Summary
Germline stem cells (GSCs) are critical for the reproduction of an organism. The self-renewal and differentiation of GSCs must be tightly controlled to avoid uncontrolled stem cell proliferation or premature stem cell differentiation. However, how the self-renewal and differentiation of GSCs are properly controlled is not fully understood. Here, we find that the novel intrinsic factor Yun is required for female GSC maintenance in Drosophila. GSCs undergo precocious differentiation due to de-repression of differentiation factor Bam by defective BMP/Dpp signaling in the absence of yun. Mechanistically, Yun associates with and stabilizes Thickveins (Tkv), the type I receptor of Dpp/BMP signaling. Finally, ectopic expression of a constitutively active Tkv (TkvQD) completely suppresses GSC loss caused by yun depletion. Collectively, these data demonstrate that Yun functions through Tkv to maintain GSC fate. Our results provide new insight into the regulatory mechanisms of how stem cell maintenance is properly controlled.
Key words: Yun, niche, BMP signaling, Tkv, Drosophila, ovary, Germline stem cell
Graphical abstract
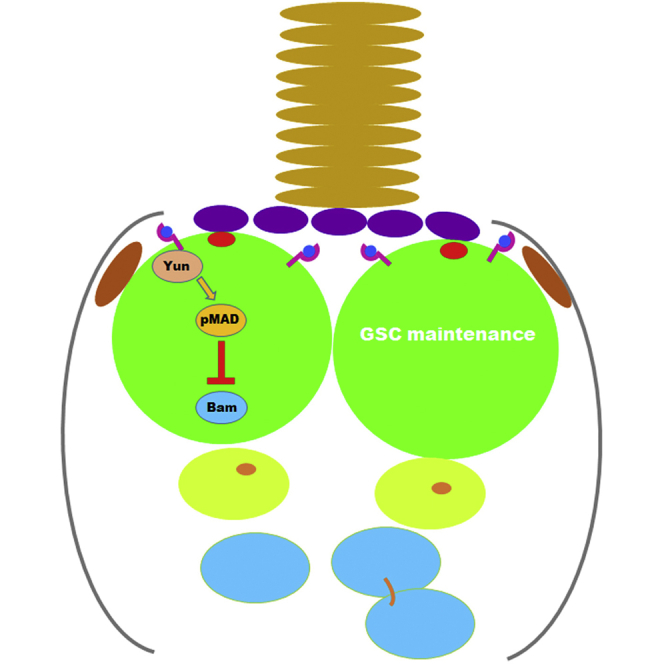
Highlights
-
•
Novel intrinsic factor Yun is required for female GSC maintenance
-
•
Yun-defective GSCs undergo differentiation due to Bam upregulation
-
•
Yun associates with and stabilizes Tkv to regulate GSC maintenance
-
•
GSC loss in the absence of yun could be rescued by constitutively active Tkv
In this article, Zhouhua Li and colleagues show that Yun maintains female germline stem cell (GSC) fate through Thickveins (Tkv). Depletion of yun in the germline causes GSC maintenance defects due to defective BMP signaling and increased Bam levels. Yun associates with and stabilizes Tkv to regulate GSC maintenance under physiological conditions.
Introduction
The self-renewal (maintenance) and differentiation of adult stem cells are critical for proper tissue homeostasis, so the balance of self-renewal and differentiation of adult stem cells must be tightly controlled. Disruption of this balanced control leads to either excessive stem cell proliferation or precocious stem cell depletion, eventually resulting in various diseases, including cancer and aging (Lin, 2008; Morrison and Spradling, 2008). Therefore, understanding of the underlying mechanisms controlling stem cell self-renewal and differentiation is important for the development of potential therapeutics for human diseases.
