Abstract
Microbial rhodopsins are the family of retinal-containing proteins that perform primarily the light-driven transmembrane ion transport and sensory functions. They are widely distributed in nature and can be used for optogenetic control of the cellular activities by light. Functioning of microbial rhodopsins results in generation of the transmembrane electric potential in response to a flash that can be measured by direct time-resolved electrometry. This method was developed by L. Drachev and his colleagues at the Belozersky Institute and successfully applied in the functional studies of microbial rhodopsins. First measurements were performed using bacteriorhodopsin from Halobacterium salinarum—the prototype member of the microbial retinal protein family. Later, direct electrometric studies were conducted with proteorhodopsin from Exiguobacterium sibiricum (ESR), the sodium pump from Dokdonia, and other proteins. They allowed detailed characterization of the charge transfer steps during the photocycle of microbial rhodopsins and provided new insights for profound understanding of their mechanism of action.
Keywords: Retinal protein, Proton transport, Photocycle, Photoelectric potential generation, Direct time-resolved electrometry
Introduction
Microbial rhodopsins are widespread membrane proteins that capture light and use its energy for transmembrane transport or signal transduction (Govorunova et al. 2017; Gushchin and Gordeliy 2018; Kandori 2020; Brown 2022). Their molecules are comprised of an opsin protein which contains seven transmembrane segments and retinal chromophore connected with a conservative lysine residue via the Schiff base linkage. Upon light absorption, retinal changes its configuration from all-trans to 13-cis. Its return to the initial state is accompanied by a series of conformational changes in the protein between several intermediates with specific spectroscopic and structural characteristics (photocycle). During the photocycle, absorbed light energy is utilized, for example, to perform ion transfers in the case of ion pumps. However, many details of this process are still unknown.
Along with rapidly developing methods of structural analysis, which mainly provide valuable static information, it is extremely important to use functional approaches with time resolution in the range from nanoseconds to hundreds of milliseconds which allow to establish the molecular mechanism of functioning of these proteins. In this regard, the direct electrometric method provides unique information that allows tracking movement of a proton inside a protein in real time during one catalytic turnover which is practically impossible using other approaches.
In this mini-review, we discuss the foundations of the method and recently published experimental results obtained using the time-resolved direct electrometry in the studies of the internal charge transfer and coupled energy conversion during the photocycle of microbial rhodopsins from different sources. Primarily we focus on ion pumps reconstructed in proteoliposomes. Alternative approaches such as immobilization of oriented membranes in gels (Dér and Keszthelyi 2001), expression of retinal proteins in oocytes (Vogt et al. 2013), and other cells that are applied in studies of channelrhodopsins (Berndt et al. 2016; Deisseroth and Hegemann 2017; Govorunova et al. 2021) are beyond the scope of this review.
Method of the time-resolved direct electrometry (capacitive potentiometry)
Direct electrometric measurement of the kinetics of membrane potential generation is based on a method developed by L. Drachev and his colleagues (Drachev et al. 1974, 1979). The studied protein is incorporated into the membrane of proteoliposomes which are then associated with the measuring collodion membrane between the compartments of the cell filled with buffer (Fig. 1A). The collodion film is formed from nitrocellulose solution in amyl acetate on a water surface. The film is dried, impregnated with solution of lecithin in decane (~ 100 mg/ml), and placed in a dismountable Teflon chamber. Chamber compartments are filled with 25 mM HEPES buffer (pH 7.5) containing 20–30 mM MgSO4. Proteoliposomes are added to the sample compartment and adhere spontaneously to the film during ∼2-h incubation at room temperature.
Fig. 1.
A Setup of the electrometric measurements. The light impulse from Nd:YAG laser, which is focused onto the collodium membrane in the measuring cell, initiates the photocycle reaction. Generation of the membrane potential is detected by two electrodes, one of which is connected to a common ground, while the other is connected via the operational amplifier to the digital oscilloscope and computer. B Kinetics of light-induced membrane electric potential generation, ∆Ψ, in proteoliposomes containing bacteriorhodopsin (BR) at pH 7.5
The measuring membrane should be sufficiently thin and possess large electric capacity (about 5 nF) and high resistance (2–3 GΩ) to detect rapid charge translocation events with time resolution of up to ~ 100 ns. A pulsed Nd:YAG laser (YG-481, Quantel, λ = 532 nm, pulse half-width 12 ns, flash energy up to 40 mJ) is used as a flash source to turn on the registration system and photocycle simultaneously in all protein molecules. In response to a flash, ∆Ψ across the proteoliposome membrane is generated. Its value is proportionally divided by the measuring membrane and, thus, can be detected by Ag/AgCl electrodes immersed in the buffer solution at different sides of the membrane. Typically, Ag/AgCl electrodes are used for measurements due to their stability, compactness, and easy manufacturing. ΔΨ generation during a single photocycle of retinal proteins usually develops within tens to hundreds of milliseconds and then fades spontaneously with a time constant of several seconds as a result of passive proton leakage through the membrane.
