Abstract
Intestinal organoids are self-organized tissue constructs, grown in vitro, that resemble the structure and function of the intestine and are often considered promising as a prospective platform for drug testing and disease modeling. Organoid development in vitro is typically instructed by exogenous cues delivered from the media, but cellular responses also depend on properties of the surrounding microenvironmental niche, such as mechanical stiffness and extracellular matrix (ECM) ligands. In recent years, synthetic hydrogel platforms have been engineered to resemble the in vivo niche, with the goal of generating physiologically relevant environments that can promote mature and reproducible organoid development. However, few of these approaches consider the importance of intestinal organoid morphology, or how morphology changes during development, as cues that may dictate organoid functionality. For example, intestinal organoids grown in vitro often lack the physical boundary conditions found in vivo that are responsible for shaping a collection of cells into developmentally relevant morphologies, resulting in organoids that often differ in structure and cellular organization from the parent organ. This disconnect relates, in part, to a lack of appropriate adaptable and programmable materials for cell culture, especially those that enable control over colony growth and differentiation in space and time (i.e., 4D materials). We posit that the future of organoid culture platforms may benefit from advances in photoadaptable chemistries and integration into biomaterials scaffolds, thereby allowing greater user-directed control over both the macro and micro-scale material properties. In this way, synthetic materials can begin to better replicate changes in the ECM during development or regeneration in vivo. Recapitulation of cellular and tissue morphological changes, along with an appreciation for the appropriate developmental timescales, should help instruct the next generation of organoid models to facilitate predictable outcomes.
Keywords: Intestinal organoids, Biomaterials, Photochemistry, Tissue Geometry
Graphical Abstract
SUMMARY
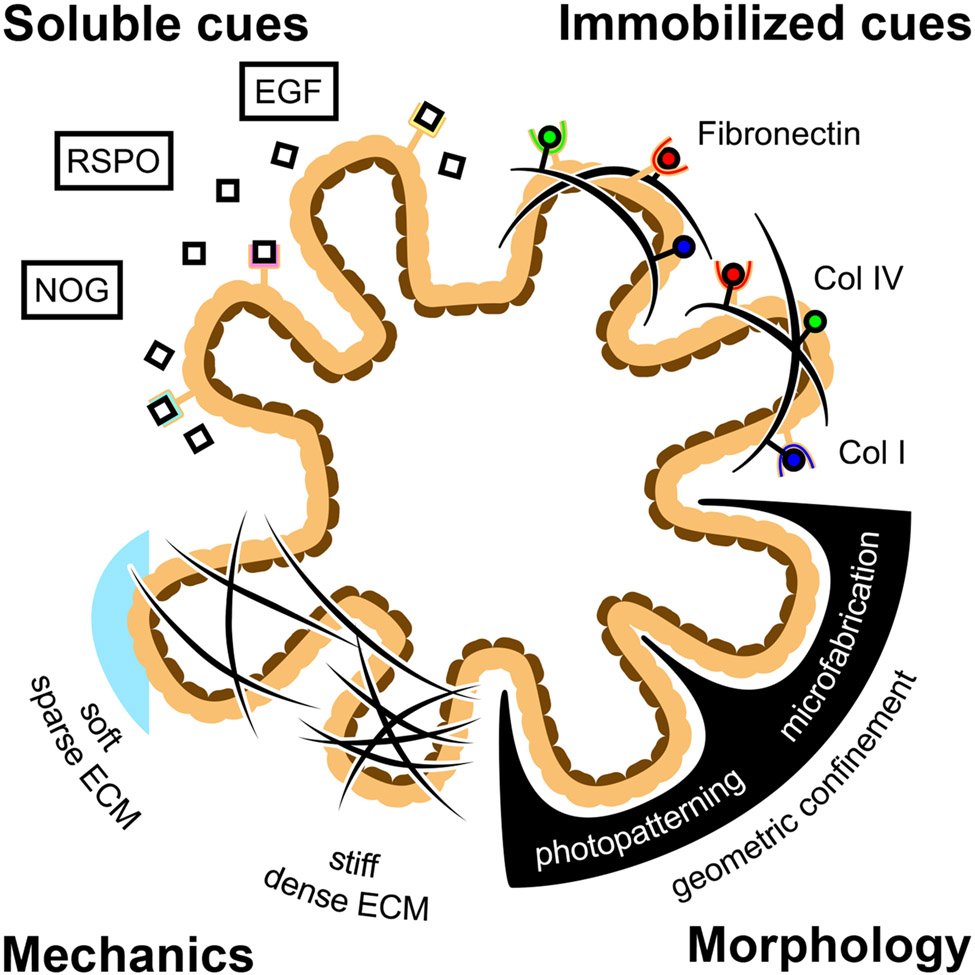
Intestinal organoids are multicellular 3D tissue constructs that self-organize and differentiate from intestinal stem cells to form relevant cellular compositions and microarchitectures in vitro that mimic the structure, function, and spatial phenotypic distributions of the intestine1. Organoids contain intestinal crypts that house intestinal stem cells and secretory Paneth cells that extend off a central, enclosed lumen, replicating the crypt-villus architecture seen in vivo. The ability to recapitulate these complex structure-function relationships in vitro enables the use of intestinal organoids as relevant replacements for animal models to approach a host of applications, including drug screening2,3, studying organ development4 and disease progression in vitro5, and sourcing transplantable tissue6. Naturally, there is substantial interest in generating more physiologically relevant intestinal organoid models with better-matched cellular diversity and functional outputs to rigorously replicate in vivo biological processes. However, the development of physiologically relevant organoid models in vitro requires recapitulating the complex mixture of signaling cues found within the in vivo microenvironmental niche. To date, a multitude of biomaterial and tissue engineering approaches have