Abstract
Tumor necrosis factor alpha (TNF-α) is a critical pro-inflammatory cytokine in a wide range of tumors and infectious diseases. This study showed for the first time that TNF-α could specifically bind to certain intracellular or circulating inflammation-related microRNAs both in vitro and in vivo. The binding sites of TNF-α to microRNAs are located at the N-terminal of TNF-α and the 3′-GGUU motif of microRNAs. TNF-α could deliver exogenous unmodified single-stranded microRNAs into recipient cells through the TNF-α receptors (TNFRs) and stabilize them from being degraded by RNase in cells. Exogenous miR-146a or let-7c delivered into HCT116 cells by TNF-α could escape from lysosomes and specifically downregulate their target genes and then affect cell proliferation and migration in vitro, as well as tumorigenesis in vivo. Based on the above findings, the concept of “non-conjugated ligand-mediated RNA delivery (ncLMRD)” was proposed, which may serve as a promising strategy for therapeutic microRNA delivery in the future.
Keywords: TNF-α, RNA binding protein, TNF-α receptor, microRNA delivery, recipient cells
Graphical abstract

Zhao et al. describes a microRNA delivery paradigm by using TNF-α as RNA binding protein, which can deliver exogenous microRNAs with functional targeting capabilities into cells. They also put forward the concept of non-conjugated ligand-mediated RNA delivery (ncLMRD), which advances current understanding of the application of ligand-receptor in RNAi therapeutics.
Introduction
RNA interference (RNAi) is an endogenous pathway for post-transcriptional virtually silencing of any gene that is triggered by double-stranded RNA, including endogenous microRNA (miRNA) and synthetic short interfering RNA (siRNA), which plays a pivotal role in cell differentiation, proliferation, and survival.1,2 Since the discovery of RNAi in mammalian cells, there have been great efforts in exploiting this powerful method in disease treatment. RNAi therapeutics, mainly including siRNAs or microRNAs, are a new class of drug molecules that can be used to treat diseases by attacking mRNA of the targeted genes in cells.3 The applications and indications of RNAi therapeutics are broad enough to include cancers, rare diseases, viral diseases, kidney diseases, cardiovascular diseases, inflammatory diseases, and metabolic diseases. An increasing number of RNAi therapeutic drugs have been approved by FDA or under clinical trials.4 Although RNAi therapeutic drugs will come to the fast track and soar in the foreseeable future, such limitations and concerns as inefficient delivery, poor in vivo stability, inappropriate biodistribution of certain tissues, stimulation of innate immune responses, and untoward side effects have to be addressed before RNAi-based therapeutics can be harnessed as a new therapeutic modality.5, 6, 7 Oligonucleotide delivery remains the bottleneck because the nucleic acids have difficulty penetrating the cell membrane. So far, a large number of viral vectors and non-viral delivery systems have been developed to circumvent these challenges. The most widely used are lipid nanoparticles (LNPs) and GalNAc systems, which were chemically conjugated and mostly targeted the liver, with great cytotoxicity and immunogenicity.8,9 Therefore, safe, natural, and effective delivery materials need to be found to bring siRNAs or microRNAs to the sites of action without adverse effects.
A previous study reported that Argonaute2 (Ago2), the key effector protein of microRNA-mediated silencing, could carry a population of circulating microRNAs independent of vesicles in human plasma, indicating that Ago2 was able to bind to microRNAs.10 Also, Vickers et al. presented evidence that high-density lipoprotein (HDL) could transport circulating microRNAs and deliver them to recipient cells with functional targeting capabilities.11 This gave us the incentive to find out whether the natural proteins, such as HDL and Ago2, could serve as delivery carriers free of the limitations of existing delivery systems, but unfortunately none of them have been effectively used as an RNAi therapeutic delivery system.
Tumor necrosis factor alpha (TNF-α), initially discovered as an antitumor protein, has been shown to be a critical pro-inflammatory cytokine involved in different kinds of diseases, including cytokine storm.12, 13, 14 As our previous study demonstrated that TNF-α had RNA chaperone activity, which could bind to random 68 nt RNAs,15,16 we were led to ask whether the natural TNF-α could bind and deliver RNA to recipient cells and serve as a natural and novel nucleic acid delivery vehicle. Our study presented evidence that TNF-α could bind to exogenous microRNAs and deliver them to recipient cells with functional targeting capabilities.
