Abstract
Secondary metabolites of bacteria are regulatory molecules that act as “info-chemicals” that control some metabolic processes in the cells of microorganisms. These molecules provide the function of bacteria communication in microbial communities. As primary producers of organic matter in the biosphere, microalgae play a central ecological role in various ecosystems. Photosynthesis is a central process in microalgae cells, and it is exposed to various biotic and abiotic factors. Various secondary metabolites of bacteria confer a noticeable regulatory effect on photosynthesis in microalgae cells. The main purpose of this review is to highlight recent experimental results that demonstrate the impact of several types of common bacterial metabolites (volatile organic compounds, non-protein amino acids, and peptides) on photosynthetic activity in cells of microalgae. The use of these molecules as herbicides can be of great importance both for practical applications and for basic research.
Keywords: Allelopathic molecules, BMAA, Chlorophyll, Microcystins, Photosystems, Phytotoxicity, Secondary metabolites, VOCs
Introduction
The German biochemist Ludwig Karl Martin Leonard Albrecht Kossel (Hartmann 2008) introduced the concept of primary and secondary metabolites. He referred to the primary metabolites as nucleic acids, proteins, lipids, and carbohydrates. Secondary metabolites are low molecular weight molecules, and they are not necessary for cell survival. Modern analytical and bioinformatics methods have made it possible to identify more than 100,000 secondary metabolites of bacteria. Among them were identified structurally diverse compounds: antibiotics, alkaloids, isoprenoids, phenolic compounds, peptides, non-protein amino acids, volatile organic compounds, and many others.
There are a growing number of databases created containing information on subsets of these compounds. For example, a database of microbial volatiles “mVOC” is now available online (http://bioinformatics.charite.de/mvoc) (Lemfack et al. 2018). The Antimicrobial Peptide Database (https://aps.unmc.edu) has about 3300 different peptides. The CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria, is available now (Jones et al., 2021). Many cyanobacterial peptides, such as microcystins and nodularin, are toxic to humans and animals and are called cyanotoxins (Merel et al. 2013; Svirčev et al. 2019). The European Multi Lake Survey dataset has been created, which contains data on environmental variables, phytoplankton pigments, and cyanotoxins (Mantzouki et al. 2018), and it was one of the first datasets containing cyanotoxins.
Other common and environmentally significant metabolites are non-protein amino acids (NPAAs). These molecules are not naturally encoded in the genetic code of living organisms. Many NPAAs found in nature are analogous to proteinogenic amino acids, and some exist as secondary metabolites in various organisms (Fichtner et al. 2017).
Currently, secondary metabolites are considered as means of communication of microorganisms in various ecosystems. The concept of microbial communication includes the complex adaptive behavior of bacteria. Microbial communication using chemical signals is a topic subject of research in microbiological ecology. This signaling, called quorum sensing (QS), leads to physiological or behavioral changes in cells and affects colonies and bacterial biofilm structures. Quorum sensing involves the secretion of “signaling” molecules (“info-chemicals”) by individual cells. When the local concentration of these molecules reaches a threshold value, cells react by turning on specific genes. Thus, individual cells can sense the local bacteria density, so that the population as a whole can perform a coordinated response (Czárán and Hoekstra, 2009).
These secondary metabolites have a pronounced allelopathic effect on competition between microorganisms and, therefore, are ecologically very important. Allelopathy is usually defined as any effect: direct or indirect, stimulating or inhibiting, mediated by a chemical compound released into the environment by a given plant or microorganism (Rice, 1984). Taking into account the role of photoautotrophic microorganisms in the biosphere, special attention is paid to metabolites produced by both phototrophic organisms themselves and metabolites of other organisms that affect photosynthesis. Typical allelochemicals for inhibiting microalgae cells are polyphenolics and their derivatives (Ren 2008; Huang et al. 2015); fatty acids/esters (Zhang et al. 2009); terpenoids and their derivatives (Wang et al. 2014); and N-containing compounds (alkaloids, anilines, amino acids, and their derivatives) (Zhu et al. 2021).
The importance of secondary metabolites for the survival of plant and algae species has been emphasized in many studies over the past decades (Wink, 1988; Heisey, 1990; Makkar et al., 2007; Blum, 2011; Pang et al., 2021). Secondary metabolites of plants play many roles, including a protective role against pathogens, pests, and herbivores and a response to environmental stresses, and intermediary interactions between organisms (Pang et al., 2021). Allelopathy (Molisch, 1937) is a common biological phenomenon in which one organism produces biochemical molecules (which are known as allelochemicals) that affect the growth, survival, development, and reproduction of other organisms in positive or negative way. These allelochemicals have beneficial or harmful effects on target organisms (Cheng and Cheng, 2015). During biochemical interaction between plants, in which the donor plant secretes a secondary metabolite, allelochemical substances, that are harmful to the growth of its neighbors (Schandry and Becker, 2020). Thus, aquatic angiosperms to counteract the strong competition for light and carbon with other primary producers, especially phytoplankton and epiphytes (Leu et al., 2002). Some allelochemicals from plants have been used as pesticides (Heisey, 1990; Leu et al., 2002). Secondary metabolites remarkably affect the photosynthesis in microalgae cells in several ways: including a decrease in the content of photosynthetic pigments (Liu et al. 2007), reducing the rate of oxygen release (Shao et al. 2009), a change in the kinetics of energy during absorption, transfer, dissipation, and distribution of energy (Zhu et al. 2010; Voronova et al. 2019), a disturbance of the electron flow in the photosynthesis system (PS) (Leu et al., 2002; Jiang et al. 2014; Voronova et al. 2019).
The main goal of this review is to highlight recent experimental results demonstrating the effect on photosynthesis in microalgae cells of several types of common bacterial metabolites, such as volatile organic compounds, non-protein amino acids, and peptides.
Volatile organic compounds and their biological effect on microalgae photosynthesis
Volatile organic compounds (VOCs) are the most common and ecologically significant groups of metabolites. Plants, fungi, and bacteria produce a large diversity of VOCs with various biological functions. These organic compounds participate in bacteria-to-bacteria, bacteria-to-plant, fungi-to-plant, and plant-to-plant communications as signaling molecules that affect the physiology and development of target organisms (Ryu et al. 2020; Xie et al. 2021; Alfiky and Weisskopf 2021; Plyuta et al 2021; Sidorova et al 2022). The database on volatile microbial compounds “mVOC” is constantly updated with new data (Lemfack et al. 2018).
Allelopathic effects of cyanobacterial VOCs on microalgae
The synthesis and release of VOCs by cyanobacteria are related to the cell energy status, which, in turn, depends on the photosynthesis and pigment synthesis that are regulated by different environment factors (Watson 2003; Zuo et al. 2018). Among these factors are the intensity of light, temperature, nutrients (availability of phosphorus, iron, and nitrogen), the level of salinity, the aerated (mixing/turbulence) or static culture, the presence of a phage infection, the cyanobacterial population density, the culture’s age (aging or apoptosis), and the presence or absence of herbivores (Koksharova, 2020).
In water ecosystems, volatile organic molecules play essential allelopathic roles in algae competition (Koksharova, 2020; Xie et al. 2021). The type of produced VOCs depends on the cyanobacterial or algal species (Lee et al. 2017). Some volatile molecules are produced by cyanobacteria and by green algae. Among them, 2,4,7-decatrienal, 6-methyl-5-hepten-2-one, and dimethyl sulfide (DMS) are detected. Noteworthy, the spectrum of synthesized VOCs by cyanobacteria differs from volatile organic compounds released by eukaryotic algae. Cyanobacteria produce 2-MIB, geosmin, β-cyclocitral, β-ionone, monoterpene alcohols, aliphatic alcohols, aliphatic fatty acids, aldehydes, and many others that act as allelopathic tools against phytoplankton. Unlike eukaryotic algae, only cyanobacteria can produce 2-methyl-isoborneol (MIB), geosmin, geraniol, isopropyl disulfide, dimethyl disulfide (DMDS), dimethyl trisulfide (DMTS), nerol, ketone, and ionone derivatives. Given that eukaryotic algae and cyanobacteria inhabit the same niches, these differences in synthesized VOCs may bring competitive superiority to both of them in the microalgae community.
