Abstract
Inflammatory bowel disease (IBD) is a group of disorders characterized by chronic inflammation in the intestine. Several studies confirmed that oxidative stress induced by an enormous amount of reactive free radicals triggers the onset of IBD. Currently, there is an increasing trend in the global incidence of IBD and it is coupled with a lack of adequate long-term therapeutic options. At the same time, progress in research to understand the pathogenesis of IBD has been hampered due to the absence of adequate animal models. Currently, the toxic chemical Dextran Sulfate Sodium (DSS) induced gut inflammation in rodents is widely perceived as a good model of experimental colitis or IBD. Drosophila melanogaster, a genetic animal model, shares ~ 75% sequence similarity to genes causing different diseases in humans and also has conserved digestion and absorption features. Therefore, in the current study, we used Drosophila as a model system to induce and investigate DSS-induced colitis. Anatomical, biochemical, and molecular analyses were performed to measure the levels of inflammation and cellular disturbances in the gastrointestinal (GI) tract of Drosophila. Our study shows that DSS-induced inflammation lowers the levels of antioxidant molecules, affects the life span, reduces physiological activity and induces cellular damage in the GI tract mimicking pathophysiological features of IBD in Drosophila. Such a DSS-induced Drosophila colitis model can be further used for understanding the molecular pathology of IBD and screening novel drugs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03349-2.
Keywords: Drosophila, Dextran sodium sulfate, Antioxidant, Smurfing, Inflammatory bowel disease
Introduction
Inflammatory bowel diseases (IBD) are chronic and life-long diseases characterized by gastrointestinal inflammation. Ulcerative Colitis (UC) and Crohn’s Disease (CD) are the two types of IBD, mostly with similar symptoms and significant overlapping genetic components. IBD symptoms can extend beyond the digestive tract but most frequently include diarrhea with blood and stomach ache. Globally, around 6.8 million cases were reported in 2017, and over the past few decades, IBD was most dominant in the western world. However, in recent times, IBD incidence has increased rapidly in many Asian countries with a consistent rising trend (Collaborators 2020; Ng et al. 2013; Yeshi et al. 2020). In Asia, males between the ages of 20–40 years are most affected by CD, which is not the case in western countries (Kwak et al. 2019; Yeshi et al. 2020; Yen et al. 2019). However, there is a lack of national registries of IBD cases in many developing countries, and therefore, detailed knowledge about the incidence and prevalence of IBD is missing. Overall, the increasing trends in IBD incidence and lack of adequate long-term therapeutic options have substantial financial burdens on the healthcare system worldwide (Yeshi et al. 2020).
The CD and UC are distinguishable with their location and nature of inflammation. UC mainly attacks colonic mucosa, and CD can be in any part of the gastrointestinal (GI) tract. However, both conditions share many clinical features in common. The fistulas, perianal disease, colonic and small bowel obstructions are common in CD. Cryptitis and crypt abscesses are observed in both UC and CD, while crypt architecture is more disturbed in the case of UC. The UC causes inflammation and ulceration of the inner lining of the colon and rectum, leading to symptoms including diarrhea, abdominal pain, fatigue, loss of appetite, weight loss, and anemia (Sobrado and Sobrado 2016; Tremaine 2011). CD mainly affects the terminal ileum, caecum, perianal area, and colon. Symptoms include chronic or nocturnal diarrhea, abdominal pain, bowel obstruction, weight loss, fever, or night sweats (Veauthier and Hornecker 2018). Different factors that contribute to the development of UC and DC include genetic susceptibility, disturbed gut microflora, environmental influences, and an abnormal immune response (de Lange and Barrett 2015; Yeshi et al. 2020; Zhang and Li 2014).
Several studies have been carried out to understand the leading cause of IBD; however, the exact cellular and molecular mechanism of IBD has not yet been fully understood. Additionally, the progress in research to understand the pathogenesis of IBD has been delayed by the absence of adequate animal models. A useful animal model of IBD should be reproducible to resemble the clinical course, therapeutic response, and inflammatory profiles of IBD. The most widely used animal models of IBD are induced by administering toxic chemicals such as Dextran Sulfate Sodium (DSS) (Kiesler et al. 2015; Elson et al. 1995). DSS causes gut inflammation that mimics similar features of ulcerative colitis and is widely perceived as a good model for experimental colitis (Gaudio et al. 1999; Strober et al. 2002).
Drosophila melanogaster, a genetic animal model, shares around 75% sequence similarity with genes causing different diseases in humans (Pandey and Nichols 2011). The different genetic tool kits, including the Gal4-UAS system to regulate gene expression levels available in Drosophila research fields, making them a versatile genetic model organism for various biological studies (Brand and Perrimon 1993; Raghu et al. 2018; Raghu and Patil 2021). In addition, many features of digestion and absorption appear to be conserved between flies and mammals (Lemaitre and Miguel-Aliaga 2013). Hence in the current study, we used Drosophila melanogaster as a model system to study DSS-induced colitis. As oxidative stress is largely implicated in the pathophysiology of IBD. We used this model to study gut inflammation, antioxidant defense levels, and their role in the survival of Drosophila upon DSS treatment.