been used to develop in vitro culture platforms that present niche-specific mechanical cues7,8, ECM interactions9, and soluble growth factors10-12 that are known to enhance intestine-specific growth and morphogenesis. While the presentation of such cues is important for generating functional and physiologically relevant organoids, engineering strategies involving intestinal organoids have largely neglected the role of morphology and, more importantly, the time-dependence of tissue morphogenesis in recapitulating the in vivo niche (Figure 1). Formation of the intestine in vivo is highly regulated by cell shape and organization, which dictate the appropriate cell-cell contacts and paracrine signaling interactions that generate gradients to promote cellular self-renewal or differentiation that are essential for proper development. Additionally, formation of functional crypt-villi architecture relies on the proper temporal regulation of these morphological cues13. However, in vitro organoid platforms often utilize isotropic materials and homogenous soluble cues in bulk media compositions that limit the proper spatial and temporal regulation of tissue morphology. To replicate the dynamic changes that occur during organoid evolution, we and others7,14,15 have focused on the development of adaptable materials that enable 4D control over material properties, thereby directing cellular interactions and organoid morphology.
Figure 1.
Engineering approaches have attempted to enhance the fidelity of intestinal organoid models by the addition of niche specific soluble signaling cues, immobilized ECM cues, and mechanical cues. Geometric confinement of intestinal organoids is an underutilized method to construct physiologically relevant organoid structures and morphologies.
While proteinaceous biomaterials (e.g., collagen, Matrigel) are routinely used for organoid culture and have led to many advancements in the field, these culture scaffolds do not allow for spatiotemporal control over material properties. Intestinal stem cells cultured in these materials experience unrestricted self-organization to produce stochastic crypt geometries that generates an uncontrolled distribution in crypt size, number, and spacing. Unintended changes that occur in organoid architecture in vitro can alter the paracrine signaling mechanisms that are essential for proper development, and may be one of the reasons that different transcriptional profiles are observed in vivo and in vitro10,16.
As an alternative approach, micropatterning of biomaterials has been used to recreate tissue architectures seen in vivo. For example, Wang et al. developed polydimethylsiloxane (PDMS) stamps to generate 2D crypt-villus structures using collagen hydrogels with tunable stiffness17. Intestinal epithelial cells growing as a monolayer on the gel surface conformed to the patterned shape, which contributed to compartmentalization of the stem cell niche. A complementary platform used laser photoablation to reconstruct the relevant 3D crypt-villus architecture in within a collagen/Matrigel hydrogel, forming an intestinal monolayer that conformed to the constructed three-dimensional patterns18. Despite significant advances in patterning intestinal epithelial monolayers, these materials strategies do not consider the dynamic nature of tissue morphogenesis. Instead, they rely on templated approaches where stem cells are restricted to static, user-defined surfaces that minimize the capacity for progressive self-organization.
