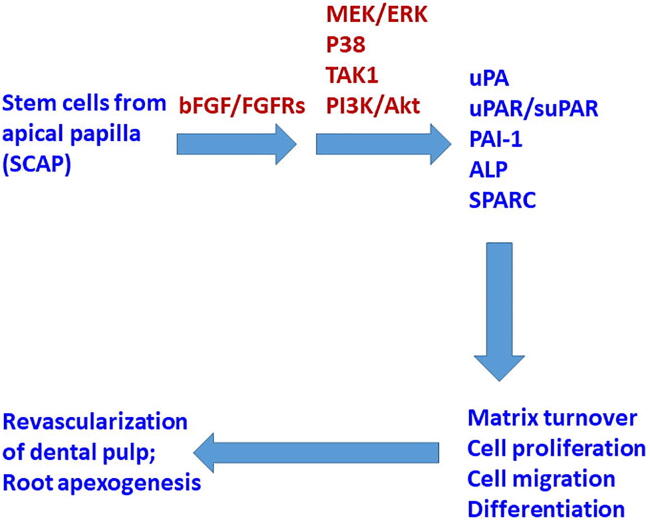
Graphical abstract
Keywords: Basic fibroblast growth factor, Matrix turnover, Plasminogen activation system, Signal transduction, Stem cells from apical papilla
Abbreviations: Akt, protein kinase B; ALP, alkaline phosphatase; BAC, β-actin; bFGF, basic fibroblast growth factor; BSA, bovine serum albumin; DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethylsulfoxide; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; FBS, fetal bovine serum; FGFR, fibroblast growth factor receptor; IGF-1, insulin-like growth factor-1; MEK, mitogen-activated protein kinase kinase; MSCs, mesenchymal stem cells; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Osx, osterix; PAI-1, plasminogen activator inhibitor-1; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; PDGF, platelet-derived growth factor; PDL, periodontal ligament; PI3k, phosphatidylinositol-4,5-bisphosphate 3-kinase SB203580 4-(4-fluorophenyl)-2- (4-methylsulfinylphenyl)-5- (4-pyridyl)-imidazole; SCAP, stem cells from apical papilla; SPARC, secreted protein acidic and rich in cysteine (osteonectin); STAT, leukemia inhibitory factor-signal transducers and activators of transcription; TAK1, TGF-α-activated kinase-1; TGF-α, transforming growth factor-α; TIMP-1, tissue inhibitor of metalloproteinase-1; U0126, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor
Highlights
-
•
bFGF induced uPA, uPAR, PAI-1 production/expression in SCAP →
-
•
bFGF induced decline of ALP and SPARC of SCAP →
-
•
The effects of bFGF are regulated by ERK, p38, TAK1 and Akt signaling →
-
•
Crucial for SCAP proliferation, matrix turnover and differentiation →
-
•
These events are important for revascularization/root apexogenesis
Abstract
Introduction
Basic fibroblast growth factor (bFGF) plays a critical role in odontoblast differentiation and dentin matrix deposition, thereby aiding pulpo-dentin repair and regeneration.
Objectives
The purpose of this study was to clarify the effects of bFGF on plasminogen activation factors, TIMP-1), ALP; and SPARC (osteonectin) expression/production of stem cells from apical papilla (SCAP) in vitro; and the involvement of MEK/ERK, p38, Akt, and TAK1 signaling.
Methods
SCAP were exposed to bFGF with/without pretreatment and co-incubation with various signal transduction inhibitors (U0126, SB203580, LY294002, and 5Z-7-oxozeaenol). The expression of FGF receptors (FGFRs), PAI-1, uPA, p-ERK, p-TAK1, and p-p38 was analyzed via immunofluorescent staining. The gene expression and protein secretion of SCAP were determined via real-time PCR and ELISA. ALP activity was evaluated via ALP staining.
Results
SCAP expressed FGFR1, 2, 3, and 4. bFGF stimulated the PAI-1, uPA, uPAR, and TIMP-1 mRNA expression (p < 0.05). bFGF induced PAI-1, uPA, and soluble uPAR production (p < 0.05) but suppressed the ALP activity and SPARC production (p < 0.05) of SCAP. bFGF stimulated ERK, TAK1, and p38 phosphorylation of SCAP. U0126 (a MEK/ERK inhibitor) and 5Z-7-oxozeaenol (a TAK1 inhibitor) attenuated the bFGF-induced PAI-1, uPA, uPAR, and TIMP-1 expression and production of SCAP, but SB203580 (a p38 inhibitor) did not. LY294002, SB203580, and 5Z-7oxozeaenol could not reverse the inhibition of ALP activity caused by bFGF. Interestingly, U0126 and 5Z-7-oxozeaenol prevented the bFGF-induced decline of SPARC production (p < 0.05).
Conclusion
bFGF may regulate fibrinolysis and matrix turnover via modulation of PAI-1, uPA, uPAR, and TIMP-1, but bFGF inhibited the differentiation (ALP, SPARC) of SCAP. These events are mainly regulated by MEK/ERK, p38, and TAK1. Combined use of bFGF and SCAP may facilitate pulpal/root repair and regeneration via regulation of the plasminogen activation system, migration, matrix turnover, and differentiation of SCAP.
Introduction
The invasion of microorganisms through dentinal tubules, deep dental caries, dental trauma, or fracture of dens evaginatus may lead to early pulpal infection and necrosis in immature permanent teeth. Infection, inflammation, and necrosis of dental pulp may further impair the development of root formation, leaving an open apex and weak dentinal walls of the tooth root that are susceptible to root fracture [1]. Clinically, apexification procedures with mechanical/chemical debridement for infection and inflammation control, calcium hydroxide dressing for apical barrier formation, or apical mineral trioxide aggregate (MTA) plugs before root canal obturation have been successfully used to treat infected dental pulp in immature permanent teeth [2]. However, the apexification method cannot restore necrotic dental pulp tissues in the root canal. Therefore, determining how to induce the revascularization/regeneration of lost dental pulp tissue in immature and mature teeth with subsequent root canal wall mineralization/thickening and apical closure is a crucial clinical issue.
Clinically, control of pulpal/root canal infection and inflammation is accomplished via mechanical debridement, irrigation with NaOCl or chlorhexidine, and root canal dressing with triple antibiotic paste or Ca(OH)2. If infection is successfully controlled, periapical pro-inflammatory M1 macrophages may shift to anti-inflammatory M2 macrophages to phagocytose bacteria and dead cells and signal tissue inflammation-regeneration coupling [3]. The cell homing method can then be applied by using revascularization/revitalization procedures to induce the formation of blood clots and the ingrowth of periapical vital tissue into the root canal and pulp space, with subsequent root apex formation and canal calcification [4]. Endodontic files are placed over the root apex to induce bleeding from the root apex and the formation of blood clots within the root canal. Collagen gels, platelet-rich plasma, chitosan, or other materials are placed into the root canal to increase the efficacy of tissue regeneration [5]. Tissue regeneration may result from the migration and proliferation of stem cells already present in apical vital tissues, apical papilla, periodontal ligament (PDL), or alveolar bone [6]. Among them, stem cells from apical papilla (SCAP) are the major population of mesenchymal stem cells (MSCs) derived from apical papilla in the developing root apex of human teeth [7].
In addition to stem cells, scaffolds and growth factors are also considered to be crucial factors for tissue regeneration. Blood clots, platelet-rich fibrin, and platelet-rich plasma contain numerous growth factors such as basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor-α (TGF-α), and many others [8]. These growth factors may be also released from dentin during treatment with Ca(OH)2, MTA, or ethylenediaminetetraacetic acid [9]. These growth factors and many other pro-inflammatory cytokines may promote wound healing and angiogenic and neurogenic responses by provoking and controlling various cell activities such as metabolism, mitogenesis, chemotaxis, and differentiation of SCAP [8]. The family members of FGFs play pivotal roles in the regulation of cell proliferation, migration,t and differentiation during organ development via the activation of various fibroblast growth factor receptors (FGFRs) [10]. bFGF may be involved in bone remodeling, osteogenesis, and cementogenesis, which are crucial for the regeneration of periodontal and periapical tissues [11], [12]. Moreover, bFGF may be involved in maintaining the differentiation capacity of MSCs as well as increasing their telomere length in various cell cultures [13], [14], [15]. However, little is known regarding the effect of bFGF on the plasminogen activation factors of SCAP.
Plasminogen activation system molecules such as plasminogen activator inhibitor-1 (PAI-1), urokinase plasminogen activator (uPA), and urokinase plasminogen activator receptor (uPAR) are crucial for the homeostasis of the extracellular matrix and wound repair and regeneration [16]. The plasminogen system is the primary fibrinolytic mechanism responsible for blood clot dissolution and stem cell mobilization [17], possibly from the root apex or bone marrow. Focal proteolysis via plasminogen activation may mediate the detachment of stem cells from their niche (root apex) by breaking cell-matrix interactions, and promote the migration of stem cells to their preferred niches (root canal/pulp chamber) and wounded areas [16].
Recently, we reported the expression of two bFGF receptors, FGFR1 and FGFR2, in SCAP [18]. The exposure of SCAP to bFGF stimulated cell proliferation as well as the mRNA expression of cdc2, cyclinB1, and tissue inhibitor of metalloproteinase-1 (TIMP-1), but not collagen type I. These events are correlated with the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signal transduction pathway [18]. bFGF has also been found to effectively stimulate the migration of SCAP [19], [20]. To further understand the roles of bFGF in clinical treatment with the apexogenesis and regeneration of the pulpo-dentin complex, we hypothesized that bFGF does not affect the plasminogen activation factors and osteogenic/odontogenic differentiation-related factors of SCAP. The purpose of this study was to investigate the influence of bFGF on plasminogen activation system molecules (PAI-1, uPA, uPAR) and differentiation (alkaline phosphatase [ALP] activity and osteonectin [secreted protein acidic and rich in cysteine, SPARC] production), two osteogenic/odontogenic markers of SCAP in vitro, and the related signaling mechanisms.
