Abstract
Chimeric antigen receptor (CAR) T cell therapy has created a paradigm shift in the treatment of hematologic malignancies but has not been as effective toward solid tumors. For such tumors, the primary obstacles facing CAR T cells are scarcity of tumor-specific antigens and the hostile and complex tumor microenvironment. Glycosylation, the process by which sugars are post-translationally added to proteins or lipids, is profoundly dysregulated in cancer. Abnormally glycosylated glycoproteins expressed on cancer cells offer unique targets for CAR T therapy as they are specific to tumor cells. Tumor stromal cells also express abnormal glycoproteins and thus also have the potential to be targeted by glycan-binding CAR T cells. This review will discuss the state of CAR T cells in the therapy of solid tumors, the cancer glycoproteome and its potential for use as a therapeutic target, and the landscape and future of glycan-binding CAR T cell therapy.
Keywords: CAR T cell therapy, glycoprotein, glycan, cancer glycobiology, tumor microenvironment, solid tumor
Graphical abstract
The use of CAR T cell therapy in solid tumors is limited by a lack of tumor-specific antigens. Abnormal glycosylation patterns on solid tumor cells and tumor stroma provide new targets for CAR T cell therapy. Novel glycan-binding components can be used to drive these CAR T cells.
Introduction
The advent of immunotherapy has revolutionized cancer treatment. Of the many immunotherapeutic approaches, immune checkpoint inhibitors and chimeric antigen receptor T cell (CAR T) therapy in particular have shown tremendous efficacy, but the latter has thus far failed in the setting of solid tumors. Immune checkpoint inhibitors (ICIs) circumvent tumor immune evasion by blocking inhibitory signaling pathways, which allows for the activation of anti-tumor T cells.1,2 Despite the success of ICIs, their use in many malignancies with high mortality rates, such as lung cancer, is limited to a select subgroup of patients and has been plagued by significant adverse effects; thus, there is an urgent need for new immunotherapeutic approaches to most solid tumors.3,4
CAR T cell therapy involves engineering a patient’s T cells to express receptors that specifically target tumor antigens and lead to tumor cell destruction. CAR T cells have created a paradigm shift in the treatment of hematologic malignancies, resulting in unprecedented outcomes in the treatment of relapsed and refractory B cell cancers.5 The lack of efficacy of CAR T cell therapies toward solid tumors is multifactorial, but a major cause has been the lack and heterogeneity of tumor-specific antigens and the immunosuppressive tumor microenvironment (TME).6
Glycosylation, the process by which sugars are added to proteins, lipids, and RNA, is substantially dysregulated in cancer. Aberrant glycoproteins on the surface of cancer cells present attractive therapeutic targets, as their expression is often limited almost exclusively to tumor cells.7,8 The field of cancer glycomics and glycoproteomics is evolving rapidly, and recent advances have the potential to greatly expand our understanding of the abnormal glycostructures on the proteins of cancer cells and their subsequent use as therapeutic targets. CAR T cells targeting tumor-specific glycosylation patterns have the potential to overcome significant obstacles in the use of CAR therapies for solid tumors. In this review, we will discuss the novel approach of glycan-directed CAR T cell therapy, including a brief summary of the challenges facing CAR T cells in solid tumors, an overview of the cancer glycoproteome and its use as a therapeutic target, and the history and future of glycan-directed CAR T cells, including novel antigen-binding moieties.
CAR T cells and solid tumors
Early clinical trials highlighted the challenge and the importance of identifying target antigens specific to solid tumor cells.9,10 However, as most solid tumors originate from normal epithelial tissue, the identification of tumor-specific antigens not expressed on healthy tissues has proven difficult, and even antigens thought to be specific to tumor cells are often expressed at low levels on non-malignant cells.11 In contrast to liquid malignancies where tumor cells circulate in blood and lymphoid tissue, allowing ample opportunity for interaction with CAR T cells, in solid tumors, CAR T cells must home to, infiltrate, and expand in discrete solid tumor sites. These essential steps are severely restricted by the hostile TME, which has many complex mechanisms of inhibiting both physiologic T cell and CAR T cell infiltration of solid tumors. Barriers to T cell trafficking and infiltration include both lack of secretion of T cell chemokines and active secretion of inhibitory chemokines and cytokines by the TME, decreased expression of adhesion molecules necessary for T cell extravasation, and extracellular matrix proteins that physically restrict T cell entry.12, 13, 14 For example, the inhibitory cytokine CXCL12, which is highly expressed by many types of tumor stroma, actively inhibits T cell migration.15 Transforming growth factor β (TGF-β), a cytokine with multifactorial effects in tumorigenesis, is present at high concentrations in the TME and promotes immune tolerance partly through T cell suppression; other inhibitory cytokines including interleukin-4 (IL-4) and IL-10 are also prevalent in the TME.16,17 Additionally, denser areas of extracellular matrix are associated with low T cell migration and proliferation, as well as increased immunomodulatory effects (compared with less dense areas).18,19 If the T cell is successful in homing to and infiltrating into the tumor, its ability to survive, engage antigen, and expand is limited by the harsh conditions of the TME, including hypoxia, oxidative stress, and production of toxic metabolites and soluble factors, all of which restrict endogenous T cell and CAR T cell survival and function.14,20 For example, hypoxic conditions in the TME have been shown to impair CAR T expansion, differentiation, and anti-tumor cytotoxicity.21,22 Lactate, a metabolic by-product of tumor cells, suppresses T cell function; the TME also contains enzymes that degrade amino acids essential for T cell function.12,23 Prostaglandin E2, an inflammatory mediator that is known to inhibit T cell proliferation and suppress CD4 cells, is produced by tumor cells and macrophages in the TME.24 The TME also contains abundant immunomodulatory cells, including T regulatory cells, tumor-associated macrophages, and myeloid-derived suppressor cells, all of which inhibit the anti-tumor effect of CAR T.14 Though many modifications to CAR T cells have been tried in an attempt to overcome these challenges, and with some benefit, none have been entirely successful in overcoming the numerous obstacles leading to successful use of CAR T cell therapy in solid tumors to produce a clinically effective and safe treatment.25, 26, 27, 28, 29
