distinct phenotypes affecting lumen inflation, stabilization, and resolution [118]. In the mouse vasculature, apical secretion of Podocalyxin, a heavily sialylated tethered mucin, promotes lumen expansion by inhibiting the interaction of opposing apical membranes, which is thought to be mediated by electrostatic repulsion of negatively charged glycans [119]. Although apical delivery of Podocalyxin is required for lumen morphogenesis in MDCK cysts [21], the mouse vasculature [119], and likely also zebrafish vasculature [16], it does not seem to be universally required in all epithelial organs that it is expressed. In the mouse kidney, for example, although Podocalyxin and its related CD34 family member Endoglycan are highly expressed at apical lumina of nephrons, double mutants of these sialomucins do not show lumen phenotypes in this tissue [120]. Because CD34 proteins have cytosolic PDZ-interacting domains that bind to and help organize apical scaffolding and polarity networks [21], Podocalyxin/Endoglycan double mutants fail to recruit the PDZ-scaffold NHERF1 to the apical pole of nephrons [120]. Given that NHERF1 is also required for lumen formation in MDCK cysts [21], it is remarkable that Podocalyxin/Endoglycan double mutants undergo lumen formation normally.
The mechanisms controlling apical trafficking of proteoglycans and mucins such as Podocalyxin have been extensively characterized in MDCK cell cysts [19–22, 121]. Early 2-cell stage cysts are initially unpolarized and localize Podocalyxin to the basolateral membrane. Cell-ECM integrin interactions establish basolateral identity and trigger internalization of Podocalyxin, which is then transported through recycling endosomes to the newly forming apical membrane (apical membrane initiation site, AMIS). Trafficking of Podocalyxin to the AMIS depends on GTPases such as Rab11 and Rab8, and domain identity of the AMIS is specified by polarity protein networks such as Par3/Par6/aPKC/CDC42/Anxa2 and phosphoinositides [19–22, 121]. These mechanisms have been reviewed in detail previously [122, 123]. In vivo studies have provided additional mechanistic insight to apical membrane proteins sorting and trafficking, demonstrating roles for Rab8 [124, 125], Rab11, [126, 127], MYO5B [128], and the vacuolar H+ATPase (V-H+ATPase) [129], among others [28, 130–132]. A forward genetic screen for mutations affecting apical membrane biogenesis in zebrafish revealed that V-H+ATPase activity is required for biosynthetic sorting of O-Glycosylated apical membrane proteins and for post-Golgi trafficking of all apical membrane proteins tested [129]. In this system, V-H+ATPase activity regulates the acidic lumen of the TGN, and manipulation of luminal pH during TGN export is sufficient to cause randomized sorting. These studies suggest that acidification of the TGN may promote apical sorting of O-glycosylated membrane proteins by controlling their oligomerization [28]. This model is supported by studies showing that oligomerization of mucins is enhanced by the mildly acidic pH levels characteristic of the TGN [133].
Importantly, some organs, such as the zebrafish intestine, undergo lumen formation before apical protein sorting is tightly regulated, and the above mentioned unbiased genetic screen for mutations affecting apical membrane protein targeting in this organ failed to yield lumen phenotypes [129]. Additionally, even in Drosophila, some tubular organs regulate apical polarization independently of canonical apical polarity factors such as Par-6 and aPKC [134]. Therefore, more in vivo studies are needed to understand the roles and requirements of apical polarization in developing organs versus cell culture models.
7. Conclusions and perspectives
The work highlighted here shows the tremendous progress made in understanding the roles of polarized transport in lumen morphogenesis. Though vastly different in scale and complexity, lumen formation throughout many different organs and animals relies heavily on the polarized targeting of membrane and secreted proteins. Common themes described in this review are that secretion promotes lumen formation by generating hydrostatic pressure in a variety of organs and organisms, and that phyla-specific genes appear to have been co-opted for regulation of lumen morphogenesis among invertebrates and vertebrates. Moreover, as argued here, self-organization principles appear to underly polarization of critical junctional complexes, ion pumps, and channels required for lumen opening and expansion in immature epithelia (Fig. 1). These insights may help to explain how single lumen formation can be achieved in newly forming epithelial organs in vivo.
While candidate-based studies of cell culture models have yielded major insights into the mechanisms of lumen morphogenesis, unbiased genetic approaches in vivo are needed to better define the roles of epithelial polarization, sorting, and trafficking in lumen formation. Of particular importance is to obtain genetic evidence on the mechanisms controlling sorting and polarization of the Na+/K+-ATPase in vivo. Drosophila and C. elegans have traditionally showcased the power of using unbiased genetics to study lumen formation, providing many detailed mechanisms described here. However, the amenability of some vertebrate models such as zebrafish and medaka to forward genetics [135, 136], sophisticated live imaging [41], and genetic engineering [137, 138] should also be leveraged to uncover vertebrate-specific mechanisms controlling lumen formation.