Material and methods
Materials
Cell culture biologicals were obtained through Life Technologies (Thermo Fisher Scientific Ltd., Waltham, MA, USA). uPA, suPAR, and SPARC enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (R&D DuoSet, Minneapolis, MN, USA). Recombinant bFGF and PAI-1 ELISA kits were bought from PeproTech Biotechnology (PeproTech Asia, Rehovot, Israel). We obtained 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126), 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-imidazole (SB203580), LY294002, and dimethylsulfoxide (DMSO) from Sigma-Aldrich Company (St. Louis, MO, USA). 5Z-7-oxozeaenol was obtained from Tocris Cookson Ltd. (Bristol, UK). Kits for total RNA isolation were obtained from Qiagen Company (Taipei, Taiwan). Polymerase chain reaction (PCR) primers for β–actin (BAC), uPA, uPAR, PAI-1, TIMP-1, and type I collagen were synthesized by Genemed Biotechnologies, Inc. (San Francisco, CA, USA).
Ethics statement
With the agreement of the Ethics Committee of National Taiwan University Hospital, informed consent forms were signed by the guardians of young patients. More than three healthy human premolars with incomplete root formation were extracted for orthodontic management (approval numbers of Institutional Review Board: 201512156RINA and 201412147RINC) and used for culture of SCAP in this study.
Culture of human SCAP
Culture of SCAP was performed using the tissue explant technique, as described previously [18], [21], [22], [23]. Briefly, apical papilla tissues were removed from the root apex and cut into small pieces (approximately 1 mm3) using a surgical knife. For culture of SCAP performed using the tissue explant technique, apical papilla tissues were placed on culture dishes in Dulbecco’s modified Eagle medium comprising 10% fetal bovine serum (FBS), 2% glutamine, and 1% penicillin. When the growth of SCAP reached confluence, they were sub-cultured. The passage numbers of SCAP between 3 and 8 were used for this study. These SCAP have been observed to express CD73, CD90, and CD105 MSC markers as analyzed by flow cytometry [21].
Cell viability assay and ELISA
To estimate cell viability, MTT assays were used as previously described [18], [24], [25], [26]. SCAP (1 × 105 cells/well) were inoculated on 24-well plates. After 24 h, the medium was decanted and cells were incubated into fresh culture medium containing solvent control (0.1% bovine serum albumin [BSA] with/without DMSO) or different concentrations of bFGF (10 to 500 ng/mL) with/without inhibitors for 5 days. The culture medium was collected for ELISA analysis of PAI-1, uPA, and suPAR, according to the manufacturer’s instructions, as previously described [23], [27]. The cell layer was employed for ALP staining or MTT assay of cell viability. Briefly, 1 mL of medium containing 20 μL of MTT (final 0.5 mg/mL) was added into each well. After incubation for 2 h, the medium was aspirated and 0.5 mL of DMSO was placed into each well to dissolve the insoluble formazan. Cell viability was estimated by reading the DMSO eluents of formazan against blanks (DMSO) at 540 nm with a Dynatech Microwell plate reader (Dynatech Laboratories, Alexandria, VA, USA) and data were expressed as a percentage of the control (as 100%).
Immunofluorescent staining
Initially, the SCAP were incubated with various concentration of bFGF (0, 10, 50, 100, 250, 500 ng/mL) for 24 h. Immunofluorescent staining of SCAP was performed as previously described [26], [28]. In short, SCAP were washed with phosphate-buffered saline (PBS) and fixed for 20 min in 4% paraformaldehyde. After permeabilization in PBST (2 mL of Tween 20 and 98 mL of PBS; PBST), cells were incubated in 1 mL of 0.3% H2O2 (v/v) for an additional 20 min. Blocking was performed by incubating cells in 250 µL of PBST comprising specific primary antibodies (FGFR1, FGFR2, FGFR3, FGFR4, p-ERK, p-p38 mitogen activated protein kinase, p-transforming growth factor β-activated kinase-1 [p-TAK1], PAI-1, and uPA; 1:100 (v/v) with 75 μL of 0.1% PBST, 300 μL of 1% BSA, and 1110 μL of PBS) overnight. Cells were subsequently washed with PBS and incubated in tetramethylrhodamine (TRITC)-conjugated secondary antibodies (prepared in 1:100 dilutions for anti-mouse and anti-rabbit, and 1:200 for anti-goat secondary antibodies) for 1 h. Finally, 300 μL of 4′,6-diamidino-2-phenylindole (DAPI, 1:1000 dilution, 500 μg/mL) was used for nuclear staining for 30 min. Immunofluorescent images were captured using an Olympus IX70 Inverted Microscope (Tokyo, Japan).
RNA extraction and reverse transcription and real-time PCR
After the treatment of SCAP with solvent control or bFGF with/without inhibitors, total RNA was isolated as previously described [16], [20], [22]. After reverse transcription with the Invitrogen SuperScriptTM III First Strand Synthesis System, the generated cDNA was amplified via real-time PCR in a reaction mixture containing SYBR master mix, primers, cDNA, and diethyl pyrocarbonate water. The reaction states were 95 °C for 2 min for 1 cycle in Stage 1, followed by 95 °C for 5 s and 60 °C for 30 s for 40 cycles in Stage 2. The sequence of PCR primers was PAI-1: ATGGGATTCAAGATTGATGA and TCAGTATAGTTGAACTTGTT, uPA: GCCCTCCTCTCCTCCAGAAGAA and GTAGACGATGTA GTCCTCCTTC, uPAR: ATGGATGCTCCTCTGAAGAG and CACAGTCTGGCAGTCATTAG [29], SPARC (osteonectin): AAGAT CCATGAGAATGAGAAG and AAAAGCGGGTGGTG CAATG [30], and BAC (internal control): TGACGGGGTCACCCACACTGTG CCCATCTA and CTAGAAGCATTG CGGTGGACGATGGAGGG. For quantification of the results, the delta/delta cycle threshold values (ΔCt = mean ΔCt [treated] ‐ mean delta (Δ) Ct [control]) were applied to determine changes in the gene expression level. The fold of alterations in the study groups compared with the control (solvent) group was determined using the 2‐ΔΔCt method. The mRNA expression of BAC was exploited as an internal control gene in all the PCR experiments.
ALP staining
Briefly, 1 × 105 SCAP were inoculated on a 24-well culture plate. After 24 h, the medium was changed and solvent control or bFGF with/without various inhibitors (U0126, SB203580, LY294002, or 5Z-7oxozeaenol added 30 min before the addition of bFGF) were added. Cells were incubated for an additional 5 days. The culture medium was saved for ELISA. The ALP activity of SCAP was estimated via ALP staining, as described previously [31], [22]. Briefly, after the medium was collected, cells were rinsed with PBS and then incubated in 200 μL of freshly prepared stock substrate solution (10 mg of fast blue 2′,5′-diethoxybenzanilide salt/50 mL of ddH2O, 3.94 g of Tris-base containing 0.015 g of naphthol AS phosphate, and 250 μL of N,N-dimethylformamide) for 30 to 60 min in the dark. Color images of ALP staining were captured under a microscope.
Effect of bFGF on SPARC production of SCAP and its regulation by signal transduction inhibitors
Briefly, 1 × 105 SCAP were plated onto a 24-well culture plate. After 24 h of attachment for cells, the medium was changed, and the solvent control or bFGF with/without various inhibitors (U0126, SB203580, LY294002, or 5Z-7oxozeaenol added 30 min before the addition of bFGF) were added. Cells were incubated for an additional 5 days. The culture medium was collected for SPARC ELISA according to the manufacturer’s instructions.
Statistical analysis
More than three independent experiments were conducted on different days and the results (mean ± standard error of the mean) were used for statistical analysis. Differences between the control and study groups were examined using a paired t-test. A p value < 0.05 was considered to show a statistically significant difference between the control and study groups.
Results
Effects of bFGF on cell viability and ALP activity of SCAP
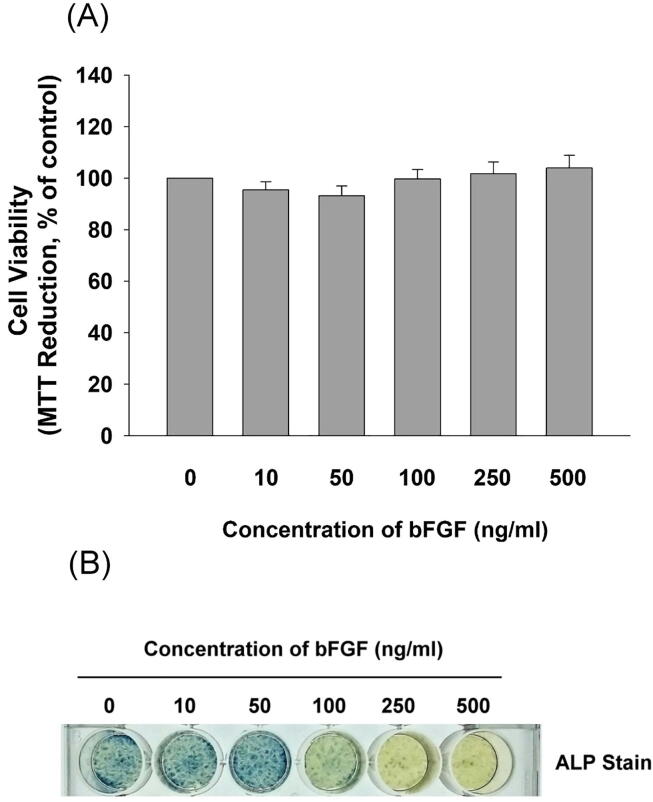
In our recent investigation of the effects of bFGF on the functions of SCAP, we observed the stimulation of SCAP cell proliferation by bFGF under serum-free conditions, but the growth-stimulating effect became less evident in the presence of 10% FBS [18]. Accordingly, near confluent SCAP (1 × 105 cells/well) were exposed to various concentrations of bFGF (10–500 ng/mL) for 5 days in fresh medium comprising 10% FBS in this study. No stimulatory effect of bFGF on the cell viability of SCAP was noted (Fig. 1A). SCAP have demonstrated the potential to differentiate into mineralized tissues [6]. In this study, we found that untreated SCAP (solvent control) exhibited ALP enzyme activity, as shown by ALP staining (Fig. 1B). After exposure to bFGF (50–500 ng/mL), decreases in ALP enzyme activities were revealed by ALP staining (Fig. 1B).