Results
TNF-α specifically binds to certain inflammation-related intracellular or circulating microRNAs
TNF-α is mainly produced by activated macrophages and many other cell types. To induce TNF-α expression, U937 was differentiated into macrophage-like cells with PMA before being stimulated with LPS. Our previous results showed that the expression of TNF-α in activated U937 cells was increased significantly as other studies did.16 Two protein bands (two forms of TNF-α) were detected: the 26 kDa membrane form (mTNF-α) and the 17 kDa soluble form (sTNF-α), as reported previously (Figure S1A). We next detected the expression of inflammation-relative microRNAs17, 18, 19, 20 in activated U937 cells by quantitative real-time PCR and found that the expressions of inflammation-related miR-146a, miR-146b, and miR-21 were upregulated both in the cell lysates16 and supernatants (Figure 1A) of activated U937 cells. However, there were no obvious changes in miR-155 or let-7c (Figure 1A). Our previous studies suggested that TNF-α was an RNA chaperone.15 To find out whether TNF-α could bind to these microRNAs, RNA binding protein immunoprecipitation (RIP) assays were performed by using a TNF-α-specific antibody. Results showed that both of mTNF-α and sTNF-α were captured from the cell lysates by anti-TNF-α antibodies, but only sTNF-α was captured from the conditioned culture medium (Figure S1A). Surprisingly, quantitative real-time PCR results showed that TNF-α could specifically capture miR-146a, miR-146b, and let-7c from both the cell lysates16 and supernatants (Figure 1B) of activated U937 cells.
Figure 1.
TNF-α specifically binds to certain inflammation-related intracellular or circulating microRNAs
(A) Quantitative real-time PCR analyzing the expression of inflammation-related microRNAs in activated U937 cell supernatants. (B) Quantitative real-time PCR analyzing the expression of inflammation-related microRNAs in the supernatants captured by TNF-α antibody assayed by RNA-IP. (C) EMSA analyzing the binding of rhTNF-α to miR-146a, miR-146b, let-7c, and miR-21 (left), and to miR-146a in a dose-dependent manner (middle), as well as the competitive assay of rhTNF-α to miR-146a (right). (D) The association constant Kd value of rhTNF-α to miR-146a measured by bio-layer interferometry (BLI) biosensor (Gator Bio.). The concentration of rhTNF-α is 50, 25, 12.5, 6, 3, 1.5, and 0.75 μg/mL from high to low. (E) The binding ratio of rhTNF-α to miR-146b, let-7c, miR-146a-antisense (miR-146a-AT), and miR-21 normalized to miR-146a. (F) The kinetic curve of natural TNF-α from activated U937 cells binding to miR-146a compared with control product by BLI biosensor. (G) The interaction docking simulation and molecular dynamics simulation for binding stability of the TNF-α homotrimer to miR-146a based on the crystal structure (PDB: 1TNF). The N-terminal and the C-terminal of TNF-α are marked with red or green arrows, respectively. (H) The binding ratio of nrhTNF, TNF-mut 4 (NH2-R2G-R6G, the mutation that decreased of basic amino acids in the N-terminal), TNF-mut 1 (NH2-A84V, complete loss of TNF-α biological activity as well as the conformational change from homotrimers to monomer), and TNF-mut 3 (nrhTNF-A84V) to miR-146a normalized to wild-type rhTNF-α. (I) The binding ratio of rhTNF-α to different 3′-GGUU mutations of microRNAs normalized to their wild types, respectively. mT, the mutation of 3′-GGUU to 3′-GGGG of miR-146a; mG, the mutation of 3′-GGUU to 3′-CCUU of miR-146a; the mutation of 3′-GGCU to 3′-CCCU of miR-146b. mGU, the mutation of 3′-UUGA to 3′-GGUU of miR-21. GU, adding GGUU to the 3′ tail of miR-21. ATGU, adding GGUU to the 3′ tail of miR-146a-AT. The sequences were listed in Table S2. (J) The binding ratio of rhTNF-α to different microRNAs after adding poly U to the 3′ tails normalized to their wild types, respectively. Poly U, UUUUUUUUUU. (K) The binding ratio of TNF-α or nrh-TNF to miR-146a or let-7c with 2′-fluoro (2′-F) modification normalized to their wild types, respectively. The association constant Kd value of each binding pair are listed in Table S1. The experiments were performed in triplicate. All data are mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
To investigate whether TNF-α binds to microRNAs directly, rhTNF-α and chemically synthesized single-stranded microRNAs labeled with biotin were used for electrophoretic mobility shift assay (EMSA). Results showed that rhTNF-α could bind to miR-146a, miR-146b, and let-7c directly in vitro (Figure 1C, left) and bind to miR-146a in a dose-dependent manner (Figure 1C, middle), but not to miR-21 (Figure 1C, left). In a competitive assay, the binding could be inhibited by the 20-fold non-labeled corresponding miR-146a (Figure 1C, right), indicating that rhTNF-α could bind to miR-146a specifically.