Acknowledgements
This work was supported by NIH grant DK121007 (M.B.) and Duke University Training Grant in Digestive Diseases and Nutrition Grant DK007568 (D.S.L). M.B. is an HHMI Faculty Scholar.
Abbreviations
- ABCC4
ATP Binding Cassette Subfamily C Member 4
- AJ
Adherens Junction
- AMIS
Apical Membrane Initiation Site
- Anxa2
Annexin A2
- AP
Adaptor Protein
- aPKC
Atypical Protein Kinase C
- cAMP
Cyclic Adenosine Monophosphate
- CDC42
Cell Division Cycle 42
- CD34
CD34 Molecule
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- CG5789
Drosophila Dmel\CG5789
- CHEs
CFTR High Expressing Cells
- Ch-LD
Chitin-binding, Low-Density Lipoprotein Particle Receptor Class A-containing deacetylase
- CHO
Chinese Hamster Ovary
- Cldn15la
Claudin15-like A
- COP-1
Coat Protein 1
- Cse1l
Chromosome Segregation 1 Like
- ECM
Extracellular Matrix
- Ehbp1
EH Domain Binding Protein 1
- ER
Endoplasmic Reticulum
- GEF
Guanine Exchange Factor
- GPI
Glycosylphosphatidylinositol
- KV
Kupffer’s Vesicle
- MDCK
Madin-Darby Canine Kidney
- Na+/K+-ATPase
Sodium-Potassium Pump
- NHERF1
Na(+)/H(+) Exchange Regulatory Cofactor NHE-RF1
- Nkcc1
Sodium-Potassium-Chloride Cotransporter 1
- Par3
Partitioning Defective 3 Homolog
- Par6
Partitioning Defective 6 Homolog
- PDZ
PSD-95, Disks Large, Zona Occludens 1
- PDZK1
PDZ Domain Containing 1
- PKA
Protein Kinase 1
- SJ
Septate Junction
- SMG
Submandibular Gland
- Tcf2
Transcription Factor 2
- TGN
Trans-Golgi Network
- VIP
Vasoactive Intestinal Peptide
- V-H+ATPase
Vacuolar-Proton ATPase
- VIPR1
Vasoactive Intestinal Peptide Receptor 1
- ZO-1
Zona Occludens 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest
The authors declare no competing interest.
References
- [1].Caceres PS, Benedicto I, Lehmann GL, Rodriguez-Boulan EJ, Directional Fluid Transport across Organ-Blood Barriers: Physiology and Cell Biology, Cold Spring Harb Perspect Biol 9(3) (2017) a027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sigurbjornsdottir S, Mathew R, Leptin M, Molecular mechanisms of de novo lumen formation, Nat Rev Mol Cell Biol 15(10) (2014) 665–76. [DOI] [PubMed] [Google Scholar]
- [3].Camelo C, Luschnig S, Cells into tubes: Molecular and physical principles underlying lumen formation in tubular organs, Curr Top Dev Biol 143 (2021) 37–74. [DOI] [PubMed] [Google Scholar]
- [4].Bernascone I, Hachimi M, Martin-Belmonte F, Signaling Networks in Epithelial Tube Formation, Cold Spring Harb Perspect Biol 9(12) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biggers JD, Bell JE, Benos DJ, Mammalian blastocyst: transport functions in a developing epithelium, Am J Physiol 255(4 Pt 1) (1988) C419–32. [DOI] [PubMed] [Google Scholar]
- [6].Dan K, Cyto-Embryology of Echinoderms and Amphibia, in: Bourne GH, Danielli JF (Eds.), Int Rev Cytol, Academic; Press1960, pp. 321–367. [DOI] [PubMed] [Google Scholar]
- [7].Slack C, Warner AE, Intracellular and intercellular potentials in the early amphibian embryo, The Journal of Physiology 232(2) (1973) 313–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Selwood L, Young GJ, Cleavage in vivo and in culture in the dasyurid marsupial Antechinus stuartii (macleay), Journal of Morphology 176(1) (1983) 43–60. [DOI] [PubMed] [Google Scholar]
- [9].Leung B, Hermann GJ, Priess JR, Organogenesis of the Caenorhabditis elegans intestine, Dev Biol 216(1) (1999) 114–34. [DOI] [PubMed] [Google Scholar]
- [10].Beitel GJ, Krasnow MA, Genetic control of epithelial tube size in the Drosophila tracheal system, Development 127(15) (2000) 3271–82. [DOI] [PubMed] [Google Scholar]
- [11].Bagnat M, Cheung ID, Mostov KE, Stainier DY, Genetic control of single lumen formation in the zebrafish gut, Nat Cell Biol 9(8) (2007) 954–60. [DOI] [PubMed] [Google Scholar]
- [12].Deng W, Nies F, Feuer A, Bocina I, Oliver D, Jiang D, Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis, Proc Natl Acad Sci U S A 110(37) (2013) 14972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tonning A, Hemphälä J, Tång E, Nannmark U, Samakovlis C, Uv A, A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea, Dev Cell 9(3) (2005) 423–30. [DOI] [PubMed] [Google Scholar]
- [14].Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA, Requirement for chitin biosynthesis in epithelial tube morphogenesis, Proceedings of the National Academy of Sciences of the United States of America 102(47) (2005) 17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lowery LA, Sive H, Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products, Development 132(9) (2005) 2057–67. [DOI] [PubMed] [Google Scholar]
