Corresponding Author
Key Words: anticoagulation, computed tomography, exercise
Cycling is a worldwide passionately practiced and followed by millions of people across the globe. While many of us see it as an enjoyable leisure activity or a healthy way to commute to and from work, for some recreational cyclists it can become an obsession. Avid recreational cyclists oftentimes put their body through such rigorous and demanding training blocks in order to achieve peak levels of performance that they can reach workloads comparable to those endured by professional cyclists. Whether it is watching cycling pros battling fierce uphill inclines every summer at the Tour de France or trying to overtake a fellow cyclist on a local hill during a weekend Café ride, cycling enthusiasts live their sport with passion and an almost religious-like devotion.
Medically speaking, however (and stripping any romanticism from our thoughts), cycling may be viewed by many as a potentially dangerous activity. Besides the risk associated with sharing roads with motorized vehicles or riding a bicycle downhill into hairpin curves at fast speeds, there is also the heavy toll many recreational cyclists put their bodies through by pushing the limits of their functional threshold power and claim the KOM (king of mountains) at their local STRAVA segment. Besides the known cardiovascular adaptations endurance athletes (including cyclists) can sustain, resulting in the “athlete’s heart” syndrome,1 the cumulative workload exerted on different muscle groups, particularly the lower train, and the repetitive muscle engagement during rides and training exercises can lead to relatively uncommon but potentially dangerous medical risks.
In this issue of JACC: Case Reports, Petek et al2 discuss the case of a 22-year-old avid cyclist (riding load of 250-300 miles per week) presenting with right lower extremity pain and swelling, who was later found to have extensive acute deep vein thrombosis (DVT) related to mechanical compression of the right common iliac vein (RCIV) from psoas muscle hypertrophy. The diagnosis was confirmed by venous Duplex ultrasound and a venous phase computed tomography scan showing moderate compression of the RCIV between the enlarged right psoas muscle, the right common iliac artery, and L5 vertebral body proximally, and between the right internal iliac artery and psoas muscle distally. The patient was initiated on anticoagulation therapy with subcutaneous enoxaparin and then transitioned to oral apixaban. He was recommended to undergo detraining by reducing his riding load for a period following his thrombotic event. Despite treatment and recommendations, 6 months later he reported ongoing right leg swelling and exertional leg pain. Repeat computed tomography showed unchanged mechanical compression of the RCIV from the hypertrophied psoas muscle and partial recanalization of the right superficial femoral vein. Studies to evaluate a hypercoagulable state revealed a homozygous factor V Leiden gene mutation. The patient ultimately underwent an invasive procedure confirming stenosis of the RCIV on contrast venography and a slitlike compression of the RCIV on an intravascular ultrasound scan. Serial balloon dilatations did not improve the vein compression, but a decision was made against placing a stent because of his young age and concerns for long-term patency.
This case illustrates an uncommon presentation of proximal DVT in a previously healthy recreational cyclist, with all components of Virchow's triad present: A hypercoagulable state from a homozygous factor V Leiden gene mutation, endothelial injury from external compression of a hypertrophied psoas muscle, and venous stasis from frequent and repetitive hip flexion associated with the cycling riding posture. Despite the rigorous evaluation by the authors,2 (including a high index of suspicion for the initial diagnosis), the adequacy of their medical care, and the importance of raising awareness about the potential association (and likely causality) of psoas muscle hypertrophy in the development of DVT in cyclists, many unanswered questions remain. Can DVT develop in cyclists solely as a result of mechanical compression of the iliac vein from a hypertrophied psoas muscle, without inherited or acquired hypercoagulability? What is the actual incidence of psoas muscle hypertrophy–related DVT in diagnosed cases of amateur and professional cyclists? Are certain types of cycling disciplines and cycling-related training activities more likely to increase the risk of DVT secondary to psoas muscle hypertrophy (track cycling vs road cycling, sprint training vs endurance training)?, Can other risk factors play a role in this scenario (eg, dehydration during training or racing or use of performance-enhancing medications or nutritional supplements)?
