Patients with cirrhosis are high risk for developing hepatocellular carcinoma (HCC) and warrant surveillance using abdominal ultrasound and α-fetoprotein.1 Those with positive surveillance results should undergo diagnostic evaluation with multiphase computed tomography (CT) or magnetic resonance imaging (MRI). The LI-RADS system is an evidence-based system to classify observations on CT or MRI in at-risk patients, ranging from LR-1 (definite benign) to LR-5 (definite HCC), with LR-3 and LR-4 observations being intermediate risk for HCC.2 LR-3 and LR-4 observations are observed on CT or MRI in more than one-fourth of patients undergoing HCC surveillance and have a high, yet variable, risk for progression to HCC.3 Approximately one-third of patients with LR-3 observations and more than two-thirds of LR-4 observations develop HCC, and surveillance strategies vary widely in practice.4,5 Variation in radiographic appearance and natural history of these observations suggests that this may be a heterogeneous group of patients; however, their histopathology has not been well described. Herein, we correlated imaging findings and explant histopathology from liver transplant recipients with at least 1 LR-3 or LR-4 observation on CT or MRI within 6 months preceding transplantation.
We conducted a retrospective cohort study of patients with cirrhosis and at least 1 LR-3 or LR-4 observation on multiphase contrast-enhanced CT or MRI, who underwent liver transplantation between January 2014 and October 2020 at UT Southwestern Medical Center. Imaging findings were abstracted from standardized radiology reporting templates, including number of LR-3 or LR-4 observations, maximum diameter of largest observation, and radiographic features (including arterial phase hyperenhancement, delayed washout) of largest observation. Explant pathology findings were independently reviewed by a liver pathologist to confirm diagnoses for purposes of this study. Univariable and multivariable logistic regression analyses were performed to identify patient-level predictors of HCC on explant, examining variables of a priori interest, including sex, cirrhosis etiology, concomitant or known history of HCC, maximum LR-3 or LR-4 diameter, and radiographic findings of index observation (arterial-phase enhancement and delayed washout), with statistical significance defined as P < .05. The study was approved by the UT Southwestern Medical Center Institutional Review Board, and analyses were conducted using STATA version 14.2 (College Station, TX).
Among 495 transplant recipients during the study period, we identified 90 eligible patients (median age, 61 years; 74.4% men), including 58 with LR-3 observations and 32 with at least 1 LR-4 observation. Patient characteristics are detailed in Supplementary Table 1. The cohort was diverse regarding race and ethnicity (55.6% non-Hispanic White, 30.0% Hispanic, and 10.0% Black), cirrhosis etiology (30.0% viremic hepatitis C, 12.2% hepatitis C postsustained virologic response, 33.3% alcohol-associated, and 13.3% nonalcoholic steatohepatitis), and liver disease severity (16.9% Child Pugh A, 32.6% Child Pugh B, 50.6% Child Pugh C). Two-thirds of the cohort (n = 60 )had a history of HCC, including 37 patients with LR-3 observations and 23 with LR-4 observations. Median sizes of the LR-3 and LR-4 observations were 1.0 and 1.6 cm, with 51.7% and 71.9% being ≥1 cm, respectively.
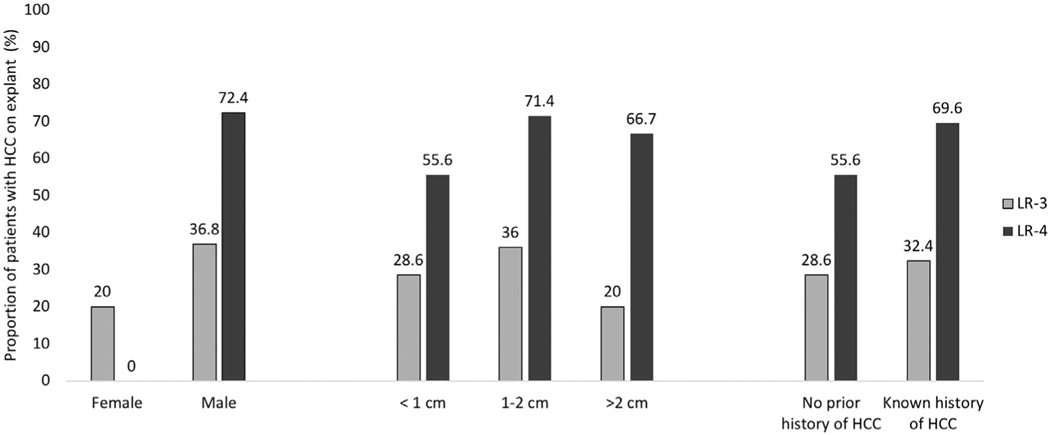
Most patients with LR-3 observations (n = 34; 58.6%) had no correlate on pathologic examination, although 18 (31.0%) were found to have HCC and 5 (10.3%) had a dysplastic nodule. Surprisingly, 1 patient with LR-3 observations was found to have angiosarcoma on explant. In contrast, most patients with LR-4 observations (n = 21; 65.6%) were found to have HCC on explant, with 2 (6.3%) having a dysplastic nodule and 9 (28.1%) having no corresponding pathologic abnormality. Results were similar when stratified by size of LR-3 or LR-4 observations. HCC was found on explant in 28.6%, 36.0%, and 20.0% of patients with LR-3 observations <1 cm, 1–2 cm, and >2 cm, respectively, compared with 55.6%, 71.4%, and 66.7% of those with LR-4 observations <1 cm, 1–2 cm, and >2 cm, respectively (Figure 1). Among 30 patients without prior HCC, 21 had LR-3 observations and 9 had LR-4 observations. Results in this subgroup were similar to the overall cohort, with 61.9% and 28.6% of patients with LR-3 observations having no pathologic correlate and HCC, respectively, in contrast to 33.3% and 55.6%, of those with LR-4 observations (Figure 1).