- [16].Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting H-G, Affolter M, Distinct Cellular Mechanisms of Blood Vessel Fusion in the Zebrafish Embryo, Current Biology 21(22) (2011) 1942–1948. [DOI] [PubMed] [Google Scholar]
- [17].Chan CJ, Hiiragi T, Integration of luminal pressure and signalling in tissue self-organization, Development 147(5) (2020). [DOI] [PubMed] [Google Scholar]
- [18].Torres-Sanchez A, Kerr Winter M, Salbreux G, Tissue hydraulics: Physics of lumen formation and interaction, Cells Dev (2021) 203724. [DOI] [PubMed] [Google Scholar]
- [19].Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K, PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42, Cell 128(2) (2007) 383–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE, A molecular network for de novo generation of the apical surface and lumen, Nat Cell Biol 12(11) (2010) 1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE, A molecular switch for the orientation of epithelial cell polarization, Dev Cell 31(2) (2014) 171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gálvez-Santisteban M, Rodriguez-Fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, Bañón-Rodríguez I, Bernascone I, Datta A, Spivak N, Young K, Slim CL, Brakeman PR, Fukuda M, Mostov KE, Martín-Belmonte F, Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells, Nature Cell Biology 14(8) (2012) 838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Datta A, Bryant DM, Mostov KE, Molecular Regulation of Lumen Morphogenesis, Current Biology 21(3) (2011) R126–R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blasky AJ, Mangan A, Prekeris R, Polarized protein transport and lumen formation during epithelial tissue morphogenesis, Annu Rev Cell Dev Biol 31 (2015) 575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schliffka MF, Maître J-L, Stay hydrated: basolateral fluids shaping tissues, Current Opinion in Genetics & Development 57 (2019) 70–77. [DOI] [PubMed] [Google Scholar]
- [26].Rodriguez-Boulan E, Macara IG, Organization and execution of the epithelial polarity programme, Nat Rev Mol Cell Biol 15(4) (2014) 225–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stoops EH, Caplan MJ, Trafficking to the apical and basolateral membranes in polarized epithelial cells, J Am Soc Nephrol 25(7) (2014) 1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levic DS, Bagnat M, Self-organization of apical membrane protein sorting in epithelial cells, FEBS J 289(3) (2022) 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim YS, Fan R, Kremer L, Kuempel-Rink N, Mildner K, Zeuschner D, Hekking L, Stehling M, Bedzhov I, Deciphering epiblast lumenogenesis reveals proamniotic cavity control of embryo growth and patterning, Sci Adv 7(11) (2021) eabe1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Navis A, Bagnat M, Developing pressures: fluid forces driving morphogenesis, Curr Opin Genet Dev 32 (2015) 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Paul SM, Ternet M, Salvaterra PM, Beitel GJ, The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system, Development 130(20) (2003) 4963–74. [DOI] [PubMed] [Google Scholar]
- [32].Ng ANY, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Dong PDS, Stainier DYR, Heath JK, Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis, Developmental Biology 286(1) (2005) 114–135. [DOI] [PubMed] [Google Scholar]
- [33].Alvers AL, Ryan S, Scherz PJ, Huisken J, Bagnat M, Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling, Development 141(5) (2014) 1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, Hoffman MP, Knox SM, Parasympathetic innervation regulates tubulogenesis in the developing salivary gland, Dev Cell 30(4) (2014) 449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Palmer MA, Nelson CM, Fusion of airways during avian lung development constitutes a novel mechanism for the formation of continuous lumena in multicellular epithelia, Dev Dyn 249(11) (2020) 1318–1333. [DOI] [PubMed] [Google Scholar]
- [36].Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, Turlier H, Maitre JL, Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst, Science 365(6452) (2019) 465–468. [DOI] [PubMed] [Google Scholar]
