Abstract
Conformational changes play an important role in the functioning of proteins and their complexes. This is also true for the pigment-protein super-complex of photosystem II (PSII). The data testify about the pH-induced macromolecular conformational changes in the water-oxidizing complex (WOC) on the donor side of PSII, the interaction between the spatial structure of WOC proteins and the distribution of cytochrome b559 redox-forms, and the electron transfer efficiency between QA and QB on the acceptor side of PSII. Changes in the protein environment near QA and QB can be observed after the removal of the bicarbonate ion associated with non-heme Fe or after the addition of herbicides binding to the QB site, which results in the suppression of the electron transfer in this site. The “locking” of the de novo assembled PSII in an inactive state until WOC activation is also accompanied by strong structural perturbations on the PSII acceptor and donor sides with the participation of Psb28 and Psb27 proteins. The triggers for degradation and replacement of damaged PSII proteins are structural changes induced by their oxidative modification and aggregation. Macromolecular changes in the antenna proteins underlie the activation of photoprotective non-photochemical quenching, which are induced by protonation of the lumenal residues of PsbS or/and Lhcsr3, as well as the phosphorylation of antenna proteins. Besides this, many smaller-scale conformational changes may occur in PSII. This review summarizes current knowledge about the possible conformational changes in proteins in the PSII super-complex and describes their proposed influence on PSII function.
Keywords: Photosystem II, Conformational changes, Water-oxidizing complex, Acceptor side, Antenna complex
Introduction
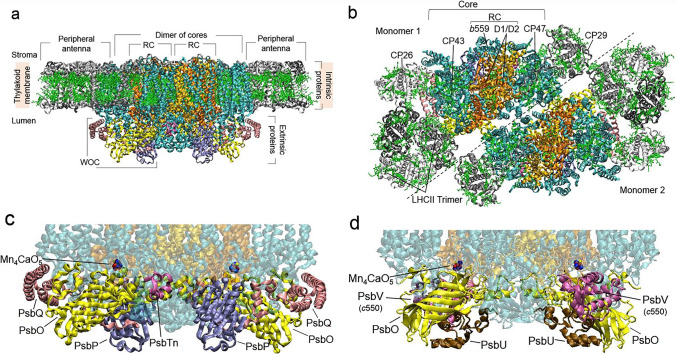
Photosystem II (PSII) is a large multi-subunit pigment-protein complex embedded in the thylakoid membrane as a dimer (Fig. 1a, b) and is present in all oxygenic photosynthesizing organisms from cyanobacteria to higher plants.
Fig. 1.
Overall structure of the spinach PSII super-complex from the thylakoid membrane (a) and stroma (b) sides. Protein composition and spatial structure of WOC in PSII of higher plants (c) and cyanobacteria (d). The figure was prepared based on structural data from the PDB (https://www.wwpdb.org) obtained by cryo-electron microscopy (a, b, c) for spinach PSII (PDB ID: 3JCU, https://doi.org/10.2210/pdb3jcu/pdb (Wei et al. 2016)) and by X-ray crystallography (d) for the cyanobacterial PSII core complex (Thermosynechococcus vulcanus) (PDB ID: 3WU2, https://doi.org/10.2210/pdb3wu2/pdb (Umena et al. 2011))
The core complex of the PSII monomer consists of the reaction center (RC), which binds the water-oxidizing complex (WOC) on the lumenal (donor) side, the core (inner) antenna, and several low-molecular-mass subunits (Fig. 1). The RC is formed by D1 (PsbA) and D2 (PsbD) proteins, which bind cofactors of the internal electron-transport chain, as well as the PsbE and PsbF proteins of cytochrome b559 (Cyt b559) that are tightly associated with the D1/D2 complex from the D2 protein side (Ferreira et al. 2004; Loll et al. 2005; Nelson and Yocum 2006; Wei et al. 2016). The main redox cofactors embedded into protein matrix of RC are the primary electron donor P680, which is formed by a couple of chlorophylls, PD1 and PD2; the accessory chlorophylls ChlD1 and ChlD2; the pheophytins PheoD1 and PheoD2; the peripheral chlorophylls PhlzD1 and PhlzD2; the primary and secondary quinone acceptors QA and QB; and non-heme Fe located between QA and QB and binding a molecule of bicarbonate (Fig. 2) (Ferreira et al. 2004; Loll et al. 2005; Nelson and Yocum 2006). The core antennae include two large chlorophyll-binding proteins CP43 (PsbC) and CP47 (PsbB) attaching to D1 and D2, respectively (Fig. 1a, b) (Ferreira et al. 2004; Loll et al. 2005; Umena et al. 2011; Wei et al. 2016). D1, D2, CP43, CP47, and Cyt b559 proteins (i.e., intrinsic subunits) of PSII have a high homology across all known photosynthesizing organisms (Wei et al. 2016).
Fig. 2.
Localization of cofactors within the protein matrix of the D1/D2 complex. The figure was prepared based on structural data from the PDB (https://www.wwpdb.org) obtained by X-ray crystallography for the cyanobacterial PSII core complex (T. vulcanus) (PDB ID: 3WU2, https://doi.org/10.2210/pdb3wu2/pdb (Umena et al. 2011))
WOC is composed mainly of three soluble proteins (extrinsic proteins) (Fig. 1c, d). Among these, only PsbO is present in all oxygenic organisms, and the two others are different. In cyanobacteria, red, diatom, and other eukaryotic algae, they are represented by the PsbV (Cyt c550) and PsbU proteins, which bind to the RC through interactions with the PsbO protein and with one another (Ferreira et al. 2004; Loll et al. 2005; Umena et al. 2011). In green algae and higher plants, these two proteins are replaced by two nonhomologous subunits, PsbP and PsbQ (Wei et al. 2016; Burton-Smith et al. 2019). The spatial structure of the PsbO protein is represented by the eight-stranded β-barrel (Ferreira et al. 2004; Wei et al. 2016; Burton-Smith et al. 2019; Muh and Zouni 2020), and in the crystal structure of cyanobacterial PSII, it was identified that PsbO stabilizes the conformation of the AB-loop and C-terminus of the D1 protein (Ferreira et al. 2004). In contrast, the proteins in pairs of PsbP and PsbV, and PsbQ and PsbU have significantly different spatial structures (Nelson and Yocum 2006) and are nonhomologous. Nevertheless, one of the main roles of PsbV and PsbP is the same: maintaining of the WOC affinity for Ca2+ and Cl− ions (Muh and Zouni 2020). In spinach PSII, the PsbP protein binds between a lumenal domain of CP43 and CP47 near the β7–β8 loop region of PsbO. At the same time, an Asp137–Glu140 loop of PsbP stabilizes the C-terminus of D1 and D2 proteins (Wei et al. 2016). The PsbQ protein is in connection with the N-terminus of PsbO, as well as interacting with CP43. The elongated N-terminus of PsbQ reaches a Lys90–Ala111 loop of PsbP (Fig. 1c) and thus provides the interconnection between the proteins (Wei et al. 2016).
Recent studies have testified that the proteins of the WOC in green algae (Chlamydomonas reinhardtii) are organized in similar manner as in higher plants (Burton-Smith et al. 2019). At the same time, the PSII from red algae and diatoms, besides the PsbO, PsbV, and PsbU subunits observed in the cyanobacterial WOC (Ferreira et al. 2004; Loll et al. 2005; Umena et al. 2011), contains also a PsbQʹ protein, which is placed on the opposite side of the major domain of PsbO (Ago et al. 2016; Nagao et al. 2019). In spite the fact that PsbQʹ and PsbQ have low homology, PsbQʹ is superimposed with spinach PsbQ and also has an interaction with a lumenal loop of CP43 (Ago et al. 2016).
The arrangement of extrinsic proteins in all organisms allows to suggest that they form a “cap” over the active center of the WOC composed of Mn, Ca, and O ions (i.e., the Mn4CaO5 cluster (Umena et al. 2011)), where the water oxidation reaction occurs. The Mn4CaO5 cluster is located on the D1 protein (Figs. 1c, d and 2) close to its lumenal CD-helix (Ferreira et al. 2004; Loll et al. 2005; Umena et al. 2011), and a barrier composed of WOC proteins may prevent its interactions with the reductants and chelating agents of the lumen bulk. Protons and molecular oxygen obtained through water oxidation are transferred out by channels formed by amino acid residues of the WOC subunits and water molecules with the participation of amino acid residues of D1 and CP43 proteins (Gabdulkhakov et al. 2009; Umena et al. 2011; Hussein et al. 2021). Simultaneously, electrons are transferred from the Mn4CaO5 cluster to photo-oxidized P680 through a redox-active Tyr161 of the D1 protein (Ferreira et al. 2004; Umena et al. 2011).
The core complex of PSII in higher plants and green algae is surrounded by a peripheral (outer) antenna or the light-harvesting complex II (LHCII). It includes monomeric and trimeric complexes of Lhcb(m) proteins (Fig. 1a, b) binding the main part of the chlorophyll and carotenoid pigments of the PSII super-complex. All of the Lhcb(m) proteins in higher plants and green algae have three transmembrane helices (A–C) and two amphipathic helices (D, E) on the lumen side parallel to the membrane (Shen et al. 2019). The proteins CP24 (absent in PSII from C. reinhardtii), CP26, and CP29 were observed to be monomeric and to connect with the core complex and LHCII trimers (Wei et al. 2016; Shen et al. 2019). In cyanobacteria and red algae, the outer antenna is represented by a large water-soluble protein complex of phycobilisome attached to the core complex on the stromal side. This includes phycobiliproteins, phycocyanin, phycoerythrin, allophycocyanin, and linker proteins (Zhang et al., 2017; Zheng et al. 2021). Little is known about the organization of the outer antenna in diatoms. It has been shown only that two homotetramers and three monomers of the fucoxanthin chlorophyll binding protein are attached to one core complex of PSII (Nagao et al., 2019).
According to the most recent data, the conformational changes in proteins play important roles in the PSII function at different levels of its organization. The present review summarizes the known and predicted results about structural modifications occurring in the proteins of the PSII super-complex during its functioning, repair, and degradation. The macromolecular perturbations and their influences are described for the core complex and the WOC in PSII from higher plants, green algae, and cyanobacteria due to the availability of a large amount of data from crystallography, cryo-electron microscopy, biophysics, biochemistry, and other high-precision approaches, while the changes in the molecule structure for the outer antenna are described mainly for PSII from higher plants and green algae.
The water-oxidizing complex
The WOC functioning is accompanied by rapid release of protons as a product of the water oxidation reaction. It was calculated, for example, that in PSII preparations from C. reinhardtii, the rate of proton removal from the WOC under light is near 75 H+/s per RC (Terentyev et al. 2019). This is obvious that the shift in the pH value is the main factor of the lumen, which can induce changes in the spatial structure of the WOC proteins. Moreover, the dependence of the O2-evolving activity of the WOC on the pH (Terentyev et al. 2019) indicates that these changes can affect even the Mn4CaO5 cluster functional state.
