Abstract
Objective:
To compare the efficacy of ultrasound-guided hyaluronic acid (HA) vs. leukocyte-poor platelet-rich plasma (LP-PRP) injection in the treatment of glenohumeral osteoarthritis.
Design:
Double-blind randomized controlled trial.
Setting:
Academic institution
Patients:
Seventy patients with chronic glenohumeral osteoarthritis were randomly assigned to receive a single injection of HA (n=36) or LP-PRP (n=34).
Interventions:
LP-PRP was processed using Harvest/TerumoBCT Clear PRP kits. Ultrasound-guided injections of 6-mL HA or 6-mL LP-PRP into the glenohumeral joint were performed. Patients, the injecting physician, and outcomes assessor were blinded to treatment assignments.
Main Outcome Measures:
Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) score, current/average numerical rating scale (NRS) pain scores, satisfaction, and side effects were assessed at 5 follow-up time points over 12 months.
Results:
Baseline characteristics were similar between groups. There were no significant between-group differences with regard to SPADI, ASES, and current/average NRS pain scores at any time point up to 12 months post-injection (p>0.05). However, significant improvements in SPADI, ASES, and current/average NRS pain scores were observed in both groups starting at 1 or 2 months (p<0.01, p<0.01, p<0.001, and p<0.01, respectively). These improvements were observed regardless of osteoarthritis severity. For patients who received LP-PRP, there was no effect of platelet yield on outcomes. Side effect and satisfaction rates were similar between groups.
Conclusions:
There were no differences in pain and functional outcomes following a single injection of LP-PRP versus HA. However, significant improvements in pain and function were observed following both treatments in patients with glenohumeral osteoarthritis.
Keywords: glenohumeral osteoarthritis, pain, shoulder, hyaluronic acid, platelet-rich plasma
Introduction
Glenohumeral osteoarthritis (OA) is the third most common type of OA and causes chronic and persistent shoulder pain.1 Primary glenohumeral OA usually affects elderly patients and is typically characterized by degenerative changes to the glenohumeral joint. Secondary glenohumeral OA is characterized by history of trauma to the shoulder or avascular necrosis.1 There is an increased risk of shoulder pain secondary to glenohumeral OA in the elderly and in athletes or patients who engage in overhead exercises.2-5
Conservative treatments for glenohumeral OA include activity modification, physical therapy, non-steroidal anti-inflammatory drugs (NSAIDs), and injectable corticosteroids.1 If these treatments fail, shoulder arthroplasty is advised and is a successful treatment option amongst elderly patients.1 However, shoulder arthroplasty is less successful for patients younger than 50 years old.6 Recently, hyaluronic acid (HA) and platelet-rich plasma (PRP) have been suggested as potential treatments for glenohumeral OA.6
HA is a glycosaminoglycan that is found in synovial joint fluid and has viscoelastic, chondroprotective, and possible anti-inflammatory properties.7 When injected intra-articularly, it promotes endogenous HA growth and production, which increases joint lubrication and decreases mechanical stress on the affected joint.8,9 Multiple studies have demonstrated improvements in pain and range of motion for 3-6 months following ≥3 HA injections to the subacromial space or glenohumeral joint.10-18 Improvements in pain and function have also been demonstrated after one or two HA injections to the glenohumeral joint.14,19-22 Although different molecular weight formulations for HA exist, there does not appear to be evidence of greater efficacy or advantage of one preparation over others in clinical studies.8,9 Furthermore, there is inconsistency in the HA and glenohumeral OA literature in terms of recruitment, sample size, injection technique, image guidance, HA type, and presence of a control group. A few studies have compared the efficacy of HA versus placebo injections to the glenohumeral joint.10,23-25 Blaine et al. showed significant improvements in shoulder pain at 13 weeks following a 3-injection series of HA, 5-injection series of HA, or placebo; larger improvements were observed with HA compared to placebo, although these were not statistically significant.10 When looking at overall treatment effect over 26 weeks, both HA groups demonstrated significant improvements in shoulder pain compared to placebo.10 Similarly, Kwon et al. found significant improvements in pain following 3 weekly injections of HA versus placebo, starting at 1 week and maintained up to 26 weeks. Mean improvements were greater following HA compared to placebo, but this was statistically significant only for patients with glenohumeral OA in the absence of concomitant pathologies.11
Alternatively, PRP involves the use of a patient’s own platelets to take advantage of autologous growth factors and is increasingly being used to treat different musculoskeletal conditions.26 Existing research has suggested benefit in pain related to knee and hip OA,27-30 but limited-to-no data are available for its effect on glenohumeral OA. One case report reported improvement in pain and function following 3 intra-articular PRP injections in a 62-year-old woman with glenohumeral OA.31 Another study comparing a single intra-articular steroid injection versus a PRP injection for glenohumeral OA demonstrated benefits of both, with a superior benefit coming from PRP.32
To our knowledge, no study in the literature has compared the efficacy of these two emerging treatments with ultrasound guidance in the treatment of glenohumeral OA. This study aimed to determine whether HA or PRP can decrease pain, restore function, and improve disability in patients suffering from chronic glenohumeral OA that is refractory to other conservative management. We hypothesized that PRP injections would be more efficacious than HA injections in patients with chronic glenohumeral OA.
Methods
Ethics
This double-blind, randomized controlled trial was approved by the Institutional Review Board. Written informed consent was obtained from all subjects prior to any research activities. This study was registered at ClinicalTrials.gov and was conducted in accordance with CONSORT guidelines.
Patient Recruitment
Patients were recruited for the study from December 2014-November 2018 by posting the study online, distributing flyers in the hospital, and receiving referrals from primary care sports medicine, orthopedic surgery, and physiatry departments. Potential patients were screened according to eligibility criteria (Table 1).
Table 1.
Study Eligibility Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
HIV: human immunodeficiency virus; NSAID: non-steroidal anti-inflammatory drug; OA: osteoarthritis; PRP: platelet-rich plasma
Treatment Allocation and Administration
Using a computer-generated, block-randomization scheme, patients were randomly assigned to receive a single injection of HA or leukocyte-poor PRP (LP-PRP) into the glenohumeral joint. Randomization assignments were concealed in opaque envelopes. Patients were asked to discontinue NSAIDs for 5 days pre-injection. Both groups underwent venipuncture for blinding purposes, with a blood draw of 61 mL under sterile conditions for the LP-PRP group only. A curtain enclosure was used to prevent patients from viewing the venipuncture and blood draw. All patients were bandaged afterwards. Patients, the treating physician, and outcomes assessors were blinded to treatment groups. Injection syringes were covered with opaque shields to preserve blinding for the patient and treating physician.