- [37].Ryan AQ, Chan CJ, Graner F, Hiiragi T, Lumen Expansion Facilitates Epiblast-Primitive Endoderm Fate Specification during Mouse Blastocyst Formation, Dev Cell 51(6) (2019) 684–697 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chan CJ, Costanzo M, Ruiz-Herrero T, Monke G, Petrie RJ, Bergert M, Diz-Munoz A, Mahadevan L, Hiiragi T, Hydraulic control of mammalian embryo size and cell fate, Nature 571(7763) (2019) 112–116. [DOI] [PubMed] [Google Scholar]
- [39].Moriwaki K, Tsukita S, Furuse M, Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos, Dev Biol 312(2) (2007) 509–22. [DOI] [PubMed] [Google Scholar]
- [40].Hoijman E, Rubbini D, Colombelli J, Alsina B, Mitotic cell rounding and epithelial thinning regulate lumen growth and shape, Nat Commun 6 (2015) 7355. [DOI] [PubMed] [Google Scholar]
- [41].Mosaliganti KR, Swinburne IA, Chan CU, Obholzer ND, Green AA, Tanksale S, Mahadevan L, Megason SG, Size control of the inner ear via hydraulic feedback, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abbas L, Whitfield TT, Nkcc1 (Slc12a2) is required for the regulation of endolymph volume in the otic vesicle and swim bladder volume in the zebrafish larva, Development 136(16) (2009) 2837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E, The clathrin adaptor AP-1A mediates basolateral polarity, Dev Cell 22(4) (2012) 811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD, A Dileucine Motif Targets E-cadherin to the Basolateral Cell Surface in Madin-Darby Canine Kidney and LLC-PK1 Epithelial Cells *, J. Biol. Chem 276(25) (2001) 22565–22572. [DOI] [PubMed] [Google Scholar]
- [45].Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E, AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells, Proc Natl Acad Sci U S A 104(5) (2007) 1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gut A, Balda MS, Matter K, The Cytoplasmic Domains of a β1 Integrin Mediate Polarization in Madin-Darby Canine Kidney Cells by Selective Basolateral Stabilization *, J. Biol. Chem 273(45) (1998) 29381–29388. [DOI] [PubMed] [Google Scholar]
- [47].Jenkins PM, Vasavda C, Hostettler J, Davis JQ, Abdi K, Bennett V, E-cadherin Polarity Is Determined by a Multifunction Motif Mediating Lateral Membrane Retention through Ankyrin-G and Apical-lateral Transcytosis through Clathrin, J. Biol. Chem 288(20) (2013) 14018–14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ, Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells, Journal of Cell Biology 130(5) (1995) 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Morton MJ, Farr GA, Hull M, Capendeguy O, Horisberger J-D, Caplan MJ, Association with β-COP Regulates the Trafficking of the Newly Synthesized Na,K-ATPase *, J. Biol. Chem 285(44) (2010) 33737–33746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tokhtaeva E, Sachs G, Vagin O, Assembly with the Na,K-ATPase α1 Subunit Is Required for Export of β1 and β2 Subunits from the Endoplasmic Reticulum, Biochemistry 48(48) (2009) 11421–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stabach PR, Devarajan P, Stankewich MC, Bannykh S, Morrow JS, Ankyrin facilitates intracellular trafficking of α1-Na+-K+-ATPase in polarized cells, American Journal of Physiology-Cell Physiology 295(5) (2008) C1202–C1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Farr GA, Hull M, Stoops EH, Bateson R, Caplan MJ, Dual pulse-chase microscopy reveals early divergence in the biosynthetic trafficking of the Na,K-ATPase and E-cadherin, Molecular Biology of the Cell 26(24) (2015) 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kryvenko V, Vagin O, Dada LA, Sznajder JI, Vadász I, Maturation of the Na,K-ATPase in the Endoplasmic Reticulum in Health and Disease, The Journal of Membrane Biology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farr GA, Hull M, Mellman I, Caplan MJ, Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells, The Journal of Cell Biology 186(2) (2009) 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nelson WJ, Veshnock PJ, Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells, The Journal of cell biology 104(6) (1987) 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dubreuil RR, Wang P, Dahl S, Lee J, Goldstein LSB, Drosophila β Spectrin Functions Independently of α Spectrin to Polarize the Na,k Atpase in Epithelial Cells, Journal of Cell Biology 149(3) (2000) 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nelson WJ, Veshnock PJ, Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells, Nature 328(6130) (1987) 533–536. [DOI] [PubMed] [Google Scholar]