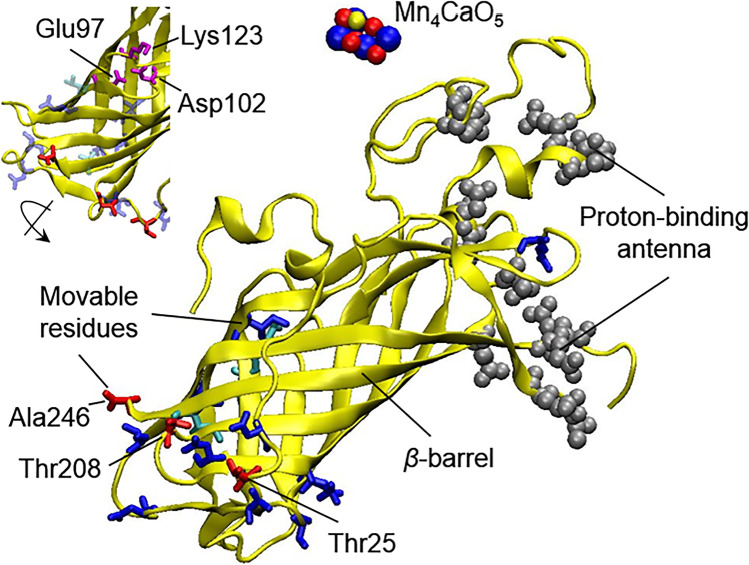
PsbO is a conserved subunit of PSII (Fig. 1c, d) and its capacity for specific conformational changes in response to a shift in pH was observed in acid–base titration experiments with isolated spinach protein (Shutova et al. 1997). It was shown that while the gross structure of the protein, as well as the state of an intrinsic S–S bridge remained unaffected, the hydrophobic core of PsbO that was hidden at a neutral pH became more accessible for the aqueous phase upon acidification, and the possibility of aromatic amino acids for rotation increased (Shutova et al. 1997; Carius et al. 2019). These changes possibly occur in the β-barrel of PsbO due to the protonation/deprotonation of amino acid residues (Carius et al. 2019), which, most likely, are represented by the carboxylic groups of glutamic and aspartic acids (Shutova et al. 1997, 2007). Under a lower pH, they may form a “proton-binding antenna” (Fig. 3), proposed by the authors for PsbO from different organisms, including C. reinhardtii, which may play a role in H+ removal to the lumen (Shutova et al. 2007; Carius et al. 2019). The combined results from crystallography and molecular dynamic simulation showed that upon deprotonation the distance between the highly conserved carboxylate dyad Glu97 and Asp102 (Fig. 3) increases, while in the protonation state they remain closer and Lys123 shows transient interactions with both Glu97 and Asp102 (Bommer et al. 2016). Thus, the protonation state may influence the local conformational structure of PsbO. In one of the latest works, the strong chemical shift changes were identified for several residues upon the pH titration, which were monitored by 2D 1H, 15N correlation spectroscopy (Gerland et al. 2020). Large changes were observed for residues toward the loops on the lumenal side of the β-barrel, with the largest effects found for Thr25, Thr208, and Ala246 (Fig. 3).
Fig. 3.
Structure of PsbO with indication of amino acid resides as gray spheres involved in the putative protein-binding antenna (Shutova et al. 2007; Carius et al. 2019), as well as resides movable upon the pH shift (Gerland et al. 2020), which are colored in cyan, blue, and red colors depending on the weak, middle, and strong effect, respectively. The insert shows a rotated PsbO molecule and residues involved in local changes upon pH shift according to Bommer et al. (2016). The figure was prepared with using of structural data from the PDB for cyanobacterial PSII (T. vulcanus) (PDB ID: 3WU2, https://doi.org/10.2210/pdb3wu2/pdb (Umena et al. 2011))
The PsbO subunit does not have a direct connection to the Mn4CaO5 cluster, and the smallest distance between them is near 17 Å. Nevertheless, a strong structural coupling between PsbO and the proteins surrounding the Mn4CaO5 cluster has been concluded for cyanobacterial PSII based on the data of the light-induced Fourier transform infrared (FTIR) spectroscopy. The method allows the detection of changes in the secondary structures of protein main chains, the protonation/deprotonation reactions of amino acid chains, and structural changes of hydrogen bounded water molecules (Debus 2015). Light-induced S2-minus-S1 FTIR difference spectra obtained for cyanobacterial PSII core complexes, which had significant changes upon removal of all WOC proteins, were restored mainly by the binding of PsbO, which indicated the recovery of the Mn4CaO5 cluster stability and the changes in protein conformation (Nagao et al. 2015). This is consistent with the restoration of the O2-evolving activity after the addition of PsbO reaching up to 60% of the maximum value obtained after the rebinding of all three WOC proteins (Shen and Inoue 1993). The binding of PsbV solely did not induce structural changes in the WOC; however, the stepwise addition of PsbV and then PsbU after PsbO binding fully recovered the FTIR spectra (Nagao et al. 2015) and restored the maximal O2-evolving activity (Shen and Inoue 1993).
At the same time, in the case of PSII from higher plants (and probably from green algae) the PsbP protein mainly affects the protein conformation near the Mn4CaO5 cluster without changing its ligand structure, while PsbO and PsbQ have minor effects (Tomita et al. 2009). Experiments with 13C-labeled PsbP showed that the signals observed in the light-induced FTIR different spectra originate not from the PsbP itself but from the PsbP-induced changes in the intrinsic core proteins of PSII. Moreover, researchers using Δ15-PsbP showed that 15 residues of the highly conserved flexible N-terminus of PsbP (Fig. 4) are crucial for the recovery of FTIR spectra (Tomita et al. 2009), as well as for Ca2+ and Cl− retention (Ifuku et al. 2005a). The depletion (or rebinding) of PsbP in PSII from higher plants and green algae also results in significant changes in the O2-evolving activity (up to 50%), which was comparable with the effect observed for the depletion/rebinding of PsbO (Miyao and Murata 1985; Ifuku et al. 2005a; Suzuki et al. 2005). It was proposed the possibility that the depletion/rebinding of PsbP can induce structural changes in PsbO (Tomita et al. 2009). The results obtained in PsbP-deficient mutant C. reinhardtii BF25 showed even the fully suppressed O2-evolving activity, as well as a ~ 20% decrease in the value of the maximal quantum yield of PSII (Nishimura et al. 2017). The same results were observed in PsbP-deficient mutants of higher plants (Ifuku et al. 2005b; Yi et al. 2007). Interestingly, the data from FTIR spectroscopy of PSII from the red algae Cyanidium caldarium, in contrast to cyanobacterial PSII, showed the main role of PsbV, but not PsbO, in the regulation of the WOC protein conformation (Uno et al. 2013), which is consistent with the role of PsbP in higher plants and green algae. PsbU and PsbQ proteins are probably involved in fine-tuning of the WOC structure.
Fig. 4.
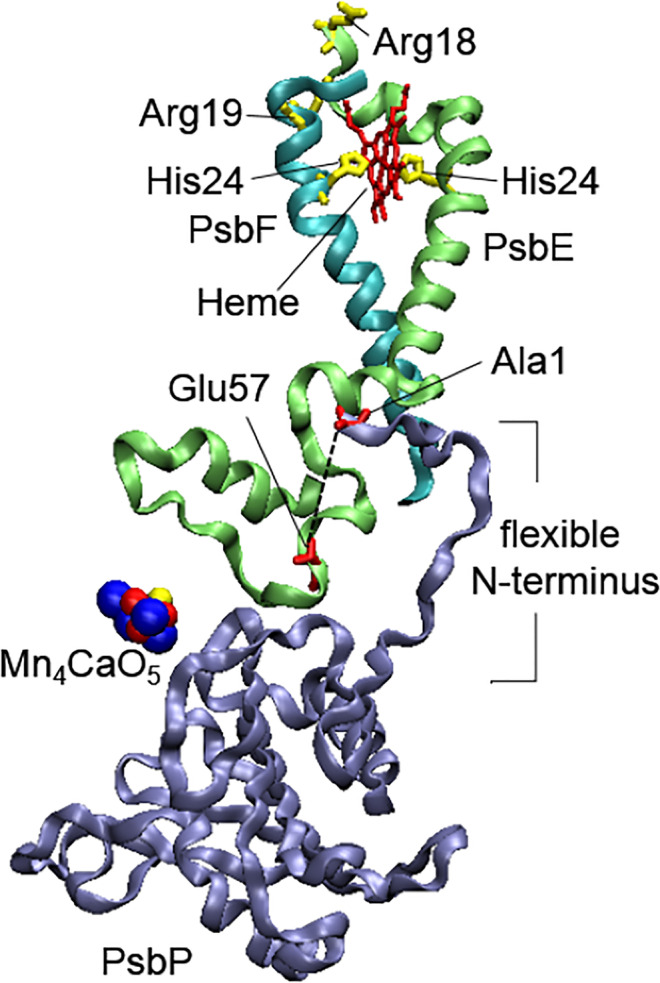
Structures of PsbP, PsbE, and PsbF proteins with the putative interaction between the flexible N-terminus of PsbP and Glu57 of PsbE shown by the dotted line. The figure was prepared with using of structural data from the PDB for PSII from green algae (C. reinhardtii) (PDB ID: 6KAC, https://doi.org/10.2210/pdb6kac/pdb (Sheng et al. 2019))
PSII-enriched preparations from green algae C. reinhardtii contain a large amount of lumenal carbonic anhydrase CAH3 located in close vicinity of WOC and affecting its activity (Villarejo et al. 2002; Shutova et al. 2008; Terentyev et al. 2019, 2020). The most recent data have shown that the CAH3 protein itself may be involved in the correct structural organization of the WOC (Shukshina and Terentyev 2021). The absence of CAH3 in the case of PSII from cia3 mutant resulted in less extent of PsbP removal upon an increase in NaCl concentrations compared with PSII from the wild type, probably due to the changes that occurred in the intermolecular interactions within the WOC, accompanied by the emergence of additional hydrophobic or electrostatic connections. At the same time, in contrast to the wild type, PSII from cia3 demonstrated the suppression of the O2-evolving activity at 50–100 mM NaCl, when PsbP removal was not detected, which suggested the presence of more pronounced conformational dynamics of the WOC proteins upon an increase in the ionic strength of the medium.
It should be noted that in some works, the opposite results have been obtained. For example, Offenbacher with co-workers observed changes in the light-induced S2-minus-S1 FTIR difference spectra in spinach PSII upon the removal of PsbO but not PsbP and PsbQ (Offenbacher et al. 2013), and Nishimura with co-workers did not observe a decrease in the O2-evolving activity upon the removal of PsbP (Nishimura et al. 2016). One of the reasons may be errors made during preparing the samples, as Ifuku and Noguchi discussed (Ifuku and Noguchi 2016).