Of note, epithelial cell layers that conform to static material structure do not experience the same forces and cell interactions that occur during dynamic tissue formation found in vivo. However, advances in material chemistry now allow for dynamic control over cellular interactions and tissue morphology, particularly adaptable materials that can change mechanical properties or material conformation in response to internal and external stimuli in a spatiotemporally controlled manner. Highlighting the importance of matrix remodeling for intestinal development19, Gjorevski et al. developed a hydrolytically degradable poly(ethylene glycol) (PEG) hydrogel and tuned the degradation rate to capture aspects of temporal matrix remodeling that promote intestinal crypt formation7. As such, the use of temporally modulated materials, such as hydrolytic or protease degradable, or stress relaxing chemistries, can be tuned to mimic temporal changes in organoids that affect development. While the use of these chemistries enables time dependent control over material properties, it is necessary to simultaneously exert spatial control to achieve physiological relevant tissue geometries. However, independently combining spatial and temporal technologies has been difficult to achieve. Traditional degradable or stress relaxing chemistries that endow temporal control over hydrogel mechanical properties affect the bulk gel, and often cannot be applied in a spatially defined manner. Similarly, technologies that introduce spatial control, such as micropatterning, do not afford temporal control as they are difficult to alter post fabrication. Instead, the application of light to modulate photosensitive chemistries offers unique spatial and temporal control in a singular hydrogel chemistry. Here, patterning of light exposure offers spatial control over material properties, while controlled shuttering of the light offers temporal control. The application of light controlled material chemistries to modulate the mechanical and biochemical properties of biomaterials (e.g., photoadaptable materials) presents unique opportunities to control organoid morphology in both space and time20.
FUTURE DIRECTIONS
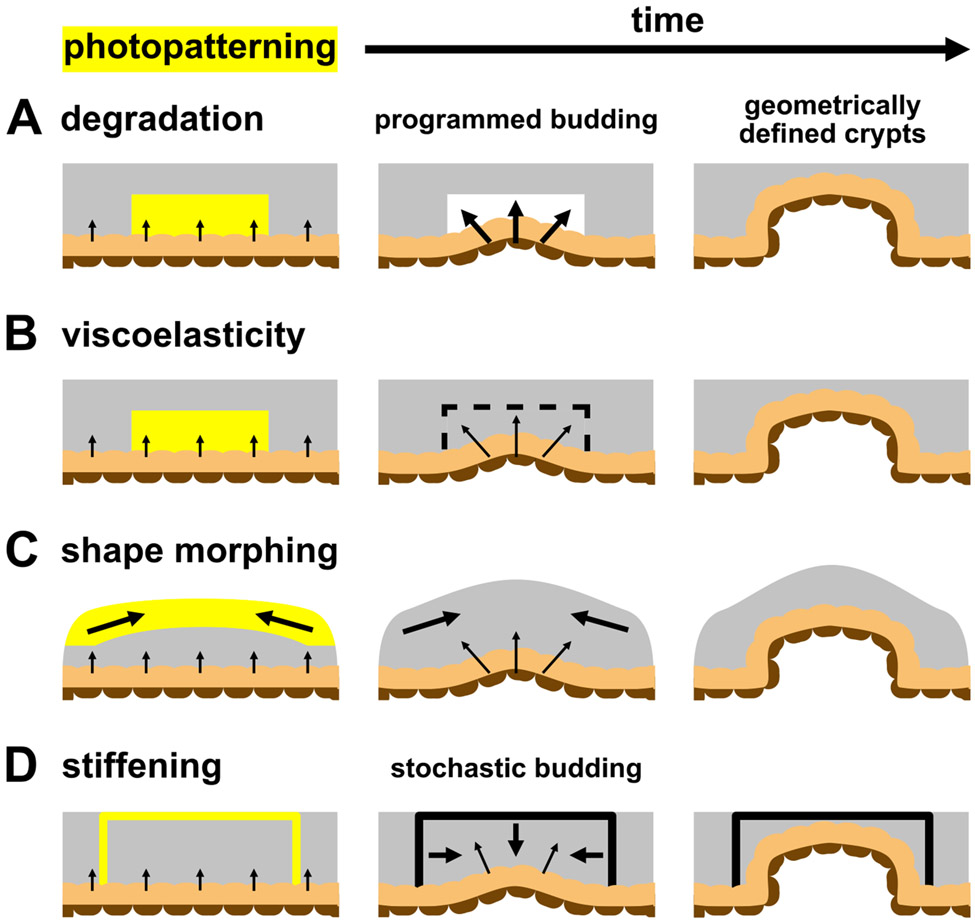
Photoadaptable biomaterials have been used extensively to modulate mechanical properties and cellular interactions in vitro21-23, and these strategies also provide many benefits for controlling intestinal organoid development. For example, two photon laser scanning confocal microscopy was used to form 3D channels within photodegradable nitrobenzyl hydrogels that promoted and controlled axon outgrowth from motor neuron bundles21. This photodegradation mechanism was subsequently adapted to intestinal organoid culture, in which photodegraded channels were used to direct the formation of physiologically relevant crypts structures within photodegraded regions and control symmetry breaking events in intestinal organoid colonies24. In combination with soluble