Abstract
Extreme endurance athletic challenges provide unique opportunities to study the cardiovascular system’s capacity for structural, functional, and hemodynamic adaptation. The authors present a case of a male subject who ran 2,469 km, with serial multiparametric cardiac magnetic resonance imaging used to demonstrate adaptive and maladaptive alterations in cardiac remodeling and myocardial tissue health. (Level of Difficulty: Advanced.)
Key Words: 4D-flow, cardiac magnetic resonance, strain analysis, tissue mapping
Abbreviations and Acronyms: 3D-MDA, 3-dimensional myocardial deformation analysis; 4D-flow, 4-dimensional flow analysis; CMR, cardiac magnetic resonance; ECV, extracellular volume; EDV, end-diastolic volume; ESV, end-systolic volume; LA, left atrium; LV, left ventricle; RV, right ventricular
Central Illustration
Rarely, individuals undertake endeavors that exceed the perceived boundaries of conventional sports, placing unusual demands on the human cardiovascular system. Such events present a unique opportunity to study the impact of extreme physiological demand on cardiac tissue health, chamber remodeling, contractile performance, and their resultant influence on cardiac hemodynamics. Contemporary cardiac magnetic resonance (CMR) imaging provides the unique capacity to study these in a single setting through the use of tissue mapping,1 3-dimensional myocardial deformation analysis (3D-MDA),2 and 4-dimensional flow analysis (4D-flow).3 In this case report, we performed serial CMR evaluations in a 37-year-old athlete who ran 2.469 km to raise awareness for rare disease research. Alterations in cardiac phenotype were compared with a concurrently recruited healthy volunteer cohort.
Learning Objectives
-
•
To describe the structural and hemodynamic alterations experienced in response to supraphysiological demands during extreme sporting activities.
-
•
To describe the relationship between serum and CMR-based markers of cardiac injury in the setting of ultramarathon running.
-
•
To recognize the versatility and value of contemporary CMR techniques to evaluate cardiovascular adaptation in the setting of endurance sports.
History of Presentation
A 37-year-old ultramarathon runner preparing to run across Canada gave consent to participate in a case study. Weight was 74 kg, height was 1.78 m (body surface area 1.91 kg/m2), and baseline blood pressure was 105/51 mm Hg. He ran 1,122 km over the first 11 days (average distance 102 km/day) leading up to a peak performance evaluation. During this period, the maximal elevation gain was 2,095 feet, with a mean cadence of 170 to 180 strides/min and mean heart rate (during moving time) of 150 to 155 beats/min. He subsequently completed 1,347 km over 21 days in the context of an injury (experienced on day 22). Following a 2-month period of rest, he underwent a convalescent evaluation. Fifteen healthy control subjects (ages 18 to 65 years, mean 44 years) with no cardiovascular or pulmonary disease were prospectively recruited and underwent baseline evaluations to establish reference values for serum biomarkers and CMR findings. The study was approved by the Conjoint Health Research Ethics Board at University of Calgary (REB 18-0664), and all subjects provided written informed consent.
Past Medical History
The case subject and healthy control subjects were nonsmokers with no known cardiovascular disease, and no history of diabetes or hypertension. Healthy control subjects were prospectively recruited from the local community and were required to be nonsedentary, but not participating in >3 h/wk of sporting activity (aerobic or isometric).
Differential Diagnosis
Not relevant to case.
Investigations
Serial CMR, bloodwork, and clinical evaluations were performed in the case subject at 4 time points: baseline (2 months before the run), prerun (2 days before, following 2 months of daily training), peak run (day 11), and convalescence (2 months following completion of event). Baseline and convalescent evaluations incrementally included gadolinium for the assessment of extracellular volume (ECV) fraction. Peak stage imaging was performed 4 hours following the cessation of activity; this was pragmatically chosen to provide a period of metabolic rest. Imaging was performed on a 3-T scanner (Prisma, Siemens Healthineers) using a standardized protocol to acquire standard cine images in long and short axes, native T1 mapping (precontrast and postcontrast), native T2 mapping, and 4D-flow.4 Commercial software (cvi42, Circle Cardiovascular Imaging) was used for all measurements except 3D-MDA, performed using previously validated software.2 Volumes were indexed to height, given the anticipated change in weight during the event.