The acceptor side and core complex proteins
The absence of the PsbP protein influences not only the WOC structural state and, as a consequence, the Mn4CaO5 cluster function, but also the efficiency of the electron transfer rate on the acceptor side of PSII. A significant decrease in the QA− oxidation rate was observed in PSII-enriched preparations after the removal of PsbP and PsbQ proteins (by NaCl-washing) (Roose et al. 2010; Semin et al. 2018) and in PsbP-deficient mutants of higher plants (Ifuku et al. 2005b; Yi et al. 2007). In addition, in a ΔPsbP tobacco mutant, the substantial accumulation of reduced QA was identified on the basis of the 1 − qP parameter calculated from chlorophyll fluorescence induction curves (Ifuku et al. 2005b). At the same time, the further removal of PsbO in the case of PSII preparations did not strongly affect the QA− oxidation rate (Roose et al. 2010).
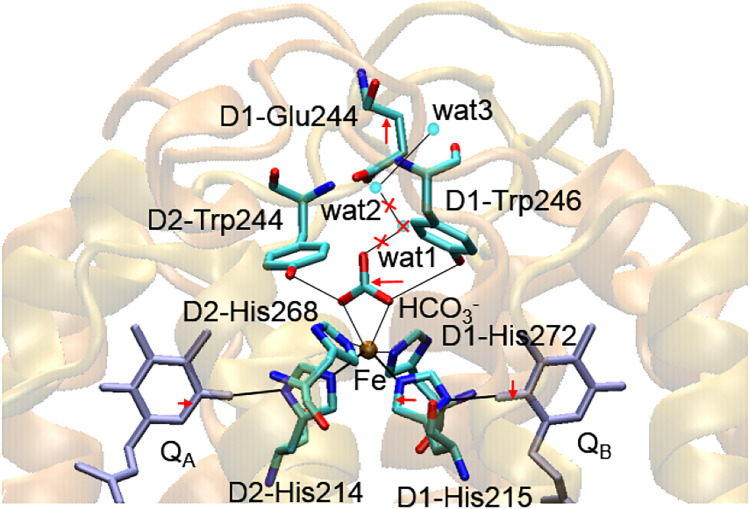
The decrease in the QA− oxidation rate was also observed upon the extraction of Ca2+ from the WOC or the replacement of Ca2+ with Sr2+ in the Mn4CaO5 cluster (Kargul et al. 2007; Semin et al. 2018), in spite the fact that the distance between the Mn4CaO5 cluster and QA is ~ 40 Å (Fig. 2). The data of X-ray crystallography of cyanobacterial PSII with a Sr-modified WOC showed minor changes in the ligand environment of the Mn4SrO5 cluster, with longer Sr-D1-Asp170 and shorter Sr-D1-Glu189 distances compared with the Mn4CaO5 cluster (Koua et al. 2013). Nevertheless, this was accompanied by a perturbation in the protein environment on the acceptor side, including in the vicinity of the QA–Fe–QB site (Fig. 5) (Koua 2019), and stabilization of S2QA− recombination (Koua et al. 2013). The analysis showed rise of a weaker H-bonds between D2-His214, D2-Trp217 and QA, a shift in the positions of the residues of D2-His214 and D1-His215 having H-bonds with non-heme Fe and QA and QB, respectively, displacement of a bicarbonate ion (~ 0.37 Å) with significant changes in the H-bonding network, including distances with D1-Thyr246 and D2-Tyr244, and broken of H-bonds with water molecules (wat-1 and wat-2) due to the loss of wat-1, etc. In addition, five Glu resides (D1-Glu242, -243, -244 and D2-Glu241, -242) shifted by ~ 0.15 Å to the stromal side upon Ca2+/Sr2+ exchange (Koua 2019).
Fig. 5.
Perturbations on the acceptor side of cyanobacterial PSII after replacement of Ca2+ by Sr2+ in the Mn4CaO5 cluster. The red arrows show the shift in positions as compared with native PSII. The black lines indicate the network of H-bonds. The red crosses indicate the breakage of the H-bond network due to the loss of the wat1 water molecule. The figure is composed according to Koua et al. (2019) with using of structural data from the PDB for cyanobacterial PSII with a modified Mn4SrO5 cluster (T. vulcanus) (PDB ID: 4IL6, https://doi.org/10.2210/pdb4il6/pdb (Koua et al. 2013)). The molecular bridge QA–D2-His214–non-heme Fe–D1-His215–QB is shown by bold lines
The Mn4CaO5 cluster site is formed by D1; thus, perturbation in the ligands on the donor side can be transferred to the QB site through the transmembrane helices of this protein and then to the QA site formed by D2 through a QA–D2-His214–non-heme Fe–D1-His215–QB molecular bridge (Fig. 5), as Kato with co-workers discussed in their works (Kato and Noguchi 2014, 2019). In 1990, Rashid and Carpentier suggested the same idea about changes in the QB site, after they observed that removal of the PsbP and PsbQ proteins made the QB site more susceptible to the herbicide atrazine (Rashid and Carpentier 1990). In addition, a recent molecular docking study showed the possible role of D1-His215 in this process (Battaglino et al. 2021).
At the same time, another way of transferring the perturbation can be realized by the involvement of the PSII small intrinsic proteins. The data from cross-linked studies showed the interconnection between the PsbP and PsbE (Cyt b559) subunits (Ido et al. 2012), which occurred via the interaction of PsbP-Ala1 at the flexible N-terminus and PsbE-Glu57 (Nishimura et al. 2016). As it was found, PsbP can affect the redox status of Cyt b559 heme located close to the stromal side (Fig. 4), and a significant reduction in the high-potential redox form was observed upon PsbP removal (Nishimura et al. 2016). The exact structural changes in Cyt b559 among different redox forms are unknown. At the same time, the single amino acid substitution on the stromal side of cyanobacterial PsbE and PsbF subunits (Chiu et al. 2013) revealed the involvement of PsbE-Arg8, PsbE-Arg18, and PsbF-Arg19, which are close to PsbE-His23 and PsbF-His24 ligating the heme, in the distribution of the redox forms of Cyt b559. Therefore, as Nishimura with co-workers suggested (Nishimura et al. 2016), the interaction of PsbP with PsbE on the PSII donor side would change the interaction between PsbE and PsbF on the stromal side of Cyt b559 altering the redox properties of the heme. The substitutions of PsbE-Arg8 and PsbF-Arg19 also reduced the electron transfer between QA and QB (Chiu et al. 2013), which indicated an interconnection between the structural state of the PsbE/PsbF stromal side and the PSII acceptor side, and suggested a transfer of the WOC conformational changes to the PSII acceptor side through the transmembrane helices of Cyt b559 subunits. This suggestion is consistent with the results obtained for PSII-enriched preparations from green algae C. reinhardtii. The perturbations in the WOC structural organization in the case of cia3 mutant (Shukshina and Terentyev 2021) was accompanied by changes in the distributions of Cyt b559 redox forms and suppression of the electron transfer between QA and QB (Terentyev et al. 2020).
The changes in the protein environment should influence the electrochemical properties of the cofactors. The redox potential (Em) of QA (QA/QA−) under different experimental conditions has been the most studied parameter in PSII for the last few decades. Through fluorescence measurements, it was identified that Em (QA/QA−) is upshifted by 100–160 mV under depletion of the Mn4CaO5 cluster or Ca+2 (Johnson et al. 1995; Krieger-Liszkay and Rutherford 1998; Allakhverdiev et al. 2011; Kato et al. 2012, 2019; Brinkert et al. 2016), or by ~ 27 mV upon Ca2+/Sr2+ exchange, suggesting the important role of Ca2+-binding site modifications (Kato et al. 2012). Besides this, the impact of WOC protein binding on the value of Em (QA/QA−) has also been discussed (Kato and Noguchi 2021). Studies using FTIR spectroscopy showed other results. The depletion of the Mn4CaO5 cluster did not change the value of Em (QA/QA−) in the case of spinach PSII and downshifted it by 10 ± 4 mV in the case of cyanobacterial PSII (Kato et al. 2019). At the same time, removal PsbP and PsbQ, and of PsbO, proteins from spinach PSII downshifted Em (QA/QA−) by ~ 14 mV and ~ 20 mV, respectively (Kato and Noguchi 2021), supporting the role of WOC protein binding on the redox properties of QA.
Changes in the value of Em (QA/QA−) were also observed upon the binding of PSII-inhibiting herbicides to the QB site, indicating the induction of conformation perturbation of the PSII acceptor side, which depends on the chemical properties of the herbicide molecules. For example, according to fluorescence measurements, the value of Em (QA/QA−) is upshifted by ~ 52 mV in the presence of DCMU (Diuron) and downshifted by ~ 45 mV and ~ 33 mV in the presence of bromoxynil and ioxynil, respectively. Interestingly, the effects of the herbicides and the Mn-depletion were superimposed (Krieger-Liszkay and Rutherford 1998). The differences in the influence of various herbicides on the value of Em (QA/QA−) were also observed by FTIR measurements (Kato and Noguchi 2021). Co-crystallization of the cyanobacterial PSII with an atrazine-type herbicide showed that this molecule can be bound to the QB-site via four H-bonds with D1-Phe265 (two bonds), D1-Ser264, and D1-Ala263, as well as non-polar interactions with D1-Met214 and D1-Leu271 (Broser et al. 2011). A molecular docking study found that herbicides with higher affinity to PSII in vitro such as DCMU, metribuzin, and terbuthylazine preferred to orient toward D1-His215 (Battaglino et al. 2021), which is involved in the QA–D2-His214–non-heme Fe–D1-His215–QB molecular bridge, as mentioned above (Fig. 5).
The non-heme Fe, in turn, is ligated by two additional His residues (D2-His268 and D1-His272), as well as a bicarbonate ion (HCO3−) as a bidentate ligand (Fig. 5) (Koua 2019). As Brinkert with co-workers showed, HCO3− depletion upshifted the Em (QA/QA−) by ~ 74 mV and slowed the QA− oxidation rate (Brinkert et al. 2016), which may have been induced by local conformational changes in the protein environment. The replacement of bicarbonate with formate upshifted the Em (QA/QA−) by only 50–53 mV (Brinkert et al. 2016; Kato and Noguchi 2021), but the stronger suppression of the QA− oxidation rate (Brinkert et al. 2016) indicated the dependence of the effect on the molecular structure of the ligand. This suggestion is consistent with the results observed during EPR studies by Deligiannakis with co-workers concerning the binding of different carboxylate anions to the non-heme-Fe (Deligiannakis et al. 1994). The possible fine-tuning mechanism of PSII function regulated by HCO3− binding/depletion at the non-heme Fe site in vivo was proposed (Brinkert et al. 2016).
The data obtained by electron microscopy with subsequent analysis of the single-particle images of spinach PSII showed the strong dependence of the core protein spatial structure on the extrinsic proteins binding to the PSII donor side. The removal of PsbP and PsbQ led to changes in the intensities of masses in the core area, with an inward shift in the positions of CP29 and CP26 by 1.2 and 0.2 nm, respectively, with accompanied movement of the S-LHCII trimer (Boekema et al. 2000). The subsequent removal of PsbO mostly resulted in a change in the S-LHCII trimer position.