Fig. 1.
Effect of bFGF on the cell viability and ALP activity of near confluent SCAP after 5 days of exposure. (A) SCAP were incubated in different concentrations of bFGF in the presence of 10% FBS for 5 days, and stained with MTT for the cell viability assay. Results of cell viability (mean ± standard error) are expressed as percentages of the control (as 100%) (B) Near confluent SCAP were incubated in different concentrations of bFGF in the presence of 10% FBS for 5 days before ALP staining. One representative ALP staining image is shown.
Effect of bFGF on the plasminogen activation-related gene expression of SCAP
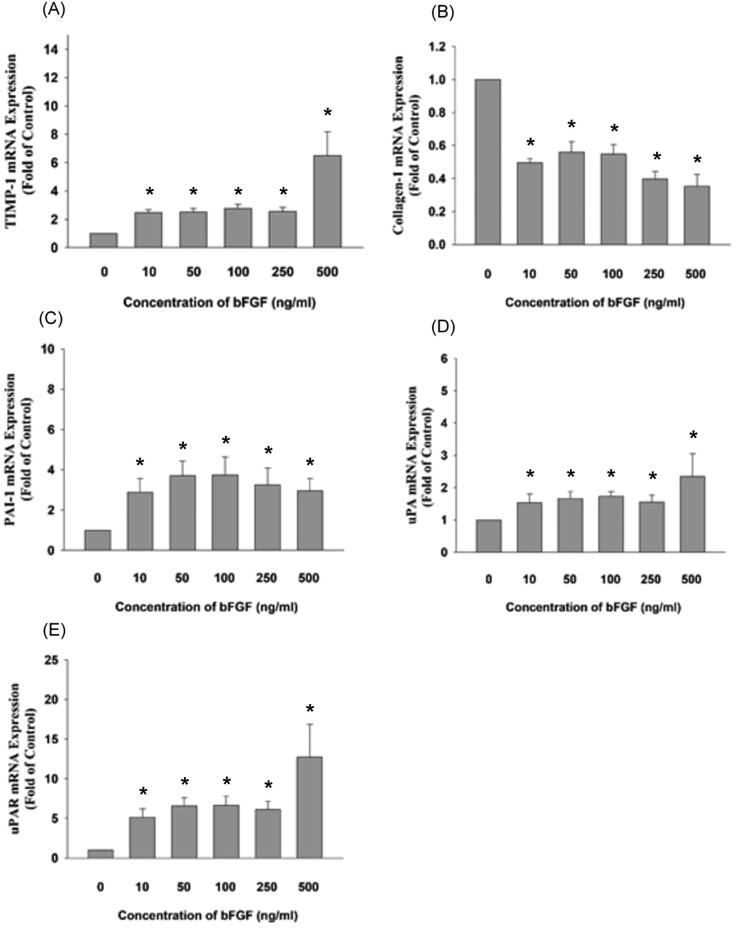
Because plasminogen activation factors may regulate cellular matrix turnover, fibrinolysis, and cell migration and proliferation [16], [17], we examined the mRNA expression of plasminogen activation factors of SCAP after exposure to bFGF via real-time PCR. We found that bFGF stimulated TIMP-1 mRNA expression to 2.4 to 6.5-fold of the control at concentrations of 10–500 ng/mL (p < 0.05) (Fig. 2A) but reduced type I collagen mRNA expression (>10 ng/mL), as revealed by real-time PCR (Fig. 2B). This is generally in accordance with our previous findings that bFGF stimulates TIMP-1 but reduces the type I collagen protein expression of SCAP [18].
Fig. 2.
Effect of bFGF on the gene expression of SCAP as revealed by real-time PCR. (A) TIMP-1 mRNA expression (n = 8), (B) Type I collagen (n = 8), (C) PAI-1 mRNA expression (n = 8), (D) uPA mRNA expression (n = 8), (E) uPAR mRNA expression (n = 8). Results are expressed as folds of the control (as 1) (mean ± standard error). *denotes a statistically significant difference (p < 0.05) compared with the solvent-treated control.
Interestingly, at concentrations of 10–500 ng/mL, bFGF stimulated PAI-1 to 2.9 to 3.7-fold of the control (p < 0.05) (Fig. 2C). bFGF also induced the uPA and uPAR mRNA expression of SCAP to 1.5 to 2.3-fold and 5.1 to 12.7-fold of the control, respectively, at concentrations of 10–500 ng/mL (p < 0.05) (Fig. 2D, 2E).
Effect of bFGF on the plasminogen activation-related protein production of SCAP
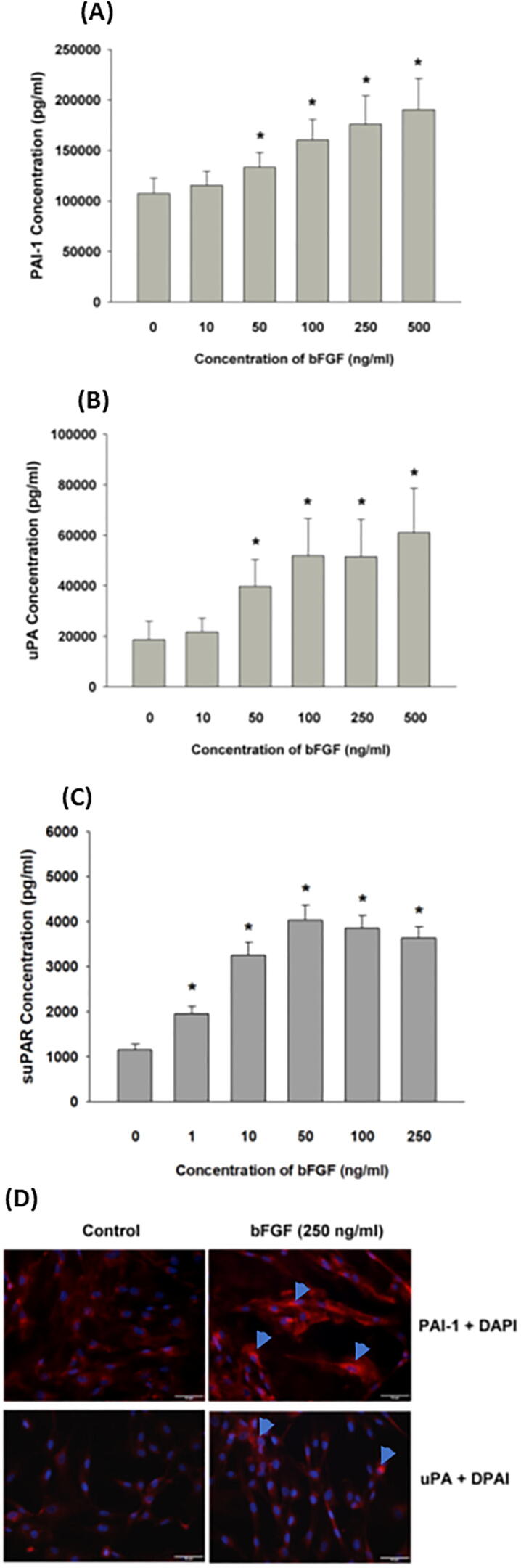
When we investigated whether bFGF stimulates plasminogen activation factors at both the transcription and translation levels, we found that the exposure of SCAP to bFGF (>50 ng/mL) stimulated PAI-1 production, as revealed by ELISA (p < 0.05) (Fig. 3A). Unexpectedly, exposure to bFGF for 5 days also induced the uPA (>50 ng/mL) and suPAR (>1 ng/mL) production of SCAP (p < 0.05) (Fig. 3B, 3C). Accordingly, bFGF also stimulated uPA and PAI-1 protein (red fluorescence) expression, as revealed by immunofluorescent staining (Fig. 3D).
Fig. 3.
Effect of bFGF on (A) PAI-1 production, (B) uPA production, and (C) suPAR production of SCAP. Results are expressed as the mean ± standard error (pg/mL). *denotes a statistically significant difference compared with the solvent control group (p < 0.05). #denotes a significant difference compared with the bFGF-treated group (p < 0.05). (D) Immunofluorescent staining of PAI-1 and uPA protein expression of control SCAP and SCAP treated with bFGF (250 ng/mL) for 24 h. One representative immunofluorescent staining image is shown. Red fluorescence: target protein expression, blue fluorescence: DAPI staining of cell nucleus.
Signaling pathways induced by bFGF in SCAP
The regulation of the functions of SCAP by bFGF may be due to the binding of bFGF to various FGFRs and subsequent activation of various signaling pathways [11], [18]. We recently reported the mRNA expression of FGFR1 and FGFR2, and the activation of p-ERK by bFGF in SCAP [18]. The activation of different FGF receptors may trigger different signaling pathways [32]. We investigated the molecular mechanisms associated with bFGF-mediated changes of SCAP and observed FGFR1, FGFR2, FGFR3, and FGFR4 protein expression, as revealed by immunofluorescent staining (red fluorescence, Fig. 4A). Accordingly, 24-h exposure to bFGF also increased the p-ERK, p-p38, and p-TAK1 protein expression in SCAP, as revealed by immunofluorescent staining (Fig. 4B).
Fig. 4.