The HA group received one 6-mL injection of low-molecular-weight (average 500,000–730,000 dalton) HA (Hyalgan, Fidia Pharma, Florham Park, NJ). Needle guidance and injection were performed by a fellowship-trained, board-certified physiatrist and sports medicine physician, who is also a registered musculoskeletal sonographer, using a 3-inch, 22-gauge spinal needle under continuous ultrasound guidance (Xporte, 15-6mHz linear array transducer, Fujifilm Sonosite Inc, Bothell, WA). A posterior medial-to-lateral in-plane approach was used. Subcutaneous local anesthetic (1% lidocaine; 3mL) was used, but was not injected into the joint to minimize confounding. Once needle placement within the joint was confirmed, the blinded contents of the syringe were injected. Arthrocentesis was attempted if effusion was present prior to injection, but no significant fluid could be returned. This may be due to the 22-gauge needle or seated position the patient was in for the procedure.
The LP-PRP group underwent the same procedure as the HA group with respect to ultrasound guidance and anatomic approach and received one 6-mL injection of LP-PRP. Prior to injection, the Harvest–TerumoBCT (Plymouth, MA) SmartPReP Clear PRP system was used to prepare the samples in accordance with the manufacturer’s instructions. Venipuncture, blood collection, and LP-PRP processing were performed in a sterile manner by a trained study team member not involved in the direct care and management of study subjects. A total of 61mL whole blood was drawn, and 1mL was stored at room temperature. The amount of time between blood draw and LP-PRP injection was less than 40min. After processing, LP-PRP samples were stored at room temperature. Whole-blood and LP-PRP samples were sent to the laboratory for complete blood differential analyses, and platelet yields were calculated.
Post-procedure, patients in both groups were instructed to take acetaminophen 500mg every 6 hours (maximum: 3g) for pain relief or were offered a week of Tramadol 50mg as needed. Ice applied over the affected joint was not allowed for 3 days, and NSAIDs were avoided for at least 6 weeks post-injection. Patients were also instructed to avoid taking baths and swimming for 2 days to minimize the risk of infection. Showers were allowed, and patients were encouraged to continue their usual daily activities, including exercises, as tolerated.
Outcomes and Data Collection
Demographics were collected at baseline (pre-injection). The Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons Society-Standardized Shoulder Assessment Form (ASES), current/average NRS pain, sleep quality, and general well-being were assessed at baseline and at 1, 2, 3, 6, and 12 months post-injection. Side effects and satisfaction were also assessed at the follow-up timepoints. Current/average NRS pain were scored on 0-10 scales (0-no pain at all; 10-worst possible pain imaginable). Sleep quality was assessed on a 0-10 scale, with 0 representing “I could not sleep at all” and 10 representing “I slept perfectly well”. General well-being was assessed on a 0-10 scale, with 0 representing “I am doing the worst ever” and 10 representing “I am doing very well”. For satisfaction, patients were asked if they would do the study treatment again (yes/no).
OA Severity Grading
OA severity was determined from radiographs, magnetic resonance imaging (MRI), or computed tomography (CT) scans by a fellowship-trained musculoskeletal radiologist with 9 years of experience. The Weinstein classification was used for radiographs, along with measuring the size of the humeral osteophytes (Samilson and Prieto classifications).33 Four stages were documented: I-normal and no joint spacing narrowing; II-minimal joint space narrowing with concentric head and glenoid; III-moderate joint space narrowing and/or early inferior osteophyte formation; and IV-severe joint space loss with osteophyte formation and loss of concentricity between glenoid and humeral head.33 Seven shoulders were upgraded from II to III if they had osteophytes >4mm.
The Walch classification was additionally used to guide the grading of glenohumeral OA for patients with CT and/or MRI.34 Mild-to-moderate OA included A1 and B1, where there is minor to no bony erosion, and severe OA included A2, B2, and B3 classifications. There were no dysplastic grade C glenoid configurations.
OA severity was graded as mild, moderate, or severe, based on the aforementioned classifications.
Statistical Analysis
The primary outcome was SPADI. Previous studies have utilized 10 points as the minimal clinically important difference (MCID) for SPADI.35 A power analysis performed a priori showed that sample sizes of 25 patients in each group are sufficient to achieve 86% power to detect a 10-point difference in SPADI score between groups, with an estimated SD of 15 points and an alpha of 0.05.
Continuous variables are reported as means and SDs in the descriptive analysis. Discrete variables are reported as frequencies and percentages. Longitudinal analysis of SPADI scores from baseline to 12 months was conducted using a linear mixed model with repeated measures. Longitudinal analyses of secondary outcomes from baseline to 12 months were performed using generalized estimating equations (GEE). All observations were analyzed using maximum likelihood estimations. The models included time as the fixed effect and observed differences between HA and LP-PRP treatment with a random intercept for each participant for random variability between participants. A linear mixed model and GEE were used to estimate crude effects of baseline platelet yield over time. These models included time*platelet-yield as the fixed effect and a random intercept for random variability between participants. Additionally, a sensitivity analysis utilizing linear mixed models and GEE models evaluated associations between OA severity (mild/moderate vs. severe) and outcomes, adjusting for age, sex, BMI, and treatment (as-treated).
Parameter estimates are reported as means, SEs, and 95% CIs. Multiple pairwise comparisons were adjusted for using Bonferroni correction. Statistical significance was defined as p<0.05. All analyses were performed with Stata, version 14.2.
Results
Patient Flow and Baseline Information
Of the 108 patients who were assessed for eligibility from December 2014–October 2019, 70 patients were consented and randomly allocated to receive HA (n=36) or LP-PRP (n=34) (Figure 1). One patient in the HA group screened out and did not receive any interventions. Two patients in the HA group received LP-PRP, and two patients in the LP-PRP group received HA.
Figure 1. CONSORT Flow Diagram.