The method of direct electrometry, referred also as capacitive potentiometry (Läuger 1991), was originally applied for the studies of bacteriorhodopsin (Drachev et al. 1974, 1981; Kalaidzidis et al. 2001). Later, this electrometric technique was successfully used to study bacterial reaction centers (Dracheva et al. 1988; Kaminskaya et al. 1986), chromatophores (Drachev et al. 1986, 1989), pigment-protein complexes of photosystems 1 and 2 (Mamedov et al. 1996; Vassiliev et al. 1997), and cytochrome oxidase (Siletsky, 2013; Siletsky et al. 1999, 2004, 2010, 2017, 2021a; Zaslavsky et al. 1995).
Bacteriorhodopsin—the first retinal protein studied by direct electrometry
Bacteriorhodopsin (BR) was discovered 50 years ago in extremely halophilic Halobacterium salinarum (Oesterhelt and Stoeckenius 1971) and became the first protein which was studied using the direct electrometric method long before its three-dimensional structure was resolved with atomic resolution (Drachev et al. 1974). The obtained results and the conclusions about the mechanism of operation of this proton pump were later successfully confirmed by numerous structural and functional studies (Luecke et al. 1999; Balashov 2000).
Measurements of the flash-induced photoelectric response of the BR-containing proteoliposomes revealed the presence of at least four components, the first three of which are associated with the charge transfer inside BR molecules (Fig. 1B). The fastest component represents the formation of a small potential difference in the direction opposite to that of other components and to the direction of potential generation induced by continuous light. Its characteristic time is < 100 ns that is less than the time resolution of the measuring system. This time constant refers to the formation of the K state with an absorption maximum of 590 nm which reflects the electrogenic displacement of the charged Schiff base (SB) caused by a change in the retinal conformation (all-trans → 13-cis) during the light absorption. In addition, a small electrogenic phase in the same direction can be resolved (τ ~1.8 μs). It corresponds to the K → L transition which reflects relaxation of the retinal microenvironment after isomerization. Taking together, these events result in a negative phase of ΔΨ generation with amplitude about 5% of the total amplitude of the photoelectric response (Kalaidzidis et al. 2001).
The characteristic time of the next electrogenic component is 25–70 μs. The direction of the electric vector turns out to be the same as in response to the continuous illumination of the sample. This phase is associated with the component of spectral changes during formation of the M state (412 nm) that corresponds to the transfer of a proton from the SB to the acceptor residue Asp85 and the simultaneous release of a proton to the extramembrane space from the Glu194 and Glu204 residues (proton releasing group, PRG). The amplitude of this electrogenic phase is ~ 20% of the total amplitude of the positive part of the photoelectric response and of the total dielectric thickness of the membrane. It corresponds to the displacement of the positive charge perpendicular to the membrane plane by ~ 8 Å including the proton transfer to Asp85 at a distance of ~ 4 Å, conformational change of Arg82 (Dickopf and Heyn 1997; Luecke et al. 2000), and the transfer of protons from PRG to the extracellular side of the membrane at another ~ 4 Å (Kaulen 2000; Kalaidzidis et al. 2001).
The next phase has the same direction and the characteristic time of 4–12 ms that approximately corresponds to the M state decay and return to the original state of the BR with an absorption maximum at 570 nm (Kaulen 2000; Kalaidzidis et al. 2001). This phase corresponds to the transfer of H+ to the SB from the bulk and involves (i) reprotonation of the SB from the internal donor Asp96 in M → N transition and (ii) subsequent reprotonation of Asp96 from the bulk (N → O transition). Deprotonation of the donor due to transient pKa decrease becomes possible due to the passage of water molecules into the vicinity of Asp96 through temporary contact with the bulk formed in the M state as a result of conformational shifts of the corresponding alpha helices (Lanyi and Luecke 2001). Reprotonation of the donor is accompanied by the closure of the contact and occurs possibly as a result of the movement of the hydroxyl ion when the deprotonated water molecule exits the vicinity of Asp96 into the bulk. It should be noted that it is extremely difficult to distinguish the electrogenic movement of a proton from the oppositely directed movement of a hydroxide ion. In addition, the millisecond electrogenic phase includes (iii) the final stage of the photocycle, namely, the electrogenic transfer of a proton from Asp85 to PRG, return of Arg82 side chain to initial conformation, and return of the retinal to its background conformation (O → BR). Overall, the amplitude of the electrogenic events listed above during the transitions M → N → O → BR is ~ 80% that is four times greater than that of the phase with τ 25–70 μs (Fig. 1B). The three stages of proton transfer that constitute the slow electrogenic phase reflect the translocation of a proton at about ~ 10 Å each. Thus, the distances of ~ 8 and 30–33 Å correspond to the microsecond and millisecond phases, respectively. At uniform value of intraprotein dielectric constant (ε), this result is in good agreement with the phase amplitude ratio of 1 to 4 (Kalaidzidis et al. 2001). The final negative phase (~ 500 ms, not shown in Fig. 1B) reflects passive leakage (discharge) through the membrane.