differentiation cues, ISCs that expanded into the photodegraded regions underwent a shape change, triggering mechanosensitive pathways to induce fate specification and crypt formation. In this way, photodegradation, and thus crypt formation, can be manipulated at any point in space and time to replicate the dynamic changes that occur during in vivo crypt formation (Figure 2A). The application of spatially patterned light could be used to generate physiologically relevant crypt spacing and dimensions to best replicate the in vivo niche and could even be tailored to accommodate species-specific13 or location-specific differences25 for advancements in personalized medicine.
Figure 2.
A) The application of patterned light to photodegradable hydrogels results in local softening that guides crypt formation into the degraded region. B) Similarly, patterned light can temporarily induce viscoelasticity to modulate intestinal epithelial shape, allowing intrinsic morphogenic programming to dictate crypt morphology. C) Irradiation of shape morphing hydrogels causes a macroscale deformation that guides crypt formation. D) Photostiffening reactions are used to generate locally stiff regions that confine intestinal crypts to the patterned shape.
While hydrogels with nitrobenzyl-containing crosslinks allow for spatiotemporal controlled crypt morphology, photodegradation kinetics are relatively slow, relying on a one-photon, one-event mechanism. In contrast, more recent research has shown that allyl sulfide hydrogels allow for amplification of photodegradation26, as well as the ability to induce viscoelastic material changes with light27. Amplification of this mechanism enhances the kinetics of photodegradation and circumvents issues with light attenuation that hinder nitrobenzyl photodegradation, and has been shown to be beneficial for bulk passaging intestinal organoids28 and bulk material degradation, leading to intestinal organoid differentiation15. By altering the photoreaction conditions, allyl sulfide-containing hydrogels can also be designed to undergo a photoinduced change in their viscoelastic properties. Marozas et al. used allyl sulfide PEG hydrogels to photopattern viscoelasticity to probe hMSC mechanosensing by following cellular process extension as a function of on-demand changes in viscoelasticity27. Similar strategies are being explored with intestinal organoids where application of photopatterned viscoelasticity can disturb the mechanical equilibrium between the expanding organoid and the surrounding hydrogel to spatiotemporally modulate epithelial shape, without altering the overall network structure (e.g., elastic modulus). Using these types of materials, researchers may be able to deconvolute the effects of cell-generated forces from the overall hydrogel structure and equilibrium mechanical properties, ultimately improving the field’s understanding of the role of intestinal organoid morphology and cell shape in directing crypt formation (Figure 2B).