Materials and methods
Reagents
Dextran sulfate sodium salt (DSS), colitis grade (36,000–50,000 Da) was purchased from MP Biomedicals, USA (CAS no. 9011-18-1). Bromophenol Blue (Merck Germany, Cat No: 108122) was used as a tracer dye. The custom-made or predesigned primers for qPCR were purchased from Thermo Fisher Scientific, India. All the other chemicals were of analytical grade from Himedia or Merck, India.
Fly culture
The wild-type Canton-S (CS) (Drosophila Stock Centre, Mysore University, India) flies were maintained on a wheat cream-agar medium at 25 °C with a relative humidity of 65–70% and 12:12 h light–dark cycle.
DSS feeding in flies
One-day-old male and female CS flies were harvested and fed with 3% DSS in 5% sucrose solution as per the method described by Amcheslavsky et al. (2009), with suitable modifications. The DSS feeding continued for 5 days, and on the 6th day, flies were shifted to a regular wheat cream-agar diet. To externally quantify the feeding success on 3% DSS, the DSS and sucrose mixture is combined with a blue food dye (Brilliant Blue FCF). For comparison, the control flies were fed with a mixture of sucrose and blue food dye. The flies with blue markings on the abdomen were used for all following experiments in control and treated conditions.
Survival assay
A total of 25 flies in triplicates were used as a control or 3% DSS-treated flies and then observed for their mortality rate for 10 days.
Gut permeability assay (Smurf phenotype)
Smurf assay was performed to quantify the feeding success on 3% DSS and to understand its toxic impact on the permeability of the GI tract (Sheng et al. 2021). The DSS and sucrose mixture in combination with 0.2% Bromophenol Blue (Merck Germany, Cat No: 108122) was fed overnight on the last day of treatment (10th Day). For comparison, the control flies were fed with a mixture of sucrose and Bromophenol Blue. The control and test flies were observed and analyzed under a stereomicroscope (Olympus Pvt Ltd, India).
Climbing assay
After 10 days of DSS feeding, the male and female flies were transferred separately to 25 ml serological pipette tubes that were cut to 20 cm in length. The top and bottom of the tube were sealed with parafilm with a few small holes to provide ventilation. The flies were habituated to the new environment by keeping tubes flat on the surface for one hour at 25 °C. The tube was tapped against a hard surface at the beginning of the experiment to place all the flies at the bottom. The time for each fly to reach the top marked point was recorded. Any flies that could not reach the top mark within 60 s were marked as failures to climb. The climbing index was calculated as the percentage of flies that reached the top mark within 60 s. 25 flies were used for each set of climbing assays and all experiments were performed in triplicates.
Measurement of GI tract length
The control and DSS-fed flies after 10 days were cold anesthetized and dissected under a stereomicroscope in 1X PBS solution. The total length of the GI tracts was imaged and measured using MagVision software (Magus Analytics, version: × 64, 3.7.7934).
ROS visualization in the GI tract
The guts were dissected and handled in a PBS medium throughout the experiment. The samples were incubated in CM-H2DCFDA (5 μM) at 25 °C for 30 min in the dark. Mounted samples were then imaged immediately under a confocal microscope (Zeiss LSM 800) at 492–495 nm excitation and 517–527 nm detection. Z-series spanning the intestinal epithelium of the posterior midgut were gathered. The single confocal sections were used to measure relative fluorescence signal intensities within the posterior midgut region using ImageJ software (Chen et al. 2021; Ramond et al. 2021).
Sample preparation for biochemical analysis
The homogenate of DSS treated and control flies were prepared as described previously (Paithankar et al. 2018). Briefly, fifty flies were taken and homogenized in 0.5 ml ice-cold extraction buffer consisting 50 mM potassium phosphate (Himedia, India) buffer, pH 7.4, 1 mM poly methyl sulphonyl fluoride, 1 mM Ethylenediaminetetraacetic acid (EDTA) (Sigma–Aldrich, India) and 0.1% Triton X-100 (Himedia, India). Homogenized samples were centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was used for protein estimation and antioxidant assays.
Protein estimation
The total protein content was estimated by the Bradford method at 595 nm using bovine serum albumin as the standard (Bradford 1976).
Antioxidant assays
The antioxidants (both enzymatic and non-enzymatic) were assayed to understand the influence of DSS in the progression of gut inflammation. After ten days of survival, the homogenates (as mentioned above) of control and treated flies were prepared and used for the following assays:
Total antioxidant capacity (TAC)
TAC assay was performed as per the method described by Prieto et al. (1999). This test is used to gauge the inhibition of reducing Mo (VI) capability to Mo (V) in the presence of the sample under acidic conditions. It reacts with phosphate to form a green-colored complex. The concentration of the formed complex is proportional to the intensity of the color and is measured at 695 nm. The TAC is measured in relation to the standard Ascorbic acid.
Superoxide dismutase activity assay (SOD)
The SOD activity was determined by pyrogallol assay (Li 2012; Ramasarma et al. 2015). The assay is based on the effect of fly lysates to inhibit the auto-oxidation of pyrogallol with respect to time. An increase in absorbance is inversely proportional to the inhibition of auto-oxidation of pyrogallol. The SOD activity was calculated using the formula described previously (Li 2012).