(A) Expression of FGFR1, FGFR2, FGFR3, and FGFR4 in SCAP. Scale bar = 20 µm) (B) Activation of p-ERK, p-p38, and p-TAK1 in SCAP after 24 h of exposure to bFGF as revealed by immunofluorescent staining (scale bar = 50 µm). One representative immunofluorescent staining image is shown. Red TRITC fluorescence: target protein expression, blue fluorescence: DAPI staining of cell nucleus.
Effect of different signal transduction inhibitors on bFGF-induced changes in the PAI-1, uPA, suPAR, and TIMP-1 mRNA expression of SCAP
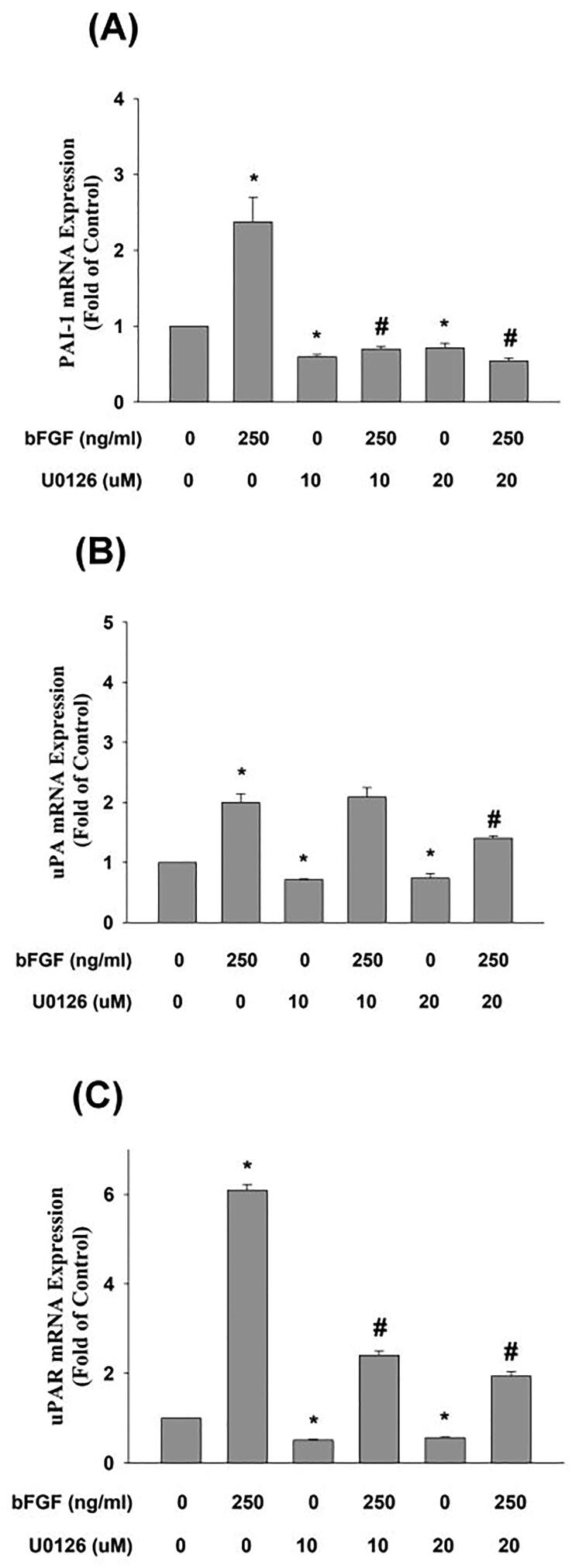
We investigated the signal transduction responsible for bFGF-induced plasminogen activation factor expression and found that U0126 effectively reduced the bFGF-induced expression of PAI-1 and uPAR mRNA (Fig. 5A, C), but an inhibitory effect on bFGF-induced uPA expression was found at 20 uM (Fig. 5B). Our previous study revealed that U0126 prevented bFGF-induced TIMP-1 expression [18].
Fig. 5.
Effect of U0126 on bFGF-induced changes in (A) PAI-1 (n = 4), (B) uPA (n = 4), (C) uPAR (n = 4) mRNA expression in SCAP as revealed by real-time PCR. Results are expressed as folds of the control (as 1) (mean ± standard error). *denotes a statistically significant difference compared with the control group. #denotes a statistically significant difference compared with the bFGF treatment group (p < 0.05).
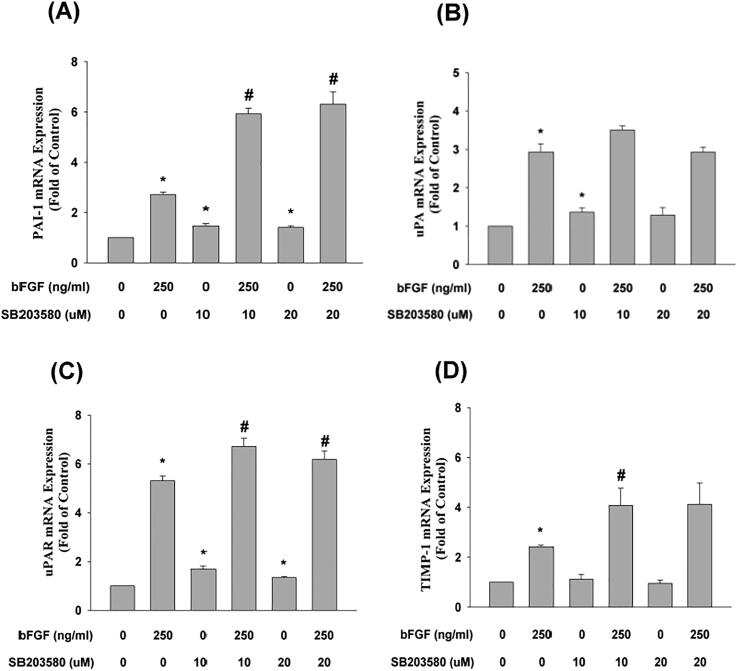
SB203580 exerted no preventive effect on bFGF-induced PAI-1 mRNA expression and even enhanced this event (Fig. 6A). Similarly, SB203580 also exerted little preventive effect on the bFGF-induced uPA, uPAR, and TIMP-1 mRNA expression of SCAP, and even enhanced this event (Fig. 6B, C, D). In addition, SB203580 could not attenuate the bFGF-induced decline of osterix (Osx) mRNA expression (data not shown).
Fig. 6.
Effect of SB203580 on bFGF-induced changes in (A) PAI-1 (n = 6), (B) uPA (n = 6), (C) uPAR (n = 6), (D) TIMP-1 (n = 6) mRNA expression in SCAP as revealed by real-time PCR. Results are expressed as folds of the control (as 1) (mean ± standard error). *denotes a statistically significant difference compared with the control group. #denotes a statistically significant difference compared with the bFGF treatment group (p < 0.05).
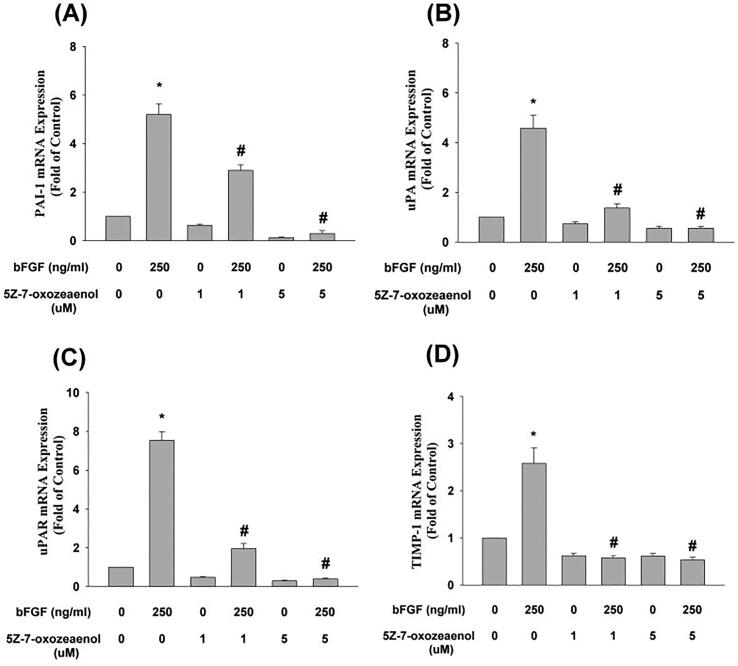
5Z-7-oxozeaenol attenuated bFGF-induced PAI-1 mRNA expression (Fig. 7A) and blocked the bFGF-induced uPA, uPAR, and TIMP-1 mRNA expression of SCAP (Fig. 7B, 7C, 7D). However, 5Z-7-oxozeaenol was not effective at suppressing the bFGF-induced decline of Osx mRNA expression (data not shown).
Fig. 7.
Effect of 5Z-7oxozeaenol on bFGF-induced changes in (A) PAI-1 (n = 8), (B) uPA (n = 10), (C) uPAR (n = 10), (D) TIMP-1 (n = 10) mRNA expression in SCAP as revealed by real-time PCR. Results are expressed as folds of the control (as 1) (mean ± standard error). *denotes a statistically significant difference compared with the control group. #denotes a statistically significant difference compared with the bFGF treatment group (p < 0.05).