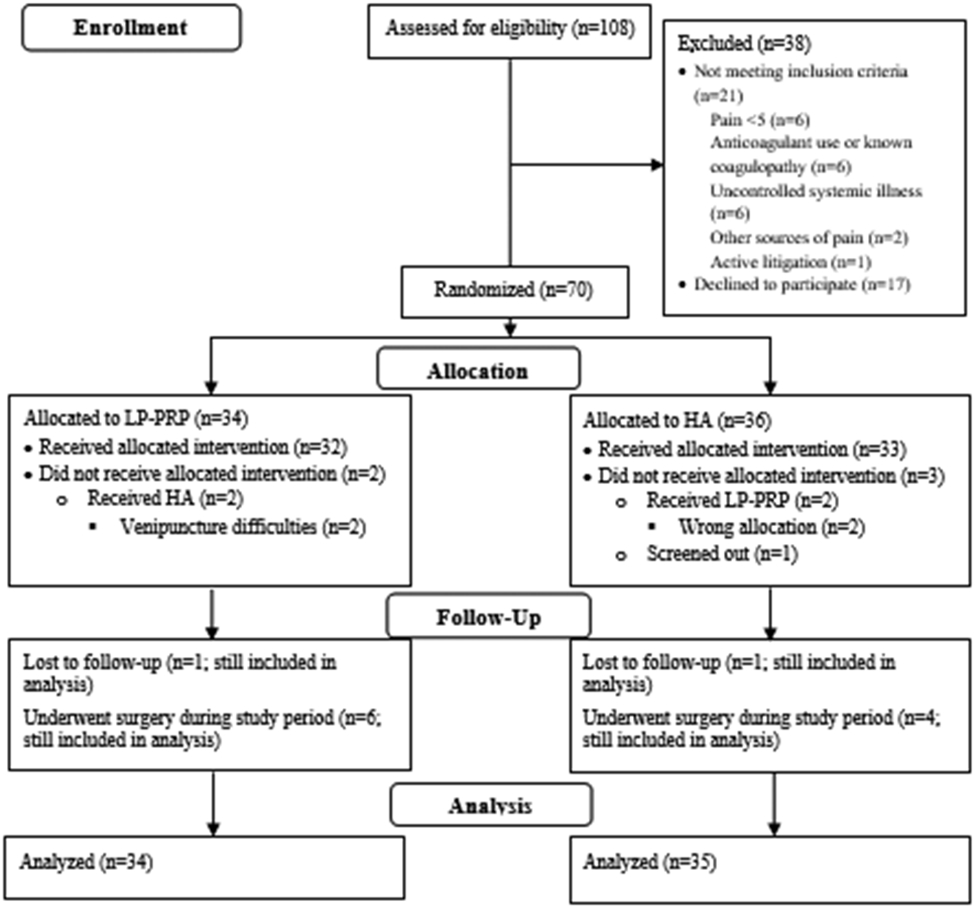
The numbers of participants who were assessed for eligibility, randomized, followed up, and analyzed are shown. HA – hyaluronic acid; LP-PRP – leukocyte-poor platelet-rich plasma.
Both intention-to-treat and as-treated analyses demonstrated similar baseline characteristics between HA and LP-PRP groups (Table 2). Most patients in both groups had severe OA. Platelet counts increased by 3.44±1.60-fold in LP-PRP samples, compared to whole-blood samples. White blood cell counts decreased from 5.45±1.58 (×109/L) in whole-blood samples to 0.21±0.79 (×109/L) in LP-PRP samples. Red blood cell counts decreased from 4.16±0.57 (×1012/L) in whole-blood samples to 0.03±0.02 (×1012/L) in LP-PRP samples.
Table 2.
Baseline Characteristics.
| Variables | HA (n=36) | LP-PRP (n=34) | P-value |
|---|---|---|---|
| Age, years; mean (SD) | 68.4 (11.9) | 69.1 (11.5) | 0.83 |
| Female sex; n (%) | 18 (50) | 20 (59) | 0.46 |
| BMI, kg/m2; mean (SD) | 27.9 (5.4) | 26.8 (5.2) | 0.37 |
| Right-sided OA; n (%) | 19 (53) | 19 (56) | 0.79 |
| Duration of symptoms, months; mean (SD) | 46 (15) | 43 (7) | 0.85 |
| Use of pain medication; n (%) | 27 (77) | 20 (69) | 0.10 |
| Severe OA; n (%) | 23 (64) | 21 (62) | 0.85 |
Abbreviations: BMI – body mass index; HA – hyaluronic acid; LP-PRP – leukocyte-poor platelet-rich plasma; OA – osteoarthritis; SD – standard deviation.
Patient-Reported Outcomes
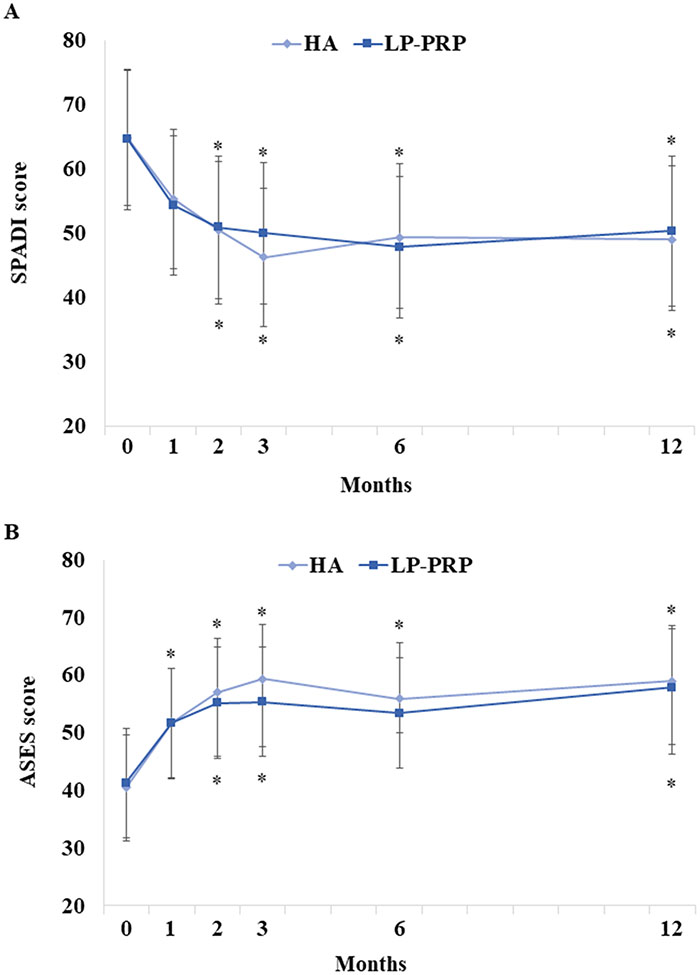
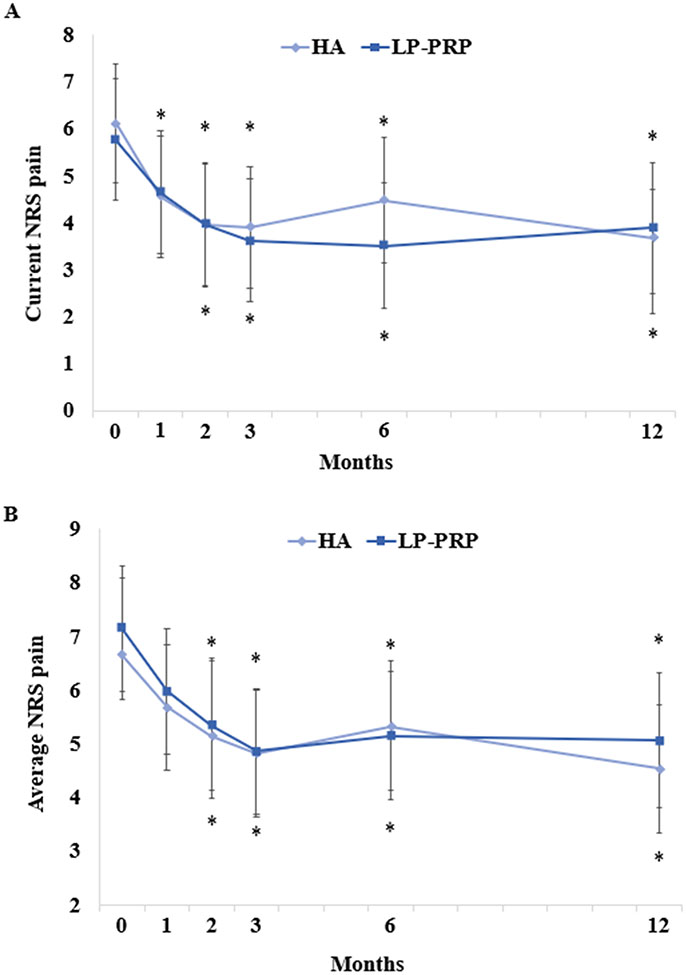
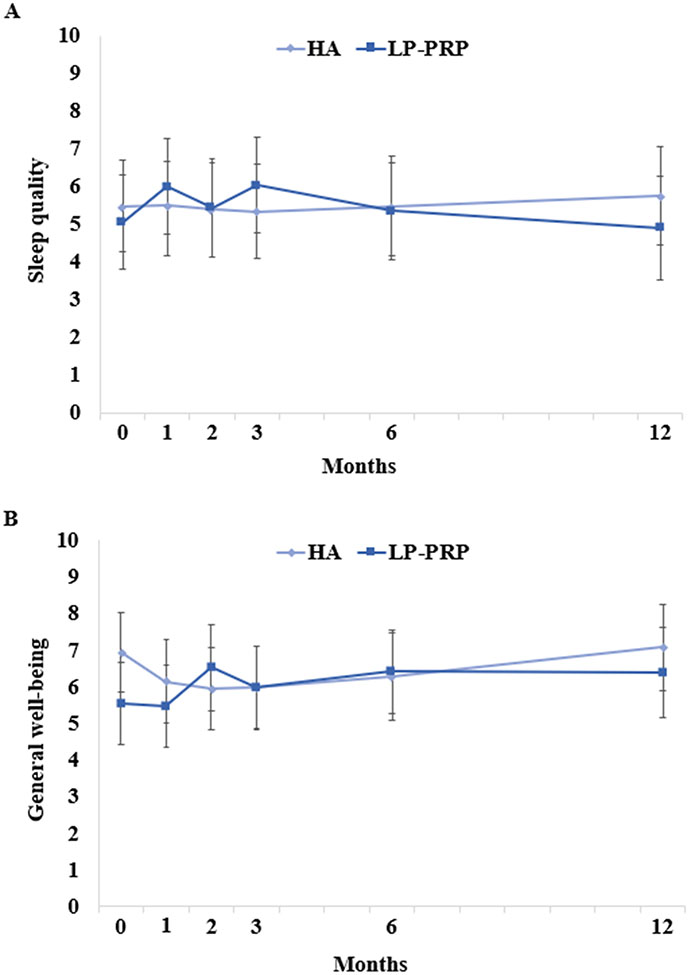
