Abstract
Background/Aims:
The extent to which nonalcoholic fatty liver disease (NAFLD) contributes to hepatocellular carcinoma (HCC) prevalence in contemporary practices and whether there are any etiologic differences in surveillance receipt, tumor stage, and overall survival (OS) remains unclear. We aimed to estimate the burden of NAFLD-related HCC and magnitude of associations with surveillance receipt, clinical presentation, and outcomes in a contemporary HCC cohort.
Methods:
In a cohort of HCC patients from the SEER-Medicare database between 2011 and 2015, we used multivariable logistic regression to identify factors associated with surveillance receipt, early-stage tumor detection, and curative treatment. Cox regression was used to identify factors associated with OS.
Results:
Among 5,098 HCC patients, NAFLD was the leading etiology, accounting for 1,813 (35.6%) of cases. Compared to those with hepatitis C-related HCC, NAFLD was associated with lower HCC surveillance receipt (adjusted odds ratio [aOR] 0.31, 95% confidence interval [CI] 0.23–0.40), lower early-stage HCC detection (aOR 0.49, 95%CI 0.40 – 0.60) and modestly worse OS (adjusted hazard ratio [aHR] 1.20, 95%CI 1.09 – 1.32). NAFLD subgroup analysis showed that early-stage HCC, absence of ascites/hepatic encephalopathy, surveillance and curative treatment receipt were associated with improved OS. NAFLD patients with coexisting liver disease were more likely to have surveillance, early-stage detection, and curative treatment, improved OS than NAFLD patients without coexisting liver diseases.
Conclusion:
NAFLD is the leading etiology of HCC among Medicare beneficiaries. Compared to other etiologies, NAFLD was associated with lower HCC surveillance receipt, early-stage detection, and modestly poorer survival. Multi-faceted interventions for improving surveillance uptake are needed to improve prognosis of patients with NAFLD-related HCC.
Keywords: Fatty liver, NASH, NAFLD, liver cancer, HCC, survival, surveillance
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world.1 The prevalence of NAFLD is over 30% in the United States (US) and its burden continues to rise in parallel with the increasing prevalence of obesity and metabolic syndrome.2 Patients with NAFLD are at increased risk for hepatocellular carcinoma (HCC).3 With a rising prevalence of NAFLD, it is recognized as an increasingly important etiology of HCC in the US,4, 5 and it is the fastest-growing etiology of HCC among liver transplant recipients.6 In a previous analysis of the Surveillance, Epidemiology and End Results (SEER) Medicare database, only 14% of all HCC diagnosed between 2004 and 2009 were attributed to NAFLD.7 However, the burden of NAFLD-associated HCC has likely increased more recently, given the steady increase in the burden of NAFLD in the general population, particularly in states with a higher prevalence of obesity.8
Currently, the extent to which NAFLD plays a role in the etiology HCC remains unclear in the US general population. Further, previous small cohort studies have suggested that patients with NAFLD-associated HCC have lower odds of cancer detection via surveillance.9, 10 This is in part due to underrecognition of cirrhosis in patients with NAFLD, resulting in a higher proportion being diagnosed at later stages resulting in worse survival.7, 9, 11 However, contemporary population-based data on NAFLD-associated HCC and its association with surveillance receipt, tumor stage and overall survival (OS) are limited. Therefore, we aimed to estimate the magnitude of NAFLD-associated HCC and etiologic differences in surveillance receipt, clinical presentation and outcomes in a population-based US cohort.
METHODS
Study Population
We conducted a population-based cohort study using the SEER-Medicare database. The linked SEER-Medicare database combines demographic, clinical, and survival information for patients with cancer from the SEER program of cancer registries with Medicare claims information on covered health services from the time of Medicare eligibility until death.12, 13 The SEER program collects data on incident cancer cases from 18 cancer registries, including state, central, metropolitan, and the Alaska Native registries, which cover 28% of the US.12, 14, 15 Medicare is the primary health insurer for approximately 94% of individuals ages 65 years and older, and roughly 90% of Medicare beneficiaries are covered by both Part A (inpatient hospitalizations, skilled nursing facility stays, home health visits, and hospice care) and Part B (outpatient visits and physician office visits/services) benefits.16
We included all Medicare beneficiaries, aged 68 years and older, who have been diagnosed with HCC (International Classification of Diseases [ICD]-Oncology-3 codes, site: C22.0 AND histology: 8170–8175) from 2011 to 2015 (Supplementary Figure 1). We limited the cohort to individuals aged 68 years and older to ensure 3 years of follow-up to allow sufficient time after Medicare enrollment for identification of risk factors and surveillance receipt. We excluded: 1) HCC cases ascertained by direct visualization without microscopic confirmation, or death certificate only; 2) patients with Medicare Part A and B enrollment fewer than 3 years; 3) patients with less than 6 months follow-up time after HCC diagnosis to ensure complete capture of HCC-directed treatment; and 4) patients enrolled in Medicare health maintenance organization (HMO) plans as these plans were not required to submit individual claims information for services to the Centers for Medicare and Medicaid Services.
Study variables
Variables of interest included sex, age, race/ethnicity (defined as non-Hispanic White, Black, Asian/Pacific Islander [API]/Others, and Hispanic), etiology of HCC, extent of tumor, types of HCC treatment, National Cancer Institute (NCI) comorbidity index, the presence of diabetes, cirrhosis, ascites, and hepatic encephalopathy, surveillance for HCC, SEER region stratified by census tract poverty level, metropolitan/nonmetropolitan counties.
To define liver disease etiology, Medicare claims ICD, 9th revision or 10th revision codes were used for hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic liver disease (ALD), NAFLD, and others (see the ICD-9 and 10 codes for each etiology in Supplementary Table 1). As NAFLD is often under-coded, patients with ICD-9 or 10 codes for obesity, type 2 diabetes, history of bariatric surgery, or both dyslipidemia and hypertension in the absence of HBV, HCV, alcohol abuse, and other known liver disease were also classified as NAFLD. For patients with multiple etiologies, etiology was classified with the following hierarchy (HCV >HBV >ALD >others >NAFLD).
Cirrhosis was defined based on ICD-9 or 10 codes from Medicare claims (Supplementary Table 1).17 We used diagnosis and procedure codes one year before HCC diagnosis to calculate the NCI Comorbidity Index as a measure of noncancer comorbidity (Supplemental methods).18 The NCI Comorbidity index was calculated after excluding liver conditions and diabetes to avoid collinearity in multivariable models.
Tumor characteristics were extracted from the SEER Patient Entitlement and Diagnosis Summary File. As SEER only provides the number of tumor nodules as a binary variable (unifocal vs. multifocal), we defined early-stage HCC as a single tumor, less than or equal to 5 cm in diameter without vascular invasion or extrahepatic metastasis.
We characterized receipt of HCC surveillance into 3 mutually exclusive categories during the 3-year period before HCC diagnosis: (1) consistent surveillance, (2) inconsistent surveillance, and (3) no surveillance according to our previous publication.19 Consistent screening was defined as having one or more abdominal ultrasound, computerized tomography (CT), or magnetic resonance imaging (MRI) per calendar year, and inconsistent screening was defined as having one or more abdominal imaging during the study period, but less than annually. Receipt of abdominal ultrasound (76700 or 76705), CT (74160, 74170, or 74177), or MRI (74182 or 74183) was identified using the Current Procedural Terminology codes.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized by standard descriptive measures (frequency and percentage for categorical variables and mean ± standard deviation for continuous variables). These characteristics were then compared using the Pearson’s chi-square test for categorical variables and the Welch’s t-test or the Mann-Whitney-Wilcoxon test for continuous variables as appropriate.