To further prove the binding kinetic characteristics of the rhTNF-α-microRNA complex, the bio-layer interferometry (BLI) biosensor (Gator Bio., CA, USA) was used to determine the binding affinity. Results showed that rhTNF-α could bind to miR-146a, miR-146b, miR-155, and let-7c with high affinities, and the association constant Kd of rhTNF-α to miR-146a was 71.6 nM (Figures 1D, 1E, and S1B; Table S1). However, rhTNF-α could not bind to miR-21 or had little binding affinity to miR-146a-antisense (miR-146a-AT) (Figures 1E and S1B; Table S1), indicating that the binding of rhTNF-α to microRNAs had selectivity.
To find out whether natural TNF-α from cells could bind to microRNAs in vitro, TNF-α extracted from the conditioned culture medium of activated U937 cells captured by anti-TNF-α antibodies was used for binding assay (Figure S1C). Results showed that natural TNF-α could also bind to miR-146a and let-7c (Figures 1F and S1D) as rhTNF-α did.
To study the binding motif of TNF-α and microRNAs, the sequences of TNF-α and microRNAs were analyzed based on previous crystal studies using the HDOCK SERVER (http://hdock.phys.hust.edu.cn) followed by molecular dynamics simulation (Discovery Studio).21,22 It was found that the binding of TNF-α to miR-146a could form a stable complex and that the N-terminal of TNF-α monomer might be crucial to the binding (Figures 1G and S1E). To have a better idea of the importance of the N-terminal of TNF-α, the N-terminal mutation, a new recombinant human tumor necrosis factor (nrhTNF) expressed in prokaryotic system (E. coli) was used in our experiment. The sequence of nrhTNF is NH2-1-7Del-P8R-S9K-D10R-L157F, exposing more basic and positively charged amino acids. nrhTNF was reported to be more effective and less toxic and was approved for clinical application.23 As was expected, the binding affinities of nrhTNF to microRNAs were much higher than those of wild-type rhTNF-α assayed by EMSA and BLI (Figures 1H and S1F; Table S1), suggesting that the basic characteristics in the N-terminal of TNF-α were crucial to the binding. Based on this hypothesis, we constructed the N-terminal-mutated TNF-α, TNF-mut 4 (NH2-R2G-R6G), which had less basic amino acids. It was found that the binding affinity of TNF-mut 4 to miR-146a decreased significantly (Figures 1H and S1G; Table S1), but the biological activity of TNF-mut 4 was not affected (Figure S1H). These results added evidence to the hypothesis that the basic amino acids in the N-terminal of TNF-α were crucial to the binding.
Given that soluble TNF-α predominantly forms a homotrimer structure in a non-covalent form, whether the binding was dependent on the homotrimer or monomer of TNF-α was investigated. A previous study has proved that the A84V mutation of TNF-α could result in the complete loss of biological activities, as well as the conformational change from homotrimers to monomer.24 Thus, two TNF-α-A84V mutants, TNF-mut 1 (NH2-A84V) and TNF-mut 3 (NH2-1-7Del-P8R-S9K-D10R-A84V-L157F), were constructed based on wild-type TNF-α and nrhTNF, respectively. Proliferation assays showed that both TNF-mut 1 and TNF-mut 3 had little biological activities compared with the wild type (Figure S1I). It is interesting that TNF-mut 1 and TNF-mut 3 had comparable or even higher binding affinities as their parental types (Figure 1H; Table S1), indicating that the binding was attributed to the monomer. Collectively, it was concluded that the binding of TNF-α to microRNAs mainly depended on the basic amino acids exposed in its N terminus rather than on the conformation, and that the binding was dependent on the monomer.