There were no between-group differences in SPADI scores (Figure 2A). However, SPADI scores significantly improved in both HA and LP-PRP groups, up to 12 months post-injection. These improvements were clinically significant starting at 2 months post-injection. The mean decreases at 12 months post-injection were 15.8 and 14.3 points in HA and LP-PRP groups, respectively. Similarly, there were no between-group differences in ASES, current NRS, and average NRS pain scores, although they significantly improved up to 12 months post-injection (Figures 2B-3). General well-being and sleep quality were unchanged over time and were similar between groups (Figure 4).
Figure 2. Patient-Reported Outcomes Following HA or LP-PRP Treatment.
Parameter estimates of (A) Shoulder Pain and Disability Index (SPADI) and (B) American Shoulder and Elbow Surgeons (ASES) scores at baseline and at 1, 2, 3, 6, and 12 months post-HA or LP-PRP are shown. Error bars are 95% confidence intervals. *P<0.05 vs. baseline.
Figure 3. Pain Outcomes Following HA or LP-PRP Treatment.
Parameter estimates of (A) current numerical rating scale (NRS) pain and (B) average NRS pain scores at baseline and at 1, 2, 3, 6, and 12 months post-HA or post-LP-PRP are shown. Error bars are 95% confidence intervals. *P<0.05 vs. baseline.
Figure 4. Sleep Quality and General Well-Being Following HA or LP-PRP Treatment.
Parameter estimates of (A) sleep quality and (B) general well-being at baseline and at 1, 2, 3, 6, and 12 months post-HA or post-LP-PRP are shown. Error bars are 95% confidence intervals. Both outcomes were measured on 0-10 scales; a higher score represented better sleep quality or better general well-being.
Side Effects and Satisfaction
Side effects rates were 3.9% and 2.7% in the HA and LP-PRP groups, respectively; weakness was the most common. At 12 months, 60% of patients in the HA group and 80% of patients in the LP-PRP group stated that they would repeat the treatment. Satisfaction rates ranged from 57–73% for both groups over the follow-up period.