While photorelaxation approaches aim to control cell and tissue shape on the microscale, other approaches can be used to influence tissue-level organization by controlling macroscale material conformations. For example, the use of folding materials, such as origami or kirigami-inspired platforms, enable dynamic transitions in shape to occur during tissue development. Viola et al. prepatterned hinges into planar ECM sheets that folded into complex 3D origami shapes in response to cell-generated tension29. MDCK cells that were adhered to these sheets formed into epithelial tubules along the patterned folds to replicate the branching epithelial network of the kidney. In this case, development of the tissue was guided by the time-dependent formation of this macroscale architecture. However, the design of synthetic systems to recapitulate important morphological changes in organoid systems using programmed material strains is underutilized30. Shape memory polymers and liquid crystal elastomers are two promising classes of materials that can switch between material conformations in response to stimuli, such as light (Figure 2C). However, synthesis of these materials often requires harsh temperature and solvent treatments, and so care must be taken to preserve the activity of sensitive bioactive ligands necessary for cell attachment and survival. The expansion and utilization of shape morphing platforms will enable more complex macro-scale morphological changes to influence tissue development.
Photostiffening is a final photochemical process with potential to modulate organoid morphology. Recently, the photoinduced gelation of an interpenetrating synthetic network was used to fabricate three-dimensional, locally stiffened regions within ECM scaffolds that physically altered the morphology of expanding organoids31 and guided the direction and shape of crypt formation32 (Figure 2D). While these on-demand photostiffening reactions provide additional means to regulate local hydrogel mechanical properties to control organoid morphology, further control can be leveraged by the utilization of photochemistries to reversibly alter mechanical properties, such as reversible cycloadditions33,34 or photoisomerizations35. The ability to repeatably and reversibly soften and stiffen local hydrogel environments has the potential to provide even greater control over organoid morphologies in time and space.
While the use of photoadaptable 4D materials offers unprecedented spatial and temporal control over organoid morphology, their use is not without limitations. Photosensitive chemistries are susceptible to issues with light absorbance such as scattering and attenuation, which reduces the fidelity and penetration depth of illuminated patterns. Additionally, cellular applications involving photoadaptable materials must ensure that the combination of light intensity, irradiation time, and wavelength are cytocompatible36. Although light-responsive systems are largely dependent on specialized chemistries, synthetically tractable approaches for many of these materials are available using inexpensive reagents. Moreover, emerging hydrogel technologies present new opportunities for sequential or repeatable degradation and crosslinking in a spatiotemporal manner34,37,38. While many advanced photochemistries that enable these properties have yet to reach the intestinal organoid community, unique mechanical conditioning involving both softening and stiffening will be made newly accessible by next-generation biomaterials incorporating this orthogonal responsiveness.
These technologies are also challenging to apply in a high-throughput manner to intestinal organoids. Photopatterning using a directed laser requires application of light on a pattern-by-pattern basis, which is not readily scalable without advanced computational assistance. While the use of photomasks can provide higher-throughput patterning, this method requires initial spatial patterning of cells to align with prefabricated photomask patterns, which can be achieved using microfabrication techniques like soft lithography and imprinting2. New technologies to deliver light, such as tomographic projection, will broaden the use of photoadaptable materials for organoid development by affording scalable 3D control over illumination and subsequent manipulation of mechanical properties and biochemical cues39,40.
A final limitation of photoadaptable materials is that the modulation of material properties to guide in vitro development is user-defined, relying on previous knowledge of developmental geometries and timescales to influence the timing and extent of irradiation. For mouse intestinal organoids, which mimic some aspects of murine intestinal development, relevant in vitro geometries and timescales can be easily derived by studying in vivo developmental dynamics of mouse models. However, knowledge of developmental dynamics may be difficult to assess in more relevant or complex species, such as humans, or in organs and tissues that are less accessible than the intestine. While mimicking changes in tissue geometry can enhance the physiological relevance of organoid models, it is clear that this approach is necessary but not sufficient for guiding organoid development in vitro to optimally recapitulate in vivo processes. Tissue generation in vivo is enhanced by the incorporation of multiple niche cues acting in concert, including ECM signaling, soluble growth factors, and microenvironmental mechanics. Thus, designing the next generation of 4D biomaterials will involve increased collaboration between engineers, chemists, biologists, and biophysicists to design, guide, and control complex cellular behaviors at precise points in space and time to build better functional tissues and organs
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (R01 DK120921) and the National Science Foundation (NSF 2033723). F.M.Y acknowledges the Department of Education Graduate Assistance in Areas of National Need (DoEd GAANN) and the National Institutes of Health (F31 DK126427) for support. Figures were generated using Affinity Publisher 1.10.1.
