Abstract
When conducting combined therapy of malignant neoplasms, treatment methods with various mechanisms of antitumor effects are used, while an additive or even synergistic effect can be realized. Combination treatment regimens are aimed at increasing the efficiency and, above all, at the complete eradication of the tumor, which can be achieved either by suppressing the survival mechanisms in PDT-resistant tumor cells or by pre-attenuation of tumor cells so that they become more susceptible to subsequent PDT. Photodynamic therapy is an approved medical technology for the treatment of various malignant neoplasms, and several precancerous and non-cancer diseases. To date, numerous data have been published on the combined use of PDT with traditional and innovative methods of treatment. This review considers research in this area in recent years.
Keywords: Photodynamic therapy, Photosensitizer, Combined cancer therapy, Nanoparticle drug delivery
Introduction
Treatment of malignant neoplasms is a challenge that cannot always be managed successfully. A variety of clinical protocols are currently used for cancer treatment. A specific treatment scheme is determined for each specific case of the disease, with consideration for the degree of the process development and localization. It may include one or more kinds of treatments. The main of these are surgery, chemotherapy, and radiation therapy. The use of alternative treatments can provide better results in some cases. Anticancer photodynamic therapy (PDT) is one of these methods (Filonenko 2021). It involves the application of photosensitizers (PS) combined with visual light of a certain wavelength for their excitation. They transfer energy to molecular oxygen to form reactive oxygen species (ROS). Of these, singlet oxygen is the main cytotoxic agent that causes necrosis of cancer tissues (Celli et al. 2010; Dąbrowski and Arnaut 2015). PDT is a high-tech medical technique for the treatment of cancer patients. Its main advantages include non-invasiveness, absence of complications that arise from surgery, and minimization of side effects characteristic of chemotherapy and radiotherapy. The use of PDT does not require long-term inpatient treatment or expensive drugs, owing to which it is a cost-efficient method.
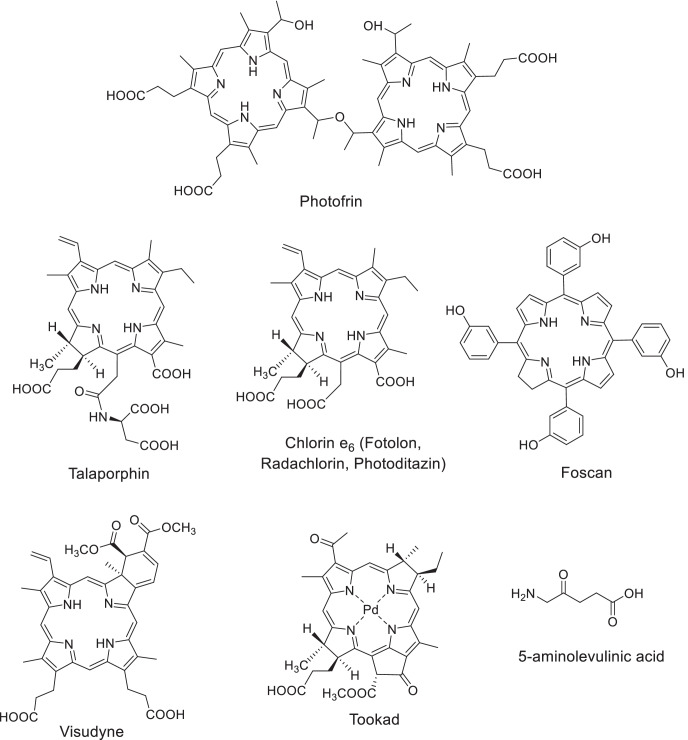
There are three generations of photosensitizers, among which compounds of the tetrapyrrole series are most widely used in PDT. The first-generation drugs include natural hematoporphyrin derivatives, namely, Photofrin II (USA-Canada) and Photogem (Russia) (Lobel et al. 2001; Moan and Peng 2003; Trindade et al. 2012; Zang et al. 2017; Hu et al. 2018). Second-generation PS with enhanced spectral characteristics were developed on the basis of chlorophyll a derivatives, i.e., Talaporphin (Japan), Photoditazin and Radachlorin (Russia), Fotolon (Belarus), etc.; synthetic chlorins—Visudyne (Canada), Foscan (UK); and phthalocyanines—Photosense (Russia), as well as a photosensitizer that absorbs in the near IR region—Tookad (Israel) (Dolmans et al. 2003; Rousset et al. 2003; Brun et al. 2004; Zverev et al. 2016; Brilkina et al. 2019). The PS are classified as third-generation drugs if their structure comprises vector molecules that ensure the targeted nature of action or based on their spectral characteristics.
The development of highly efficient photosensitizers and optimization of their dosage forms made is possible to make significant progress in the fluorescent diagnostics (FD) and in the PDT of malignant neoplasms (Fig. 1). To date, numerous data have been published on the combined use of PDT with traditional and innovative methods of treatment. This review considers research in this area in recent years.
Fig. 1.
Photosensitizers used in clinical practice for the PDT of cancer
Combined chemotherapy and PDT for cancer treatment
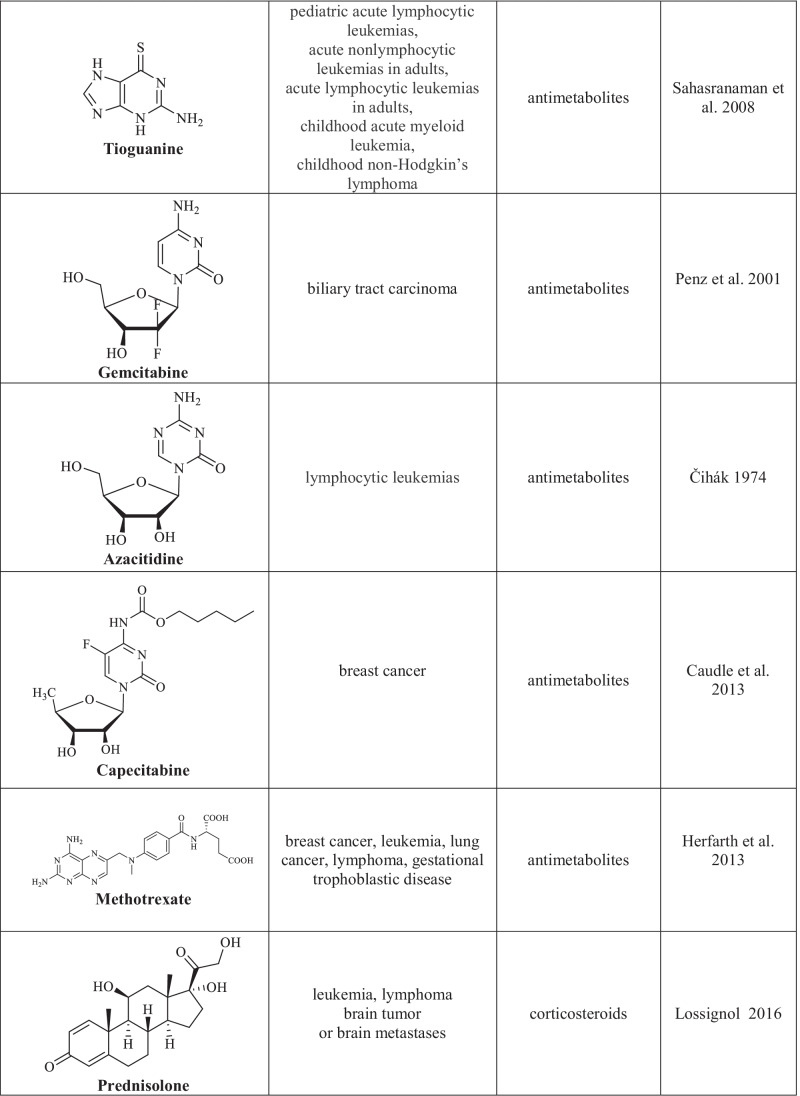
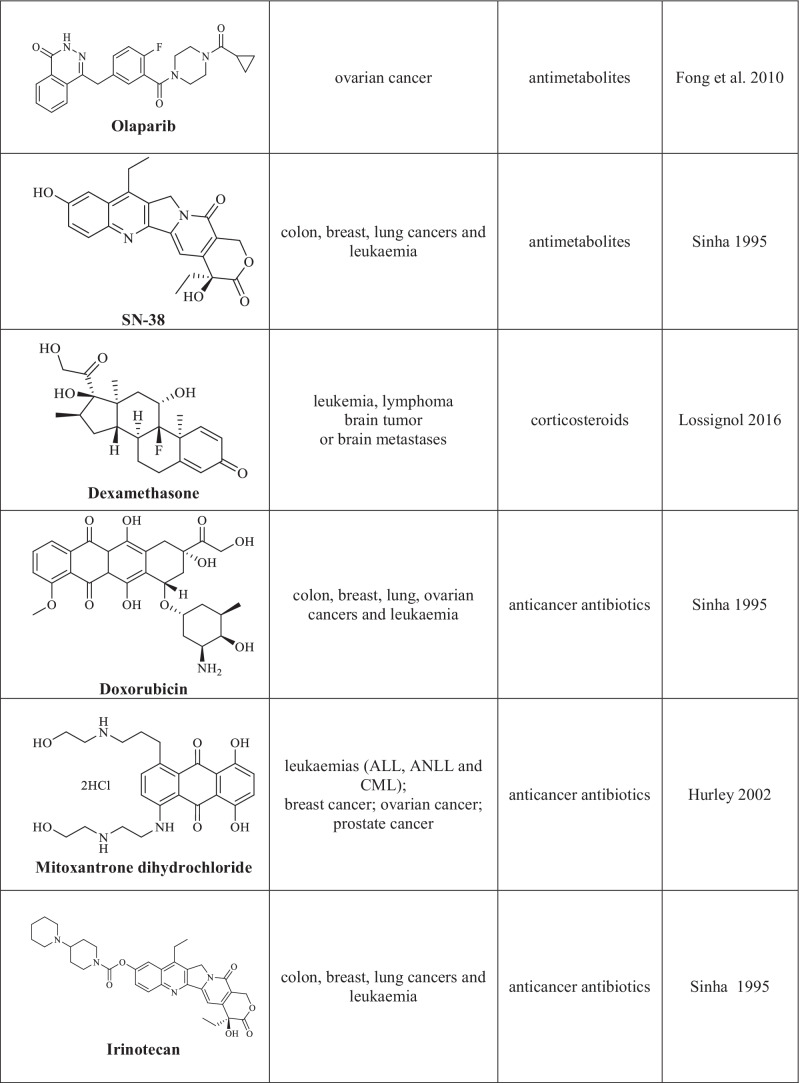
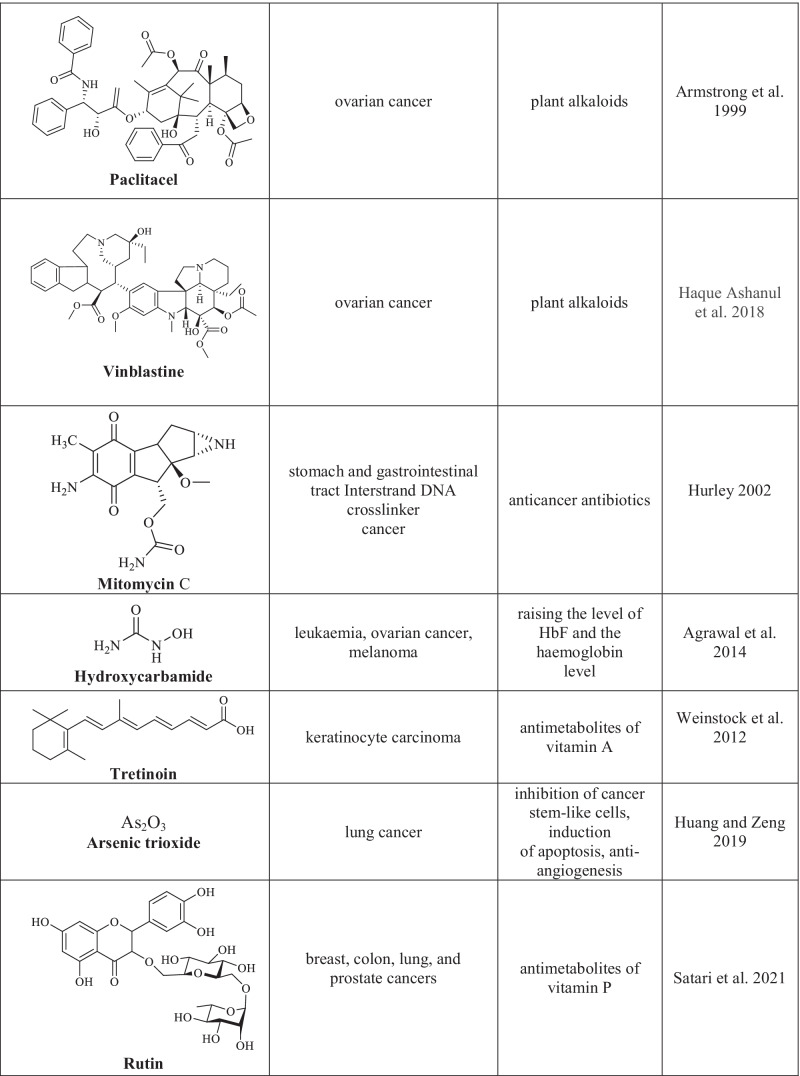
Chemotherapy is one of the basic and efficient methods for the treatment of oncological diseases (Schirrmacher 2019). Its main advantage is the direct cytotoxic effect attained by using chemotherapy drugs which, unfortunately, feature many side effects due to their impact on normal cells. Chemotherapy drugs (cytostatic agents) are classified according to the mechanism of their effect on cancer cells that results in the arrest of their proliferation (Chu 2017). The following groups of chemotherapy agents are distinguished: drugs with alkylating action of various chemical groups, antimetabolites, plant alkaloids, corticosteroids, and other chemotherapy drugs with various action mechanisms (Table 1) (Sinha 1995; Hurley 2002; Asuncion 2014; Isah 2016; Lossignol 2016; Gao et al. 2020; Amjad et al. 2022).