ESR, a retinal protein from Exiguobacterium sibiricum
ESR is a light-driven proton pump from the Gram-positive psychrotrophic bacterium Exiguobacterium sibiricum that belongs to the proteorhodopsin (PR) family (Petrovskaya et al. 2010). In contrast to other proton pumping microbial rhodopsins, ESR contains Lys96 at the position corresponding to internal proton donor to the SB (Fig. 2). In BR and PR, this position is occupied with carboxylic residues Asp or Glu, correspondingly. We have shown that the K96A substitution resulted in dramatic decrease in the rate of the SB reprotonation and strong pH dependence of the decay of the M intermediate in the mutant (Balashov et al. 2013). The obtained data clearly demonstrated that Lys96 in ESR acts as a proton donor to the SB. Other structural and functional features of ESR are common to proteorhodopsins including close interaction of the His57 residue with the primary proton acceptor Asp85 and absence of the PRG that explains late proton release in this protein (Balashov et al. 2012; Gushchin et al. 2013).
Fig. 2.
Structural comparison of the SB-proximal regions in BR (A) and ESR (B) (PDB entries 1C3W (Luecke et al. 1999) and 4HYJ (Gushchin et al. 2013))
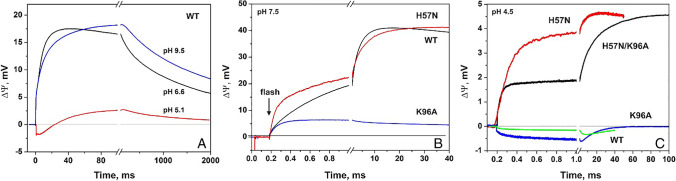
To elucidate the mechanism of ESR functioning, we performed extensive characterization of the proton transfer reactions in this protein with the use of the direct electrometry approach. In the wild type ESR, electrical potential change ΔΨ produced by a single flash increases up to 30 ms and then slowly decreases due to the passive discharge of the membrane and returns to the baseline during several seconds (Fig. 3A). The overall amplitude of the response was comparable to that of BR; however, the analysis of the kinetic components revealed substantial differences in their time constants and relative contribution (Siletsky et al. 2016). The first negative electrogenic phase of ΔΨ generation which corresponds to the formation of the K and L states was detected in ESR only at pH 5.1. At neutral and alkaline pH values, it is obscured by the fast (~ 3 μs) positive phases which correspond to the M state formation (deprotonation of the SB). Their relative contribution (6%) is three times smaller than in BR due to the absence of the early proton release to the bulk.
Fig. 3.
Kinetics of light-induced membrane electric potential generation, ∆Ψ, in proteoliposomes containing: A Wild type ESR at indicated pH. B Wild type ESR, K96A, and H57N mutants at pH 7.5. The ESR and H57N traces are normalized by the total amplitude. C Wild type ESR, H57N, H57N/K96A, and K96A mutants at pH 4.5. The H57N and H57N/K96A traces are normalized by the total amplitude. Plots are from Siletsky et al. (2016, 2021a, b), with modifications
The major contribution to ΔΨ generation in ESR (72%) is provided by two millisecond electrogenic components (~0.6 and ~3.4 ms at pH 7.5) which correspond to proton transfer from the bulk to the SB. They correlate with the decay of the M state which proceeds in transitions M ↔ N1 → N2/O. The slowest electrogenic component (~18.4 ms, 22%) reflects recovery of the initial state of ESR which is accompanied by deprotonation of Asp85 and proton release to the bulk.
At pH 5.1, the photoelectric response of ESR is substantially inhibited (Fig. 3A) due to partial protonation of the primary acceptor, Asp85 (Balashov et al. 2012). Increase of pH from 6.6 to 9.5 results in deceleration of the M state decay and corresponding kinetic components of the photoelectric response in ESR (Fig. 3A). This is explained by the fact that the proton donor Lys96 is initially unprotonated and proton uptake from the bulk takes place before reprotonation of the SB (Balashov et al. 2013). Interestingly, at pH 8.4 additional electrogenic component with τ ~ 0.23 ms was resolved with relative contribution 11% which does not correspond to any absorption changes. We suggested that this component reflects the proton transfer from the bulk to Lys96 (protonation of the donor) while the subsequent phase with ~ 4.5 ms corresponds to the proton transfer from Lys96 to the SB (Siletsky et al. 2016).