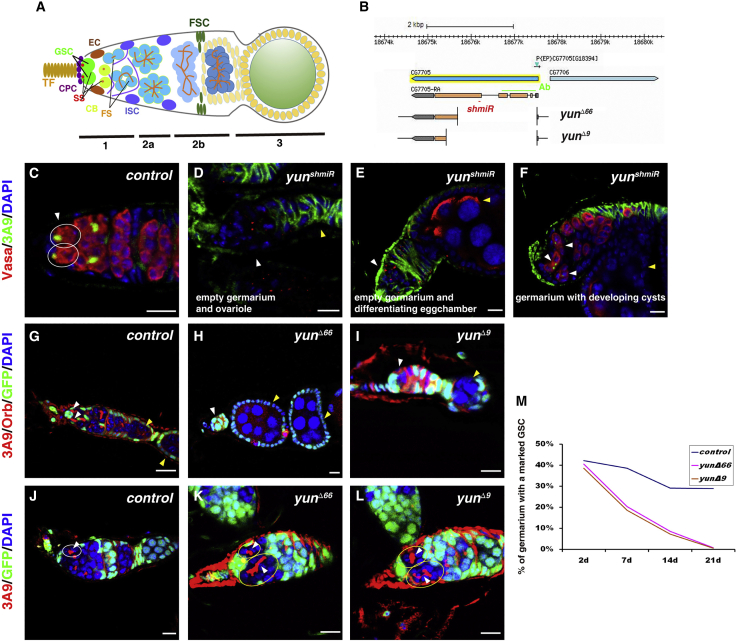
The adult Drosophila ovary is an excellent system to study the regulation of stem cell self-renewal and differentiation in vivo (Hsu et al., 2019; Ishibashi et al., 2020; Zhang and Cai, 2020). A structure, termed germarium, locates at the anterior end of a Drosophila ovariole, which contains the GSC niche, GSCs, and developing cysts (Figure 1A). Two or three GSCs are located in the niche at the anterior tip of the germarium, which is composed of several types of somatic stromal cells including terminal filaments (TFs), cap cells (CPCs), and escort cells (ECs). GSCs can be easily identified by their location and the intracellular organelle known as the spectrosome: they physically contact with cap cells and contain an anteriorly anchored spherical-shaped spectrosome (Figure 1A). GSCs normally undergo asymmetric self-renewing divisions, producing a self-renewed GSC daughter and a differentiating daughter, called a cystoblast (CB). CB is displaced from the niche (one cell diameter away from cap cells and containing a randomly localized spectrosome) to initiate differentiation. CB undergoes four rounds of synchronized divisions with incomplete cytokinesis, producing a 2- to 16-cell developing cyst. The developing cyst contains a branched organelle (fusome) that interconnects individual cystocytes (Lin et al., 1994). The niche utilizes several extrinsic signals, such as bone morphogenetic protein (BMP) (Dpp and Gbb), Upd cytokine, and Wnt, to maintain GSC self-renewal (Luo et al., 2015; Wang et al., 2008a; Xie and Spradling, 1998, 2000). The short-range BMP/Dpp signaling is the major niche signal, which functions in one cell diameter in the niche to maintain GSC self-renewal and repress the transcription of the differentiation factor Bam (Bag-of-marbles) (Chen and McKearin, 2003; Losick et al., 2011; Tabata and Takei, 2004; Tu et al., 2020). Upon binding of BMP/Dpp ligands to the hetero-dimeric receptor composed of type I receptor (like Tkv) and type II receptor (like Punt), Tkv is activated and results in the phosphorylation of MAD (Mothers against dpp, co-SMAD in Drosophila, pMAD), pMAD forms a complex with Med (Medea, R-SMAD in Drosophila), and they translocate in the nucleus of GSCs to regulate the expression of their target genes such as Dad (Daughters against dpp) (Araujo et al., 2011; Chen and McKearin, 2003; Decotto and Spradling, 2005; Losick et al., 2011; Tabata and Takei, 2004; Xie and Spradling, 1998, 2000). Additionally, niche-expressing E-cadherin is required to anchor GSCs in the niche for long-term maintenance (Song and Xie, 2002). Somatic and germline cells deploy several mechanisms acting in concert to spatially restrict BMP/Dpp signaling within the niche (Chen et al., 2010; Guo and Wang, 2009; Harris and Ashe, 2011; Hayashi et al., 2009; Liu et al., 2010; Losick et al., 2011; Luo et al., 2015; Ting, 2013; Xia et al., 2010, 2012). As BMP/Dpp signaling is inactivated in multiple ways in CBs, bam expression is de-repressed, which further drives germline cell differentiation through multiple independent mechanisms (Casanueva and Ferguson, 2004; Chen and McKearin, 2003; Chen et al., 2011; Fu et al., 2015; Li et al., 2009, 2013; McKearin and Ohlstein, 1995; McKearin and Spradling, 1990; Ohlstein and McKearin, 1997; Pan et al., 2014; Shen et al., 2009; Song et al., 2004). However, how BMP/Dpp signal is precisely controlled in GSCs for GSC maintenance is not fully understood.
Figure 1.
Yun is required for GSC maintenance
(A) Schematic cartoon of the germarium. TF: terminal filament, CPC: cap cell; GSC: germline stem cell; CB: cystoblast; EC: escort cell; SS: spectrosome; FS: fusome; ISC: inner sheath cell; FSC: follicle stem cell.
(B) The schematic cartoon of yun reagents used.
(C) Vasa (red) and 3A9 (green) in control germarium (white ovals and arrowhead).
(D–F) Germaria of nos > yunshmiR (white and yellow arrowheads).
(G) 3A9 and Orb (red) in marked control clones induced from larva.
(H and I) 3A9 and Orb (red) in yun mutant mosaic germline clones induced from larva (white and yellow arrowheads).
(J) 3A9 in marked control clones induced in adult flies (white oval and arrowhead).
(K and L) 3A9 in yun mutant mosaic germline clones induced in adult flies (yellow ovals and white arrowheads).
(M) Percentages of the germaria carrying a marked WT or yun mutant GSC clone over time (2, 7, 14, and 21 days after ACI). Three replicates. Scale bars: 10 μm.