Terpenoids represent the largest group of specialized (secondary) metabolites of plants (Yazaki et al. 2017). These naturally occurring chemical compounds are very diverse in chemical structure. Terpenoids are biosynthetically derived from the mevalonic acid and isopentenyl pyrophosphate pathways (Latif et al. 2017). Microcystis cells produce β-cyclocitral and eucalyptol that help these cyanobacteria to dominate over green algae in water ecosystems (Sun et al. 2020) and inhibit Chlorophyta, for example, Chlamydomonas reinhardtii. Another example is the cyanobacterium M. flos-aquae which produce eucalyptol and limonene that inhibit cell division of eukaryotic green algae Chlorella vulgaris. It was found that in the nitrogen-free medium, the concentrations of Chl a and Chl b were remarkably reduced in the presence of limonene and eucalyptol (Xu et al. 2017) (Table 1). These terpenoids reduce the PSII reaction center concentration and inhibit electron transport in PSII in green algae Chlorella vulgaris (Zhao et al. 2016). Limonene and eucalyptol facilitate dissipation of the absorbed light energy as heat. These VOCs cause degradation of photosynthetic pigments and disturb photosynthesis in eukaryotic algae cells; therefore, they are remarkably effective allelopathic compounds of cyanobacteria.
Table 1.
Effects of bacterial metabolites on photosynthesis in microalgae cells
| Reference | Target | Secondary metabolites | Source of metabolites | Effects and growth conditions |
|---|---|---|---|---|
| VOCs | ||||
| Xu et al (2017) | Chlorella vulgaris | Limonene and eucalyptol | M. flos-aquae | VOCs inhibit cell division. Chl a and Chl b amount was reduced remarkably in the nitrogen-free medium |
| Zuo et al (2018) | Chlamydomonas reinhardtii | Limonene and eucalyptol | M. flos-aquae | Inhibition of photosynthesis was detected in the absence of phosphorus in the growth medium |
| Zhao et al (2016) | Chlorella vulgaris | Eucalyptol and limonene | Pure chemicals | These VOCs reduce the concentration of PS2 reaction centers and inhibit the quantum production and electron transport in PS2. The VOCs promote the dissipation of the absorbed light energy as heat. These terpenoids induce the degradation of photosynthetic pigments and reduce photosynthetic abilities in algae |
| Voronova et al. (2019) | Synechococcus sp. PCC 7942 |
Ketones 2-Nonanone and 2-undecanone |
Pure chemicals | Ketones strongly inhibit electron transport through PS2 in cyanobacterial cells in vivo. The ketones decrease the quantum yield of primary PS2 photo-reactions and alter the PS2 chlorophyll fluorescence induction curves. VOCs inhibit electron transfer from QA to QB, and electron transport at the donor side of PS2 |
| Chen et al., (2019) | Chlamydomonas reinhardtii | Linalool and α-terpineol | Pure chemicals | The photosynthetic pigments gradually degrade, and Fv/Fm value gradually declines towards zero |
| Sun et al., (2020) | Chlamydomonas reinhardtii | Eucalyptol and β-cyclocitral | Pure chemicals | The photosynthetic pigments gradually degrade, and Fv/Fm value gradually declines |
| Liu et al., (2021) | Chlamydomonas reinhardtii | β-Ionone (0.2 mM), limonene (0.2 mM), and longifolene (0.4 mM) | Pure chemicals | Photosynthetic pigments in C. reinhardtii cells gradually degrade, and Fv/Fm value gradually decreases and disappears at 24 h |
| NPAAs, peptides, MCs | ||||
| Downing et al., (2015) | Synechocystis PCC 6803 |
BMAA MCs |
Pure chemical | The toxins resulted in a decrease in the electron flux through PS2. BMAA inhibits electron transfer within the plastoquinone pool. A model is proposed for the assumed physiological roles of MC and BMAA, being the prevention of damage related to high-intensity light, whether in the absence or presence of nitrogen |
| Koksharova et al., (2020a) | Nostoc sp. PCC 7120 | BMAA | Pure chemical | Under nitrogen starvation, exogenous BMAA strongly downregulates the main proteins of PSI, some proteins of the pigment complexes, and plastocyanin (petE), as well as three enzymes of chlorophyll metabolism. However, one protein of PS2 (13 kDa protein, psbW) and protein petC (cytochrome b6-f complex iron–sulfur subunit) are upregulated |
| Koksharova et al., (2020b) | Nostoc sp. PCC 7120 | BMAA | Pure chemical | In cells grown in nitrogen-replete media, five proteins involved in photosynthesis were downregulated and two proteins (cpcB and cpcG4) were slightly upregulated in the presence of BMAA. Downregulated proteins are cytochrome c-550 (psbV, all0259), subunit IV of PS1 (psaE, asr4319), PS2 protein CP47 (psbB, all0138), phycobilisome core component apcF (all2327), and the beta subunit of ATP synthase F0F1 (all5039) |
| Koksharova et al., (2021) | Nostoc sp. PCC 7120 | BMAA | Pure chemical | During diazotrophic cell growth, BMAA reduces the expression of 18 proteins related to photosynthesis (proteins of PS1 and PS2, cytochrome b6f complex, antenna pigment complexes) |
| Banin et al., (2001) | Coral symbiotic algae (zooxanthellae) | Toxin P is a linear, proline-rich dodecapeptide | Coral pathogen bacterium Vibrio shiloi | Peptide inhibits the photosynthesis of coral symbiotic algae (zooxanthellae) |
| Omidi et al., (2017) (review) | MCs | Microcystis spp. | (1) Adaptation to light intensities in cyanobacteria. (2) Direct relationship between MCs content and chlorophyll a content. (3) Localization of MCs on the thylakoids’ membrane. (4) Protection against oxidative stress in cyanobacterial cells. (5) Lower photosynthesis efficiency and chlorophyll content of Chlamydomonas microsphaera | |
Recently, a volatile short-chain apocarotenoid β-cyclocitral has been discovered as a new biologically active compound in various organisms. This VOC is formed during the process of enzymatic or non-enzymatic oxidation of the carotenoid β-carotene (Havaux 2020). The effect of different concentrations of pure chemical β-cyclocitral on the cells of cyanobacteria M. aeruginosa PCC 7005 and M. aeruginosa PCC 7820 and diatoms Nitzschia palea was studied. Diatoms are much more sensitive to β-cyclocitral (Chang et al 2011). Currently, the detection of endogenous β-cyclocytral in cyanobacterium Microcystis spp. cell extracts is being optimized and an analytical methodology is being developed (Yamashita et al 2020).
Thus, cyanobacteria in environments under conditions of nutrient restriction (in the absence of nitrogen or phosphorus) increase the production of VOCs, and those compounds might be used to compete for the scarce resources due to their allelopathic effect on neighbors. For example, when Chlorella vulgaris cells were exposed to the VOCs from cyanobacteria Microcystis flosaquae, the cell growth of these green algae was inhibited significantly in a nitrogen-free medium in terms of cell density, Chl a content, Chl b content, and Fv/Fm value. These allelochemical compounds may provide cyanobacteria with an advantage in the fight for resources in nutrient-poor environments (Xu et al., 2017). The mechanisms of action of VOCs on photosynthetic pigments currently are unknown. It is known that eucalyptol can inhibit the K + channels in snail neurons. In algae and higher plants, the blockage of K + channels leads to an imbalance of Na + /K + in cells, which leads to disruption of intracellular metabolism and affects cell growth (Xu et al., 2017). Thus, elevated levels of VOCs from cyanobacterial cells under nitrogen-limited conditions were used to poison other algae in order to preserve the advantages of cyanobacteria under conditions of competition for resources, to promote the formation of a dominant species, and to promote blooming.