Studies of the ESR mutants provided valuable information about the contribution of the specific amino acid residues to the photocycle and proton transfer reactions (Balashov et al. 2012, 2013). Due to the unusual lysine residue acting as a proton donor in ESR, the K96A mutant was the first object of our study (Siletsky et al. 2019). Unexpectedly, the kinetics of ΔΨ generation in this mutant demonstrated disappearance of the major positive electrogenic phases in the millisecond range (Fig. 3B). As a result, the overall amplitude of the K96A photoelectric response was almost ten times smaller in comparison with the wild type ESR. In our earlier work, deceleration of the M state decay in the K96A by two orders of magnitude was revealed by light-induced absorption change measurements (Balashov et al. 2013); however, corresponding electrogenic phases should be detectable even in the case of such slowing.
The time constant of the second millisecond phase, ~25 ms, reflects the fast component of the M decay (SB reprotonation) in the mutant. Its negative sign implies that the source of a proton is from the extracellular side of the protein. pH dependence of photoelectric response of the K96A revealed that two pathways compete for the SB reprotonation in the mutant. At low pH, the proton uptake through the cytoplasmic channel is more efficient while at pH 8.5 the alternative source dominates. The obtained data point to the significance of back reactions in the photocycle of ESR and possibly other proteorhodopsins. Apparently, in the absence of a donor, reprotonation of the SB mainly occurs from the extracellular side, presumably in reverse transfer from the acceptor site His57-Asp85 (Siletsky et al. 2019).
These assumptions were further confirmed by the studies of the H57N and H57N/K96A mutants (Siletsky et al. 2021b). The characteristic feature of the H57N mutant is the increase of the relative contribution of the electrogenic phases which are associated with the formation of the M state (28% vs 6% in the wild type, Fig. 3B). In the kinetics of the absorption changes, significant acceleration of the corresponding photocycle transitions in comparison with the wild type ESR was revealed. The obtained data point to connection of the slow phases of the SB deprotonation in ESR with His57. The decay of M and especially the M → N2/O transition was also accelerated in the H57N.
In the double H57N/K96A mutant, the rate of the M state decay at neutral pH is ten times slower than in the K96A. This complicates kinetic analysis of the associated electrogenic phases because their time constants exceed the characteristic time of the passive discharge of the membrane (~1 s). However, at pH 6.5, their relative contribution is ten times larger than in the single K96A mutant. This indicates the absence of back reactions in this mutant in contrast to the K96A and specifies the role of His57 residue as a plausible source of a proton during its reverse transfer from Asp85 to the SB. Increase of the photoelectric response in H57N and H57N/K96A mutants at the acidic pH range (Fig. 3C) correlates with elevated pumping efficiency of these variants in Escherichia coli cells in comparison with the wild type ESR and the K96A mutant at low pH (Siletsky et al. 2021b) in agreement with effect of H57N mutation on the pKa of Asp85 in ESR (Balashov et al. 2012).
Taken together, studies of ΔΨ generation in ESR and its mutant variants and their comparison with BR shed light on the architecture of the proton conducting channel in this retinal protein and the details of its functioning in various conditions. First, the significance of Lys96 as an efficient proton donor in ESR even at low pH was additionally demonstrated. Evidently, its role is not limited to acceleration of proton delivery to the SB as it was shown earlier but also includes prevention of the reverse transfer reactions from the extracellular side of the protein. Second, interaction between His57 and the primary acceptor Asp85 was shown to have the global influence on the kinetics and direction of the proton transfer reactions in ESR. Protonation state of His57 indirectly regulates the rate of the M state formation and decay through modulation of Asp85 pKa (Balashov et al. 2012). Increased pumping efficiency and amplitude of the photoelectric response of H57N and H57N/K96A mutants at acidic pH confirms that His57-Asp85 interaction determines the low pH limit of ESR functioning.
Sodium rhodopsin
A new group of microbial retinal proteins that perform light-driven transmembrane cation transport and contain a characteristic sequence motif NDQ includes the sodium pumping rhodopsins (NaRs) (Inoue et al. 2013; Balashov et al. 2014). Recently, the NaR from the marine flavobacterium Dokdonia sp. was studied by the direct electrometric method and the electrogenic Na+-dependent stages of its photocycle were determined (Bogachev et al. 2016). The sequence of the intermediate products of NaR photocycle and their optical and kinetic characteristics in general are similar to BR; however, they are not identical. The light absorption by NaR leads to the formation of a long-wave intermediate K (NaR519 + hv → K585), which turns into a short-wave (L-M), which then relaxes to its initial state. The third stage ((L450 ↔ M495) → O585) is the only one that depends on Na+ concentration, indicating its association with Na+ binding.