Factors associated with (1) surveillance receipt, (2) early-stage HCC at diagnosis, and (3) curative treatment receipt were identified using univariate and multivariable logistic regression. Patients who had no follow-up period after diagnosis or died during the same calendar month of HCC diagnosis (0 month follow-up) were excluded from survival analyses (n=710). Survival probabilities were estimated using the Kaplan-Meier method and compared using the log-rank test. Factors associated with OS were determined using univariate and multivariable Cox-proportional hazards regression. Etiology of liver disease and demographic variables (except age at diagnosis) satisfied the proportional hazard assumption as demonstrated by the Schoenfeld residuals test. Subgroup analysis was performed to assess the factors associated with clinical outcomes in patients with NAFLD. We first performed this analysis using a strict definition of NAFLD alone and then used a broader, more inclusive definition of NAFLD in which patients were allowed to have coexisting liver diseases. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) and Stata 16.1 (StataCorp, College Station, TX, USA) software with two-sided tests and a significance level of 0.05.
RESULTS
Patient Characteristics
Of 5,098 HCC patients, NAFLD was the leading etiology, accounting for 35.6% of cases (Table 1). About two-thirds of patients were male and the mean age was 76.8 years. Compared to non-NAFLD patients, those with NAFLD were older. Overall, 74.8% of patients with HCC had cirrhosis. The proportion of patients with cirrhosis varied by etiology, with a higher proportion among patients with ALD (90.4%) and HCV (89.0%) and a lower proportion in those with HBV (80.9%), NAFLD (57.9%), and other/no (53.9%) etiologies.
Table 1.
Demographic, clinical and treatment characteristics of HCC patients.
| Characteristics | Total (n= 5,098) | NAFLD (n= 1,813) | HCV (n= 1,715) | ALD (n= 895) | HBV (n= 246) | Others/None (n= 429) | P Value |
|---|---|---|---|---|---|---|---|
| Male Sex , n (%) | 3,438 (67.44) | 1,177 (64.92) | 1,088 (63.44) | 742 (82.91) | 166 (67.48) | 265 (61.77) | <0.001 |
| Age, mean (SD) | 76.75 (6.23) | 78.63 (6.34) | 75.10 (5.69) | 75.42 (5.37) | 75.98 (5.93) | 78.66 (6.94) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | ||||||
| Non-Hispanic White | 3,224 (63.24) | 1,331 (73.41) | 848 (49.45) | 663 (74.08) | 52 (21.14) | 330 (76.92) | |
| Non-Hispanic Black | 390 (7.65) | 71 (3.92) | 240 (13.99) | 46 (5.14) | ** | ** | |
| Non-Hispanic API/Others | 810 (15.89) | 204 (11.25) | 372 (21.69) | 35 (3.91) | 161 (65.45) | 38 (8.86) | |
| Hispanic | 674 (13.22) | 207 (11.42) | 255 (14.87) | 151 (16.87) | ** | ** | |
| Census Poverty Level, n (%) | 0.001 | ||||||
| 0% to <5% poverty | 1,011 (19.83) | 364 (20.08) | 312 (18.19) | 186 (20.78) | 53 (21.54) | 96 (22.38) | |
| 5% to <10% poverty | 1,192 (23.38) | 449 (24.77) | 359 (20.93) | 237 (26.48) | 54 (21.95) | 93 (21.68) | |
| 10% to <20% poverty | 1,560 (30.6) | 569 (31.38) | 535 (31.2) | 239 (26.7) | 73 (29.67) | 144 (33.57) | |
| 20% to 100% poverty | 1,335 (26.19) | 431 (23.77) | 509 (29.68) | 233 (26.03) | 66 (26.83) | 96 (22.38) | |
| Rural-Urban Counties, n (%) | <0.001 | ||||||
| Metro > 1 million | 3,029 (59.42) | 993 (54.77) | 1,143 (66.65) | 504 (56.31) | 181 (73.58) | 208 (48.48) | |
| Metro 250k to1 million | 1,028 (20.16) | 380 (20.96) | 321 (18.72) | 190 (21.23) | 42 (17.07) | 95 (22.14) | |
| Metro < 250k | 414 (8.12) | 165 (9.1) | 110 (6.41) | 87 (9.72) | ** | ** | |
| Non-Metro/Rural | 627 (12.3) | 275 (15.17) | 141 (8.22) | 114 (12.74) | ** | ** | |
| NCI comorbidity index, n (%) | <0.001 | ||||||
| Low (0 to 2) | 3,842 (75.36) | 1,274 (70.27) | 1,349 (78.66) | 652 (72.85) | 209 (84.96) | 358 (83.45) | |
| Moderate (>2 to 4) | 725 (14.22) | 301 (16.60) | 214 (12.48) | 142 (15.87) | 24 (9.76) | 44 (10.26) | |
| High (>4) | 531 (10.42) | 238 (13.13) | 152 (8.86) | 101 (11.28) | 13 (5.28) | 27 (6.29) | |
| Diabetes , n (%) | 3,512 (68.89) | 1,533 (84.56) | 1,054 (61.46) | 643 (71.84) | 157 (63.82) | 125 (29.14) | <0.001 |
| Cirrhosis, n (%) | 3,815 (74.83) | 1,050 (57.92) | 1,526 (88.98) | 809 (90.39) | 199 (80.89) | 231 (53.85) | <0.001 |
| Ascites, n (%) | 2,213 (43.41) | 631 (34.8) | 817 (47.64) | 527 (58.88) | 106 (43.09) | 132 (30.77) | <0.001 |
| Hepatic encephalopathy, n (%) | 875 (17.16) | 181 (9.98) | 369 (21.52) | 246 (27.49) | 33 (13.41) | 46 (10.72) | <0.001 |
| Early-stage HCC*, n (%) | 967 (18.97) | 235 (12.96) | 420 (24.49) | 180 (20.11) | 61 (24.8) | 71 (16.55) | <0.001 |
| Treatment type, n (%) | <0.001 | ||||||
| Curative treatment | |||||||
| Tumor ablation | 589 (11.55) | 120 (6.62) | 292 (17.03) | 105 (11.73) | 35 (14.23) | 37 (8.62) | |
| Liver resection | 467 (9.16) | 182 (10.04) | 132 (7.7) | 57 (6.37) | 49 (19.92) | 47 (10.96) | |
| Liver transplant | 111 (2.18) | 20 (1.1) | 57 (3.32) | 20 (2.23) | ** | ** | |
| Non-curative Treatment | |||||||
| Chemoembolization | 777 (15.24) | 238 (13.13) | 312 (18.19) | 151 (16.87) | 38 (15.45) | 38 (8.86) | |
| Radioembolization | 225 (4.41) | 71 (3.92) | 74 (4.31) | 43 (4.8) | 15 (6.1) | 22 (5.13) | |
| Other Radiation | 132 (2.59) | 51 (2.81) | 46 (2.68) | 24 (2.68) | ** | ** | |
| Systemic treatment | 675 (13.24) | 227 (12.52) | 250 (14.58) | 114 (12.74) | 35 (14.23) | 49 (11.42) | |
| Others/best supportive care | 2122 (41.62) | 904 (49.86) | 552 (32.19) | 381 (42.57) | 62 (25.2) | 223 (51.98) |
ALD, alcoholic liver disease; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Single & ≤5 cm tumor without vascular invasion or metastasis.
