Abstract
Papillomaviruses are viruses with double-stranded DNA that are epitheliotropic and non-enveloped that infects cutaneous epithelial and mucosal cells in a species-specific way in several higher vertebrate species and cause cellular growth."There are around 100 different human papillomaviruses (HPVs)", as "more than 150 HPV types have been isolated and fully sequenced". We classify the probability of cancer development following viral infection with each HPV genotype into two types: “low-risk” and “high-risk.” As a result, HPV diagnosis is a critical component of HPV genotype identification and characterization. Based on its activities, we may classify the HPV genome into three regions: the long control region (LCR) or the non-coding upstream regulatory region (URR), the late (L) region, and the early (E) region. Functional proteins are mostly static things that are not inflexible; they have undergone both local and global movements at various times and time ranges. The structural differences between HPV16 and 18 discovered by molecular modeling of the E6 oncoprotein were associated with their carcinogenic characteristics. Similarly, the E6 protein has two sets of C-X-X-C motifs that play significant roles in transformation, transcriptional activation, interactions, and immortalization with other proteins of cells in the host environment. Here, we review the literature regarding the protein mechanisms associated with HPV and how they cause cancer. Unless otherwise noted, it described all protein activities in terms of HPV proteins. The term “papillomaviruses” refers to groups of papillomavirus proteins that have a characteristic in common. HPV proteins can study the genetic influences on pathogenicity and the therapeutic applications of genomics. The future study provides a potential advancement in HPV infections and malignant illnesses to improve preventive and treatment strategies. Patients have been able to conquer this condition using a range of therapies and vaccines that were projected to be effective and robust enough to put an end to the ailment completely. In cancer prevention strategies, HPV vaccination is one of the most effective. It is safe, efficient, and long-lasting.
Keywords: Human Papillomavirus, Carcinogenesis, Viral proteins, Pathogenesis
Introduction
Viruses are intracellular parasites that come in different shapes and cause potential harm to humankind. Few viruses may cause infections, also have oncogenic potential and cause life-threatening cancers. They vary in their transmission and may have genetic material such as DNA or RNA. There are a few viruses that are sexually transmitted, such as the Human immunodeficiency virus (HIV), Human sarcoma virus (HSV), and Human Papilloma Virus (HPV), and pose global health concerns. HPV is a cancer-causing virus that belongs to the papillomaviridae family. It causes genital warts, plantar and palmar warts, cancer of the vulva, cervix, testis, penile, oropharyngeal cancers, and frequent papillomatosis of the lungs globally [63]. The most prevailing sexually spread HPV infection spreads from one person to another by skin-to-skin contact, mucosal skin contact, which is frequent during sexual activity [35]. They mainly categorized it into high risk and low risk depending on the infection and cancer-causing ability. The intensity of the viral infection and the kind of malignancy that is causing the cutaneous or mucosal infection determine whether an infection is high-risk or low-risk.The low-risk and the high-risk types are linked with pre-neoplastic lesions and other cancers. Chronic infection by HPV in a person disrupts cell cycle pathways and leads to cancer. The high-risk human papillomaviruses are HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, which are related to malignant tumors of the oropharynx, neck, and neck cancer, in addition to malignancies of the male and female reproductive system. HPV 6, 11, and other low-risk genotypes are linked to benign skin lesions, papilloma, and warts [10].The human papillomavirus genome’s E6 and E7 genes are transcribed to produce essential proteins for viral replication. They also influence the p53 pathway, the retinoblastoma protein, and the overexpression of the p16 protein during carcinogenesis.
Compared to HPV-negative tumors, HPV-positive tumors had considerably fewer chromosomal mutations, according to ploidy studies [45]. The increased risk of contracting an HPV infection can be due to sexual intercourse at a young age, increased use of marijuana, oral sexual intercourse, and the patient’s history of having genital warts. In addition, patients with Fanconi anemia and HIV have an elevated probability of developing oropharyngeal cancer caused by the human papillomavirus. The nucleotide sequence homology of the L1 gene is the utmost sealed portion of the viral gene encodes for the capsid used to classify HPV [52]. It may be possible to expand the scope of immunotherapy by targeting HPV oncoproteins T cells using genetic programming with minimal adverse effects.
Epidemiology
The most prevalent cause of death worldwide is cancer [39]. Globally, about 38,000 instances of head and neck cancer are caused by HPV. In Tanzania, East Africa, research detected HPV genotypes in other anatomical sites such as fingertips. They compared the genotype concordance with the samples from genital and oral regions, showing a likelihood of autoinoculation in unvaccinated adolescent girls. This oncogenic virus accounts for around 5% of all malignancies globally, and 600,000 cancer occurrences are linked with HPV [3]. Scientists have identified that it increases the prevalence of HPV 16 in oropharyngeal cancer compared with cervical cancer. Both HPV 16 and HPV 18 contribute to 85% of malignancies in the head and neck region, and other types such as HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 constitute 90% of cases. Despite reducing cigarette and alcohol usage, which are independent predictors of oropharyngeal cancer, HPV exposure on the mucosal surface and seropositivity caused the increased occurrence of oral squamous tumor cells. Exposure induces carcinogenesis to a high-risk form of HPV, most often HPV 16, and can occur independently of tobacco or alcohol use. Specific molecular markers distinguish between HPV positive and negative oropharyngeal cancer [58, 23].
Patients with oropharyngeal cancer who tested positive for HPV had a 28% lower chance of dying and a 49% lower chance of relapse. Oropharyngeal carcinoma results from epigenetic aberrations, the immunological state of the person infected with HPV, and genetic variants that predispose a person to become infected with HPV and develop cancer [23]. The extensive population-based epidemiological assessment of the U.S. population showed an incidence of HPV-positive oropharyngeal squamous cell carcinoma of 4.62 per 100,000 people, as per the study conducted by Mahal et al. [44]. Cervical cancer causes the deaths of almost 68,000 Indians each year. There is a 432.2 million percent probability of acquiring cancer for every 15-year-old Indian girl. It is the most common kind of tumor in women between the ages of 15 and 44. According to the World Health Organization (WHO), Indian women are twice as likely as those in Bangladesh, Sri Lanka, or Iran to get cervical cancer when age is considered, with a prevalence rate of 22%.