Effect of different signaling inhibitors on the bFGF-induced decline of ALP activity in SCAP
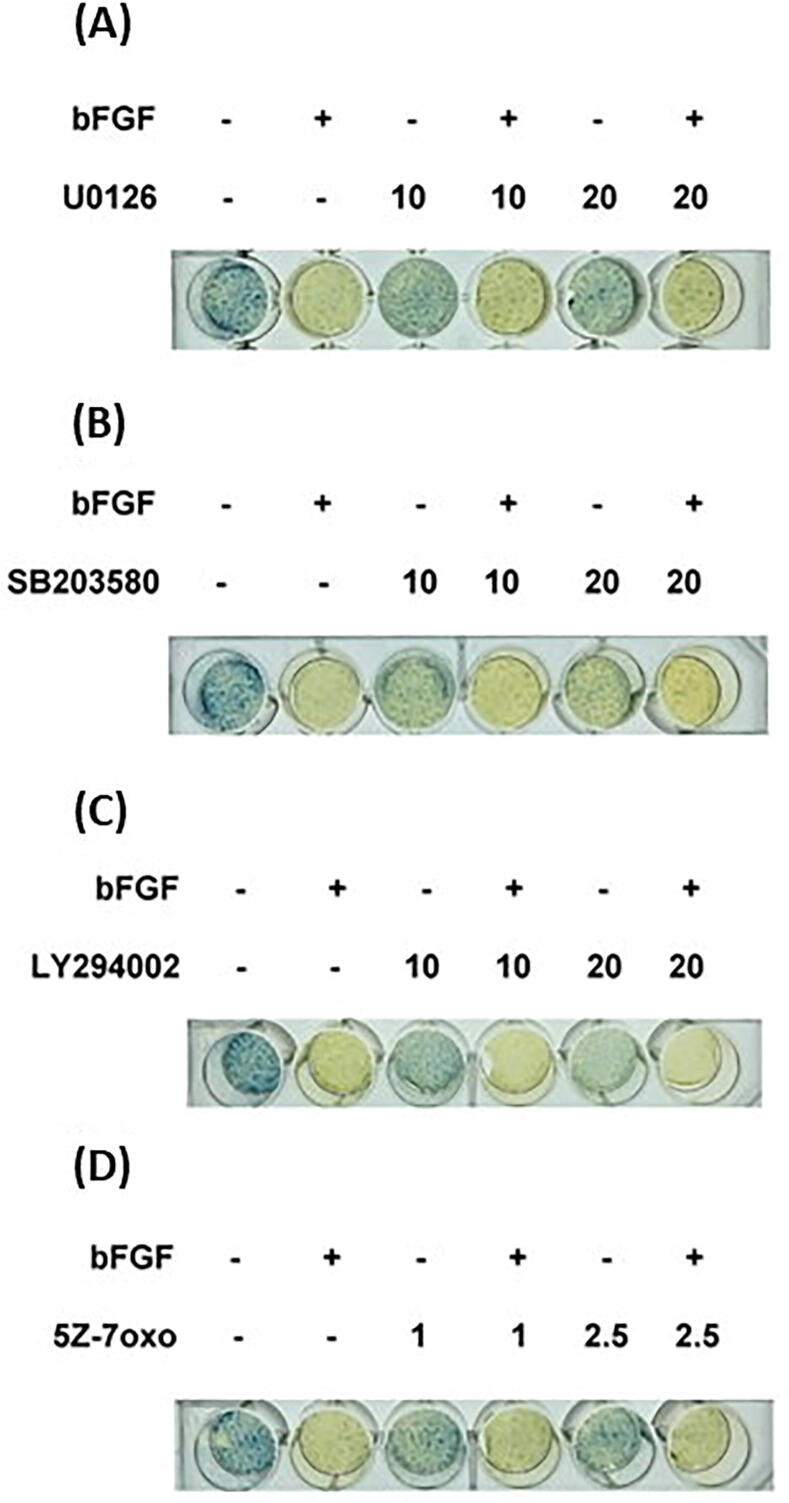
Because ALP and SPARC are two differentiation markers of osteoblasts and odontoblasts [33], we further studied the effect of bFGF on the ALP activities and SPARC production of SCAP. As compared to the solvent control, ALP activity was inhibited by bFGF at concentrations higher than 50 µg/mL. We investigated the possible pathways mediating the bFGF-induced event and found that U0126 exerted no marked effect on or only mildly reversed the inhibitory effect of bFGF on the ALP activity of SCAP (Fig. 8A). SB203580, LY294002, and 5Z-7oxozeaenol by themselves inhibited the ALP activities of SCAP. They were also unable to prevent the bFGF-induced decrease in ALP activities in SCAP (Fig. 8B, C, D).
Fig. 8.
Effect of (A) U0126, (B) SB203580, (C) LY294002, and (D) 5Z-7oxozeaenol on the bFGF-induced decline in ALP activity in SCAP. One representative ALP staining image is shown. *denotes a statistically significant difference compared with the control group. #denotes a statistically significant difference compared with the bFGF treatment group (p < 0.05).
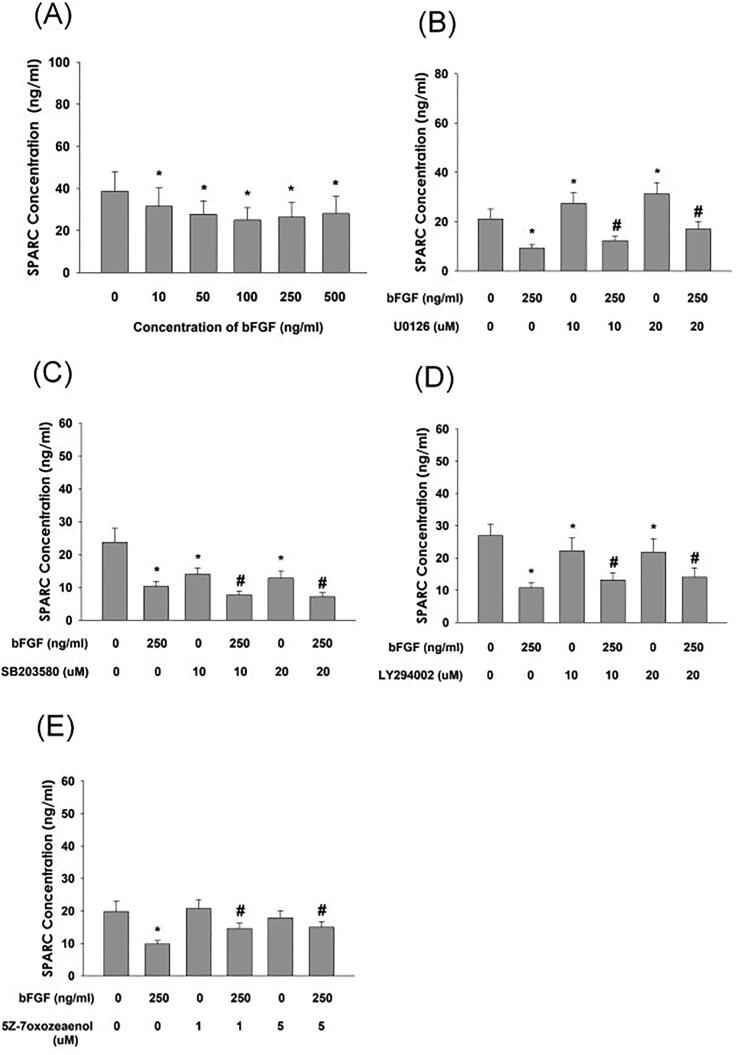
Effect of bFGF on SPARC production and its regulation by various signaling inhibitors in SCAP
bFGF inhibited the SPARC (osteonectin) production of SCAP at concentrations>10 ng/mL (Fig. 9A). Interestingly, 5Z-7oxozeaenol, U0126, and LY294002 partly attenuated the bFGF-induced decline of SPARC production of SCAP (Fig. 9B, D, E). Conversely, the bFGF-induced decrease in SPARC production in SCAP was prevented by SB203580 (Fig. 9C).
Fig. 9.
Effect of bFGF on the SPARC (osteonectin) production of SCAP and its regulation by various inhibitors. (A) Effect of bFGF on SPRAC production and its regulation by (B) U0126, (C) SB203580, (D) LY294002, and (E) 5Z-7oxozeaenol. Results are expressed as the mean ± standard error. *denotes a statistically significant difference compared with the control group. #denotes a statistically significant difference compared with the bFGF treatment group (p < 0.05).
In summary, bFGF stimulated the expression and production of plasminogen activation factors (PAI-1, uPA, and uPAR). SCAP expressed all four FGFRs (FGFR1, 2, 3, and 4). bFGF stimulated the phosphorylation and activation of TAK1, ERK, and p38. bFGF reduced ALP activity as well as SPARC expression and production in SCAP. The activation of TAK1 and ERK signaling may mediate both bFGF-induced TIMP-1 and plasminogen activation factors and the bFGF-induced decline in SPARC production in SCAP. However, these inhibitors exerted little preventive effect on the bFGF-induced decline in ALP activity.
Discussion
We tested whether bFGF would exert little effect on the proliferation, plasminogen activation factors, and osteogenic/odontogenic differentiation markers of SCAP and found that bFGF stimulated the expression/production of PAI-1, uPA, uPAR, and TIMP-1 but inhibited ALP and SPARC in SCAP. Moreover, bFGF exerted little stimulatory effect on the viability of SCAP in the presence of 10% FBS, which supported the findings of our previous report [18]. bFGF stimulated the proliferation of SCAP under serum-free conditions [18]. bFGF also stimulates cell stemness, DNA synthesis, and the proliferation of dental pulp cells, but inhibits apoptosis [10], [31], [34], [35], indicating the general growth-stimulating effect of bFGF.