Cells with less than 11 patients were suppressed. To avoid retrieval of number in the suppressed cells from the column/row totals, cells in another category with the lowest numbers were also suppressed.
NAFLD etiology is associated with lower HCC surveillance
Approximately two-thirds of patients (64.6%) had any surveillance (inconsistent or consistent), but only 15.2% of patients had consistent surveillance. Surveillance receipt varied by etiology and was less frequent in NAFLD (Figure 1A). This proportion increased when the analysis was restricted to the subgroup of patients with cirrhosis, with 70.4% of patients receiving surveillance, but surveillance receipt was still lowest in patients with NAFLD (Figure 1B). Among those who had any surveillance, 67.3% had at least one or more CT or MRI as a surveillance test. The proportion who had CT or MRI imaging were similar between NAFLD and non-NAFLD (64.9% vs. 68.3%, P=0.06). NAFLD was associated with a lower likelihood of consistent HCC surveillance receipt (reference: HCV) in multivariable (adjusted OR [aOR]: 0.22, 95% CI: 0.17–0.28) analyses (Table 2). Compared to HCV, NAFLD remained associated with a lower likelihood of any HCC surveillance receipt (consistent or inconsistent) in multivariable (aOR: 0.37, 95% CI: 0.32–0.44) analyses (Table 2). In subgroup analyses, results remained consistent when restricted to the subgroup of patients with cirrhosis (Supplementary Table 2).
Figure 1. HCC surveillance receipt by etiology.
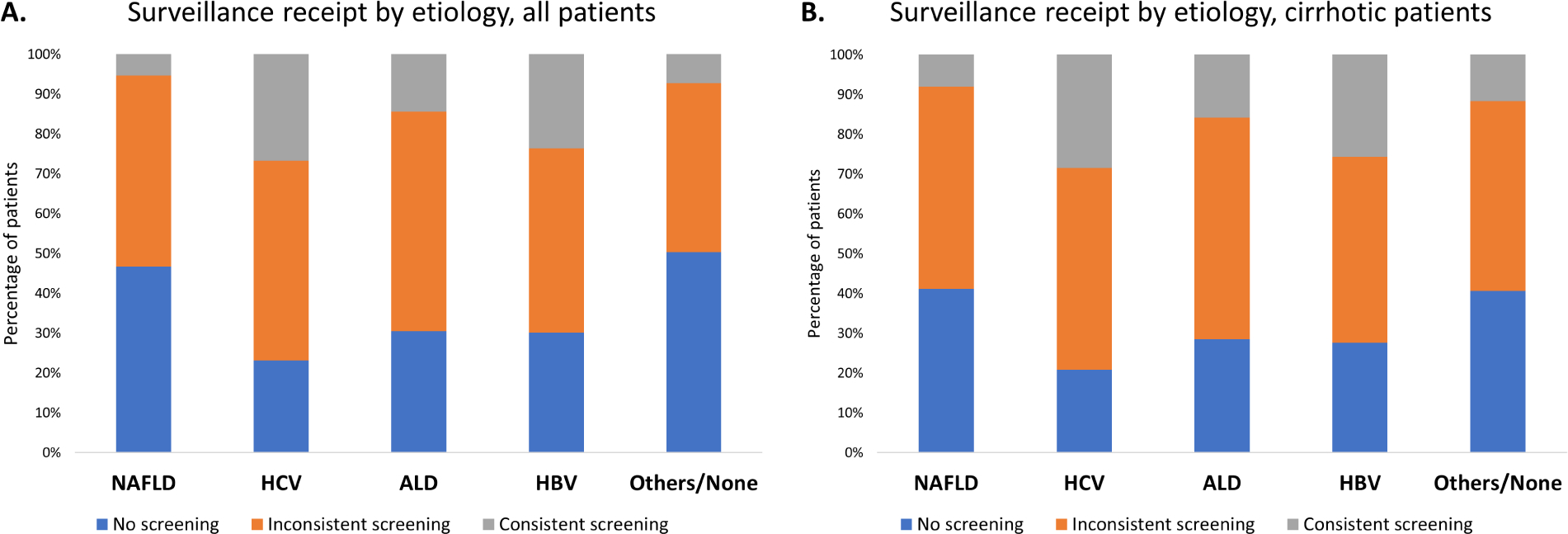
(A) All HCC patients.
(B) HCC patients with cirrhosis.
A low proportion of individuals with NAFLD-associated HCC had surveillance.
ALD, alcoholic liver disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Table 2.
Factors associated with receipt of HCC surveillance
| Consistent Surveillance | Any (Consistent or Inconsistent) Surveillance | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |||||
| Characteristics | OR (95% CI) | P value | aOR (95% CI) | P value | OR (95% CI) | P value | aOR (95% CI) | P value |
| Female Sex (Ref. Male) | 1.82 (1.56 to 2.13) | <0.001 | 1.89 (1.59 to 2.24) | <0.001 | 1.41 (1.25 to 1.60) | <0.001 | 1.51 (1.32 to 1.73) | <0.001 |
| Age | 0.95 (0.93 to 0.96) | <0.001 | 0.97 (0.95 to 0.98) | <0.001 | 0.96 (0.95 to 0.97) | <0.001 | 0.98 (0.97 to 0.99) | <0.001 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | ref | ref | ref | ref | ref | ref | ref | ref |
| Non-Hispanic Black | 1.13 (0.84 to 1.54) | 0.42 | 0.74 (0.53 to 1.04) | 0.09 | 0.84 (0.68 to 1.04) | 0.12 | 0.61 (0.48 to 0.78) | <0.001 |
| Non-Hispanic API/Others | 2.24 (1.85 to 2.71) | <0.001 | 1.59 (1.26 to 1.99) | <0.001 | 1.39 (1.18 to 1.64) | <0.001 | 1.14 (0.94 to 1.38) | 0.19 |
| Hispanic | 1.59 (1.28 to 1.99) | <0.001 | 1.11 (0.87 to 1.42) | 0.39 | 1.52 (1.26 to 1.82) | <0.001 | 1.17 (0.96 to 1.42) | 0.13 |
| Census Poverty Level | ||||||||
| <5% | ref | ref | ref | Ref | ref | ref | ref | ref |
| 5% to <10% | 0.88 (0.70 to 1.11) | 0.28 | 0.91 (0.71 to 1.16) | 0.44 | 0.88 (0.74 to 1.06) | 0.17 | 0.87 (0.72 to 1.04) | 0.13 |
| 10% to <20% | 0.88 (0.71 to 1.09) | 0.23 | 0.85 (0.67 to 1.07) | 0.17 | 0.86 (0.72 to 1.01) | 0.07 | 0.80 (0.67 to 0.96) | 0.02 |
| 20% to 100% | 0.86 (0.69 to 1.07) | 0.18 | 0.83 (0.64 to 1.06) | 0.14 | 0.91 (0.77 to 1.08) | 0.30 | 0.85 (0.70 to 1.03) | 0.10 |
| Rural-Urban | ||||||||
| Metro > 1 million | ref | ref | ref | ref | ref | ref | ref | ref |
| Metro 250k to 1 million | 0.74 (0.60 to 0.90) | 0.003 | 0.83 (0.67 to 1.03) | 0.10 | 0.90 (0.77 to 1.04) | 0.15 | 0.99 (0.85 to 1.16) | 0.91 |
| Metro < 250k | 0.52 (0.37 to 0.73) | <0.001 | 0.67 (0.47 to 0.96) | 0.03 | 0.93 (0.75 to 1.15) | 0.49 | 1.10 (0.87 to 1.38) | 0.44 |
| Non-Metro/Rural | 0.54 (0.41 to 0.71) | <0.001 | 0.77 (0.57 to 1.04) | 0.09 | 0.72 (0.60 to 0.85) | <0.001 | 0.90 (0.74 to 1.09) | 0.29 |