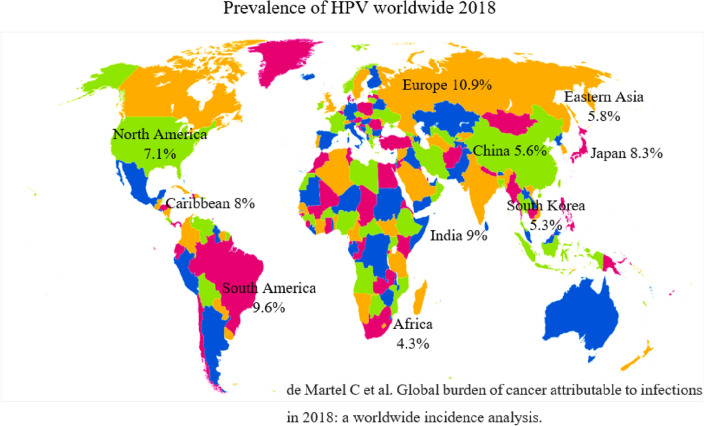
Cervical cancer screening rates vary between developed and developing countries, ranging from 1% in Bangladesh to 73% in Brazil. Africans account for 43% of cervical cancer cases; 15% occur in the United States; 10% occur in Eastern Asia; 6% occur in Australia; 4% occur in Western Asia; 15% occur in the Caribbean; and 27% occur in Melanesia [9, 37]. In 2018, the HPV and associated cancer incidence rate were 10.9% in Europe, 9.6% in South America, 7.1% in North America, 8% in the Caribbean, 4.3% in Africa, 9.6% in South East Asia, 9% in India, 7.9% in South Central Asia, 5.3% in South Korea, 8.3% in Japan, 5.6% in China, 5.8% in Eastern Asia [46] (Fig. 1). The incidence of cervical cancer among women aged 15 and over in India is 432.20 million. The problem of cervical cancer affects 122,844 women every year, and 67,477 of them lose their lives to it. According to the ICO Information Centre on Human Papilloma Virus (HPV) in India, around 7.9 percent of women in the general population are likely to be infected with cervical HPV at any given moment, and HPV 16 or 18 is responsible for 84.1 percent of invasive cervical malignancies [26].
Fig. 1.
Incidence rate of human papilloma virus worldwide
HPV distribution meta-analysis
When stratified by histological and country type, the prevalence of HPV in females with invasive cervical cancer (ICC) is estimated at around 85.4 percent; CIN, low- and high-grade squamous intraepithelial lesions (LSIL and HSIL), respectively, were estimated at 59.2 percent and 71.3 percent. In women with CIN, I/LSIL, the HPV prevalence was 54.4 percent, compared to 73.4 percent in women with CIN II-III. They found Asian women to have a greater prevalence of HPV positive than African women, which is intriguing found to have a greater prevalence of HPV positive than African women, which is intriguing. Moreover, 34, 8% of the women with ASCUS lesions had HPV. They found the infections in 12.2 percent of ICC patients, 13.5 percent had CIN II-III/HSIL, 24.8 percent had CIN I/LSIL, and 10.5 percent had ASCUS. HPV 16, 18, 45, 31, 33, 35, 6/11, 58, 52, and 59 were the 10 most prevalent HPV genotypes in ICC cases, followed by HPV 39, 66, 68, and 56, 73, and 51. Cervical cancers caused by HPV 16 and 18 were the most frequent in all nations. African women with ICC (83.3%) had a higher rate of HPV 16 and 18 infections than Asian women with ICC (70.8 percent) [64].
Role and severity of HPV in various cancer
HPV infection in the mouth is now recognized to induce oropharyngeal cancer. Despite the fact that oral HPV has a low frequency in the general population 4.5%, research has revealed that HPV 16 is responsible for 95 percent of HPV-related head and neck malignancies. Oropharyngeal malignancies caused by HPV are most commonly seen towards the base of the tongue and in the tonsils. The rising frequency of HPV-related oropharyngeal cancer has been linked to an increase in the number of sexual partners and younger persons. The relationship between sexual practices and HPV-associated oropharyngeal cancers implies that sexual partners of cervical cancer patients may be at a higher risk of getting HPV-associated oropharyngeal cancers. To assess the viability of reducing radiation doses in the treatment of HPV-associated oropharyngeal cancer, many clinical studies are now being conducted [16–50].HPV is widely known to play a role in the development of anal cancers. It has however become increasingly common over the past several decades. Anal cancer is more common among homosexual men and persons infected with the Human Immunodeficiency Virus (HIV). Though there is growing evidence that anal intra epithelial neoplasia (AIN) is a precursor to anal cancer, the data comes mostly from small studies with only a 5–10-year follow-up. It is necessary to conduct larger studies to determine whether AIN progresses to anal malignancies and whether it affects treatment outcomes [66].
Human Papillomavirus structural and functional properties
Human Papilloma Viruses are papilloma viridian with a diameter of 52–55 nm, are icosahedral in form, non-enveloped, and contain 8000 base pairs of double-stranded DNA. It contained eight genes inside HPV’s circular genome, including genes expressed in the early and late life cycle phases [24]. These genomic areas originated in the template or coding strand of double-stranded DNA: ten open-ended reading frames; the early region comprises up to seven open-reading frames (ORFs), for example, E1, E2, E4, E5, E6, and E7, which have genes encoding viral regulatory proteins. “E” and a number value showing the ORF length denotes each ORF in the early region, and in the late region, “L” genes encode viral capsid proteins. The origin of replication and transcriptional control sequences is in the lengthy control region, upstream regulatory region, and non-coding region [2]. There are over 200 HPV types that have been sequencing have finally. There are five diverse genera into which they categorize the viruses based on tissue tropism and subsequent host pathogenesis. The five genus kinds are ∝ -papillomavirus, β-papillomavirus, γ-papillomavirus, µ-papillomavirus, ν-papillomavirus. The International Committee on Taxonomy of Viruses (ICTV) papillomavirus research group provides papillomavirus nomenclature. Some HPV types, alpha papillomaviruses, are cancerous and cause cervical cancer and malignancies of the anal, vulvar, penile, vaginal, and oral cavity areas, while others cause benign lesions and warts [34] (Fig. 2).
Fig. 2.

Structure of HPV
Alpha papilloma virus
Infectious agents cause more than 15% of human malignancies, with HPV infection accounting for more than one-third of them. Cervical cancer, as well as vaginal, vulvar, anal, rectal, penile, and oropharyngeal cancer, is strongly linked to HPVs. In the International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans published in 2012, 12 HPVs were declared carcinogenic (Group 1), and another 13 were classified as probably or possibly carcinogenic (Group 2A and B) based on limited evidence and/or their close phylogenetic placement with other carcinogenic HPVs [57, 38].
Beta Papilloma virus
HPV strains from the beta gene family have long been disputed for their connection to skin keratinocyte carcinomas (KC), specifically squamous cell carcinoma. In people with epidermodysplasia verruciformis (EV) and the beta-HPV variants, two of these variants may cause cancer, according to the IARC. The evidence is not strong enough to prove that these viruses cause cancer in healthy people, however. The etiology of KCs has remained a mystery for quite some time because beta-HPVs, unlike alpha-HPVs, do not contribute to tumor maintenance. A dominant HPV type has not been consistently linked to KC, and they are not integrated into tumor cells. It has been demonstrated, however, that beta-HPV contributes to carcinogenesis [36, 59].