Glycosylation and the cancer glycoproteome
Glycosylation, the process by which saccharides are enzymatically linked to other saccharides, proteins, lipids, and RNA, is a key mechanism involved in regulating many complex biological processes. Aberrant glycosylation, specifically protein glycosylation, is known to be a hallmark of malignancy (Figure 1) and plays important roles in regulating crucial oncogenic processes including malignant transformation, invasion, metastasis, angiogenesis, and immune evasion.7,30,31 Protein glycosylation is the most common post-translational protein modification, with over 50% of all human proteins estimated to be glycosylated.32 This process occurs in the Golgi apparatus and endoplasmic reticulum and is mediated by a complex array of glycosyltransferases. In the cytoplasm and nucleus, intracellular glycosylation of proteins occurs by the addition of O-linked GlcNAc to serine (Ser)/threonine (Thr) residues, akin to protein phosphorylation. This is a dynamic and reversible process catalyzed by two enzymes: O-GlcNAc transferase, which adds GlcNAc, and O-GlcNAcase, which removes GlcNAc. For secreted and membrane glycoproteins, the two most common types of protein glycosylation are N- and O-linked glycosylation. N-linked glycosylation occurs via nitrogen linkages at asparagine (Asn) residues to generate a glycosylamide in the consensus sequence Asn-X-Ser/Thr (where X is any amino acid except proline) and is initiated by the oligosaccharyltransferase complex. O-linked glycosylation, which occurs via oxygen linkages at hydroxyl groups of Ser, Thr, and, less commonly, tyrosine (Tyr) residues, is initiated by a variety of GalNAc transferase isoenzymes that generate O-glycosidic bonds of the sugar GalNAc to amino acids.33 Both processes can undergo numerous further modifications, creating an incredibly complex, fluid glycoproteome.7,8
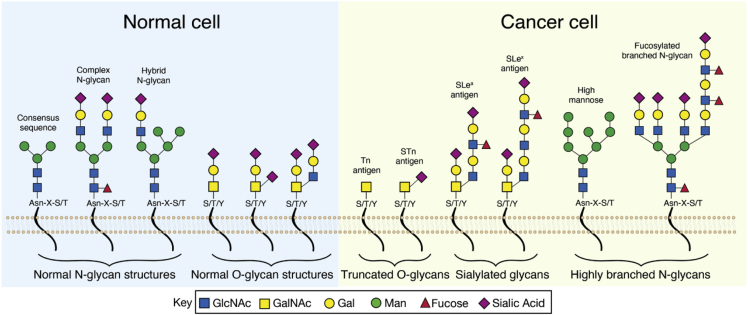
Figure 1.
Glycosylation patterns in normal and malignant cells
Left panel depicts glycosylation patterns found on normal cell-surface glycoproteins, including N-linked glycosylation, which occurs via nitrogen linkages at asparagine (Asn) residues in the consensus sequence Asn-X-serine (S)/threonine (T) (where X is any amino acid except proline), and O-linked glycosylation, which occurs via oxygen linkages at hydroxyl groups of S, T or, much less commonly, tyrosine (Y) residues. Right panel depicts abnormal glycosylation patterns found on cancer cells. These include the truncated O-glycans Tn and STn, sialylated glycans including sialyl Lewis A (sLeA) and sialyl Lewis x (sLeX), and highly branched N-glycans including high-mannose and fucosylated branched N-glycans.
Tumor cells display profound differences in glycosylation patterns compared with normal cells, and these differences are known to regulate numerous oncogenic processes. There are two primary categories of aberrant glycosylation in malignant cells: incomplete synthesis, which tends to occur early in cancer and which involves truncation of normal glycosylation structures, and neo-synthesis, which often occurs in later stages of cancer and involves the expression of de novo glycosylation patterns.31 These processes are mediated by glycosyltransferases, whose expression is known to be altered in the TME.34 The most common abnormal glycosylation patterns in cancer are fucosylation, sialylation, O-glycan truncation, and increased N-glycan branching. Highly fucosylated glycans including the fucose-containing Lewis blood group antigens and their sialylated counterparts are often overexpressed on cancer cell surfaces and have been associated with poor prognosis in multiple cancer types. For example, the antigen sialyl Lewis A (sLeA), which is detected by the serological assay CA19-9, functions as both a diagnostic and prognostic biomarker in a wide range of malignancies, including gastric, pancreatic, and ovarian cancers, but is useful only in those patients (∼90%) that are Lewis positive.35, 36, 37
Both increased complex branching of N-glycans and abnormal truncation of O-glycans have been identified in many types of cancers and contribute to tumorigenesis and metastasis. High-mannose-type N-glycans, which have 5–9 mannose molecules, are present physiologically early in the glycan biosynthetic pathway, but for most glycoproteins, the mannose residues are normally clipped off in the Golgi apparatus before the mature glycoprotein is secreted, leaving only 3 mannose residues in the complex N-glycans. This often does not occur in cancer cells, and the presence of high mannose promotes metastasis in several cancer types, including breast cancer, colorectal cancer, and cholangiocarcinoma.38, 39, 40 High mannose is also abundantly present on tumor stromal cells but not on normal stromal cells.40 Abnormally truncated O-glycans are also commonly found on cells of solid tumors and protect cancer cells from apoptosis, as well as promoting invasion and metastasis. Examples include two of the most common cancer-associated glycans: α-linked GalNAc to Ser/Thr residues (Tn or CD175) and its sialylated form, Sialyl Tn (STn or CD175s), which have been associated with malignant transformation in pancreatic cancer and poor prognosis in patients with gastric cancer.41, 42, 43, 44 The Notch signaling pathway, which has multifactorial roles in oncogenesis and involves O-linked fucose residues in a different type of O-glycosylation, demonstrates several different types of abnormal glycosylation patterns in cancer.45
Glycosylation is also known to play significant roles in mediating tumor immune evasion. Glycosylation of programmed death ligand-1 (PD-L1), which is an important mediator of cancer cell immune escape and the target of many ICIs, plays key roles in its activation and degradation.46 PD-L1 is a heavily glycosylated protein, and inhibition of PD-L1 glycosylation promoted PD-L1 degradation, which enhanced efficacy in mouse models of breast cancer.47 Removal of glycan moieties from PD-L1 in tumor tissue samples can improve anti-PD-L1 antibody binding and has been proposed as a better way to identify patients who may benefit from PD-L1-targeted therapies.48 The clinical importance of abnormal glycoproteins is highlighted by the increasing number of glycoproteins currently in use as cancer biomarkers, including AFP, beta-hCG, CA19-9, CEA, and PSA.34 Many of these abnormal glycoproteins are present only on malignant cells, making them promising potential therapeutic targets.