Despite to the fact that, according to spectral and structural data, the sodium binding site is presumably located near the SB (in the middle of the membrane dielectric thickness), the (L ↔ M) → O transition is accompanied by relatively small electrogenesis, about 15% of the charge transfer through the entire thickness of the membrane. Currently, it can be ruled out that this site resides at a larger distance from the SB. According to Kovalev et al. (2020a), the sodium ion binds to Asp116 close to the SB. An interesting explanation suggests a different mechanism of sodium ion transfer through the membrane due to its larger size compared to the proton. Presumably, Na+ moves through a deep and narrow water-filled pathway, characterized by high dielectric permittivity and ending near the SB and Asp116. This pathway remains open during the entire transition (L ↔ M) → O. The last event in the photocycle of Na+-transporting rhodopsin is the transition O → NaR which accounts for about 70% of the total electrogenesis. This stage does not depend on the sodium concentration that corresponds to the transfer of Na+ from its binding site to the outer surface of the membrane through a hydrophobic protein site (with low permittivity). The photocycle of sodium pumps involves deprotonation of the SB and subsequent reprotonation (Balashov et al. 2014) so the electrical phases reflect movements not only of sodium ions but also of H+. A single mutation at the donor site that replaces Gln with Asp converts sodium pump into a proton pump (Mamedov et al. 2016).
Heliorhodopsins, the nontransporting retinal proteins with inverted topology
A new family of heliorhodopsins (HeRs) with inverted membrane topology and unknown function has recently been discovered (Pushkarev et al. 2018). Unlike other known retinal proteins, HeRs have N-terminal ends facing the cytoplasm. The structure of bacterial HeR 48C12 was resolved in two states with a resolution of 1.5 Å (Kovalev et al. 2020b) and revealed noticeable differences from all known rhodopsins. In contrast to outward-directed retinal proton pumps, inner half of the extracellular part of HeR is completely hydrophobic while the cytoplasmic part contains a SB cavity (SBC) surrounded by charged residues and containing a cluster of water molecules, presumably being the primary acceptor of protons from the SB.
In the kinetics of generation of the HeRs membrane potential, an increase in the ΔΨ (~ 9 μs, 70%; ~ 30 μs, 30%) coincides with the formation of the M state in the photocycle and corresponds to the SB deprotonation. After that, a drop in the membrane potential difference is observed with the first component (~ 0.5 ms, ~ 10–20% of the maximum potential) coinciding with the decay of M to O1 state which corresponds to the SB reprotonation and the proton movement in the opposite direction. The next component of the overall decay of the electric potential (~ 500 ms) correlates with spectroscopic transitions from O1 to the ground state of HeR. This is accompanied by the movement of the charged residues to the ground state. Unlike in the above mentioned proton pumps (Drachev et al. 1974, 1981; Siletsky et al. 2016), where similar experiments have shown that when a proton is translocated, it always moves in one specific direction (inside the proteoliposomes in the case of BR) during the entire photocycle, in case of HeR the direction of the proton movement is reversed on reprotonation of the SB. The results of this time-resolved study are in favor of the hypothesis that the SBC plays the role of a collective primary proton acceptor in HeRs (Kovalev et al. 2020b).
Conclusions and perspectives
Despite the significant progress achieved after the three-dimensional structures were analyzed and a number of key residues in proton-conducting pathways were established, the molecular mechanisms in microbial rhodopsin family remain largely unexplained. This is especially true of new proteins, retinal-containing proton and sodium pumps and rhodopsins with unknown functions discovered in recent years. In this regard, the time-resolved electrometric studies of ion pumping and elucidation of the real time charge transfer kinetics in these proteins are extremely important. The details of the functioning in retinal protein family could be further used for optimization of these molecular energy-converting devices for biotechnological and optogenetic applications.
Acknowledgements
The authors are grateful to V. Kurashev for the help in preparing Fig. 1.