Pathogenesis of HPV encoded proteins
Usually, the basal cell splits and produces two offspring cells, one of which remains in the basal layer, and the other climbs upward and undergoes significant variation. When cells differentiate, they change their characteristics as well. Human papillomavirus entering cells involves many links, cellular conformational changes, and receptor flipping. HPV genomes exist as episomes or extrachromosomal segments after infection of the cell. They internalize the viral particle through a distinct endocytic process. It moves on to the nucleus after passing via the Golgi complex and the subviral complex [14]. It divided the HPV genome into early and late regions. The late region includes the L1 and L2 genes, while the early region has the E1, E2, E3, E4, E5, E6, and E7 genes (Table 1). Only one strand of dsDNA is the template for the expression of genes that encode diverse viral DNA transcripts. The genetic material of HPV typically integrates into a host-cell chromosome during carcinogenesis. Each site is unique because the virus integrates in an unpredictable manner [27]. Genes undergo a course of translation and produce proteins that aid in the viral replication cycle and oncogenicity.
Table 1.
HPV protein and their functions
| HPV Protein | Protein Name | Function | Organism | References |
|---|---|---|---|---|
| E1 | Replication protein E1 |
It is highly conserved among the HPV types and commonly decoded during early expression of HPV infection It is necessary for viral DNA replication because it helps E2 put together a Helicase complex, ATPase, and an ATP-binding protein |
HPV 31 | [6] |
| E2 | Regulatory protein E2 |
It is multifunctional proteins mostly associated with viral replication and transcription Depending on the promoter, it acts as an activator of transcription or as a repressor of viral genome The functions of E2 proteins are disrupted by mutation or the integration of DNA Binds E1 to help initiate viral DNA unwinding and replication, a viral transcription factor |
HPV 16 | [33] |
| E3 | Ubiquitin-protein ligase TRIM68 | Based on its ubiquitin ligase activity, it serves as a coactivator for the androgen receptor | HPV 31 | [68] |
| E4 | Protein E4 |
Plays a role in viral DNA post-transcriptional modification Commonly, E4 proteins have been expressed in the late stages of infection In the virus replication cycle, the expression of the E4 protein is mostly confined to the middle and the upper layers of the epithelium |
HPV 8 | [71] |
| E5 | Probable protein E5 |
E5 is one of the major oncoproteins in HPV-mediated carcinogenesis, however, β-HPV does not encode for E5 Growth factor receptors are activated when cells undergo transformation It is commonly expressed in the early stages of HPV infection that is evident by the E5 protein-mediated increased immortalization of human keratocytes by E6 and E7 |
HPV 16 | [18] |
| E6 | Protein E6 |
The principal role of E6 oncoproteins is to promote the ubiquitin-dependent proteasome degradation of p53 in HPV-infected cells, that demonstrate a neoplastic effect Immortalizes primary keratinocytes via binding to and directing p53's ubiquitin-mediated degradation and working in conjunction with E7 |
HPV 16 | [12] |
| E7 | Protein E7 |
In HPV-induced Cervical carcinoma, E7 oncoprotein plays a crucial role in promoting cervical dysplasia causing dysregulated cell cycle Co-operates with E6 to immortalise primary cells by binding retinoblastoma protein and activating E2F transcription factor |
HPV 16 | [51] |
The genes of the early region L1 and L2 code for proteins that form the capsid. The capsid shell contains 360 L1 monomers organized into 72 pentamers, also known as capsomeres, in each capsid. According to studies, there are about 72 molecules of L2 in each capsid, organized as one L2 copy per capsomere [15]. The L1 protein is a vital structural protein of about ~ 55 kD synthesized by L1 gene translation, resulting in the main capsid protein via the virus particle interacting with the heparin sulfate proteoglycan receptors on the cell surface of keratinocyte cells. The viral capsid comprises 72 pentameric L1 protein capsomeres. 360 copies of the L1 protein are required to produce the capsid of the human papillomavirus and are arranged in the shape of a pentamer [62]. It proteolytically clove the capsid proteins before entering the cell by proteolytic enzymes, allowing the viral particles to enter the cell quickly. Capsid proteins undergo conformational changes when they connect with the Heparin sulfate proteoglycan receptors. A cyclophilin first exposes L2 protein—B-mediated conformational changes in viral capsid, which are then degraded by Kallikrein-8, furin, and PC5/6. A secondary receptor on the target cell’s plasma membrane enables it to attach to it [32, 8]. It directly exposed the basal cell layer owing to micro-abrasions; there is an upregulation of the syndecan receptor, which leads to the internalization or absorption of human papillomavirus particles in vivo [43].
Syndecans are the most abundant heparin sulfate proteoglycan receptors in human papillomavirus target cells, epithelial cells [1]. L2 serves as the minor capsid protein, and it composed this polypeptide of 500 amino acids, and its molecular mass is 55 kDa. The capsid also includes an unspecified number of L2 proteins. However, up to 72 L2 proteins they have further estimated that to be present in a single capsid. The functions of the L2 protein are to provide stability, aid in viral entry and localization of viral particles in the cell, and form the capsid. L2 promotes HPV infection via an interaction between the L2 protein’s N terminus and an unknown cell surface receptor. The study conducted by Laura et al. suggests that the minor capsid protein has a significant impact because they have identified L2 as a vital protein as it drives immune evasion of HPV16 through the interaction between the body’s cells and the virus [72] (Fig. 3).
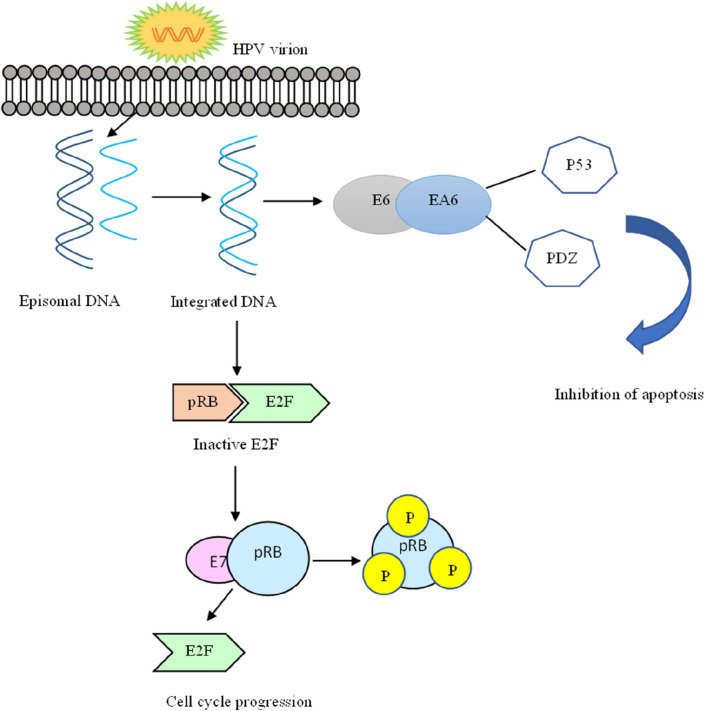
Fig. 3.