| NCI comorbidity index | ||||||||
| Low (0 to 2) | ref | ref | ref | ref | ref | ref | ref | ref |
| Moderate (>2 to 4) | 0.77 (0.61 to 0.97) | 0.03 | 0.86 (0.67 to 1.11) | 0.25 | 1.30 (1.10 to 1.55) | 0.002 | 1.43 (1.19 to 1.71) | <0.001 |
| High (>4) | 0.82 (0.63 to 1.07) | 0.14 | 1.01 (0.76 to 1.35) | 0.92 | 1.37 (1.13 to 1.67) | 0.002 | 1.54 (1.25 to 1.90) | <0.001 |
| Etiology | ||||||||
| HCV | ref | ref | Ref | ref | ref | ref | ref | ref |
| NAFLD | 0.15 (0.12 to 0.19) | <0.001 | 0.22 (0.17 to 0.28) | <0.001 | 0.34 (0.30 to 0.40) | <0.001 | 0.37 (0.32 to 0.44) | <0.001 |
| ALD | 0.46 (0.37 to 0.57) | <0.001 | 0.53 (0.42 to 0.67) | <0.001 | 0.68 (0.57 to 0.82) | <0.001 | 0.67 (0.55 to 0.81) | <0.001 |
| HBV | 0.84 (0.62 to 1.15) | 0.29 | 0.77 (0.55 to 1.08) | 0.13 | 0.70 (0.52 to 0.94) | 0.02 | 0.70 (0.51 to 0.96) | 0.03 |
| Others/None | 0.21 (0.15 to 0.31) | <0.001 | 0.35 (0.24 to 0.53) | <0.001 | 0.30 (0.24 to 0.37) | <0.001 | 0.44 (0.34 to 0.56) | <0.001 |
| Diabetes | 1.09 (0.92 to 1.28) | 0.33 | 1.27 (1.05 to 1.53) | 0.01 | 1.43 (1.26 to 1.61) | <0.001 | 1.55 (1.35 to 1.79) | <0.001 |
| Cirrhosis | 6.04 (4.47 to 8.14) | <0.001 | 3.46 (2.49 to 4.81) | <0.001 | 2.64 (2.32 to 3.00) | <0.001 | 2.07 (1.75 to 2.46) | <0.001 |
| Ascites | 1.41 (1.21 to 1.64) | <0.001 | 0.78 (0.66 to 0.94) | 0.007 | 1.29 (1.15 to 1.45) | <0.001 | 0.69 (0.59 to 0.80) | <0.001 |
| Hepatic encephalopathy | 2.30 (1.93 to 2.75) | <0.001 | 1.68 (1.38 to 2.04) | <0.001 | 2.07 (1.75 to 2.45) | <0.001 | 1.43 (1.19 to 1.72) | <0.001 |
ALD, alcoholic liver disease; aOR, adjusted odds ratio; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute; OR, odds ratio; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
NAFLD is associated with lower early-stage HCC detection, curative therapy receipt, and worse overall survival
Overall, 19.0% of patients presented with early-stage HCC and 22.9% received potentially curative treatment. A lower proportion of patients with NAFLD had early-stage HCC (13.0%) and received potentially curative treatment (17.7%) (Table 1). In multivariable analyses, NAFLD was associated with lower early-stage HCC (aOR: 0.49, 95% CI: 0.40–0.60) and curative treatment receipt (aOR: 0.75, 95% CI: 0.62–0.91) compared to those with HCV-related HCC (Table 3).
Table 3.
Factors associated with early-stage detection and curative treatments among HCC patients.
| Early-stage HCC* | Curative treatments** | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |||||
| Characteristics | OR (95% CI) | P value | aOR (95% CI) | P value | OR (95% CI) | P value | aOR (95% CI) | P value |
| Female sex (Ref. Male) | 1.45 (1.25 to 1.68) | <0.001 | 1.52 (1.31 to 1.77) | <0.001 | 1.20 (1.05 to 1.38) | 0.009 | 1.22 (1.05 to 1.42) | 0.01 |
| Age | 0.97 (0.96 to 0.99) | <0.001 | 0.98 (0.97 to 1.00) | 0.008 | 0.93 (0.92 to 0.94) | <0.001 | 0.93 (0.92 to 0.94) | <0.001 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | ref | ref | ref | ref | ref | ref | ref | ref |
| Non-Hispanic Black | 0.69 (0.51 to 0.93) | 0.02 | 0.52 (0.37 to 0.72) | <0.001 | 0.62 (0.47 to 0.83) | 0.001 | 0.50 (0.36 to 0.68) | <0.001 |
| Non-Hispanic API/Others | 1.29 (1.07 to 1.56) | 0.008 | 0.98 (0.79 to 1.22) | 0.87 | 1.66 (1.40 to 1.96) | <0.001 | 1.28 (1.05 to 1.58) | 0.02 |
| Hispanic | 1.19 (0.97 to 1.46) | 0.10 | 1.01 (0.81 to 1.25) | 0.94 | 0.85 (0.69 to 1.05) | 0.13 | 0.74 (0.59 to 0.93) | 0.01 |
| Census Poverty Level | ||||||||
| <5% | ref | ref | ref | ref | ref | ref | ref | ref |
| 5% to <10% | 0.92 (0.75 to 1.14) | 0.46 | 0.94 (0.76 to 1.17) | 0.59 | 0.74 (0.61 to 0.89) | 0.002 | 0.76 (0.62 to 0.94) | 0.01 |
| 10% to <20% | 0.96 (0.78 to 1.17) | 0.67 | 0.97 (0.79 to 1.19) | 0.76 | 0.66 (0.55 to 0.79) | <0.001 | 0.65 (0.53 to 0.79) | <0.001 |