- [58].Nakamura Y, Ochi Y, Satoh T, Satoh AK, Rab10, Crag and Ehbp1 regulate the basolateral transport of Na+K+ATPase in Drosophila photoreceptors, Journal of Cell Science 133(7) (2020) jcs238790. [DOI] [PubMed] [Google Scholar]
- [59].Satoh T, Inagaki T, Liu Z, Watanabe R, Satoh AK, GPI biosynthesis is essential for rhodopsin sorting at the trans-Golgi network in Drosophila photoreceptors, Development 140(2) (2013) 385–394. [DOI] [PubMed] [Google Scholar]
- [60].Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M, The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase, Journal of Cell Biology 110(1) (1990) 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cereijido M, Contreras RG, Shoshani L, Larre I, The Na+-K+-ATPase as self-adhesion molecule and hormone receptor, Am J Physiol Cell Physiol 302(3) (2012) C473–81. [DOI] [PubMed] [Google Scholar]
- [62].Shoshani L, Contreras RG, Roldán ML, Moreno J, Lázaro A, Balda MS, Matter K, Cereijido M, The Polarized Expression of Na+,K+-ATPase in Epithelia Depends on the Association between β-Subunits Located in Neighboring Cells, Molecular Biology of the Cell 16(3) (2004) 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I, Tight junction and polarity interaction in the transporting epithelial phenotype, Biochimica et Biophysica Acta (BBA) - Biomembranes 1778(3) (2008) 770–793. [DOI] [PubMed] [Google Scholar]
- [64].McNeill H, Ozawa M, Kemler R, Nelson WJ, Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity, Cell 62(2) (1990) 309–316. [DOI] [PubMed] [Google Scholar]
- [65].Padilla-Benavides T, Roldán ML, Larre I, Flores-Benitez D, Villegas-Sepúlveda N, Contreras RG, Cereijido M, Shoshani L, The polarized distribution of Na+,K+-ATPase: role of the interaction between {beta} subunits, Mol Biol Cell 21(13) (2010) 2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wilson PD, Devuyst O, Li X, Gatti L, Falkenstein D, Robinson S, Fambrough D, Burrow CR, Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform, Am J Pathol 156(1) (2000) 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vagin O, Turdikulova S, Sachs G, Recombinant addition of N-glycosylation sites to the basolateral Na,K-ATPase beta1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells, J Biol Chem 280(52) (2005) 43159–67. [DOI] [PubMed] [Google Scholar]
- [68].Lobato-Alvarez JA, Roldan ML, Lopez-Murillo TD, Gonzalez-Ramirez R, Bonilla-Delgado J, Shoshani L, The Apical Localization of Na(+), K(+)-ATPase in Cultured Human Retinal Pigment Epithelial Cells Depends on Expression of the beta2 Subunit, Front Physiol 7 (2016) 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Palmgren MG, Nissen P, P-Type ATPases, Annual Review of Biophysics 40(1) (2011) 243–266. [DOI] [PubMed] [Google Scholar]
- [70].Huang Y, Takar M, Best JT, Graham TR, Conserved mechanism of phospholipid substrate recognition by the P4-ATPase Neo1 from Saccharomyces cerevisiae, Biochim Biophys Acta Mol Cell Biol Lipids 1865(2) (2020) 158581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cereijido M, Contreras RG, Shoshani L, Cell Adhesion, Polarity, and Epithelia in the Dawn of Metazoans, Physiological Reviews 84(4) (2004) 1229–1262. [DOI] [PubMed] [Google Scholar]
- [72].Nejsum LN, Nelson WJ, A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity, Journal of Cell Biology 178(2) (2007) 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim N-H, Lee A-Y, Reduced Aquaporin3 Expression and Survival of Keratinocytes in the Depigmented Epidermis of Vitiligo, Journal of Investigative Dermatology 130(9) (2010) 2231–2239. [DOI] [PubMed] [Google Scholar]
- [74].Login FH, Jensen HH, Pedersen GA, Koffman JS, Kwon T-H, Parsons M, Nejsum LN, Aquaporins differentially regulate cell-cell adhesion in MDCK cells, The FASEB Journal 33(6) (2019) 6980–6994. [DOI] [PubMed] [Google Scholar]
- [75].Tokhtaeva E, Sachs G, Souda P, Bassilian S, Whitelegge JP, Shoshani L, Vagin O, Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β₁ subunits, J Biol Chem 286(29) (2011) 25801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BLM, Bennett V, Ankyrin-G Is a Molecular Partner of E-cadherin in Epithelial Cells and Early Embryos *, J. Biol. Chem 282(36) (2007) 26552–26561. [DOI] [PubMed] [Google Scholar]