Pathogenesis of HPV in carcinogenesis
Proteins associated with HPV
A single DNA strand is used to transcribe the eight ORFs seen in all HPV genomes. Necessary proteins (E1–E7) necessary for viral reproduction are found in the early (E) and late (L) portions of the genome, whereas cis-elements requisite for viral DNA transcription and replication are found in the long control region, which is primarily non-coding. Various promoters transcribe viral L and E proteins at different stages during infection. The presence of two proteins in HPV, E1, and E2, helps to identify replication sources. E2 is in charge of viral gene expression. E4 is thought to be engaged in the late phases of the viral life cycle, whereas E5 is intricate in both the early and late steps. Several cell cycle regulators, including the E6 and E7 proteins, are targeted by these two proteins, especially p105Rb and p53. Viral E6 and E7 are intricate in maintaining viral episomes and the re-entry of cells into the S phase throughout the viral life cycle. The L1 and L2 proteins produce capsomers, which subsequently form icosahedral capsids around the viral DNA during the formation of progeny virions [19].
E1 replication protein
E1 is the only protein with an enzymatic activity that includes helicase or ATPase activity. It is the most significant protein when compared with other gene products. The E1 protein’s primary function is to assist viral replication and interact with other replication machinery components. Histone H1 and Ini1/hSNF5 (a part of the chromatin remodeling complex SWI/SNF that promotes viral replication) are two other biological components that interact with the E1 protein. Amplification of the viral genome is thought to be promoted by E1 throughout the prolific stage of the viral life cycle, which arises in the uppermost distinguished epithelial layers by increasing the copy number and maintaining the viral episome number after infection of the keratinocyte in the basal layer. In order to start and catalyze viral DNA synthesis, this ATP-dependent hexameric helicase unwinds the viral genome’s origin of replication (ori) and DNA prior to replication forks [5]. HPV E1 proteins triggered a decrease in four immune response genes (CCL5, RSAD2, IFNB1, and IFNL1). E1 of HPV type 16, 18, and 11 proteins reduce IFNβ1 and IFNλ1 expression [11]. It is not uncommon for viral genomes to be integrated to affect the gene that codes for the E1 replicative helicase, which disrupts the E2 gene upstream. It highly regulated the E1 protein in HPV infection because excessive E1 production might cause DNA damage and a growth halt. The over amplification of the integration area is another way that E1 expression might contribute to localized genomic instability. A selective growth advantage might be gained by disrupting the E1 gene, leading to clonal proliferation [61] (Fig. 4).
Fig. 4.
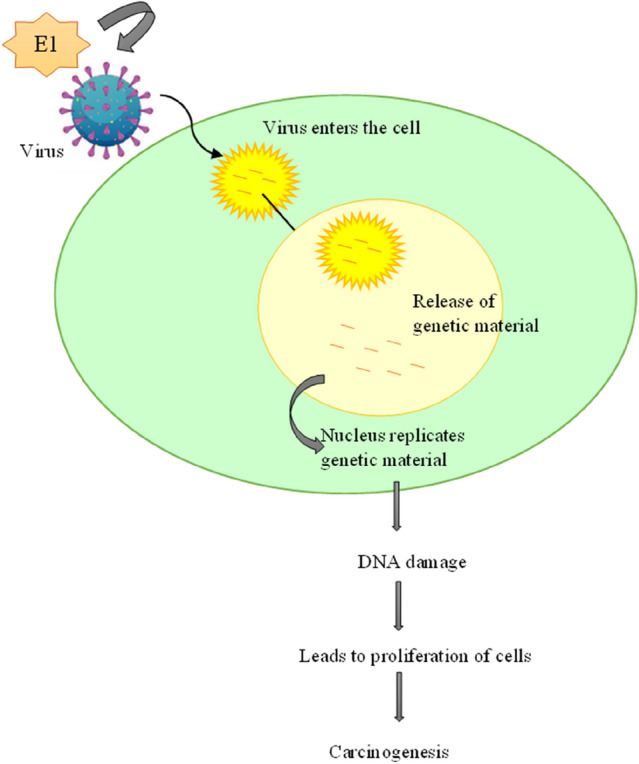
Role of E1 in HPV
E2 regulatory protein
E2 is a regulatory multifunctional protein that interacts with E1 and helps it to bind to the viral origin. They found the viral replication origin in the Upstream Regulatory Region (URR), a non-coding area of the viral genome [42]. The E2 protein is functional and comprises three domains: the Serine and Arginine-rich Hinge region, the C-terminal DNA-binding dimerization domain, and the N-terminal transactivation domain. The transactivation domain of the E2 protein interacts with the E1 viral protein and several host cellular proteins and has various roles in DNA replication, transcription, post-transcriptional regulation, and many other roles [41–31]. HPV genome integrates into the human genome; as a result, the E2 gene is most disruptive, resulting in the deletion of the E2 gene, which has linked to carcinogenesis. The expression of the genes E6 and E7 of the HPV’s viral genome is consistent in all HPV-associated cancers. The E6 associates with an ubiquitin-protein ligase E6AP target, the p53 ubiquitination and proteolysis, whereas the E7 binds to the pRB and inactivates its function, which plays a significant role in tumor suppression. Because E2 can suppress the expression of E6 or E7, when the E2 disruption of a gene, the unrestricted expression of E6 and E7 occurs, leading to carcinogenesis [56].
Ubiquitin E3
The E3 gene, which is found in the early region of the HPV genome, is only present in a limited subset of papillomavirus variations, such as HPV 1, 11, 16, 31, and 33 [55]. The E1, E2, and E3 belong to the ubiquitin–proteasome pathway (UPS). It is an essential metabolic pathway with significant roles in the cell cycle, apoptotic mechanisms, and DNA repair in response to damage. UPS selectively degrades misfolded proteins, controls the activity, and maintains the correct proteins intracellularly [68]. The sequential action of ubiquitination and deubiquitination enzymes is a vital part of the ubiquitination proteasome system. It transferred the ubiquitin to the target protein in 3 steps: activation of ubiquitin, and it binds to the ubiquitin-activating enzyme E1. ubiquitin transfer from E1 to E2 (ubiquitin-conjugating enzyme); interaction between E2 and E3, resulting in ubiquitin transfer to lysine amino acid residues on the target protein by the E3 ubiquitin-protein ligase and the formation of polyubiquitin chains; proteasomal protein degradation of target protein substrates with polyubiquitin chains attached via lysine 48 residue by the 26 S proteasome [21].