Funding
The work was supported by Russian Scientific Foundation Grant №22–14-00104.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergey A. Siletsky, Email: siletsky@genebee.msu.su
Sergei P. Balashov, Email: balashov@uci.edu
Lada E. Petrovskaya, Email: lpetr65@yahoo.com
References
- Balashov SP (2000) Protonation reactions and their coupling in bacteriorhodopsin. Biochim Biophys Acta (BBA)-Bioenergetics 1460:75–94. 10.1016/S0005-2728(00)00131-6 [DOI] [PubMed]
- Balashov SP, Petrovskaya LE, Lukashev EP, Imasheva ES, Dioumaev AK, Wang JM et al (2012) Aspartate-histidine interaction in the retinal schiff base counterion of the light-driven proton pump of Exiguobacterium sibiricum. Biochemistry 51:5748–5762. 10.1021/bi300409m [DOI] [PMC free article] [PubMed]
- Balashov SP, Petrovskaya LE, Imasheva ES, Lukashev EP, Dioumaev AK, Wang JM et al (2013) Breaking the carboxyl rule: lysine 96 facilitates reprotonation of the Schiff base in the photocycle of a retinal protein from Exiguobacterium sibiricum. J Biol Chem 288:21254–21265. 10.1074/jbc.M113.465138 [DOI] [PMC free article] [PubMed]
- Balashov SP, Imasheva ES, Dioumaev AK, Wang JM, Jung K-H, Lanyi JK (2014) Light-driven Na+ pump from Gillisia limnaea: a high-affinity Na+ binding site is formed transiently in the photocycle. Biochemistry 53:7549–7561. 10.1021/bi501064n [DOI] [PMC free article] [PubMed]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, et al. Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci USA. 2016;113:822–829. doi: 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogachev AV, Bertsova YV, Verkhovskaya ML, Mamedov MD, Skulachev VP (2016) Real-time kinetics of electrogenic Na(+) transport by rhodopsin from the marine flavobacterium Dokdonia sp. PRO95. Sci Rep 6:21397. 10.1038/srep21397 [DOI] [PMC free article] [PubMed]
- Brown LS. Light-driven proton transfers and proton transport by microbial rhodopsins – A biophysical perspective. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2022;1864:183867. doi: 10.1016/j.bbamem.2022.183867. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Hegemann P. The form and function of channelrhodopsin. Science. 2017;357:eaan5544. doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dér A, Keszthelyi L. Charge motion during the photocycle of bacteriorhodopsin. Biochem Mosc. 2001;66:1234–1248. doi: 10.1023/A:1013179101782. [DOI] [PubMed] [Google Scholar]
- Dickopf S, Heyn MP. Evidence for the first phase of the reprotonation switch of bacteriorhodopsin from time-resolved photovoltage and flash photolysis experiments on the photoreversal of the M-intermediate. Biophys J. 1997;73:3171–3181. doi: 10.1016/S0006-3495(97)78343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachev LA, Jasaitis AA, Kaulen AD, Kondrashin AA, Liberman EA, Nemecek IB et al (1974) Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin. Nature 249:321–324. https://doi.org/10.1038/249321a0 [DOI] [PubMed]
- Drachev LA, Kaulen AD, Semenov AY, Severina II, Skulachev VP. Lipid-impregnated filters as a tool for studying the electric current-generating proteins. Anal Biochem. 1979;96:250–262. doi: 10.1016/0003-2697(79)90580-3. [DOI] [PubMed] [Google Scholar]
- Drachev LA, Kaulen AD, Khitrina LV, Skulachev VP. Fast stages of photoelectric processes in biological membranes. I Bacteriorhodopsin Eur J Biochem. 1981;117:461–470. doi: 10.1111/j.1432-1033.1981.tb06361.x. [DOI] [PubMed] [Google Scholar]
- Drachev L, Kaminskaya O, Konstantinov A, Mamedov M, Samuilov V, Semenov AY et al (1986) Effects of electron donors and acceptors on the kinetics of the photoelectric responses in Rhodospirillum rubrum and Rhodopseudomonas sphaeroides chromatophores. Biochimica et Biophysica Acta (BBA)-Bioenergetics 850:1–9. 10.1016/0005-2728(86)90002-2
- Drachev L, Kaurov B, Mamedov M, Mulkidjanian AY, Semenov AY, Shinkarev V et al (1989) Flash-induced electrogenic events in the photosynthetic reaction center and bc 1 complexes of Rhodobacter sphaeroides chromatophores. Biochimica et Biophysica Acta (BBA)-Bioenergetics 973:189–197. 10.1016/S0005-2728(89)80421-9