Table 1.
Cytotoxic agents used in the chemotherapy of cancer
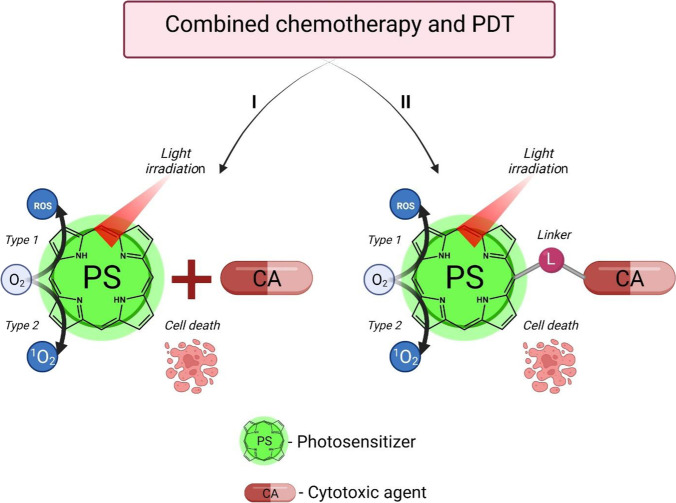
The use of photodynamic therapy combined with chemotherapy proved to be highly efficient in both in vitro and in vivo studies and in clinical practice. Analysis of research publications makes it possible to distinguish two approaches, one of which involves the sequential application of two different types of action on the tumor, while the second approach implies the development of new combined-action drugs (Fig. 2).
Fig. 2.
Approaches to the combined use of photosensitizers and chemotherapy agents in oncology
PDT and chemotherapy applied have drawbacks and limitations in monotherapy mode. Currently, combination therapy is actively used to enhance the efficiency of anticancer treatment in clinical practice. It involves the use of two different types of action on a tumor. Such a multimodal approach makes it possible to achieve better curing results due to the synergy effect. Here we review recent studies in the field of PDT combined with other methods of cancer treatment.
A study was conducted that focused on the cytostatic effect of doxorubicin combined with the photodynamic action on tumor cells. Sulfonated zinc phthalocyanine was used as the PS (Aniogo et al. 2017). Cells of mammary adenocarcinoma MCF-7 were incubated with doxorubicin, after which sulfonated zinc phthalocyanine was added in various concentrations in combination with irradiation by a diode laser (681 nm, 5 J/cm2). The studies showed that the combined chemotherapeutic and photodynamic impact efficiently suppressed the proliferation of tumor cells, though the doxorubicin dose was decreased twofold. An important advantage of the combination therapy is that the multidrug resistance of tumor cells is overcome. For example, the human melanoma cell line SK-MEL-3, which features chemoresistance, was used to show that the effect of doxorubicin in the monotherapy mode is low, while if the chemotherapy drug and photodynamic effect induced by the use of zinc phthalocyanine and irradiation are combined, the mechanisms of cell resistance to doxorubicin were blocked, autophagy was activated, the cell cycle was arrested, and a decrease in the ability of cells for migration and enhanced apoptosis was observed (Doustvandi et al. 2019).
Studies were published in which new chemotherapy drugs were explored. These drugs showed enhanced efficiency in combination with PDT. The efficiency of flavonoid (rutoside) was estimated as a new chemotherapy agent (Kopustinskiene et al. 2020) that acts as a pro-oxidant and causes the oxidative stress of tumor cells. The combined effect of rutoside with PDT, where methylene blue was used as a PS, on the lung cancer cell line A375 was studied (Satari et al. 2021). A combination of PDT and rutoside was shown to cause apoptosis and arrest of the cell cycle of tumor cells. As a result, the authors consider this combination as a new approach to the treatment of melanoma.
Data were reported that the therapeutic use of new cytotoxic drugs can be expanded if they are used in combination with PDT. For example, olaparib, a drug registered in 2014 by the European Medicines Agency (EMA) and Food and Drug Administration (FDA) for the monotherapy of certain types of ovarian, breast, and prostate cancer, was studied by M. Tanaka et al. as a new drug for the treatment of stomach cancer in combination with PDT (Tanaka et al. 2021). Studies in vitro on cell lines MKN45 and in vivo showed that olaparib efficiently enhanced the antitumor effect of PDT with talaporphine without significant side effects. The authors noted that they successfully reduced the light dose by a factor of 4 compared with the standard dose for talaporphine (from 100 J/cm2 to 25 J/cm2), which apparently decreased the side effects in combination therapy. The authors believe that this study is a promising result for the development of the strategy of stomach cancer treatment with olaparib.
Of interest are studies on combining cisplatin chemotherapy with PDT. Tae-Gyu Ahn et al. estimated the combined action of cisplatin and photodynamic therapy for EMT6 breast carcinoma in in vivo experiments on nude mice (Ahn et al. 2019). Analysis of tumor tissue transcriptomes showed that the expression of genes associated with the inflammatory response increased, while the expression of Fn1 decreased in the combination therapy group compared to the PDT group. It was assumed that the increased inflammatory response in the combination therapy group compared to the PDT group led to an enhancement of the antitumor effect.
Yet another article provided data on the efficiency of the combination treatment of rats with M-1 sarcoma (Kaplan et al. 2016) using Fotolon and cisplatin as the photodynamic and cytotoxic agents, respectively. Various treatment modes and drug doses were used. The effect of treatment was estimated by the absolute rate of tumor growth, inhibition of tumor growth, and complete tumor regression. It was found that the most effective treatment mode involved photodynamic therapy with irradiation in 2 h after administration of the photosensitizer and subsequent administration of cisplatin 1 and 4 days after the PDT with a 2.5 mg/kg total dose of the chemotherapy drug. By the end of the study, complete tumor regression was noted in 88.9% of tumor-bearing animals and inhibition of tumor growth was observed in 99.9% of all the cases. The conclusion was made that the combination of photodynamic therapy with cisplatin therapy is more efficient than monotherapy, as it leads to a synergy effect and favors a reduction in the cisplatin dose.
In combined chemo- and photodynamic therapy, anticancer drugs of different classes are sometimes used simultaneously (polychemotherapy). Most often, this approach is used to treat advanced oncological diseases, as well as nosologies that poorly respond to conventional treatments. Two independent research teams headed by C. Büning and T. Weismüller studied PDT combined with chemotherapy in patients with unresectable cholangiocarcinoma (Wentrup et al. 2016; Gonzalez-Carmona et al. 2019). PDT was performed by endoscopic retrograde cholangiopancreatography. Porphyrin derivatives were used as photosensitizers at standard doses of 1.5 to 2.5 mg/kg, depending on the formulation. Total body chemotherapy was carried out using a combination of drugs (gemcitabine and cisplatin; gemcitabine and oxaliplatin; gemcitabine and capecitabine; cisplatin and irinotecan), or monotherapy with gemcitabine or 5-FU (5-Fluorouracil) was performed. An increase in median survival in combination therapy by a factor of about 1.5 with respect to PDT monotherapy, or by a factor of 2 with respect to monochemotherapy was noted.
D.A. Granov et al. conducted clinical studies on the efficacy of combination therapy in advanced stages of bile duct cancer (Polysalov et al. 2018). Radachlorin or Fotolon were used as photosensitizers. Chemotherapy was carried out a few days after PDT by the scheme: on day 1, rituximab was administered intravenously and, on day 2, intravenous infusion of gemcitabine and oxaliplatin was performed (López et al. 2008). The study showed that the combination of locoregional therapy methods for unresectable Klatskin tumors with intraductal PDT and chemotherapy improved the prognosis of the disease and the quality of life of patients, and also increased the median survival and average life expectancy, and, in some cases, allowed stage IV tumor pathomorphosis to be achieved.
Lung cancer that is often an unresectable type of cancer is poorly treatable with monotherapy. Clinical studies were conducted to estimate the safety and efficacy of combined treatment of non-small cell and other types of lung cancer, even at terminal stages, including chemotherapy and endobronchial PDT. A.L. Akopov et al. compared the results of treatment of two groups of patients with centrally located non-small cell lung cancer. Group A received first-line chemotherapy with endobronchial PDT, while group B only received chemotherapy. PDT was carried out using photosensitizers of chlorin series at a dose of 1 mg/kg body weight (Simone et al. 2012; Akopov et al. 2014). Based on the results, it was concluded that combining endobronchial PDT with chemotherapy improves the treatment outcomes and survival of patients with lung cancer compared to monochemotherapy (Simone et al. 2012; Kimura et al. 2015; Shafirstein et al. 2016).
R. Mahmood et al. studied the effects of various vitamin A analogs used as a neoadjuvant agent by combining the administration of chemotherapy drugs (doxorubicin and methotrexate) with photodynamic impact (5-aminolevulinic acid or Photogem) (Mahmood et al. 2020) in experiments in vitro on rhabdomyosarcoma cells. It was shown that the use of vitamin A as a neoadjuvant agent reduced the efficacy of the cytotoxic effect by suppressing the induction of oxidative stress.
The combination of PDT and chemotherapy is now widely used in veterinary medicine. The treatment of periorbital equine sarcoids in horses is a challenging problem if monotherapy is used. H. Gehlen et al. developed a combination therapy that leads to complete regression of the disease in 87% cases where irradiation of the tumor surface with a diode laser using methylene blue as a PS was combined with the intratumoral administration of mitomycin (Marginter et al. 2021).