The use of high-speed atomic force microscopy allowed the visualization of the WOC structural dynamics in a recent study (Tokano et al. 2020). The results demonstrated that the removal of WOC proteins liberated the lumenal domain of CP43 between Pro304 and Pro400, which belong to the large extrinsic loop E (Fig. 6c). Observations demonstrated that the domain underwent up-and-down fluctuations. CP47 has a similar domain between Pro270 and Pro447, the maximum of which was also well detectable by the same method; however, it did not show any structural changes. The authors suggested that this difference may be due to the involvement of CP43-Glu354 and CP43-Glu357 in a direct interaction with the Mn4CaO5 cluster, the status of which can affect the CP43 lumenal loop conformational state (Tokano et al. 2020). The CP43 protein is in close association with the CP26 protein, and conformational changes in both of them can be interconnected. The data of cross-linking studies indeed showed multiple interactions of PsbP and PsbQ with both CP43 and CP26 proteins (Ido et al. 2014). Thus, conformational changes in the WOC can affect not only the PSII acceptor side but also spatial structures and positions of the PSII core antenna proteins.
Fig. 6.
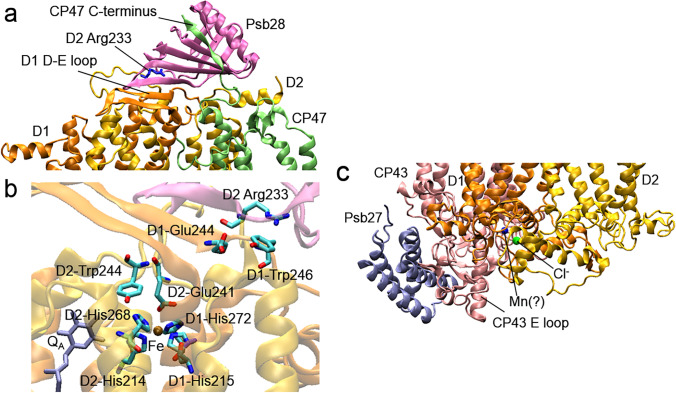
Structural organization of the acceptor (a, b) and donor (c) sides of PSII in the presence of Psb27 and Psb28 proteins, respectively. b Changes in the orientation of amino acid residues near non-heme Fe, as well as replacement of the bicarbonate ion by D2-Glu241 on the PSII acceptor side. c Proposed stabilization of the CP43 lumenal loop by Psb27 and detected positions of the Cl.− ion, as well as an unknown ion in the place of the Mn4CaO5 cluster. The figure was prepared according to Zabret et al. (2021) with using of structural data from the PDB for cyanobacterial PSII (T. vulcanus) (PDB ID: 7NHP, https://doi.org/10.2210/pdb7nhp/pdb (Zabret et al. 2021))
De novo assembled photosystem II
The process of PSII super-complex assembly during biogenesis includes the stepwise actions of more than 20 auxiliary proteins (Heinz et al. 2016). Among these, Psb28 and Psb27 are involved in the last stages of PSII assembly and the induction of photosynthetic activity through strong conformational perturbations of the acceptor and the donor sides of PSII, respectively. Despite the fact that both proteins were originally identified in PSII preparations from Synechocystis sp. PCC 6803, their orthologs have also been found in higher plants (Mabbitt et al. 2014).
Data from a mass spectrometry-based cross-linking study obtained from cyanobacterial PSII (Thermosynechococcus elongatus) showed the interconnections between Psb28-Lys8 and the N-termini of PsbE and PsbF, as well as Psb28-Ala2 and the N-terminus of PsbF with predicted cross-link distances of 10–15 Å (Weisz et al. 2017). The proposed model placed the CP47-associated Psb28 protein ~ 9–12 Å directly above the heme of Cyt b559 and 23–27 Å from the redox-active aromatic ring of the quinone QB, and the possibility of the induced structural perturbation in the QB site area was discussed. However, in recent work based on cryo-electron microscopy, the position of Psb28 was significantly altered (Zabret et al. 2021). According to the data, Psb28 binds to PSII directly above the QB, forming a β-hairpin structure involving the central β-sheet of Psb28, the C-terminus of CP47, and the D-E loop of D1 (Fig. 6a). As a result, conformational changes occur in the D-E loop of D1, which prevent binding of the C-terminus of CP43 to the PSII. At the same time, the movement of D2-Arg233 also suggests structural changes in the D-E loop of D2. The structural perturbation also alters the positions of residues involved in the ligation of a bicarbonate ion in mature PSII: D1-Glu244 and D1-Tyr246 are displaced significantly, while D2-Glu241 even replaces a bicarbonate molecule and becomes a ligand to non-heme Fe (Fig. 6b). As expected, this results in strong suppression of the QA− oxidation rate (Zabret et al. 2021). Psb28 probably helps CP47 join the RC and protects the CP47-RC complex from photodamage, but Psb28 has to be released to CP43 can completely bind to the RC, and Psb27, in turn, can attach to the PSII donor side for the last step in PSII biogenesis (Weisz et al. 2017; Zabret et al. 2021).
Fig. 7.
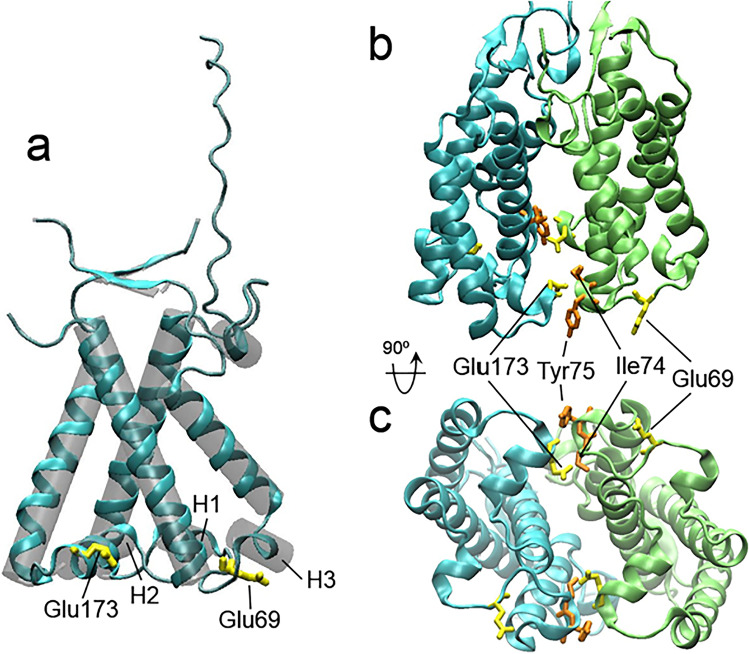
Structures of the monomer (a) and dimer (b, c) of spinach PsbS obtained at acidic pH. The positions of pH-sensing lumen-faced Glu residues are shown in yellow, and the positions of residues involved in hydrogen bonding with Glu173 in the dimer are shown in orange. The figure was prepared with using of structural data from the PDB for spinach PsbS (PDB ID: 4RI2, https://doi.org/10.2210/pdb4ri2/pdb (Fan et al. 2015))
The Psb27 protein is associated with the lumenal loops C and E of CP43 (Fig. 6c) (Liu et al. 2011; Huang et al. 2021; Zabret et al. 2021), and a weak binding of Psb27 to PSII was suggested (Huang et al. 2021). Nevertheless, data from cryo-electron microscopy studies showed significant structural differences on the donor side of Psb27-PSII that did not contain the WOC proteins and the Mn4CaO5 cluster, compared with the mature PSII (Huang et al. 2021; Zabret et al. 2021). Thus, some CP43 residues involved in the interaction with PsbO were changed in their side orientations (CP43-Ser330, Ser348, Ser362, Leu377) or shifted toward the PsbO side (the region of CP43-Arg343 to Gly347), others obtained increased flexibility (the region of CP43-Gln332 to Lys339). CP47 underwent structural changes in the loop region between CP47-Arg384 and Arg385, and the D2 C-terminus turned toward the lumenal side. In addition, the D1 C-terminus from D1-Arg334 (a loop) was not visible (Huang et al. 2021). It is assumed that the presence of Psb27 forms a structural conflict between the residues of PsbO and the PSII core complex proteins, preventing the attachment of PsbO (or its release during PSII repair) to the binding site (Huang et al. 2021; Zabret et al. 2021). Recent research identified even a small overlap between Psb27 and the WOC subunits binding sites (Zabret et al. 2021). In addition, in the presence of Psb27, slight differences in the helices positions were observed for PsbE, PsbF, and PsbZ low molecular weight proteins, as well as an increase in the flexibility of the CP47 C-terminus and the N-termini of PsbE and PsbF (Huang et al. 2021).
Huang with co-workers discussed that the conformational rearrangement of the CP43 lumenal domain induced by incorporation of the Mn4CaO5 cluster may lead to the dissociation of Psb27 (Huang et al. 2021). Indeed, as Tokano with co-workers proposed (Tokano et al. 2020), photoassembly of the Mn4CaO5 cluster fixes the CP43 lumenal domain via the interaction with CP43-Glu354 and CP43-Glu357 (Tokano et al. 2020; Zabret et al. 2021), which allows the binding of PsbO and other WOC proteins locking the CP43 luminal domain (Tokano et al. 2020). Thus, the role of Psb27 can be to limit the conformational fluctuation of the CP43 lumenal domain to facilitate Mn4CaO5 cluster assembly, which induces, in turn, the binding of WOC proteins.
Protein damage
The action of high light on PSII, resulting in the suppression of its photosynthetic activity, mostly leads to damage of the D1 core protein, which is probably triggered by reactive oxygen species (Aro et al. 1993; Yamamoto et al. 2008; Cheregi et al. 2016). Tandem mass spectrometry showed that the oxidation of D1-His332 coordinated with Mn1 of the Mn4CaO5 cluster induces a cascade of oxidative events with the modification of numerous residues in the D1 and D2 proteins on the PSII donor side, accompanied by a 40–60% decrease in the O2-evolving activity (Kale et al. 2017). At the same time, the oxidative modification of D1-Glu130 and D1-Trp131, which are in close vicinity to PheoD1, as well as D2-Glu242, D2-Tyr244, and D2-Ser245, which are located in the D-E loop of D2 and are in close vicinity of non-heme Fe and QA, was detected. FTIR spectroscopy data indicated that after high light treatment of PSII preparations, the amount of α-helices relative to β-sheets decreased (He et al. 1991; Zhang et al. 1997), while under anaerobic conditions, this change was less pronounced (He et al. 1991; Aro et al. 1993). The results of SDS-PAGE and western blot analysis identified a decrease in the electrophoretic mobility of the main D1 and D2 bands and their intensity. These occurred together with an increase in the intensity of the high molecular weight diffuse band (60–70 kDa) and the appearance of a new low weight band (~ 40 kDa), both of which reacted with the antibody raised against D1 (He et al. 1991; Kettunen et al. 1996). Thus, high light treatment induces conformational changes in D1 and D2, as well as the formation of aggregates and partial degradation of D1.