Serum biomarkers are summarized in Table 1, these captured at time of CMR (each stage for the case subject and at time of single reference CMR for healthy control subjects). Significant elevations in troponin T and N-terminal pro–B-type natriuretic peptide (NT-proBNP) were observed at peak exercise, associated with concurrent reduction in hemoglobin and hematocrit. All values normalized in convalescence, except from hemoglobin, which remained lower than baseline.
Table 1.
Serial Clinical and Serum Markers Captured During Extreme Endurance Event With Comparison to Healthy Control Cohort
| Healthy Cohort (n = 15) | Subject |
||||
|---|---|---|---|---|---|
| Baseline (−2 Months) | Prerun (−2 Days) | Peak Run (11 Days) | Convalescence (+2 Months) | ||
| Clinical characteristics | |||||
| Age, y | 44.4 ± 14.6 | 37 | |||
| Male | 9 (60) | 1 (100) | |||
| Height, m | 1.74 ± 0.08 | 1.78 | |||
| Weight, kg | 75.8 ± 13.1 | 74.0 | 70.3 | 70.0 | 72.0 |
| BSA, kg/m2 | 1.91 ± 0.20 | 1.91 | 1.86 | 1.87 | 1.9 |
| Systolic blood pressure, mm Hg | 111.1 ± 12.3 | 104 | 114 | 114 | 107 |
| Diastolic blood pressure, mm Hg | 69.8 ± 9.2 | 51 | 61 | 61 | 51 |
| Heart rate, beats/min | 57.8 ± 11.2 | 51 | 58 | 74 | 67 |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hyperlipidemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diabetes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Serum biomarkers | |||||
| Troponin T, ng/L | 5.0 ± 4.1 | 3 | 4 | 37 | 3 |
| NT-proBNP, pg/mL | 29.2 ± 28.3 | 25 | 85 | 782 | 25 |
| LDL cholesterol, mmol/L | 2.59 ± 0.84 | 2.97 | 2.97 | 1.38 | 2.28 |
| HDL cholesterol, mmol/L | 1.45 ± 0.45 | 2.21 | 2.21 | 1.66 | 1.56 |
| Triglycerides, mmol/L | 1.74 ± 1.48 | 1.11 | 1.11 | 1.37 | 1.86 |
| Creatinine, mmol/L | 84.1 ± 13.2 | 96 | 96 | 98 | 80 |
| Hemoglobin, mg/dL | 146.4 ± 8.3 | 160 | 160 | 131 | 148 |
| Hematocrit, % | 43.3 ± 2.1 | 47 | 47 | 39 | 43 |
Values are mean ± SD, n, or n (%). As a descriptive study of serial evaluations performed in a single subject no additional statistical analyses were required nor appropriate to perform. Peak endurance activity serum biomarker measurements represented in bold.
BSA = body surface area; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
As shown in Table 2, baseline CMR showed chamber volumes were similar to control subjects with the exception of increased indexed right ventricular (RV) end-diastolic volume (EDV) and end-systolic volume (ESV), suggesting chronic remodeling. Mild inferior RV insertion site fibrosis was observed on late gadolinium enhancement imaging. Prerun CMR, performed following 2 months of daily 40-km marathon training, showed matched elevations in EDV and ESV for the left ventricle (LV), RV, and right atrium, with the left atrium (LA) showing a slight imbalance leading to mild reduction in the LA ejection fraction (global and booster components). At peak run (day 11) marked increases in EDV and ESV were observed for all chambers. Although being balanced for LV and RV, the atria demonstrated mild drops in ejection fraction.
Table 2.