Effect of bFGF on ALP activity and differentiation - ALP activity is high in mineralized tissue cells such as osteoblasts and odontoblasts and is considered to be an early marker of osteogenic and odontogenic differentiation [36]. bFGF has been shown to affect osteogenesis depending on the maturation condition of osteoblasts. In immature pre-osteoblast cells, short-term exposure to bFGF stimulates cell growth, but inhibits type I collagen, ALP activity, and osteocalcin. Conversely, bFGF improves cellular differentiation in mature osteoblasts [11]. In SCAP, Wu et al. demonstrated that bFGF (5 ng/mL) inhibited the mRNA expression of ALP and osteocalcin in differentiation medium over 2 weeks. Furthermore, ALP activity was significantly lower in the presence of bFGF after 8 days. However, 1-week pretreatment with bFGF before induction enhanced subsequent osteogenic differentiation [14]. These studies suggest that the effects of bFGF on cell differentiation depend on the specific timing and cell developmental stage. Similarly, bFGF (10–500 ng/mL) has been shown to inhibit the ALP activity of human dental pulp cells [31], [34]. In our experiment, the ALP activity of SCAP was obviously inhibited by bFGF (>10 ng/mL) after 5 days of exposure, suggesting that bFGF may modulate the differentiation of SCAP. However, the effects of bFGF on ALP under different experimental conditions can be tested further in the future.
Expression and role of FGFRs – Members of the FGF family are noted to affect cell metabolism, proliferation, survival, migration, differentiation during embryonic development, organogenesis, and tissue repair/regeneration. These events are differentially regulated through receptor activation and signaling [32]. Four FGF receptors (FGFR1, 2, 3, and 4), are differentially expressed in different kinds of tissues and organs [10], but upstream stimulators and downstream signaling molecules (e.g., phospholipase C gamma, leukemia inhibitory factor-signal transducers, activators of transcription [STAT], mitogen-activated protein kinase [MAPK], and phosphatidylinositol-4,5-bisphosphate 3-kinase [PI3K]-AKT) mediating different FGFR activation show some differences and remain to be clarified [10], [32], particularly in SCAP. We recently demonstrated FGFR1 and FGFR2 mRNA expression in SCAP and human dental pulp cells [18], [31]. In this study, we further delineated the protein expression of FGFR1, 2, 3, and 4 in SCAP. In bone tissue, knock-down of FGFR1 encouraged the differentiation of osteoblasts, implicating the role of FGFR1 in down-regulating bone formation. Deletion of FGFR2 also led to a decrease in bone density. On the other hand, FGFR3 is involved in chondrogenesis [11]. Signaling of FGF via the differential activation of FGFRs is crucial to maintain the pluripotency of stem cells via stimulation of effector molecules including SOX-2, Oct3/4, Klf4, nanog, and c-myc [32]. The differential roles of four activated FGFRs in SCAP should be further addressed.
Effect of bFGF on TIMP-1 and collagen – The main role of TIMP1 is reported to be reducing matrix degradation and controlling extracellular matrix turnover [37]. TIMP-1 also has cytokine-like functions and may influence cell growth, apoptosis, differentiation, and angiogenesis, possibly via receptor binding and the activation of cell signaling [38]. Recently, TIMP-1 has been shown to stimulate stem cell migration and promote tissue regeneration [39]. Little is known about the effect of bFGF on TIMP-1 in pulp cells and SCAP. Silverio-Ruiz et al. showed that bFGF (1 and 10 ng/mL) reduced TIMP1 and collagen I expression but enhanced matrix metalloproteinase-2 (MMP2) in human periodontal ligament cells after 72 h of incubation [40]. Conversely, Hakki et al. showed that bFGF (10 ng/mL) exerted no effect on TIMP1, collagen I, or MMP2 expression in periodontal ligament cells after 14 days of exposure [41]. We previously reported that bFGF induced the TIMP-1 expression and reduced the type I collagen protein expression of human SCAP [18], [31]. Similarly, bFGF also reduced the type I collagen level of human dental pulp cells [18], [42]. In this study, we also found that bFGF elevated TIMP-1 and reduced type I collagen mRNA expression in SCAP. The difference in expression of TIMP-1 and collagen by bFGF may be due to cell type, treatment period, or other unknown factors and should be further investigated.
Effect of bFGF on plasminogen activation factors - Limited knowledge is available on the expression of plasminogen activation system molecules in SCAP and their regulation by bFGF. Blood/fibrin clots contain various growth factors such as PDGF, bFGF, insulin-like growth factor-1, vascular endothelial growth factor, and stromal cell-derived factor-1 [43]. In addition, plasminogen is generated by the liver and circulates at a concentration of approximately 2 µM or higher in plasma and interstitial fluid [16]. Plasminogen activation factors (uPA, uPAR, and PAI-1) are the major fibrinolytic factors of stem cells for the stepwise proteolysis/dissolution of blood clots, growth factor availability, signal transduction, angiogenesis, immune modulation, and the migration/proliferation/homing of stem cells from apical papilla of the root apex and possibly also from bone marrow after tissue injury and inflammation [16], [17]. In this study, bFGF stimulated uPA, uPAR/suPAR, and PAI-1 mRNA expression and protein production in SCAP. The binding of uPA to membrane-bound uPAR or soluble uPAR may trigger the production of plasmin in the microenvironment. PAI-1 and plasmin may stimulate cell motility by regulating the interaction between vitronectin and uPAR for cell adhesion, which is due to the binding of PAI-1 to the uPA/uPAR complex on the cell surface and the detachment of uPA/uPAR from vitronectin [16]. Endocytic clearance of the complex integrin, uPA, uPAR, and PAI-1 also leads to the detachment of integrins from the extracellular matrix and enhances cell migration [44]. suPAR may directly bind to G protein-coupled receptor formyl peptide receptor-like 1 and induce cell migration [45]. Stimulation of the migration of MSCs by granulocyte colony-stimulating factor was abrogated in uPAR-/- mice [46]. These events may contribute to pericellular proteolysis and the subsequent migration and proliferation of SCAP into the pulp/root canal, which are crucial for clinical success. This can partly explain the stimulation of SCAP migration by bFGF [19], [20]. Interestingly, bFGF also stimulated the migration bone marrow and dental pulp stem cells in vitro [47], [48], and pulp recellularization and revascularization in endodontically treated human teeth placed into the dorsum of experimental rats in vivo [47].
Clinically, the induction of revascularization for immature teeth with an open root apex and pulp necrosis typically leads to favorable treatment outcomes. However, little is known about the subsequent repair and regeneration processes after blood clot induction. Whether the stem cells for pulpal repair/regeneration are derived from the blood stream or from the migration of SCAP is unclear. For the migration of SCAP into the root canal/pulp chamber, the focal fibrinolysis of blood clots, proliferation, and matrix deposition and differentiation are necessary for clinical success. This can be differentially regulated by numerous growth factors that promote the proliferation and migration of SCAP, as well as plasminogen activation system molecules. During wound healing, dynamic remodeling of the extracellular matrix is crucial for cell migration. In addition to fibrin clot dissolution, plasminogen activation/the fibrinolytic system is crucial for stem cell recruitment, growth factor bioavailability, and the functional consequence of tissue regeneration in the microenvironment [16].
Signal transduction of bFGF on plasminogen activation factors - The binding of uPA to uPAR may elicit many signal transduction pathways, including MEK/ERK, PI3K/protein kinase-B (Akt), Rho/Rho-associated protein kinase, and c-jun N-terminal kinase/p38 to mediate various cellular downstream events [16]. The signaling pathways after the binding of bFGF to four FGFRs have mainly been reported as Ras-MAPK, PI3K/Akt, phospholipase C-/protein kinase-C, and STAT [49], but the activation of TAK1 has also been reported [50]. However, the signal transduction responsible for bFGF-induced plasminogen activation system molecules remains to be clarified. Only MEK/ERK and p38 have been reported to associate with Chishao or lipopolysaccharide-induced bFGF and uPA production in Schwann cells and cardiac fibroblasts [51], [52]. The synergistic induction of TIMP-1 expression in human fibroblasts by all-trans retinoic acid and bFGF was attenuated by the inhibition of MEK/ERK [53]. In this study, U0126 and 5Z-7-oxozeaenol exerted preventive effects on bFGF-induced changes in PAI-1, uPA, uPAR, and TIMP-1 expression of SCAP, but SB203580 and LY294002 did not, suggesting the differential contribution of MEK/ERK and TAK1 signaling.
Signal transduction of bFGF on ALP and SPARC - The regulation mechanism of ALP in various cell types has been investigated. The signaling pathway regulated by bFGF on ALP remains to be clarified, particularly in SCAP. Osathanon et al. found that bFGF (20 ng/mL) attenuated ALP mRNA expression and mineralized tissue formation through MEK signaling in PDL stem cells and SHED. Adding an FGFR inhibitor (SU5402, 10 μM) or MEK inhibitor (PD98059, 40 μM) could reverse the reduction [54]. In this study, the inhibitory influence of bFGF (250 ng/mL) on ALP activity was mildly prevented by U0126 (MEK/ERK inhibitor), but not reversed by inhibitors of p-38, TAK1, or PI3K/Akt. Another study reported a preventive effect of pertussis toxin against the bFGF-induced decline of ALP in ROS 17/2.8 rat osteosarcoma cells [55], implicating the involvement of G-protein coupled receptor. Interestingly, we found that exposure of SCAP to bFGF also inhibited the production of SPARC (osteonectin). Similarly, bFGF has been shown to inhibit SPARC in human dental pulp cells [34]. Both ALP and SPARC are mineralized markers of odontoblasts and osteoblasts [33], [42]. SCAP have been shown to express various mineralization markers such as osteocalcin, osteonectin, and osteopontin. Vitamin D3 and cannabidiol may stimulate their expression in SCAP [56]. No prior report has addressed the influence of bFGF on the osteonectin expression/production of SCAP. In this study, bFGF reduced the osteonectin/SPARC production of SCAP. In dental pulp cells, bFGF has been shown to suppress Type I collagen, ALP activity, and SPARC expression [57]. A similar effect of bFGF on murine MC3T3 osteoblasts has also been reported [58]. Intriguingly, we found that the inhibitory effect of bFGF on SPARC production in SCAP can be attenuated by blocking MEK/ERK, TAK1, and PI3K/Akt signaling. These results suggest the differential roles of various signaling pathways by bFGF on the mineralization processes of SCAP.