Similar FTIR spectral changes and the formation of aggregates were observed after thermo-induced damage of PSII (He et al. 1991; Komayama et al. 2007). Yamamoto with co-workers also suggested a similar mechanism of D1 damage under photo- and heat-induced inhibition of PSII (Yamamoto et al. 2008). Heat stress resulted in destabilization of the QB site, probably due to the conformational change in the structure of the D-E loop of D1 (Yamane et al. 1998; Yoshioka et al. 2006). This led to an alteration in the kinetics of chlorophyll fluorescence, reflecting the QA to QB electron transfer efficiency (Yamane et al. 1998) and even induced D1 cleavage by FtsH proteases recognizing denatured stromal loops of the protein (Yoshioka et al. 2006). As was shown, the binding of herbicides (DCMU, bromoxynil, ioxynil) to the QB site prevented D1 cleavage, probably by fixing the structure of the QB site (Yoshioka et al. 2006) and the similarity of these data to those observed in studies with excessive illumination of PSII was mentioned.
Heat and high light stresses result in the structural perturbation of the PSII donor side, especially in the case of the D1 protein, which are accompanied by the release of all WOC proteins, as well as Mn (Henmi et al. 2004; Komayama et al. 2007; Yamamoto et al. 2008). Similar to the D1 protein, the oxidative damage of PsbO and PsbP occurs under these conditions, which may even lead to a loss of the capability of these proteins to rebind to PSII (Henmi et al. 2004), probably due to their inability to re-fold the correct tertiary structure.
Besides this, the formation of aggregates between D1 and the surrounding proteins has been observed, which can significantly disrupt the spatial structures of proteins in such complexes. The aggregates were identified by researchers in preparations from both higher plants (He et al. 1991; Kettunen et al. 1996; Komayama et al. 2007) and cyanobacteria (Lupínková and Komenda 2007), and the D1/CP43 complex was the most possible product in this case. Generation of a D1/D2 heterodimer, and cross-linking between the DE-loop of D1 (the D1-Phe239 to D1-Glu244 region) and the N-terminal serine of PsbE (Cyt b559) were also obtained (Komayama et al. 2007; Yamamoto et al. 2008; Huesgen et al., 2009). Anyway, PSII with such aggregates undergo rapid degradation by proteases (Yamamoto et al. 2008; Cheregi et al. 2016).
The phosphorylation of the N-terminal residues of D1, D2, CP43, and PsbH by the protein kinase STN8 upon exposure to high light is a well observed process (Bonardi et al. 2005; Tikkanen et al. 2008; Puthiyaveetil and Kirchhoff 2013). As is the case for D1, it marks the protein for degradation (Aro et al. 1993; Rantala et al. 2020), but the opposite results have also been obtained (Bonardi et al. 2005). It was shown that PSII core protein phosphorylation can facilitate PSII super-complex disassembly (Tikkanen et al. 2008; Fristedt and Vener 2011), which should be realized by conformational changes in the proteins, however, their exact structural modifications have not been studied yet.
Induction of non-photochemical quenching
A number of conformational perturbations occur in the large protein complex of PSII outer antenna under the induction of non-photochemical quenching (NPQ) of chlorophyll fluorescence, which is involved in the photoprotection of PSII by dissipating absorbed light energy or redistributing it between the two photosystems (Ruban 2016). The major and most rapid component of NPQ in higher plants and green algae is qE (energy-dependent quenching), which forms within seconds and is triggered by a decrease in lumenal pH, which results in LHCII switching from the absorbed to the dissipating state (Ruban 2012, 2016).
In higher plants, qE is connected with the LHCII-integrated PsbS protein, which belongs to the LHC superfamily but does not contain pigments (Fan et al. 2015). It is assumed that PsbS acts as a molecular pH sensor via two symmetrically arranged lumen-faced Glu residues (Glu69 and Glu173 in spinach (Fan et al. 2015), or Glu122 and Glu226 in Arabidopsis (Li et al. 2004)), which are located within or close to the lumen-exposed loops of the protein (Fig. 7). Both residues are equivalent in pH response and qE induction, since E122Q and E226Q Arabidopsis mutants had a significantly reduced NPQ response, while the double E122Q/E226Q mutant did not induce NPQ at all (Li et al. 2004). The activation of PsbS, as proposed, is associated with reversible monomerization of the PsbS dimer under a decrease in pH (Bergantino et al. 2003), but according to the data obtained by Fan with co-workers the dimeric form of PsbS is maintained at a low pH (Fan et al. 2015). The authors also showed in the obtained crystal structure of PsbS (Fig. 7b, c) that the pH-sensing Glu173 in each monomer is important (together with other residues within a molecule) for dimerization at a low pH by forming two hydrogen bounds with the Ile74 and Tyr75 of another monomer (i.e., four per dimer) on the lumenal side of the protein (Fan et al. 2015). The intermolecular hydrogen bonds can be altered under a shift in pH to neutral values, which can result in conformational changes of the whole PsbS molecule. Indeed, the earlier elution of PsbS from a gel-filtration column at a neutral then at an acidic pH was detected. The increase in pH also allowed the cross-linking of a pair of lumenal lysine residues (Lys164) belonging to different monomers by cross-linkers with space arms longer than 15.4 Å. This indicates an increase in the gap at the lumenal side of the protein, probably due to weakening or even disruption of the intermolecular hydrogen bonds there as a result of the deprotonation of Glu173 at a pH above 7.0. It was concluded that the activation of PsbS occurred mainly through conformational changes in the protein molecule but not the monomer–dimer transition (Fan et al. 2015). Liguori with co-workers based on data of a molecular dynamics simulation predicted the unfolding of the small luminal H3 helical element of PsbS (Fig. 7a) containing Ile74 and Tyr75, which form hydrogen bonds with Glu173, into a loop element under an increase in pH up to 7–8 (Liguori et al. 2019). This result is consistent with the data obtained by NMR and vibrational spectroscopies on the PsbS from moss Physcomitrella patens (Krishnan-Schmieden et al. 2021). Besides this, it has been shown that another small luminal H2 helical element of PsbS containing Glu173 (Fig. 7a) is relocated from the aqueous into the membrane phase upon a decrease in pH. It can be suggested that the pH-dependent conformational changes in PsbS, located in the interface region between the PSII core complex and LHCII (Bergantino et al. 2003), can significantly influence the interactions between them and, as a consequence, the absorbed energy transfer from the antenna to the RC.
In C. reinhardtii, another protein named Lhcsr3 (light-harvesting complex stress-related) is essential for qE induction under a decrease in lumenal pH (Ballottari et al., 2016), while the presence of PsbS in cells after high light treatment was also observed (Redekop et al. 2020). In contrast to PsbS, Lhcsr3 is a pigment binding protein (Fig. 8) containing 6–7 chlorophylls and 2–3 carotenoids (xanthophyll) (Bonente et al. 2011). The crystal structure of Lhcsr3 has not been resolved yet, and the pH-induced conformational changes are not as well studied as those of PsbS. The involvement of acidic residues of the protein lumenal side in qE induction has mostly been investigated with a series of mutants (Ballottari et al., 2016; Camargo et al. 2021). According to the results obtained and the suggestions, the protonation of lumen-exposed Glu221, Glu224, and Asp117 (Fig. 8) can change the chlorophyll-carotenoid interaction (Camargo et al. 2021) and supports a lutein radical cation formation within the protein (Ballottari et al., 2016), while the protonation of acidic residues in the C-terminal loop probably changes the chlorophyll-chlorophyll interaction (Camargo et al. 2021).
Fig. 8.
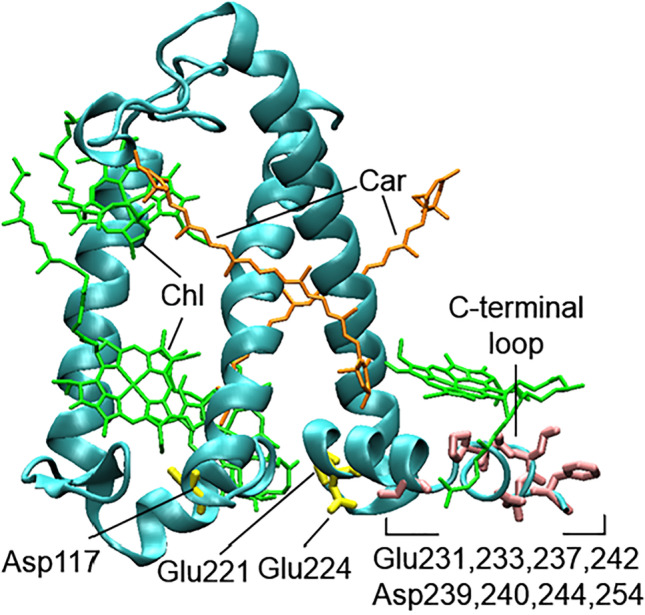
Structural model of Lhcsr3 based on the crystal structure of CP29 (PDB ID: 3PL9, https://doi.org/10.2210/pdb3pl9/pdb (Pan et al. 2011)). Chlorophyll and carotenoid molecules are shown in green and orange, respectively. Protonated residues are shown in yellow and pink. The figure was prepared according to Camargo et al. (2021) and Ballottari et al. (2016)
The second on timescale component of NPQ is state transitions (ST) formed in 10 min and resulted in the detachment of LHCII (or outer antenna) from PSII and even their relocation to photosystem I (Ruban 2012). The phosphorylation of LHCII proteins by STN7 kinase in higher plants and STT7 kinase in green algae is the initial step of ST. As known, functionally the phosphorylation adds a negative charge to the amino acid side chain (Grieco et al., 2016), which can induce conformation perturbations in protein molecules. Fluorescence lifetime imaging microscopy showed that phosphorylation indeed led to the dissociation of LHCII from PSII in C. reinhardtii and even to the formation of energy-dissipative aggregates (Iwai et al. 2010). The disassembly of LHCII from the super-complex can be also facilitated by phosphorylation of PSII core proteins by STT8 kinase (Tikkanen et al. 2008; Fristedt and Vener 2011), which can probably increase the conformational discrepancy between two multi-protein complexes.
The ST is observed also in cyanobacterial cells, which contain the phycobilisome complexes bound to the stromal surface of the thylakoid membrane, instead of LHCII (Calzadilla and Kirilovsky 2020). The amount of phycobilisomes involved in ST in Synechocystis was estimated to be around 13% (Chukhutsina et al. 2015). The involvement of the phosphorylation of four residues (Ser22, Ser49, Thr94, and Ser154) of the phycocyanin β-subunit in ST induction has been proposed (Chen et al. 2015). However, a study with construction of single mutants for all known kinases in Synechocystis (Calzadilla et al. 2019) showed that the phosphorylation reactions are not essential for ST in cyanobacteria. Thus, the mechanism of ST induction, as well as the accompanying conformational changes in antenna proteins in cyanobacteria, is still unknown.