Bacterial VOCs inhibit cyanobacterial photosynthesis
In addition to algae and cyanobacteria, many other bacteria do synthesize VOCs. VOCs, including ketones produced by widespread bacteria strains of Pseudomonas and Serratia, inhibit the development of various microorganisms, including single-celled model cyanobacteria Synechocystis sp. PCC 6803, Synechococcus sp. PCC 7942, and filamentous cyanobacteria Anabaena sp. PCC 7120 (Popova et al 2014). Bacterial ketones can inhibit photosynthesis processes in cyanobacterial cells as was experimentally demonstrated in vivo (Voronova et al. 2019). The authors found that 2-nonanon and 2-undecanon inhibit electron transport via photosystem 2 (PS2) in Synechococcus sp. PCC 7942 cells. In this work, different methods of photosynthesis investigation have been applied: the high-resolution spectroscopy based on the study of the light-induced chlorophyll fluorescence kinetics, variable fluorescence relaxation kinetics, absorption spectroscopy in the visible spectral region, emission fluorescence spectroscopy, and photoinduced P700 turnover kinetics. The cell treatment by ketones reduces the quantum yield of primary PS2 photoreactions and changes the fluorescence induction curves of chlorophyll of PS2. It was demonstrated that ketones inhibit electron transfer from QA to QB, and electron transport on the donor side of PS2. These VOCs modify the process of energy transfer from the antenna complex to the PS2 reaction center and thereby increase both the quantum yield of chlorophyll fluorescence and the lifetime of the excited state of chlorophyll. The authors suggested that these ketones can act as allelopathic tools for regulating the number of microbial populations in their competition for limited nutrition in the soil ecosystem (Voronova et al. 2019).
There are many unresolved issues in the research field of VOCs and their biological significance for their producing organisms and target organisms, such as:
The spatial and temporal analysis of cell-bound and dissolved VOCs;
The molecular regulation of VOCs production and perception;
The mechanisms underlying the resistance of cyanobacteria themselves to high concentrations of VOCs;
The molecular regulatory signals that induce the synthesis of VOCs in cells under specific conditions.
The application of experimental tools of mutagenesis and gene cloning, biophysical methods, and “-omics” techniques will allow going deeper into the understanding of the mechanisms of VOC biological activity for various bacteria and algae.
Impact of non-proteinogenic amino acids on photosynthetic activity in cyanobacteria
It is known that non-proteinogenic amino acids (NPAAs) can also be biologically active molecules. They are synthesized by microorganisms and plants (Vranova et al. 2011). Non-proteinogenic (non-protein) amino acids are natural amino acids, their amides, amino acids, which, as a rule, are not part of proteins. About 400 non-protein amino acids are known. They are considered modified protein amino acids. NPAAs are obtained from protein amino acids as a result of elongation or reduction of the carbon chain (addition or removal of CH2- or CH-fragments); hydrogenation and dehydrogenation; hydroxylation; and amination. Non-proteinogenic amino acids participate in the formation of proteinogenic amino acids and serve as a spare form of nitrogen and sulfur. They are a transport form of nitrogen and can perform various protective functions (such as binding ammonia that accumulates during the breakdown of proteins), and they serve as an organic nitrogen reserve pool in ecosystems (Casagrande and Given 1980). NPAAs play an essential role in the adaptation of organisms to the environment. So, a number of NPAAs are phytosiderophores (Shenker et al. 2001). They can chelate Zn or Fe and increase their uptake by microorganisms. NPAs in microorganisms serve as building blocks for the synthesis of small bioactive peptides (Walsh et al. 2013; Nunn and Codd 2019).
For several NPAAs, for example, m-Tyrosine, γ-aminobutyric acid (GABA), and β-N-methylamine-L-alanine (BMAA), it was shown the presence of strong allelochemical impact on plants’ early growth and development (Brenner et al. 2000, 2009; Zer et al. 2020; Jander et al. 2020; Li et al. 2021; Balfagón et al. 2022). For example, many legume plants accumulate large amounts of canavanine (a structural analog of arginine) or other NPAAs, since they not only act as protective metabolites, but also serve to store nitrogen in seeds. Canavanine can be toxic to plants due to its indirect inhibition of nitric oxide biosynthesis, which leads to the formation of differently nitrated proteins and to disruption of the antioxidant system in tomato roots. Another NPAA, 1-aminocyclopropane carboxylate (ACC), functions as a plant signaling molecule. Physiological processes in plants that are directly influenced by ACC include stomatal development, cell wall biosynthesis, stress responses, and pathogen interactions (Jander et al. 2020). ACC is the direct precursor of ethylene, which is a gaseous hormone regulating a wide of developmental and stress-related processes in plants. Gamma-aminobutyric acid (GABA) acts as a signal in the transformation of plant genes mediated by Agrobacterium tumefaciens and in plant development, especially in the elongation of pollen tubes, root growth, fruit ripening, and seed germination (Li et al. 2021). GABA plays a key role in the acclimation of plants to a combination of two abiotic stresses: high light and heat stress, potentially by promoting autophagy (Balfagón et al. 2022).
The non-proteinogenic amino acid BMAA is synthesized by microalgae (cyanobacteria and diatoms) and accumulates in food chains, which may lead to the development of neurodegenerative diseases in humans (Lobner et al. 2007; Popova and Koksharova 2016; Nunn 2017). However, the functional significance of this amino acid in the metabolism of microalgae themselves remains not fully understood.
BMAA (β-N-methylamine-L-alanine) negatively affects the development of seedlings in Arabidopsis plants in the light conditions. One of the possible mechanisms underlying BMAA’s harmful effect on higher organisms could pertain to its competitive binding to various glutamate receptors (Brenner et al. 2000, 2009). It was shown that BMAA inhibits the growth of unicellular and filamentous cyanobacteria (Downing et al. 2015; Downing and Downing 2016; Berntzon et al. 2013). The addition of micromolar amounts of exogenous BMAA to cyanobacterial cells switches the expression of genes responsible for nitrogen fixation in filamentous diazotrophic cyanobacteria Anabaena/Nostoc sp. PCC 7120 under conditions of excess and absence of nitrogen in the growth medium (Popova et al. 2018a,b). BMAA addition affects the number of photosynthetic pigments and the expression of many different photosynthetic electron transport chain components in cyanobacterial cells (Koksharova et al., 2020a, 2020b, 2021) (Table 1).
Furthermore, it was shown, by using a proteomics approach, that an exogenous BMAA in micromolar amounts changes the expression of many proteins involved in various metabolic processes in the filamentous nitrogen-fixing cyanobacteria Nostoc sp. PCC 7120 grown under three different physiological conditions (Koksharova et al., 2020a, 2020b, 2021). These conditions differed in relation to nitrogen concentration in the mineral growth medium BG11 (Rippka et al. 1979):
Diazotrophic conditions, when cyanobacterial cells are forced to fix atmospheric nitrogen;
Conditions of nitrogen excess in the growth medium;
Transitional conditions, when cells are transferred from a nitrogen-replete medium to a poor medium without nitrogen (nitrogen starvation conditions).
BMAA impact on photosynthesis in cyanobacterial cells grown under diazotrophic conditions
It was shown that in nitrogen-limited (diazotrophic) growth conditions, BMAA affected 19 proteins involved in photosynthesis and 6 proteins that participate in oxidative phosphorylation in Nostoc sp. PCC 7120 (Koksharova et al. 2021). In the presence of BMAA, dramatic reduction was observed in the expression of 18 proteins related to photosynthesis. These proteins are present in the composition of photosystem complexes 1 or 2 (PS1 and PS2), in the cytochrome b6f complex, and in the antenna pigment complexes (Fig. 1, Table 2). BMAA addition to cyanobacterial cells leads to the downregulation of two proteins of PS1 reaction center (subunits IV and XI). Besides that, BMAA downregulates four proteins of PS2 (D1, D2, CP47, psbO) and five proteins that are components of the phycobilisome light-harvesting antennae of PS2. Additionally, four enzymes involved in the porphyrin and chlorophyll metabolism were also downregulated. Among the downregulated proteins were identified ferredoxin-NADP( +) reductase (petH) and two subunits of the cytochrome b6-f complex (petB and petC). Also, reduced expression of the H subunit of the NAD(P)H-quinone oxidoreductase and five subunits of ATP-complex was observed (Koksharova et al. 2021). Only one protein, cytochrome c6 (alr4251), was found upregulated in BMAA treated cells. Cytochrome (Cyt) c6 transfers electrons between the Cytb6-f complex and PS1 in the thylakoid’s lumen of cyanobacteria and green algae (Reyes-Sosa et al. 2011). It was shown that this protein is the main respiratory and photosynthetic soluble electron donor in heterocysts of Anabaena sp. PCC 7120 (Torrado et al. 2019). The upregulation of Cyt c6 under BMAA treatment could be considered a kind of compensatory mechanism in the respiratory electron transport and photosynthetic electron transport, while many other photosynthetic proteins were being downregulated.