Catalase activity assay
The catalase activity was determined using an earlier reported method (Sinha 1972; Iyyaswamy and Rathinasamy 2012). In this assay, the fly homogenate was used as a source of enzyme and made to react with H2O2 for different time intervals (0–90 s). Followed by reaction with potassium dichromate-acetic acid mixture at 100 °C for 10 min. A green color that developed was measured at 610 nm. A standard curve was developed using H2O2 and used for measuring the catalase activity.
Glutathione peroxidase assay (GPx)
The GPx activity was measured as per the method described previously (Rotruck et al. 1973) with minor modifications (Iyyaswamy and Rathinasamy 2012). A difference in absorbance due to the conversion of oxidized glutathione to reduced form was measured at 412 nm and then used to calculate the enzyme activity.
Analysis of inflammatory cytokine and antioxidant enzymes by qPCR
Total RNA from fly GI tract samples was isolated using TRIzol™ reagent according to the manufacturer’s instructions (Thermo Fisher Scientific, India). Reverse transcription-PCR (RT-PCR) was performed using 1 μg of total RNA with random primers using PrimeScript™ 1st strand cDNA synthesis Kit (Takara Bio India Pvt. Ltd.) in a Wee-32™ thermal cycler (Himedia, India) as per the manufacturer’s protocol. The qPCR was performed for upd2, upd3, Catalase, SOD, PHGPx and RpL32 enlisted in Table 1 according to the manufacturer’s instructions using Premix Ex Taq™ DNA Polymerase or TB Green® Advantage® qPCR reagent (Takara Bio India Pvt. Ltd.; Cat# RR039A; 639676) on a Quantstudio 5 thermal cycler (Thermo Fisher Scientific, India). All studies were performed in triplicates. The results were expressed as fold change (2−ΔΔCT) by calculating the expression of a target gene to the housekeeping gene (RpL32) and then normalized to the untreated controls.
Table 1.
Primers used for qPCR
| Gene id | Assay ID # or Reference or primer sequences |
|---|---|
| Catalase | Dm02398859_s1 |
| Superoxide dismutase 1 (SOD) | Dm01841588_m1 |
| Glutathione peroxidase (PHGPx) | Dm01832545_g1 |
| Ribosomal Protein L32 (RpL32) | Dm02151827_g1 |
| Unpaired 2 (upd2) |
Forward—5' AGCAGAAGAGCCTCAACGAG 3' Reverse—5' CTGGCGTGTGAAAGTTGAGA 3' |
| Unpaired 3 (upd3) |
Forward- 5' ATCGCGACCTGCAGATTTAC 3' Reverse- 5' TGTACAGCEGGTTGGTCAGG 3' |
| Actinobacteria | Bacchetti De Gregoris et al. (2011) |
| Bacteroidetes | |
| Firmicutes | |
| α-Proteobacteria | |
| γ-Proteobacteria | |
| Universal bacterial primer |
Profiling of gut microbiome by qPCR
The DNA from the gut was isolated as per the manufacturer’s protocol using the genomic DNA extraction kit (NucleoSpin® Tissue XS kit, Macherey–Nagel Inc, USA). The qPCR was performed for the gut microbiome panel enlisted in Table 1 using predesigned primers and protocols described elsewhere (Bacchetti De Gregoris et al. 2011) using TB Green® Advantage® qPCR premix reagent (Takara Bio India Pvt. Ltd.; Cat# 639676) on a Quantstudio 5 thermal cycler (Thermo Fisher Scientific, India). All assays were performed in triplicates. The results were expressed as fold change (2−ΔΔCT) by calculating the expression of a target gene to the housekeeping gene (Universal bacterial primer) and then normalized to the untreated controls.
Statistical analysis
All the experiments were performed in triplicates (n = 3), and the results were expressed as mean ± Standard deviation. An unpaired two-tailed t-test was performed to compare the mean of each treated group with the mean of the control group using GraphPad prism software (Graph Pad Software Inc., San Diego, CA, USA).
Results and discussion
In the present study, an attempt was made to utilize Drosophila melanogaster as an experimental model to investigate the correlation between life span and antioxidant status upon exposure to DSS, a chemical colitogen that induces epithelial damage (Okayasu et al. 1990; Chassaing et al. 2014). The gut of Drosophila is anatomically similar to the human intestine (Apidianakis and Rahme 2011; Capo et al. 2019). Essentially, the posterior midgut and hindgut of Drosophila are functionally analogous to the human small intestine and the colon, respectively (Micchelli and Perrimon 2006). Hence, Drosophila can be distinctly used to study and characterize the pathways involving gut immunity, tissue regeneration, and homeostasis, especially those that occur akin to human diseases such as IBD and cancer (Apidianakis and Rahme 2011).
DSS is known to cause disruption in the intestinal barrier, and thus may impact the survival of the flies (Wang et al. 2021; Howard et al. 2019; Amcheslavsky et al. 2009). Hence, we examined whether this is an indicator for tracing the influence of DSS in the flies. Our results indicate that upon feeding 3% DSS for five days and subsequent transfer to a regular wheat cream diet, only 45% of male (Fig. 1a) and 27% of female (Fig. 1b) flies had the ability to survive during the study. Fatigue is a significant clinical problem in IBD patients (Borren et al. 2019; van Langenberg and Gibson 2010; Nocerino et al. 2020; Qazi 2020). Studies indicate that in humans, > 80% of active disease patients show signs of fatigue (Borren et al. 2019).