Okamoto et al. developed a protocol for the treatment of carcinomas in cats and dogs using combination therapy (Osaki et al. 2019). Cisplatin was used as the chemotherapeutic agent, and photodynamic therapy was conducted with 5-aminolevulinic acid. The authors were the first to find a correlation between the intracellular accumulation of protoporphyrin IX and the relative levels of uroporphyrinogen III synthase (UROS) mRNA in various sarcomas in which the suppression of the UROS protein stimulates the biosynthesis of protoporphyrin IX, thus favoring an enhancement of the efficacy of combination therapy.
The main difficulty encountered by doctors in the implementation of combined chemo- and photodynamic therapy is the choice of the optimal time of administration and doses of drugs with different mechanisms of action on the tumor. Another approach that is currently under development involves the use of drugs that combine chemotherapeutic and photodynamic subunits in the same molecule.
Combining a cytotoxic agent and a PS in one molecule may enhance the synergistic antitumor effect, overcome drug resistance, and reduce the therapeutic doses of the drug. As a result, side effects may be diminished.
This approach implies that both the photodynamic and cytostatic agents are simultaneously delivered to the zone of interest, owing to which the pharmacokinetics of the dual-action drug becomes more predictable. In addition, this approach makes it possible to monitor the accumulation of the chemotherapeutic agent due to fluorescence of the photosensitizer. Compounds of the porphyrin series are known to have affinity to tumor tissues and thus exhibit a directed (targeted) effect (Tojo et al. 2022).
The strategy of covalent addition of a cytotoxic agent to a photosensitizer is extremely promising and extends the capabilities of antitumor therapy. Fedorov et al. recently published a review on this topic (Otvagin et al. 2022). In this review, we will consider some features of this approach.
A pigment molecule can be covalent-bound to a cytotoxic agent via a linker or without it. The latter option is possible if the photosensitizer does not affect the mechanism of cytotoxic action of the antitumor agent, which, in turn, does not quench the generation of reactive oxygen species. Conjugates of this kind based on photosensitizers and cytotoxic compounds of platinum, gold, and tin were reported (Song et al. 2002, 2003; Lottner et al. 2004; Battogtokh et al. 2012; Naik et al. 2014; Le et al. 2021; Tikhonov et al. 2021; Toubia et al. 2021).
Guo et al. (2017b) report the synthesis of conjugates of chlorin e6 with artesunate, a semi-synthetic derivative of artemisinin which is used for malaria treatment. The use of drugs containing artemisinin derivatives (artemisinin combination drugs) is currently the standard in the treatment of tropical malaria worldwide. The structure of artesunate comprises an endoperoxide bridge, which in the presence of divalent iron is cleaved by the Fenton reaction with generation of reactive oxygen species, which can induce the apoptosis of tumor cells (Li et al. 2015; Letis et al. 2017). In other words, the action of artesunate is similar to that of a photosensitizer. The authors prepared chlorins comprising from one to three artesunate molecules. It was shown that both photoinduced and “dark” toxicity increased with respect to the original chlorin e6, thus indicating a synergistic effect of the photosensitizer and artesunate.
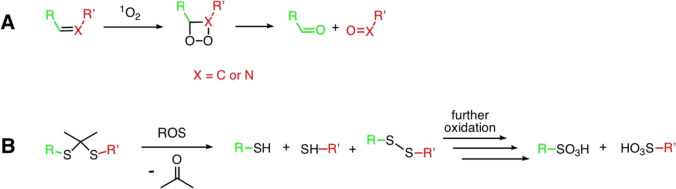
The use of linkers is required both for distancing the photoactive and cytotoxic moieties and for creating prodrugs. To this end, pH-sensitive linkers, enzyme-sensitive linkers, and linkers that are degraded by reactive oxygen species are used. The latter are of particular interest for application in PDT. Molecules containing C = C or C = N double bonds (Rudshteyn et al. 2014; Ghosh et al. 2015) can act as such linkers. The mechanism of cleavage of such linkers is schematically shown in Fig. 3A. You et al. published a number of papers on the creation of conjugates of photosensitizers with various cytotoxic agents using an aminoacrylate linker, which was shown to successfully implement the approach called “photo-unclick chemistry.” They developed conjugates based on porphyrins and phthalocyanines with paclitaxel and combretastatin A4 and performed their biological tests in vitro and in vivo (Bio et al. 2013, 2014, 2017; Hossion et al. 2013; Rajaputra et al. 2016; Thapa et al. 2016). The prodrug itself was shown to be significantly less toxic than the parent cytotoxic agent. Irradiation with light was followed by both release of the chemotherapeutic drug and cell death due to photodynamic exposure.
Fig. 3.
Alkenyl (A) and thioketal (B) linker degradation via ROS generated by PS
Thioketals are yet another type of linkers sensitive to singlet oxygen, which have been shown to be promising tools in the creation of prodrugs (Fig. 3B) (Lamb and Barbas 2015; Liu and Thayumanavan 2020). Zhang et al. obtained conjugates of 5,10,15,20-tetraphenylporphyrin with gemcitabine (Liu et al. 2016). In one case, they used a thioketal-based linker, and in the other case, an alkyl linker not affected by singlet oxygen. Porphyrin conjugation was shown to increase the stability and decrease the cytotoxicity of gemcitabine. Owing to this, such conjugates can be used as prodrugs with controllable release of the cytotoxic agent. In addition, due to the presence of a hydrophobic porphyrin molecule in the conjugate, accumulation of the latter in the cells is higher compared to polar gemcitabine. This conjugate showed significant antitumor efficacy in vitro and in vivo in PDT accompanied by release of gemcitabine due to the cleavage of the thioketal linker. In a study of a conjugate with a non-cleavable alkyl linker, specific activity only due to the photodynamic effect was observed.
Combination of PDT and surgery
Surgery is the oldest and one of traditional approaches to cancer treatment. However, it is usually combined with other types of treatment such as radio-, chemo-, or immunotherapy. Using photodynamic therapy combined with surgery can increase the efficacy of disease management. There have been published many studies on combined PDT and surgery in different types of tumors (Caesar et al. 2015; Hao et al. 2022).
Wang et al. studied sequential PDT and surgery for squamous cell carcinoma of the lip which is conventionally treated by surgery or radiotherapy. PDT benefits preservation of unaffected tissues and that can improve the cosmetic result. The authors have used topical 5-aminolevulinic acid in combination with superficial resection surgery for invasive squamous cell lip carcinoma. They reported tumor-free result at 12 months follow-up without patient disability (Yan et al. 2020).
Facial basal cell carcinoma is a common skin cancer occurring on open skin areas (face, head, neck, hands) in which PDT can be useful. Wang et al. in their study have used Mohs surgery (a micrographic surgery which is an optimal method in the field of skin cancers). Essentially it is a preserving tumor removal, where the resection quality is controlled by on site histology. The authors have performed surgery alone and in combination with PDT in 86 patients. They found that the 2-year recurrence rate was 3.8% in surgery and 1.16%—when combined with topical PDT (Zhang et al. 2020).
The PDT and surgical combination can also be used for treatment of Bowen disease—squamous cell skin carcinoma in situ. Ana Gabriela Salvio et al. reported a case of slowly progressing left arm tumor. The treatment consisted of four PDT courses using 5-aminolevulinic acid followed by surgical tumor removal. Pre-operational PDT has provided 80% tumor size reduction that resulted in faster surgery, more efficient healing process, and reduced recurrence risk. Surgery alone would require a skin draft leading to possible postoperative complications and worse cosmetic effect (Salvio et al. 2020).
Kuroda et al. studied the efficacy of intra-operational PDT using Talaporfin in patients with glioblastoma. They reported no tumor cells reaching depths of 9–18 mm from the surface where PDT had been applied. However, viable tumor tissues were observed beyond or around the therapeutic tissue depth of PDT (Akimoto et al. 2019). Clinical recurrence rate of gliomas is high and can reach 100% in glioblastomas. Li et al. showed that when using PDT combined with surgery the recurrence rate reduced to 31.58% that is significantly lower than in surgery alone (Keskin and Çiloğlu 2021). Combined fluorescence-guided surgery and photodynamic therapy for glioblastoma also showed better efficacy when compared to traditional methods in neurooncology (Teng et al. 2020). Therefore, additional PDT is a potent method in local control of this cancer type.
Efficacy and safety of neo-adjuvant pre-operative PDT were demonstrated in pre-clinical studies in advanced non-small cell lung cancer (Abrahamse and Hamblin 2016), breast cancer (extra-mammary Paget disease) (Gao et al. 2015), and mesothelioma (Friedberg et al. 2003).
Combination of PDT and radiation therapy
PDT and subsequent radiotherapy are also considered an efficient combination in cancer treatment. Radiotherapy is required in approximately 60–70% of oncological patients. Its efficacy alone can reach over 90% but only in patients with early stages of skin cancer, cervical cancer, lymphoma, and nasopharyngeal cancer (Gunaydin et al. 2021). However, PDT + radiotherapy combination demonstrates higher clinical efficacy than PDT or radiation alone. Calzavara et al. have demonstrated that the treatment rate of esophageal cancer is enhanced when using adjuvant radiotherapy following PDT compared to both of these methods used alone (Calzavara et al. 1990). In a study in Japan, PDT + external beam radiation therapy for roentgen-negative lung cancer showed complete response in most of the patients (Imamura et al. 1994). Another study showed that sequential PDT and high dose brachytherapy in bronchogenic carcinoma are safe and improve the survival rate without recurrency and major adverse events in most patients (Freitag et al. 2004).
The combination of radiotherapy and PDT in patients with progressive tumors can improve life quality, lead to symptom relief, and increase in life expectancy. Yi-shan Wang et al. studied the effectiveness of PDT and radiotherapy combination in 90 cases including 32 patients with gastric carcinoma, 12—with esophageal cancer; 24—rectal cancer; 8—bladder cancer; 6—cervical cancer; and 8—superficial tumors. It is noteworthy that the patients included in this study were after operation failure or without surgery indications because of aggressive tumor progression. The authors reported that PDT combined with regional intensity modulated conformed radiotherapy relieved the symptoms and improved the life quality in advanced carcinoma patients offering palliative help to those patients in whom surgical, radio- and chemotherapy were ineffective (Na 2009).
Takahashi and Ghoodrzi in their studies found that PDT can be used as a sensitizer to make tumor cells more sensible to radiotherapy; therefore, it improves the treatment outcomes and reduces the dose and time of radiation (Ghoodarzi et al. 2016).
PDT has high efficacy, low toxicity, and good selectiveness in cancer treatment and its mechanisms differ from those of radiotherapy. Whereas these two methods do not have cross-resistance, this offers a worthwhile opportunity for their combination.
Combination of PDT with radionuclide therapy and diagnostics
Another area of application of porphyrin photosensitizers involves the inclusion of radioactive isotopes in their composition in order to deliver the latter to the tumor tissue for radionuclide diagnostics and therapy. The use of radiopharmaceuticals makes it possible to obtain images of zones with abnormal metabolism, which enables the visualization of tumors, inflammation, or thrombosis, thereby overcoming the limitations and drawbacks of fluorescence diagnostics, including fluorescence decay, in the diagnosis and treatment of deep-lying and/or large tumors. It also expands the capabilities of radical treatment of cancer patients suffering from concomitant diseases, reduce hospitalization time to 2 days, and ensure a high quality of life for a patient.