| 20% to 100% | 0.93 (0.75 to 1.14) | 0.46 | 0.98 (0.78 to 1.22) | 0.85 | 0.68 (0.57 to 0.83) | <0.001 | 0.72 (0.58 to 0.89) | 0.003 |
| Rural-Urban | ||||||||
| Metro > 1 million | ref | ref | ref | ref | ref | ref | ref | ref |
| Metro 250k to 1 million | 0.93 (0.78 to 1.12) | 0.44 | 0.96 (0.80 to 1.15) | 0.65 | 0.98 (0.83 to 1.16) | 0.83 | 1.02 (0.85 to 1.22) | 0.84 |
| Metro < 250k | 0.76 (0.57 to 1.00) | 0.048 | 0.82 (0.61 to 1.09) | 0.18 | 0.73 (0.56 to 0.94) | 0.02 | 0.88 (0.66 to 1.16) | 0.37 |
| Non-Metro/Rural | 0.74 (0.58 to 0.93) | 0.01 | 0.82 (0.64 to 1.05) | 0.11 | 0.68 (0.55 to 0.85) | 0.001 | 0.77 (0.60 to 0.98) | 0.04 |
| NCI comorbidity index | ||||||||
| Low (0 to 2) | ref | ref | ref | ref | ref | ref | ref | ref |
| Moderate (>2 to 4) | 0.73 (0.58 to 0.90) | 0.004 | 0.79 (0.63 to 0.99) | 0.04 | 0.66 (0.54 to 0.80) | <0.001 | 0.80 (0.64 to 0.99) | 0.04 |
| High (>4) | 0.91 (0.72 to 1.15) | 0.41 | 1.06 (0.83 to 1.35) | 0.65 | 0.40 (0.30 to 0.52) | <0.001 | 0.48 (0.36 to 0.64) | <0.001 |
| Etiology | ||||||||
| HCV | ref | ref | ref | ref | ref | ref | ref | ref |
| NAFLD | 0.46 (0.39 to 0.55) | <0.001 | 0.49 (0.40 to 0.60) | <0.001 | 0.55 (0.47 to 0.65) | <0.001 | 0.75 (0.62 to 0.91) | 0.003 |
| ALD | 0.78 (0.64 to 0.95) | 0.01 | 0.86 (0.69 to 1.06) | 0.15 | 0.65 (0.54 to 0.79) | <0.001 | 0.77 (0.62 to 0.95) | 0.02 |
| HBV | 1.02 (0.75 to 1.39) | 0.92 | 0.99 (0.71 to 1.37) | 0.96 | 1.53 (1.16 to 2.03) | 0.003 | 1.32 (0.96 to 1.81) | 0.09 |
| Others/None | 0.61 (0.46 to 0.81) | 0.001 | 0.68 (0.51 to 0.92) | 0.01 | 0.68 (0.53 to 0.88) | 0.003 | 0.90 (0.67 to 1.19) | 0.45 |
| Diabetes | 0.98 (0.84 to 1.13) | 0.75 | 1.13 (0.96 to 1.33) | 0.16 | 0.97 (0.85 to 1.12) | 0.72 | 1.17 (0.99 to 1.37) | 0.06 |
| Cirrhosis | 1.58 (1.33 to 1.88) | <0.001 | 1.54 (1.25 to 1.90) | <0.001 | 1.24 (1.06 to 1.45) | 0.006 | 1.28 (1.05 to 1.56) | 0.01 |
| Ascites | 0.76 (0.66 to 0.88) | <0.001 | 0.56 (0.47 to 0.66) | <0.001 | 0.62 (0.54 to 0.70) | <0.001 | 0.56 (0.48 to 0.66) | <0.001 |
| Hepatic encephalopathy | 1.12 (0.93 to 1.34) | 0.22 | 1.03 (0.85 to 1.26) | 0.74 | 0.76 (0.63 to 0.91) | 0.003 | 0.74 (0.60 to 0.91) | 0.004 |
| Early-stage HCC * | N/A | N/A | N/A | N/A | 3.64 (3.14 to 4.23) | <0.001 | 3.25 (2.77 to 3.81) | <0.001 |
ALD, alcoholic liver disease; aOR, adjusted odds ratio; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute; OR, odds ratio; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Single lesion ≤5 cm without vascular invasion or metastasis.
Local ablation, resection, and liver transplant.
The median OS of the entire cohort was 15 months (IQR: 6–39 months). Median OS was 12 (IQR: 5–32 months), 20 (IQR: 8–48 months), 24 (IQR: 8–66 months), 14 (IQR: 6–31 months), and 15 (IQR: 6–38 months) months for HCC patients with NAFLD, HCV, HBV, ALD, other/none etiology, respectively (P<0.001; Figure 2). NAFLD was associated with worse OS in univariate (hazard ratio: 1.37, 95% CI: 1.27–1.49) and multivariable analyses (adjusted hazard ratio: 1.20, 95% CI: 1.09–1.32). (Table 4). There was a consistent association between NAFLD etiology and worse survival across most subgroups, although survival appeared similar in subgroups with consistent surveillance, early-stage HCC, and those who underwent curative treatment (Supplementary Figure 2). In multivariable analysis, other factors associated with worse OS included older age, lower socioeconomic status, lack of HCC surveillance, advanced tumor stage, presence of ascites and hepatic encephalopathy, and noncurative treatment (Table 4).
Figure 2. Overall survival, stratified by HCC etiology.
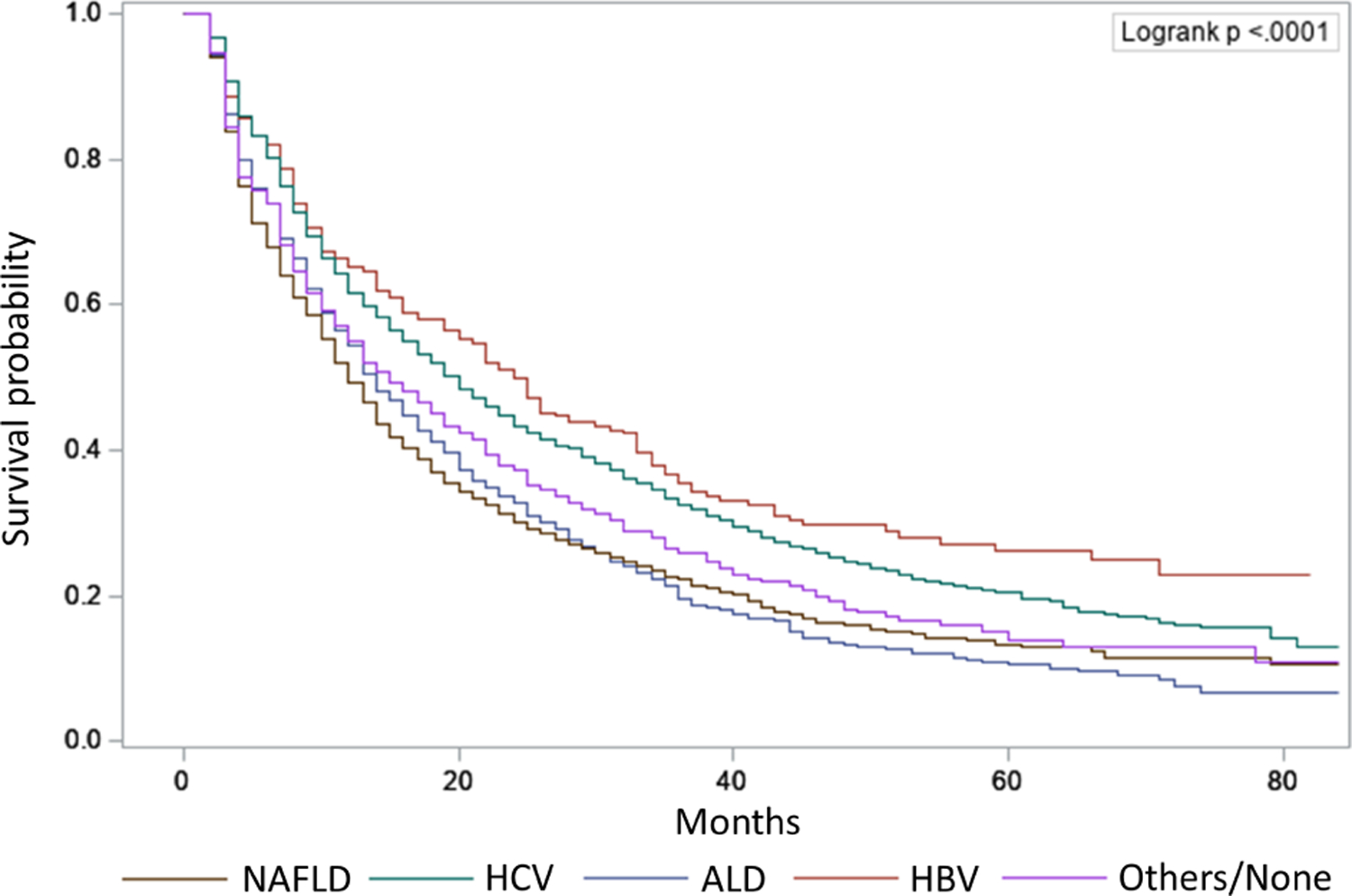
Patients with NAFLD had the shortest median overall survival among subgroups with different etiologies. ALD, alcoholic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Table 4.