To learn more about the binding preference of TNF-α to which kinds of microRNAs, the sequences of microRNAs by charge, polar, and secondary structure were analyzed. According to the binding affinities and the interaction docking simulation of TNF-α homotrimer to miR-146a (Figures 1E and 1G), the sequences of related microRNAs were analyzed, with the finding that the 3′-GGUU, which exists in both the 3′ tails of miR-146a and let-7c, might be important for the bindings. Thus, the 3′ tail of miR-146a was mutated from GGUU to GGGG (miR-146a-mT) or CCUU (miR-146a-mG), or the 3′ tail of miR-146b was mutated from GGCU to CCCU (miR-146b-mG), and it was found that the binding affinities decreased as expected (Figure 1I; Tables S1 and S2). It was intriguing that miR-21 and miR-146a-AT, which had little binding affinities to rhTNF-α, increased their binding abilities after mutating from UUGA to GGUU (miR-21-mGU) in the 3′ tail or adding GGUU (miR-21-GU, 146a-ATGU) to their 3′ tails (Figure 1I; Tables S1 and S2). There was one report of the role of poly U in RNA recognition.25 To investigate whether poly U could improve the binding affinities of TNF-α to microRNAs, we added poly U to the 3′ tail of microRNAs and found that the binding ability increased considerably (Figure 1J; Table S1). Furthermore, the analysis of the binding of nrhTNF to different microRNAs revealed that nrhTNF bound to all mutant microRNAs with high affinities (Figure S1J), suggesting again that the basic characteristics of the TNF-α N terminus were crucial to the binding. As the microRNAs used in the binding assay were the chemically synthesized single-stranded microRNAs without modification, we then tested whether TNF-α could bind to microRNAs with 2′-fluoro (2′-F) modifications. The results showed that TNF-α, including nrh-TNF, could bind to 2′-F-modified single-stranded miR-146a with higher binding affinities than unmodified single-stranded miR-146a (Figure 1K; Table S1).
TNF-α-mediated internalization of exogenous microRNAs into colorectal cancer cells
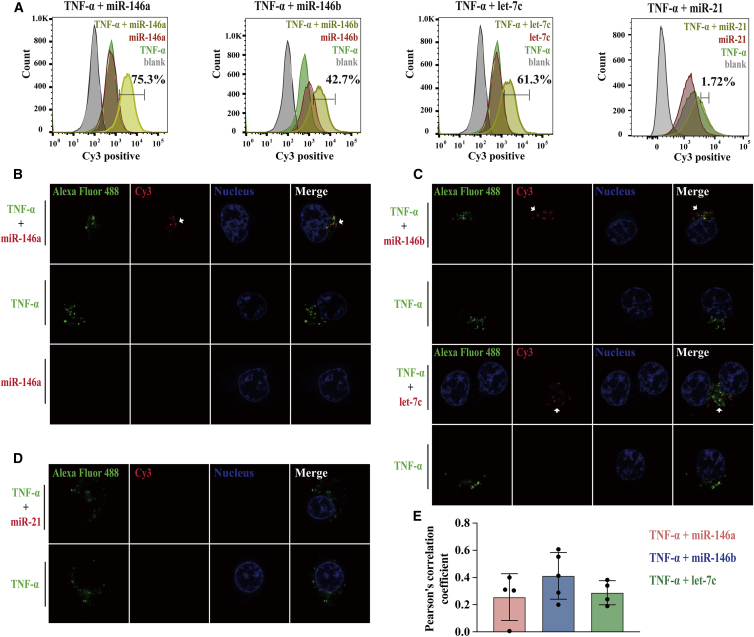
To investigate whether the rhTNF-α-microRNA complex could be internalized into HCT116 cells, rhTNF-α labeled with green fluorescence (Alexa Fluor 488) and microRNAs labeled with Cy3 were used in the experiments. The experiment began by studying whether different concentrations of rhTNF-α could cause toxic effects that led to cell membrane leakage and cell death using a trypan blue membrane leakage assay. The results showed that the cell viability did not change when cells were incubated with different concentrations of rhTNF-α for a prolonged period of 24 h (Figure S2A). The tight junction protein (ZO-1) was also tested to determine whether tight junctions were disrupted under this condition. Confocal images of HCT116 cells showed that there was little disruption of ZO-1 in TNF-α-treated cells (Figure S2B), indicating the membrane permeability remained intact. Next, we found that both rhTNF-α alone and the rhTNF-α-microRNA complex could be internalized into cells analyzed by flow cytometry and the percentage of positive cells in rhTNF-α group was almost 99% (Figure S2C), as was reported previously.26 The percentage of Cy3-positive cells was increased by 40%–70% with addition of rhTNF-α, indicating that rhTNF-α could bind to and transport miR-146a, miR-146b, or let-7c into cells efficiently (Figures 2A and S2D), with the exception of miR-21, which could not be bound or transported into cells (Figures 2A and S2D). Visualization of the intracellular rhTNF-α-microRNA complex under a confocal microscope led to the finding that microRNAs could be delivered into live cells by rhTNF-α and that rhTNF-α alone could internalized into cells, as detected using flow cytometry (Figure S2E); however, microRNAs alone could not enter cells (Figure 2B). The delivery of microRNAs by rhTNF-α could be observed as early as 5 h after incubation with the TNF-α-microRNA complex. Examination of different rhTNF-α-microRNA complexes found that only microRNAs binding to rhTNF-α could be delivered into cells (Figure 2C); but this was not the case for microRNAs that could not bind to TNF-α, such as miR-21 (Figure 2D). Furthermore, the microRNAs delivered into live cells by rhTNF-α became co-localized with their “carrier” to some extent (Figure 2E). Given the above robust evidence, it was concluded that TNF-α could transport certain exogenous microRNAs into HCT116 cells.