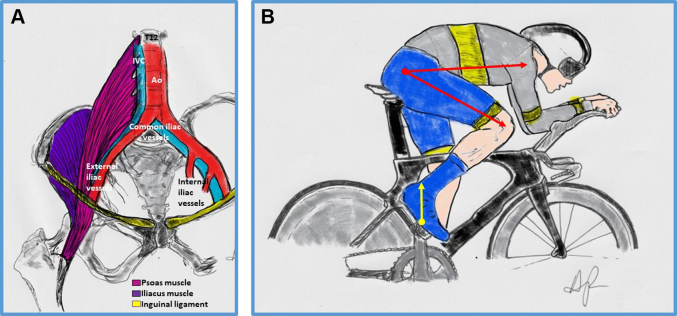
Psoas muscle hypertrophy is reported in the medical literature as the cause of a wide variety of mechanical compression issues in runners, cyclists, and other endurance athletes, including colonic spams,3 ureteral compression,4 and lower extremity edema resulting from obstruction of lymphatic drainage.5 External Iliac artery (EIA) endofibrosis is a well-documented complication of psoas hypertrophy in trained recreational and competitive cyclists.6 Hypertrophy of other muscle groups engaged during cycling has also been linked with development of unprovoked DVT, including the gastrocnemius in cases of popliteal vein entrapment syndrome.7 Thus, a modified Virchow triad (the cyclist triad), including mechanical compression from psoas muscle hypertrophy, repetitive trauma during pedaling and hip flexion posture on the bike (Figures 1A and 1B), and hypercoagulability from inherited coagulopathies, dehydration or unregulated nutritional supplement or medication use could create the “perfect storm” for iliac vein DVT in cyclists. In a study of 33 competitive athletes (17 cyclists, 8 triathletes, and 2 runners), the single most important anatomical factor associated with symptomatic EIA endofibrosis was the psoas muscle transverse cross-sectional area at the L5 level on the affected side, when compared with the nonaffected contralateral side. It has been postulated that the development of clipless pedals (where the shoe is attached to the pedal by using a spring retention system) has changed the biomechanics of modern-day cycling, by allowing for more pedaling power generation through forceful hip flexion in addition to hip extension and thus increasing the risk of psoas muscle hypertrophy.8 The unilateral nature of most clinically reported cases of EIA endofibrosis (and probably also for iliac DVT, as in the case reported by Petek et al2) is likely a marker of asymmetry in psoas muscle hypertrophy due to preferential repetitive stress on the affected limb. Uneven power force readings and pedaling differences between both legs (dominant vs nondominant) are commonly seen in recreational and competitive cyclists and may vary due to pedaling cadence, exercise intensity and duration, and type of activity (competition vs training).9
Figure 1.
Biomechanical Adaptation of the Hip Flexors and Iliac Vessel Entrapment From Psoas Muscle Hypertrophy
(A) Repetitive hip flexion and engagement of the psoas muscle during training and cycling can result in muscle hypertrophy, with subsequent compression of adjacent vascular structures leading to iliac artery endofibrosis or deep vein thrombosis of the iliac vein on the affected side. (B) The biomechanics of cycling affecting psoas muscle hypertrophy include increased vector forces during hip flexion (yellow arrow) from clip-on pedaling style (upward stroke motion and power transmission) and hyperflexion of the hip joint at the highest point of the pedaling stroke (red arrows) favored by extreme aerodynamic riding positions, as seen during time trialing and triathlon disciplines. Ao = aorta; IVC = inferior vena cava.
To understand the biomechanical adaptation of the lower train muscles to repetitive training more clearly, Ema et al10 prospectively compared the muscular volumes of individual lower body muscle groups by magnetic resonance imaging between competitive and untrained age- and body size–-matched individuals, at baseline and following a period of training for the competitive cyclists group. These investigators identified a nonhereditary training effect of skeletal muscle adaptation (muscle volume hypertrophy) in several lower extremity muscles, including the hip flexors, particularly the psoas muscle, after 6 months of competitive training.10
This interesting case report highlights a potential cause of DVT in both professional and recreational cyclists, which must not be overlooked when such individuals present with unilateral lower extremity edema or signs and symptoms suggestive of DVT. A high index of clinical suspicion is necessary and subsequent imaging is required to confirm the diagnosis of psoas hypertrophy and mechanical compression of the iliac vein. As seen in this case and other reported cases of DVT in athletes, evaluation for a possible hypercoagulable state is also recommended, as oftentimes these patients have more than 1 predisposing factor. Treatment should aim at mechanical and/or pharmacologic recanalization of the affected vein, systemic anticoagulation, and adequate physiotherapy and detraining to allow for a prompt recovery. Surgical options are common practice for patients with symptomatic EIA endofibrosis, with reported excellent results. On the contrary, for cases of compression-related proximal DVT, definitive treatment is less clear, and long-term outcomes may unfortunately affect the individual’s ability to continue to practice their sport at a competitive or even recreational level, especially given the risks associated with systemic anticoagulation in the context of a sport prone to falls and severe injuries. Although there have been no reports of pulmonary embolism as the cause of sudden death among recreational and professional cyclists from autopsy case series,11, 12, 13 the potential risks of proximal DVT, large clot burden, and subsequent risk for hemodynamically significant pulmonary embolism or development of pulmonary hypertension over recurrent thromboembolic events cannot be underestimated. There is no definitive answer regarding what would be considered a safe level of training load to avoid psoas muscle hypertrophy because this threshold may be different across individuals with differences in body adaptation to the training stress. However, it appears that a combination of repetitive cycling (high training and/or racing loads), hyperflexion of the hip joint favored by modern-day aerodynamic bicycles and cycling positioning, and current pedal motion techniques favoring hip flexion loading all contribute to the lower train biomechanical load that ultimately leads to psoas muscle hypertrophy and its mechanical complications.