Factors associated with overall survival among HCC patients.
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | P value | aHR (95% CI) | P value |
| Female Sex (Ref. Male) | 0.86 (0.80 to 0.92) | <0.001 | 0.93 (0.86 to 1.00) | 0.050 |
| Age | 1.03 (1.02 to 1.03) | <0.001 | 1.02 (1.01 to 1.02) | <0.001 |
| Race/ethnicity | ||||
| Non-Hispanic White | ref | ref | ref | ref |
| Non-Hispanic Black | 1.01 (0.89 to 1.15) | 0.86 | 0.98 (0.86 to 1.13) | 0.81 |
| Non-Hispanic API/Others | 0.65 (0.59 to 0.72) | <0.001 | 0.83 (0.74 to 0.92) | 0.001 |
| Hispanic | 0.94 (0.85 to 1.04) | 0.22 | 0.87 (0.79 to 0.97) | 0.009 |
| Census Poverty Level | ||||
| <5% | ref | Ref | ref | ref |
| 5% to <10% | 1.14 (1.03 to 1.26) | 0.01 | 1.05 (0.95 to 1.16) | 0.33 |
| 10% to <20% | 1.12 (1.02 to 1.24) | 0.02 | 1.05 (0.95 to 1.15) | 0.36 |
| 20% to 100% | 1.14 (1.03 to 1.26) | 0.009 | 1.11 (1.00 to 1.24) | 0.050 |
| Rural-Urban | ||||
| Metro > 1 million | Ref | ref | ref | ref |
| Metro 250k to 1 million | 1.15 (1.06 to 1.25) | 0.001 | 1.16 (1.06 to 1.26) | 0.001 |
| Metro < 250k | 1.23 (1.09 to 1.39) | 0.001 | 1.04 (0.91 to 1.17) | 0.58 |
| Non-Metro/Rural | 1.28 (1.15 to 1.42) | <0.001 | 1.09 (0.98 to 1.22) | 0.11 |
| NCI comorbidity index | ||||
| Low (0 to 2) | ref | ref | ref | ref |
| Moderate (>2 to 4) | 1.23 (1.11 to 1.35) | <0.001 | 1.08 (0.98 to 1.19) | 0.14 |
| High (>4) | 1.60 (1.43 to 1.78) | <0.001 | 1.28 (1.14 to 1.43) | <0.001 |
| Etiology | ||||
| HCV | ref | ref | ref | ref |
| NAFLD | 1.37 (1.27 to 1.49) | <0.001 | 1.20 (1.09 to 1.32) | <0.001 |
| ALD | 1.36 (1.24 to 1.50) | <0.001 | 1.10 (0.99 to 1.21) | 0.08 |
| HBV | 0.87 (0.73 to 1.03) | 0.10 | 1.08 (0.91 to 1.28) | 0.39 |
| Others/None | 1.21 (1.06 to 1.38) | 0.004 | 1.09 (0.95 to 1.25) | 0.24 |
| Diabetes | 1.10 (1.02 to 1.18) | 0.01 | 1.01 (0.93 to 1.09) | 0.80 |
| Cirrhosis | 1.06 (0.98 to 1.14) | 0.16 | 1.01 (0.91 to 1.11) | 0.90 |
| Ascites | 1.78 (1.67 to 1.91) | <0.001 | 1.82 (1.68 to 1.97) | <0.001 |
| Hepatic encephalopathy | 1.54 (1.41 to 1.68) | <0.001 | 1.44 (1.31 to 1.58) | <0.001 |
| Surveillance Type | ||||
| No | ref | ref | ref | ref |
| Inconsistent | 0.68 (0.63 to 0.73) | <0.001 | 0.75 (0.70 to 0.81) | <0.001 |
| Consistent | 0.50 (0.45 to 0.55) | <0.001 | 0.66 (0.58 to 0.73) | <0.001 |
| Early-stage HCC * | 0.46 (0.42 to 0.50) | <0.001 | 0.57 (0.52 to 0.62) | <0.001 |
| Curative treatment ** | 0.27 (0.25 to 0.30) | <0.001 | 0.32 (0.29 to 0.35) | <0.001 |
aHR, adjusted hazard ratio; ALD, alcoholic liver disease; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Single lesion ≤5 cm without vascular invasion or metastasis.
Local ablation, resection, and liver transplant.
NAFLD subgroup analysis
We assessed predictors for surveillance, early-stage cancer detection, treatment, and OS among patients with NAFLD (n=1,813). Cirrhosis was the strongest predictor of surveillance receipts (Supplementary Table 3). Similar to the results in the overall cohort, female sex and younger age were associated with higher surveillance receipt and higher early-stage detection (Supplementary Table 3–4). Black race was associated with lower likelihood of early-stage HCC and curative treatment receipt (Supplementary Table 3–4). Early-stage HCC, absence of ascites and hepatic encephalopathy, receipt of surveillance and curative treatment receipt were associated with improved OS (Supplementary Table 5).
Next, we investigated the potential impact of coexisting liver diseases on clinical outcomes. In this analysis, we used an inclusive definition of NAFLD, including those with coexisting liver diseases [NAFLD+HCV (n=1,330), NAFLD+alcohol (n=768), NAFLD+HBV (n=193), and NAFLD+other liver diseases (n=158)]. NAFLD with coexisting liver diseases was associated with higher surveillance receipt, higher early-stage detection, and higher curative treatment than those with NAFLD alone (Supplementary Table 6–7). Patients with coexisting liver diseases have similar to modestly improved OS compared to those with NAFLD alone (Supplementary Table 8).