- Dracheva SM, Drachev LA, Konstantinov AA, Semenov AY, Skulachev VP, Arutjunjan AM, et al. Electrogenic steps in the redox reactions catalyzed by photosynthetic reaction-centre complex from Rhodopseudomonas viridis. Eur J Biochem/ FEBS. 1988;171:253–264. doi: 10.1111/j.1432-1033.1988.tb13784.x. [DOI] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Li H, Spudich JL. Microbial Rhodopsins: Diversity, Mechanisms, and Optogenetic Applications. Annu Rev Biochem. 2017;86:845–872. doi: 10.1146/annurev-biochem-101910-144233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Spudich JL. Emerging Diversity of Channelrhodopsins and Their Structure-Function Relationships. Front Cell Neurosci. 2021;15:800313. doi: 10.3389/fncel.2021.800313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushchin I, Chervakov P, Kuzmichev P, Popov AN, Round E, Borshchevskiy V, et al. Structural insights into the proton pumping by unusual proteorhodopsin from nonmarine bacteria. Proc Natl Acad Sci USA. 2013;110:12631–12636. doi: 10.1073/pnas.1221629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushchin I, Gordeliy V (2018) Microbial Rhodopsins. In: Harris, J., Boekema, E. (eds) Membrane Protein Complexes: Structure and Function. Subcellular Biochemistry, vol 87, pp 19-56. Springer, Singapore. 10.1007/978-981-10-7757-9_2 [DOI] [PubMed]
- Inoue K, Ono H, Abe-Yoshizumi R, Yoshizawa S, Ito H, Kogure K, et al. A light-driven sodium ion pump in marine bacteria. Nat Commun. 2013;4:1678. doi: 10.1038/ncomms2689. [DOI] [PubMed] [Google Scholar]
- Kalaidzidis I, Kaulen A, Radionov A, Khitrina L. Photoelectrochemical сycle of bacteriorhodopsin. Biochemistry (Moscow) 2001;66:1220–1233. doi: 10.1023/A:1013127117712. [DOI] [PubMed] [Google Scholar]
- Kaminskaya OP, Drachev LA, Konstantinov AA, Semenov AY, Skulachev VP (1986) Electrogenic reduction of the secondary quinone acceptor in chromatophores of Rhodospirillum rubrum: rapid kinetics measurements. FEBS Lett 202:224–228. 10.1016/0014-5793(86)80691-3
- Kandori H. Biophysics of rhodopsins and optogenetics. Biophys Rev. 2020;12:355–361. doi: 10.1007/s12551-020-00645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen AD (2000) Electrogenic processes and protein conformational changes accompanying the bacteriorhodopsin photocycle. Biochim Biophys Acta (BBA)-Bioenergetics 1460:204–219. 10.1016/S0005-2728(00)00140-7 [DOI] [PubMed]
- Kovalev K, Astashkin R, Gushchin I, Orekhov P, Volkov D, Zinovev E, Marin E, Rulev M, Alekseev A, Royant A, Carpentier P, Vaganova S, Zabelskii D, Baeken C, Sergeev I, Balandin T, Bourenkov G, Carpena X, Boer R, Maliar N, Borshchevskiy V, Büldt G, Bamberg E, Gordeliy V (2020a) Molecular mechanism of light-driven sodium pumping. Nature Communications 11(1):2137. 10.1038/s41467-020-16032-y [DOI] [PMC free article] [PubMed]
- Kovalev K, Volkov D, Astashkin R, Alekseev A, Gushchin I, Haro-Moreno JM et al (2020b) High-resolution structural insights into the heliorhodopsin family. Proc Natl Acad Sci USA 117:4131–4141. 10.1073/pnas.1915888117 [DOI] [PMC free article] [PubMed]
- Lanyi JK, Luecke H. Bacteriorhodopsin. Curr Opin Str Biol. 2001;11:415–419. doi: 10.1016/S0959-440X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Läuger P (1991) Electrogenic ion pumps. Sinauer Associates, Sunderland
- Luecke H, Schobert B, Richter H-T, Cartailler J-P, Lanyi JK. Structure of bacteriorhodopsin at 1.55 Å resolution. J Mol Biol. 1999;291:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]
- Luecke H, Schobert B, Cartailler J-P, Richter H-T, Rosengarth A, Needleman R, et al. Coupling photoisomerization of retinal to directional transport in bacteriorhodopsin. J Mol Biol. 2000;300:1237–1255. doi: 10.1006/jmbi.2000.3884. [DOI] [PubMed] [Google Scholar]
- Mamedov M, Gadzhieva R, Gourovskaya K, Drachev L, Semenov AY. Electrogenicity at the donor/acceptor sides of cyanobacterial photosystem I. J Bioenerg Biomembr. 1996;28:517–522. doi: 10.1007/BF02110441. [DOI] [PubMed] [Google Scholar]