Figure 2.
TNF-α-mediated internalization of exogenous microRNAs into colorectal cancer cells
(A) Flow cytometry fluorescence distribution histograms show the internalization of rhTNF-α, rhTNF-α-microRNA complex, microRNA into HCT116 cells. rhTNF-α was labeled with Alexa Fluor 488 (shown in green). MicroRNAs were labeled with Cy3 (shown in red). Blank groups are shown in gray. The percentage of positive cells is shown in yellow (microRNAs delivered into cells by rhTNF-α). (B–D) The internalization of microRNA, rhTNF-α, and rhTNF-α-microRNA complex into HCT116 cells visualized under a laser scanning confocal microscope (LSM Zeiss 880) using a 63×/1.4 NA objective. (B) miR-146a; (C) up, miR-146b; (C) down, let-7c; (D) miR-21. rhTNF-α was labeled with Alexa Fluor 488 (shown in green). MicroRNAs were labeled with Cy3 (shown in red and marked with arrows). The nuclei were stained with Hoechst (shown in blue). (E) Pearson’s correlation coefficient analysis of the degree of co-localization between TNF-α and microRNAs for co-localization assays. The experiments were performed in triplicate. All data are mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
The TNF-α-microRNA complex delivered into recipient cells is mediated by TNFRs
To improve the rate of cellular uptake, more efforts are being made to target ligands of specific cells to induce receptor-mediated endocytosis.27 However, there are few reports available on receptor-mediated endocytosis of microRNAs. TNF-α exerts its effects by binding as a trimer to two cell membrane TNF-α receptors (TNFRs), TNFR1 (TNF-R55, 55 kDa) and TNFR2 (TNF-R75, 75 kDa), which are present on the membrane of all cell types except erythrocytes (The Human Protein Atlas).28 To find out whether the TNF-α-microRNA complex delivered into recipient cells was mediated by TNFRs, TNFR1 high-expression cell lines, A549 and HUVEC, as well as TNFR low-expression cell lines, HEK293T and HT-29, were investigated to assess the delivery efficiency of the TNF-α-microRNA complex. Results showed that rhTNF-α delivered far more microRNAs into A549 or HUVEC cells than into HEK293T and HT-29 cells, and the statistics of mean fluorescence intensity (MFI) showed that microRNAs internalized into TNFR low-expression cell lines decreased significantly compared with those into the high-expression lines (Figure 3A), indicating that TNFR might have been involved in the delivery process. We further knocked down TNFR1 by siRNA and found that the internalization of both rhTNF-α and microRNAs decreased dramatically, as shown by both imaging and MFI statistics (Figure 3B; Figure S3A). Similar results were observed in TNFR1-knockdown HCT116 cells by using rhTNF-α-delivered miR-146a labeled with biotin by EMSA assay (data not shown). As two types of TNFRs were expressed in colorectal cancer HCT116 cells (Figure S3B), both TNFR1 and TNFR2 were knocked out using CRISPR-Cas9 technology. Western blot and Sanger sequencing were carried out to determine the knockout efficiency (Figures S3C and S3D,;Table S4). Laser scanning confocal microscopy results showed that, without the two TNFRs, neither rhTNF-α nor microRNAs could be internalized into cells, (Figures 3C and S3E). For further study, we constructed TNF-mutant 2 (NH2-V91G), whose mutation could cause dysfunction of receptor recognition.24 The binding affinities of TNF-mutant 2 to microRNAs did not change (Figure S3F), but the bioactivity decreased, as assessed by proliferation assay (Figure S3G). LSCM results showed that TNF-mutant 2 lost the ability to delivery microRNAs into cells, as well as the ability to internalize itself into cells (Figure 3D). The above results indicated that the TNF-α-microRNA complex delivered into recipient cells was mediated by TNFRs and could be interrupted by blocking the TNFRs. Finally, the effect of Adalimumab, a TNF-α antagonistic antibody,29 on blocking the access of rhTNF-α and the rhTNF-α-microRNA complex to HCT116 cells was investigated. Results showed that Adalimumab could compete with exogenous sTNF-α to bind to receptors and keep the exogenous rhTNF-α or the rhTNF-α-microRNA complex out of the cells, resulting in the accumulation of rhTNF-α or the complex all around the cell membrane (Figures 3E and S3H). These results indicated that TNF-α-mediated delivery of microRNAs could also be interrupted by blocking TNF-α itself with specific antibodies.