DISCUSSION
In this large population-based cohort study of US Medicare beneficiaries, NAFLD was the leading cause of HCC. Further, NAFLD was associated with a lower likelihood of receiving HCC surveillance, likely due to a lower proportion of patients with underlying cirrhosis. As a result, NAFLD was associated with lower early-stage HCC detection and curative treatment receipt than other liver disease etiologies. NAFLD was an independent predictor of worse OS after adjusting for demographic and socioeconomic characteristics, medical comorbidities, tumor stage, surveillance, and type of treatment although the strength of association was modest. Subgroup analyses among NAFLD patients showed that early-stage HCC, absence of severe liver diseases (ascites, hepatic encephalopathy), surveillance and curative treatment receipts showed a strong association with improved OS. Finally, NAFLD with coexisting liver diseases is associated with increased surveillance, early-stage HCC detection and curative treatment receipt compared to those with NAFLD alone.
With the rapidly rising burden of NAFLD in the US, NAFLD is considered as one of the leading etiologies of HCC.5 A recent study from the Texas HCC Consortium showed that NAFLD was the underlying cause of cirrhosis in 23% of patients overall and 34% in the Hispanic subgroup.4 NAFLD is also the most rapidly rising etiology of HCC among liver transplant registrants.6 Similar to the results from the United Network for Organ Sharing (UNOS) database,6 we showed that NAFLD is the leading etiology of HCC between 2011–2015 among Medicare beneficiaries in the US. This represents a significant increase in the burden of NAFLD-associated HCC, as it was previously the third most common etiology of HCC after HCV and alcohol in a SEER-Medicare study that included patients diagnosed between 2004 and 2009.1
Consistent surveillance was performed in a very small proportion of patients (15.2%), with higher surveillance among those with viral etiology (26.8% for HCV and 23.6% for HBV) and lower in NAFLD (5.3%). While the association between etiology and surveillance receipt was discordant in previous studies,20, 21 we demonstrated that NAFLD is associated with lower surveillance receipt compared to other etiologies after adjusting for demographics, socioeconomic status, and medical comorbidities. Recently, Singal et al. summarized a conceptual model for the HCC screening continuum and reasons for surveillance failure.22 One of the key reasons for the low surveillance in HCC compared to other cancers (e.g., breast or colon cancer) is difficulty with identification of high-risk populations. 22 It is noteworthy that 42.1% of HCC patients attributable to NAFLD have no cirrhosis, and this group of patients is not included in recommended surveillance populations.5 This may partly explain why NAFLD is associated with lower surveillance receipt. Despite the low incidence of HCC in noncirrhotic NAFLD3, the burden of HCC will continue to increase given its high prevalence in the general population. Thus risk stratification for HCC in noncirrhotic NAFLD with low cost, high accurate biomarkers will be important to identify high-risk patients even in the absence of cirrhosis who might benefit from the surveillance program.23
We also observed that surveillance receipt is less frequent in the subset of NAFLD patients with cirrhosis. We believe that lower surveillance receipt in NAFLD is in part due to higher prevalence of unrecognized cirrhosis among cirrhotics in addition to a higher proportion of patients without cirrhosis. A population-based study from Olmsted County showed that 41% of cirrhotic patients had unrecognized cirrhosis at HCC diagnosis and unrecognized cirrhosis was highest in NAFLD (62%).9 While the proportion of unrecognized cirrhosis (24.6%) was lower in a Veterans Administration (VA) hospital study, NAFLD was associated with a 4.8-fold increased likelihood of having unrecognized cirrhosis compared to HCV.11 A higher proportion of unrecognized cirrhosis, particularly in NAFLD, is partly due to a lack of screening program for NAFLD in contrast to HCV or HBV. Furthermore, symptoms and laboratory/imaging changes from early cirrhosis are often subtle, thus identification of eligible candidates for HCC surveillance is difficult, particularly in patients with NAFLD. Further effort to facilitating tailored strategies to maximize the effectiveness of HCC surveillance for NAFLD is an urgent need. Artificial intelligence-guided recognition of NAFLD-related cirrhosis via laboratory and imaging-based algorithms may improve identification of appropriate candidates for surveillance implementation. For example, the NAFLD ridge score is a machine learning-based model that can detect NAFLD with 87% accuracy using commonly available blood test results.24 Similarly, machine learning models have been used to detect cirrhosis using routine clinical parameters with 90% accuracy.25
We observed that HCC patients with NAFLD had a lower likelihood of presenting at early-stage disease. Similar results were seen in the previous studies.1, 26 More advanced HCC stage in NAFLD is likely due to a lower surveillance implementation as well as limited accuracy of surveillance tests. For example, the accuracy of ultrasound and alpha-fetoprotein, the two most commonly used surveillance tests, is lower in patients with NAFLD.27–29 This might lead to failure of surveillance test to detect early-stage HCC in NAFLD.30 NAFLD was inversely associated with curative treatment receipt likely attributed to a lower proportion of patients with early-stage HCC and increased medical comorbidities. Impact of surveillance test (US, CT/MRI, novel blood-based biomarkers) on clinical outcomes in NAFLD associated cirrhosis and HCC should be further investigated in a future study.
Association between NAFLD etiology and OS has been controversial in HCC.7, 21, 26 While a previous analysis with SEER-Medicare database showed that NAFLD is associated with 21% increased risk of mortality7, a recent single-center study of 92 NAFLD-related HCC showed no association between NAFLD (vs. viral etiology) and OS. An Italian multicenter study with propensity score matching analysis showed no significant difference in survival between NAFLD and HCV-related HCC.26 Our results showed that survival was shorter in the NAFLD-related HCC group, albeit modest. While this association could be due to residual confounders, such as older patients, increased medical comorbidities, larger tumor burden, worse outcome in NAFLD-related HCC could be due to different underlying tumor biology. It is well known that the underlying molecular pathogenesis of HCC varies by underlying etiology.31 Multiples studies have demonstrated a difference in the rate of tumor progression and treatment response by underlying etiology of HCC.32–34 Of note, survival appeared similar between NAFLD and HCV in several subgroups with consistent surveillance, early-stage HCC, and those who underwent curative treatment, suggesting that early HCC detection via surveillance and curative treatment receipt might mitigate the prognostic disadvantage observed in those with NAFLD-related HCC. However, the prognostic impact of NAFLD should be interpreted with caution in view of only mild effect size (HR of 1.2) and lack of adjustment for granular clinical data. Finally, NAFLD subgroup analysis showed that prognostic factors are comparable between overall HCC patients and NAFLD subgroup. NAFLD patients without coexisting chronic liver diseases had lower surveillance and early-stage detection than those with coexisting liver disease; this subgroup analysis supports that worse outcomes among NAFLD patients may be mediated by underrecognition of underlying liver disease and cirrhosis.