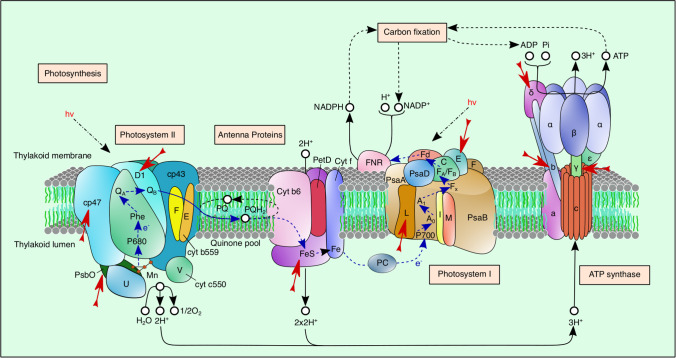
Fig. 1.
The diagram shows photosynthetic protein complexes and the BMAA’s influence on the protein components of PS1, PS2, cytochrome b6/f complex, and ATP synthase that was observed in cyanobacterial cells grown in diazotrophic conditions. Red arrows indicate downregulated proteins under BMAA treatment. The diagram has been adapted with modifications from Koksharova et al. (2021). The diagram was performed by using the Inkscape graphics program
Table 2.
BMAA impacts on proteins involved in photosynthesis in cells of cyanobacterium Nostoc sp. PCC 7120 grown in different growth conditions (according to experimental data obtained in Koksharova et al., 2020a, 2020b, 2021)
| Growth conditions | Antenna proteins, ferredoxins, and cytochromes | PS1 | PS2 | CO2-concentrating mechanism | CO2 fixation | Synthesis of chlorophyll | Photophosphorylation |
|---|---|---|---|---|---|---|---|
| Nitrogen-free environment (nitrogen fixation) |
cpcA cpcB apcD cpcG1 cpcG2 cpcG4 pecB petH petB petC cytA (cytC6)* |
psaE psaL |
psbA psbB psbD psbO |
CcmM CmpD |
HemC HemH Protochlorophyllide reductase Magnesium-protoporphyrin IX Monomethyl ester (oxidative) cyclase |
ATP synthase subunits A, B, δ, γ, β NAD(P)H-quinone oxidoreductase subunit H |
|
| Nitrogen replete medium |
cpcB cpcG4 apcF psbV (cytC-550) |
psaE | psbB |
CmpA CmpK CmpK |
RbcL Rca RbcS |
atpB | |
| Nitrogen starvation |
petC (cytB6/F) petE cpcA apcD |
psaA psaB psaF |
psbW | CcmM | RbcL | Delta-aminolevulinic acid dehydratase, glutamyl-tRNA synthetase, geranylgeranyl hydrogenase |
*Up-shifted proteins are bold. All other proteins are down-shifted at BMAA presence
BMAA impact on cyanobacterial photosynthesis under nitrogen starvation
Several proteins involved in photosynthesis were downregulated or upregulated in the presence of BMAA under nitrogen starvation condition when cells were transferred from a nitrogen-replete medium to a nitrogen-free growth medium (Table 2). Among the downregulated proteins were the main proteins of PS1, some proteins of pigment complexes, and plastocyanin (petE). PsaA (PS1 P700 chlorophyll a apoprotein A1) was downregulated almost threefold and PsaB (PS1 P700 chlorophyll a apoprotein A2) was downregulated almost fourfold. These two proteins are encoded by two genes that are coexpressed, alr5154 and alr5155 (http://alcodb.jp/cyano/PCC7120/alr5154/list). At the same time, one protein of PS2 (13 kDa protein, psbW) and petC protein (cytochrome b6-f complex iron–sulfur subunit) were upregulated (Koksharova et al. 2020a). In the case of Arabidopsis, it was shown that without the PsbW protein, the ordered rows of semicrystalline macrodomains of PSII-LHCII supercomplexes cannot be formed. This in turn leads to a decrease in the efficiency of energy transfer between PSII units, and to a delayed regulation response of PSII upon light stress (García-Cerdán et al. 2010).
BMAA also affects chlorophyll metabolism in cyanobacterial cells. Three enzymes, delta-aminolevulinic acid dehydratase (EC: 4.2.1.24), glutamyl-tRNA synthetase (EC: 6.1.1.17), and geranylgeranyl hydrogenase (EC: 1.3.1.111), are involved in chlorophyll metabolism. They are strongly downregulated by BMAA (Koksharova et al. 2020a) (Table 2). Exogenous BMAA downregulates cyanobacterial proteins that participate in nitrogen fixation, carbon assimilation, and photosynthesis, which leads to cyanobacterial cell starvation for nitrogen, carbon, and energy.
BMAA affects weakly photosynthetic proteins in nitrogen-replete cyanobacterial cells
In nitrogen-replete cells of Nostoc, photosynthetic proteins were only slightly affected by BMAA (Table 2) in comparison to a very strong BMAA impact on PS1 and PS2 proteins in nitrogen-starved cyanobacterial cells. In cells grown in nitrogen-replete media, five proteins involved in photosynthesis were downregulated and two proteins (cpcB and cpcG4) were slightly upregulated in the presence of BMAA (Table 2) (Koksharova et al. 2020b). Among the downregulated proteins were found cytochrome c-550 (psbV, all0259), subunit IV of PS1 (psaE, asr4319), PS2 protein CP47 (psbB, all0138), phycobilisome core component apcF (all2327), and the beta subunit of ATP synthase F0F1 (all5039). However, this physiological state of cyanobacterial cells cannot be defined as chlorosis compared with the strong chlorosis cells suffers under BMAA treatment during nitrogen starvation conditions (Koksharova et al. 2020a; 2021).
A regulatory effect of BMAA on carbon dioxide assimilation in cyanobacterial cells
Exogenous BMAA impacts the process of carbon dioxide assimilation and the carbon concentrating mechanism in cyanobacterial cells (Koksharova et al., 2020a, 2020b, 2021). In cyanobacteria, the key CO2 fixing enzyme is ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) (Tabita and Colletti, 1979). In cyanobacterial cells, effective carbon fixation with RuBisCO is based on the ability to concentrate inorganic carbon (Ci) near the active center of RuBisCO (Badger and Price, 2003). Signal molecule 2-OG is used by cyanobacteria as a reporter of the cellular C/N balance in such a way that at a low nitrogen content (in conditions of the high levels of 2-OG), the activity of CCM will be downshifted. This fact explains the decrease in the rate of CO2 fixation in conditions of nitrogen deficiency (Forchhammer and Lüddecke 2015). In the proteomics studies, it was demonstrated that exogenous BMAA addition disturbed regulation of CO2 fixation in nitrogen-replete Nostoc cells. Moreover, BMAA is differently influenced proteins that are involved in the process of CO2 fixation in nitrogen-starved cells (Koksharova et al. 2020a) and in nitrogen-replete cells of Nostoc (Koksharova et al. 2020b). It was discovered that two proteins, RbcL (alr1524) and CcmM (all0865), that are involved in CO2 fixation, were downregulated by exogenous BMAA in nitrogen-starved cells of Nostoc. However, in nitrogen-replete cells, RbcL (alr1524), CcmK (all0868), and a number of other proteins were upregulated by BMAA. Such a BMAA regulatory effect could be explained by the PII protein involvement in carbon fixation regulation (Forchhammer and Selim 2019). BMAA impacts the amount of regulatory PII protein in various growth conditions (Koksharova et al., 2020a, 2020b).