OA Severity and Patient-Reported Outcomes
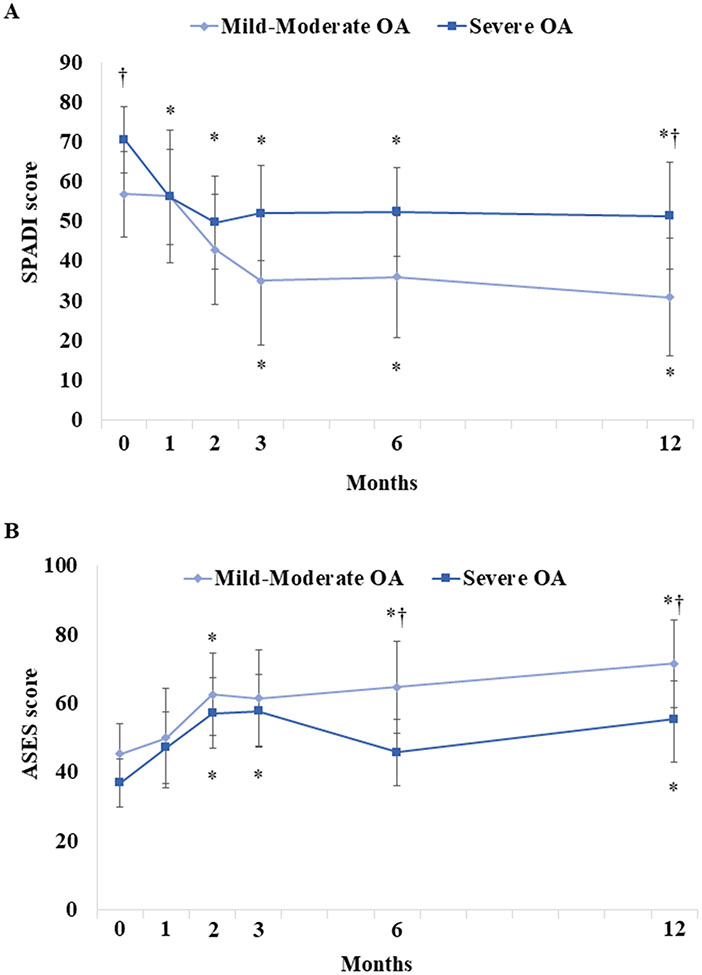
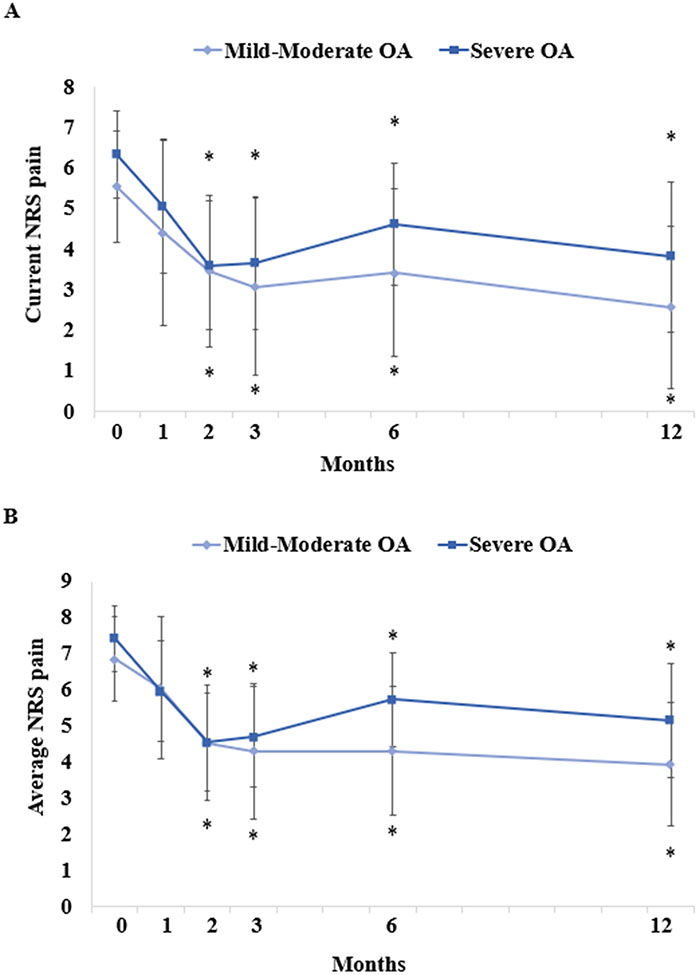
SPADI scores in the severe OA group were significantly higher at baseline (70.6[95% CI: 62.3–78.8] vs. 56.9[46.1–67.7]; p=0.030) and 12 months post-injection (51.4[95% CI: 37.9–64.9] vs. 30.8[16.0–45.7]; p=0.022), compared to those in the mild/moderate OA group; no other differences between mild/moderate and severe OA groups were observed (Figure 5A). ASES scores in the severe OA group were significantly lower at 6 months (45.8[95% CI: 36.1–55.5] vs. 64.7[51.4–78.0]; p=0.007) and 12 months only (55.5[95% CI: 42.7–66.4] vs. 71.5[58.7–84.2]; p=0.034), compared to the mild/moderate OA group (Figure 5B). Current/average NRS pain scores were similar between mild/moderate and severe OA groups (Figure 6).
Figure 5. Patient-Reported Outcomes By OA Severity Following Treatment.
Parameter estimates of (A) SPADI and (B) ASES scores in patients with mild-moderate osteoarthritis (OA) vs. severe OA are shown. Error bars are 95% confidence intervals. *P<0.05 vs. baseline; †P<0.05 vs. mild-moderate OA.
Figure 6. Pain Outcomes by OA Severity Following Treatment.
Parameter estimates of (A) current NRS pain and (B) average NRS pain in patients with mild-moderate OA vs. severe OA are shown. Error bars are 95% confidence intervals. *P<0.05 vs. baseline.
Platelet Yield and Patient-Reported Outcomes
Additional analyses evaluated the effect of platelet yield on patient-reported outcomes in patients who received a single injection of LP-PRP. Overall, there were no effects of platelet yield on SPADI, ASES, current NRS pain, and average NRS pain scores at any of the follow-up time points in the crude model (Table 3).
Table 3.