Classification of the E3 ubiquitin ligases is based on their substrates into two leading families: RING finger E3 ubiquitin ligases and Homologous to E6-associated protein C-terminus (HECT). It produced cellular ubiquitin ligase E6AP because of the E3A or UBE3A gene transcription and belongs to the HECT family. HECT E3 ligases directly link cancer and neurodegenerative disorders and play a role in developing other diseases [65]. The HPV E6 protein interacts with the conserved LxxLL motif on the N terminus of the E6AP. The complex of E6 and E6AP further recruits the p53 tumor suppressor protein. The ubiquitination of p53 makes it a target for proteasomal degradation, promoting carcinogenesis as there is less tumor suppressor. The UBE3A gene is on chromosome 15, locus q11-13 [60]. A mutation in the E6AP motif reveals that the association of elimination of association prevents targeting [7]. The low-risk types encode a little of diverse E6 and do not target the p53 protein for proteolysis. The deactivation of suppressor proteins by the viral oncoprotein induces cell transformation and cancer [30]. Proteomic analysis conducted by Subbaiah et al. indicated an overproduction of EDD1 (E3 ligase), which interacts with Tat-interacting protein (TIP60). It reduced the production of TIP60 in patients who have cancer. Apoptosis and central dogma have a role in the TIP60protein. The study’s results show that, through the proteasome pathway, E3 ligase down-regulates TIP60. It is also one way that E3 ligases influence carcinogenesis mechanisms [67]. TIP60 has a tumour suppressor function independent of its p53 acetylation function [4], and TIP60 degradation allows HPV to increase cell survival and proliferation [40].
E4 proteins with HPV
E4 ORF is between the E2 ORF and varies in size according to the HPV types. It expressed the protein product in the upper middle epithelial layers, disrupts cell cycle mechanisms and arrests cell growth for viral amplification, regulates the late region genes L1 and L2, and aids in releasing virions. The high-risk E4 proteins being deposited as amyloid fibers. This makes it a biomarker that shows infection [20, 13]. The E4 gene product that produce as a fusion protein E1^E4 because of mRNA in which the initiation codon and first few amino acids are derived from the E1 ORF as a leucine cluster motif, which allows it for self-association, resulting in the manipulation of host cell architecture. The E4-associated induction of apoptosis may be related to the suppression of the activity of E6 and E7 while cells migrate from the base to the middle layers of the epithelia in infection with the high-risk type HPV16. As the affected cell undergoes ultimate maturation, cellular kinases and proteases physically and physiologically change the 16E1^E4 protein [73].
E5 proteins with HPV
It produced the E5 proteins because of the transcription and translation of the HPV E5 gene. The locus of the E5 gene is present at the 3’ end of the viral genome’s early site, and they translated it via splicing mRNA that starts upstream of the E2 gene [17]. Intracellular communication between the target host cell protein and the E5 protein of HPV is vital and serves excellent importance, as this communication mechanism helps the virus from detection by the immune system and cellular transformation. It stops receptor-mediated apoptotic cell death, which is indispensable for the protein’s function. Integrating the viral genome into the target cell results in the deletion or loss of E2 and E5 genes [69].E5 engages in pro-carcinogenic activities such as EGF-mediated cell proliferation, suppression of apoptosis produced by Tumor Necrosis Factor Ligand (TNFL), and CD95 ligand (CD95L) modulation of genes concerned with cell adhesion and cell motility. HPV16 E5 can bind to and block the role of EVER1, EVER2, and ZnT-1, leading to MTF-1 transcriptional suppression. The E5 form is expressed by high-risk human papillomaviruses (hrHPVs), which contribute to lesion progression and tumour formation [25] (Fig. 5).
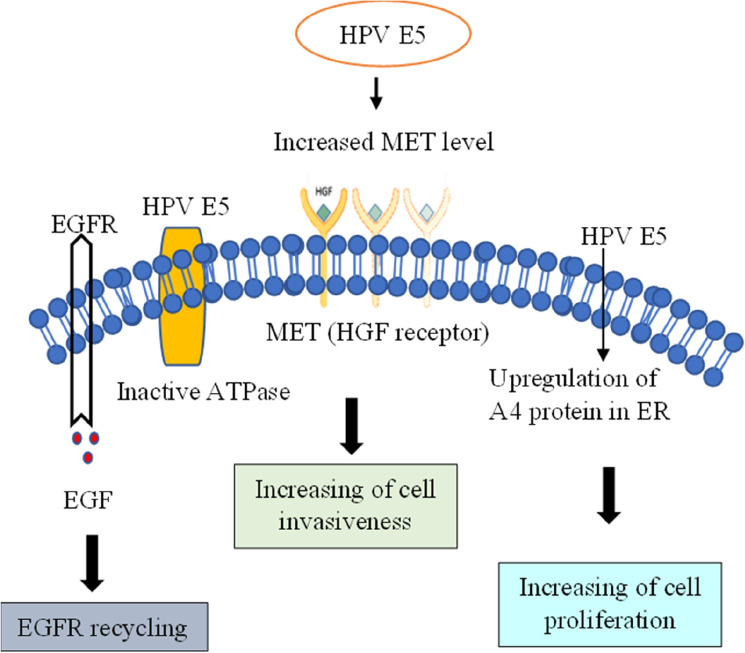
Fig. 5.
Role of E5 in HPV
HPV 16 E5 relies on specific critical functions known as kinase signalling to transform cells. Most studies connect HPV E5 transformation to the epidermal growth factor (EGF) receptor, rather than the PDGF-b receptor. HPVs primarily target epithelial cells, which have a high number of EGF receptors but no PDGF receptors [16]. They hypothesized E5 to work by interacting with and regulating the function of host proteins. In the instance of the HPV 16 type, the E5 proteins interact with various proteins, including ErbB4, a receptor for growth factors involved in cell division and apoptotic processes. The E5 protein performs its function by interacting with a variety of proteins and regulatory processes. The cell culture model investigations show that EGFR hyper-activation occurs within the cells containing E5, but the activation mechanism is unknown. To remove a viral infection from the body, the arm of the immune response that is cell-mediated recognizes the foreign viral peptides presented by the MHC molecule. The E5 protein of HPV induces down regulation of MHC class 1 molecules on the cell surface by targeting HLA-A and HLA-B. It therefore removed them from the cell surface.