Figure 3.
TNF-α-microRNA complex delivered into recipient cells was mediated by TNFRs
(A) The visualization of rhTNF-α delivering miR-146a into TNFR high-expression cell lines (upper: left, A549; right, HUVEC) and TNFR low-expression cell lines (lower: left, HEK293T; right, HT-29) captured under an LSCM using a 63×/1.4 NA objective. Mean fluorescence intensity (MFI) of cells as determined by ImageJ software. (B) The decreased miR-146a delivery abilities of rhTNF-α after knocking down TNFR1 by siRNA in HCT116 cells. The left panel shows the merged images, and the individual images are shown on the right. MFI of cells as determined by ImageJ software. NC, negative control. (C) The visualization of miR-146a delivered by rhTNF-α into TNFR1/2 knockout cell lines constructed by CRISPR-Cas9 technology captured under an LSCM using a 63×/1.4 NA objective. (D) The decreased miR-146a delivery abilities of TNF-mut 2 captured under an LSCM using a 63×/1.4 NA objective. (E) The visualization of rhTNF-α delivering miR-146a into HCT116 cells after treatment with Adalimumab. rhTNF-α was labeled with Alexa Fluor 488 (shown in green). MicroRNAs were labeled with Cy3 (shown in red and marked with arrows). The nuclei were stained with Hochest (shown in blue). The cell membrane was stained with DiD (shown in magenta). The experiments were performed in triplicate. All data are mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns, not significant.
TNF-α transfers exogenous microRNAs to recipient cells with functional targeting capabilities both in vitro and in vivo
The microRNAs used in this delivery study are single-stranded microRNAs without chemical modifications, and are different from the double-stranded RNA mimics or modified RNAs widely used for RNAi research. Thus, the integrity of microRNAs co-existing with rhTNF-α in different concentration of serum was examined in vitro. The results showed that miR-146a alone was degraded rapidly in 1% and 5% sera, but at a much lower speed when accompanied by TNF-α (Figure 4A), indicating that TNF-α was capable of improving the stability of microRNAs in serum. As the exogenous substances are swallowed and degraded by lysosomes once inside live cells, we investigated whether the TNF-α-delivered exogenous microRNAs in recipient cells could escape from lysosomes and retain their biological function. Firstly, the subcellular location of miR-146a transferred by rhTNF-α was identified. It was found that the co-localization of miR-146a transferred into cells by rhTNF-α with lysosomes decreased over time (Figure 4B), as did the incubation time of the TNF-α-microRNA complex (from 1 to 24 h), and the Pearson’s correlation coefficient of lysosome and miR-146a ranged from 0.91 to 0.13 (Figure S4A), indicating that miR-146a could escape from lysosomes. Then, the biological functions of microRNAs delivered by rhTNF-α into HCT116 cells were examined. It was found that rhTNF-α alone could inhibit colony formation, but that the TNF-α+miR146a complex had significantly less activity than TNF-α alone in suppressing colony formation (Figure 4C). Migration assay showed that the rhTNF-α-miR-146a complex could increase the migratory abilities of HCT116 cells but not for miR-146a alone (Figure 4D). More studies showed that the expression of NUMB and TRAF6, which were the targets of miR-146a reported previously,30,31 was also specifically downregulated by the rhTNF-α-miR-146a complex (Figure 4E). To exclude the possible synergistic effect of TNF-α with miR-146a, we chose let-7c as another delivery microRNA, which was widely reported as a tumor growth suppressor in colorectal cancer,32 and proved to be able to bind to TNF-α with high affinity. Results showed that the TNF-α-let-7c complex not only inhibits the colony formation and migratory abilities further (Figures S4B and S4C), but also downregulates the expression of LIN28B (Figure 4F), which was the specific target of let-7c reported previously.33,34 It was interesting to find that the addition of let-7c to the rhTNF-α-miR-146a complex could further decrease the effects of the rhTNF-α-miR-146a complex on colony formation and migration, which was consistent with the onco-miR effect of miR-146a and the anti-miR effect of let-7c (Figures 4G and 4H).