Results and discussion
We identified a novel factor that we named Yun (“luck” in Chinese) in a large-scale RNAi screen for regulators of adult intestinal stem cell maintenance, proliferation, and differentiation (Liu et al., 2022; Ren et al., 2022; Zhao et al., 2022a, 2022b). yun (CG7705) encodes a novel protein of 592 aa without any known domain and motif (Figures 1B and S1A). During the course of our study, another group showed that it is implicated in cell proliferation in larval brain and spermatogenesis and named it diamond (dind) (Graziadio et al., 2018), but its mechanism in brain cell proliferation and spermatogenesis remained unexplored, and it is unclear whether Yun functions in GSCs regulation. As Yun could be detected in female germline cells, including GSCs, we asked whether Yun plays any role in GSC regulation (Figure S1B). Depleting yun in GSCs using a functional yunshmiR by nosGal4 in germline resulted in almost complete elimination of germline cells and GSCs, indicating that Yun may be required for GSC maintenance (Figures 1B, S1C, and S1D) (Zhao et al., 2022b). Detailed examinations showed that most of the nos > yunshmiR germaria was devoid of germline cells compared with control germaria (Figures 1C–1E). And no GSCs were observed in those nos > yunshmiR germaria containing a few germline cells, indicating that these germline cells are developing cysts (Figures 1F and S1E–S1I). GSC loss could be due to defective GSC self-renewal or apoptosis. However, no increased apoptosis was observed in yun-depleted germline cells, excluding the possibility that yun-defective GSCs are lost due to apoptosis (Figures S1J–S1L).
To further confirm that Yun intrinsically controls GSC maintenance, we performed mosaic clonal analysis of yun null mutants using Flp/FRT system (Figure 1B) (Zhao et al., 2022b). We first examined the mosaic clones that were induced at larval stages in adult animals. Control GSC clones underwent constant self-renewal divisions and produced developing egg chambers; although no GSCs were observed in yun mutant clones, many empty germaria were followed by differentiating egg chambers containing yun mutant germline cells, indicating that yun-defective GSCs could not be maintained and underwent differentiation (Figures 1G–1I and S1M–S1P). We then induced mosaic clones in adult flies. Marked control GSCs were constantly observed, while yun mutant GSCs could not maintain their stem cell fate and differentiated (Figures 1J–1L and S1Q–S1S). We further investigated GSC maintenance by examining the percentage of marked GSCs at different time points after clone induction (ACI). Compared with the marked control GSCs that still remained in the niche 2 and 3 weeks ACI, respectively, yun mutant GSCs were rapidly lost, and no marked yun mutant GSCs could be observed 3 weeks ACI (Figure 1M). Collectively, these data indicate that Yun is intrinsically required for GSC maintenance.
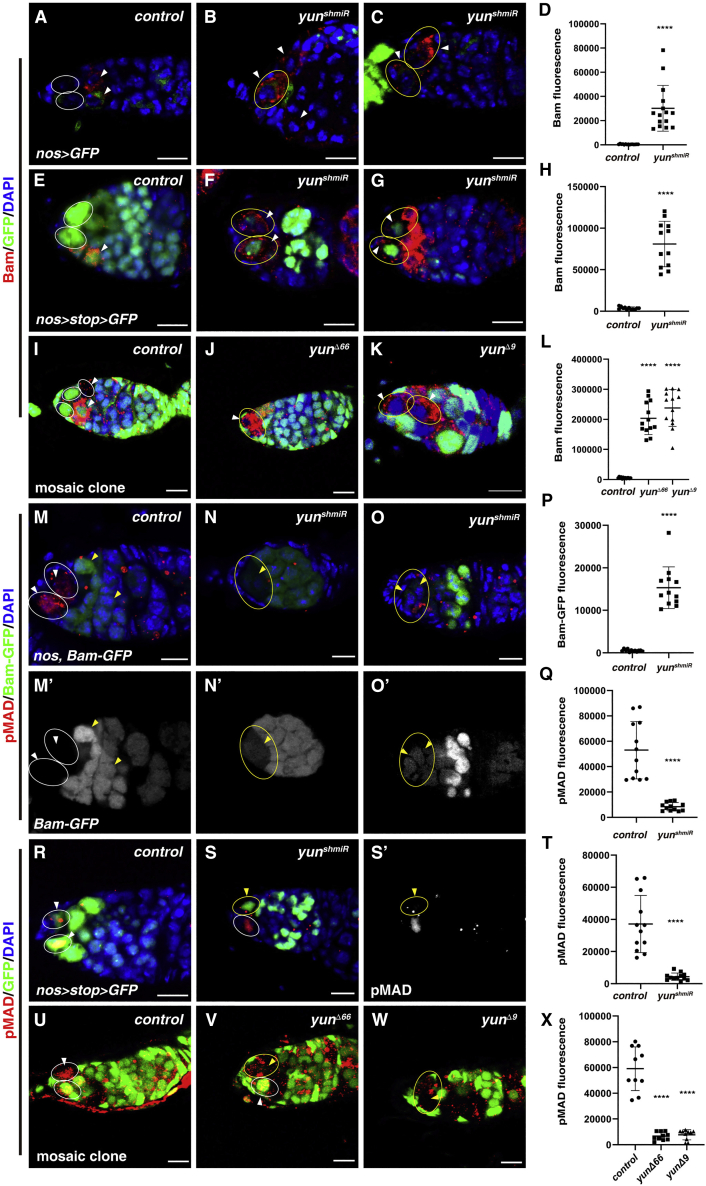
The differentiation factor Bam plays major role in GSC self-renewal and differentiation (McKearin and Spradling, 1990). We then examined the expression of Bam in the absence of yun using Bam antibody and a bam-GFP reporter, respectively (Chen and McKearin, 2003). Bam protein could not be detected in control GSCs, while Bam protein was detected within the nos > yunshmiR niche, indicating that elevated Bam protein may cause the loss of yun-defective GSCs (Figures 2A–2D). Furthermore, Bam protein was detected within the niche of nos > stop > Gal4>yunshmiR germaria, which bypassed the possible side effects of early-on depletion of yun using nosGal4 (Figures 2E-2H) (Ma et al., 2014). Moreover, Bam protein was detected in yun mutant GSCs within the niche (Figures 2I–2L). bam-GFP is repressed in control GSCs, while it was detected in putative GSCs in the nos > yunshmiR niche (Figures 2M−2P and S2A–S2D). Altogether, these data show that upregulation of Bam in yun-defective GSCs is likely the cause of GSC loss. Consistently, simultaneous depletion of Bam in nos > yunshmiR germaria completely rescued GSC loss observed in nos > yunshmiR germaria, and nos > BamRNAi, yunshmiR germaria were identical to nos > BamRNAi germaria (Figure S2E). Collectively, these data show that Yun is intrinsically required for GSC maintenance by repressing Bam expression.