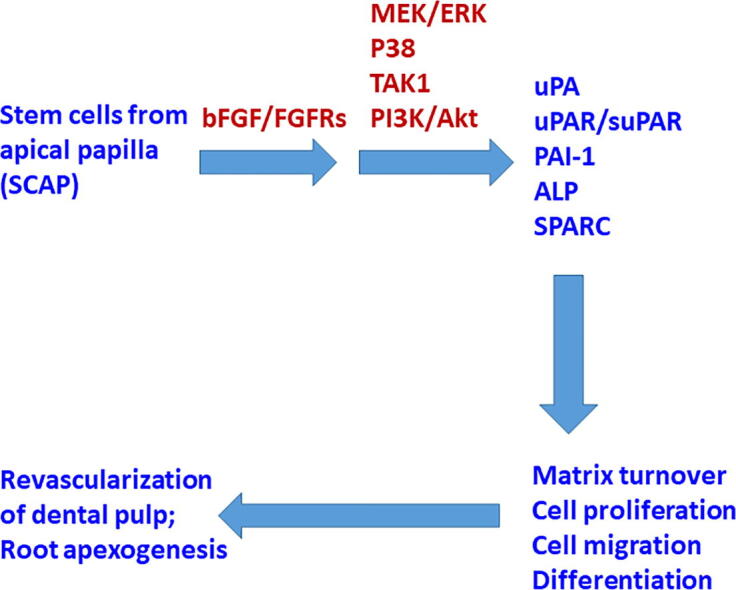
Summary – The results of this study indicated that SCAP expressed FGFR1, FGFR2, FGFR3, and FGFR4. In addition to p-ERK, bFGF alone also activated the p-TAK1 and p-p38 signaling pathways. Exposure of SCAP to bFGF increased PAI-1, uPA, uPAR, and TIMP-1, but reduced type I collagen expression under our culture conditions (Fig. 10). bFGF also suppressed the ALP activities and SPARC expression/production of SCAP. These results implicate the possible role of bFGF in the regulation of growth, migration, matrix turnover, and differentiation of SCAP (Fig. 10). However, more studies are necessary to evaluate the effect of bFGF on SCAP in the mineralization medium to further understand its role and interaction with other chemicals (e.g., vitamin C, β-glycerophosphate, dexamethasone) or growth factors such as TGF-α and bone morphogenetic proteins in mineralization processes.
Fig. 10.
Proposed mechanisms for clinical revascularization/apexogenesis in an immature open apex tooth, and the involvement of bFGF-induced receptor activation, signal transduction, and plasminogen activation system molecules, to regulate the cell proliferation, matrix turnover, migration, and differentiation of SCAP.
Conclusion
In conclusion, the endogenous or exogenous addition of bFGF in conjunction with scaffolds and SCAP may be useful to provoke the healing, repair, and regeneration of lost dental pulp in revascularization procedures via the autocrine or paracrine regulation of the plasminogen activation system molecules and TIMP-1 of adjacent cells. Understanding the effects of bFGF on SCAP and related signal transduction pathways is useful for future clinical therapies to promote apexogenesis and pulpo-dentin complex regeneration.
CRediT authorship contribution statement
Mei-Chi Chang: Conceptualization, Methodology, Resources. Nai-Yuan Chen: Investigation, Data curation. Jen-Hao Chen: Validation, Writing – review & editing. Wei-Ling Huang: Resources, Visualization. Chi-Yu Chen: Investigation, Data curation. Chih-Chia Huang: . Yu-Hwa Pan: Resources. Hsiao-Hua Chang: Conceptualization, Methodology, Resources, Supervision. Jiiang-Huei Jeng: Conceptualization, Methodology, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This study is supported by the Ministry of Science and Technology, Taipei, Taiwan (MOST104-2314-B-255- 010-MY3, MOST106-2314-B-002-033-MY2, MOST106-2314-B-002-034-MY2, MOST107-2314- B-255-009-MY3, MOST107-2314-B-255-008-MY2, MOST108-2314 -B-002-043-MY3, MOST110-2314-B255-002-MY3. MOST110-2314-B-255-003-MY3) and Chang Gung Memorial Hospital (CMRPF1G0101, CMRPF1G0102, CMRPF1F0071, CMRPF1H0061, CMRPF1H0062, CMRPF1H0063, CMRPF3E0022, CMRPF3E0023, NMRPF3E0041, NMRPF3E0042, NMRPF3E0043, NMRPF3H0061, NMRPF3H0062, NMRPF3H0071, NMRPF3H0072, NMRPF3H0073, NMRPF3L0031, NMRPF3L0041, CMRPF1K0071, CMRPF1K0072). The authors declare no conflict of interest regarding this manuscript.
Compliance with ethics requirements
This study was approved by the Ethics Committee of National Taiwan University Hospital (201512156RINA and 201412147RINC).
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Hsiao-Hua Chang, Email: hhhchang@ntu.edu.tw.
Jiiang-Huei Jeng, Email: jhjeng@ntu.edu.tw, jhjeng@kmu.edu.tw.
References
- 1.Andreasen J.O., Farik B., Munksgaard E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18(3):134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim S.G., Malek M., Sigurdsson A., Lin L.M., Kahler B. Regenerative endodontics: a comprehensive review. Int Endod J. 2018;51(12):1367–1388. doi: 10.1111/iej.12954. [DOI] [PubMed] [Google Scholar]
- 3.Zaky S.H., Shehabeldiin M., Ray H., Sfeir C. The role of inflammation modulcation in dental pulp regeneration. Eur Cell Mater. 2021;41:184–193. doi: 10.22203/eCM.v041a13. [DOI] [PubMed] [Google Scholar]
- 4.Chueh L., Huang G. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32(12):1205–1213. doi: 10.1016/j.joen.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Mittal N., Parashar V. Regenerative evaluation of immature roots using PRF and artificial scaffolds in necrotic permanent teeth: A clinical study. J Contem Dent Pract. 2019;20:720–726. [PubMed] [Google Scholar]
- 6.Huang G.-J., Sonoyama W., Liu Y.i., Liu H.e., Wang S., Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonoyama W., Liu Y.i., Yamaza T., Tuan R.S., Wang S., Shi S., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao J., An N.a., Ouyang X. Quantification of growth factors in different platelet concentrates. Platelets. 2017;28(8):774–778. doi: 10.1080/09537104.2016.1267338. [DOI] [PubMed] [Google Scholar]
- 9.Cooper P.R., Holder M.J., Smith A.J. Inflammation and Regeneration in the Dentin-Pulp Complex: A Double-edged Sword. J Endodont. 2014;40(4):S46–S51. doi: 10.1016/j.joen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Liu K., Yu S., Ye L., Gao B. The regenerative potential of bFGF in dental pulp repair and regeneration. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.680209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marie P.J. Fibroblast growth factor signaling controlling bone formation: An update. Gene. 2012;498(1):1–4. doi: 10.1016/j.gene.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 12.Qian J., Jiayuan W., Wenkai J., Peina W., Ansheng Z., Shukai S., et al. Basic fibroblastic growth factor affects the osteogenic differentiation of dental pulp stem cells in a treatment-dependent manner. Int Endod J. 2015;48(7):690–700. doi: 10.1111/iej.12368. [DOI] [PubMed] [Google Scholar]
- 13.Li B., Qu C., Chen C., Liu Y., Akiyama K., Yang R., et al. Basic fibroblast growth factor inhibits osteogenic differentiation of stem cells from human exfoliated deciduous teeth through ERK signaling. Oral Dis. 2012;18(3):285–292. doi: 10.1111/j.1601-0825.2011.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Huang G.-J., He W., Wang P., Tong Z., Jia Q., et al. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod. 2012;38(5):614–622. doi: 10.1016/j.joen.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morito A., Kida Y., Suzuki K., Inoue K., Kuroda N., Gomi K., et al. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009;72(1):51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- 16.Heissig B., Dhahri D., Eiamboonsert S., Salama Y., Shimazu H., Munakata S., et al. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell Mol Life Sci. 2015;72(24):4759–4770. doi: 10.1007/s00018-015-2035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong Y., Hoover-Plow J. The plasminogen system in regulating stem cell mobilization. J Biomed Biotechnol. 2012;2012:1–7. doi: 10.1155/2012/437920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang M.-C., Chen C.-Y., Chang Y.-C., Zhong B.-H., Wang Y.-L., Yeung S.-Y., et al. Effect of bFGF on the growth and matrix turnover of stem cells from human apical papilla: Role of MEK/ERK signaling. J Formos Med Assoc. 2020;119(11):1666–1672. doi: 10.1016/j.jfma.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi N., Hayashi Y., Murakami M., Alvarez F.J., Horibe H., Iohara K., et al. Similar in vitro effects and pulp Regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015;21(1):113–122. doi: 10.1111/odi.12227. [DOI] [PubMed] [Google Scholar]
- 20.Fayazi S., Takimoto K., Diogenes A. Comparative evaluation of chemotactic factor effect on migration and differentiation of stem cells of the apical papilla. J Endod. 2017;43(8):1288–1293. doi: 10.1016/j.joen.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Chang H.-H., Chang M.-C., Wu I.-H., Huang G.-F., Huang W.-L., Wang Y.-L., et al. Role of ALK5/Smad2/3 and MEK1/ERK signaling in transforming growth factor beta-1-modulated growth, collagen turnover, and differentiation of stem cells from apical papilla of human tooth. J Endod. 2015;41(8):1272–1280. doi: 10.1016/j.joen.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Chang H.H., Chen I.L., Wang Y.L., Chang M.C., Tsai Y.L., Lan W.C., et al. Regulation of the regenerative activity of dental pulp stem cells from exfoliated deciduous teeth (SHED) of children by TGF-1 is associated with ALK5/Smad2, TAK1, p38 and MEK/ERK signaling. Aging. 2020;12:21253–21272. doi: 10.18632/aging.103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang M.C., Chang H.H., Hsieh W.C., Huang W.L., Lian Y.C., Jeng P.Y., et al. Effects of transforming growth factor-beta1 on plasminogen activation in stem cells from the apical papilla: role of activating receptor-like kinase 5/Smad2 and mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling. Int Endod J. 2020;53:647–659. doi: 10.1111/iej.13266. [DOI] [PubMed] [Google Scholar]
- 24.Jeng J.H., Hsieh C.C., Lan W.H., Chang M.C., Lin S.K., Hahn L.J., et al. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol Toxicol. 1998;14:383–389. doi: 10.1023/a:1007591426267. [DOI] [PubMed] [Google Scholar]