Fig. 1.
Effect of 3% DSS on the survival (Kaplan–Meier curve) [(a) Male and (b) Female flies] and (c) negative geotactic behavior in Drosophila melanogaster. [Sample n = 75]
In flies, the negative geotaxis measures the innate response to climbing vertically when exposed to an external stimulus such as forcing to the bottom of the vial by tapping. This involved measuring the number of flies that could climb a distance of 20 cm in 60 s. Several studies have shown that negative geotactic activity was influenced by various physiological indicators such as age, oxidative stress, bacterial infections, temperature, etc. (Simon et al. 2006; Linderman et al. 2012; Rhodenizer et al. 2008). We hypothesized that the climbing assay would be affected by the induction of gut inflammation influencing the physiological activity of flies. Our study observed that there was indeed a significant reduction in climbing capacity in DSS-treated flies (Fig. 1c). Furthermore, among the flies that survived 5 days post DSS treatment, 23% of males and 10% of females were unsuccessful in covering the experimental distance in the given time, indicating that most of the flies were facing fatigue. Several studies have shown that DSS-fed mice had lowered voluntary activity (Weegh et al. 2020). Natural negative geotaxis movement is an innate response and a good indicator of healthy flies (Linderman et al. 2012). Hence, the study was carried out to gauge their physical movement. The results show that the DSS-fed flies significantly reduced climbing behavior, implying sickness.
DSS is known to disrupt the intestinal barrier, and thus to verify the structural damage of the fly gut, a Smurf assay was performed (Martins et al. 2018; Rera et al. 2011; Sheng et al. 2021). Bromophenol Blue was fed along with the diet and observed for its leakage through the disrupted intestinal epithelium (Fig. 2a) and b). In the natural course of food ingestion, the dye is filled in the digestive tract (Fig. 2a). However, in the case of DSS-fed flies, the dye gets leaked into the abdominal cavity due to the loss of integrity in the intestinal epithelial barrier (Fig. 2b). Also, the survival assay showed that the DSS-treated flies had a shorter lifespan than the untreated flies. Moreover, upon dissecting the digestive tract from the fly and measuring the length, it was found that the DSS-treated flies had significantly shorter lengths than the untreated flies (Fig. 2c–e). The gut length was found to have shortened by ~ 30% as compared to the untreated control. All these features are similar to studies in mice where reports show that DSS-induced colon shortening, reduced life span, and increased gut permeability upon feeding DSS thus are regarded as hallmarks for assessment of colonic inflammation (Chassaing et al. 2014; Kaushal et al. 2014; Cochran et al. 2020). Altogether these findings show that treatment with 3% DSS for 5 days induced loss in intestinal barrier integrity and fatigue leading to death in flies which is relatively similar to the clinical observations that were reported in mice.
Fig. 2.
Smurf assay: Assessment of intestinal permeability upon treatment with 3% DSS. (a, b, control, and DSS-treated flies, respectively). e Analysis of gut length at day 10 (c, d, control, and DSS-treated flies, respectively). h Measurement of ROS upon treatment with CM-H2DCFDA (f, g, control, and DSS-treated flies, respectively). [Sample n = 5]
ROS molecules such as oxygen radicals (Superoxide, hydroxyl) and non‐radicals (hypochlorous acid, singlet oxygen, and hydrogen peroxide) have deleterious effects on the cells. DSS has been shown to increase ROS generation and induce inflammatory responses within a cell (Bhattacharyya et al. 2014). Hence to confirm the presence of ROS, DSS-fed flies were dissected and the relative fluorescence intensity (RFI) was measured and compared to the untreated control. From the analysis, it was observed that a tenfold increase in the RFI was measured (Fig. 2f–h). ROS accrual leads to the damage of specific genes involved in cell growth and differentiation or may cause changes in the levels of the antioxidant enzyme expression and their activity. From our study as well as others it is evident that there is an increase in RFI upon staining with H2DCFDA also expression of IL-6-like cytokine, i.e., upd3 and to a lesser extent, upd2 were upregulated due to the treatment of DSS. This upregulation may be attributed to the activation of several injury responses including JNK/STAT signaling (Wright et al. 2011). In Drosophila, this critical inflammatory response is known to activate STAT92E (analogous to STAT5 in humans) to facilitate regeneration and homeostasis in the gut (Zeidler et al. 2000; Arbouzova and Zeidler 2006).
The survival assay found that irrespective of gender, there was a significant deviation in the survival capacity between the untreated and DSS-treated flies. Hence, the homogenates of the untreated and DSS-treated flies were analyzed for antioxidant activities. Among the antioxidant mechanisms, there are two forms, namely, enzymatic and non-enzymatic (Nimse and Pal 2015). The SOD, Catalase, and GPx are all part of the enzymatic antioxidant defense system that fights against oxidative damage (Nimse and Pal 2015). The antioxidant enzymes work by the multistep process where reactive oxidative products are converted to hydrogen peroxide and water, thereby preventing cellular damage. The antioxidants are well-known scavengers of free radicals (Lobo et al. 2010). Moreover, a cumulative effect of both enzymatic and non-enzymatic antioxidants determines the survival of an organism (Frei et al. 1988). Hence in this study, we have measured the levels of enzymatic (SOD, Catalase, and GPx) and non-enzymatic antioxidants that may have directly or indirectly contributed to the difference in the survival capacity of untreated and DSS-treated flies.