Serial CMR Markers During Extreme Endurance Event With Comparison to Healthy Control Cohort
| Healthy Cohort (n = 15) | Subject |
||||
|---|---|---|---|---|---|
| Baseline (−2 Months) | Prerun (−2 Days) | Peak Run (+11 Days) | Convalescence (+2 Months) | ||
| Chamber volumes and global function | |||||
| LV EDV/height, mL/m | 92.3 ± 15.7 | 87.1 | 97.2 | 103.3 | 96.1 |
| LV ESV/height, mL/m | 34.0 ± 8.9 | 26.4 | 29.8 | 30.0 | 37.8 |
| LV CO, L/min | 5.7 ± 0.8 | 7.7 | 8.3 | 10.1 | 8.0 |
| LV EF, % | 64.1 ± 6.2 | 70 | 69 | 71 | 61 |
| LV mass/height, g/m | 61.5 ± 13.5 | 68.5 | 72.5 | 74.4 | 71.7 |
| RV EDV/height, mL/m | 99.7 ± 18.6 | 109.6 | 108.4 | 132.2 | 116.7 |
| RV ESV/height, mL/m | 43.3 ± 13.6 | 46.1 | 42.1 | 52.8 | 53.3 |
| RV CO, L/min | 5.7 ± 0.6 | 7.9 | 8.2 | 11 | 8.6 |
| RV EF, % | 57.3 ± 6.6 | 58 | 61 | 60 | 54 |
| LAVol max/height, mL/m | 38.2 ± 12.3 | 34.8 | 30.3 | 49.4 | 32.2 |
| LAVol min/height, mL/m | 17.9 ± 8.2 | 15.7 | 12.8 | 20.6 | 12.3 |
| LA EF, % | 63.5 ± 7.5 | 70 | 66 | 58 | 68 |
| LA booster EF, % | 24.6 ± 5.9 | 19 | 16 | 15 | 18 |
| RAVol max/height, mL/m | 57.0 ± 17.6 | 53.4 | 54.3 | 78.6 | 57.2 |
| RAVol min/height, mL/m | 29.8 ± 8.0 | 27.9 | 29.2 | 43.7 | 30.2 |
| RA EF, % | 45.6 ± 8.4 | 62 | 65 | 61 | 60 |
| 3D myocardial deformation analysis | |||||
| 3D peak systolic strain amplitude | |||||
| Circumferential | −15.2 ± 1.9 | −15.6 | −14.4 | −17.9 | −16.3 |
| Longitudinal | −14.3 ± 1.9 | −18.4 | −15.3 | −16.3 | −15.3 |
| 3D time to peak systolic strain, % cycle | |||||
| Circumferential | 40.7 ± 4.0 | 42.5 | 42.5 | 34.2 | 44.8 |
| Longitudinal | 41.4 ± 4.3 | 44.4 | 40.8 | 34.8 | 48.3 |
| 3D peak diastolic strain rate, 1/s | |||||
| Circumferential | −0.9 ± 0.1 | −1.4 | −1.1 | −1.5 | −1.3 |
| Longitudinal | −1.0 ± 1.0 | −1.5 | −1.3 | −1.5 | −1.1 |
| Diastolic wall thickness, mm | |||||
| Septal wall | 6.1 ± 0.9 | 6.7 | 6.4 | 8.0 | 7.1 |
| Lateral wall | 6.1 ± 0.9 | 6.7 | 6.4 | 8.0 | 7.1 |
| 4D-flow analysis | |||||
| LV flow component analysis | |||||
| Direct flow, % | 63 ± 20 | 64 | 67 | 50 | 60 |
| Delayed ejection, % | 11 ± 9 | 17 | 13 | 10 | 7 |
| Retained flow, % | 11 ± 9 | 17 | 13 | 10 | 7 |
| Residual volume, % | 16 ± 11 | 6 | 7 | 30 | 26 |
| Tissue mapping analysis | |||||
| Native T2 mapping | |||||
| Septal, ms | 42.1 ± 2.5 | 39.6 | 37.7 | 40.6 | 36.7 |
| Lateral, ms | 40.9 ± 3.7 | 38.4 | 38.7 | 38.4 | 38.2 |
| Native T1 mapping | |||||
| Septal, ms | 1,183.0 ± 40.0 | 1,204.0 | 1,185.6 | 1,260.4 | 1,191.0 |
| Lateral, ms | 1,166.2 ± 29.1 | 1,204.3 | 1,136.2 | 1,267.1 | 1,190.6 |
| Postcontrast T1 mapping | |||||
| Septal, ms | 657.0 ± 39.8 | 657.2 | — | — | 635.0 |
| Lateral, ms | 661.2 ± 39.3 | 665.1 | — | — | 645.7 |
| ECV, MOLLI | |||||
| Septal, % | 26.7 ± 1.9 | 28 | — | — | 31 |
| Lateral, % | 25.7 ± 2.2 | 28 | — | — | 30 |
Values are mean ± SD or n. Measurements were indexed to height, this being selected as a more stable reference for comparison of serial measurements throughout periods of extreme activity (associated with weight loss).