- [77].Vasquez CG, Vachharajani VT, Garzon-Coral C, Dunn AR, Physical basis for the determination of lumen shape in a simple epithelium, Nat Commun 12(1) (2021) 5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Saint-Criq V, Gray MA, Role of CFTR in epithelial physiology, Cellular and Molecular Life Sciences 74(1) (2017) 93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Castellani C, Assael BM, Cystic fibrosis: a clinical view, Cellular and Molecular Life Sciences 74(1) (2017) 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sarmah B, Latimer AJ, Appel B, Wente SR, Inositol polyphosphates regulate zebrafish left-right asymmetry, Dev Cell 9(1) (2005) 133–45. [DOI] [PubMed] [Google Scholar]
- [81].Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ, Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut, Development 132(6) (2005) 1247–1260. [DOI] [PubMed] [Google Scholar]
- [82].Navis A, Marjoram L, Bagnat M, Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish, Development 140(8) (2013) 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rathbun LI, Colicino EG, Manikas J, O’Connell J, Krishnan N, Reilly NS, Coyne S, Erdemci-Tandogan G, Garrastegui A, Freshour J, Santra P, Manning ML, Amack JD, Hehnly H, Cytokinetic bridge triggers de novo lumen formation in vivo, Nat Commun 11(1) (2020) 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA, PDZ Domain Interaction Controls the Endocytic Recycling of the Cystic Fibrosis Transmembrane Conductance Regulator*, J. Biol. Chem 277(42) (2002) 40099–40105. [DOI] [PubMed] [Google Scholar]
- [85].Bidaud-Meynard A, Bossard F, Schnúr A, Fukuda R, Veit G, Xu H, Lukacs GL, Transcytosis maintains CFTR apical polarity in the face of constitutive and mutation-induced basolateral missorting, Journal of Cell Science 132(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Singh AK, Riederer B, Krabbenhöft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U, Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice, J Clin Invest 119(3) (2009) 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moyer BD, Denton J, Karlson KH, Reynolds D, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M, Stanton BA, A PDZ-interacting domain in CFTR is an apical membrane polarization signal, Journal of Clinical Investigation 104(10) (1999) 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang S, Yue H, Derin RB, Guggino WB, Li M, Accessory Protein Facilitated CFTR-CFTR Interaction, a Molecular Mechanism to Potentiate the Chloride Channel Activity, Cell 103(1) (2000) 169–179. [DOI] [PubMed] [Google Scholar]
- [89].Holleran JP, Zeng J, Frizzell RA, Watkins SC, Regulated recycling of mutant CFTR is partially restored by pharmacological treatment, Journal of Cell Science 126(12) (2013) 2692–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL, Regulation of plasma membrane recycling by CFTR, Science 256(5056) (1992) 530–2. [DOI] [PubMed] [Google Scholar]
- [91].Berger HA, Anderson MP, Gregory RJ, Thompson S, Howard PW, Maurer RA, Mulligan R, Smith AE, Welsh MJ, Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel, J Clin Invest 88(4) (1991) 1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bagnat M, Navis A, Herbstreith S, Brand-Arzamendi K, Curado S, Gabriel S, Mostov K, Huisken J, Stainier DY, Cse1l is a negative regulator of CFTR-dependent fluid secretion, Curr Biol 20(20) (2010) 1840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jakab RL, Collaco AM, Ameen NA, Characterization of CFTR High Expresser cells in the intestine, Am J Physiol Gastrointest Liver Physiol 305(6) (2013) G453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, Rajagopal J, A revised airway epithelial hierarchy includes CFTR-expressing ionocytes, Nature 560(7718) (2018) 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, Jaffe AB, A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte, Nature 560(7718) (2018) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Inokuchi M, Nakamura M, Miyanishi H, Hiroi J, Kaneko T, Functional classification of gill ionocytes and spatiotemporal changes in their distribution after transfer from seawater to freshwater in Japanese seabass, J Exp Biol 220(Pt 24) (2017) 4720–4732. [DOI] [PubMed] [Google Scholar]
- [97].Hsu HH, Lin LY, Tseng YC, Horng JL, Hwang PP, A new model for fish ion regulation: identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes), Cell Tissue Res 357(1) (2014) 225–43. [DOI] [PubMed] [Google Scholar]