Conclusion
Besides the macromolecular perturbations described above, the significant development of techniques and approaches has now allowed researchers to study conformational changes occurring even at much lower levels of organization of the PSII pigment-protein super-complex. For example, by a method of serial femtosecond crystallography, the conformational changes in PSII from T. vulcanus upon a S1–(S2)–S3 transition in the WOC were determined (Kupitz et al. 2014). The structures of dark-adapted (S1) and two-flash-illuminated (S3) PSII from T. vulcanus at room temperature were obtained from crystals of PSII dimers by femtosecond pulses of an X-ray free electron laser (Young et al. 2016). In recent work, a high-resolution structure of PSII from T. vulcanus provided a better visualization of possible H2O and H+ channels within the WOC with the identification of the water molecules and amino acid residues involved (Hussein et al. 2021). In addition, two-dimensional fluorescence lifetime correlation spectroscopy was used to resolve the conformational dynamics of Lhcsr3 and Lhcsr1 proteins upon a shift in pH to establish the participants (pigments) of quenching (Manna and Schlau-Cohen 2022). Molecular dynamics simulations have been used to study conformational changes in PSII during charge separation (Kulik et al. 2020), the pathway for quenching in the antenna (Papadatos et al. 2017) including PsbS dynamics (Liguori et al. 2019), and more. The new knowledge about the conformational changes at all levels of PSII organization can significantly improve our understanding of the molecular mechanisms of its functioning, regulation, and protection, which can be a useful tool for optimizing photosynthesis to increase its productivity.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ago H, Adachi H, Umena Y, Tashiro T, Kawakami K, Kamiya N, Tian L, Han G, Kuang T, Liu Z, Wang F, Zou H, Enami I, Miyano M, Shen J-R. Novel features of eukaryotic photosystem II revealed by its crystal structure analysis from a red alga. J Biol Chem. 2016;291:5676–5687. doi: 10.1074/jbc.M115.711689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Tsuchiya T, Watabe K, Kojima A, Los DA, Tomo T, Klimov VV, Mimuro M. Redox potentials of primary electron acceptor quinone molecule (QA)− and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d. Proc Natl Acad Sci. 2011;108:8054–8058. doi: 10.1073/pnas.1100173108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta - Bioenerg. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Ballottari M, Truong TB, De Re E, Erickson E, Stella GR, Fleming GR, Bassi R, Niyogi KK. Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J Biol Chem. 2016;291:7334–7346. doi: 10.1074/jbc.M115.704601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglino B, Grinzato A, Pagliano C. Binding properties of photosynthetic herbicides with the QB site of the D1 protein in plant photosystem II: a combined functional and molecular docking study. Plants. 2021;10:1501. doi: 10.3390/plants10081501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantino E, Segalla A, Brunettaeardo E, Rigoni F, Giacometti GM, Szabò I, A. Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc Natl Acad Sci. 2003;100:15265–15270. doi: 10.1073/pnas.2533072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekema EJ, van Breemen JFL, van Roon H, Dekker JP. Conformational changes in photosystem II supercomplexes upon removal of extrinsic subunits. Biochemistry. 2000;39:12907–12915. doi: 10.1021/bi0009183. [DOI] [PubMed] [Google Scholar]
- Bommer M, Bondar A-N, Zouni A, Dobbek H, Dau H. Crystallographic and computational analysis of the barrel part of the PsbO protein of photosystem II: carboxylate–water clusters as putative proton transfer relays and structural switches. Biochemistry. 2016;55:4626–4635. doi: 10.1021/acs.biochem.6b00441. [DOI] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature. 2005;437:1179–1182. doi: 10.1038/nature04016. [DOI] [PubMed] [Google Scholar]
- Bonente G, Ballottari M, Truong TB, Morosinotto T, Ahn TK, Fleming GR, Niyogi KK, Bassi R. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 2011;9:e1000577. doi: 10.1371/journal.pbio.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkert K, De Causmaecker S, Krieger-Liszkay A, Fantuzzi A, Rutherford AW. Bicarbonate-induced redox tuning in photosystem II for regulation and protection. Proc Natl Acad Sci. 2016;113:12144–12149. doi: 10.1073/pnas.1608862113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broser M, Glöckner C, Gabdulkhakov A, Guskov A, Buchta J, Kern J, Müh F, Dau H, Saenger W, Zouni A. Structural basis of cyanobacterial photosystem II inhibition by the herbicide terbutryn. J Biol Chem. 2011;286:15964–15972. doi: 10.1074/jbc.M110.215970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Smith RN, Watanabe A, Tokutsu R, Song C, Murata K, Minagawa J. Structural determination of the large photosystem II–light-harvesting complex II supercomplex of Chlamydomonas reinhardtii using nonionic amphipol. J Biol Chem. 2019;294:15003–15013. doi: 10.1074/jbc.RA119.009341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzadilla PI, Kirilovsky D. Revisiting cyanobacterial state transitions. Photochem Photobiol Sci. 2020;19:585–603. doi: 10.1039/C9PP00451C. [DOI] [PubMed] [Google Scholar]
- Calzadilla PI, Zhan J, Sétif P, Lemaire C, Solymosi D, Battchikova N, Wang Q, Kirilovsky D. The cytochrome b6f complex is not involved in cyanobacterial state transitions. Plant Cell. 2019;31:911–931. doi: 10.1105/tpc.18.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FVA, Perozeni F, Valbuena GDLC, Zuliani L, Sardar S, Cerullo G, D’Andrea C, Ballottari M. The role of acidic residues in the C terminal tail of the LHCSR3 protein of Chlamydomonas reinhardtii in non-photochemical quenching. J Phys Chem Lett. 2021;12:6895–6900. doi: 10.1021/acs.jpclett.1c01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carius AB, Rogne P, Duchoslav M, Wolf-Watz M, Samuelsson G, Shutova T. Dynamic pH-induced conformational changes of the PsbO protein in the fluctuating acidity of the thylakoid lumen. Physiol Plant. 2019;166:288–299. doi: 10.1111/ppl.12948. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhan J, Chen Y, Yang M, He C, Ge F, Wang Q. Effects of phosphorylation of β subunits of phycocyanins on state transition in the model cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015;56:1997–2013. doi: 10.1093/pcp/pcv118. [DOI] [PubMed] [Google Scholar]
- Cheregi O, Wagner R, Funk C. Insights into the cyanobacterial Deg/HtrA proteases. Front Plant Sci. 2016;7:694. doi: 10.3389/fpls.2016.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-F, Chen Y-H, Roncel M, Dilbeck PL, Huang J-Y, Ke S-C, Ortega JM, Burnap RL, Chu H-A. Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b559 in photosystem II. Biochim Biophys Acta - Bioenerg. 2013;1827:507–519. doi: 10.1016/j.bbabio.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Chukhutsina V, Bersanini L, Aro E-M, van Amerongen H. Cyanobacterial light-harvesting phycobilisomes uncouple from photosystem I during dark-to-light transitions. Sci Rep. 2015;5:14193. doi: 10.1038/srep14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus RJ. FTIR studies of metal ligands, networks of hydrogen bonds, and water molecules near the active site Mn4CaO5 cluster in photosystem II. Biochim Biophys Acta - Bioenerg. 2015;1847:19–34. doi: 10.1016/j.bbabio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Deligiannakis Y, Petrouleas V, Diner BA. Binding of carboxylate anions at the non-heme Fe(II) of PS II. I. Effects on the QA−Fe2+ and QAFe3+ EPR spectra and the redox properties of the iron. Biochim Biophys Acta - Bioenerg. 1994;1188:260–270. doi: 10.1016/0005-2728(94)90044-2. [DOI] [Google Scholar]
- Fan M, Li M, Liu Z, Cao P, Pan X, Zhang H, Zhao X, Zhang J, Chang W. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat Struct Mol Biol. 2015;22:729–735. doi: 10.1038/nsmb.3068. [DOI] [PubMed] [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- Fristedt R, Vener AV. High light induced disassembly of photosystem II supercomplexes in Arabidopsis requires STN7-dependent phosphorylation of CP29. PLoS ONE. 2011;6:e24565. doi: 10.1371/journal.pone.0024565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabdulkhakov A, Guskov A, Broser M, Kern J, Muh F, Saenger W, Zouni A. Probing the accessibility of the Mn4Ca cluster in photosystem II: channels calculation, noble gas derivatization, and cocrystallization with DMSO. Structure. 2009;17:1223–1234. doi: 10.1016/j.str.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Gerland L, Friedrich D, Hopf L, Donovan EJ, Wallmann A, Erdmann N, Diehl A, Bommer M, Buzar K, Ibrahim M, Schmieder P, Dobbek H, Zouni A, Bondar A, Dau H, Oschkinat H. pH-dependent protonation of surface carboxylate groups in PsbO enables local buffering and triggers structural changes. ChemBioChem. 2020;21:1597–1604. doi: 10.1002/cbic.201900739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Jain A, Ebersberger I, Teige M. An evolutionary view on thylakoid protein phosphorylation uncovers novel phosphorylation hotspots with potential functional implications. J Exp Bot. 2016;67:3883–3896. doi: 10.1093/jxb/erw164. [DOI] [PubMed] [Google Scholar]
- He WZ, Newell WR, Haris PI, Chapman D, Barber J. Protein secondary structure of the isolated photosystem II reaction center and conformational changes studied by Fourier transform infrared spectroscopy. Biochemistry. 1991;30:4552–4559. doi: 10.1021/bi00232a027. [DOI] [PubMed] [Google Scholar]
- Heinz S, Liauw P, Nickelsen J, Nowaczyk M. Analysis of photosystem II biogenesis in cyanobacteria. Biochim Biophys Acta - Bioenerg. 2016;1857:274–287. doi: 10.1016/j.bbabio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Henmi T, Miyao M, Yamamoto Y (2004) Release and Reactive-Oxygen-Mediated Damage of the Oxygen-Evolving Complex Subunits of PSII during Photoinhibition. Plant Cell Physiol 45:243–250. 10.1093/pcp/pch027 [DOI] [PubMed]