CMR = cardiac magnetic resonance; CO = cardiac output; ECV = extracellular volume; EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; LA = left atrium; LV = left ventricle; LAVol = left ventricular volume; RA = right atrium; RAVol = right ventricular volume; RV = right ventricle; MOLLI = Modified Look-Locker Imaging.
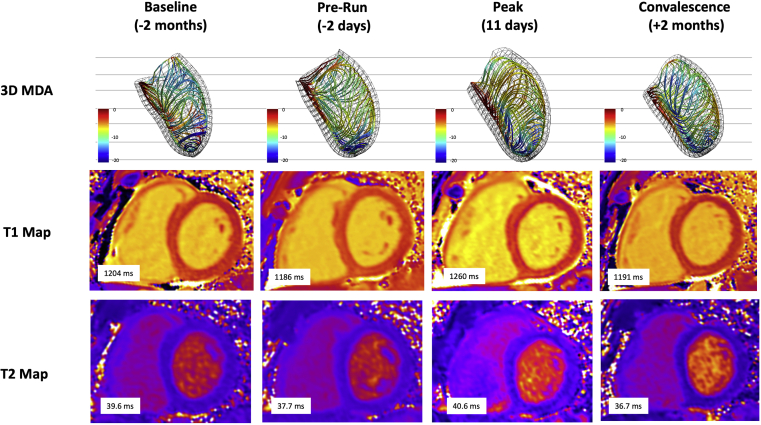
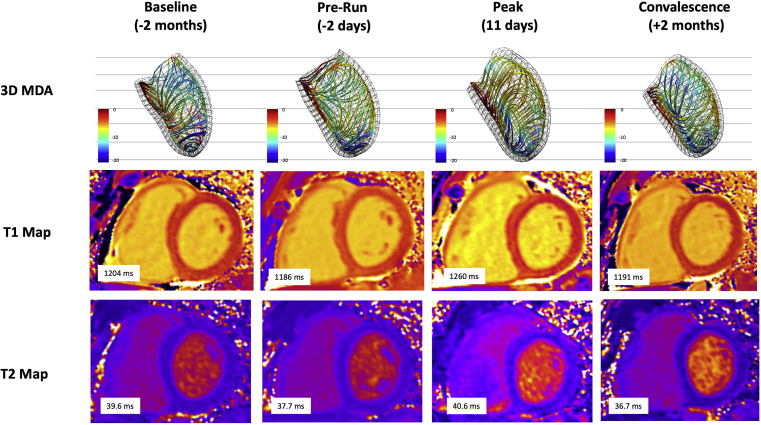
As shown in Table 2 and Figure 1, tissue mapping showed native T1, and to a lesser degree T2, transiently increased during the peak stage. These values normalized in convalescence. However, despite normalization, a 3% absolute increase in septal ECV (calculated using serum hematocrit) was observed at the convalescent evaluation. No visible changes were observed on late gadolinium enhancement imaging.