Figure 2.
Yun represses GSC differentiation
(A) Bam (red) in control germarium (GSCs, white ovals).
(B and C) Bam (red) in nos > yunshmiR germarium (GSCs, white ovals).
(D) Quantification of Bam fluorescence in control and nos > yunshmiR GSCs. Mean ± SEM is shown. n = 15. ∗∗∗∗p < 0.0001.
(E) Bam (red) in nos > stop > GFP germarium (GSCs, white ovals).
(F and G) Bam (red) in nos > stop > yunshmiR germarium (yellow ovals and white arrowheads).
(H) Quantification of Bam fluorescence in control and nos > stop > yunshmiR GSCs. Mean ± SEM is shown. n = 12. ∗∗∗∗p < 0.0001.
(I) Bam (red) in marked control clones (oval) induced in adult flies.
(J and K) Bam (red) in marked yun mutant clones (yellow ovals).
(L) Quantification of Bam fluorescence in control and yun mutant GSCs. Mean ± SEM is shown. n ≥ 12. ∗∗∗∗p < 0.0001.
(M) pMAD (red) and Bam-GFP (green) in control germarium (GSCs, white ovals). Bam-GFP channel is showed separately in black and white.
(N and O) pMAD (red) and Bam-GFP (green) in nos > yunshmiR germarium (GSCs, yellow ovals).
(P) Quantification of Bam-GFP fluorescence in control and nos > yunshmiR GSCs. Mean ± SEM is shown. n = 12. ∗∗∗∗p < 0.0001.
(Q) Quantification of pMAD fluorescence in control and nos > yunshmiR GSCs. Mean ± SEM is shown. n = 12. ∗∗∗∗p < 0.0001.
(R) pMAD (red, white arrowheads) in marked nos > stop GSCs (white ovals).
(S) pMAD (red, arrowheads) in marked nos > stop > yunshmiR GSCs (yellow oval). pMAD channel is shown separately.
(T) Quantification of pMAD fluorescence in control and nos > stop > yunshmiR GSCs. Mean ± SEM is shown. n = 12. ∗∗∗∗p < 0.0001.
(U) pMAD (red, white arrowhead) in marked control GSCs (white ovals).
(V and W) pMAD (red, white arrowheads) in marked yun mutant clones (yellow ovals).
(X) Quantification of pMAD fluorescence in control and yun mutant GSCs. Mean ± SEM is shown. n = 10. ∗∗∗∗p < 0.0001. Scale bars: 10 μm.
Niche-derived BMP/Dpp signaling is necessary and sufficient for GSC self-renewal and Bam suppression (Casanueva and Ferguson, 2004; Chen and McKearin, 2003; Kai and Spradling, 2003; Song et al., 2004; Xie and Spradling, 1998, 2000). Next, we determined the activation of Dpp signaling in yun-defective GSCs. The levels of pMAD were strongly diminished in nos > yunshmiR and nos > stop > yunshmiR germaria compared with those in control GSCs (Figures 2M−2O, 2Q–2T, and S3A–S3C). The levels of pMAD were also dramatically reduced in marked yun mutant GSCs compared with those in control GSCs (Figures 2U–2X). The expression of Dad-lacZ, the downstream target of Dpp signaling, was almost undetectable in nos > yunshmiR germline cells compared with that of control (Figures S3D–S3F). The expression of Dad-lacZ in yun mutant GSCs was also dramatically diminished (Figures S3G–S3H′). Previous study showed that adhesion of GSCs to cap cells by E-cadherin is important for GSC maintenance (Song and Xie, 2002), so we asked whether the levels of E-cadherin were affected upon Yun depletion. The results showed that no reductions in the levels of E-cadherin were observed in the nos > yunshmiR niche compared with those in the control, indicating that the loss of yun-defective GSCs is not caused by reduced adhesion of GSCs to cap cells (Figures S3I and S3J′). Altogether, these data show Yun is intrinsically required for Dpp signaling activation to suppress Bam expression for GSC maintenance.