REFERENCES
- (1).Sato T; Clevers H Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science (80-. ). 2013, 340 (6137), 241–260. 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- (2).Brandenberg N; Hoehnel S; Kuttler F; Homicsko K; Ceroni C; Ringel T; Gjorevski N; Schwank G; Coukos G; Turcatti G; Lutolf MP High-Throughput Automated Organoid Culture via Stem-Cell Aggregation in Microcavity Arrays. Nat. Biomed. Eng 2020. 10.1038/s41551-020-0565-2. [DOI] [PubMed] [Google Scholar]
- (3).Yoshida S; Miwa H; Kawachi T; Kume S; Takahashi K Generation of Intestinal Organoids Derived from Human Pluripotent Stem Cells for Drug Testing. Sci. Rep 2020, 10 (1), 1–11. 10.1038/s41598-020-63151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sumigray KD; Terwilliger M; Lechler T Morphogenesis and Compartmentalization of the Intestinal Crypt. Dev. Cell 2018, 45 (2), 183–197.e5. 10.1016/j.devcel.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Dekkers JF; Wiegerinck CL; De Jonge HR; Bronsveld I; Janssens HM; De Winter-De Groot KM; Brandsma AM; De Jong NWM; Bijvelds MJC; Scholte BJ; Nieuwenhuis EES; Van Den Brink S; Clevers H; Van Der Ent CK; Middendorp S; Beekman JM A Functional CFTR Assay Using Primary Cystic Fibrosis Intestinal Organoids. Nat. Med 2013, 19 (7), 939–945. 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- (6).Watson CL; Mahe MM; Múnera J; Howell JC; Sundaram N; Poling HM; Schweitzer JI; Vallance JE; Mayhew CN; Sun Y; Grabowski G; Finkbeiner SR; Spence JR; Shroyer NF; Wells JM; Helmrath MA An in Vivo Model of Human Small Intestine Using Pluripotent Stem Cells. Nat. Med 2014, 20 (11), 1310–1314. 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gjorevski N; Sachs N; Manfrin A; Giger S; Bragina ME; Ordóñez-Morán P; Clevers H; Lutolf MP Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539 (7630), 560–564. 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- (8).Hunt DR; Klett KC; Mascharak S; Wang H; Gong D; Lou J; Li X; Cai PC; Suhar RA; Co JY; LeSavage BL; Foster AA; Guan Y; Amieva MR; Peltz G; Xia Y; Kuo CJ; Heilshorn SC Engineered Matrices Enable the Culture of Human Patient-Derived Intestinal Organoids. Adv. Sci 2021, 8 (10), 1–12. 10.1002/advs.202004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hernandez-Gordillo V; Kassis T; Lampejo A; Choi G; Gamboa ME; Gnecco JS; Brown A; Breault DT; Carrier R; Griffith LG Fully Synthetic Matrices for in Vitro Culture of Primary Human Intestinal Enteroids and Endometrial Organoids. Biomaterials 2020, 254 (May), 120125. 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fujii M; Matano M; Toshimitsu K; Takano A; Mikami Y; Nishikori S; Sugimoto S; Sato T Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23 (6), 787–793.e6. 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- (11).Czerwinski M; Holloway EM; Tsai YH; Wu A; Yu Q; Wu J; Walton KD; Childs C; Glass I; Treutlein B; Camp JG; Spence JR In Vitro and in Vivo Development of the Human Intestinal Niche at Single Cell Resolution. bioRxiv 2020, No. 1, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yin X; Farin HF; Van Es JH; Clevers H; Langer R; Karp JM Niche-Independent High-Purity Cultures of Lgr5 + Intestinal Stem Cells and Their Progeny. Nat. Methods 2014, 11 (1), 106–112. 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Walton KD; Mishkind D; Riddle MR; Tabin CJ; Gumucio DL Blueprint for an Intestinal Villus: Species-Specific Assembly Required. Wiley Interdiscip. Rev. Dev. Biol 2018, 7 (4), 1–19. 10.1002/wdev.