Figure 1.
CMR Evaluations Using 3D-MDA and Tissue Mapping
Septal views of 3D-MDA demonstrating regional reduction in circumferential strain amplitude during peak stage (3D strain lines projected in the dominant direction of local deformation). Serial T1 maps showing transient elevation in T1 at peak stage. Serial T2 maps showing modest transient elevation in T2. 3D = 3-dimensional; 3D-MDA = 3-dimensional myocardial deformation analysis; CMR = cardiac magnetic resonance.
3D-MDA revealed a transient elevation in LV septal and lateral wall thickness at the peak stage (Figure 1). Ventricular dilation was accompanied by diastolic flattening of the interventricular septum. Despite a subtle global rise in circumferential strain amplitude at the peak stage (Table 2), 3D analysis revealed regional reductions in septal regions (Figure 1). Time to peak systolic strain shortened significantly, consistent with prolonged total diastolic filling times.
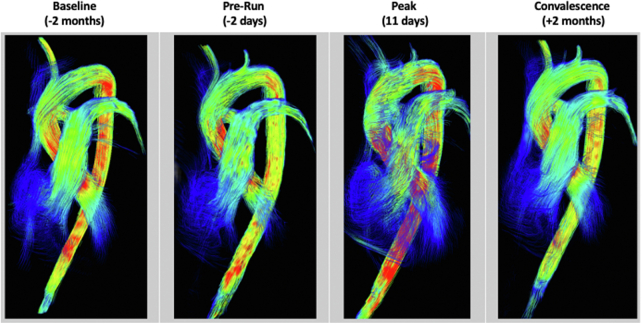
4D-flow demonstrated a loss of laminar flow at the peak stage with turbulence in the RV, main pulmonary artery, and ascending thoracic aorta by streamline analysis (Figure 2). Flow component analyses of the LV, summarized in Table 2, showed a reduction in direct flow and increase in residual volume, indicating loss in hemodynamic efficiency.
Figure 2.
Serial 4D-Flow CMR Analysis at All Time Points
Streamline visualization showing loss of laminar flow and turbulence in right-sided chambers and great vessels at the peak stage. 4D-flow = 4-dimensional flow analysis; CMR = cardiac magnetic resonance.
Management
The subject stopped running on day 32, 10 days following a progressive injury experienced on day 22. He did not participate in further training for 2 months, leading up to a convalescent evaluation. No other interventions were undertaken.
Discussion
This case study combined serial serum biomarkers and multiparametric CMR to document dynamic alterations in cardiac tissue, biomechanical performance, and cardiac hemodynamics during extreme endurance running. At the time of peak performance, a significant rise in serum markers of tissue injury were associated with increased tissue water content and multichamber dilation. Despite globally preserved contractility, regional reductions in septal strain and loss of hemodynamic efficiency were observed. At convalescence, a 3% increase in septal ECV was seen.
Prior CMR studies evaluating the influence of endurance sports on cardiac tissue health have largely focused on marathon duration events5 or the cumulative effects of such activities on cardiac remodeling.6 In the former, markers of acute tissue injury have failed to demonstrate significant alterations at time of marathon completion,7 being consistent with our observations following daily marathon (40 km) training. However, upon escalation in workload above training conditions, we saw a transient rise in native T1, marginal rise in T2 (not exceeding upper limits of reference population), and concurrent increase in LV mass. Collectively, these findings support expansion in tissue water content. However, whether this is in response to cellular injury, increased capillary permeability, elevated blood volume, or a combination remains uncertain. The observed elevation in serum troponin may evoke the former, however, prior work has suggested such elevation may reflect only an increase in cellular permeability.8
Substantial biventricular dilation was seen at the peak stage with respective increases in LV and RV EDV of 21% and 22% (vs baseline), respectively. Given no global reduction in contractile function, these changes led to a 31% increase in resting cardiac output. However, chamber dilation came with appreciable cost, resulting in abnormal intracardiac hemodynamics. Prominent turbulence was observed by 4D-flow imaging with reduced efficiencies confirmed by LV flow component analysis. With increasing turbulent energy loss an escalating mismatch between cardiac energy expenditure and forward cardiac output would be anticipated. This was evidenced by the increase seen in LV residual volume (from 6% to 30%) and the reduction in LA booster phase ejection fraction, the latter consistent with elevated LV filling pressures. Overall, these findings support that the rate and degree of chamber dilation experienced during athletic activity are important determinants of peak cardiovascular performance. These observations also provide clinical relevance to our understanding of cardiovascular disease states associated with chamber dilation, highlighting physiological tradeoffs for this adaptive mechanism that are anticipated to result in progressive reductions in cardiac efficiency and exercise capacity.