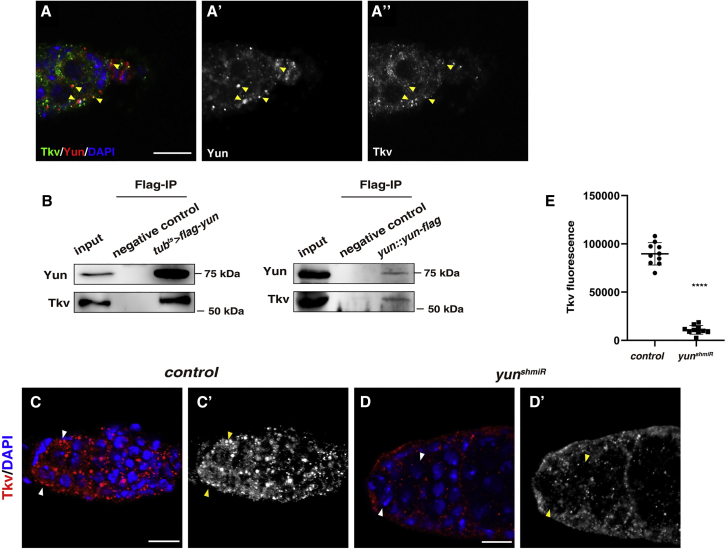
How does Yun regulate GSC self-renewal through Dpp signaling? Since Dpp signaling is defective in the absence of Yun, we asked whether Yun functions through the key components of the Dpp signaling pathway. Interestingly, we found the subcellular localization of Yun in GSCs is similar to that of Tkv, and Yun often co-localizes with Tkv (Figure 3A and S4A). Similar phenomena were observed between endogenous Yun and GFP-tagged Tkv under its endogenous promoter in the germarium (Figure S4A). These data suggest that Yun may function through Tkv to maintain GSC fate. We found that Yun and Tkv could not be detected in the recycling endosomes at the same time, indicating that the receptor recycling pathway may not account for the GSC maintenance defects observed in the absence of yun (Figure S4B). Furthermore, the coimmunoprecipitation results showed that both overexpressed and endogenous Yun associate with endogenous Tkv (Figure 3B). We then examined whether Yun affects Tkv levels. Interestingly, the levels of Tkv in the niche of nos > yunshmiR germaria were dramatically reduced compared with those in control GSCs, indicating that Yun affects Tkv protein levels (Figures 3C and 3D′) (Luo et al., 2015). We also examined whether Tkv affects the levels of Yun. However, the levels of Yun proteins were largely unaffected in the absence of tkv, indicating that Tkv is not likely to affect the levels of Yun proteins (Figure S4C). Collectively, these data indicate that Yun functions through Tkv to regulate Dpp signaling, thereby maintaining GSC fate.
Figure 3.
Yun interacts with and stabilizes Tkv
(A) Some endogenous Yun puncta (red) co-localize with endogenous Tkv (green) (yellow arrowheads). Yun and Tkv channels are shown separately in black and white.
(B) Endogenous Tkv associates with both overexpressed and endogenous Yun by coimmunoprecipitation.
(C) Tkv (red) in control GSCs (white arrowheads). Tkv channel is showed separately in black and white.
(D) The levels of Tkv (red) are dramatically reduced in nos > yunshmiR germarium (white arrowheads).
(E) Quantification of Tkv fluorescence in control and nos > yunshmiR GSCs. Mean ± SEM is shown. n = 10. ∗∗∗∗p < 0.0001. Scale bars: 10 μm.