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cruz-Acuña R; Quirós M; Farkas AE; Dedhia PH; Huang S; Siuda D; García-Hernández V; Miller AJ; Spence JR; Nusrat A; García AJ Synthetic Hydrogels for Human Intestinal Organoid Generation and Colonic Wound Repair. Nat. Cell Biol 2017, 19 (11), 1326–1335. 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hushka EA; Yavitt FM; Brown TE; Dempsey PJ; Anseth KS Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids. Adv. Healthc. Mater 2020, 1901214, 1–9. 10.1002/adhm.201901214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mead BE; Ordovas-Montanes J; Braun AP; Levy LE; Bhargava P; Szucs MJ; Ammendolia DA; MacMullan MA; Yin X; Hughes TK; Wadsworth MH; Ahmad R; Rakoff-Nahoum S; Carr SA; Langer R; Collins JJ; Shalek AK; Karp JM Harnessing Single-Cell Genomics to Improve the Physiological Fidelity of Organoid-Derived Cell Types. BMC Biol. 2018, 16 (1), 1–24. 10.1186/s12915-018-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang Y; Gunasekara DB; Reed MI; DiSalvo M; Bultman SJ; Sims CE; Magness ST; Allbritton NL A Microengineered Collagen Scaffold for Generating a Polarized Crypt-Villus Architecture of Human Small Intestinal Epithelium. Biomaterials 2017, 128, 44–55. 10.1016/j.biomaterials.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nikolaev M; Mitrofanova O; Broguiere N; Geraldo S; Dutta D; Tabata Y; Elci B; Gjorevski N; Clevers H; Lutolf MP Homeostatic Mini-Intestines through Scaffold-Guided Organoid Morphogenesis. Nature 2020, in Press (June 2018). 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- (19).Bonnans C; Chou J; Werb Z Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol 2014, 15 (12), 786–801. 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Brown TE; Anseth KS Spatiotemporal Hydrogel Biomaterials for Regenerative Medicine. Chem. Soc. Rev 2017, 46 (21), 6532–6552. 10.1039/C7CS00445A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).McKinnon DD; Brown TE; Kyburz KA; Kiyotake E; Anseth KS Design and Characterization of a Synthetically Accessible, Photodegradable Hydrogel for User-Directed Formation of Neural Networks. Biomacromolecules 2014, 15 (7), 2808–2816. 10.1021/bm500731b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Rosales AM; Vega SL; DelRio FW; Burdick JA; Anseth KS Hydrogels with Reversible Mechanics to Probe Dynamic Cell Microenvironments. Angew. Chemie - Int. Ed 2017, 56 (40), 12132–12136. 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Brown TE; Silver JS; Worrell BT; Marozas IA; Yavitt FM; Günay KA; Bowman CN; Anseth KS Secondary Photocrosslinking of Click Hydrogels to Probe Myoblast Mechanotransduction in Three Dimensions. J. Am. Chem. Soc 2018, 140 (37), 11585–11588. 10.1021/jacs.8b07551. [DOI] [PubMed] [Google Scholar]
- (24).Gjorevski N; Nikolaev M; Brown TE; Brandenberg N; DelRio F; Yavitt FM; Liberali P; Anseth KS; Lutolf MP Tisse Geometry Drives Deterministic Organoid Patterning. Science (80-. ). 2022, 9021. 10.1126/science.aaw9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Middendorp S; Schneeberger K; Wiegerinck CL; Mokry M; Akkerman RDL; van Wijngaarden S; Clevers H; Nieuwenhuis EES Adult Stem Cells in the Small Intestine Are Intrinsically Programmed with Their Location-Specific Function. Stem Cells 2014, 32 (5), 1083–1091. [DOI] [PubMed] [Google Scholar]