A chronic viral infection starts carcinogenic processes. HPV16 E5 may hinder programmed cell death in response to variability, including Fas ligand and TRAIL. It can also decrease apoptosis following UV-B irradiation via a mechanism involving ERK1/2, MAPK, and PI3-kinase. Targeting the pro-apoptotic regulator BAX also prevented reactive oxygen species-induced apoptosis. Incorporating the genetic material of HPV within-host chromosomes is frequently related to HPV-induced malignancy. As a result, E5 is unlikely to be a viable therapeutic aim in impending phases of illness [53].
E6 proteins with HPV
The first open reading frame downstream of the non-coding region undergoes transcription and translation to produce the HPV E6 protein of the high-risk alpha genera comprising 150 amino acids. Two conserved internal repeats of a sequence of nucleotides distinguished it. HPV E6 oncoprotein interacts with numerous cellular proteins, causing cellular change and, as a result, the production of malignancy. The E6 primary cellular target is p53, which is followed by interactions with critical cellular proteins involved in apoptotic processes, cell proliferation, cell migration, antiviral response, DNA repair, and genomic instability. The interaction of E6 with MCM7 (replication licensing factor) enables the early G1 phase arrest point to be overridden by chromosomal aberrations [74]. Another function is the capacity to stimulate telomerase and ensues through a transactivation cascade comprising interactions between Myc and NFX1-9. The action of E6 fulfilled significant functions in growth stimulus by the instigation of telomerase and assumed inhibition of the degradation of SRC family kinases. Other disruptions in cell G protein signaling cause a negative regulation of the mTOR signaling pathway. HR-HPV E6 inhibits the transactivation and initiation of interferon β via interacting with interferon-regulatory factor 3. The initiation of cytokine production and the recognition of viral genome material is prevented as HR-HPV E6 inhibits transcription of TLRs. Investigating telomerase functions and genome instability can provide more information about HPV-related carcinogenesis [70, 75].
E7 proteins with HPV
The E7 gene sequence when transcribed and translated into E7 protein, the E7 gene sequence. This polypeptide contains 100 amino acids, is acidic in nature, and is small in size.The E7 protein allows the reproduction of viruses in cells that are no longer dividing by the interruption of closely associated processes such as cell proliferation, cellular differentiation. E7 can exist in both the cytoplasm and the nucleus. The high affinity of binding between E7 protein and retinoblastoma (Rb) protein of the high-risk type is comparatively higher than that of the low-risk type. The Rb protein serves as a tumor suppressor and is accountable for the G1 barrier, which blocks the progression into the S phase-cell cycle. Rab2/p130, Rb/p105, and P107 are members of the Rb family [74, 48]. These are called pocket proteins; the pocket domains comprise significant sequences for tumor suppressor function. The binding of Rb with E2F (transcription factor) is essential to suppress the expression of replication enzymes in a normal physiological condition. However, during HPV infection, the interaction between Rb and E7 results in cell cycle progression. This binding results in the release of E2F in transcriptionally active forms, which promote cell proliferation. The oncoprotein and host protein (tumor suppressor) lead to cellular transformation [28]. The exact sequence of the E7 gene must be present to induce carcinogenesis by binding. A study conducted by Mirabello et al. found that benign lesions or warts had more mutations in the E7 gene than neoplastic lesions. Human APOBEC3 may cause mutations in the sequence of the E7 gene, which introduces mutations in the viral DNA to restrict the human papillomavirus [49] (Fig. 6). There is evidence that HPV viral oncogenes E6 and E7 contribute significantly to HPV-induced cervical cancer. Invasive tumors and subsets of high-grade lesions show enhanced expression caused by integraation of the viral DNA into host genomes [54].
Fig. 6.
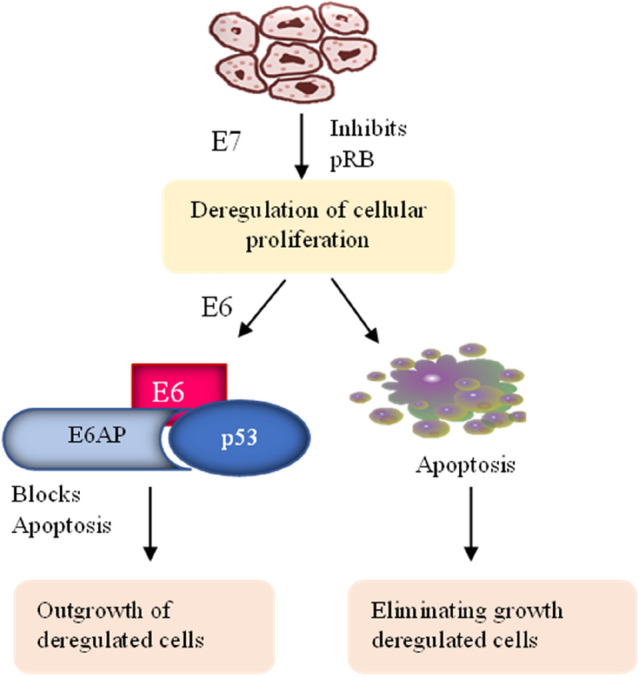
Role of E6 and E7 HPV proteins
Conclusion
HPVs are a significant cause of illness and death globally. Around 5% of all malignancies in the world and 600,000 cancer occurrences are linked with HPV.Identifying the many HPV strains that cause disease has resulted in improved diagnostic, screening, and preventative measures for the medical community. As the infected cell is forcibly driven to resume DNA replication, E6 performs several functions, interfering with several cellular pathways to establish a conducive environment for viral replication and neutralizing the cellular surveillance mechanisms that are turned on. Oncogenic HPV strains share an ancestral lineage, according to the most recent research. The precise genetic basis for HPV oncogenicity is complicated and will need new analytic techniques. As a model for non-recombinant genome evolution, pathogenicity, genetic factors, and genomic applications for therapeutic use, the HPV genome may be studied. Large-scale investigations will offer protein interactions of HPV high-risk genotypes, and advanceevaluations with progressing tumours (Stage III, IV) will be necessary to validate these present findings, for future HPV research, this data will serve as a baselinevaccination programs. We should screen women with a family history of cancer for HPV at regular intervals and genetic testing for oncogenes. Improved knowledge of the infection’s genomic features and microenvironment may lead to novel therapeutic interventions, such as preventative vaccinations. In addition, the genomic studies of this virus might help cure existing lesions and limit the risk of HPV-related malignancies.
Acknowledgements
The authors thank the Chettinad Academy of Research Education for the constant support and encouragement.