As discussed above, one of the key contributing factors that inhibits many immunotherapies for solid tumors is the complex TME, and this theme also plays out in the realm of glycosylation. Various cues from TME stromal components drive the crosstalk between cancer-associated glycan biosynthesis and aggressive disease progression.30 Soluble factors such as TGF-β and IL-10 secreted from stromal cells have been shown to influence glycosylation patterns on endothelial cells and tumor cells in the TME.49 Furthermore, stromal cells, including fibroblasts, contribute to a hypoxic environment that promotes glycan remodeling.21,50 Recent studies characterizing stromal fibroblasts within the tumor compared with fibroblasts surrounding the tumor now indicate distinct phenotypes and glycosylation patterns. Glycoproteomic analysis revealed unique O-glycoslylation patterns on tumor-associated fibroblasts (TAFs; located on the leading edge of tumors, or “peri-tumor fibroblasts”) compared with cancer-associated fibroblasts (CAFs; found within tumors), which induced epithelial-mesenchymal transition in cancer cells and promoted a migratory role of stroma cells in lung adenocarcinoma.51 In colorectal carcinoma, mass spectrometry imaging highlighted distinct N-glycosylation signatures on stroma at the interface of tumor versus distant stroma. Results showed that stroma at the interface exhibited similar glycosylation to the cancer itself, characterized by an abundance of high-mannose N-glycans, whereas distant stroma expressed a very low abundance of high-mannose N-glycans.40 Similar results were also found in breast cancer where high-mannose and fucosylated N-glycans were prominent on cancer and cancer-associated stroma but not on normal stroma and epithelium.52 These studies indicate that the TME influences the glycan signatures on not only tumors but also the supporting adjacent stroma, expanding the potential targets to disrupt the solid TME using glycan-targeted therapies.
Though abnormalities in cancer cell glycosylation were first identified over 50 years ago, and important work in characterizing the cancer glycome and glycoproteome has been done since that time, the approaches used in this work have significant limitations and have largely focused on the identification of individual proteins and glycosylation sites.32,53, 54, 55 Because the glycoproteome results from a large host of post-translational modifications due to a wide range of enzymes, substrates, and residues, specific glycoproteins are often present at only low levels, and there is constant flux in response to changes in the intracellular and extracellular environments. Thus, both sophisticated enrichment strategies and high-throughput analysis are required for accurate characterization. Recently, a new quantitative approach was developed utilizing hydrophilic interaction chromatography for enrichment, followed by nano-liquid chromatography electrospray ionization tandem mass spectrometry for peptide fragmentation and novel algorithms for glycan and glycoprotein identification.56 This strategy enables the identification of intact glycopeptides on a proteomic scale, allowing the characterization of complex N- or O-glycosites on an incredibly large number of glycoproteins found in and on each individual cell. This approach opens the door to a previously inaccessible, comprehensive understanding of the cancer glycoproteome, including identification of specific glycostructures that distinguish cancer cells from normal cells and can therefore serve as targets for CAR T cells and other therapies.
“Sweet CARs”
Most cell-surface antigens undergo post-translational modification by covalently linked glycans that can greatly influence recognition by immune cells.57 Furthermore, glycosylation can affect processing of proteins to be presented on major histocompatibility complex (MHC) molecules which are recognized by T cell receptors (TCRs). The use of glycoproteins as antigenic targets for CAR T cell therapy (so-called “sweet CARs”) holds enormous promise, as CARs recognize antigens independent of MHC. To bring this therapy forward, however, the inherent limitations of generating antibody-derived, anti-glycan single-chain variable fragments (scFvs) will need to be addressed. The cancer glycoproteome presents a host of antigens unique to tumor cells, the lack of which has thus far been an Achilles’ heel of CAR T cell therapy for solid tumors. As described above, though our understanding of these tumor-specific glycoproteins remains somewhat primitive, new techniques have recently been developed that will allow for a more complete understanding. Recently, a CAR T cell targeting an abnormally truncated O-glycan that is not expressed in healthy tissue, the Tn antigen, and which is present on a cancer-associated, overexpressed cell-membrane protein, MUC1, was shown to be effective in mouse models of leukemia and pancreatic cancer.58 The Tn-MUC1 epitope was not detected on healthy cells, and no on-target/off-tumor effects were observed, reinforcing the idea that these aberrant cancer glycans can serve as targets highly specific to cancer cells. Notably, the CAR T cell in this study was effective against multiple types of cancer, including leukemia, pancreatic, and breast cancer cell lines, all expressing the abnormal Tn-MUC1 epitope, indicating that glycan-targeting CAR T cells could be used across cancer types and act as a “universal CAR”—an approach that, though much sought-after, has thus far proved elusive and would be a highly significant advance in the field of CAR T cell therapy.59 Indeed, the Tn-MUC1 targeting CAR T cell is currently being studied in a phase I clinical trial for several different types of solid tumors.60
GD2, a disialoganglioside, has also been a target for many immunotherapies. This molecule is a carbohydrate-containing sphingolipid (glycosphingolipid) with two sialic acid residues attached to three other monosaccharides that facilitate cell-to-cell adhesion. Expression of GD2 is minimal in normal tissue, and restricted to peripheral nerves, brain, and melanocytes, but is upregulated on almost all neuroblastomas and melanomas, as well as on sarcomas and glioblastomas.61 Because of this discriminatory expression pattern, GD2 was recognized as one of the top cancer antigen targets by the NCI for therapeutic vaccine development.62 Anti-GD2 monoclonal antibodies have been used to induce antibody-dependent cell cytotoxicity (ADCC) and complement-mediated cytotoxicity by recognizing the extracellular sugar residues on the surface of neuroblastoma cells.63 CAR T cells targeting GD2 are currently being developed to treat patients with high-grade glioma,64 osteosarcoma, and neuroblastoma,65,66 as well as many sarcomas and other solid tumors.67,68
Pancreatic cancer cells have substantially altered glycosylation patterns on their surface proteins that are potential targets for immunotherapy.69 Interestingly, N-glycans expressed on glycoproteins of pancreatic cancer cells were recently reported to have inhibitory effects on CAR T cells targeting a CD44 variant that is expressed in multiple cancer types via interference with immune synapse formation and reduction in cytotoxicity. Inhibition of N-glycan synthesis enhanced anti-tumor effects of CAR T in mouse models of pancreatic cancer.70 This approach emphasizes the complexity of the cancer glycoproteome and the diversity of glycans as therapeutic targets, as will be discussed further below.