- (26).Brown TE; Marozas IA; Anseth KS Amplified Photodegradation of Cell-Laden Hydrogels via an Addition–Fragmentation Chain Transfer Reaction. Adv. Mater 2017, 29 (11). 10.1002/adma.201605001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Marozas IA; Cooper-White JJ; Anseth KS Photo-Induced Viscoelasticity in Cytocompatible Hydrogel Substrates. New J. Phys 2019, 21 (4), 0–12. 10.1088/1367-2630/ab1309. [DOI] [Google Scholar]
- (28).Yavitt FM; Brown TE; Hushka EA; Brown ME; Gjorevski N; Dempsey PJ; Lutolf MP; Anseth KS The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and Their Application as a Degradable Scaffold for Organoid Passaging. Adv. Mater 2020, 32 (30), 1905366. 10.1002/adma.201905366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Viola JM; Porter CM; Gupta A; Alibekova M; Prahl LS; Hughes AJ Guiding Cell Network Assembly Using Shape-Morphing Hydrogels. Adv. Mater 2020, 32 (31). 10.1002/adma.202002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kim J; Hayward RC Mimicking Dynamic in Vivo Environments with Stimuli-Responsive Materials for Cell Culture. Trends Biotechnol. 2012, 30 (8), 426–439. 10.1016/j.tibtech.2012.04.003. [DOI] [PubMed] [Google Scholar]
- (31).Urciuolo A; Poli I; Brandolino L; Raffa P; Scattolini V; Laterza C; Giobbe GG; Zambaiti E; Selmin G; Magnussen M; Brigo L; De Coppi P; Salmaso S; Giomo M; Elvassore N Intravital Three-Dimensional Bioprinting. Nat. Biomed. Eng 2020, 4 (9), 901–915. 10.1038/s41551-020-0568-z. [DOI] [PubMed] [Google Scholar]
- (32).Elvassore N; Urciuolo A; Giobbe G Four-Dimensional Hydrogel-in-Hydrogel Bioprinting for the Spatiotemporal Control of Organoid and Organotypic Cultures. [Google Scholar]
- (33).Günay KA; Ceccato TL; Silver JS; Bannister KL; Bednarski OJ; Leinwand LA; Anseth KS PEG–Anthracene Hydrogels as an On-Demand Stiffening Matrix To Study Mechanobiology. Angew. Chemie 2019, 131 (29), 10017–10021. 10.1002/ange.201901989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Truong VX; Li F; Ercole F; Forsythe JS Wavelength-Selective Coupling and Decoupling of Polymer Chains via Reversible [2 + 2] Photocycloaddition of Styrylpyrene for Construction of Cytocompatible Photodynamic Hydrogels. ACS Macro Lett. 2018, 7 (4), 464–469. 10.1021/acsmacrolett.8b00099. [DOI] [PubMed] [Google Scholar]
- (35).Rosales AM; Rodell CB; Chen MH; Morrow MG; Anseth KS; Burdick JA Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjug. Chem 2018, 29 (4), 905–913. 10.1021/acs.bioconjchem.7b00802. [DOI] [PubMed] [Google Scholar]
- (36).Ruskowitz ER; Deforest CA Proteome-Wide Analysis of Cellular Response to Ultraviolet Light for Biomaterial Synthesis and Modification. ACS Biomater. Sci. Eng 2019, 5 (5), 2111–2116. 10.1021/acsbiomaterials.9b00177. [DOI] [PubMed] [Google Scholar]
- (37).Pelloth JL; Tran PA; Walther A; Goldmann AS; Frisch H; Truong VX; Barner-Kowollik C Wavelength-Selective Softening of Hydrogel Networks. Adv. Mater 2021, 2102184, 2102184. 10.1002/adma.202102184. [DOI] [PubMed] [Google Scholar]
- (38).Truong VX; Bachmann J; Unterreiner A-N; Blinco JP; Barner-Kowollik C Wavelength-Orthogonal Stiffening of Hydrogel Networks with Visible Light. Angew. Chemie Int. Ed 2022. 10.1002/anie.202113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Loterie D; Delrot P; Moser C High-Resolution Tomographic Volumetric Additive Manufacturing. Nat. Commun 2020, 11 (1), 1–6. 10.1038/s41467-020-14630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bernal PN; Delrot P; Loterie D; Li Y; Malda J; Moser C; Levato R Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater 2019, 31 (42). 10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]