Thus, this non-proteinogenic amino acid not only affects the proteins involved in the light reactions of photosynthesis, but also leads to changes in the reactions of photosynthesis in the dark, including CO2 fixation and carbon metabolism. It has been hypothesized that BMAA can be used by phytoplankton organisms in various ecosystems as a possible allelopathic tool for controlling the population of cyanobacterial cells during a period of intense competition for nitrogen and other resources (Koksharova et al. 2020a; 2021).
The studies of the effect of non-protein amino acids on the photosynthesis of microalgae have just begun. The detailed molecular mechanisms causing these changes in the electron transfer chain of photosynthesis should be investigated in vivo and in vitro using biophysical and genetic methods. Taking into consideration the prevalence of non-protein amino acids in nature, it is necessary to continue research on their effect on microbial cells. The use of these molecules as herbicides can be of great importance both for practical applications and for fundamental research. The use of biophysical methods to study photosynthesis processes, along with methods of mutagenesis, transcriptomics, and proteomics, will help to understand the regulatory mechanisms of the allelopathic action of non-protein amino acids on the primary metabolic processes in microalgae cells.
Cyanobacterial peptides and photosynthesis
Cyanobacteria have important ecological roles in the carbon and nitrogen cycles, of which Spirulina, Anabaena, Nostoc, Oscillatoria, and Microcystis genera are particularly important because they produce many secondary metabolites with various chemical structures: peptides, alkaloids, and polysaccharides (Singh 2016). It is common for bacteria to weaponize peptides, which allows them to defend their place in ecosystems and regulate their relationships with symbionts or competitors. Up till now, there is a limited amount of research done, which is dedicated to the study of bacterial peptides action on the photosynthesis process. For example, the coral-bleaching bacteria Vibrio shiloi secretes an extracellular peptide, referred to as toxin P, which inhibits photosynthesis of coral symbiotic algae (zooxanthellae) (Banin et al. 2001). Right now, the majority of research on cyanobacterial peptides focuses on their great ecological significance and their toxicity towards animals and humans. It just turned out that they also affect photosynthesis.
Peptides account for more than 60% of the known biologically active compounds produced by cyanobacteria (Chlipala et al. 2011). Cyanobacterial peptides consist of cyclic and linear non-ribosomal peptides, which may also contain non-protein residues and post-translational modifications (Welker and Von Döhren 2006). The 1990s–2000s were marked by the appearance of ever-growing records of cyanopeptide studies not only for microcystins but also for nodularin, aeruginosins, anabaenopeptins, cyanopeptolins, microgynins, microviridins, and aerucyclamides (Janssen 2019; Zervou et al. 2021). One common feature observed for cyanopeptides is the inhibition of enzymes. Cyanopeptides inhibit various proteases in the nanomolar range (IC50). Frequently, protease inhibition was reported for cyanopeptolins, anabaenopeptins, aeruginosins, and microginins with the lower inhibitory activity threshold (IC50 values) reaching the nanomolar range, which is comparable to the concentrations observed during bloom events (Janssen 2019).
Microcystins (MCs) are cyclic heptapeptides produced by nonribosomal peptide synthetases (Dawson 1998). MCs are highly bioactive molecules. These cyclic peptides are involved in bloom formation due to their influence on growth, aggregation, photosynthesis, and biodiversity. Intracellular and extracellular functions of MCs are discussed in detail in the review (Omidi et al., 2017). These cyclic peptides increase the efficiency of photosynthesis in the cells producing them and, at the same time, inhibit photosynthesis in competitor algae cells (Table 1). A comparison between a wild type and a mutant, unable to synthesize MCs, allowed (Hesse et al., 2001) to find out that there is positive correlation between MC amount and chlorophyll concentration. In this work, the authors suggested that microcystins could play a role in light adaptation process. The use of the immunogold-labeling technique revealed the fact that most of the MCs are localized on the thylakoid membranes of the cell, and less in the nucleoplasm region. Physically, more than two-thirds of MCs were attached to thylakoid membranes (Shi et al., 1995; Young et al., 2005, 2008).
Cyanobacteria take advantage of most of the effects produced by microcystins (Sedmak and Eleršek 2006). MCs affect the target species by photosynthesis inhibition, growth inhibition, and oxidative stress induction (Gantar et al. 2008; Legrand et al. 2003; Omidi et al. 2017; Campos et al. 2021; Máthé et al. 2021; Zhang et al. 2022). Cyanobacteria apply MCs as allelopathic instruments to oust a diatomic algae population (Keating 1978). The allelopathic function of MCs has also been observed as growth inhibition of various algae species such as Chlamydomonas, Haematococcus, Navicula, and Cryptomonas, as well as cyanobacteria (Babica et al., 2006; Kaebernick and Neilan 2001; Leão et al. 2009; Singh et al. 2001). Moreover, the phytotoxicity of microcystins affects the morphology, photosynthetic efficiency, and antioxidant system of agricultural plants (Zhang et al. 2022).
The studies conducted with the joint cultivation of two cyanobacteria are fascinating and informative. These experiments allow researchers to advance in simulating natural conditions. For example, interspecific interactions of two cyanobacterial species producing MCs were studied: M. aeruginosa CPCC299 and Planktothrix agardhii NIVA-CYA 126 (Ngwa et al. 2014). It was shown that the presence of competing cyanobacteria negatively affected growth and the transcripts of Mcy gene for both species in mixed cultures when compared to monoculture.
The use of cyclic heptapeptides to inhibit growth and photosynthesis, together with the induction of oxidative stress in other aquatic species of the phytoplankton community, allows cyanobacteria to dominate over other community members. Cyanobacterial peptides play an important role in the regulation of toxin synthesis, in controlling the number of their own populations and populations of competing species, in the process of cyanobacteria to changing light conditions, and the availability of nutrients in the growth medium.
Conclusions and perspectives
Bacteria produce a large amount of the different secondary metabolites. Many of them are biosynthesized under different stress conditions. Some metabolites are produced during nutrient deficiency in the growth environment, and these compounds suppress competitors in the competition for the limited resources. These molecules can affect photosynthesis in microalgae cells. Metabolites help control the growth and density of algae populations, acting as “info-chemicals.”
This area of research is relatively young, and we are only taking the first steps. There are still many fascinating questions that require answering. Researchers strive to understand:
The regulation of metabolite production and perception;
The processes underlying the resistance of the bacteria themselves to the high concentrations of secreted metabolites;
The molecular signals that induce the metabolite synthesis in cells under certain conditions;
The molecular mechanisms which trigger and regulate their extracellular release;
The detailed molecular mechanisms of metabolite impacts on different photosynthetic proteins;
The regulation of interactions between organisms in symbioses, and many other questions. The use of various experimental biophysical tools, as well as methods of genomics and bioinformatics, mutagenesis and gene cloning, transcriptomics, proteomics, and metabolomics will provide a deep understanding of the mechanisms of regulation of photosynthesis by secondary metabolites in microalgae cells.
Acknowledgements
The authors were inspired to write this review by the work of the VIII Congress of the Russian Photobiological Society, All-Russian Conference “Modern problems of Photobiology.” The authors are grateful to the unknown reviewers for valuable comments that contributed to the improvement of the text of the manuscript.
Author contribution
Koksharova Olga had the idea for the article, performed the literature search and data analysis, and drafted and critically revised the work. Safronov Nikolai critically revised the work and made an illustration. All authors read and approved the final manuscript.
Funding
The study was supported by state program no. AAAA-A17-117120570011–4 (“Investigation of the mechanism of energy conversion by photosynthesis enzymes”).
Declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alfiky A, Weisskopf L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J Fungi. 2021;7(1):61. doi: 10.3390/jof7010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiga M (2021) Bacterial communication. Biol Philos 36(4). 10.1007/s10539-021-09814-1
- Babica P, Bláha L, Maršálek B. Exploring the natural role of microcystins – a review of effects on photoautotrophic organisms. J Phycol. 2006;42:9–20. doi: 10.1111/j.1529-8817.2006.00176.x. [DOI] [Google Scholar]
- Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54:609–622. 10.1093/jxb/erg076 [DOI] [PubMed]
- Balfagón D, Gómez-Cadenas A, Rambla JL, Granell A, de Ollas C, Bassham DC, Mittler R, Zandalinas SI. γ-Aminobutyric acid plays a key role in plant acclimation to a combination of high light and heat stress. Plant Physiol. 2022 doi: 10.1093/plphys/kiac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Khare SK, Naider F, Rosenberg E. Proline-rich peptide from the coral pathogen Vibrio shiloi that inhibits photosynthesis of zooxanthellae. Appl Environ Microbiol. 2001;67:1536–1541. doi: 10.1128/AEM.67.4.1536-1541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntzon L, Erasmie S, Celepli N, Eriksson J, Rasmussen U, Bergman B. BMAA inhibits nitrogen fixation in the cyanobacterium Nostoc sp. PCC 7120. Mar Drugs. 2013;11:3091–3108. doi: 10.3390/md11083091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum U (2011) Plant–plant allelopathic interactions. Plant-Plant Allelopathic Interact 1–7. 10.1007/978-94-007-0683-5_1
- Brenner ED, Martinez-Barboza N, Clark AP, Liang QS, Stevenson DW, Coruzzi GM. Arabidopsis mutants resistant to S(+)-beta-methyl-alpha, beta-diaminopropionic acid, a cycad-derived glutamate receptor agonist. Plant Physiol. 2000;124:1615–1624. doi: 10.1104/pp.124.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner ED, Feinberg P, Runko S, et al. A mutation in the Proteosomal Regulatory Particle AAA-ATPase-3 in Arabidopsis impairs the light-specific hypocotyl elongation response elicited by a glutamate receptor agonist, BMAA. Plant Mol Biol. 2009;70:523–533. doi: 10.1007/s11103-009-9489-7. [DOI] [PubMed] [Google Scholar]
- Campos A, Redouane EM, Freitas M, Amaral S, Azevedo T, Loss L, Máthé C, Mohamed ZA, Oudra B, Vasconcelos V. Impacts of microcystins on morphological and physiological parameters of agricultural plants: a review. Plants (Basel) 2021;10:639. doi: 10.3390/plants10040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande DJ, Given PH. Geochemistry of amino acids in some Florida peat accumulation-II. Amino acid distributions. Geochim Cosmochim Acta. 1980;44:1493–1507. doi: 10.1016/0016-7037(80)90114-3. [DOI] [Google Scholar]
- Chang DW, Hsieh ML, Chen YM, Lin TF, Chang JS. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to β-cyclocitral. J Hazard Mater. 2011;185:1214–1220. doi: 10.1016/j.jhazmat.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Chen Y, Weng Y, Zhou M, Meng Y, Liu J, Yang L, Zuo Z. Linalool- and α-terpineol-induced programmed cell death in Chlamydomonas reinhardtii. Ecotoxicol Environ Saf. 2019;167:435–440. doi: 10.1016/j.ecoenv.2018.10.062. [DOI] [PubMed] [Google Scholar]
- Cheng F, Cheng Z (2015) Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci 6. 10.3389/fpls.2015.01020 [DOI] [PMC free article] [PubMed]
- Chlipala GE, Mo S, Orjala J. Chemodiversity in freshwater and terrestrial cyanobacteria - a source for drug discovery. Curr Drug Targets. 2011;12:1654–1673. doi: 10.2174/138945011798109455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czárán T, Hoekstra RF. Microbial communication, cooperation and cheating: quorum sensing drives the evolution of cooperation in bacteria. PLoS ONE. 2009;4(8):e6655. doi: 10.1371/journal.pone.0006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RM. The toxicology of microcystins. Toxicon. 1998;36:953–962. doi: 10.1016/S0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Downing TG, Phelan RR, Downing S. A potential physiological role for cyanotoxins in cyanobacteria of arid environments. J Arid Environ. 2015;112:147–151. doi: 10.1016/j.jaridenv.2014.02.005. [DOI] [Google Scholar]
- Downing S, Downing TG. The metabolism of the non proteinogenic amino acid β-N-methylamino-L-alanine (BMAA) in the cyanobacterium Synechocystis PCC 6803. Toxicon. 2016;115:41–48. doi: 10.1016/j.toxicon.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Fichtner M, Voigt K (1861) Schuster S (2017) The tip and hidden part of the iceberg: proteinogenic and non-proteinogenic aliphatic amino acids. Biochim Biophys Acta Gen Subj 1 Pt A:3258–3269. 10.1016/j.bbagen.2016.08.008 [DOI] [PubMed]
- Forchhammer K, Lüddecke J. Sensory properties of the P II signalling protein family. FEBS J. 2015;283:425–437. doi: 10.1111/febs.13584. [DOI] [PubMed] [Google Scholar]
- Forchhammer K, Selim KA. Carbon/nitrogen homeostasis control in cyanobacteria. FEMS Microbiol Rev. 2019;44:33–53. doi: 10.1093/femsre/fuz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantar M, Berry JP, Thomas S, et al. Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol Ecol. 2008;64:55–64. doi: 10.1111/j.1574-6941.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cerdán JG, Kovács L, Tóth T, et al. The PsbW protein stabilizes the supramolecular organization of II in higher plants. Plant J. 2010;65:368–381. doi: 10.1111/j.1365-313x.2010.04429.x. [DOI] [PubMed] [Google Scholar]
- Hartmann T. The lost origin of chemical ecology in the late 19th century. Proc Natl Acad Sci U S A. 2008;105:4541–4546. doi: 10.1073/pnas.0709231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M. β-Cyclocitral and derivatives: emerging molecular signals serving multiple biological functions. Plant Physiol Biochem. 2020;155:35–41. doi: 10.1016/j.plaphy.2020.07.032. [DOI] [PubMed] [Google Scholar]
- Heisey RM. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima) Am J Bot. 1990;77:662–670. doi: 10.2307/2444812. [DOI] [Google Scholar]
- Hesse K, Dittmann E, Bӧrner T. Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol Ecol. 2001;37:39–43. doi: 10.1016/s0168-6496(01)00142-8. [DOI] [Google Scholar]
- Huang H, Xiao X, Ghadouani A, Wu J, Nie Z, Peng C, Xu X, Shi J. Effects of natural flavonoids on photosynthetic activity and cell integrity in Microcystis aeruginosa. Toxins (Basel) 2015;7:66–80. doi: 10.3390/toxins7010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Kolukisaoglu U, Stahl M, Yoon GM. Editorial: Physiological aspects of non-proteinogenic amino acids in plants. Front Plant Sci. 2020;11:519464 . doi: 10.3389/fpls.2020.519464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM-L. Cyanobacterial peptides beyond microcystins – a review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019;151:488–499. doi: 10.1016/j.watres.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Peiyong G, Chang C, Gao L, Li S, Wan J. Effects of allelochemicals from Ficus microcarpa on Chlorella pyrenoidosa. Braz Arch Biol Technol. 2014;57:595–605. doi: 10.1590/S1982-88372014000100018. [DOI] [Google Scholar]
- Jones MR, Pinto E, Torres MA, Dörr F, Mazur-Marzec H, Szubert K, … Janssen EM-L (2021) CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res 196:117017. 10.1016/j.watres.2021.11701 [DOI] [PubMed]