- Huang G, Xiao Y, Pi X, Zhao L, Zhu Q, Wang W, Kuang T, Han G, Sui S-F, Shen J-R. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc Natl Acad Sci. 2021;118:1–9. doi: 10.1073/pnas.2018053118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesgen PF, Schuhmann H, Adamska I. Deg/HtrA proteases as components of a network for photosystem II quality control in chloroplasts and cyanobacteria. Res Microbiol. 2009;160:726–732. doi: 10.1016/j.resmic.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Hussein R, Ibrahim M, Bhowmick A, Simon PS, Chatterjee R, Lassalle L, Doyle M, Bogacz I, Kim I-S, Cheah MH, Gul S, de Lichtenberg C, Chernev P, Pham CC, Young ID, Carbajo S, Fuller FD, Alonso-Mori R, Batyuk A, Sutherlin KD, Brewster AS, Bolotovsky R, Mendez D, Holton JM, Moriarty NW, Adams PD, Bergmann U, Sauter NK, Dobbek H, Messinger J, Zouni A, Kern J, Yachandra VK, Yano J. Structural dynamics in the water and proton channels of photosystem II during the S2 to S3 transition. Nat Commun. 2021;12:6531. doi: 10.1038/s41467-021-26781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido K, Kakiuchi S, Uno C, Nishimura T, Fukao Y, Noguchi T, Sato F, Ifuku K. The conserved His-144 in the PsbP protein is important for the interaction between the PsbP N-terminus and the Cyt b559 subunit of photosystem II. J Biol Chem. 2012;287:26377–26387. doi: 10.1074/jbc.M112.385286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido K, Nield J, Fukao Y, Nishimura T, Sato F, Ifuku K. Cross-linking evidence for multiple interactions of the PsbP and PsbQ proteins in a higher plant photosystem II supercomplex. J Biol Chem. 2014;289:20150–20157. doi: 10.1074/jbc.M114.574822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Nakatsu T, Shimamoto R, Yamamoto Y, Ishihara S, Kato H, Sato F. Structure and function of the PsbP protein of photosystem II from higher plants. Photosynth Res. 2005;84:251–255. doi: 10.1007/s11120-004-7160-3. [DOI] [PubMed] [Google Scholar]
- Ifuku K, Yamamoto Y, Ono T, Ishihara S, Sato F. PsbP protein, but not PsbQ protein, is essential for the regulation and stabilization of photosystem II in higher plants. Plant Physiol. 2005;139:1175–1184. doi: 10.1104/pp.105.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Noguchi T (2016) Structural coupling of extrinsic proteins with the oxygen-evolving center in photosystem II. Front Plant Sci 710.3389/fpls.2016.00084 [DOI] [PMC free article] [PubMed]
- Iwai M, Yokono M, Inada N, Minagawa J. Live-cell imaging of photosystem II antenna dissociation during state transitions. Proc Natl Acad Sci. 2010;107:2337–2342. doi: 10.1073/pnas.0908808107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GN, Rutherford AW, Krieger A. A change in the midpoint potential of the quinone QA in photosystem II associated with photoactivation of oxygen evolution. Biochim Biophys Acta - Bioenerg. 1995;1229:202–207. doi: 10.1016/0005-2728(95)00003-2. [DOI] [Google Scholar]
- Kale R, Hebert AE, Frankel LK, Sallans L, Bricker TM, Pospíšil P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of photosystem II. Proc Natl Acad Sci. 2017;114:2988–2993. doi: 10.1073/pnas.1618922114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargul J, Maghlaoui K, Murray JW, Deak Z, Boussac A, William Rutherford A, Vass I, Barber J. Purification, crystallization and X-ray diffraction analyses of the T. elongatus PSII core dimer with strontium replacing calcium in the oxygen-evolving complex. Biochim Biophys Acta - Bioenerg. 2007;1767:404–413. doi: 10.1016/j.bbabio.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Kato Y, Noguchi T. Long-range interaction between the Mn4CaO5 cluster and the non-heme iron center in photosystem II as revealed by FTIR spectroelectrochemistry. Biochemistry. 2014;53:4914–4923. doi: 10.1021/bi500549b. [DOI] [PubMed] [Google Scholar]
- Kato Y, Noguchi T. Effects of stromal and lumenal side perturbations on the redox potential of the primary quinone electron acceptor QA in photosystem II. Biochemistry. 2021;60:3697–3706. doi: 10.1021/acs.biochem.1c00624. [DOI] [PubMed] [Google Scholar]
- Kato Y, Shibamoto T, Yamamoto S, Watanabe T, Ishida N, Sugiura M, Rappaport F, Boussac A. Influence of the PsbA1/PsbA3, Ca2+/Sr2+ and Cl−/Br− exchanges on the redox potential of the primary quinone QA in photosystem II from Thermosynechococcus elongatus as revealed by spectroelectrochemistry. Biochim Biophys Acta - Bioenerg. 2012;1817:1998–2004. doi: 10.1016/j.bbabio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Kato Y, Ohira A, Nagao R, Noguchi T. Does the water-oxidizing Mn4CaO5 cluster regulate the redox potential of the primary quinone electron acceptor QA in photosystem II? A study by Fourier transform infrared spectroelectrochemistry. Biochim Biophys Acta - Bioenerg. 2019;1860:148082. doi: 10.1016/j.bbabio.2019.148082. [DOI] [PubMed] [Google Scholar]
- Kettunen R, Tyystjarvi E, Aro EM. Degradation pattern of photosystem II reaction center protein D1 in intact leaves (the major photoinhibition-induced cleavage site in D1 polypeptide is located amino terminally of the DE loop) Plant Physiol. 1996;111:1183–1190. doi: 10.1104/pp.111.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komayama K, Khatoon M, Takenaka D, Horie J, Yamashita A, Yoshioka M, Nakayama Y, Yoshida M, Ohira S, Morita N, Velitchkova M, Enami I, Yamamoto Y. Quality control of photosystem II: cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids. Biochim Biophys Acta - Bioenerg. 2007;1767:838–846. doi: 10.1016/j.bbabio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Koua FHM. Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 cluster site and the possible long-range interactions. Biomolecules. 2019;9:371. doi: 10.3390/biom9080371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koua FHM, Umena Y, Kawakami K, Shen J-R. Structure of Sr-substituted photosystem II at 2.1 Å resolution and its implications in the mechanism of water oxidation. Proc Natl Acad Sci. 2013;110:3889–3894. doi: 10.1073/pnas.1219922110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Rutherford AW. Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: relevance to photodamage and phytotoxicity. Biochemistry. 1998;37:17339–17344. doi: 10.1021/bi9822628. [DOI] [PubMed] [Google Scholar]
- Krishnan-Schmieden M, Konold PE, Kennis JTM, Pandit A. The molecular pH-response mechanism of the plant light-stress sensor PsbS. Nat Commun. 2021;12:2291. doi: 10.1038/s41467-021-22530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik N, Kutý M, Řeha D. The study of conformational changes in photosystem II during a charge separation. J Mol Model. 2020;26:75. doi: 10.1007/s00894-020-4332-9. [DOI] [PubMed] [Google Scholar]
- Kupitz C, Basu S, Grotjohann I, Fromme R, Zatsepin NA, Rendek KN, Hunter MS, Shoeman RL, White TA, Wang D, James D, Yang JH, Cobb DE, Reeder B, Sierra RG, Liu H, Barty A, Aquila AL, Deponte D, Kirian RA, Bari S, Bergkamp JJ, Beyerlein KR, Bogan MJ, Caleman C, Chao TC, Conrad CE, Davis KM, Fleckenstein H, Galli L, Hau-Riege SP, Kassemeyer S, Laksmono H, Liang M, Lomb L, Marchesini S, Martin AV, Messerschmidt M, Milathianaki D, Nass K, Ros A, Roy-Chowdhury S, Schmidt K, Seibert M, Steinbrener J, Stellato F, Yan L, Yoon C, Moore TA, Moore AL, Pushkar Y, Williams GJ, Boutet S, Doak RB, Weierstall U, Frank M, Chapman HN, Spence JCH, Fromme P. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature. 2014;513:261–265. doi: 10.1038/nature13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem. 2004;279:22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- Liguori N, Campos SRR, Baptista AM, Croce R. Molecular anatomy of plant photoprotective switches: the sensitivity of PsbS to the environment, residue by residue. J Phys Chem Lett. 2019;10:1737–1742. doi: 10.1021/acs.jpclett.9b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Huang RYC, Chen J, Gross ML, Pakrasi HB. Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates. Proc Natl Acad Sci. 2011;108:18536–18541. doi: 10.1073/pnas.1111597108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- Lupínková L, Komenda J. Oxidative modifications of the photosystem II D1 protein by reactive oxygen species: from isolated protein to cyanobacterial cells. Photochem Photobiol. 2007;79:152–162. doi: 10.1111/j.1751-1097.2004.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Mabbitt PD, Wilbanks SM, Eaton-Rye JJ. Structure and function of the hydrophilic photosystem II assembly proteins: Psb27, Psb28 and Ycf48. Plant Physiol Biochem. 2014;81:96–107. doi: 10.1016/j.plaphy.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Manna P, Schlau-Cohen GS. Photoprotective conformational dynamics of photosynthetic light-harvesting proteins. Biochim Biophys Acta - Bioenerg. 2022;1863:148543. doi: 10.1016/j.bbabio.2022.148543. [DOI] [PubMed] [Google Scholar]
- Miyao M, Murata N. The Cl− effect on photosynthetic oxygen evolution: interaction of Cl− with 18-kDa, 24-kDa and 33-kDa proteins. FEBS Lett. 1985;180:303–308. doi: 10.1016/0014-5793(85)81091-7. [DOI] [Google Scholar]
- Muh F, Zouni A. Structural basis of light-harvesting in the photosystem II core complex. Protein Sci. 2020;29:1090–1119. doi: 10.1002/pro.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R, Tomo T, Noguchi T. Effects of extrinsic proteins on the protein conformation of the oxygen-evolving center in cyanobacterial photosystem II as revealed by Fourier transform infrared spectroscopy. Biochemistry. 2015;54:2022–2031. doi: 10.1021/acs.biochem.5b00053. [DOI] [PubMed] [Google Scholar]
- Nagao R, Kato K, Suzuki T, Ifuku K, Uchiyama I, Kashino Y, Dohmae N, Akimoto S, Shen J-R, Miyazaki N, Akita F. Structural basis for energy harvesting and dissipation in a diatom PSII–FCPII supercomplex. Nat Plants. 2019;5:890–901. doi: 10.1038/s41477-019-0477-x. [DOI] [PubMed] [Google Scholar]
- Nelson N, Yocum CF. Structure and function of photosystems I and II. Annu Rev Plant Biol. 2006;57:521–565. doi: 10.1146/annurev.arplant.57.032905.105350. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nagao R, Noguchi T, Nield J, Sato F, Ifuku K. The N-terminal sequence of the extrinsic PsbP protein modulates the redox potential of Cyt b559 in photosystem II. Sci Rep. 2016;6:21490. doi: 10.1038/srep21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Sato F, Ifuku K. In vivo system for analyzing the function of the PsbP protein using Chlamydomonas reinhardtii. Photosynth Res. 2017;133:117–127. doi: 10.1007/s11120-017-0370-2. [DOI] [PubMed] [Google Scholar]