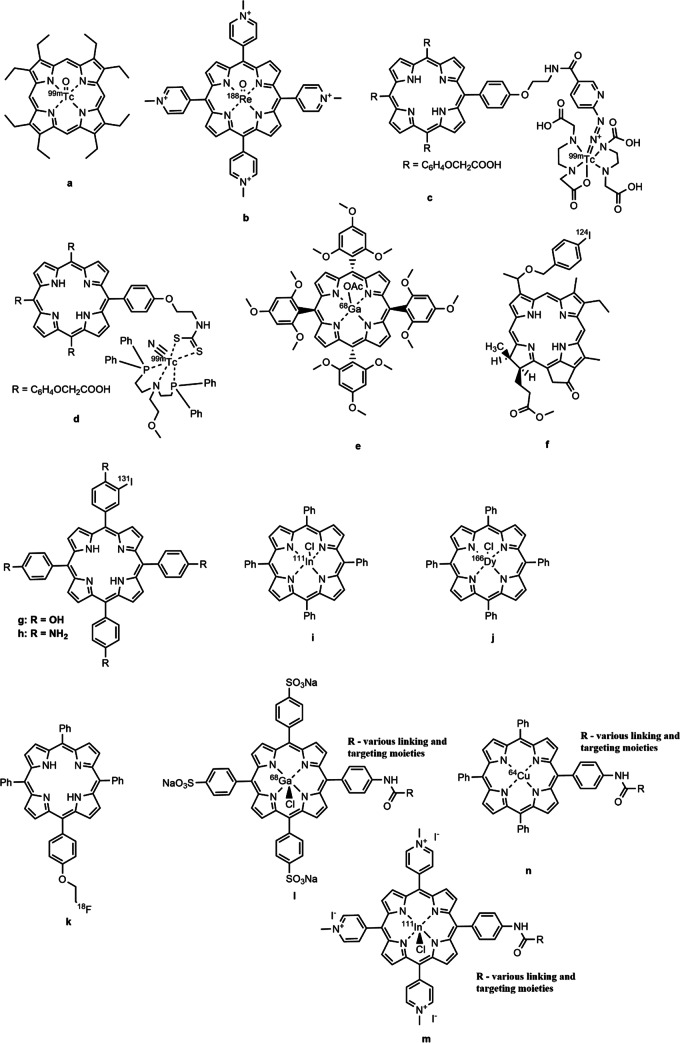
As shown above, porphyrins and their derivatives can accumulate in tumor cells. The specific mechanism of this behavior is not fully understood, but the reasons for the high tumor affinity of porphyrin-like compounds were described in a review by Osterloh and Vicente (2002). In addition, due to the closely spaced four nitrogen atoms in the macrocycle, these compounds can chelate atoms of various metals. Some metal complexes are already used in clinical practice (McFarland et al. 2020). Metal atoms that cannot be introduced into a macrocyclic structure for some reason, can be chelated with exocyclic complexing agents attached to the carrier molecule (Guleria et al. 2019; Yang et al. 2020). Aside from incorporation of radioactive metal isotopes, it is possible to chemically modify the carrier molecule and introduce an atom of a radioactive non-metal element into its structure (Simões et al. 2015; Srivatsan et al. 2020) (Fig. 4).
Fig. 4.
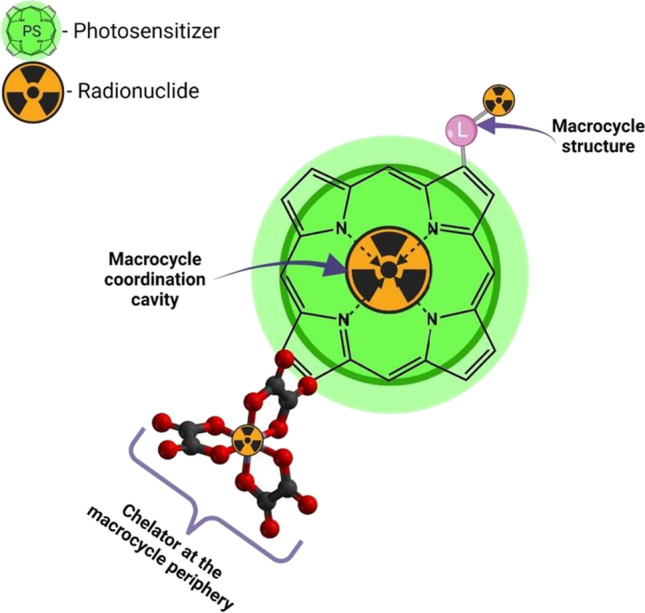
Variants for introducing radioactive isotopes into molecules of porphyrins and their hydrogenated analogs
The radioisotopes chelated by macrocyclic tetrapyrrole compounds can be tentatively classified into two groups: diagnostic and therapeutic. The former compounds are used in such diagnostic methods as single photon emission computerized tomography (SPECT) and positron emission tomography (PET). SPECT uses isotopes with a γ-radiation energy of 100–200 keV, among which 67 Ga, 99mTc, 111In, 123I, and 201Tl are used most widely, 99mTc being the most common due to its availability and low radiation energy. For PET, compounds containing short-lived 11C, 18F, 15O, 13 N, 68 Ga, 82Rb, 68Ge, and 82Sr isotopes are used in most cases. The 18F isotope is most used in radiolabeled chemical compounds for PET diagnosis due to its longer half-life and low energy of emitted positrons compared to the other isotopes. The most widely used isotopes in therapy include 32P, 90Y, 169Er, 89Sr, 153Sm, 177Lu, 188Re, and 131I (Qaim 2017).
Sanad et al. (2021) used octaethylporphyrin and 99mTc isotope obtained from a 99Mo/99mTc generator to produce a radio tracer (Fig. 5a). Studies on tumor-bearing mice showed that the metal complexes obtained mainly accumulated in the kidneys and tumor tissues. The concentration in the latter was found to be 4.4 times higher than in healthy tissues, and excretion from the kidneys occurred faster than from the tumor. They also noted that the suggested radioactive metal complexes featured more selective accumulation in tumor tissues compared to the previously reported 99mTc(V)-dimercaptosuccinic acid and the 177Lu-labeled conjugate of 5-carboxymethyleneoxyphenyl-10,15,20-tri(p-N-methylpyridyl)porphyrin with p-aminobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid.
Fig. 5.
Porphyrinoid compounds containing a radioactive isotope for the therapy and diagnosis of tumors
In addition to 99mTc, the 188Re isotope is also used to prepare radiotracers with porphyrins (Sanad et al. 2019). The biodistribution of the 5,10,15,20-(tetra-N-methyl-4-pyridyl)porphyrin complex with 188Re (Fig. 5b) was studied in Swiss Albino mice with Ehrlich ascites carcinoma. The results obtained showed a high stability of the compounds obtained in blood plasma for 48 h. As early as in 5 min after intravenous administration, the above compound is rapidly distributed throughout the organs. The highest concentration in the tumor was observed 30 min after the administration, while the concentration of the substance in tumor tissues was 7.5 times higher than in healthy ones. A positive feature of the complex is that it is rapidly excreted from the body: its concentration in most organs decreased noticeably within 2 h after the administration.
The creation of metal complexes based on porphyrin compounds is not limited to the chelation of radioactive metals in the internal cavity of the macrocycle. Methods for modifying the latter have been developed, including the introduction of chelating fragments at the molecule periphery. For example, Guleria et al. (2019) reported the creation of two modified porphyrin complexes with 99mTc based on 5-(p-aminopropyleneoxyphenyl)-10,15,20-tris-(p-carboethoxymethyleneoxyphenyl)-porphyrin. In these compounds, the radioactive metal atom is bound to the porphyrin by external chelation with hydrazinonicotinic acid (Fig. 5c) and bis-[(2-dimethylphosphino)ethyl]-methoxyethylamine) (Fig. 5d). Both compounds exhibited high stability in the blood plasma for 3 h after the administration; however, the difference in the degree of binding of these two complexes to blood plasma proteins was 30%. The choice of an external chelating fragment for a metal atom was shown to strongly affect the accumulation of a complex in a tumor and its pharmacokinetic characteristics, which must be taken into account in synthesizing new compounds. Fazaeli et al. (2019) studied a complex of 5,10,15,20-tetrakis(2,4,6-trimethoxyphenyl)porphyrin with the 68 Ga isotope (Fig. 5e). This metal is used in radioisotope diagnostics by positron emission tomography. The compound obtained in the study showed good solubility in aqueous media due to its polar nature. The metal complex accumulated in the chest and abdominal region and was excreted by the liver and kidneys. It is noted that the accumulation of this compound in the tumor was higher than that of similar compounds with other porphyrin moieties, in particular, tetraphenylporphyrin, 68 Ga-labeled tetraphenylporphyrin, and 68 Ga-labeled 5,10,15,20-tetrakis(pentafluoro-13-phenyl)porphyrin. At the same time, the “dark” cytotoxicity of this complex was lower than that of other metal porphyrinates, which makes the suggested compound a promising theranostic agent for PDT and PET.
124I is another promising isotope for creating radioactively labeled photosensitizers. Srivatsan et al. (2020) reported a chlorophyll a derivative: 124I-labeled methyl-3[(1′-m(iodo-benzyloxy)ethyl]-3-devinyl-pyropheophorbide a (Fig. 5f) comprising the 124I isotope covalent-bound to it, which makes it possible to visualize tumors and their metastases in various organs using PET.
In addition to the 124I isotope, studies are underway on the use of another isotope, 131I. For example, Song et al. (2018), using 131I-labeled 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin (Fig. 5g) and 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (Fig. 5h) as examples, showed that both these compounds were well accumulated in tumor cells, and could also be used not only in photodynamic therapy but also in radionuclide therapy. It is important to note that the therapeutic effect of compounds of this kind is superior to that of photodynamic or radionuclide therapy alone.
A complex of synthetic 5,10,15,20-tetraphenylporphyrin with 111In (Fig. 5i) was suggested as a candidate for application in SPECT/PDT. The results presented by Fazaeli et al. (2016) have shown that this compound and its homologs can be used to prepare radiotracers for SPECT, while the radiation doses from it do not exceed those of widely used radiopharmaceuticals. However, the active accumulation of tetraphenylporphyrin in the skin leads to an enhanced radiation exposure of skin.
An interesting study dealt with the creation of an in vivo generator of radioactive 166Ho from 166Dy (Salek et al. 2020). The authors synthesized 5,10,15,20-tetraphenylporphyrin whose inner macrocycle sphere contained the 166Dy radioisotope (Fig. 5j) that can spontaneously form the 166Ho isotope via beta-decay, and the resulting metal complex was administered to laboratory animals. This approach made it possible to avoid contaminating the animal organism with radioactive long-living complexes of isotopes formed during direct production of 166Ho from 165Ho by irradiation of the latter with neutrons. Studies on the biodistribution of this radio complex have shown that it is weakly accumulated in the liver and is rapidly excreted from the body by kidneys.
Simões et al. (2015) reported the synthesis and characterization of 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin (Fig. 5k) for use in PET. It was noted that the maximum absorption of the studied compound by tumor tissues, which occurred in 45 min after the administration, amounted to 2.5% of the total administered dose of the compound. Results of an in vivo PET study show that the substance features low absorption in the brain, lungs, and muscle tissues, but a noticeable accumulation of the substance occurs in the liver, which indicates the route of its excretion from the body. The authors note that they continue the necessary studies aimed at improving the biodistribution and cellular uptake of compounds of this type.
Comprehensive studies on the synthesis and properties of charged (Fig. 5l, m) and neutral (Fig. 5n) metal complexes of porphyrins with 111In, 64Cu, and 68 Ga radioisotopes were carried out by Ciaffaglione et al. (2021). Based on the studies of a series of compounds that included charged or neutral porphyrins, a radioactive isotope, and a vector molecule, the authors showed that the ability of compounds to form complexed and the biodistribution throughout the body strongly depended on the nature of the initial porphyrin, while addition of a targeted fragment led to a significant increase in the affinity of the radio complex to tumors. It is noted that such compounds are of interest for diagnostics, including the PET and SPECT techniques.
It can be concluded from the results presented above that porphyrins and their derivatives are actively used in the preparation of radioactively labeled compounds for both diagnostics and monotherapy, as well as for the theranostics of malignant neoplasms. In this case, porphyrins serve as a chelate-forming basis, and their inherent affinity to tumors makes it possible to deliver a radioisotope to the tumor zone, thus implementing radionuclide therapy and diagnostics.