Funding Support and Author Disclosures
The author has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Sharma S., Merghani A., Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J. 2015;36(23):1445–1453. doi: 10.1093/eurheartj/ehv090. [DOI] [PubMed] [Google Scholar]
- 2.Petek B.J., Soong C.-P., Buckley A.J., et al. Acute deep vein thrombosis in a cyclist with iliac vein compression from psoas muscle hypertrophy. J Am Coll Cardiol Case Rep. 2022;4(17):1080–1085. doi: 10.1016/j.jaccas.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson D.J., Khan A.N., Shreeve D.R. Psoas muscle hypertrophy: mechanical cause for “jogger’s trots?”. Br Med J (Clin Res Ed) 1985;291(6498):787–788. doi: 10.1136/bmj.291.6498.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramesmayer C., Mitterberger M., Oberhammer L., Kunit T., Lusuardi L. Lower ureteral compression through external vascular elongation in a cyclist: a case report. Int J Surg Case Rep. 2021;83 doi: 10.1016/j.ijscr.2021.106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manoharan A., Pitney M.R. Bilateral pelvic masses in a long-distance cyclist. Postgrad Med J. 1988;64(758):977–978. doi: 10.1136/pgmj.64.758.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim C.S., Gohel M.S., Shepherd A.C., Davies A.H. Iliac artery compression in cyclists: mechanisms, diagnosis and treatment. Eur J Vasc Endovasc Surg. 2009;38(2):180–186. doi: 10.1016/j.ejvs.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Kean J., Pearton A., Fell J.W., et al. Deep vein thrombosis in a well-trained masters cyclist, is popliteal vein entrapment syndrome to blame? J Thromb Thrombolysis. 2019;47(2):301–304. doi: 10.1007/s11239-018-1796-x. [DOI] [PubMed] [Google Scholar]
- 8.Fisher A.T., Tran K., Dossabhoy S.S., Sorondo S., Fereydooni A., Lee J.T. Ann Vasc Surg; Published online May 30, 2022. Anatomic factors contributing to external iliac artery endofibrosis in high performance athletes. [DOI] [PubMed] [Google Scholar]
- 9.Carpes F.P., Mota C.B., Faria I.E. On the bilateral asymmetry during running and cycling - a review considering leg preference. Phys Ther Sport. 2010;11(4):136–142. doi: 10.1016/j.ptsp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Ema R., Wakahara T., Yanaka T., Kanehisa H., Kawakami Y. Unique muscularity in cyclists’ thigh and trunk: A cross-sectional and longitudinal study. Scand J Med Sci Sports. 2016;26:782–793. doi: 10.1111/sms.12511. [DOI] [PubMed] [Google Scholar]
- 11.Suárez-Mier M.P., Aguilera B. Causes of sudden death during sports activities in Spain. Rev Esp Cardiol. 2002;55(4):347–358. [PubMed] [Google Scholar]
- 12.de Noronha S.V., Sharma S., Papadakis M., Desai S., Whyte G., Sheppard M.N. Aetiology of sudden cardiac death in athletes in the United Kingdom: a pathological study. Heart. 2009;95(17):1409–1414. doi: 10.1136/hrt.2009.168369. [DOI] [PubMed] [Google Scholar]
- 13.Raschka C., Parzeller M., Kind M. Organ pathology causing sudden death in athletes. International study of autopsies (Germany, Austria, Switzerland)] Med Klin (Munich) 1999;94(9):473–477. doi: 10.1007/BF03044938. [DOI] [PubMed] [Google Scholar]