In engineering glycan-binding CAR T, one important consideration is the genesis of the glycan-targeting antigen-recognition element (the binder), which has traditionally been the scFv of a monoclonal antibody.5 The generation of glycan-targeting monoclonal antibodies has largely been done in mouse models. This approach has a number of limitations, the most significant of which is that the human and murine glycomes share many common glycans, thus human glycans may not be appropriately antigenic in murine models due to self-tolerance.71,72 Another limitation is the difficulty in developing antibodies with sufficient specificity to the target glycan. Intriguing new strategies have emerged to overcome these problems. Lampreys, ancient members of the agnathan (jawless vertebrate) group, have a glycome distinct from that of humans and mice and are capable of producing highly specific anti-mammalian glycan antibodies called variable lymphocyte receptors (VLRs). These VLRs have a unique structure that presents some advantages over monoclonal antibodies, including structural differences that allow for more specific binding.73 This knowledge led to the creation of VLR-immunoglobulin (Ig) chimeras, called smart anti-glycan reagents (SAGRs), which can be generated on a large scale by cloning lamprey lymphocyte cDNA into yeast surface display libraries and which have demonstrated the ability to bind unique glycan epitopes.74 This approach has been used to generate high-affinity anti-carbohydrate antibodies able to distinguish between exquisitely subtle differences in glycan structures and holds promise for generating anti-tumor glycan antibodies that could be used as the antigen-recognition component of sweet CARs.
Lectins represent another potential class of glycan-binding elements. Lectins are carbohydrate-specific proteins that have evolved over millions of years to bind carbohydrate moieties with high specificity, which makes them uniquely poised for precision targeting of glycans. Indeed, glycan-lectin interactions mediate various essential cellular processes in normal cells and are involved in many of the oncogenic activities of the cancer-associated glycans discussed above.75 Lectins have been used as tools in characterizing the glycome, including in lectin-based microarrays, and are also used to detect abnormal glycostructures in cancer diagnostics and prognostics.76, 77, 78 Due to their higher degree of specificity, most lectins that can potentially target tumor-associated glycans are of plant origin, and thus the immunogenicity of these lectins is a concern.31,79 Recently, a recombinant human-derived lectin was developed with the ability to specifically bind to the Tn antigen. This lectin, macrophage galactose lectin (MGL), which is normally expressed on the surface of dendritic cells and macrophages, has known specificity for the Tn antigen, a glycan abundantly expressed across cancer types.80,81 Recombinant MGL fragments were able to bind with high specificity to colon and lung cancer cell lines and with no observed toxicity in a mouse model of colon cancer.82 It is unclear whether this lectin can be used to drive a CAR T cell, as it has some inherent immunosuppressive properties.82
A recently developed “lectibody” containing Avaren, a high-mannose-binding actinomycete-derived lectin, fused to a human IgG1 Fc (AvFc), demonstrated selective binding to breast, colon, and lung cancer cell lines.83 AvFc treatment slowed tumor growth in a subcutaneous model of A549 lung cancer and induced cancer cell cytotoxicity at nanomolar concentrations. Studies suggested that AvFc recognizes high-mannose expression on N-glycans of the epidermal growth factor receptor (EGFR) and the insulin-like growth factor 1 receptor (IGF1R) glycoproteins and results in reduced downstream signaling. This study highlights the potential use of lectins derived from plants in hybrid drugs. A recombinant protein derived from malaria, rVAR2, is also being developed for cancer binding, as it recognizes a specific form of the glycosaminoglycan chondroitin sulfate that is recognized by malarial parasites and used to enter placental cells but is also found on diverse types of cancer cells.84
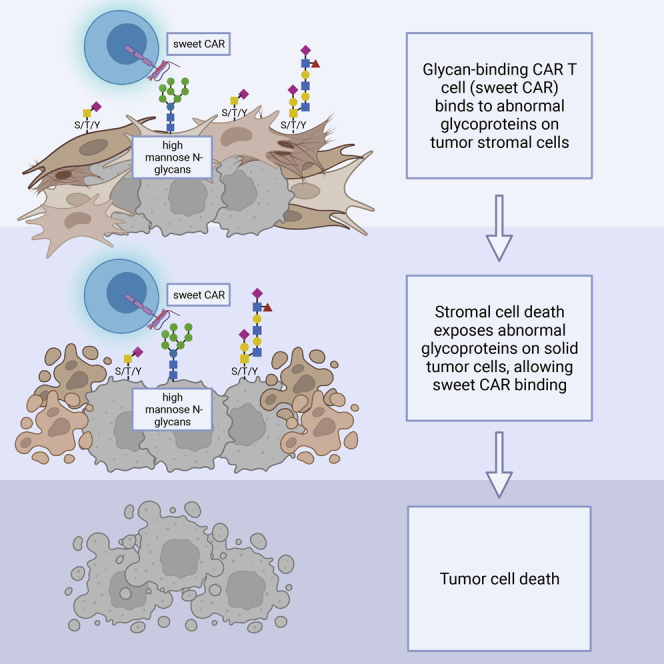
If CAR constructs can be made with adequate surface expression and proper folding, we propose that lectins can be used to guide CAR T cells to the abnormal glycosylation found on the surface proteins of solid tumors. Recently, a CAR T utilizing a lectin antigen-binding moiety targeting globotriaosylceramide (Gb3), a glycosphingolipid highly expressed across several cancer types, demonstrated cytotoxicity against both solid tumor and lymphoma cells.85 Along these lines, several years ago we demonstrated that two functions of a lectin, in this case mitogenicity and antiviral activity, can be separated through targeted molecular engineering of a lectin and can be understood at the atomic level.86 These studies resulted in a non-mitogenic, well-tolerated broad-spectrum antiviral agent based on the banana lectin BanLec. The molecularly modified BanLec, termed H84T BanLec, attacks the crucial surface proteins covered with high mannose that are found on many pathogenic viruses, thus blocking viral entry into human host cells. H84T Banlec is a highly effective antiviral agent and is well tolerated when given systemically to animals, and antibodies to the lectin do not appear to interfere with safety or efficacy.87,88 As noted above, high-mannose N-glycans are also abundantly present on the surface of many cancer cells, as well as on tumor stromal cells, but not on normal cells, making proteins containing high mannose intriguing therapeutic CAR targets.40,86,89 In support of the possibility of treating cancer with a lectin-driven CAR construct, we recently generated a CAR-natural killer (NK) utilizing H84T BanLec as the antigen-binding component and showed that it reduced infection of cells by a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus expressing high mannose on its viral envelope.90
In view of the above, our group has generated the first CAR T that is guided by a lectin to a tumor in vivo; H84T BanLec serves as the antigen-binding moiety (H84T-CAR T). Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease characterized by aberrant N-glycan expression, including the presence of high mannose on tumor cell surfaces.69,91,92 One of the primary barriers to effective therapy for pancreatic cancer is its dense desmoplastic stroma.93 Uniquely, H84T-CAR T disrupts the supportive stroma in PDAC as well, and this CAR T construct has demonstrated potent anti-tumor activity against PDAC in pre-clinical models both in vitro and in vivo.94 Though off-target effects are always a concern in any therapeutic modality, pre-clinical data also support the safety of this approach. No on-target/off-tumor or off-target toxicities have been observed with the use of H84T BanLec in models of viral infection in vitro or in extensive in vivo studies.86, 87, 88,95 We have observed a similar lack of these toxicities in preliminary studies using H84T-CAR T in PDAC tumor in vivo models. Similarly, we found no evidence for cytokine release syndrome or other cytokine-mediated toxicities.94 This encouraging lack of preclinical toxicity will nonetheless have to be replicated in dose-escalation studies of H84T-CAR T cells in the clinic. If safety and efficacy are indeed confirmed in clinical studies, this novel therapeutic approach could overcome two of the primary obstacles to CAR T therapy for solid tumors in general: lack of tumor-specific antigens and inability of CAR T to penetrate the TME. As high-mannose N-glycans are found on the surface of multiple other solid tumors and the associated stroma, H84T-CAR T therapy holds the potential to be useful because of its dual targeting and allows for broad applicability, as most solid tumors include stroma with a more stereotyped and sustained array of antigens.