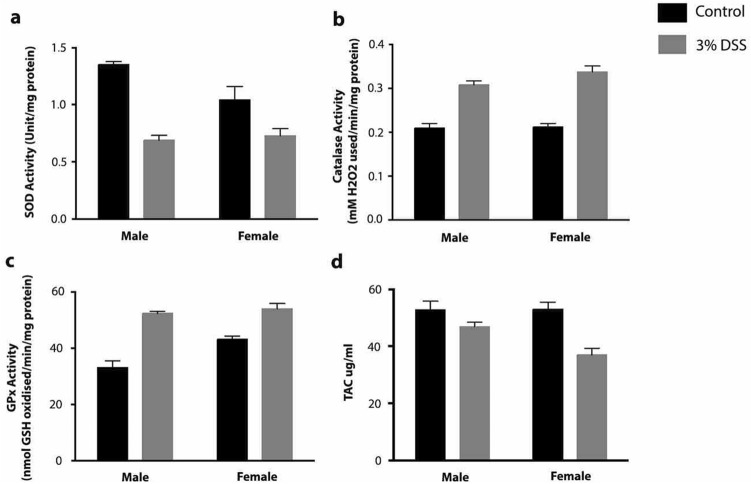
The TAC assay was performed by the phosphomolybdenum method described by Prieto et al. (1999). This method is shown to be a good indicator for understanding the role of low molecular weight antioxidants present within the flies. From the study, it was observed that untreated males and females had 52.84 ± 2.89 μg/ml and 53 ± 2.43 μg/ml, respectively. However, upon DSS treatment, the TAC levels were significantly reduced to 46.6 ± 1.6 μg/ml and 36.97 ± 2.36 μg/ml in male and female flies, respectively (Fig. 3d). Hence, a 12–30% reduction in the TAC in treated flies may have contributed to a decline in the survival capacity of these flies. The SODs are ubiquitous metalloenzymes that act as the first line of defense against ROS (Bannister et al. 1987; Wang et al. 2018). It acts on the superoxide radicals generated in the biological system and converts them to H2O2 and further to H2O and O2 aided by the catalase and peroxidase enzymes. However, the SOD activity was significantly reduced by ~ 50% in males and ~ 31% in DSS treatment in females (Fig. 3a). In contrast, qPCR analysis indicated that there was significant overexpression of mRNA by 7–8 folds in males and females, respectively (Fig. 4a, b). Although the reason for the increase is still unknown. A lowered enzyme activity may be a likely inducer for pro-inflammatory immune responses contributing to oxidative stress in DSS-fed flies.
Fig. 3.
Antioxidant status in Drosophila melanogaster upon 3% DSS treatment. a SOD, b Catalase, c GPx and d Total antioxidant assay using digestive tract samples. All the assays were performed in triplicates
Fig. 4.
qPCR analysis for the antioxidant enzymes (SOD, Catalase, PHGPx) and inflammatory cytokines (udp2 and upd3) from the digestive tract of Control [C] and DSS-treated flies [D]. All the assays were performed in triplicates
Catalase aids in protection against oxidative stress (Nandi et al. 2019). The study observed that there was a significant increase in catalase activity upon DSS treatment. The increase was ~ 47% in males and ~ 58% in females, respectively (Fig. 3b). The mRNA expression was a similar trend to the enzyme activity, where the male and female flies showed a 3 to 4 fold increase in mRNA expression compared to the control (Fig. 4a, b).
The glutathione peroxidases are vital enzymes that, along with SOD and catalase, help scavenge H2O2 formed during the metabolic process (He et al. 2017). In this study on DSS treatment, the GPx activity was significantly increased by ~ 58% in males and ~ 25% in females, respectively (Fig. 3c). Moreover, there was a <1-fold change in the mRNA levels in the DSS-treated flies (Fig. 4a, b).
Oxidative stress is well documented in inflammatory bowel disease (IBD), where increased ROS and reduced antioxidant levels have been attributed to the cause of mucosal inflammation that leads to chronic tissue damage (Bhattacharyya et al. 2014; Kaushal et al. 2014). The colon is more susceptible to oxidative damage because of the relatively low amount of antioxidants available in the mucosa (Wang et al. 2020). On the other hand, the non-enzymatic antioxidants usually interrupt the chain reactions of reactive free radicals, thereby preventing the damage. As numerous non-enzymatic antioxidants contribute to cellular function. In the present study, we performed a total antioxidant assay to evaluate the overall performance of the cellular antioxidant defense upon treatment with DSS. From the study, it was evident that irrespective of gender, treatment with DSS caused a significant reduction in the total antioxidant levels in the digestive tract of Drosophila. Further studies looking at the activity of antioxidant enzymes revealed that there was a drastic reduction in the activity of SOD in the lysates of the digestive tract. However, the catalase and GPx had increased activities in both male and female flies. Catalase is one of the intracellular antioxidants that are present and released into circulation upon oxidative burst. Catalase is primarily involved in converting hydrogen peroxide to water. However, catalase did not have any correlation with disease progression. Studies in IBD patients have shown that there are increased levels of catalase activity during the onset of the disease (Rana et al. 2014). This probably is a compensatory mechanism associated with the cell to circumvent the inflammatory process. Another enzyme, namely, GPx is also involved in reducing hydrogen peroxide and organic peroxides forming water. Even this enzyme was found to be elevated in the plasma of IBD (pediatric) patients and DSS-treated mouse models (Tham et al. 2002; Tian et al. 2017; Hoffenberg et al. 1997; Thomas et al. 1994). However, the identity of this elevated activity is still not clear. The expression of these enzymes at the transcriptional level was analyzed by isolating the total RNA from the digestive tract. The qPCR analysis shows an increase in the mRNA expression of the SOD, catalase, and PHGPx enzymes (Fig. 4a, b).