- [98].Kim K, Lane EA, Saftien A, Wang H, Xu Y, Wirtz-Peitz F, Perrimon N, Drosophila as a model for studying cystic fibrosis pathophysiology of the gastrointestinal system, Proc Natl Acad Sci U S A 117(19) (2020) 10357–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hayashi S, Kondo T, Development and Function of the Drosophila Tracheal System, Genetics 209(2) (2018) 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Behr M, Riedel D, Schuh R, The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila, Dev Cell 5(4) (2003) 611–20. [DOI] [PubMed] [Google Scholar]
- [101].Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ, Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control, Journal of Cell Biology 164(2) (2004) 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Genova JL, Fehon RG, Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila, Journal of Cell Biology 161(5) (2003) 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Paul SM, Palladino MJ, Beitel GJ, A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control, Development 134(1) (2007) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang S, Jayaram SA, Hemphälä J, Senti KA, Tsarouhas V, Jin H, Samakovlis C, Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea, Curr Biol 16(2) (2006) 180–5. [DOI] [PubMed] [Google Scholar]
- [105].Moussian B, Schwarz H, Bartoszewski S, Nüsslein-Volhard C, Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster, Journal of Morphology 264(1) (2005) 117–130. [DOI] [PubMed] [Google Scholar]
- [106].Araújo SJ, Aslam H, Tear G, Casanova J, mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development—Analysis of its role in Drosophila tracheal morphogenesis, Developmental Biology 288(1) (2005) 179–193. [DOI] [PubMed] [Google Scholar]
- [107].Luschnig S, Bätz T, Armbruster K, Krasnow MA, serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila, Curr Biol 16(2) (2006) 186–94. [DOI] [PubMed] [Google Scholar]
- [108].Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C, Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila, Dev Cell 13(2) (2007) 214–25. [DOI] [PubMed] [Google Scholar]
- [109].Forster D, Armbruster K, Luschnig S, Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila, Curr Biol 20(1) (2010) 62–8. [DOI] [PubMed] [Google Scholar]
- [110].Luschnig S, Uv A, Luminal matrices: an inside view on organ morphogenesis, Exp Cell Res 321(1) (2014) 64–70. [DOI] [PubMed] [Google Scholar]
- [111].Öztürk-Çolak A, Moussian B, Araújo SJ, Casanova J, A feedback mechanism converts individual cell features into a supracellular ECM structure in Drosophila trachea, eLife 5 (2016) e09373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dong B, Hannezo E, Hayashi S, Balance between apical membrane growth and luminal matrix resistance determines epithelial tubule shape, Cell Rep 7(4) (2014) 941–50. [DOI] [PubMed] [Google Scholar]
- [113].Husain N, Pellikka M, Hong H, Klimentova T, Choe KM, Clandinin TR, Tepass U, The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina, Dev Cell 11(4) (2006) 483–93. [DOI] [PubMed] [Google Scholar]
- [114].Abrams EW, Mihoulides WK, Andrew DJ, Fork head and Sage maintain a uniform and patent salivary gland lumen through regulation of two downstream target genes, PH4alphaSG1 and PH4alphaSG2, Development 133(18) (2006) 3517–27. [DOI] [PubMed] [Google Scholar]
- [115].Syed ZA, Bouge AL, Byri S, Chavoshi TM, Tang E, Bouhin H, van Dijk-Hard IF, Uv A, A luminal glycoprotein drives dose-dependent diameter expansion of the Drosophila melanogaster hindgut tube, PLoS Genet 8(8) (2012) e1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, Gilmour D, Luminal signalling links cell communication to tissue architecture during organogenesis, Nature 515(7525) (2014) 120–4. [DOI] [PubMed] [Google Scholar]
- [117].Gill HK, Cohen JD, Ayala-Figueroa J, Forman-Rubinsky R, Poggioli C, Bickard K, Parry JM, Pu P, Hall DH, Sundaram MV, Integrity of Narrow Epithelial Tubes in the C. elegans Excretory System Requires a Transient Luminal Matrix, PLoS Genet 12(8) (2016) e1006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Cohen JD, Sparacio AP, Belfi AC, Forman-Rubinsky R, Hall DH, Maul-Newby H, Frand AR, Sundaram MV, A multi-layered and dynamic apical extracellular matrix shapes the vulva lumen in Caenorhabditis elegans, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Strilic B, Eglinger J, Krieg M, Zeeb M, Axnick J, Babal P, Muller DJ, Lammert E, Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels, Curr Biol 20(22) (2010) 2003–9. [DOI] [PubMed] [Google Scholar]