From prerace to peak race, we observed a rise in the peak diastolic strain rate despite a relatively longer total diastolic filling period (ie, reduced time to peak systolic strain). This finding may reflect an augmentation of early active diastolic filling or “untwisting” of the LV, an effective strategy to mitigate rising left atrial pressures. Of importance, prior work by Scott et al9 has suggested this valuable mechanism may become muted over time in trained endurance athletes, consistent with diastolic dysfunction.
Follow-Up
A repeat CMR evaluation was performed 2 months following completion of the endurance event, this being a deconditioning period given that injury prevented meaningful participation in athletic activity. At this time, only partial normalization was seen in chamber volumes despite normalization of tissue edema markers, and a 3% rise in septal ECV was documented.
Conclusions
In this unique case of a 37-year-old ultramarathon runner, we leveraged advanced multiparametric, multidimensional CMR to document the limits of short-term cardiovascular adaptation. Overall, this case elegantly demonstrated the consequences of abrupt and extreme escalations in physiological demand on myocardial tissue health, contractility, chamber remodeling, and flow dynamics.
Funding Support and Author Disclosures
The work was funded in part by an unrestricted research grant from the Calgary Health Foundation (to Dr White). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Haaf P., Garg P., Messroghli D.R., Broadbent D.A., Greenwood J.P., Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satriano A., Afzal Y., Sarim Afzal M., et al. Neural-network-based diagnosis using 3-dimensional myocardial architecture and deformation: demonstration for the differentiation of hypertrophic cardiomyopathy. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.584727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H., Sheitt H., Wilton S.B., White J.A., Garcia J. Left ventricular flow distribution as a novel flow biomarker in atrial fibrillation. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.725121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer C.M., Barkhausen J., Bucciarelli-Ducci C., Flamm S.D., Kim R.J., Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22(1):17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahir E., Scherz B., Starekova J., et al. Acute impact of an endurance race on cardiac function and biomarkers of myocardial injury in triathletes with and without myocardial fibrosis. Eur J Prev Cardiol. 2020;27:94–104. doi: 10.1177/2047487319859975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Silva A., Bhuva A.N., van Zalen J., et al. Cardiovascular remodeling experienced by real-world, unsupervised, young novice marathon runners. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.00232. 232-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aengevaeren V.L., Froeling M., Hooijmans M.T., et al. Myocardial injury and compromised cardiomyocyte integrity following a marathon run. J Am Coll Cardiol Img. 2020;13:1445–1447. doi: 10.1016/j.jcmg.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Aengevaeren V.L., van Kimmenade R.R.J., Ordóñez-Llanos J., et al. Cardiac biomarker kinetics and their association with magnetic resonance measures of cardiomyocyte integrity following a marathon run: implications for postexercise biomarker testing. J Am Heart Assoc. 2021;10(13) doi: 10.1161/JAHA.120.020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott J.M., Esch B.T., Haykowsky M.J., et al. Effects of high intensity exercise on biventricular function assessed by cardiac magnetic resonance imaging in endurance trained and normally active individuals. Am J Cardiol. 2010;106:278–283. doi: 10.1016/j.amjcard.2010.02.037. [DOI] [PubMed] [Google Scholar]