Nanoparticles for co-delivery of chemotherapeutic drugs and photosensitizers
The use of nanoparticles (NPs) as a transport for antitumor drugs is widely known, since this approach makes it possible to enhance the accumulation of drugs in a tumor and in its microenvironment and, as a result, reduce the required dose and overall toxicity (Yao et al. 2020). The enhanced accumulation of nanosized objects in the tumor focus is primarily associated with two reasons: the enhanced permeability and retention (EPR) effect and more active capture of nanostructures by tumor cells (Maeda et al. 2000; Koo et al. 2011). The variety of materials and the ability to control the shape and size in the preparation process enable choosing the “ideal deliverer” for a particular drug and a particular disease. The great potential for surface modification of NPs makes their use in drug delivery even more promising. In particular, as reported in many studies, chemical modification of nanotransporters with vector molecules, monoclonal antibodies or their fragments facilitates the selective accumulation in various tumors. An immense role in the development of nanocontainers for drug delivery is attributed to their surface (in the case of liposomes, micelles, lipid NPs, and other types of NPs without solid cores) or coatings. Modification of NP surfaces with natural and synthetic polymers prevents aggregation and rapid removal of NPs from the body (stealth coatings), increases drug-loading capacity, and can trigger the release of a drug in the zone of interest under the impact of external triggers, such as acidity of the medium, the presence of certain bio-molecules, temperature, and irradiation with light.
Numerous works have been published, in which the efficacy of active or passive targeting was shown in in vivo and in vitro studies.
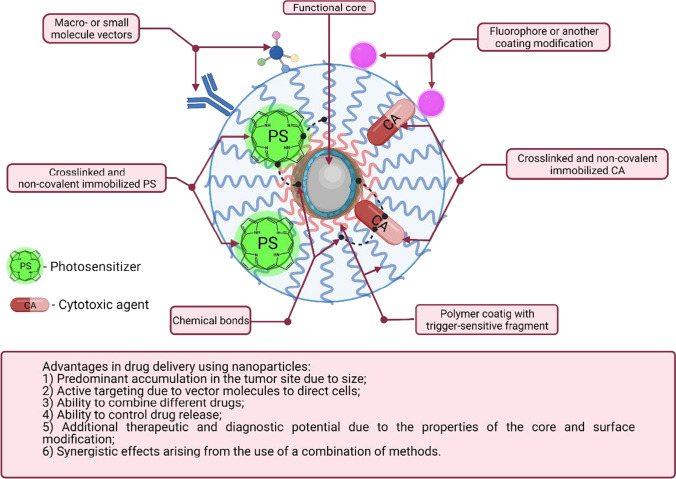
In combined chemo- and photodynamic therapy, the use of nanotransporters is the most common way of co-delivery of two or more different drugs, including photosensitizers. The possible advantages of using NPs to combine chemotherapeutic agents and photosensitizers are illustrated in Fig. 6, including enhanced accumulation and selectivity of action in tumor cells due to active and passive targeting of NPs and the ability to concurrently deliver different drugs to the zone of interest. The list of advantages also includes the following: (1) the ability to control the release of drugs in a tumor under the impact of certain endogenous and external factors; (2) additional therapeutic or diagnostic possibilities that emerge if nanoparticles made of functional materials are used; and (3) enhanced efficacy of a certain method of therapy due to synergy.
Fig. 6.
Schematic representation of a nanotransporter loaded with a PS and a cytotoxic agent
The passive targeting strategy is based on the enhanced permeability and retention (EPR) effect (Shi et al. 2020). It is traditionally believed that the accumulation in a tumor mediated by the EPR effect is due to nanoparticles circulating for a long time in the bloodstream, the hydrodynamic diameter of which exceeds the renal clearance threshold and which can leave the tumor vessels. Even the very attachment of a PS and a chemotherapeutic agent to a nanosized carrier in a covalent way or due to non-covalent interactions significantly increases the efficacy of therapy, since the major fraction of administered drugs would participate in the therapy process due to reduction in losses in transportation (Lin et al. 2021).
Ways to expand the use of already known approaches for the selective accumulation of a drug in a tumor due to the EPR effect were studied in recent works (Shi et al. 2020). The efficacy of nanotransporters for the delivery of various chemotherapeutic drugs to adenocarcinoma of the pancreatic ducts with a mild EPR effect was shown (Liu et al. 2019). In this case, the enhanced accumulation of nanoparticles in the tumor is associated with transcytosis. The efficacy of ultra-small nanoparticles with a diameter of up to 10 nm was shown in a number of works (Bort et al. 2020; Xu et al. 2020). Another line of research in the application of nanoparticles for drug delivery due to the EPR effect involves enhancing this effect using ultrasonic and hyperthermic (Dhaliwal and Zheng 2019), sonodynamic (Duan et al. 2020), and physiological (Tsioumpekou et al. 2020) impact on angiogenesis.
Active targeting can be used as a complementary strategy to passive drug delivery based on the EPR effect. Antibodies, antibody fragments, and peptides are used most commonly as ligands that target nanoparticles to a tumor (Garbuzenko et al. 2019; Zou et al. 2019; Dammes and Peer 2020; de Bruijn et al. 2020).
The concept of targeting is not limited to the delivery of drugs to the zone of interest. The amount of drugs carried by a nanoparticle and their release in the zone of interest can be controlled using various coatings. Two groups of triggers that cause the release of drugs from nanoparticles can be distinguished.
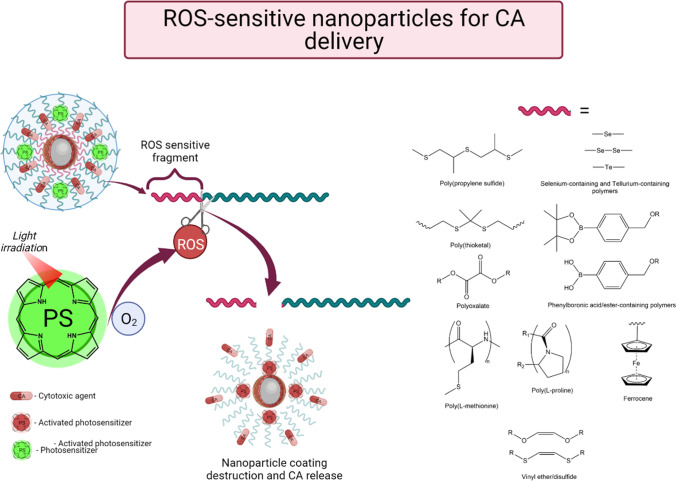
First, these are exogenous or external triggers, such as an external magnetic field that can cause local overheating or motion of nanoparticles, ultrasonic exposure and light irradiation that cause the generation of singlet oxygen if a photosensitizer is available in the formulation. Second, endogenous triggers, for example, pH changes in the tumor microenvironment that consists of relatively healthy tissues, overexpression of specific enzymes that break ester or peptide bonds by tumor cells, an increased content of glutathione that is able to reduce the disulfide fragment, etc. (Senapati et al. 2018). However, in recent works researchers were most interested in the concept in which a PS irradiated with light and causing generation of ROS (reactive oxygen forms) performs as a trigger for the destruction of a nanoparticle and release of an active cytotoxic agent (Fig. 7). In this approach, the selectivity of the therapeutic effect is enhanced not only due to passive or active targeting and preferential accumulation of the drug in the tumor, but also due to the selectivity of irradiation.
Fig. 7.
Use of ROS-sensitive nanoparticles for targeted delivery and controlled release of drugs
It has been shown in a number of works that titanium oxide nanoparticles themselves could act as a photosensitizer (Hou et al. 2019; Zheng et al. 2019; Jiao et al. 2020). Wei Guo et al. successfully developed a multifunctional platform for combined photodynamic, photothermal, and drug therapy based on TiO2 nanoparticles coated with polydopamine (Guo et al. 2017a). The study demonstrated the synergistic action of various types of therapy both in vitro and in vivo. The efficacy of titanium oxide nanoparticles coated with polydopamine and loaded with doxorubicin irradiated with light at a wavelength of 808 nm was almost 8 times higher than that of doxorubicin and 7 times higher than that of unloaded titanium oxide nanoparticles against epithelial cells of human breast cancer MDA-MB-231. Mice inoculated with MDA-MB-231 tumors exhibited 100% tumor regression within 14 days of observation. This effect is explained by the combined action of the ROS produced upon irradiation of titanium oxide NPs and doxorubicin that is released upon destruction of the polydopamine coating due to local heating of NPs. To confirm this hypothesis, we carried out confocal fluorescence microscopy of cells incubated with samples of doxorubicin-loaded NPs with and without light irradiation. Colocalization of signals of doxorubicin and DAPI nuclear stain is more pronounced under irradiation, which confirms the release of the chemotherapeutic agent from the complex containing the particles. This approach to drug release control was called “on–off.”
Another example of increasing the efficacy of a cytotoxic agent was presented by Yu et al. An interesting feature of this study is that the cytotoxic effect of the chemotherapeutic agent is enhanced in case of PDT-induced hypoxia (Yu et al. 2022). Honglian Yu et al. implemented an interesting approach to combining 4,4,4,4-(porphine-5,10,15,20-tetrayl)tetrakis(benzoic acid) and an experimental chemotherapeutic agent, tirapazamine. First, Yu’s group prepared nanoparticles in the form of organometallic cores by coordinating Fe3+ of the porphyrin metal complex. To increase the stability and biocompatibility of NPs, they were coated with silk fibroin by covalent binding it with PS carboxyl groups. Next, the obtained NPs were loaded with tirapazamine via non-covalent interactions. It was expected that as NPs enter tumor cells with an elevated level of glutathione, NPs would be destroyed due to reduction of Fe3+ to Fe2+, release of the cytotoxic agent, and a decrease in the antioxidative defense of the cell, thus increasing its vulnerability to oxidative stress. A study on the physicochemical properties of the resulting NPs confirmed the dependence of the drug release on the concentration of glutathione and showed the generation of •OH in a medium comprising H2O2 and ROS upon porphyrin irradiation. Estimation of the therapeutic efficacy of the above-described NPs on the breast cancer cell line 4T1 and mice with inoculated tumors of the same genesis showed a synergistic therapeutic effect from the combined action of PDT and hypoxia induced by tirapazamine. Thus, using a combined nanodrug, inhibition of the antioxidative defense of the cell and an increase in the intracellular content of ROS due to PDT and Fenton-like activity of Fe2+, which can lead to irreversible damage to tumor cells, were achieved. The efficacy of this combination is confirmed by the complete regression of tumor cells in vitro and tumors in vivo.
However, the main strategy for the development of delivery systems by combining PS and chemotherapeutic drugs is to incorporate fragments that are destroyed by ROS with release and/or activation of cytotoxic agents into structural components of nanosized carriers. To this end, polymers containing ROS-sensitive fragments are used, such as poly(propylene sulfides), selenium and organotellurium fragments, thioketals, phenylboronic acids and esters, oxalic acid esters, poly(L)-methionine, poly-(L)-proline, ferrocenes, vinyl ethers and disulfides (Cao et al. 2021).
Yi et al. (2021) obtained liposomal platforms based on cholesterol, phospholipids, and polyethylene glycol loaded with indocyanine green and a modified fragment containing pinacolyl phenylboronate. It was demonstrated that PS photoactivation resulted in liposome destruction and conversion from an inactive boron-containing doxorubicin derivative to an active metabolite. The activity of the inactivated liposomal form of doxorubicin in tests on human embryonic kidney cells HEK293 was shown to be 133.5 times lower than that of the free form. The concept of controlled doxorubicin activation by irradiation and photoexcitation of indocyanine green was confirmed on human breast cancer epithelial cells MDA-MB-231. A decrease in the IC50 of the liposomal inactivated form of doxorubicin with respect to the free form of doxorubicin by a factor of almost 3.5 was shown. To study the kinetics of doxorubicin release and death of tumor cells, a study was performed on HEK293 cells incubated with liposomal and free doxorubicin forms using confocal fluorescence microscopy. It was shown that doxorubicin accumulated with time in the cell nuclei after irradiation. In vivo studies showed a significantly higher efficacy of the liposomal form of doxorubicin under irradiation compared to the control groups, and a 94.9% inhibition of tumor growth was attained.