Past reports have shown in Drosophila there are three unpaired (upd) cytokines that are released in response to injury or infection. Amongst them, upd2 is known to be produced in progenitors as well as in enterocytes and has additive effects on upd3 during the regeneration of epithelial cells (Osman et al. 2012; Jiang et al. 2016). Whereas, upd3 is expressed in enterocytes and is strongly induced during injury or infection (Osman et al. 2012; Jiang et al. 2016; Zhou et al. 2013; Chakrabarti et al. 2016). Therefore, qPCR analyses for upd2 and upd3 were carried out to assess the activation of inflammatory pathways in the gut. From the analysis, it was observed that upd2 and upd3 were relatively overexpressed in DSS-treated gut samples compared to the untreated controls (Fig. 4c, d). Moreover, the expression of upd3 was relatively higher (20–30 folds) than upd2 (up to five folds). Thus, like previous reports, our study indicates that upd3 and to a lesser extent, upd2 are upregulated and likely to react to systemic wound response upon exposure to DSS.
The intestine is the primary digestive organ that absorbs nutrients and is exposed to several stressor agents (Bonfini et al. 2016). One among them is the microorganisms that are ingested or commensals which contribute to the host immune immunity. The gut microflora has a potential impact on the physiology and metabolism of an organism (Gilbert et al. 2018). Dysbiosis of gut bacteria has been implicated in chronic conditions such as IBD and neuroinflammation in humans (Gilbert et al. 2018). While the gut microbiome of Drosophila does not fully represent the mammalian intestine. Knowing that the taxon-specific microbial composition will help in investigating the functional pathways that can affect gut homeostasis (Bacchetti De Gregoris et al. 2011). The Drosophila intestine is mainly composed of bacteria from the phyla Actinobacteria, Firmicutes, Bacteroidetes, α-Proteobacteria, and γ-Proteobacteria as reported by various studies (Chandler et al. 2011; Broderick and Lemaitre 2012). From the analysis of the gut samples, it was observed that there was overexpression of Actinobacteria, Bacteroidetes, Firmicutes, α-proteobacteria, γ-proteobacteria when compared to the untreated controls (Sup Fig. 1). Although overexpression of all the phyla was observed, the fold change was different among each other. For instance, Actinobacteria showed an 80–90-fold increase in expression upon DSS treatment. Followed by α-Proteobacteria which showed a 40–50 times fold increase in expression of bacterial DNA than the untreated controls. In the human context, these 4 major phyla have been extensively studied (Khanna and Tosh 2014). Of them, during inflammatory conditions such as IBD (Crohn’s disease and ulcerative colitis), Proteobacteria has been shown to drastically increase in mammalian models and humans (Shin et al. 2015; Sartor 2008; Rehman et al. 2010; Gophna et al. 2006). Although in Drosophila, microbial dysbiosis during DSS treatment has not been previously studied. But our preliminary analyses do highlight the fact that proinflammatory bacteria mainly belonging to phylum Proteobacteria may have a vital role in the progression of the disease.
Conclusions
In conclusion, the present study demonstrates that DSS treatment in Drosophila melanogaster causes gut inflammation similar to that of human patients suffering from IBD. This study also revealed that lowered antioxidant status along with upregulation of ROS and IL-6-like cytokines were similar to ulcerative colitis patients and likely a potential cause for such a clinical manifestation in flies. Moreover, other clinical parameters such as weakness, impairment in physical mobility, shortening of lifespan, and reduced digestive gut length were representative of human IBD pathophysiology. However, Drosophila lacks adaptive immunity; but their genetic makeup, distinct architecture, and similar physiology resembling the digestive tract of vertebrates make them useful. More importantly, Drosophila shares conserved signaling mechanisms for human intestinal pathophysiology, making them a convenient model for studying IBD.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure S1: qPCR analysis for the gut microbiota (bacterial phyla specific analysis) using the digestive tract of control and DSS-treated flies. All the assays were performed in triplicates (TIF 3843 KB)
Acknowledgements
The authors thank the DST-PURSE facility, Mangalore University, for the confocal microscopy. Dr. Shamprasad Varija Raghu is grateful to DBT Ramalingaswami Fellowship. The authors thank UGC-SAP Funded Facility at the Department of Applied Zoology, Mangalore University for qPCR analysis and Dr. Anurag Sharma, NUCSER, Nitte (Deemed to be University), Mangalore for providing the upd2 and upd3 primers.