Conclusions and future directions
While CAR T therapy has transformed the treatment of certain hematologic malignancies, its use in solid tumors has thus far had limited success, and significant barriers remain to the creation of CAR T cell therapy that is both efficacious and safe for these malignancies. The cancer glycoproteome presents a novel opportunity to develop therapies to treat solid cancers. Though our understanding of the cancer glycoproteome is incomplete, a significant number of glycoproteins unique to solid tumors have already been identified. These abnormal glycoproteins, which are largely only expressed on malignant cells and not on healthy tissues, are excellent antigenic targets for CAR T cells. With the recent advent of innovative new technologies in glycoproteomics, a more comprehensive understanding of aberrant glycosylation patterns in cancer, including new therapeutic targets, is on the horizon and can guide this approach (Figure 2).
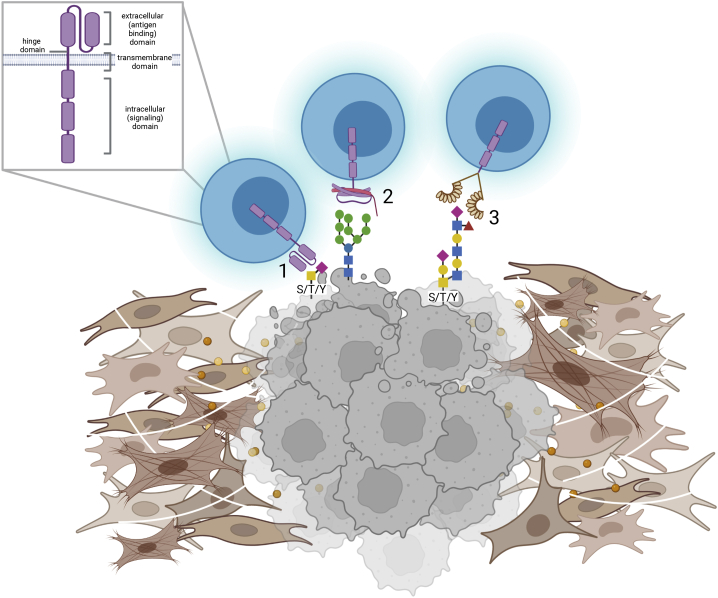
Figure 2.
Sweet CAR design
Depicted are CAR T cells with a traditional scFv extracellular domain (1), lectin extracellular domain (2), or smart anti-glycan reagent (SAGR; generated from lamprey antibodies) extracellular domain (3), binding to representative abnormal glycoproteins on the surface of cancer cells (in gray), surrounded by the tumor microenvironment (in brown). Inset shows CAR domains.
The generation of glycan-binding antibodies has also been hampered by significant obstacles. New approaches to generating glycan-specific antibodies, including utilizing the lamprey model, and the use of non-antibody glycan binders, such as lectins, present exciting new approaches in this field. Glycan-binding CAR T cells have shown preliminary successes in targeting and treating solid malignancies without significant adverse effects. These unique CAR T cells may be able to mitigate some of the most significant barriers to the use of CAR T cells in solid malignancies, including the identification of tumor-specific antigens, limiting on-target/off-tumor effects, and the successful navigation of the hostile TME. Individual glycan-targeting CAR constructs might also prove effective against multiple cancers that share the same abnormal glycosylation patterns on their surface proteins. Therefore, sweet CARs present a compelling new approach for the treatment of solid tumors.
Acknowledgments
M.K.M. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number 5T32HL092332-17 supervised by Dr. Helen Heslop and the National Cancer Institute under the award 5PO1CA094237-15. C.L.B. is a St. Baldrick’s Foundation Scholar, has pending patent applications describing the use of engineered T and NK cells to enhance tumor targeting, including the use of H84T-BanLec effector cell targeting of SARS-CoV-2, and has received research funding from Merck, Sharp, and Dohme, Inc., Kiadis Pharma, and Bristol Myers Squibb. W.W. is supported by the University of Michigan. M.P.d.M. is supported by National Cancer Institute grant U01-CA224145 and NIH grants R01-CA271510 and R01-CA224145. J.M.P. is supported by a Canada 150 Chair and the T. von Zastrow Foundation. R.D.C. is supported by NIH grant R01GM140201. M.K.B. is supported by National Cancer Institute grant nos. P50CA126752 and P01CA094237, by Stand Up To Cancer (SU2C)/American Association for Cancer Research (AACR) 604817 Meg Vosburg T-Cell Lymphoma Dream Team, and the Leukemia and Lymphoma Society. SU2C is a program of the Entertainment Industry Foundation administered by the AACR. M.K.B. is a co-founder with equity at Tessa Therapeutics and Marker Therapeutics and is on the scientific advisory boards for Bluebird Bio, Turnstone, Tessa Therapeutics, Marker Therapeutics, Allogene, Walking Fish, Memgen, KUUR, Bellicum Pharmaceuticals, Tscan, Poseida, and Abintus. D.M.M. was supported by a grant from the Forbes Institute of the Rogel Cancer Center at the University of Michigan and is an inventor on University of Michigan patents concerning H84T BanLec. Figures 1 and 2 and the graphical abstract were created with BioRender.com.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Malcolm K. Brenner, Email: mbrenner@bcm.edu.