Another example of the use of ROS-sensitive nanostructures was presented by Chen et al. (2019). They developed a drug delivery system that was destroyed by ROS based on a dextran copolymer conjugated with phenylboronic acid pinacol ester. Doxorubicin was used as a cytotoxic agent, while chlorin e6 played the role of a PS. The rapid release of doxorubicin upon irradiation with light at a wavelength of 655 nm was confirmed in two independent experiments. Using transmission electron microscopy, the destruction of chlorin e6 polymer NPs upon irradiation was shown based on the decrease in their size and the change in their morphology. The release of doxorubicin in vitro was determined by fluorimetry and confocal microscopy on melanoma cell line B16–F10. It was shown that the release of doxorubicin was insignificant without irradiation; however, upon photoexcitation of chlorin e6, fluorescence rapidly developed, and doxorubicin gradually accumulated in the cell nuclei. In addition, the synergistic effect of the action of the PS and the chemotherapeutic agent was confirmed by calculations made according to the Chou and Talalay method (Chou and Talalay 1984; Chou 2006).
Xu et al. (2021) reported yet another example of the use of ROS-sensitive materials for drug delivery systems. Their team obtained nanoparticles of a copolymer of lactic and glycolic acids (PLGA) bound to polyethylene glycol through a thioketal moiety that is unstable in the presence of ROS. In addition, similar NPs were obtained and additionally modified with an RGD peptide to give them targeting properties. The NPs were loaded with a synthetic PS based on benzodithiophene and chemotherapeutic drug paclitaxel. The activation of the immune system after PDT was studied. To do so, the nanodrug was injected in mice with two inoculated tumors, but only one of the latter was irradiated. Complete regression of the light-irradiated tumor and significant inhibition of growth of the non-irradiated tumor were demonstrated.
Selenium compounds also found use in the development of controlled release systems. Yang et al. (2022) obtained diselenidosilanes and NPs based on these compounds. Methylene blue and doxorubicin PS were loaded together into polyethylene glycol-modified mesoporous silicon NPs containing diselenide bridges destroyed by the ROS, which are formed upon PS irradiation. Due to the release of methylene blue, further formation of ROS occurs that is accompanied by doxorubicin release. The synergistic effect of PDT and doxorubicin was demonstrated both in vitro and in vivo, and systemic administration of the resulting NPs followed by PS photoexcitation in mice with 4T1 mammary tumor resulted not only in complete disappearance of the tumors but also in an increase in the antitumor immune response.
PS photoactivation followed by formation of ROS can be used not only to control the release of drugs in the zone of interest, but also to increase the accumulation of NPs by enhancing the EPR effect. To confirm this fact, Zhen et al. (2014) used a therapy mode that included sequential administration of PS, PDT, and administration of Doxil, a liposomal form of doxorubicin. A phthalocyanine-based PS containing a vector for targeting the tumor’s vascular system was used in the study. After PDT, the destruction of the vascular system of 4T1 tumors was estimated by various methods: scanning electron microscopy, confocal fluorescence microscopy, magnetic resonance imaging, and histological methods for analyzing vessel morphology. The antitumor activity of Doxil was compared in mice with inoculated 4T1 tumors, which either did or did not undergo photodynamic exposure. The experiment showed a significantly higher efficacy of the combination of Doxil and PDT compared to Doxil monotherapy.
Conclusion
The use of combined and complex therapy for cancer is steadily expanding. The increased interest in the multicomponent treatment of patients with malignant neoplasms is due to the fact that scientific data on their pathogenesis have been accumulated, as well as the continuous improvement of radiation and drug treatment methods. The concept of “combined approach” includes the use of two fundamentally different methods of treatment (for example, surgery and radiation; surgery and chemotherapy; radiation therapy and chemotherapy). Currently, the main in oncology are three methods of treatment (radiation, surgery, chemotherapy).
In contrast to the above basic methods of treatment, photodynamic therapy is characterized by low invasiveness, high selectivity of lesions, low toxicity of administered drugs, and no risk of severe local and systemic complications of treatment. Since the antitumor effects of this type of treatment are due to a combination of direct cell photodamage, including necrosis and induced apoptosis, destruction of the tumor vasculature and activation of the immune response, this method of treatment has fundamental advantages over the three main ones.
Multimodal therapy of oncological patients allows to overcome the disadvantages of one method or another at the expense of the advantages of another. As was shown in separate chapters of this review, the combination of PDT with chemotherapy, surgery, and radiation therapy leads to an additive effect, which is expressed in an increase in the effectiveness of treatment. Thus, the low selectivity of the action of chemotherapy drugs is compensated by the selective accumulation of photosensitizers in tumors of various origins, especially if two drugs are combined into a single structure.
The main limitation of PDT in terms of the depth of the photodamaging effect can be compensated by a combination with radiation therapy, which provides for the complete suppression of the viability of a malignant tumor by creating an absorbed dose of ionizing radiation in the irradiated focus, necessary for the destruction of this tumor.
The current treatment protocols in oncology, including preoperative, postoperative, and intraoperative PDT, confirm the successful combination of a radical surgical approach and a “sanitizing” photodynamic effect in the tumor area.
The rapid development of molecular biology and biochemical research methods has provided extensive information on cellular and subcellular targets involved in oxidative stress–induced cytotoxicity after PDT. Therefore, the developers of innovative targeted photosensitizers have panels of biologically active substances, the introduction of which implements the target-oriented delivery of any constructs to the tumor lesion zone.
The clinical use of PDT has significantly expanded in recent years due to the undoubted advantages of the method, and the key factor in its development is the search for new photosensitizers, including multimodal ones with different mechanisms of cytotoxic action.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ostroverkhov Petr Vasilievich, Popov Alexander Alexandrovich, Kirin Nikita Sergeevich, Pogorilyy Viktor Alexeyevich, and Suvorov Nikita Vladimirovich. The article sections had been written by the following authors: Abstract, Introduction, and Conclusion (Grin Mikhail Alexandrovich, Filonenko Elena Vyacheslavovna); Combined chemotherapy and PDT for cancer treatment (Pogorilyy Viktor Alexeyevich, Suvorov Nikita Vladimirovich); Combination of PDT and surgery (Kirin Nikita Sergeevich); Combination of PDT and radiation therapy (Anna Igorevna Sazonova); Combination of PDT with radionuclide therapy and diagnostics (Popov Alexander Alexandrovich); Nanoparticles for co-delivery of chemotherapeutic drugs and photosensitizers (Ostroverkhov Petr Vasilievich); and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation (project № 0706-2020-0019), the Russian Foundation for Basic Research (№ 20-33-90289), and the Russian Science Foundation (№ 21-13-00078).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abrahamse H, Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem J. 10.1042/BJ20150942 [DOI] [PMC free article] [PubMed]
- Agrawal RK, Patel RK, shah V, et al. (2014) Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 10.1007/s12288-013-0261-4 [DOI] [PMC free article] [PubMed]
- Ahn TG, Jung JM, Lee EJ, Choi JH. Effects of cisplatin on photosensitizer-mediated photodynamic therapy in breast tumor-bearing nude mice. Obstet Gynecol Sci. 2019 doi: 10.5468/ogs.2019.62.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto J, Fukami S, Suda T, et al. First autopsy analysis of the efficacy of intra-operative additional photodynamic therapy for patients with glioblastoma. Brain Tumor Pathol. 2019 doi: 10.1007/s10014-019-00351-0. [DOI] [PubMed] [Google Scholar]
- Akopov A, Rusanov A, Gerasin A, et al. Preoperative endobronchial photodynamic therapy improves resectability in initially irresectable (inoperable) locally advanced non small cell lung cancer. Photodiagnosis Photodyn Ther. 2014 doi: 10.1016/j.pdpdt.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Amjad MT, Chidharla A, Kasi A (2022) Cancer chemotherapy. Treasure Island (FL) [PubMed]
- Aniogo EC, George BPA, Abrahamse H. In vitro combined effect of Doxorubicin and sulfonated zinc Phthalocyanine–mediated photodynamic therapy on MCF-7 breast cancer cells. Tumor Biol. 2017 doi: 10.1177/1010428317727278. [DOI] [PubMed] [Google Scholar]
- Armstrong, D. K. (2002). Randomized phase III study of intravenous (IV) paclitaxel and cisplatin versus IV paclitaxel, intraperitoneal (IP) cisplatin and IP paclitaxel in optimal stage III epithelial ovarian cancer (OC): a Gynecologic Oncology Group trial (GOG172). In Proc Am Soc Clin Oncol.