Funding
SVR is grateful to the Department of Biotechnology (DBT), Government of India for financial support as DBT-Ramalingaswami Re-entry fellowship (102/IFD/SAN/1998/2015-16).
Declarations
Conflict of interest
The authors declare that they have no conflict of interests, financial or otherwise.
Ethical standards
The current study does not involve any human participants. The use of wild type Drosophila melanogaster in the current studies is approved by the Institutional Ethical committee (MU/AZ/1091/IAEC/2016-17).
Consent to participate
All authors have seen the manuscript and approved to submit the manuscript.
Footnotes
Nishal Keshav, Ramyalakshmi Ammankallu and Shashidhar contributed equally to this work.
Contributor Information
Avinash Kundadka Kudva, Email: avinash.kudva@gmail.com.
Shamprasad Varija Raghu, Email: shamprasadvarijaraghu@gmail.com.
References
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011;86(3):351–356. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Bannister JV, Bannister WH, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22:111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfini A, Liu X, Buchon N. From pathogens to microbiota: how Drosophila intestinal stem cells react to gut microbes. Dev Comp Immunol. 2016;64:22–38. doi: 10.1016/j.dci.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol. 2019;16:247–259. doi: 10.1038/s41575-018-0091-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2021;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo F, Wilson A, Di Cara F. The Intestine of Drosophila melanogaster: an emerging versatile model system to study intestinal epithelial homeostasis and host-microbial interactions in humans. Microorganisms. 2019;7(9):336. doi: 10.3390/microorganisms7090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Dudzic JP, Li X, Collas EJ, Boquete JP, Lemaitre B. Remote control of intestinal stem cell activity by haemocytes in Drosophila. PLoS Genet. 2016;12:e1006089. doi: 10.1371/journal.pgen.1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JA, Lang J, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:152511–152514. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Su R, Ni S, Liu Y, Huang J, Li G, Wang Q, Zhang X, Yang Y. Context-dependent responses of Drosophila intestinal stem cells to intracellular reactive oxygen species. Redox Biol. 2021;39:101835. doi: 10.1016/j.redox.2020.101835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran KE, Lamson NG, Whitehead KA. Expanding the utility of the dextran sulfate sodium (DSS) mouse model to induce a clinically relevant loss of intestinal barrier function. PeerJ. 2020;8:e8681. doi: 10.7717/peerj.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun. 2015;64:91–100. doi: 10.1016/j.jaut.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, et al. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen Van Zanten SJO. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Hoffenberg EJ, Deutsch J, Smith S, Sokol RJ. Circulating antioxidant concentrations in children with inflammatory bowel disease. Am J Clin Nutr. 1997;65:1482–1488. doi: 10.1093/ajcn/65.5.1482. [DOI] [PubMed] [Google Scholar]
- Howard AM, LaFever KS, Fenix AM, Scurrah CR, Lau KS, Burnette DT, et al. DSS-induced damage to basement membranes is repaired by matrix replacement and crosslinking. J Cell Sci. 2019 doi: 10.1242/jcs.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyyaswamy A, Rathinasamy S. Effect of chronic exposure to aspartame on oxidative stress in brain discrete regions of albino rats. J Biosci. 2012;37:679–688. doi: 10.1007/s12038-012-9236-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Tian A, Jiang J. Intestinal stem cell response to injury: lessons from Drosophila. Cell Mol Life Sci. 2016;73:3337–3349. doi: 10.1007/s00018-016-2235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennett MJ, Desai D, et al. Crucial role of macrophage selenoproteins in experimental colitis. J Immunol. 2014;193:3683–3692. doi: 10.4049/jimmunol.1400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Tosh PK. A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014;89:107–114. doi: 10.1016/j.mayocp.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, et al. Emerging trends of inflammatory bowel disease in South Korea: a nationwide population-based study. J Gastroenterol Hepatol. 2019;34:1018–1026. doi: 10.1111/jgh.14542. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric Food Chem. 2012;60:6418–6424. doi: 10.1021/jf204970r. [DOI] [PubMed] [Google Scholar]
- Linderman JA, Chambers MC, Gupta AS, Schneider DS. Infection-related declines in chill coma recovery and negative geotaxis in Drosophila melanogaster. PLoS One. 2012;7:e41907. doi: 10.1371/journal.pone.0041907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RR, McCracken AW, Simons MJP, Henriques CM, Rera M. How to Catch a Smurf?—ageing and beyond… in vivo assessment of intestinal permeability in multiple model organisms. Bio Protoc. 2018 doi: 10.21769/BioProtoc.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Nandi A, Yan LJ, Jana CK, Das N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev. 2019;2019:9613090. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145(158–165):e152. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- Nocerino A, Nguyen A, Agrawal M, Mone A, Lakhani K, Swaminath A. Fatigue in inflammatory bowel diseases: etiologies and management. Adv Ther. 2020;37:97–112. doi: 10.1007/s12325-019-01151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, Tsai YC, Lemaitre B. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J Cell Sci. 2012;125:5944–5949. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- Paithankar JG, Raghu SV, Patil RK. Concomitant changes in radiation resistance and trehalose levels during life stages of Drosophila melanogaster suggest radio-protective function of trehalose. Int J Radiat Biol. 2018;94:576–589. doi: 10.1080/09553002.2018.1460499. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Qazi T. Fatigue in inflammatory bowel disease: a problematic ailment. Curr Opin Gastroenterol. 2020;36:284–294. doi: 10.1097/MOG.0000000000000644. [DOI] [PubMed] [Google Scholar]