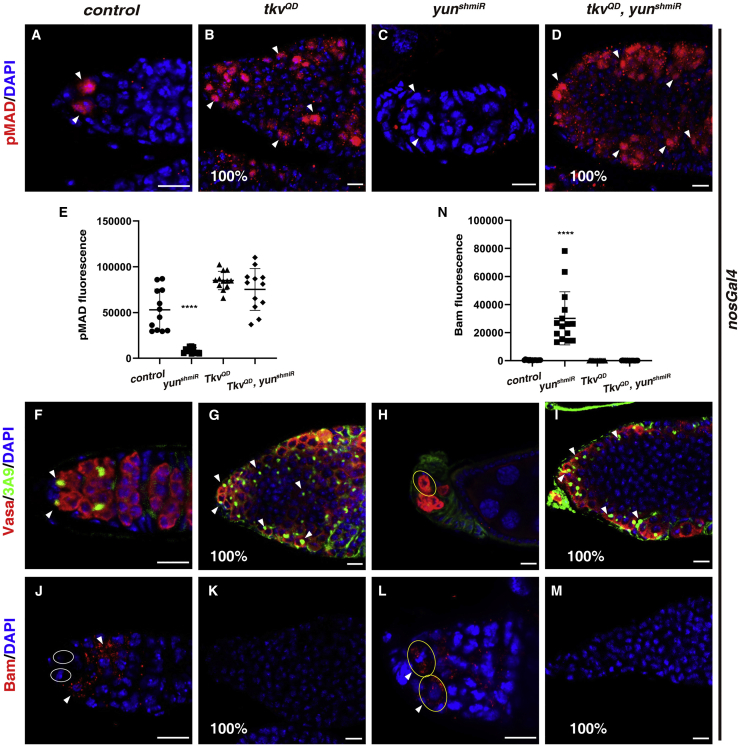
To further confirm this conclusion, we carried out rescue experiments. Expression of a constitutively active Tkv (tkvQD) resulted in strong Dpp signaling activation in the germ cells (Figures 4A and 4B). Compared with diminished Dpp signaling in the nos > yunshmiR germaria, Dpp signaling was ectopically activated in all nos > tkvQD, yunshmiR ovarioles, identical to that of nos > tkvQD ovarioles (Figures 4A–4E). The levels of Yun proteins were significantly eliminated in germline cells of nos > tkvQD, yunshmiR ovarioles (Figure S4D). These data support the notion that Yun functions through Tkv to regulate GSC self-renewal. Consistently, the germaria and developing follicles in nos > tkvQD, yunshmiR ovarioles were filled with GSCs and GSC-like cells, identical to those of nos > tkvQD ovarioles (Figures 4F–4I). Moreover, precocious bam expression observed in nos > yunshmiR GSCs was completely suppressed in nos > tkvQD, yunshmiR ovarioles, identical to nos > tkvQD ovarioles (Figures 4J–4N). Altogether, these data show that Yun acts through Tkv to ensure Dpp signaling in GSCs, thereby maintaining GSC fate within the niche.
Figure 4.
Yun functions through Tkv to maintain GSC
(A) GSCs (by pMAD in red, white arrowheads) in control germarium.
(B) nos > tkvQD germaria contain numerous GSC-like cells (white arrowheads).
(C) No GSCs (by pMAD in red, white arrowheads) can be detected in nos > yunshmiR germarium.
(D) nos > tkvQD, yunshmiR germaria contain numerous GSC-like cells (white arrowheads).
(E) Quantification of pMAD fluorescence in GSCs with indicated genotypes. Mean ± SEM is shown. n = 12. ∗∗∗∗p < 0.0001.
(F) Vasa (red) and 3A9 (green) in control germarium (white arrowheads).
(G) Vasa (red) and 3A9 (green) in nos > tkvQD germarium. All germaria contain numerous GSC-like cells (white arrowheads).
(H) Vasa (red) and 3A9 (green) in nos > yunshmiR germarium that contains developing cysts (yellow oval).
(I) Vasa (red) and 3A9 (green) in nos > tkvQD, yunshmiR germarium. All germaria contain numerous GSC-like cells (white arrowheads).
(J) Bam (red, white arrowhead) in control germarium.
(K) Bam (red) is barely detected in all nos > tkvQD germaria.
(L) Bam (red, white arrowheads) is expressed in putative GSCs (yellow ovals) in the niche of nos > yunshmiR germarium.
(M) Bam (red) is barely detected in all nos > tkvQD, yunshmiR germaria.
(N) Quantification of Bam fluorescence in GSCs with indicated genotypes. Mean ± SEM is shown. n = 15. ∗∗∗∗p < 0.0001. Scale bars: 10 μm.
As the major niche-derived extrinsic signal, Dpp is spatially restricted within the niche to ensure GSC maintenance and proper GSC lineage development. To achieve such a tight spatial control of Dpp activity, multiple strategies are deployed (Chen et al., 2011). Extrinsically, cap cell-expressed glypican Dally limits Dpp diffusion to confine high Dpp concentrations within the niche (Guo and Wang, 2009; Hayashi et al., 2009; Liu et al., 2010). Viking, the type IV collagen, sequesters Dpp around GSCs to limit the functional range of Dpp (Wang et al., 2008b). Escort cell-expressed Tkv functions as a receptor sink/trap to remove excess diffusible Dpp to restrict Dpp activity locally within the niche (Luo et al., 2015; Xu et al., 2018). Intrinsically, different mechanisms were identified to promote Dpp signal activation in GSCs but rapidly dampen it in CBs (Chen et al., 2011). These mechanisms involved the post-translational regulation of Dpp signaling components in CBs: (1) the degradation of activated Tkv by the Fused-Smurf complex (Xia et al., 2010), (2) the translation repression of MAD by the Brat-Pumilo complex (Harris et al., 2011), and (3) the regulation of Sax levels by miR-184 (Iovino et al., 2009). It is also reported that Bam acts redundantly with Smurf to turn down Dpp signaling through an unknown mechanism (Casanueva and Ferguson, 2004). Inside GSCs, a variety of mechanisms are deployed to ensure proper Dpp signal activation for GSC self-renewal. Many intrinsic factors have been identified to regulate Dpp signaling. Petola and TSC1/2 (tuberous sclerosis complex ½) are required to maintain Dpp signaling but are dispensable for bam repression (Sun et al., 2010; Xi et al., 2005). Lissencephaly-1 (Lis-1) directly binds to MAD to stabilize it and facilitate its phosphorylation, thereby regulating Dpp signaling (Chen et al., 2010). Here we provide evidence that Yun associates with and stabilizes Tkv to ensure Dpp signal activation for GSC maintenance. These data suggest that in GSCs the Dpp signaling pathway is regulated at multiple levels to ensure GSC maintenance.