- Asuncion MM. Antimetabolite treatment for pancreatic cancer. Chemother Open Access. 2014 doi: 10.4172/2167-7700.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battogtokh G, Liu HB, Bae SM, et al. Synthesis of di-pyropheophorbide-a-platinum(II) complex and the in vitro cytotoxicity against TC-1 tumor cells. J Porphyr Phthalocyanines. 2012 doi: 10.1142/S1088424612500782. [DOI] [Google Scholar]
- Bio M, Rajaputra P, Nkepang G, et al. Site-specific and far-red-light-activatable prodrug of combretastatin A-4 using photo-unclick chemistry. J Med Chem. 2013 doi: 10.1021/jm400139w. [DOI] [PubMed] [Google Scholar]
- Bio M, Rajaputra P, Nkepang G, You Y. Far-red light activatable, multifunctional prodrug for fluorescence optical imaging and combinational treatment. J Med Chem. 2014 doi: 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio M, Rajaputra P, Lim I, et al. Efficient activation of a visible light-activatable CA4 prodrug through intermolecular photo-unclick chemistry in mitochondria. Chem Commun. 2017 doi: 10.1039/c6cc09994g. [DOI] [PubMed] [Google Scholar]
- Bort G, Lux F, Dufort S, et al. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics. 2020 doi: 10.7150/thno.37543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist’s perspective. Surg Clin North Am. 2001 doi: 10.1016/S0039-6109(05)70141-9. [DOI] [PubMed] [Google Scholar]
- Brilkina AA, Dubasova LV, Sergeeva EA, et al. Photobiological properties of phthalocyanine photosensitizers Photosens. Holosens and Phthalosens: a Comparative in Vitro Analysis J Photochem Photobiol B Biol. 2019 doi: 10.1016/j.jphotobiol.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Brisse H, Orbach D, Lassau N, et al. Portal vein thrombosis during antineoplastic chemotherapy in children: report of five cases and review of the literature. Eur J Cancer. 2004 doi: 10.1016/j.ejca.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Brun PH, DeGroot JL, Gudgin Dickson EF, et al. Determination of the in vivo pharmacokinetics of palladium-bacteriopheophorbide (WST09) in EMT6 tumour-bearing Balb/c mice using graphite furnace atomic absorption spectroscopy. Photochem Photobiol Sci. 2004 doi: 10.1039/b403534h. [DOI] [PubMed] [Google Scholar]
- Caesar L, van Doeveren TEM, Tan IB, et al. The use of photodynamic therapy as adjuvant therapy to surgery in recurrent malignant tumors of the paranasal sinuses. Photodiagnosis Photodyn Ther. 2015 doi: 10.1016/j.pdpdt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Calzavara F, Tomio L, Corti L, et al. Oesophageal cancer treated by photodynamic therapy alone or followed by radiation therapy. J Photochem Photobiol B Biol. 1990 doi: 10.1016/1011-1344(90)85086-C. [DOI] [PubMed] [Google Scholar]
- Cao Z, Li D, Wang J, Yang X. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.05.023. [DOI] [PubMed] [Google Scholar]
- Caudle KE, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013 doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli JP, Spring BQ, Rizvi I, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010 doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gao Y, Li Y, et al. Synergistic chemo-photodynamic therapy mediated by light-activated ROS-degradable nanocarriers. J Mater Chem B. 2019 doi: 10.1039/c8tb03030h. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006 doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984 doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chu E (2017) Cancer Chemotherapy. In: Katzung BG (ed) Basic & clinical pharmacology, 14e. McGraw-Hill Education, New York, NY
- Ciaffaglione V, Waghorn PA, Exner RM, et al. Structural investigations, cellular imaging, and radiolabeling of neutral, polycationic, and polyanionic functional metalloporphyrin conjugates. Bioconjug Chem. 2021 doi: 10.1021/acs.bioconjchem.0c00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihák A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974 doi: 10.1159/000224981. [DOI] [PubMed] [Google Scholar]
- Dąbrowski JM, Arnaut LG. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem Photobiol Sci. 2015 doi: 10.1039/c5pp00132c. [DOI] [PubMed] [Google Scholar]
- Dammes N, Peer D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics. 2020 doi: 10.7150/thno.37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn HS, Mashayekhi V, Schreurs TJL, et al. Acute cellular and vascular responses to photodynamic therapy using EGFR-targeted nanobody-photosensitizer conjugates studied with intravital optical imaging and magnetic resonance imaging. Theranostics. 2020 doi: 10.7150/thno.37949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal A, Zheng G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: designing a new perspective in nanomedicine delivery. Theranostics. 2019 doi: 10.7150/thno.37204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003 doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Doustvandi MA, Mohammadnejad F, Mansoori B, et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn Ther. 2019 doi: 10.1016/j.pdpdt.2019.08.027. [DOI] [PubMed] [Google Scholar]
- Duan L, Yang L, Jin J, et al. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics. 2020 doi: 10.7150/thno.37593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazaeli Y, Shanehsazzadeh S, Lahooti A, et al. Preclinical dosimetric estimation of [111In] 5, 10, 15, 20-tetra phenyl porphyrin complex as a possible imaging/PDT agent. Radiochim Acta. 2016 doi: 10.1515/ract-2015-2444. [DOI] [Google Scholar]
- Fazaeli Y, Hosseini MA, Shahabinia F, Feizi S. 68Ga-5, 10, 15, 20-Tetrakis (2, 4, 6-trimethoxy phenyl) porphyrin: a novel radio-labeled porphyrin complex for positron emission tomography. J Radioanal Nucl Chem. 2019 doi: 10.1007/s10967-019-06465-1. [DOI] [Google Scholar]
- Filonenko EV (2021) Clinical implementation and scientific development of photodynamic therapy in Russia in 2010–2020. Biomed Photonics. (In Russ.) 10.24931/2413-9432-2021-9-4-4-22
- Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- Freitag L, Ernst A, Thomas M, et al. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax. 2004 doi: 10.1136/thx.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JS, Mick R, Stevenson J, et al. A phase I study of Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann Thorac Surg. 2003 doi: 10.1016/S0003-4975(02)04474-0. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang XC, Wang WS, et al. Efficacy and safety of topical ALA-PDT in the treatment of EMPD. Photodiagnosis Photodyn Ther. 2015 doi: 10.1016/j.pdpdt.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Gao Y, Shang Q, Li W, et al. Antibiotics for cancer treatment: a double-edged sword. J Cancer. 2020 doi: 10.7150/jca.47470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko OB, Kuzmov A, Taratula O, et al. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019 doi: 10.7150/thno.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoodarzi R, Changizi V, Montazerabadi AR, Eyvazzadaeh N. Assessing of integration of ionizing radiation with Radachlorin-PDT on MCF-7 breast cancer cell treatment. Lasers Med Sci. 2016 doi: 10.1007/s10103-015-1844-0. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Minnis M, Ghogare AA, et al. Photoactive fluoropolymer surfaces that release sensitizer drug molecules. J Phys Chem B. 2015 doi: 10.1021/acs.jpcb.5b00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Carmona MA, Bolch M, Jansen C, et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment Pharmacol Ther. 2019 doi: 10.1111/apt.15050. [DOI] [PubMed] [Google Scholar]
- Guleria M, Das T, Vats K, et al. Preparation and evaluation of 99mTc-labeled porphyrin complexes prepared using PNP and HYNIC cores: studying the effects of core selection on pharmacokinetics and tumor uptake in a mouse model. Medchemcomm. 2019 doi: 10.1039/c8md00559a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin G, Gedik ME, Ayan S (2021) Photodynamic therapy for the treatment and diagnosis of cancer–a review of the current clinical status. Front. Chem. [DOI] [PMC free article] [PubMed]
- Guo W, Wang F, Ding D, et al. TiO2-x based nanoplatform for bimodal cancer imaging and NIR-triggered chem/photodynamic/photothermal combination therapy. Chem Mater. 2017 doi: 10.1021/acs.chemmater.7b03241. [DOI] [Google Scholar]
- Guo X, Wang L, Wang S, et al. Synergistic antiproliferative effect of chemo-phototherapy: synthesis and photodynamic activity evaluation of novel Chlorin e6-artesunate conjugates as antiproliferative agents. Bioorganic Med Chem Lett. 2017 doi: 10.1016/j.bmcl.2017.08.055. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chung CK, Yu Z, et al (2022) Combinatorial therapeutic approaches with nanomaterial-based photodynamic cancer therapy. Pharmaceutics 1410.3390/pharmaceutics14010120 [DOI] [PMC free article] [PubMed]
- Haque A, Rahman MA, Faizi MSH, Khan MS. Next generation antineoplastic agents: a review on structurally modified vinblastine (VBL) analogues. Curr Med Chem. 2018 doi: 10.2174/0929867324666170502123639. [DOI] [PubMed] [Google Scholar]
- Herfarth HH, Long MD, Isaacs KL (2012) Methotrexate: underused and ignored? Dig Dis 30 Suppl. 10.1159/000342735 [DOI] [PMC free article] [PubMed]
- Hossion AML, Bio M, Nkepang G, et al. Visible light controlled release of anticancer drug through double activation of prodrug. ACS Med Chem Lett. 2013 doi: 10.1021/ml3003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Deng K, Wang M, et al. Hydrogenated titanium oxide decorated upconversion nanoparticles: facile laser modified synthesis and 808 nm near-infrared light triggered phototherapy. Chem Mater. 2019 doi: 10.1021/acs.chemmater.8b03762. [DOI] [Google Scholar]
- Hu DR, Chen LJ, Qu Y, et al. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics. 2018 doi: 10.7150/thno.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zeng YC. A candidate for lung cancer treatment: arsenic trioxide. Clin Transl Oncol. 2019 doi: 10.1007/s12094-019-02054-6. [DOI] [PubMed] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002 doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Imamura S, Kusunoki Y, Takifuji N, et al. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer. 1994 doi: 10.1002/1097-0142(19940315)73:6<1608::AID-CNCR2820730611>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Isah T. Anticancer alkaloids from trees: development into drugs. Pharmacogn Rev. 2016 doi: 10.4103/0973-7847.194047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Zhang W, Zhang L, et al. Rational design of oxygen deficient TiO2-x nanoparticles conjugated with chlorin e6 (Ce6) for photoacoustic imaging-guided photothermal/photodynamic dual therapy of cancer. Nanoscale. 2020 doi: 10.1039/c9nr09423g. [DOI] [PubMed] [Google Scholar]
- Kaplan MA, Galkin VN, Romanko YS, et al (2016) Combination photodynamic therapy sarcomas M-1 in combination with chemotherapy. Radiat Risk. 10.21870/0131-3878-2016-25-4-90-99
- Keskin G, Çiloğlu M. Efficacy of antimicrobial photodynamic therapy and Er, Cr:YSGG laser-activated irrigation on dentinal tubule penetration of MTA-based root canal sealer: a confocal microscopy study. Photodiagnosis Photodyn Ther. 2021;36:102584. doi: 10.1016/j.pdpdt.2021.102584. [DOI] [PubMed] [Google Scholar]
- Kimura M, Miyajima K, Kojika M, et al. Photodynamic therapy (PDT) with chemotherapy for advanced lung cancer with airway stenosis. Int J Mol Sci. 2015 doi: 10.3390/ijms161025466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Huh MS, Sun IC, et al. In vivo targeted delivery of nanoparticles for theranosis. Acc Chem Res. 2011 doi: 10.1021/ar2000138. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020 doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb BM, Barbas CF. Selective arylthiolane deprotection by singlet oxygen: a promising tool for sensors and prodrugs. Chem Commun. 2015 doi: 10.1039/c4cc09040c. [DOI] [PubMed] [Google Scholar]
- Le NA, Babu V, Kalt M, et al. Photostable platinated bacteriochlorins as potent photodynamic agents. J Med Chem. 2021 doi: 10.1021/acs.jmedchem.1c00052. [DOI] [PubMed] [Google Scholar]
- Letis AS, Seo EJ, Nikolaropoulos SS, et al. Synthesis and cytotoxic activity of new artemisinin hybrid molecules against human leukemia cells. Bioorganic Med Chem. 2017 doi: 10.1016/j.bmc.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang W, Liu Y, et al. The biological characteristics of a novel camptothecin-artesunate conjugate. Bioorganic Med Chem Lett. 2015 doi: 10.1016/j.bmcl.2014.10.048. [DOI] [PubMed] [Google Scholar]
- Lin L, Song X, Dong X, Li B (2021) Nano-photosensitizers for enhanced photodynamic therapy. Photodiagnosis Photodyn. Ther. 10.1016/j.pdpdt.2021.102597 [DOI] [PubMed]
- Liu B, Thayumanavan S. Mechanistic investigation on oxidative degradation of ROS-responsive thioacetal/thioketal moieties and their implications. Cell Reports Phys Sci. 2020 doi: 10.1016/j.xcrp.2020.100271. [DOI] [Google Scholar]
- Liu LH, Qiu WX, Li B, et al. A red light activatable multifunctional prodrug for image-guided photodynamic therapy and cascaded chemotherapy. Adv Funct Mater. 2016 doi: 10.1002/adfm.201602541. [DOI] [Google Scholar]