David M. Markovitz, Email: dmarkov@med.umich.edu.
References
- 1.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;11:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Onoi K., Chihara Y., Uchino J., Shimamoto T., Morimoto Y., Iwasaku M., Kaneko Y., Yamada T., Takayama K. Immune checkpoint inhibitors for lung cancer treatment: a review. J. Clin. Med. 2020;9:E1362. doi: 10.3390/jcm9051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 6.Ma S., Li X., Wang X., Cheng L., Li Z., Zhang C., Ye Z., Qian Q. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019;15:2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 8.Stowell S.R., Ju T., Cummings R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamers C.H.J., Sleijfer S., Vulto A.G., Kruit W.H.J., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 10.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson L.A., June C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27:38–58. doi: 10.1038/cr.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosti P., Maher J., Arnold J.N. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front. Immunol. 2018;9:1104. doi: 10.3389/fimmu.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu T.R., Fonseca N.A., Gonçalves N., Moreira J.N. Current challenges and emerging opportunities of CAR-T cell therapies. J. Control. Release. 2020;319:246–261. doi: 10.1016/j.jconrel.2019.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Garcia A., Palazon A., Noguera-Ortega E., Powell D.J., Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front. Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feig C., Jones J.O., Kraman M., Wells R.J.B., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L., et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo N., Hayakawa S., Takigawa M., Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon H., Franciszkiewicz K., Damotte D., Dieu-Nosjean M.C., Validire P., Trautmann A., Mami-Chouaib F., Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczek D.E., Larsen A.M.H., Thorseth M.L., Carretta M., Kalvisa A., Siersbæk M.S., Simões A.M.C., Roslind A., Engelholm L.H., Noessner E., et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer. 2019;7:68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38:473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Petrova V., Annicchiarico-Petruzzelli M., Melino G., Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berahovich R., Liu X., Zhou H., Tsadik E., Xu S., Golubovskaya V., Wu L. Hypoxia selectively impairs CAR-T cells in vitro. Cancers (Basel) 2019;11:E602. doi: 10.3390/cancers11050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno R., Kawada K., Sakai Y. Prostaglandin E2/EP signaling in the tumor microenvironment of colorectal cancer. Int. J. Mol. Sci. 2019:E6254. doi: 10.3390/ijms20246254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 26.Craddock J.A., Lu A., Bear A., Pule M., Brenner M.K., Rooney C.M., Foster A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruana I., Savoldo B., Hoyos V., Weber G., Liu H., Kim E.S., Ittmann M.M., Marchetti D., Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juillerat A., Marechal A., Filhol J.M., Valogne Y., Valton J., Duclert A., Duchateau P., Poirot L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 2017;7:39833. doi: 10.1038/srep39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz S.C., Point G.R., Cunetta M., Thorn M., Guha P., Espat N.J., Boutros C., Hanna N., Junghans R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016;23:142–148. doi: 10.1038/cgt.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peixoto A., Relvas-Santos M., Azevedo R., Santos L.L., Ferreira J.A. Protein glycosylation and tumor microenvironment alterations driving cancer hallmarks. Front. Oncol. 2019;9:380. doi: 10.3389/fonc.2019.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silsirivanit A. Glycosylation markers in cancer. Adv. Clin. Chem. 2019;89:189–213. doi: 10.1016/bs.acc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Franc V., Heck A.J.R. Glycoproteomics: a balance between high-throughput and in-depth analysis. Trends Biotechnol. 2017;35:598–609. doi: 10.1016/j.tibtech.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Schjoldager K.T., Narimatsu Y., Joshi H.J., Clausen H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas D., Rathinavel A.K., Radhakrishnan P. Altered glycosylation in cancer: a promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer. 2021;1875:188464. doi: 10.1016/j.bbcan.2020.188464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marrelli D., Pinto E., De Stefano A., de Manzoni G., Farnetani M., Garosi L., Roviello F. Preoperative positivity of serum tumor markers is a strong predictor of hematogenous recurrence of gastric cancer. J. Surg. Oncol. 2001;78:253–258. doi: 10.1002/jso.1163. [DOI] [PubMed] [Google Scholar]
- 36.Parikh D.A., Durbin-Johnson B., Urayama S. Utility of serum CA19-9 levels in the diagnosis of pancreatic ductal adenocarcinoma in an endoscopic ultrasound referral population. J. Gastrointest. Cancer. 2014;45:74–79. doi: 10.1007/s12029-013-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J., Yu J., Song X., Mi H. Serum CA125, CA199 and CEA combined detection for epithelial ovarian cancer diagnosis: a meta-analysis. Open Med. 2017;12:131–137. doi: 10.1515/med-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Leoz M.L.A., Young L.J.T., An H.J., Kronewitter S.R., Kim J., Miyamoto S., Borowsky A.D., Chew H.K., Lebrilla C.B. High-mannose glycans are elevated during breast cancer progression. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.002717. M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park D.D., Phoomak C., Xu G., Olney L.P., Tran K.A., Park S.S., Haigh N.E., Luxardi G., Lert-Itthiporn W., Shimoda M., et al. Metastasis of cholangiocarcinoma is promoted by extended high-mannose glycans. Proc. Natl. Acad. Sci. USA. 2020;117:7633–7644. doi: 10.1073/pnas.1916498117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyaval F., van Zeijl R., Dalebout H., Holst S., van Pelt G., Fariña-Sarasqueta A., Mesker W., Tollenaar R., Morreau H., Wuhrer M., Heijs B. N-glycomic signature of stage II colorectal cancer and its association with the tumor microenvironment. Mol. Cell. Proteomics. 2021;20:100057. doi: 10.1074/mcp.RA120.002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sewell R., Bäckström M., Dalziel M., Gschmeissner S., Karlsson H., Noll T., Gätgens J., Clausen H., Hansson G.C., Burchell J., Taylor-Papadimitriou J. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 42.Radhakrishnan P., Dabelsteen S., Madsen F.B., Francavilla C., Kopp K.L., Steentoft C., Vakhrushev S.Y., Olsen J.V., Hansen L., Bennett E.P., et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA. 2014;111:E4066–E4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura F., Sato Y., Hirakawa M., Yoshida M., Ono M., Osuga T., Okagawa Y., Uemura N., Arihara Y., Murase K., et al. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric Cancer. 2016;19:85–97. doi: 10.1007/s10120-014-0454-z. [DOI] [PubMed] [Google Scholar]