- Kaebernick M, Neilan BA. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol Ecol. 2001;35:1–9. doi: 10.1111/j.1574-6941.2001.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Koksharova OA (2020) Cyanobacterial VOCs as allelopathic tools. In: Weisskopf L, Piechulla B, Ryu CM (eds) Bacterial volatile compounds as mediators of airborne interactions. Springer, Singapore, pp 257–280. 10.1007/978-981-15-7293-7_11
- Koksharova OA, Butenko IO, Pobeguts OV, Safronova NA, Govorun VM. The first proteomic study of Nostoc sp. PCC 7120 exposed to cyanotoxin BMAA under nitrogen starvation. Toxins. 2020;12:310. doi: 10.3390/toxins12050310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksharova OA, Butenko IO, Pobeguts OV, Safronova NA, Govorun VM. Proteomic insights into starvation of nitrogen-replete cells of Nostoc sp. PCC7120 under BMAA treatment. Toxins. 2020;12:372. doi: 10.3390/toxins12060372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksharova OA, Butenko IO, Pobeguts OV, Safronova NA, Govorun VM. β-N-Methylamino-L-Alanine (BMAA) Causes severe stress in Nostoc sp. PCC 7120 cells under diazotrophic conditions: a proteomic study. Toxins. 2021;13:325. doi: 10.3390/toxins13050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating KI. Blue-green algal inhibition of diatom growth: transition from mesotrophic to eutrophic community structure. Science. 1978;199:971–973. doi: 10.1126/science.199.4332.971. [DOI] [PubMed] [Google Scholar]
- Latif S, Chiapusio G, Weston LA. Allelopathy and the role of allelochemicals in plant defence. Adv Bot Res. 2017;82:19–54. doi: 10.1016/bs.abr.2016.12.001. [DOI] [Google Scholar]
- Legrand C, Rengefors K, Fistarol GO, et al. Allelopathy in phytoplankton – biochemical, ecological and evolutionary aspects. Phycologia. 2003;42:406–419. doi: 10.2216/i0031-8884-42-4-406.1. [DOI] [Google Scholar]
- Lee J, Rai PK, Jeon YJ, Kim K-H, Kwon EE. The role of algae and cyanobacteria in the production and release of odorants in water. Environ Pollut. 2017;227:252–262. doi: 10.1016/j.envpol.2017.04.058. [DOI] [PubMed] [Google Scholar]
- Lemfack MC, Gohlke BO, Toguem SMT, Preissner S, Piechulla B, Preissner R. mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res. 2018;46(D1):D1261–D1265. doi: 10.1093/nar/gkx1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão PN, Vasconcelos MT, Vasconcelos VM. Allelopathy in freshwater cyanobacteria. Crit Rev Microbiol. 2009;35:271–282. doi: 10.3109/10408410902823705. [DOI] [PubMed] [Google Scholar]
- Leu E, Krieger-Liszkay A, Goussias C, Gross EM. Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II. Plant Physiol. 2002;130(4):2011–2018. doi: 10.1104/pp.011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dou N, Zhang H, Wu C. The versatile GABA in plants. Plant Signal Behav. 2021;16(3):1862565. doi: 10.1080/15592324.2020.1862565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhou P, Tian J, Jiang S. Effect of pyrogallol on the growth and pigment content of cyanobacteria-blooming toxic and nontoxic Microcystis aeruginosa. Bull Environ Contam Toxicol. 2007;78:499–502. doi: 10.1007/s00128-007-9096-8. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu Q, Ye B, Zhu K, Yin J, Zheng T, Xu S, Sun Q, Li Y, Zuo Z. Programmed cell death of Chlamydomonas reinhardtii induced by three cyanobacterial volatiles β-ionone, limonene and longifolene. Sci Total Environ. 2021;762:144539 . doi: 10.1016/j.scitotenv.2020.144539. [DOI] [PubMed] [Google Scholar]
- Lobner D, Piana PMT, Salous AK, Peoples RW. β-N-Methylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar HPS, Siddhuraju P, Becker K (2007) Plant secondary metabolites. Methods in Molecular Biology™. doi:10.1007/978-1-59745-425-4 [DOI] [PubMed]
- Mantzouki E, et al. A European Multi Lake Survey dataset of environmental variables, phytoplankton pigments and cyanotoxins. Sci Data. 2018;5:180226 . doi: 10.1038/sdata.2018.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Máthé C, M-Hamvas M, Vasas G, Garda T, Freytag C. Subcellular alterations induced by cyanotoxins in vascular plants-a review. Plants (Basel) 2021;10:984. doi: 10.3390/plants10050984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merel S, et al. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int. 2013;59:303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Molisch H. Der Einfluss einer Pflanze auf die andere: Allelopathie. Jena: Fischer; 1937. [Google Scholar]
- Ngwa FF, Madramootoo CA, Jabaji S. Comparison of cyanobacterial microcystin synthetase (mcy) E gene transcript levels, mcy E gene copies, and biomass as indicators of microcystin risk under laboratory and field conditions. Microbiol Open. 2014;3:411–425. doi: 10.1002/mbo3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn PB. 50 years of research on α-amino-β-methylaminopropionic acid (β-methylaminoalanine) Phytochemistry. 2017;144:271–281. doi: 10.1016/j.phytochem.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Nunn PB, Codd GA. Environmental distribution of the neurotoxin l-BMAA in Paenibacillus species. Toxicol Res. 2019 doi: 10.1039/c9tx00203k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi A, Esterhuizen-Londt M, Pflugmacher S. Still challenging: the ecological function of the cyanobacterial toxin microcystin – what we know so far. Toxin Rev. 2017;37:87–105. doi: 10.1080/15569543.2017.1326059. [DOI] [Google Scholar]
- Pang Z, Chen J, Wang T, et al. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci. 2021;12:621276 . doi: 10.3389/fpls.2021.62127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyuta VA, Chernikova AS, Sidorova DE, Kupriyanova EV, Koksharova OA, Chernin LS, Khmel IA. Modulation of Arabidopsis thaliana growth by volatile substances emitted by Pseudomonas and Serratia strains. World J Microbiol Biotechnol. 2021;37:82. doi: 10.1007/s11274-021-03047-w. [DOI] [PubMed] [Google Scholar]
- Popova AA, Koksharova OA, Lipasova VA, Zaitseva JV, Katkova-Zhukotskaya OA, Eremina SI, … Khmel IA (2014) Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed Res Int 2014:1–11. 10.1155/2014/125704 [DOI] [PMC free article] [PubMed]
- Popova AA, Koksharova OA. Neurotoxic non-proteinogenic amino acid β-N-methylamino-L-alanine and its role in biological systems. Biochemistry. 2016;81:794–805. doi: 10.1134/S0006297916080022. [DOI] [PubMed] [Google Scholar]
- Popova A, Rasmussen U, Semashko T, Govorun V, Koksharova O. Stress effects of cyanotoxin β-methylamino-L-alanine (BMAA) on cyanobacterial heterocyst formation and functionality. Environ Microbiol Rep. 2018;10:369–377. doi: 10.1111/1758-2229.12647. [DOI] [PubMed] [Google Scholar]
- Popova A, Semashko T, Kostina N, Rasmussen U, Govorun V, Koksharova O (2018b) The cyanotoxin BMAA induces heterocyst specific gene expression in Anabaena sp. PCC 7120 under repressive conditions. Toxins 10. doi:10.3390/toxins10110478. [DOI] [PMC free article] [PubMed]
- Ren S (2008) Allelopathy in sustainable agriculture and forestry. Springer Science (Business Media), New York. 10.1007/978-0-387-77337-7
- Reyes-Sosa FM, Gil-Martínez J, Molina-Heredia FP (2011) Cytochrome c 6-like protein as a putative donor of electrons to photosystem I in the cyanobacterium Nostoc sp. PCC 7119. Photosynth Res 110:61–72. 10.1007/s11120-011-9694-5 [DOI] [PubMed]
- Rice EL (1984) Allelopathy, 2nd edn. Academic Press, New York, p 422. 10.1016/S0031-9422(00)80730-X
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. 10.1099/00221287-111-1-1