- Liu X, Jiang J, Meng H. Transcytosis - an effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics. 2019 doi: 10.7150/thno.38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel J, MacDonald IJ, Ciesielski MJ, et al. 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: Implications for photodynamic therapy. Lasers Surg Med. 2001 doi: 10.1002/lsm.10001. [DOI] [PubMed] [Google Scholar]
- López A, Gutiérrez A, Palacios A, et al. GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: a phase II study. Eur J Haematol. 2008 doi: 10.1111/j.1600-0609.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Lossignol D. A little help from steroids in oncology. J Transl Intern Med. 2016 doi: 10.1515/jtim-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottner C, Knuechel R, Bernhardt G, Brunner H. Combined chemotherapeutic and photodynamic treatment on human bladder cells by hematoporphyrin-platinum(II) conjugates. Cancer Lett. 2004 doi: 10.1016/j.canlet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000 doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Khurshid A, Yousaf MS, et al. Effect of vitamin A as a neoadjuvant agent in chemotherapy and photodynamic therapy of Rhabdomyosarcoma cells. Photodiagnosis Photodyn Ther. 2020 doi: 10.1016/j.pdpdt.2020.102088. [DOI] [PubMed] [Google Scholar]
- Marginter D, Tóth J, Buijs L, Gehlen H (2021) Multimodal therapy of the periorbital equine sarcoid. Pferdeheilkunde. 10.21836/PEM20210308
- McFarland SA, Mandel A, Dumoulin-White R, Gasser G. Metal-based photosensitizers for photodynamic therapy: the future of multimodal oncology? Curr Opin Chem Biol. 2020 doi: 10.1016/j.cbpa.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J, Peng Q (2003) An outline of the hundred-year history of PDT. Anticancer Res. [PubMed]
- Moore AY. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J Dermatolog Treat. 2009 doi: 10.3109/09546630902789326. [DOI] [PubMed] [Google Scholar]
- Na S (2009) Photodynamic therapy combined with IMCRT for cancer:a clinical study
- Naik A, Rubbiani R, Gasser G, Spingler B. Visible-light-induced annihilation of tumor cells with platinum-porphyrin conjugates. Angew Chemie - Int Ed. 2014 doi: 10.1002/anie.201400533. [DOI] [PubMed] [Google Scholar]
- Osaki T, Yokoe I, Sunden Y, et al. Efficacy of 5-aminolevulinic acid in photodynamic detection and photodynamic therapy in veterinary medicine. Cancers (basel) 2019 doi: 10.3390/cancers11040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh J, Vicente MGH. Mechanisms of porphyrinoid localization in tumors. J Porphyr Phthalocyanines. 2002 doi: 10.1142/S1088424602000373. [DOI] [Google Scholar]
- Otvagin VF, Kuzmina NS, Kudriashova ES, et al. Conjugates of porphyrinoid-based photosensitizers with cytotoxic drugs: current progress and future directions toward selective photodynamic therapy. J Med Chem. 2022;65:1695–1734. doi: 10.1021/acs.jmedchem.1c01953. [DOI] [PubMed] [Google Scholar]
- Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol off J Eur Soc Med Oncol. 2001 doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- Polysalov VN, Gapbarov AC, Polekhin AS, Granov DA (2018) Combined treatment for Klatskin non-resectable tumor using intra-flow photodynamic therapy and regional chemotherapy. Vopr Onkol. 10.37469/0507-3758-2018-64-4-485-489
- Qaim SM. Nuclear data for production and medical application of radionuclides: present status and future needs. Nucl Med Biol. 2017 doi: 10.1016/j.nucmedbio.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Rajaputra P, Bio M, Nkepang G, et al. Anticancer drug released from near IR-activated prodrug overcomes spatiotemporal limits of singlet oxygen. Bioorganic Med Chem. 2016 doi: 10.1016/j.bmc.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset N, Bourré L, Thibaud S. Sensitizers in photodynamic therapy. In: Patrice T, editor. Photodynamic therapy. The Royal Society of Chemistry; 2003. pp. 59–80. [Google Scholar]
- Rudshteyn B, Castillo Á, Ghogare AA, et al (2014) Theoretical study of the reaction formalhydrazone with singlet oxygen. Fragmentation of the C=N bond, ene reaction and other processes. Photochem Photobiol. 10.1111/php.12199 [DOI] [PMC free article] [PubMed]
- Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008 doi: 10.1007/s00228-008-0478-6. [DOI] [PubMed] [Google Scholar]
- Salek N, Vosoughi S, Bahrami Samani A, et al. Radiolabeled TPP with 166Dy/166Ho in vivo generator as a novel therapeutic agent. J Radioanal Nucl Chem. 2020 doi: 10.1007/s10967-020-07364-6. [DOI] [Google Scholar]
- Salvio AG, Stringasci MD, Barreto Requena M, Bagnato VS. Photodynamic therapy in combination with surgery for the treatment of an extensive squamous cell carcinoma in situ - a case report. Photodiagnosis Photodyn Ther. 2020 doi: 10.1016/j.pdpdt.2020.101700. [DOI] [PubMed] [Google Scholar]
- Sanad MH, Farag AB, Saleh GM. Radiosynthesis and biological evaluation of 188Re-5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphyrin complex as a tumor-targeting agent. Radiochemistry. 2019 doi: 10.1134/S106636221903010X. [DOI] [Google Scholar]
- Sanad MH, Gizawy MA, Motaleb MA, et al. A comparative study of stannous chloride and sodium borohydride as reducing agents for the radiolabeling of 2,3,7,8,12,13,17,18-Octaethyl-21H,23H-Porphine with Technetium-99m for tumor imaging. Radiochemistry. 2021 doi: 10.1134/S1066362221040159. [DOI] [Google Scholar]
- Satari A, Ghasemi S, Habtemariam S, et al (2021) Rutin: a flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evidence-based Complement. Altern. Med. 10.1155/2021/9913179 [DOI] [PMC free article] [PubMed]
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review) Int J Oncol. 2019 doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafirstein G, Battoo A, Harris K, et al. Photodynamic therapy of non-small cell lung cancer narrative review and future directions. Ann Am Thorac Soc. 2016 doi: 10.1513/AnnalsATS.201509-650FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020 doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AVC, Pinto SMA, Calvete MJF, et al (2015) Synthesis of a new 18F labeled porphyrin for potential application in positron emission tomography. in vivo imaging and cellular uptake. RSC Adv. 10.1039/c5ra16103g
- Simone CB, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis. 2012 doi: 10.3978/j.issn.2072-1439.2011.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha BK. Topoisomerase inhibitors: a review of their therapeutic potential in cancer. Drugs. 1995 doi: 10.2165/00003495-199549010-00002. [DOI] [PubMed] [Google Scholar]
- Song R, Kim YS, Sohn YS. Synthesis and selective tumor targeting properties of water soluble porphyrin-Pt(II) conjugates. J Inorg Biochem. 2002 doi: 10.1016/S0162-0134(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Song R, Kim YS, Lee CO, Sohn YS. Synthesis and antitumor activity of DNA binding cationic porphyrin-platinum(II) complexes. Tetrahedron Lett. 2003 doi: 10.1016/S0040-4039(03)00022-4. [DOI] [Google Scholar]
- Song H, Wang G, Wang J, et al. 131I-labeled 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin and 5,10,15,20-tetrakis(4-aminophenyl)porphyrin for combined photodynamic and radionuclide therapy. J Radioanal Nucl Chem. 2018 doi: 10.1007/s10967-018-5735-2. [DOI] [Google Scholar]
- Srivatsan A, Pera P, Joshi P, et al. Highlights on the imaging (nuclear/fluorescence) and phototherapeutic potential of a tri-functional chlorophyll-a analog with no significant toxicity in mice and rats. J Photochem Photobiol B Biol. 2020 doi: 10.1016/j.jphotobiol.2020.111998. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sasaki M, Suzuki T, et al. Combination of talaporfin photodynamic therapy and Poly (ADP-Ribose) polymerase (PARP) inhibitor in gastric cancer. Biochem Biophys Res Commun. 2021 doi: 10.1016/j.bbrc.2020.12.073. [DOI] [PubMed] [Google Scholar]
- Teng CW, Amirshaghaghi A, Cho SS, et al. Combined fluorescence-guided surgery and photodynamic therapy for glioblastoma multiforme using cyanine and chlorin nanocluster. J Neurooncol. 2020 doi: 10.1007/s11060-020-03618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P, Li M, Bio M, et al. Far-red light-activatable prodrug of paclitaxel for the combined effects of photodynamic therapy and site-specific paclitaxel chemotherapy. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.5b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov S, Ostroverkhov P, Suvorov N, et al (2021) Tin carboxylate complexes of natural bacteriochlorin for combined photodynamic and chemotherapy of cancer è. Int J Mol Sci 2210.3390/ijms222413563 [DOI] [PMC free article] [PubMed]
- Tojo T, Niiuchi A, Kondo T, Yuasa M. Evaluation of the correlation between porphyrin accumulation in cancer cells and functional porphyrin positions of the phenyl group. ChemMedChem. 2022;17:e202100636. doi: 10.1002/cmdc.202100636. [DOI] [PubMed] [Google Scholar]
- Toubia I, Nguyen C, Diring S, et al. Study of cytotoxic and photodynamic activities of dyads composed of a zinc phthalocyanine appended to an organotin. Pharmaceuticals. 2021 doi: 10.3390/ph14050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade FZ, Pavarina AC, Ribeiro APD, et al. Toxicity of photodynamic therapy with LED associated to Photogem®: An in vivo study. Lasers Med Sci. 2012 doi: 10.1007/s10103-011-0909-y. [DOI] [PubMed] [Google Scholar]
- Tsioumpekou M, Cunha SI, Ma H, et al. Specific targeting of PDGFRβ in the stroma inhibits growth and angiogenesis in tumors with high PDGF-BB expression. Theranostics. 2020 doi: 10.7150/thno.37851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock MA, Bingham SF, DiGiovanna JJ, et al. Tretinoin and the prevention of keratinocyte carcinoma (basal and squamous cell carcinoma of the Skin): a Veterans Affairs randomized chemoprevention trial. J Invest Dermatol. 2012 doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]
- Wentrup R, Winkelmann N, Mitroshkin A, et al. Photodynamic therapy plus chemotherapy compared with photodynamic therapy alone in hilar nonresectable cholangiocarcinoma. Gut Liver. 2016;10:470–475. doi: 10.5009/gnl15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu H, Huang J, et al. Probing and enhancing ligand-mediated active targeting of tumors using sub-5 nm ultrafine iron oxide nanoparticles. Theranostics. 2020 doi: 10.7150/thno.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zheng Q, Cheng X, et al. Chemo-photodynamic therapy with light-triggered disassembly of theranostic nanoplatform in combination with checkpoint blockade for immunotherapy of hepatocellular carcinoma. J Nanobiotechnology. 2021 doi: 10.1186/s12951-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wang P, Li L, et al. Surgery sequential with 5-Aminolevulinic acid photodynamic therapy for lip squamous cell carcinoma: two cases reports. Photodiagnosis Photodyn Ther. 2020 doi: 10.1016/j.pdpdt.2020.102043. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang D, Li J, et al. A mitochondrion-targeting Mn(ii)-terpyridine complex for two-photon photodynamic therapy. Chem Commun. 2020 doi: 10.1039/d0cc02051f. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen F, Xu N, et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials. 2022;281:121368. doi: 10.1016/j.biomaterials.2022.121368. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhou Y, Liu L, et al (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 10.3389/fmolb.2020.00193 [DOI] [PMC free article] [PubMed]
- Yi H, Lu W, Liu F, et al. ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J Nanobiotechnology. 2021 doi: 10.1186/s12951-021-00877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Y, Zhang Z, et al. Silk fibroin-capped metal-organic framework for tumor-specific redox dyshomeostasis treatment synergized by deoxygenation-driven chemotherapy. Acta Biomater. 2022 doi: 10.1016/j.actbio.2021.11.009. [DOI] [PubMed] [Google Scholar]
- Zang L, Zhao H, Fang Q, et al. Photophysical properties of sinoporphyrin sodium and explanation of its high photo-activity. J Porphyr Phthalocyanines. 2017 doi: 10.1142/S1088424617500055. [DOI] [Google Scholar]
- Zhang J, Lu Y, Zhang X, et al. Clinical efficacy of Mohs surgery combined with topical photodynamic therapy for facial basal cell carcinoma. J Cancer Res Ther. 2020 doi: 10.4103/jcrt.JCRT_987_19. [DOI] [PubMed] [Google Scholar]
- Zhen Z, Tang W, Chuang YJ, et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano. 2014 doi: 10.1021/nn501134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Wang W, Wu F, et al. Zwitterionic polymer-gated Au@TiO2 core-shell nanoparticles for imaging-guided combined cancer therapy. Theranostics. 2019 doi: 10.7150/thno.35418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Wei Y, Sun Y, et al. Cyclic RGD-functionalized and disulfide-crosslinked iodine-rich polymersomes as a robust and smart theranostic agent for targeted CT imaging and chemotherapy of tumor. Theranostics. 2019 doi: 10.7150/thno.37184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zverev VV, Makarov OV, Khashukoeva AZ, et al. In vitro studies of the antiherpetic effect of photodynamic therapy. Lasers Med Sci. 2016 doi: 10.1007/s10103-016-1912-0. [DOI] [PubMed] [Google Scholar]