Figure 4.
TNF-α transfers exogenous microRNAs to recipient cells with functional targeting capabilities both in vitro and in vivo
(A) Serum degradation assay of miR-146a alone or the rhTNF-α-miR-146a complex in different concentrations of sera for 5 h. Lanes 1 and 2 are the control group without sera for 0 h. miR-146a was labeled with biotin. (B) Representative images of lysosome escaping of miR-146a delivered by rhTNF-α after incubation for 5 h (upper) and 15 h (lower) captured under an LSCM using a 63×/1.4 NA objective (left). The statistic of Pearson’s correlation coefficient analysis of the degree of co-localization between lysosome and miR-146a is shown on the right. Lysosome labeled with LysoTracker shown in green; TNF-α, unlabeled; miR-146a labeled with Cy3 shown in red. (C) The colony formation abilities of cells with exogenous miR-146a delivered by rhTNF-α. Representative images (left) and colony formation rates to blank (right) are shown. (D) Migration assay of HCT116 incubated with rhTNF-α, miR-146a, rhTNF-α-microRNA complex, and blank, respectively. Representative images (left) and statistics (right) of migration assay are shown. (E and F) Western blot analysis of miR-146a targeting NUMB and TRAF6 (E) and let-7c targeting LIN28B (F). RiM, transfection regent, RNAiMAX. β-Actin was used as an internal control. Bands were quantified with ImageJ software. (G) The counteract effect of exogenous let-7c delivered by rhTNF-α in HCT116 cells assayed by colony formation. Representative images (left) and colony formation rates to blank (right) are shown. (H) The counteract effect of exogenous let-7c delivered by rhTNF-α in HCT116 cells assayed by migration. Representative images (left) and statistics (right) of migration assay were shown. (I) HCT116 cells were subcutaneously injected into BALB/c nude mice for observation of tumor growth. The microRNAs treatment was conducted as indicated. The tumor volumes (left) were measured at the indicated time points and images of tumors from nude mice at autopsy are presented (right). In (C), (D), (G), and (H) the experiments were performed in triplicate. All data are mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
To further determine whether exogenous microRNAs delivered by TNF-α could affect tumorigenesis in vivo, HCT116 cells were subcutaneously injected into nude mice and tumor growth monitored. When the tumor volumes reached about 80 ± 30 mm3, intratumoral injection with rhTNF-α, microRNAs, or the rhTNF-α-microRNA complex was conducted. As expected, the rhTNF-α-miR-146a complex could promote tumor growth compared with rhTNF-α or microRNAs alone, while the rhTNF-α-let-7c complex could significantly inhibit tumor growth (Figure 4I). The tumorigenesis of TNFR knockout cells in vivo (Figures S3C and S3D) was also determined. The onco-miR or anti-miR effects of microRNAs delivered by TNF-α into TNFR knockout cells was not as significant as those in the wild type (Figure S4D), further illustrating that the internalization of the TNF-α-microRNA complex into recipient cells was mediated by TNFRs.
Taken together, our results demonstrated that exogenous microRNAs delivered by TNF-α had functional targeting capabilities both in vitro and in vivo, indicating the possibility of TNF-α application as a natural microRNA delivery carrier.
Discussion
In this study, it was found for the first time that TNF-α could bind to exogenous microRNAs and deliver them into recipient cells, functioning through TNFR, and a novel nucleic acid delivery paradigm in gene therapy by utilizing a natural protein is proposed (Figure 5). Several advantages can be speculated for TNF-α if used as a microRNA delivery carrier. First, TNF-α is a natural protein that will not induce auto-antibodies in the human body. Second, TNF-α could bind to microRNAs with high affinity. Third, TNF-α was found to stabilize unmodified single-stranded microRNAs and transfer them into recipient cells, which indicated that TNF-α acting as a molecular chaperone can prevent the degradation of microRNAs from RNase in some way. Without TNF-α, the unmodified single-stranded microRNAs were degraded quickly in 5% sera (Figure 4A), and there were no significant phenotypic changes between cells transfected with unmodified single-stranded microRNA and the control groups (data not shown). It is also worth noting that the ability of TNF-α stabilizing unmodified single-stranded microRNAs is limited, as the effects of the TNF-α-microRNA complex on the colony formation of HCT116 cells in medium with sera were not changed significantly. Previous studies have found that circulating microRNAs are transported and protected from plasma ribonucleases by their carriers, which are delivered to recipient cells with function, thus reducing target gene expression and altering cellular phenotype.35 These findings can help support our hypothesis. More importantly, TNF-α could deliver single-stranded microRNAs into recipient cells and escape from lysosomes, inducing the silencing of target mRNAs (Figure 4).