- Raghu SV, Mohammad F, Chua JY, Lam JSW, Loberas M, Sahani S, et al. A zinc-finger fusion protein refines Gal4-defined neural circuits. Mol Brain. 2018;11:46. doi: 10.1186/s13041-018-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu SV, Patil R. GAL4-UAS system for genetic labeling and visualization of specific regions of brain. Experiments with Drosophila for Biology Courses, Indian Academy of Sciences. 2021;1:233–237. [Google Scholar]
- Ramasarma T, Rao AVS, Devi MM, Omkumar RV, Bhagyashree KS, Bhat SV. New insights of superoxide dismutase inhibition of pyrogallol autoxidation. Mol Cell Biochem. 2015;400:277–285. doi: 10.1007/s11010-014-2284-z. [DOI] [PubMed] [Google Scholar]
- Ramond E, Jamet A, Ding X, Euphrasie D, Bouvier C, Lallemant L, He X, Arbibe L, Coureuil M, Charbit A. Reactive oxygen species-dependent innate immune mechanisms control methicillin-resistant Staphylococcus aureus virulence in the Drosophila larval model. Mbio. 2021;12:e00276-21. doi: 10.1128/mBio.00276-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana SV, Sharma S, Prasad KK, Sinha SK, Singh K. Role of oxidative stress and antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–571. [PMC free article] [PubMed] [Google Scholar]
- Rehman A, Lepage P, Nolte A, Hellmig S, Schreiber S, Ott SJ. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J Med Microbiol. 2010;59:1114–1122. doi: 10.1099/jmm.0.021170-0. [DOI] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol. 2008;43:739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Sheng X, Zhu Y, Zhou J, Yan L, Du G, Liu Z, Chen H. Antioxidant effects of caffeic acid lead to protection of Drosophila intestinal stem cell aging. Front Cell Dev Biol. 2021;9:735483. doi: 10.3389/fcell.2021.735483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends in Biotech. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Simon AF, Liang DT, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev. 2006;127:647–651. doi: 10.1016/j.mad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sobrado CW, Sobrado LF. Management of acute severe ulcerative colitis: a clinical update. Arq Bras Cir Dig. 2016;29:201–205. doi: 10.1590/0102-6720201600030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Su H, Kang Q, Wang H, Yin H, Duan L, Liu Y, et al. Changes in expression of p53 and inflammatory factors in patients with ulcerative colitis. Exp Ther Med. 2019;17:2451–2456. doi: 10.3892/etm.2019.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res. 2002;51:641–646. doi: 10.1203/00006450-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Miller V, Shenkin A, Fell GS, Taylor F. Selenium and glutathione-peroxidase status in pediatric health and gastrointestinal-disease. J Pediatr Gastr Nutr. 1994;19:213–219. doi: 10.1097/00005176-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine WJ. Diagnosis and treatment of indeterminate colitis. Gastroenterol Hepatol (NY) 2011;7:826–828. [PMC free article] [PubMed] [Google Scholar]
- van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:131–143. doi: 10.1111/j.1365-2036.2010.04347.x. [DOI] [PubMed] [Google Scholar]
- Veauthier B, Hornecker JR. Crohn's disease: diagnosis and management. Am Fam Physician. 2018;98:661–669. [PubMed] [Google Scholar]
- Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Chen Y, Zhang XY, Lu YP, Chen HX. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J Funct Foods. 2020;75:104248. doi: 10.1016/j.jff.2020.104248. [DOI] [Google Scholar]
- Wang Y, Wen R, Liu D, Zhang C, Wang ZA, Du Y. Exploring effects of chitosan oligosaccharides on the DSS-induced intestinal barrier impairment in vitro and in vivo. Molecules. 2021 doi: 10.3390/molecules26082199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weegh N, Funer J, Janke O, Winter Y, Jung C, Struve B, et al. Wheel running behaviour in group-housed female mice indicates disturbed wellbeing due to DSS colitis. Lab Anim. 2020;54:63–72. doi: 10.1177/0023677219879455. [DOI] [PubMed] [Google Scholar]
- Wright VM, Vogt KL, Smythe E, Zeidler MP. Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal. 2011;23:920–927. doi: 10.1016/j.cellsig.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019;17:54–62. doi: 10.5217/ir.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med. 2020 doi: 10.3390/jcm9051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler MP, Bach EA, Perrimon N. The roles of the Drosophila JAK/STAT pathway. Oncogene. 2000;19:2598. doi: 10.1038/sj.onc.1203482. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Rasmussen A, Lee S, Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev Biol. 2013;373:383–393. doi: 10.1016/j.ydbio.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: qPCR analysis for the gut microbiota (bacterial phyla specific analysis) using the digestive tract of control and DSS-treated flies. All the assays were performed in triplicates (TIF 3843 KB)