Effect of Platelet Yield on Patient-Reported Outcomes.
| Timepoint | Slope (SE) | 95% CI | P-value |
|---|---|---|---|
| SPADI | |||
| Baseline | REF | ||
| 1 Month | −1.4 (2.1) | −5.6, 2.7 | 0.50 |
| 2 Months | 0.0 (2.1) | −4.1, 4.2 | 0.99 |
| 3 Months | 0.6 (2.1) | −3.5, 4.8 | 0.77 |
| 6 Months | −2.1 (2.1) | −6.3, 2.1 | 0.33 |
| 12 Months | 3.7 (2.4) | −1.0, 8.4 | 0.12 |
| ASES score | |||
| Baseline | REF | ||
| 1 Month | 3.1 (2.2) | −1.2, 7.4 | 0.16 |
| 2 Months | 0.0 (2.2) | −4.4, 4.2 | 0.96 |
| 3 Months | 2.4 (2.2) | −1.9, 6.7 | 0.28 |
| 6 Months | 2.7 (2.2) | −1.7, 7.0 | 0.23 |
| 12 Months | −2.2 (2.3) | −6.7, 2.4 | 0.35 |
| Current NRS Pain | |||
| Baseline | REF | ||
| 1 Month | 0.0 (0.3) | −0.6, 0.7 | 0.95 |
| 2 Months | 0.5 (0.3) | −0.1, 1.2 | 0.11 |
| 3 Months | 0.0 (0.3) | −0.6, 0.7 | 0.92 |
| 6 Months | 0.2 (0.3) | −0.5, 0.8 | 0.61 |
| 12 Months | 0.6 (0.3) | −0.1, 1.3 | 0.07 |
| Average NRS Pain | |||
| Baseline | REF | ||
| 1 Month | −0.1 (0.3) | −0.7, 0.5 | 0.75 |
| 2 Months | 0.2 (0.3) | −0.4, 0.8 | 0.57 |
| 3 Months | −0.2 (0.3) | −0.7, 0.4 | 0.59 |
| 6 Months | −0.1 (0.3) | −0.7, 0.5 | 0.65 |
| 12 Months | 0.3 (0.3) | −0.3, 0.9 | 0.34 |
Abbreviations: ASES – American Shoulder and Elbow Surgeons; CI – confidence interval; NRS – numerical rating scale; REF – reference; SPADI – Shoulder Pain and Disability Index.
Need for Surgery
Sixteen patients (22.9%) underwent total shoulder replacements following their study injections. Ten had surgery during the study period, and 6 had surgery after their 12-month follow-ups. Only baseline SPADI score was significantly different between patients who did and did not need surgery (73.7±14.0 and 62.1±16.0, respectively; p=0.011) (Table 4).
Table 4.
Comparison of Baseline Characteristics Between Participants With and Without Need for Surgery
| Variables | No Surgery (n=53) | Surgery (N=16) | P-value |
|---|---|---|---|
| Age, years; mean (SD) | 68.8 (12.0) | 68.2 (11.3) | 0.86 |
| Female sex; n (%) | 25 (48) | 11 (69) | 0.15 |
| BMI, kg/m2; mean (SD) | 27.2 (4.8) | 28.6 (6.6) | 0.38 |
| Right-sided OA; n (%) | 30 (58) | 8 (50) | 0.59 |
| Duration of symptoms, months; mean (SD) | 45 (75) | 30 (14) | 0.19 |
| Use of pain medication; n (%) | 33 (65) | 12 (75) | 0.10 |
| Severe OA; n (%) | 32 (62) | 10 (63) | 0.94 |
| Platelet yield, fold increase; mean (SD) | 3.4 (1.8) | 3.5 (0.8) | 0.91 |
| SPADI score; mean (SD) | 62.1 (16.0) | 73.7 (14.1) | 0.011 |
Abbreviations: BMI – body mass index; HA – hyaluronic acid; LP-PRP – leukocyte-poor platelet-rich plasma; OA – osteoarthritis; SD – standard deviation; SPADI – Shoulder Pain and Disability Index
Discussion
Results from this randomized controlled trial demonstrated no significant differences in outcomes between patients who received a single injection of HA versus a single injection of LP-PRP. Improvements in SPADI, NRS pain, and ASES scores were observed over time in both groups, regardless of OA severity. In patients receiving LP-PRP, platelet yield had no effect on patient-reported outcomes throughout the follow-up period. Sixteen patients underwent surgery; these patients had higher SPADI scores at baseline, demonstrating more disability. Injection-related side effects were uncommon.
Although the effects of conservative treatments for glenohumeral OA have been well characterized,1 evidence for some treatments remains inconclusive, leading one to consider emerging treatments. HA has shown efficacy in knee OA,36 and the use of HA for glenohumeral OA has shown promising results, although there are variabilities in factors such as image guidance.14 A recent meta-analysis of 7 prospective and retrospective studies demonstrated improvements in pain and functional outcomes following intra-articular HA injections for the treatment of glenohumeral OA. The same meta-analysis also reported pain improvements in the control group, with the authors concluding that further studies are needed to evaluate the efficacy of HA.14 Two studies have shown larger reductions in pain following HA injections versus placebo injections, although these were not statistically significant at the primary-outcome endpoints.10,11 In the current study, we observed significant improvements in SPADI, ASES, and NRS pain scores over a 12-month period following a single injection of HA into the glenohumeral joint. All improvements were clinically important and supported previous studies showing that HA injections result in improvements in patients with glenohumeral OA.10-12,14,17-22,25
Another emerging treatment is PRP, which has shown efficacy in various musculoskeletal conditions, including rotator cuff pathology and “periarthritis shoulder”.37,38 Only one study has investigated the efficacy of PRP in the treatment of glenohumeral OA. In a randomized controlled trial, Saif et al. demonstrated improvements in quality-of-life and pain scores following intra-articular injections with PRP or steroids, with greater improvements observed with PRP.32 Similarly, results from our study showed statistically and clinically significant improvements in SPADI, ASES, and NRS pain scores following a single injection of LP-PRP. We chose to use LP-PRP instead of leukocyte-rich PRP, as LP-PRP has been shown to promote chondrogenesis and lead to better cartilage repair, compared to leukocyte-rich PRP.39 Furthermore, leukocyte-rich PRP has been suggested to cause increased inflammation in the acute period following treatment.40,41 Interestingly, platelet yield did not appear to have an effect on the outcomes over time. This may be due to variations in PRP preparation or the patient’s physiological status, both of which may affect the efficacy of PRP.42 It has also been shown that PRP, when prepared in the same manner, may elicit different effects in the same individual.43
Contrary to our study hypothesis that LP-PRP would be more effective than HA, there were no differences in outcomes between LP-PRP and HA groups at the follow-up timepoints. Improvements in outcomes following either single injections of LP-PRP or HA were sustained up to 12 months post-injection. Side effect rates for both treatments were low, and satisfaction rates were similar. This suggests that both treatments are associated with increasing function and decreasing shoulder pain and disability. No other study has compared these two treatments for glenohumeral OA. Further studies investigating longer-term outcomes with a larger sample size are warranted to confirm these findings.