Abbreviations
- HPV
Human Papilloma virus
- URR
Upstream regulatory region
- LCR
Long control region
- HSV
Human Sarcoma virus
- HIV
Human immuno virus
- WHO
World health organization
- ICC
Invasive cervical cancer
- LSIC
Low grade squamous intraepithelial lesions
- HSIC
High grade squamous intraepithelial lesions
- ASCUS
Atypical squamous cells of undetermined significance
- ORF
Open reading frames
Funding
Not applicable.
Data availability and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest to report.
Consent to participate
Not applicable.
Consent for publication
All authors have read and approved the manuscript.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aksoy P, Gottschalk EY, Meneses PI. HPV entry into cells. Mutat Res Rev Mutat Res. 2017 doi: 10.1016/j.mrrev.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apt D, Watts RM, Suske G, Bernard HU. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV-16 promoter. Virology. 1996 doi: 10.1006/viro.1996.0530. [DOI] [PubMed] [Google Scholar]
- 3.Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: a growing global problem. Int J Appl Basic Med Res. 2016 doi: 10.4103/2229-516X.179027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassi C, Li YT, Khu K, Mateo F, Baniasadi PS, Elia A, Mason J, Stambolic V, Pujana MA, Mak TW, Gorrini C. The acetyltransferase Tip60 contributes to mammary tumorigenesis by modulating DNA repair. Cell Death Differ. 2016 doi: 10.1038/cdd.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergvall M, Melendy T, Archambault J. The E1 proteins. Virology. 2013 doi: 10.1016/j.virol.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergvall M, Melendy T, Archambault J. The E1 proteins. Virology. 2013;445(1–2):35–56. doi: 10.1016/j.virol.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brimer N, Lyons C, Vande Pol SB. Association of E6AP (UBE3A) with human papillomavirus type 11 E6 protein. Virology. 2007 doi: 10.1016/j.virol.2006.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronnimann MP, Calton CM, Chiquette SF, Li S, Lu M, Chapman JA, Bratton KN, Schlegel AM, Campos SK. Furin cleavage of L2 during papillomavirus infection: minimal dependence on cyclophilins. J Virol. 2016 doi: 10.1128/JVI.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J. Human papillomavirus and related diseases in Kenya. Summary report. 2016.
- 10.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003 doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Muñoz LJ, Manzo-Merino J, Muñoz-Bello JO, Olmedo-Nieva L, Cedro-Tanda A, Alfaro-Ruiz LA, Hidalgo-Miranda A, Madrid-Marina V, Lizano M. The Human Papillomavirus (HPV) E1 protein regulates the expression of cellular genes involved in immune response. Sci Rep. 2019 doi: 10.1038/s41598-019-49886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celegato M, Messa L, Bertagnin C, Mercorelli B, Loregian A. Targeted disruption of E6/p53 binding exerts broad activity and synergism with paclitaxel and topotecan against HPV-transformed cancer cells. Cancers. 2022;14(1):193. doi: 10.3390/cancers14010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celewicz A, Celewicz M, Michalczyk M, Rzepka R. Perspectives in HPV secondary screening and personalized therapy basing on our understanding of HPV-related carcinogenesis pathways. Mediators Inflamm. 2020 doi: 10.1155/2020/2607594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira C, SamperioVentayol P, Vogeley C, Schelhaas M. Kallikrein-8 proteolytically processes human papillomaviruses in the extracellular space to facilitate entry into host cells. J Virol. 2015 doi: 10.1128/JVI.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiGiuseppe S, Bienkowska-Haba M, Guion LGM, Keiffer TR, Sapp M. Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J Virol. 2017 doi: 10.1128/JVI.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMaio D, Mattoon D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene. 2001 doi: 10.1038/sj.onc.1204915. [DOI] [PubMed] [Google Scholar]
- 17.DiMaio D, Petti LM. The E5 proteins. Virology. 2013 doi: 10.1016/j.virol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiMaio D, Petti LM. The E5 proteins. Virology. 2013;445(1–2):99–114. doi: 10.1016/j.virol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006 doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 20.Doorbar J. The E4 protein; structure, function and patterns of expression. Virology. 2013 doi: 10.1016/j.virol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Đukić A, Lulić L, Thomas M, Skelin J, Bennett Saidu NE, Grce M, Banks L, Tomaić V. HPV oncoproteins and the ubiquitin proteasome system: a signature of malignancy? Pathogens. 2020 doi: 10.3390/pathogens9020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53(Suppl 1):S5–11. doi: 10.1016/j.ypmed.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299–309. [PMC free article] [PubMed] [Google Scholar]
- 24.Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 doi: 10.1128/JVI.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Freitas AC, de Oliveira THA, Barros MR, Jr, Venuti A. hrHPV E5 oncoprotein: immune evasion and related immunotherapies. J Exp Clin Cancer Res. 2017 doi: 10.1186/s13046-017-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan S, Subbiah VN, Michael JC. Associated factors with cervical pre-malignant lesions among the married fisher women community at Sadras, Tamil Nadu. Asia Pac J Oncol Nurs. 2015;2(1):42–50. doi: 10.4103/2347-5625.146223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao G, Smith DI. Human papillomavirus and the development of different cancers. Cytogenet Genome Res. 2016 doi: 10.1159/000458166. [DOI] [PubMed] [Google Scholar]
- 28.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006 doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 29.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002 doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 31.Graham SV. Human papillomavirus E2 protein: linking replication, transcription, and RNA processing. J Virol. 2016 doi: 10.1128/JVI.00502-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci (Lond) 2017 doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 33.Grm HS, Massimi P, Gammoh N, Banks L. Crosstalk between the human papillomavirus E2 transcriptional activator and the E6 oncoprotein. Oncogene. 2005;24(33):5149–5164. doi: 10.1038/sj.onc.1208701. [DOI] [PubMed] [Google Scholar]
- 34.Harden ME, Munger K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017 doi: 10.1016/j.mrrev.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houlihan CF, Baisley K, Bravo IG, Pavón MA, Changalucha J, Kapiga S, De Sanjosé S, Ross DA, Hayes RJ, Watson-Jones D. Human papillomavirus DNA detected in fingertip, oral and bathroom samples from unvaccinated adolescent girls in Tanzania. Sex Transm Infect. 2019 doi: 10.1136/sextrans-2018-053756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howley PM, Pfister HJ. Beta genus papillomaviruses and skin cancer. Virology. 2015;479–480:290–296. doi: 10.1016/j.virol.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hull R, Mbele M, Makhafola T, Hicks C, Wang SM, Reis RM, Mehrotra R, Mkhize-Kwitshana Z, Kibiki G, Bates DO, Dlamini Z. Cervical cancer in low and middle-income countries. Oncol Lett. 2020 doi: 10.3892/ol.2020.11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Huma. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 39.Jayavardhini B, Kumar C, Vijayashree R, Vedakumari SW. Recent Advancements in Nanomedicine for Cancer Diagnosis. NanoBioMedicine.:63.