- 25.Chang M.C., Uang B.J., Wu H.L., Lee J.J., Hahn L.J., Jeng J.H. Inducing the cell cycle arrest and apoptosis of oral KB carcinoma cells by hydroxychavicol: roles of glutathione and reactive oxygen species. Br J Pharmacol. 2002;135:619–630. doi: 10.1038/sj.bjp.0704492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M.-C., Wang T.-M., Chien H.-H., Pan Y.-H., Tsai Y.-L., Jeng P.-Y., et al. Effect of butyrate, a bacterial by-product, on the viability and ICAM-1 expression/production of human vascular endothelial cells: Role in infectious pulpal/periapical diseases. Int Endod J. 2022;55(1):38–53. doi: 10.1111/iej.13614. [DOI] [PubMed] [Google Scholar]
- 27.Chang M.C., Chang H.H., Lin P.S., Huang Y.A., Chan C.P., Tsai Y.L., et al. Effects of TGF-beta1 on plasminogen activation in human dental pulp cells: Role of ALK5/Smad2, TAK1 and MEK/ERK signalling. J Tissue Eng Regen Med. 2018;12:854–863. doi: 10.1002/term.2339. [DOI] [PubMed] [Google Scholar]
- 28.Chang M.-C., Tang C.-M., Lin Y.-H., Liu H.-C., Wang T.-M., Lan W.-C., et al. Toxic mechanisms of Roth801, Canals, microparticles and nanoparticles of ZnO on MG-63 osteoblasts. Mater Sci Eng C Mater Biol Appl. 2021;119:111635. doi: 10.1016/j.msec.2020.111635. [DOI] [PubMed] [Google Scholar]
- 29.Dr Petro G., Tavian D., Copeta A., Portolani N., Giulini S.M., Barlati S. Expression of urokinase-type plasminogen activator (u-PA), uPA receptor, and tissue-type PA messenger RNAs in human hepatocellular carcinoma. Cancer Res. 1998;58:2234–2239. [PubMed] [Google Scholar]
- 30.Jiang X., Liu F., Wang Y., Gao J. Secreted protein acidic and rich in cysteine promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells and acquisition of cancer stem cell phenotypes. J Gasteroenterol Hepatol. 2019;34(10):1860–1868. doi: 10.1111/jgh.14692. [DOI] [PubMed] [Google Scholar]
- 31.Chang Y.-C., Chang M.-C., Chen Y.-J., Liou J.-U., Chang H.-H., Huang W.-L., et al. Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. J Endod. 2017;43(6):936–942. doi: 10.1016/j.joen.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Mossahebi-Mohammadi M., Quan M.Y., Zhang J.S., Li X.K. FGF signaling pathway: A key regulator of stem cell pluripotency. Front Cell Dev Biol. 2020;8:79. doi: 10.3389/fcell.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg M., Njeh A., Uzunoglu E. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators Inflamm. 2015;2015:1–11. doi: 10.1155/2015/347649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiba H., Nakamura S., Shirakawa M., Nakanishi K., Okamoto H., Satakeda H., et al. Effects of basic fibroblast growth factor on proliferation, the expression of osteonectin (SPARC) and alkaline phosphatase, and calcification in cultures of human pulp cells. Dev Biol. 1995;170(2):457–466. doi: 10.1006/dbio.1995.1229. [DOI] [PubMed] [Google Scholar]
- 35.Luo L., Zhang Y., Chen H., Hu F., Wang X., Xing Z., et al. Effects and mechanisms of basic fibroblast growth factor on the proliferation and regenerative profiles of cryopreserved dental pulp stem cells. Cell Prolif. 2021;54(2) doi: 10.1111/cpr.v54.210.1111/cpr.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.H., Yoon D.S., Kim H.O., Lee J.W. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem Cells Dev. 2012;21(16):2958–2968. doi: 10.1089/scd.2011.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arpino V., Brock M., Gill S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44-46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71(4):659–672. doi: 10.1007/s00018-013-1457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sendon-Lago J., Seoane S., Martinez-Ordoñez A., Eiro N., Saa J., Vizoso F.J., et al. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp Eye Res. 2019;180:110–121. doi: 10.1016/j.exer.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Silverio-Ruiz K.G., Martinez A.E.T., Garlet G.P., Barbosa C.F., Silva J.S., Cicarelli R.M.B., et al. Opposite effects of bFGF and TGF-β on collagen metabolism by human periodontal ligament fibroblasts. Cytokine. 2007;39(2):130–137. doi: 10.1016/j.cyto.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Hakki S.S., Hakki E.E., Nohutcu R.M. Regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by basic fibroblast growth factor and dexamethasone in periodontal ligament cells. J Periodont Res. 2009;44(6):794–802. doi: 10.1111/j.1600-0765.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 42.Shiba H., Fujita T., Doi N., Nakamura S., Nakanishi K., Takemoto T., et al. Differential effects of various growth factors and cytokines on the synthesis of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cystein (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol. 1998;174:194–205. doi: 10.1002/(SICI)1097-4652(199802)174:2<194::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Shoji T., Nakasa T., Yoshizuka M., Yamasaki T., Yasunaga Y., Adachi N., et al. Comparison of fibrin clots derived from peripheral blood and bone marrow. Connect Tissue Res. 2017;58(2):208–214. doi: 10.1080/03008207.2016.1215443. [DOI] [PubMed] [Google Scholar]
- 44.Czekay R.P., Aertgeerts K., Curriden S.A., Loskutoff D.J. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resnati M., Pallavicini I., Wang J.M., Oppenheim J., Serhan C.N., Romano M., et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99(3):1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallabhaneni KC, Tkachuk S, Kiyan Y, Shushakova N, Haller H, Romano M, et al. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc Res 2011;90:113–21 [DOI] [PubMed]
- 47.Schmidt A., Ladage D., Schinköthe T., Klausmann U., Ulrichs C., Klinz F.-J., et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24(7):1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki T., Lee C.H., Chen M., Zhao W., Fu S.Y., Qi J.J., et al. Induced migration of dental pulp stem cells for in vitro pulp regeneration. J Dent Res. 2011;90:1013–1018. doi: 10.1177/0022034511408426. [DOI] [PubMed] [Google Scholar]
- 49.Xie Y., Zinkle A., Chen L., Mohammadi M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nature Rev Rheumatol. 2020;16(10):547–564. doi: 10.1038/s41584-020-0469-2. [DOI] [PubMed] [Google Scholar]
- 50.Salazar L., Kashiwada T., Krejci P., Meyer A.N., Casale M., Hallowell M., et al. Fibroblast growth factor receptor 3 interacts with and activates TGFbeta-activated kinase 1 tyrosine phosphorylation and NfkappaB signaling in multiple myeloma and bladder cancer. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0086470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang K.S., Lu M.J., Chen Y.S., Tsai F.J., Kuo W.W., Cheng Y.C., et al. Proliferative effects pf Chishao on Schwann cells are FGF-uPA, and ERK- and JNK-dependent. Am J Chin Med. 2009;37:1191–1202. doi: 10.1142/S0192415X09007594. [DOI] [PubMed] [Google Scholar]
- 52.Chen L.-C., Shibu M.A., Liu C.-J., Han C.-K., Ju D.-T., Chen P.-Y., et al. ERK1/2 mediates the lipopolysaccharide-induced upregulation of FGF-2, uPA, MMP-2, MMP-9 and cellular migration in cardiac fibroblasts. Chem Biol Interact. 2019;306:62–69. doi: 10.1016/j.cbi.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Bigg H.F., McLeod R., Waters J.G., Cawston T.E., Clark I.M. Mechanisms of induction of human tissue inhibitors of metalloproteinases-1 (TIMP-1) gene expression by all-trans retinoic acid in combination with basic fibroblast growth factor. Eur J Biochem. 2000;267:4150–4156. doi: 10.1046/j.1432-1327.2000.01459.x. [DOI] [PubMed] [Google Scholar]
- 54.Osathanon T., Nowwarote N., Manokawinchoke J., Pavasant P., Osathanon T., et al. bFGF and JAGGED1 regulate alkaline phosphatase expression and mineralization in dental tissue-derived mesenchymal stem cells. J Cell Biochem. 2013;114:2551–2561. doi: 10.1002/jcb.24602. [DOI] [PubMed] [Google Scholar]
- 55.Rodan S.B., Wesolowski G., Yoon K., Rodan G.A. Opposing effects of fibroblast growth factor and pertussis toxiin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. Biol Chem. 1989;264:19934–19941. [PubMed] [Google Scholar]
- 56.Papagerakis P., Berdal A., Mesbah M., Peuchmaur M., Malaval L., Nydegger J., et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30(2):377–385. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- 57.Petrescu N.B., Jurj A., Sorițău O., Lucaciu O.P., Dirzu N., Raduly L., et al. Cannabidiol and vitamin D3 impact on osteogenic differentiation of human dental mesenchymal stem cells. Medicina (Kaunas) 2020;56(11):607. doi: 10.3390/medicina56110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delany A.M., Canalis E. Basic fibroblast growth factor destabilizes osteonectin mRNA in osteoblasts. Am J Physiol. 1998;274(3):C734–C740. doi: 10.1152/ajpcell.1998.274.3.C734. [DOI] [PubMed] [Google Scholar]