This study has several limitations. Not all patients completed the follow-up questionnaires. Ten patients underwent surgery during their follow-up period; as per the study design, follow-ups were discontinued for these patients, but they were still included in the statistical analyses. This was likely because most patients had severe OA. Further studies are needed to compare outcomes in the mild/moderate OA population, although results from the current study showed improvements in both mild/moderate and severe OA cohorts. This could be because radiological abnormalities do not always correlate with clinical findings.44 Furthermore, platelet counts could not be determined in a few whole-blood and LP-PRP samples, due to clotting, which can be caused by inadequate inverting of the tube after collection. However, there was no association between platelet yield and outcomes in this cohort. Additionally, there was no control group in the study. Placebo effects should be considered as well.
In conclusion, both LP-PRP and HA were associated with improvements in pain, disability, and functional impairments related to glenohumeral OA in this cohort of patients, and there were no differences between treatments. Similar findings were observed regardless of OA severity. In patients receiving LP-PRP, there was no association between platelet yield and outcomes. In both groups, side effects were uncommon, and satisfaction rates were high. Altogether, these findings suggest that both LP-PRP and HA can be considered as viable treatments in patients with glenohumeral OA of varying degrees of severity. Future studies should investigate different types of PRP, compare different injection volumes, or include a placebo group to further elucidate the efficacy of HA and PRP in the treatment of glenohumeral OA.
Acknowledgments:
This study was supported by the Richard ERF Materson New Investigator Grant from the Foundation for PM&R (to J.K.) and Harvest–Terumo BCT (donation of kits). REDCap use was supported by grant number UL1TR002384 from the National Center for Advancing Translational Sciences of the National Institutes of Health. Thank you to Rachel Rothman for assisting with manuscript preparation.
References
- 1.Ansok CB, Muh SJ. Optimal management of glenohumeral osteoarthritis. Orthop Res Rev. 2018; 10:9–18. doi: 10.2147/orr.S134732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber A, Lehtinen JT, Warner JJ. Glenohumeral osteoarthritis in active patients: diagnostic tips and complete management options. Phys Sportsmed. Apr 2003; 31(4):33–40. doi: 10.3810/psm.2003.04.312 [DOI] [PubMed] [Google Scholar]
- 3.Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. May 4 2011; 93(9):885–892. doi: 10.2106/jbjs.J.00960 [DOI] [PubMed] [Google Scholar]
- 4.Weldon EJ 3rd, Richardson AB. Upper extremity overuse injuries in swimming. A discussion of swimmer's shoulder. Clin Sports Med. Jul 2001; 20(3):423–438. doi: 10.1016/s0278-5919(05)70260-x [DOI] [PubMed] [Google Scholar]
- 5.Maquirriain J, Ghisi JP, Amato S. Is tennis a predisposing factor for degenerative shoulder disease? A controlled study in former elite players. Br J Sports Med. May 2006; 40(5):447–450. doi: 10.1136/bjsm.2005.023382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi LA, Piuzzi NS, Shapiro SA. Glenohumeral Osteoarthritis: The Role for Orthobiologic Therapies: Platelet-Rich Plasma and Cell Therapies. JBJS Rev. Feb 2020; 8(2):e0075. doi: 10.2106/jbjs.Rvw.19.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockmeier SF, Shaffer BS. Viscosupplementation therapy for osteoarthritis. Sports Med Arthrosc Rev. Sep 2006; 14(3):155–162. doi: 10.1097/00132585-200609000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Strauss EJ, Hart JA, Miller MD, et al. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. Aug 2009; 37(8):1636–1644. doi: 10.1177/0363546508326984 [DOI] [PubMed] [Google Scholar]
- 9.Colen S, Haverkamp D, Mulier M, et al. Hyaluronic acid for the treatment of osteoarthritis in all joints except the knee: what is the current evidence? BioDrugs. Apr 1 2012; 26(2):101–112. doi: 10.2165/11630830-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10.Blaine T, Moskowitz R, Udell J, et al. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial. A multicenter study. J Bone Joint Surg Am. May 2008; 90(5):970–979. doi: 10.2106/jbjs.F.01116 [DOI] [PubMed] [Google Scholar]
- 11.Kwon YW, Eisenberg G, Zuckerman JD. Sodium hyaluronate for the treatment of chronic shoulder pain associated with glenohumeral osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. J Shoulder Elbow Surg. May 2013; 22(5):584–594. doi: 10.1016/j.jse.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 12.Merolla G, Sperling JW, Paladini P, et al. Efficacy of Hylan G-F 20 versus 6-methylprednisolone acetate in painful shoulder osteoarthritis: a retrospective controlled trial. Musculoskelet Surg. Dec 2011; 95(3):215–224. doi: 10.1007/s12306-011-0138-3 [DOI] [PubMed] [Google Scholar]
- 13.Tagliafico A, Serafini G, Sconfienza LM, et al. Ultrasound-guided viscosupplementation of subacromial space in elderly patients with cuff tear arthropathy using a high weight hyaluronic acid: prospective open-label non-randomized trial. Eur Radiol. Jan 2011; 21(1):182–187. doi: 10.1007/s00330-010-1894-4 [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Thayaparan A, Horner N, et al. Outcomes of hyaluronic acid injections for glenohumeral osteoarthritis: a systematic review and meta-analysis. J Shoulder Elbow Surg. Mar 2019; 28(3):596–606. doi: 10.1016/j.jse.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 15.Di Giacomo G, de Gasperis N. Hyaluronic Acid Intra-Articular Injections in Patients Affected by Moderate to Severe Glenohumeral Osteoarthritis: A Prospective Randomized Study. Joints. Sep 2017; 5(3):138–142. doi: 10.1055/s-0037-1605389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Giacomo G, De Gasperis N. The role of hyaluronic acid in patients affected by glenohumeral osteoarthritis. J Biol Regul Homeost Agents. Oct-Dec 2015; 29(4):945–951. [PubMed] [Google Scholar]
- 17.Silverstein E, Leger R, Shea KP. The use of intra-articular hylan G-F 20 in the treatment of symptomatic osteoarthritis of the shoulder: a preliminary study. Am J Sports Med. Jun 2007; 35(6):979–985. doi: 10.1177/0363546507300256 [DOI] [PubMed] [Google Scholar]