- Ryu C-M, Weisskopf L, Piechulla B (Eds) (2020) Bacterial volatile compounds as mediators of airborne interactions. Springer Nature Singapore Singapore, 320, 10.1007/978-981-15-7293-7
- Schandry N, Becker C. Allelopathic plants: models for studying plant–interkingdom interactions. Trends Plant Sci. 2020;25:176–185. doi: 10.1016/j.tplants.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Sedmak B, Eleršek T. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb Ecol. 2006;51:508–515. doi: 10.1007/s00248-006-9045-9. [DOI] [PubMed] [Google Scholar]
- Shao J, Wu X, Li R. Physiological responses of Microcystis aeruginosa PCC7806 to nonanoic acid stress. Environ Toxicol. 2009;24:610–617. doi: 10.1002/tox.20462. [DOI] [PubMed] [Google Scholar]
- Shenker M, Fan TWM, Crowley DE. Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants. J Environ Qual. 2001;30:2091–2098. doi: 10.2134/jeq2001.2091. [DOI] [PubMed] [Google Scholar]
- Shi L, Carmichael WW, Miller I. Immuno-gold localization of hepatotoxins in cyanobacterial cells. Arch Microbiol. 1995;163:7–15. doi: 10.1007/BF00262197. [DOI] [PubMed] [Google Scholar]
- Sidorova DE, Plyuta VA, Padiy DA, Kupriyanova EV, Roshina NV, Koksharova OA, Khmel IA. The effect of volatile organic compounds on different organisms: agrobacteria, plants and insects. Microorganisms. 2022;10:69. doi: 10.3390/microorganisms10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Tyagi MB, Arvind Kumar JK, et al. Antialgal activity of a hepatotoxin-producing cyanobacterium Microcystis aeruginosa. World J Microbiol Biotechnol. 2001;17:15–22. doi: 10.1023/A:1016622414140. [DOI] [Google Scholar]
- Singh JS (2016) Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. 10.3389/fmicb.2016.00529 [DOI] [PMC free article] [PubMed]
- Sun Q, Zhou M, Zuo Z (2020) Toxic mechanism of eucalyptol and β-cyclocitral on Chlamydomonas reinhardtii by inducing programmed cell death. J Hazard Mater 389:121910. 10.1016/j.jhazmat.2019.121910 [DOI] [PubMed]
- Svirčev Z, Lalić D, Bojadžija Savić G, Tokodi N, et al. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch Toxicol. 2019 doi: 10.1007/s00204-019-02524-4. [DOI] [PubMed] [Google Scholar]
- Tabita FR, Colletti C. Carbon dioxide assimilation in cyanobacteria: regulation of ribulose, 1,5-bisphosphate carboxylase. J Bacteriol. 1979;140:452–458. doi: 10.1128/jb.140.2.452-458.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrado A, Ramírez-Moncayo C, Navarro JA, Mariscal V, Molina-Heredia FP. Cytochrome c6 is the main respiratory and photosynthetic soluble electron donor in heterocysts of the cyanobacterium Anabaena sp. PCC 7120. Biochim Biophys Acta BBA Bioenergy. 2019;1860:60–68. doi: 10.1016/j.bbabio.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Voronova EN, Konyukhov IV, Koksharova OA, Popova AA, Pogosyan SI, Khmel IA, Rubin AB. Inhibition of cyanobacterial photosynthetic activity by natural ketones. J Phycol. 2019 doi: 10.1111/jpy.12861. [DOI] [PubMed] [Google Scholar]
- Vranova V, Rejsek K, Skene KR, Formanek P. Non-protein amino acids: plant, soil and ecosystem interactions. Plant Soil. 2011;342:31–48. doi: 10.1007/s11104-010-0673-y. [DOI] [Google Scholar]
- Walsh CT, O’Brien RV, Khosla C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew Chem Int Ed. 2013;52:7098–7124. doi: 10.1002/anie.201208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhu H, Zhang L, Xue W, Yuan B. Identification of antialgal compounds from the aquatic plant Elodea nuttallii. Allelopath J. 2014;34:207–213. [Google Scholar]
- Watson SB. Cyanobacterial and eukaryotic algal odor compounds: signals or by-products? A review of their biological activity. Phycologia. 2003;42:332–350. doi: 10.2216/i0031-8884-42-4-332.1. [DOI] [Google Scholar]
- Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theoret Appl Genetics. 1988;75:225–233. doi: 10.1007/BF00303957. [DOI] [Google Scholar]
- Welker M, Von Döhren H. Cyanobacterial peptides-nature’s own combinatorial biosynthesis. FEMS Microbiol Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- Xie Y, Tian L, Han X, Yang Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae. 2021;7:278. doi: 10.3390/horticulturae7090278. [DOI] [Google Scholar]
- Xu Q, Yang L, Yang W, Bai Y, Hou P, Zhao J, et al. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol Environ Saf. 2017;135:191–200. doi: 10.1016/j.ecoenv.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Yamashita R, Bober B, Kanei K, Arii S, Tsuji K, Harada KI. Analytical technique optimization on the detection of β-cyclocitral in Microcystis species. Molecules. 2020;25:832. doi: 10.3390/molecules25040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K, Arimura G, Ohnishi T. ‘Hidden’ terpenoids in plants: their biosynthesis, localization and ecological roles. Plant Cell Physiol. 2017;58:1615–1621. doi: 10.1093/pcp/pcx123. [DOI] [PubMed] [Google Scholar]
- Young FM, Thomson C, Metcalf JS, et al. Immunogold localization of microcystins in cryosectioned cells of Microcystis. J Struct Biol. 2005;151:208–214. doi: 10.1016/j.jsb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Young FM, Morrison LF, James J, et al. Quantification and localization of microcystins in colonies of a laboratory strain of Microcystis (Cyanobacteria) using immunological methods. Eur J Phycol. 2008;43:217–225. doi: 10.1080/09670260701880460. [DOI] [Google Scholar]
- Zer H, Mizrahi H, Malchenko N, Avin-Wittenberg T, Klipcan L, Ostersetzer-Biran O. The phytotoxicity of meta-tyrosine is associated with altered phenylalanine metabolism and misincorporation of this non-proteinogenic Phe-analog to the plant’s proteome. Front Plant Sci. 2020;11:140. doi: 10.3389/fpls.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervou S-K, Moschandreou K, Paraskevopoulou A, et al. Cyanobacterial toxins and peptides in Lake Vegoritis. Greece Toxins. 2021;13(6):394. doi: 10.3390/toxins13060394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zheng C, He M, Wu A, Nie L. Inhibition on algae of fatty acids and the structure-effect relationship. China Environ Sci. 2009;29:274–279. [Google Scholar]
- Zhang Y, Vo Duy S, Munoz G, Sauvé S. Phytotoxic effects of microcystins, anatoxin-a and cylindrospermopsin to aquatic plants: a meta-analysis. Sci Total Environ. 2022;810:152104 . doi: 10.1016/j.scitotenv.2021.152104. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yang L, Zhou L, Bai Y, Wang B, Hou P, Xu Q, YangW ZZ. Inhibitory effects of eucalyptol and limonene on the photosynthetic abilities in Chlorella vulgaris (Chlorophyceae) Phycologia. 2016;55:696–702. doi: 10.2216/16-38.1. [DOI] [Google Scholar]
- Zhu J, Liu B, Wang J, Gao Y, Wu Z. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat Toxicol. 2010;98:196–203. doi: 10.1016/j.aquatox.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Zhu X, Dao G, Tao Y, Zhan X, Hu H. A review on control of harmful algal blooms by plant-derived allelochemicals. J Hazard Mater. 2021;401:123403 . doi: 10.1016/j.jhazmat.2020.123403. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Yang Y, Xu Q, Yang W, Zhao J, Zhou L. Effects of phosphorus sources on volatile organic compound emissions from Microcystis flos-aquae and their toxic effects on Chlamydomonas reinhardtii. Environ Geochem Health. 2018;40:1283. doi: 10.1007/s10653-017-0055-y. [DOI] [PubMed] [Google Scholar]