Dpp signaling also acts as a short-range signal to maintain self-renewal of male GSCs, another well-established system to stem cell biology (Kawase et al., 2004; Shivdasani and Ingham, 2003). We are interested to examine whether Yun is also required for male GSC maintenance. Interestingly, Yun is not expressed in the testis niche, and no obvious defects were observed upon yun depletion in male GSCs (not shown). These data suggest that although Dpp signaling is required in both niches, these two niches utilize different mechanisms to ensure GSC self-renewal.
Experimental procedures
Fly lines and cultures
Flies were maintained on standard media at 25°C. Crosses were raised at 18°C in humidity-controlled incubators or as noted. Information for alleles and transgenes used can be found either in FlyBase or as noted.
Immunostainings and fluorescence microscopy
The following primary antibodies were used: mouse mAb anti-ɑ-Spectrin (3A9, 1:50, Developmental Studies Hybridoma Bank [DSHB]), mouse mAb anti-Orb (4H8, 1:50, DSHB), mouse mAb anti-Bam (Bag-of-marbles, 1:10, DSHB), rabbit anti-Vasa (d-260, Santa Cruz, 1:200, Cat No: sc-30210), rabbit anti-Vasa (1:2000, this study), rabbit mAb anti-pMAD3 (Epitomics, 1:200, Cat No: ab92698), rabbit and rat anti-Tkv (1:2,000, generous gifts from Dr Yu Cai) (Luo et al., 2015), rabbit anti-Yun (1:1,000) (Zhao et al., 2022a, 2022b), and rabbit anti-lacZ (1:5,000, Cappel, Cat No: 55,978). All images were captured by a Zeiss LSM780 confocal microscope and processed by Adobe Photoshop and Illustrator.
Author contributions
Z.H.L. conceived the study and designed the experiments. H.Z., Z.R.L., R.K., and L.S. performed experiments and data analysis. R.M. and X.R. assisted with experiments. Z.H.L. wrote the manuscript.
Acknowledgments
We thank Cai Y, Pastor-Pareja J, Chen D, Wang Z, Xie T, and Ni J for reagents, the Bloomington Stock Center, Tsinghua Fly Center for fly stocks, and DSHB for antibodies. We are grateful for the comments by Cai Y. This work is supported by grants from the National Natural Science Foundation of China (92054109, 31972893, and 31471384), Beijing Municipal Commission of Education (KZ201910028040), the Project of Graduate Student Academic Innovation, Capital Normal University (010-2255074).
Conflicts of interest
The authors declare no competing interests.
Published: August 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.07.014.
Supplemental information
References
- Araujo H., Fontenele M.R., da Fonseca R.N. Position matters: variability in the spatial pattern of BMP modulators generates functional diversity. Genesis. 2011;49:698–718. doi: 10.1002/dvg.20778. [DOI] [PubMed] [Google Scholar]
- Casanueva M.O., Ferguson E.L. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- Chen D., McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen S., Kaneko S., Ma X., Chen X., Ip Y.T., Xu L., Xie T. Lissencephaly-1 controls germline stem cell self-renewal through modulating bone morphogenetic protein signaling and niche adhesion. Proc. Natl. Acad. Sci. USA. 2010;107:19939–19944. doi: 10.1073/pnas.1008606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang S., Xie T. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr. Opin. Genet. Dev. 2011;21:684–689. doi: 10.1016/j.gde.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Decotto E., Spradling A.C. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Fu Z., Geng C., Wang H., Yang Z., Weng C., Li H., Deng L., Liu L., Liu N., Ni J., Xie T. Twin promotes the maintenance and differentiation of germline stem cell lineage through Modulation of multiple pathways. Cell Rep. 2015;13:1366–1379. doi: 10.1016/j.celrep.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Graziadio L., Palumbo V., Cipressa F., Williams B.C., Cenci G., Gatti M., Goldberg M.L., Bonaccorsi S. Phenotypic characterization of diamond (dind), a Drosophila gene required for multiple aspects of cell division. Chromosoma. 2018;127:489–504. doi: 10.1007/s00412-018-0680-y. [DOI] [PubMed] [Google Scholar]
- Guo Z., Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Ashe H.L. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12:519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Pargett M., Sutcliffe C., Umulis D., Ashe H.L. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev. Cell. 2011;20:72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kobayashi S., Nakato H. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-J., Bahader M., Lai C.-M. Molecular control of the female germline stem cell niche size in Drosophila. Cell. Mol. Life Sci. 2019;76:4309–4317. doi: 10.1007/s00018-019-03223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino N., Pane A., Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev. Cell. 2009;17:123–133. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ishibashi J.R., Taslim T.H., Ruohola-Baker H. Germline stem cell aging in the Drosophila ovary. Curr. Opin. Insect Sci. 2020;37:57–62. doi: 10.1016/j.cois.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Kai T., Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. USA. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E., Wong M.D., Ding B.C., Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Li Y., Minor N.T., Park J.K., McKearin D.M., Maines J.Z. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc. Natl. Acad. Sci. USA. 2009;106:9304–9309. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang Q., Carreira-Rosario A., Maines J.Z., McKearin D.M., Buszczak M. Mei-p26 cooperates with Bam, Bgcn and Sxl to promote early germline development in the Drosophila ovary. PLoS One. 2013;8:e58301. doi: 10.1371/journal.pone.0058301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J. Cell Biol. 2008;180:257–260. doi: 10.1083/jcb.200712159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yue L., Spradling A.C. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Liu F., Zhao H., Kong R., Shi L., Li Z., Ma R., Zhao H., Li Z. Derlin-1 and TER94/VCP/p97 are required for intestinal homeostasis. J Genet Genomics. 2022;49:195–207. doi: 10.1016/j.jgg.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Liu M., Lim T.M., Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci. Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- Losick V.P., Morris L.X., Fox D.T., Spradling A. Drosophila stem cell niches: a Decade of Discovery suggests a Unified View of stem cell regulation. Dev. Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Wang H., Fan C., Liu S., Cai Y. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J. Cell Biol. 2015;209:595–608. doi: 10.1083/jcb.201409142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang S., Do T., Song X., Inaba M., Nishimoto Y., Liu L.-p., Gao Y., Mao Y., Li H., et al. Piwi is required in multiple cell types to control germline stem cell lineage development in the Drosophila ovary. PLoS One. 2014;9:e90267. doi: 10.1371/journal.pone.0090267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin D., Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- McKearin D.M., Spradling A.C. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Pan L., Wang S., Lu T., Weng C., Song X., Park J.K., Sun J., Yang Z.H., Yu J., Tang H., et al. Protein competition switches the function of COP9 from self-renewal to differentiation. Nature. 2014;514:233–236. doi: 10.1038/nature13562. [DOI] [PubMed] [Google Scholar]
- Ren X., Zhao H., Shi L., Li Z., Kong R., Ma R., Jia L., Lu S., Wang J.H., Dong M.Q., et al. Phosphorylation of Yun is required for stem cell proliferation and tumorigenesis. Cell Prolif. 2022;55:e13230. doi: 10.1111/cpr.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Weng C., Yu J., Xie T. eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc. Natl. Acad. Sci. USA. 2009;106:11623–11628. doi: 10.1073/pnas.0903325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani A.A., Ingham P.W. Regulation of stem cell maintenance and Transit Amplifying cell proliferation by TGF-β signaling in Drosophila spermatogenesis. Curr. Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Song X., Wong M.D., Kawase E., Xi R., Ding B.C., McCarthy J.J., Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Song X., Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Quan Z., Zhang B., Wu T., Xi R. TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development. 2010;137:2461–2469. doi: 10.1242/dev.051466. [DOI] [PubMed] [Google Scholar]
- Tabata T., Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Ting X. Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: concerted actions of niche signals and intrinsic factors. Wiley Interdiscip Rev Dev Biol. 2013;2:261–273. doi: 10.1002/wdev.60. [DOI] [PubMed] [Google Scholar]
- Tu R., Duan B., Song X., Xie T. Dlp-mediated Hh and Wnt signaling interdependence is critical in the niche for germline stem cell progeny differentiation. Sci. Adv. 2020;6:eaaz0480. doi: 10.1126/sciadv.aaz0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li Z., Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J. Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Harris R.E., Bayston L.J., Ashe H.L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Xi R., Doan C., Liu D., Xie T. Pelota controls self-renewal of germline stem cells by repressing a Bam-independent differentiation pathway. Development. 2005;132:5365–5374. doi: 10.1242/dev.02151. [DOI] [PubMed] [Google Scholar]
- Xia L., Jia S., Huang S., Wang H., Zhu Y., Mu Y., Kan L., Zheng W., Wu D., Li X., et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–990. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Xia L., Zheng X., Zheng W., Zhang G., Wang H., Tao Y., Chen D. The niche-dependent Feedback Loop generates a BMP activity gradient to determine the germline stem cell fate. Curr. Biol. 2012;22:515–521. doi: 10.1016/j.cub.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Xie T., Spradling A.C. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T., Spradling A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Xu R., Li J., Zhao H., Kong R., Wei M., Shi L., Bai G., Li Z. Self-restrained regulation of stem cell niche activity by niche components in the Drosophila testis. Dev. Biol. 2018;439:42–51. doi: 10.1016/j.ydbio.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Zhang H., Cai Y. Signal transduction pathways regulating Drosophila ovarian germline stem cells. Curr. Opin. Insect Sci. 2020;37:1–7. doi: 10.1016/j.cois.2019.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao H., Ren X., Kong R., Shi L., Li Z., Wang R., Ma R., Zhao H., Liu F., Chang H.C., et al. Auxilin regulates intestinal stem cell proliferation through EGFR. Stem Cell Rep. 2022;17:1120–1137. doi: 10.1016/j.stemcr.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shi L., Li Z., Kong R., Ren X., Ma R., Jia L., Ma M., Lu S., Xu R., et al. The Yun/Prohibitin complex regulates adult Drosophila intestinal stem cell proliferation through the transcription factor E2F1. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2111711119. e2111711119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.