- Offenbacher AR, Polander BC, Barry BA. An intrinsically disordered photosystem II subunit, PsbO, provides a structural template and a sensor of the hydrogen-bonding network in photosynthetic water oxidation. J Biol Chem. 2013;288:29056–29068. doi: 10.1074/jbc.M113.487561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Xiaowei, Li Mei, Wan Tao, Wang Longfei, Jia Chenjun, Hou Zhiqiang, Zhao Xuelin, Zhang Jiping, Chang Wenrui. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nature Structural & Molecular Biology. 2011;18(3):309–315. doi: 10.1038/nsmb.2008. [DOI] [PubMed] [Google Scholar]
- Papadatos S, Charalambous AC, Daskalakis V. A pathway for protective quenching in antenna proteins of photosystem II. Sci Rep. 2017;7:2523. doi: 10.1038/s41598-017-02892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthiyaveetil S, Kirchhoff H. A phosphorylation map of the photosystem II supercomplex C2S2M2. Front Plant Sci. 2013;4:459. doi: 10.3389/fpls.2013.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M, Rantala S, Aro E-M. Composition, phosphorylation and dynamic organization of photosynthetic protein complexes in plant thylakoid membrane. Photochem Photobiol Sci. 2020;19:604–619. doi: 10.1039/D0PP00025F. [DOI] [PubMed] [Google Scholar]
- Rashid A, Carpentier R. The 16 and 23 kDa extrinsic polypeptides and the associated Ca2+ and Cl– modify atrazine interaction with the photosystem II core complex. Photosynth Res. 1990;24:221–227. doi: 10.1007/BF00032309. [DOI] [PubMed] [Google Scholar]
- Redekop P, Rothhausen N, Rothhausen N, Melzer M, Mosebach L, Dülger E, Bovdilova A, Caffarri S, Hippler M, Jahns P. PsbS contributes to photoprotection in Chlamydomonas reinhardtii independently of energy dissipation. Biochim Biophys Acta - Bioenerg. 2020;1861:148183. doi: 10.1016/j.bbabio.2020.148183. [DOI] [PubMed] [Google Scholar]
- Roose JL, Frankel LK, Bricker TM. Documentation of significant electron transport defects on the reducing side of photosystem II upon removal of the PsbP and PsbQ extrinsic proteins. Biochemistry. 2010;49:36–41. doi: 10.1021/bi9017818. [DOI] [PubMed] [Google Scholar]
- Ruban AV. Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP. The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta - Bioenerg. 2012;1817:167–181. doi: 10.1016/j.bbabio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Semin BK, Davletshina LN, Mamedov MD. Effect of different methods of Ca2+ extraction from PSII oxygen-evolving complex on the QA− oxidation kinetics. Photosynth Res. 2018;136:83–91. doi: 10.1007/s11120-017-0441-4. [DOI] [PubMed] [Google Scholar]
- Shen JR, Inoue Y. Binding and functional properties of two new extrinsic components, cytochrome c-550 and a 12-kDa protein, in cyanobacterial photosystem II. Biochemistry. 1993;32:1825–1832. doi: 10.1021/bi00058a017. [DOI] [PubMed] [Google Scholar]
- Shen L, Huang Z, Chang S, Wang W, Wang J, Kuang T, Han G, Shen J-R, Zhang X. Structure of a C2S2M2N2-type PSII–LHCII supercomplex from the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci. 2019;116:21246–21255. doi: 10.1073/pnas.1912462116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Xin, Watanabe Akimasa, Li Anjie, Kim Eunchul, Song Chihong, Murata Kazuyoshi, Song Danfeng, Minagawa Jun, Liu Zhenfeng. Structural insight into light harvesting for photosystem II in green algae. Nature Plants. 2019;5(12):1320–1330. doi: 10.1038/s41477-019-0543-4. [DOI] [PubMed] [Google Scholar]
- Shukshina AK, Terentyev VV. Involvement of carbonic anhydrase CAH3 in the structural and functional stabilization of the water-oxidizing complex of photosystem II from Chlamydomonas reinhardtii. Biochem Mosc. 2021;86:867–877. doi: 10.1134/S0006297921070075. [DOI] [PubMed] [Google Scholar]
- Shutova T, Irrgang K-D, Shubin V, Klimov VV, Renger G. Analysis of pH-Induced structural changes of the isolated extrinsic 33 kilodalton protein of photosystem II. Biochemistry. 1997;36:6350–6358. doi: 10.1021/bi963115h. [DOI] [PubMed] [Google Scholar]
- Shutova T, Klimov VV, Andersson B, Samuelsson G. A cluster of carboxylic groups in PsbO protein is involved in proton transfer from the water oxidizing complex of photosystem II. Biochim Biophys Acta. 2007;1767:434–440. doi: 10.1016/j.bbabio.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Shutova T, Kenneweg H, Buchta J, Nikitina J, Terentyev V, Chernyshov S, Andersson B, Allakhverdiev SI, Klimov VV, Dau H, Junge W, Samuelsson G. The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J. 2008;27:782–791. doi: 10.1038/emboj.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ohta H, Enami I. Cross-reconstitution of the extrinsic proteins and photosystem II complexes from Chlamydomonas reinhardtii and Spinacia oleracea. Photosynth Res. 2005;84:239–244. doi: 10.1007/s11120-004-7760-y. [DOI] [PubMed] [Google Scholar]
- Terentyev VV, Shukshina AK, Shitov AV. Carbonic anhydrase CAH3 supports the activity of photosystem II under increased pH. Biochim Biophys Acta - Bioenerg. 2019;1860:582–590. doi: 10.1016/j.bbabio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Terentyev VV, Shukshina AK, Ashikhmin AA, Tikhonov KG, Shitov AV. The main structural and functional characteristics of photosystem-II-enriched membranes isolated from wild type and cia3 mutant Chlamydomonas reinhardtii. Life. 2020;10:63. doi: 10.3390/life10050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Nurmi M, Kangasjärvi S, Aro E-M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim Biophys Acta - Bioenerg. 2008;1777:1432–1437. doi: 10.1016/j.bbabio.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tokano T, Kato Y, Sugiyama S, Uchihashi T, Noguchi T. Structural dynamics of a protein domain relevant to the water-oxidizing complex in photosystem II as visualized by high-speed atomic force microscopy. J Phys Chem B. 2020;124:5847–5857. doi: 10.1021/acs.jpcb.0c03892. [DOI] [PubMed] [Google Scholar]
- Tomita M, Ifuku K, Sato F, Noguchi T. FTIR evidence that the PsbP extrinsic protein induces protein conformational changes around the oxygen-evolving Mn cluster in photosystem II. Biochemistry. 2009 doi: 10.1021/bi9006308. [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen J-R, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- Uno C, Nagao R, Suzuki H, Tomo T, Noguchi T. Structural coupling of extrinsic proteins with the oxygen-evolving center in red algal photosystem II As revealed by light-induced FTIR difference spectroscopy. Biochemistry. 2013;52:5705–5707. doi: 10.1021/bi4009787. [DOI] [PubMed] [Google Scholar]
- Villarejo A, Shutova T, Moskvin O, Forssén M, Klimov VV, Samuelsson G. A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J. 2002;21:1930–1938. doi: 10.1093/emboj/21.8.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature. 2016;534:69–74. doi: 10.1038/nature18020. [DOI] [PubMed] [Google Scholar]
- Weisz DA, Liu H, Zhang H, Thangapandian S, Tajkhorshid E, Gross M, Pakrasi HB. Mass spectrometry-based cross-linking study shows that the Psb28 protein binds to cytochrome b 559 in photosystem II. Proc Natl Acad Sci. 2017;114:2224–2229. doi: 10.1073/pnas.1620360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, Takenaka D, Yamashita A, Nijo N, Inagawa K, Morita N, Sasaki T, Yamamoto Y. Quality control of photosystem II: impact of light and heat stresses. Photosynth Res. 2008;98:589–608. doi: 10.1007/s11120-008-9372-4. [DOI] [PubMed] [Google Scholar]
- Yamane Y, Kashino Y, Koike H, Satoh K. Effects of high temperatures on the photosynthetic systems in spinach: oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth Res. 1998;57:51–59. doi: 10.1023/A:1006019102619. [DOI] [Google Scholar]
- Yi X, Hargett SR, Liu H, Frankel LK, Bricker TM. The PsbP protein is required for photosystem II complex assembly/stability and photoautotrophy in Arabidopsis thaliana. J Biol Chem. 2007;282:24833–24841. doi: 10.1074/jbc.M705011200. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Uchida S, Mori H, Komayama K, Ohira S, Morita N, Nakanishi T, Yamamoto Y. Quality control of photosystem II. J Biol Chem. 2006;281:21660–21669. doi: 10.1074/jbc.M602896200. [DOI] [PubMed] [Google Scholar]
- Young ID, Ibrahim M, Chatterjee R, Gul S, Fuller FD, Koroidov S, Brewster AS, Tran R, Alonso-Mori R, Kroll T, Michels-Clark T, Laksmono H, Sierra RG, Stan CA, Hussein R, Zhang M, Douthit L, Kubin M, de Lichtenberg C, Vo Pham L, Nilsson H, Cheah MH, Shevela D, Saracini C, Bean MA, Seuffert I, Sokaras D, Weng T-C, Pastor E, Weninger C, Fransson T, Lassalle L, Bräuer P, Aller P, Docker PT, Andi B, Orville AM, Glownia JM, Nelson S, Sikorski M, Zhu D, Hunter MS, Lane TJ, Aquila A, Koglin JE, Robinson J, Liang M, Boutet S, Lyubimov AY, Uervirojnangkoorn M, Moriarty NW, Liebschner D, Afonine PV, Waterman DG, Evans G, Wernet P, Dobbek H, Weis WI, Brunger AT, Zwart PH, Adams PD, Zouni A, Messinger J, Bergmann U, Sauter NK, Kern J, Yachandra VK, Yano J. Structure of photosystem II and substrate binding at room temperature. Nature. 2016;540:453–457. doi: 10.1038/nature20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabret J, Bohn S, Schuller SK, Arnolds O, Möller M, Meier-Credo J, Liauw P, Chan A, Tajkhorshid E, Langer JD, Stoll R, Krieger-Liszkay A, Engel BD, Rudack T, Schuller JM, Nowaczyk MM. Structural insights into photosystem II assembly. Nat Plants. 2021;7:524–538. doi: 10.1038/s41477-021-00895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yamamoto Y, Ishikawa Y, Zhang W, Fischer G, Wydrzynski T. A Fourier transform infrared spectroscopic study of the effects of hydrogen peroxide and high light on protein conformations of photosystem II. Photosynth Res. 1997;52:215–223. doi: 10.1023/A:1005805510602. [DOI] [Google Scholar]
- Zhang J, Ma J, Liu D, Qin S, Sun S, Zhao J, Sui S-F. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature. 2017;551:57–63. doi: 10.1038/nature24278. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zheng Z, Li X, Wang G, Zhang K, Wei P, Zhao J, Gao N. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat Commun. 2021;12:5497. doi: 10.1038/s41467-021-25813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]