- 44.Lisowska E. Tn antigens and their significance in oncology. Acta Biochim. Pol. 1995;42:11–17. [PubMed] [Google Scholar]
- 45.Pakkiriswami S., Couto A., Nagarajan U., Georgiou M. Glycosylated Notch and cancer. Front. Oncol. 2016;6:37. doi: 10.3389/fonc.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C.W., Lim S.O., Chung E.M., Kim Y.S., Park A.H., Yao J., Cha J.H., Xia W., Chan L.C., Kim T., et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201.e10. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H.H., Wang Y.N., Xia W., Chen C.H., Rau K.M., Ye L., Wei Y., Chou C.K., Wang S.C., Yan M., et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36:168–178.e4. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler K.B., Costello C.E., Rahimi N. Glycosylation in the tumor microenvironment: implications for tumor angiogenesis and metastasis. Cells. 2019;8:E544. doi: 10.3390/cells8060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croci D.O., Cerliani J.P., Dalotto-Moreno T., Méndez-Huergo S.P., Mascanfroni I.D., Dergan-Dylon S., Toscano M.A., Caramelo J.J., García-Vallejo J.J., Ouyang J., et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard G., Marques F.J.G., Karacosta L.G., Zhang W., Bermudez A., Riley N.M., Mehl L.C., Benson J.A., Shrager J.B., Bertozzi C.R., et al. Multi-omics analysis of fibroblasts from the invasive tumor edge reveals that tumor-stroma crosstalk induces O-glycosylation of the CDK4-pRB Axis. bioRxiv. 2021 doi: 10.1101/2021.05.28.446229. Preprint at. 446229. [DOI] [Google Scholar]
- 52.Ščupáková K., Adelaja O.T., Balluff B., Ayyappan V., Tressler C.M., Jenkinson N.M., Claes B.S., Bowman A.P., Cimino-Mathews A.M., White M.J., et al. Clinical importance of high-mannose, fucosylated, and complex N-glycans in breast cancer metastasis. JCI Insight. 2021;6:e146945. doi: 10.1172/jci.insight.146945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas D.R., Scott N.E. Glycoproteomics: growing up fast. Curr. Opin. Struct. Biol. 2021;68:18–25. doi: 10.1016/j.sbi.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Tian Y., Zhang H. Glycoproteomics and clinical applications. Proteomics Clin. Appl. 2010;4:124–132. doi: 10.1002/prca.200900161. [DOI] [PubMed] [Google Scholar]
- 55.Ladenson R.P., Schwartz S.O., Ivy A.C. Incidence of the blood groups and the secretor factor in patients with pernicious anemia and stomach carcinoma. Am. J. Med. Sci. 1949;217:194–197. doi: 10.1097/00000441-194902000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Stadlmann J., Taubenschmid J., Wenzel D., Gattinger A., Dürnberger G., Dusberger F., Elling U., Mach L., Mechtler K., Penninger J.M. Comparative glycoproteomics of stem cells identifies new players in ricin toxicity. Nature. 2017;28:538–542. doi: 10.1038/nature24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfert M.A., Boons G.J. Adaptive immune activation: glycosylation does matter. Nat. Chem. Biol. 2013;9:776–784. doi: 10.1038/nchembio.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Posey A.D., Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B., Stone J.D., Madsen T.D., Schreiber K., Haines K.M., et al. Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Y., Schreiber K., Wolf S.P., Wen F., Steentoft C., Zerweck J., Steiner M., Sharma P., Shepard H.M., Posey A., et al. Multiple cancer-specific antigens are targeted by a chimeric antigen receptor on a single cancer cell. JCI Insight. 2019;4:130416. doi: 10.1172/jci.insight.130416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.U.S. National Library of Medicine . 2019. A Study of CART-TnMUC1 in Patients with TnMUC1-Positive Advanced Cancers. [Google Scholar]
- 61.Nazha B., Inal C., Owonikoko T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M., Matrisian L.M. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sait S., Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev. Anticancer Ther. 2017;17:889–904. doi: 10.1080/14737140.2017.1364995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.U.S. National Library of Medicine C7R-GD2.CAR T Cells for Patients with GD2-Expressing Brain Tumors (GAIL-B) https://clinicaltrials.gov/ct2/show/NCT04099797?term=04099797&draw=2&rank=1 ClinicalTrials.gov identifier: NCT04099797.
- 65.U.S. National Library of Medicine Testing a New Immune Cell Therapy, GD2-Targeted Modified T-Cells (GD2CART) Children, Adolescents, and Young Adults with Relapsed/Refractory Osteosarcoma and Neuroblastoma, the GD2-CAR PERSIST Trial. https://clinicaltrials.gov/ct2/show/NCT04539366?term=04539366&draw=2&rank=1 ClinicalTrials.gov Identifier: NCT04539366.
- 66.U.S. National Library of Medicine Study of CAR T-Cells Targeting the GD2 with IL-15+iCaspase9 for Relapsed/Refractory Neuroblastoma or Relapsed/Refractory Osteosarcoma. https://clinicaltrials.gov/ct2/show/NCT03721068?term=03721068&draw=2&rank=1 ClinicalTrials.gov Identifier: NCT03721068.
- 67.U.S. National Library of Medicine GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children with Neuroblastoma (GINAKIT2) https://clinicaltrials.gov/ct2/show/NCT03294954?term=03294954&draw=2&rank=1 ClinicalTrials.gov Identifier: NCT03294954.
- 68.U.S. National Library of Medicine Anti-GD2 CAR T Cells in Pediatric Patients Affected by High Risk and/or Relapsed/Refractory Neuroblastoma or Other GD2-Positive Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT03373097?term=03373097&draw=2&rank=1 ClinicalTrials.gov Identifier: NCT03373097.