- Mamedov MD, Mamedov AM, Bertsova YV, Bogachev AV. A single mutation converts bacterial Na+-transporting rhodopsin into an H+ transporter. FEBS Lett. 2016;590:2827–2835. doi: 10.1002/1873-3468.12324. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Petrovskaya LE, Lukashev EP, Chupin VV, Sychev SV, Lyukmanova EN, Kryukova EA, et al. Predicted bacteriorhodopsin from Exiguobacterium sibiricum is a functional proton pump. FEBS Lett. 2010;584:4193–4196. doi: 10.1016/j.febslet.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Pushkarev A, Inoue K, Larom S, Flores-Uribe J, Singh M, Konno M, et al. A distinct abundant group of microbial rhodopsins discovered using functional metagenomics. Nature. 2018;558:595–599. doi: 10.1038/s41586-018-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siletsky SA. Steps of the coupled charge translocation in the catalytic cycle of cytochrome c oxidase. Front Biosci (Landmark Edition) 2013;18:36–57. doi: 10.2741/4086. [DOI] [PubMed] [Google Scholar]
- Siletsky S, Kaulen AD, Konstantinov AA. Resolution of electrogenic steps coupled to conversion of cytochrome c oxidase from the peroxy to the ferryl-oxo state. Biochemistry. 1999;38:4853–4861. doi: 10.1021/bi982614a. [DOI] [PubMed] [Google Scholar]
- Siletsky SA, Pawate AS, Weiss K, Gennis RB, Konstantinov AA. Transmembrane charge separation during the ferryl-oxo→ oxidized transition in a nonpumping mutant of cytochrome c oxidase. J Biol Chem. 2004;279:52558–52565. doi: 10.1074/jbc.M407549200. [DOI] [PubMed] [Google Scholar]
- Siletsky SA, Zhu J, Gennis RB, Konstantinov AA. Partial steps of charge translocation in the nonpumping N139L mutant of Rhodobacter sphaeroides cytochrome c oxidase with a blocked D-channel. Biochemistry. 2010;49:3060–3073. doi: 10.1021/bi901719e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siletsky SA, Mamedov MD, Lukashev EP, Balashov SP, Dolgikh DA, Rubin AB et al (2016) Electrogenic steps of light-driven proton transport in ESR, a retinal protein from Exiguobacterium sibiricum. Biochim Biophys Acta (BBA)-Bioenergetics 1857:1741–1750. 10.1016/j.bbabio.2016.08.004 [DOI] [PubMed]
- Siletsky SA, Belevich I, Belevich NP, Soulimane T, Wikström M. Time-resolved generation of membrane potential by ba3 cytochrome c oxidase from Thermus thermophilus coupled to single electron injection into the O and OH states. Biochim Biophys Acta (BBA)-Bioenergetics. 2017;1858:915–926. doi: 10.1016/j.bbabio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Siletsky SA, Mamedov MD, Lukashev EP, Balashov SP, Dolgikh DA, Rubin AB et al (2019) Elimination of proton donor strongly affects directionality and efficiency of proton transport in ESR, a light-driven proton pump from Exiguobacterium sibiricum. Biochim Biophys Acta (BBA)-Bioenergetics 1860:1–11. 10.1016/j.bbabio.2018.09.365 [DOI] [PubMed]
- Siletsky SA, Soulimane T, Belevich I, Gennis RB, Wikström M (2021a) Specific inhibition of proton pumping by the T315V mutation in the K channel of cytochrome ba3 from Thermus thermophilus. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1862:148450. 10.1016/j.bbabio.2021.148450 [DOI] [PubMed]
- Siletsky SA, Lukashev EP, Mamedov MD, Borisov VB, Balashov SP, Dolgikh DA et al (2021b) His57 controls the efficiency of ESR, a light-driven proton pump from Exiguobacterium sibiricum at low and high pH. Biochim Biophys Acta (BBA)-Bioenergetics 1862:148328. 10.1016/j.bbabio.2020.148328 [DOI] [PubMed]
- Vassiliev IR, Jung Y-S, Mamedov MD, AYu S, Golbeck JH. Near-IR absorbance changes and electrogenic reactions in the microsecond-to-second time domain in photosystem I. Biophys J. 1997;72:301–315. doi: 10.1016/S0006-3495(97)78669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A, Wietek J, Hegemann P. Gloeobacter rhodopsin, limitation of proton pumping at high electrochemical load. Biophys J. 2013;105:2055–2063. doi: 10.1016/j.bpj.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky D, Smirnova I, Siletsky S, Kaulen A, Millett F, Konstantinov A. Rapid kinetics of membrane potential generation by cytochrome c oxidase with the photoactive Ru (II)-tris-bipyridyl derivative of cytochrome c as electron donor. FEBS Lett. 1995;359:27–30. doi: 10.1016/0014-5793(94)01443-5. [DOI] [PubMed] [Google Scholar]