- 40.Jha S, Vande Pol S, Banerjee NS, Dutta AB, Chow LT, Dutta A. Destabilization of TIP60 by human papillomavirus E6 results in attenuation of TIP60-dependent transcriptional regulation and apoptotic pathway. Mol Cell. 2010 doi: 10.1016/j.molcel.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurg R. DNA replication-current advances. London: IntechOpen; 2011. The role of E2 proteins in papillomavirus DNA replication. [Google Scholar]
- 42.Laaneväli A, Ustav M, Ustav E, Piirsoo M. E2 protein is the major determinant of specificity at the human papillomavirus origin of replication. PLoS ONE. 2019 doi: 10.1371/journal.pone.0224334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010 doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahal BA, Catalano PJ, Haddad RI, Hanna GJ, Kass JI, Schoenfeld JD, Tishler RB, Margalit DN. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019 doi: 10.1158/1055-9965.EPI-19-0038. [DOI] [PubMed] [Google Scholar]
- 45.Marklund L, Hammarstedt L. Impact of HPV in oropharyngeal cancer. J Oncol. 2011 doi: 10.1155/2011/509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020 doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 47.McBride AA. The papillomavirus E2 proteins. Virology. 2013 doi: 10.1016/j.virol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin-Drubin ME, Münger K. The human papillomavirus E7 oncoprotein. Virology. 2009 doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirabello L, Yeager M, Yu K, Clifford GM, Xiao Y, Zhu B, Cullen M, Boland JF, Wentzensen N, Nelson CW, Raine-Bennett T, Chen Z, Bass S, Song L, Yang Q, Steinberg M, Burdett L, Dean M, Roberson D, Mitchell J, Lorey T, Franceschi S, Castle PE, Walker J, Zuna R, Kreimer AR, Beachler DC, Hildesheim A, Gonzalez P, Porras C, Burk RD, Schiffman M. HPV16 E7 genetic conservation is critical to carcinogenesis. Cell. 2017 doi: 10.1016/j.cell.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirghani H, Amen F, Blanchard P, Moreau F, Guigay J, Hartl DM, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int J Cancer. 2015;136:1494–1503. doi: 10.1002/ijc.28847. [DOI] [PubMed] [Google Scholar]
- 51.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 52.Mühr L, Eklund C, Dillner J. Towards quality and order in human papillomavirus research. Virology. 2018 doi: 10.1016/j.virol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Müller M, Prescott EL, Wasson CW, Macdonald A. Human papillomavirus E5 oncoprotein: function and potential target for antiviral therapeutics. Futur Virol. 2015;10(1):27–39. doi: 10.2217/fvl.14.99. [DOI] [Google Scholar]
- 54.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento KC. Avaliação da presença do Papilomavírushumano (HPV) no sangueperiférico de mulheres com lesõesintraepiteliais de alto grau e baixograu (Master's thesis, Universidade Federal de Pernambuco).
- 56.Nishimura A, Ono T, Ishimoto A, Dowhanick JJ, Frizzell MA, Howley PM, Sakai H. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J Virol. 2000 doi: 10.1128/jvi.74.8.3752-3760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 58.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014 doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quint KD, Genders RE, de Koning MN, Borgogna C, Gariglio M, Bouwes Bavinck JN, Doorbar J, Feltkamp MC. Human Beta-papillomavirus infection and keratinocyte carcinomas. J Pathol. 2015;235:342–354. doi: 10.1002/path.4425. [DOI] [PubMed] [Google Scholar]
- 60.Sailer C, Offensperger F, Julier A, Kammer KM, Walker-Gray R, Gold MG, Scheffner M, Stengel F. Structural dynamics of the E6AP/UBE3A-E6-p53 enzyme-substrate complex. Nat Commun. 2018 doi: 10.1038/s41467-018-06953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakakibara N, Mitra R, McBride AA. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J Virol. 2011 doi: 10.1128/JVI.00541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sapp M, Bienkowska-Haba M. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 2009 doi: 10.1111/j.1742-4658.2009.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019 doi: 10.15585/mmwr.mm6833a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shoja Z, Farahmand M, Hosseini N, Jalilvand S. A meta-analysis on human papillomavirus type distribution among women with cervical neoplasia in the WHO eastern mediterranean region. Intervirology. 2019 doi: 10.1159/000502824. [DOI] [PubMed] [Google Scholar]
- 65.Sluimer J, Distel B. Regulating the human HECT E3 ligases. Cell Mol Life Sci. 2018 doi: 10.1007/s00018-018-2848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanley MA, Winder DM, Sterling JC, Goon PK. HPV infection, anal intra-epithelial neoplasia (AIN) and anal cancer: Current issues. BMC Cancer. 2012;12:398. doi: 10.1186/1471-2407-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subbaiah VK, Zhang Y, Rajagopalan D, Abdullah LN, Yeo-Teh NS, Tomaić V, Banks L, Myers MP, Chow EK, Jha S. E3 ligase EDD1/UBR5 is utilized by the HPV E6 oncogene to destabilize tumor suppressor TIP60. Oncogene. 2016 doi: 10.1038/onc.2015.268. [DOI] [PubMed] [Google Scholar]
- 68.Tu Y, Chen C, Pan J, Xu J, Zhou ZG, Wang CY. The Ubiquitin Proteasome Pathway (UPP) in the regulation of cell cycle control and DNA damage repair and its implication in tumorigenesis. Int J Clin Exp Pathol. 2012;5(8):726–738. [PMC free article] [PubMed] [Google Scholar]
- 69.Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, Borzacchiello G. Papillomavirus E5: the smallest oncoprotein with many functions. Mol Cancer. 2011 doi: 10.1186/1476-4598-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace NA, Galloway DA. Novel functions of the human papillomavirus E6 oncoproteins. Annu Rev Virol. 2015 doi: 10.1146/annurev-virology-100114-055021. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Meyers C, Wang H-K, Chow LT, Zheng Z-M. Construction of a full transcription map of human papillomavirus type 18 during productive viral infection. J Virol. 2011;85(16):8080–8092. doi: 10.1128/JVI.00670-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JW, Roden RB. L2, the minor capsid protein of papillomavirus. Virology. 2013 doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yajid AI, Zakariah MA, Mat Zin AA, Othman NH. Potential role of E4 protein in human papillomavirus screening: a review. Asian Pac J Cancer Prev. 2017 doi: 10.22034/APJCP.2017.18.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005 doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002. doi: 10.1038/nrc798. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.