- 69.Lumibao J.C., Tremblay J.R., Hsu J., Engle D.D. Altered glycosylation in pancreatic cancer and beyond. J. Exp. Med. 2022:e20211505. doi: 10.1084/jem.20211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greco B., Malacarne V., De Girardi F., Scotti G.M., Manfredi F., Angelino E., Sirini C., Camisa B., Falcone L., Moresco M.A., et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci. Transl. Med. 2022;14:eabg3072. doi: 10.1126/scitranslmed.abg3072. [DOI] [PubMed] [Google Scholar]
- 71.Kawashima H. Generation of anti-sulfated glycan antibodies using sulfotransferase-deficient mice. Methods Mol. Biol. 2013;1022:51–60. doi: 10.1007/978-1-62703-465-4_5. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida Y., Furukawa J.I., Naito S., Higashino K., Numata Y., Shinohara Y. Quantitative analysis of total serum glycome in human and mouse. Proteomics. 2016;11:2747–2758. doi: 10.1002/pmic.201500550. [DOI] [PubMed] [Google Scholar]
- 73.McKitrick T.R., Eris D., Mondal N., Aryal R.P., McCurley N., Heimburg-Molinaro J., Cummings R.D. Antibodies from lampreys as smart anti-glycan reagents (SAGRs): perspectives on their specificity, structure, and glyco-genomics. Biochemistry. 2020;59:3111–3122. doi: 10.1021/acs.biochem.9b01015. [DOI] [PubMed] [Google Scholar]
- 74.McKitrick T.R., Goth C.K., Rosenberg C.S., Nakahara H., Heimburg-Molinaro J., McQuillan A.M., Falco R., Rivers N.J., Herrin B.R., Cooper M.D., Cummings R.D. Development of smart anti-glycan reagents using immunized lampreys. Commun. Biol. 2020;3:91. doi: 10.1038/s42003-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kremsreiter S.M., Kroell A.S.H., Weinberger K., Boehm H. Glycan-lectin interactions in cancer and viral infections and how to disrupt them. Int. J. Mol. Sci. 2021:10577. doi: 10.3390/ijms221910577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva M.L.S. Lectin biosensors in cancer glycan biomarker detection. Adv. Clin. Chem. 2019;93:1–61. doi: 10.1016/bs.acc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Lopes N., Correia V.G., Palma A.S., Brito C. Cracking the breast cancer glyco-code through glycan-lectin interactions: targeting immunosuppressive macrophages. Int. J. Mol. Sci. 2021;22:1972. doi: 10.3390/ijms22041972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry N.L., Hayes D.F. Cancer biomarkers. Mol. Oncol. 2012;6:140–146. doi: 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poiroux G., Barre A., van Damme E.J.M., Benoist H., Rougé P. Plant lectins targeting O-glycans at the cell surface as tools for cancer diagnosis, prognosis and therapy. Int. J. Mol. Sci. 2017;18:E1232. doi: 10.3390/ijms18061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heger L., Balk S., Lühr J.J., Heidkamp G.F., Lehmann C.H.K., Hatscher L., Purbojo A., Hartmann A., Garcia-Martin F., Nishimura S.I., et al. CLEC10A is a specific marker for human CD1c. Front. Immunol. 2018;9:744. doi: 10.3389/fimmu.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Vliet S.J., van Liempt E., Saeland E., Aarnoudse C.A., Appelmelk B., Irimura T., Geijtenbeek T.B.H., Blixt O., Alvarez R., van Die I., van Kooyk Y. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int. Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 82.Bulteau F., Thépaut M., Henry M., Hurbin A., Vanwonterghem L., Vivès C., Le Roy A., Ebel C., Renaudet O., Fieschi F., Coll J.L. Targeting tn-antigen-positive human tumors with a recombinant human macrophage galactose C-type lectin. Mol. Pharm. 2022;19:235–245. doi: 10.1021/acs.molpharmaceut.1c00744. [DOI] [PubMed] [Google Scholar]
- 83.Oh Y.J., Dent M.W., Freels A.R., Zhou Q., Lebrilla C.B., Merchant M.L., Matoba N. Antitumor activity of a lectibody targeting cancer-associated high-mannose glycans. Mol. Ther. 2022;30:1523–1535. doi: 10.1016/j.ymthe.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salanti A., Clausen T.M., Agerbæk M.Ø., Al Nakouzi N., Dahlbäck M., Oo H.Z., Lee S., Gustavsson T., Rich J.R., Hedberg B.J., et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell. 2015;28:500–514. doi: 10.1016/j.ccell.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Römer W., Meléndez A.V., Cárdenas R.M.H.V., Lagies S., Siukstaite L., Thomas O.S., Weber W., Kammerer B., Minguet S. Novel lectin-based chimeric antigen receptors target Gb3-positive tumour cells. bioRxiv. 2022 doi: 10.21203/rs.3.rs-1327761/v1. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swanson M.D., Boudreaux D.M., Salmon L., Chugh J., Winter H.C., Meagher J.L., André S., Murphy P.V., Oscarson S., Roy R., et al. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell. 2015;163:746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Covés-Datson E.M., Dyall J., DeWald L.E., King S.R., Dube D., Legendre M., Nelson E., Drews K.C., Gross R., Gerhardt D.M., et al. Inhibition of ebola virus by a molecularly engineered banana lectin. PLoS Negl. Trop. Dis. 2019;13:e0007595. doi: 10.1371/journal.pntd.0007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Covés-Datson E.M., King S.R., Legendre M., Gupta A., Chan S.M., Gitlin E., Kulkarni V.V., Pantaleón García J., Smee D.F., Lipka E., et al. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc. Natl. Acad. Sci. USA. 2020;117:2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Covés-Datson E.M., King S.R., Legendre M., Swanson M.D., Gupta A., Claes S., Meagher J.L., Boonen A., Zhang L., Kalveram B., et al. Targeted disruption of pi-pi stacking in Malaysian banana lectin reduces mitogenicity while preserving antiviral activity. Sci. Rep. 2021;11:656. doi: 10.1038/s41598-020-80577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christodoulou I., Rahnama R., Ravich J.W., Seo J., Zolov S.N., Marple A.N., Markovitz D.M., Bonifant C.L. Glycoprotein targeted CAR-NK cells for the treatment of SARS-CoV-2 infection. Front. Immunol. 2021;12:763460. doi: 10.3389/fimmu.2021.763460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17:487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu H., Xiao K., Tian Z. Benchmark of site- and structure-specific quantitative tissue N-glycoproteomics for discovery of potential N-glycoprotein markers: a case study of pancreatic cancer. Glycoconj. J. 2021;38:213–231. doi: 10.1007/s10719-021-09994-8. [DOI] [PubMed] [Google Scholar]
- 93.Watt J., Kocher H.M. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology. 2013;2:e26788. doi: 10.4161/onci.26788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKenna M.K., Brenner D., Brenner B., et al. Targeting tumors and the tumor microenvironment with banana lectin expressing T cells. Mol. Ther. 2022;30:579. [Google Scholar]
- 95.Lloyd M.G., Liu D., Legendre M., Markovitz D.M., Moffat J.F. H84T BanLec has broad spectrum antiviral activity against human herpesviruses in cells, skin, and mice. Sci. Rep. 2022;12:1641. doi: 10.1038/s41598-022-05580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]