Figure 1.
Proportion of patients with HCC on explant, stratified by LR-3 versus LR-4 observations.
Presence of HCC on explant was associated with having an LR-4 versus LR-3 observation and male sex but not associated with cirrhosis etiology, observation diameter, or radiographic features in univariable analyses. In multivariable analysis, presence of HCC continued to be associated with male sex (odds ratio, 3.86; 95% confidence interval, 1.14–13.1) and LR-4 versus LR-3 observation (odds ratio, 3.32; 95% confidence interval, 1.29–8.62) (Supplementary Table 2). HCC was found in 21 (72.4%) of 29 LR-4 observations in men, 14 (36.8%) of 38 LR-3 observations in men, 0 of 3 LR-4 observations in women, and 4 (20.0%) of 20 LR-3 observations in women.
In summary, we found one-third of patients with LR-3 observations and two-thirds of those with LR-4 observations had HCC on histopathology; however, more than half with LR-3 and more than one-fourth with LR-4 observations had no pathologic correlate, suggesting these observations may be perfusion variants on imaging or regenerative cirrhotic nodules. Interestingly, we found HCC risk was not associated with LR-3 or LR-4 maximum diameter, highlighting this clinical feature may not be an accurate determinant of who warrants close observation. These data highlight a need for better risk stratification tools for patients with LR-3 or LR-4 observations, which can help tailor surveillance intensity to optimize overall value.6 Oversurveillance of low-risk patients, leading to potential screening-related harms, and undersurveillance of high-risk patients, leading to missed cancers, both present significant clinical challenges.7,8 Blood-based biomarkers, clinical risk models, and radiomics approaches seem promising for risk stratification in patients with cirrhosis, but these approaches need to be specifically validated in patients with LR-3 or LR-4 observations.9,10 Our study’s limitations, including moderate interrater reliability of LI-RADS interpretation and imperfect radiologic-pathologic colocalization of observations, are balanced by its strengths, including independent review of pathology specimens and our patient-level analysis, which better informs clinical management of patients than prior observation-level analyses. Multicenter studies with larger cohorts of patients with LR-3 or LR-4 observations that have paired imaging, histopathology, and serum samples should be conducted to identify and validate diagnostic biomarkers that may improve surveillance strategies in this group of patients.
Supplementary Material
Acknowledgments
Corporate Authorship: Takeshi Yokoo, MD, Arjmand Mufti, MD, Yujin Hoshida, MD, PhD, Adam C. Yopp, MD, MS, Hao Zhu, MD, and Travis Browning, MD.
Funding
This study was conducted with support from National Institutes of Health Grant U01 CA230694. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Conflicts of interest
These authors disclose the following: Nicole E. Rich has served as a consultant for AstraZeneca. Takeshi Yokoo receives research support from Siemens Healthineers, Guerbet, Bracco, Bayer, and GE. Amit G. Singal has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL. Travis Browning has served as a consultant for Change Healthcare. The other authors disclose no conflicts.
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.03.009.
Contributor Information
COLIN DUNN, Department of Internal Medicine, UT Southwestern, Medical Center Dallas, Texas.
BO LIN, Department of Pathology, UT Southwestern Medical, Center Dallas, Texas.
NICOLE E. RICH, Department of Internal Medicine, UT Southwestern, Medical Center Dallas, Texas.
MADHUKAR S. PATEL, Department of Surgery, UT Southwestern Medical, Center Dallas, Texas.
PURVA GOPAL, Department of Pathology, UT Southwestern Medical, Center Dallas, Texas.
AMIT G. SINGAL, Department of Internal Medicine, UT Southwestern, Medical Center Dallas, Texas.
References
- 1.Singal AG, et al. J Hepatol 2020;72:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang A, et al. Clin Gastroenterol Hepatol 2019;17:1228–1238. [DOI] [PubMed] [Google Scholar]
- 3.Konerman M, et al. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvind A, et al. Clin Gastroenterol Hepatol 2022. 10.1016/j.cgh.2021.11.042. [DOI] [Google Scholar]
- 5.Tanabe M, et al. Radiology 2016;281:129–139. [DOI] [PubMed] [Google Scholar]
- 6.Goossens N, et al. Clin Transl Gastroenterol 2017;8:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf E, et al. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, et al. Clin Gastroenterol Hepatol 2021 1932;19:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujiwara N, et al. Med (N Y) 2021;2:836–850. [Google Scholar]
- 10.Harding-Theobald E, et al. Aliment Pharmacol Ther 2021; 54:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.