- [120].Yang Z, Zimmerman SE, Tsunezumi J, Braitsch C, Trent C, Bryant DM, Cleaver O, Gonzalez-Manchon C, Marciano DK, Role of CD34 family members in lumen formation in the developing kidney, Dev Biol 418(1) (2016) 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Román-Fernández Á, Roignot J, Sandilands E, Nacke M, Mansour MA, McGarry L, Shanks E, Mostov KE, Bryant DM, The phospholipid PI(3,4)P(2) is an apical identity determinant, Nat Commun 9(1) (2018) 5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Overeem AW, Bryant DM, van ISC, Mechanisms of apical-basal axis orientation and epithelial lumen positioning, Trends Cell Biol 25(8) (2015) 476–85. [DOI] [PubMed] [Google Scholar]
- [123].Jewett CE, Prekeris R, Insane in the apical membrane: Trafficking events mediating apicobasal epithelial polarity during tube morphogenesis, Traffic (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, Harada A, Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis, J Cell Sci 127(Pt 2) (2014) 422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, Harada R, Harada A, The Rab8 GTPase regulates apical protein localization in intestinal cells, Nature 448(7151) (2007) 366–9. [DOI] [PubMed] [Google Scholar]
- [126].Sobajima T, Yoshimura S.-i., Iwano T, Kunii M, Watanabe M, Atik N, Mushiake S, Morii E, Koyama Y, Miyoshi E, Harada A, Rab11a is required for apical protein localisation in the intestine, Biology Open 4(1) (2015) 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Knowles BC, Weis VG, Yu S, Roland JT, Williams JA, Alvarado GS, Lapierre LA, Shub MD, Gao N, Goldenring JR, Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes, Journal of Cell Science 128(8) (2015) 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Weis VG, Knowles BC, Choi E, Goldstein AE, Williams JA, Manning EH, Roland JT, Lapierre LA, Goldenring JR, Loss of MYO5B in Mice Recapitulates Microvillus Inclusion Disease and Reveals an Apical Trafficking Pathway Distinct to Neonatal Duodenum, Cellular and Molecular Gastroenterology and Hepatology 2(2) (2015) 131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Levic DS, Ryan S, Marjoram L, Honeycutt J, Bagwell J, Bagnat M, Distinct roles for luminal acidification in apical protein sorting and trafficking in zebrafish, J Cell Biol 219(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V, Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis, Development 139(11) (2012) 2071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Shafaq-Zadah M, Brocard L, Solari F, Michaux G, AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine, Development 139(11) (2012) 2061–2070. [DOI] [PubMed] [Google Scholar]
- [132].Delacour D, Koch A, Ackermann W, Parco IE-L, Elsässer H-P, Poirier F, Jacob R, Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo, Journal of Cell Science 121(4) (2008) 458–465. [DOI] [PubMed] [Google Scholar]
- [133].Javitt G, Khmelnitsky L, Albert L, Bigman LS, Elad N, Morgenstern D, Ilani T, Levy Y, Diskin R, Fass D, Assembly Mechanism of Mucin and von Willebrand Factor Polymers, Cell 183(3) (2020) 717–729 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chen J, Sayadian AC, Lowe N, Lovegrove HE, St Johnston D, An alternative mode of epithelial polarity in the Drosophila midgut, PLoS Biol 16(10) (2018) e3000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ryan S, Willer J, Marjoram L, Bagwell J, Mankiewicz J, Leshchiner I, Goessling W, Bagnat M, Katsanis N, Rapid identification of kidney cyst mutations by whole exome sequencing in zebrafish, Development 140(21) (2013) 4445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Mittal N, Yoon SH, Enomoto H, Hiroshi M, Shimizu A, Kawakami A, Fujita M, Watanabe H, Fukuda K, Makino S, Versican is crucial for the initiation of cardiovascular lumen development in medaka (Oryzias latipes), Scientific Reports 9(1) (2019) 9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Levic DS, Yamaguchi N, Wang S, Knaut H, Bagnat M, Knock-in tagging in zebrafish facilitated by insertion into non-coding regions, Development 148(19) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Seleit A, Aulehla A, Paix A, Endogenous protein tagging in medaka using a simplified CRISPR/Cas9 knock-in approach, bioRxiv (2021) 2021.07.29.454295. [DOI] [PMC free article] [PubMed] [Google Scholar]