- 18.Weil AJ. High molecular weight hyaluronan for treatment of chronic shoulder pain associated with glenohumeral arthritis. Med Devices (Auckl). 2011; 4:99–105. doi: 10.2147/mder.S22423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKee MD, Litchfield R, Hall JA, et al. NASHA hyaluronic acid for the treatment of shoulder osteoarthritis: a prospective, single-arm clinical trial. Med Devices (Auckl). 2019; 12:227–234. doi: 10.2147/mder.S189522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porcellini G, Merolla G, Giordan N, et al. Intra-articular glenohumeral injections of HYADD®4-G for the treatment of painful shoulder osteoarthritis: a prospective multicenter, open-label trial. Joints. Jul-Sep 2015; 3(3):116–121. doi: 10.11138/jts/2015.3.3.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brander VA, Gomberawalla A, Chambers M, et al. Efficacy and safety of hylan G-F 20 for symptomatic glenohumeral osteoarthritis: a prospective, pilot study. Pm r. Apr 2010; 2(4):259–267. doi: 10.1016/j.pmrj.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 22.Noël E, Hardy P, Hagena FW, et al. Efficacy and safety of Hylan G-F 20 in shoulder osteoarthritis with an intact rotator cuff. Open-label prospective multicenter study. Joint Bone Spine. Dec 2009; 76(6):670–673. doi: 10.1016/j.jbspin.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 23.Kwon JW, Lee JW, Kim SH, et al. Cervical interlaminar epidural steroid injection for neck pain and cervical radiculopathy: effect and prognostic factors. Skeletal Radiol. May 2007; 36(5):431–436. doi: 10.1007/s00256-006-0258-2 [DOI] [PubMed] [Google Scholar]
- 24.Colen S, Geervliet P, Haverkamp D, et al. Intra-articular infiltration therapy for patients with glenohumeral osteoarthritis: A systematic review of the literature. Int J Shoulder Surg. Oct 2014; 8(4):114–121. doi: 10.4103/0973-6042.145252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Giacomo G, de Gasperis N. Glenohumeral osteoarthritis treatment with a single hyaluronic acid administration: clinical outcomes. J Biol Regul Homeost Agents. Mar-Apr 2021; 35(2):657–661. doi: 10.23812/20-457-l [DOI] [PubMed] [Google Scholar]
- 26.Navani A, Li G, Chrystal J. Platelet Rich Plasma in Musculoskeletal Pathology: A Necessary Rescue or a Lost Cause? Pain Physician. Mar 2017; 20(3):E345–e356. [PubMed] [Google Scholar]
- 27.Sampson S, Reed M, Silvers H, et al. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. Dec 2010; 89(12):961–969. doi: 10.1097/PHM.0b013e3181fc7edf [DOI] [PubMed] [Google Scholar]
- 28.Gobbi A, Karnatzikos G, Mahajan V, et al. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. Mar 2012; 4(2):162–172. doi: 10.1177/1941738111431801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. Feb 2013; 41(2):356–364. doi: 10.1177/0363546512471299 [DOI] [PubMed] [Google Scholar]
- 30.Sánchez M, Guadilla J, Fiz N, et al. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford). Jan 2012; 51(1):144–150. doi: 10.1093/rheumatology/ker303 [DOI] [PubMed] [Google Scholar]
- 31.Freitag J. The Effect of Photoactivated Platelet-Rich Plasma Injections in the Novel Treatment of Shoulder Osteoarthritis. Int J Case Rep Images. 2014; 5:546–552. [Google Scholar]
- 32.Saif DS, Serag DM, El Tabl MA. Comparative study between platelet-rich plasma injection and steroid injection in mild–moderate shoulder osteoarthritis and their relation to quality of life. Egypt Rheumatol Rehabil. 2019/January/01 2019; 46(1):55–61. doi: 10.4103/err.err_17_18 [DOI] [Google Scholar]
- 33.Elsharkawi M, Cakir B, Reichel H, et al. Reliability of radiologic glenohumeral osteoarthritis classifications. J Shoulder Elbow Surg. Aug 2013; 22(8):1063–1067. doi: 10.1016/j.jse.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 34.Vo KV, Hackett DJ, Gee AO, et al. Classifications in Brief: Walch Classification of Primary Glenohumeral Osteoarthritis. Clin Orthop Relat Res. 2017; 475(9):2335–2340. doi: 10.1007/s11999-017-5317-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carette S, Moffet H, Tardif J, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo-controlled trial. Arthritis Rheum. Mar 2003; 48(3):829–838. doi: 10.1002/art.10954 [DOI] [PubMed] [Google Scholar]
- 36.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. Jul 18 2014; 5(3):351–361. doi: 10.5312/wjo.v5.i3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson DM, Eng C, Makovitch S, et al. Non-operative orthobiologic use for rotator cuff disorders and glenohumeral osteoarthritis: A systematic review. J Back Musculoskelet Rehabil. 2021; 34(1):17–32. doi: 10.3233/bmr-201844 [DOI] [PubMed] [Google Scholar]
- 38.Kothari SY, Srikumar V, Singh N. Comparative Efficacy of Platelet Rich Plasma Injection, Corticosteroid Injection and Ultrasonic Therapy in the Treatment of Periarthritis Shoulder. J Clin Diagn Res. 2017; 11(5):RC15–RC18. doi: 10.7860/JCDR/2017/17060.9895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Yin W, Zhang Y, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep. 2017; 7:43301–43301. doi: 10.1038/srep43301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dragoo JL, Braun HJ, Durham JL, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. Jun 2012; 40(6):1274–1281. doi: 10.1177/0363546512442334 [DOI] [PubMed] [Google Scholar]
- 41.Le ADK, Enweze L, DeBaun MR, et al. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med. 2018; 11(4):624–634. doi: 10.1007/s12178-018-9527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuffler DP. Variables affecting the potential efficacy of PRP in providing chronic pain relief. J Pain Res. 2018; 12:109–116. doi: 10.2147/JPR.S190065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuffler DP. Differing efficacies of autologous platelet-rich plasma treatment in reducing pain following rotator-cuff injury in a single patient. J Pain Res. 2018; 11:2239–2245. doi: 10.2147/JPR.S169647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kircher J, Morhard M, Magosch P, et al. How much are radiological parameters related to clinical symptoms and function in osteoarthritis of the shoulder? Int Orthop. 2010; 34(5):677–681. doi: 10.1007/s00264-009-0846-6 [DOI] [PMC free article] [PubMed] [Google Scholar]