We acknowledge that our study has several limitations, including those inherent to large administrative datasets. First, we used ICD-9 and ICD-10 codes to define the underlying etiology of HCC, and NAFLD can often be under-coded. To address this limitation, we defined NAFLD using a broader list of ICD-9 and ICD-10 codes including related metabolic conditions (e.g., diabetes, obesity, hyperlipidemia), as in prior studies.7 We used a hierarchy to classify patients into one etiology. While this approach allows to have a pure group of NAFLD-associated HCC, those with NAFLD and other coexisting liver disease (e.g., alcohol) would have been classified as non-NAFLD associated HCC. Thus, the results likely under-estimated the true burden of NAFLD-associated HCC. Second, SEER-Medicare lacks granularity, including data on liver disease severity (e.g., Model for End-stage Liver Disease, Child-Pugh Score) which is an important prognostic factor in patients with HCC. In addition, the SEER staging system is less clinically applicable compared to the Barcelona Clinic Liver Cancer (BCLC) staging system, the most commonly used staging system for HCC. We addressed this limitation by defining early-stage cancer based on single and small tumor size (≤5 cm) without vascular invasion or metastasis as opposed to using SEER stage classifications (localized, regional, distant) which are less clinically relevant in HCC. Finally, Medicare population represents older individuals and the study results might not be generalizable to younger patients with HCC. This bias may be particularly evident among patients with HBV as their average age of HCC diagnosis is much younger. The older population in this study may have also had lower eligibility for curative treatments such as liver transplantation than younger HCC patients. However, we believe this is an important population to study given the aging demographic of patients with HCC. Despite these limitations, this is the largest contemporary cohort of patients to describe NAFLD as the leading etiology of HCC in Medicare-population and its association with surveillance utilization, early-stage detection, curative treatment receipt, and OS.
In conclusion, our study shows that NAFLD is the leading etiology of HCC among Medicare beneficiaries in the United States. These results emphasize that HCC risk stratification of non-cirrhotic NAFLD and early recognition of cirrhosis among patients with NAFLD will be critical to increase surveillance implementation and earlier detection of HCC, resulting in increased utilization of curative treatment and improved survival.
Supplementary Material
BACKGROUND
NAFLD is emerging as a major etiology of HCC. We aimed to estimate the burden of NAFLD-related HCC and magnitude of associations with surveillance receipt, clinical presentation, and outcomes.
FINDINGS
NAFLD was the leading etiology of HCC among Medicare beneficiaries in the United States. NAFLD was associated with lower HCC surveillance receipt, lower early-stage cancer detection, and modestly worse overall survival.
IMPLICATIONS FOR PATIENT CARE
Multi-faceted interventions are urgently needed to improve surveillance uptake, which can lead to early-stage cancer detection, curative treatment and improving prognosis of patients with NAFLD-related HCC.
Acknowledgments
Financial support:
Dr. Karim’s research is funded in part by a fellowship supported by the Cancer Prevention and Research Institute of Texas (CPRIT) grant award RP170259 (to Mohammad A. Karim, PhD; PI: Shine Chang, PhD and Sanjay Shete, PhD).
Dr. Singal’s research is funded by National Institute of Health R01 MD012565, U01 CA230694, and R01 CA256977.
Dr. Karim and Dr. Kum’s research is funded in part by the Population Informatics Lab, and the Texas Virtual Data Library (ViDaL) at Texas A&M University.
Dr. Yang’s research is supported by American College of Gastroenterology Junior Faculty Development Award, Department of Defense Peer Reviewed Cancer Research Program Career Development Award (CA191051).
The American College of Gastroenterology, Department of Defense, and National Institute of Health had no role in the collection of data; the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
List of Abbreviations:
- ALD
alcoholic liver disease
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- OS
overall survival
- SEER
Surveillance, Epidemiology and End Results
- US
United States
Footnotes
Conflict of interest:
Dr. Yang provides a consulting service for Exact Sciences and Gilead Sciences and Eisai.
Dr. Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and TARGET RWE.
Dr Rich as served as consultant for AstraZeneca.
Dr. Noureddin has been on the advisory board for 89BIO, Gilead, Intercept, Pfizer, Novo Nordisk, Blade, EchoSens, Fractyl, Terns, Siemens and Roche diagnostic; MN has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Pfizer, Shire, Viking and Zydus; Dr. Noureddin is a minor shareholder or has stocks in Anaetos, Rivus Pharma and Viking.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease – A global public health perspective. Journal of Hepatology 2019;70:531–544. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–568. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients With NonAlcoholic Fatty Liver Disease. Gastroenterology 2018;155:1828–1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Kanwal F, Feng Z, et al. Risk Factors for Cirrhosis in Contemporary Hepatology Practices-Findings From the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020;159:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019;17:748–755.e3. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723–30. [DOI] [PubMed] [Google Scholar]
- 8.Lee YT, Wang JJ, Luu M, et al. State-Level Hepatocellular Carcinoma Incidence and Association with Obesity and Physical Activity in the United States. Hepatology 2021. [DOI] [PubMed] [Google Scholar]
- 9.Yang JD, Ahmed Mohammed H, Harmsen WS, et al. Recent Trends in the Epidemiology of Hepatocellular Carcinoma in Olmsted County, Minnesota: A US Population-based Study. J Clin Gastroenterol 2017;51:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker M, El-Serag HB, Sada Y, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Alimentary pharmacology & therapeutics 2016;43:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:Iv-3–18. [DOI] [PubMed] [Google Scholar]
- 13.Enewold L, Parsons H, Zhao L, et al. Updated Overview of the SEER-Medicare Data: Enhanced Content and Applications. JNCI Monographs 2020;2020:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Overview of the SEER Program. Available at: seer.cancer.gov/about/overview.html. Accessed January 2, 2021.
- 15.National Cancer Institute. List of SEER Registries. Available at: seer.cancer.gov/about/overview.html. Accessed January 2, 2021.
- 16.Centers for Medicare and Medicaid Services. CMS Program Statistics: 2017 Medicare Enrollment Section. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2017/2017_Enrollment.html. Accessed July 7, 2021.
- 17.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther 2008;27:274–82. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. NCI Comorbidity Index overview. Available at: https://healthcaredelivery.cancer.gov/seermedicare/_considerations/comorbidity.html. Accessed April 1, 2016.
- 19.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf E, Rich NE, Marrero JA, et al. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hester CA, Rich NE, Singal AG, et al. Comparative Analysis of Nonalcoholic Steatohepatitis-Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw 2019;17:322–329. [DOI] [PubMed] [Google Scholar]
- 22.Singal AG, Lok AS, Feng Z, et al. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara N, Kobayashi M, Fobar AJ, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (N Y) 2021;2:836–850.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip TC, Ma AJ, Wong VW, et al. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther 2017;46:447–456. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Hu Z-D, Liu X-F, et al. An MLP classifier for prediction of HBV-induced liver cirrhosis using routinely available clinical parameters. Disease markers 2013;35:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016;63:827–38. [DOI] [PubMed] [Google Scholar]
- 27.Yang JD, Dai J, Singal AG, et al. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol Biomarkers Prev 2017;26:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenberger H, Chong N, Fetzer DT, et al. Dynamic Changes in Ultrasound Quality for Hepatocellular Carcinoma Screening in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong N*SH, Yekkaluri S, Fetzer D, Rich NE, Yokoo T, Gopal P, Manwaring C, Quirk L, Singal AG. Association between ultrasound quality and test performance for HCC surveillance in patients with cirrhosis: a retrospective cohort study. Alimentary Pharmacology and Therapeutics 2022. [DOI] [PubMed] [Google Scholar]
- 31.Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017;169:1327–1341.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol 2017;67:999–1008. [DOI] [PubMed] [Google Scholar]
- 34.Rich NE, John BV, Parikh ND, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology 2020;72:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