Figure 5.
A schematic diagram of the concept of “non-conjugated ligand-mediated RNA delivery (ncLMRD)” by TNF-α
(A) TNF-α can bind to and deliver microRNA into recipient cells and escape from lysosome, thus reducing target gene expressions and altering cellular phenotype. ncLMRD, a promising strategy for therapeutic application, is proposed here as a communication mode to modulate cell phenotypes at distance. (B) The binding of TNF-α to microRNA mainly depends on the N-terminal of TNF-α, and on the monomer rather than on the homotrimer structure of TNF-α. But the monomer forms of TNF-α could not bind to the receptor and thus is unable to be internalized into cells. (C) TNF-α could not bind to the double-stranded microRNAs. (D) The delivery of microRNAs into recipient cells by TNF-α is mediated by TNFRs. The blocking of the interaction of TNF-α with TNFR, or the deletion of TNFR, will abolish the delivery.
siRNAs are artificially synthesized double-stranded RNA molecules and elicit RNAi response upon binding to their target transcript based on sequence complementarity through an RNA-induced silencing complex-mediated process associated with the Dicer and Argonaute family. MicroRNAs, as endogenous single-stranded short non-coding RNAs, can regulate target gene expression with a mechanism similar to synthetic siRNA. Duplex microRNA mimics are often effectively used in modulation of gene expression both in vitro and in vivo. As potential therapeutic agents, double-stranded RNA molecules have such drawbacks as poor ability to enter target tissues in vivo, off-target effects by passenger strand, and high commercial cost.36, 37, 38 In this respect, scientists have developed several strategies for using single-stranded siRNAs or microRNAs as therapeutics that fulfill the direct and final step of RNAi.39, 40, 41, 42 In this study, we found that TNF-α could bind to single-stranded wild-type microRNAs and had little binding affinity with double-stranded microRNAs (data not shown). To protect more stable microRNAs from degradation, chemically modified microRNAs were also used for TNF-α binding assay. It was found that the binding affinity was quite distinct when single-stranded microRNAs were modified differently. TNF-α could bind to microRNAs with full-length of 2′-F modification with higher affinities compared with unmodified microRNAs, but not to full-length of 2′-OMe modification and partial thioketone modification along with cholesterol in the 3′ tail (data not shown). Strangely, single-stranded microRNAs with full-length 2′-F modification could be delivered to recipient cells by TNF-α, but could not silence the target gene expression because of their transfection with transfection reagents (data not shown). The reason is not clear and needs to be investigated in the future.
To explore the sequence specificity of microRNAs binding to TNF-α, the sequences of microRNAs used in our study were analyzed, resulting in the finding that the 3′ tail GGUU was of importance, especially the base U. Adding poly (U) tails to microRNAs could increase the binding affinities of TNF-α to microRNAs to some extent. It is interesting that wild-type TNF-α could not bind to miR-146a antisense or miR-21, but could bind to those oligonucleotides with poly (U) tails, thus indicating the importance of base U. However, a common oligonucleotide sequence or “motif” was not found in microRNAs that could specifically bind to TNF-α with high affinity. More studies are needed to obtain an explicit binding model using crystal structure analysis and bioinformatics in the future.
To explore the binding motif or key amino acids of TNF-α to microRNAs, several mutants of TNF-α were employed for binding assays. It was found that the N-terminal of TNF-α was important for the binding of TNF-α to microRNAs. nrhTNF (NH2-1-7Del-P8R-S9K-D10R-L157F) and TNF-mut 4 (NH2-R2G-R6G) showed distinct binding affinities, illustrating the importance of the basic amino acids in the N-terminal of TNF-α. The interaction docking simulation of TNF-α homotrimer to miR-146a (Figure 1G) also supported the speculation that the N-terminal of TNF-α was associated with microRNA binding. Previous studies showed that trimeric TNF-α could bind to cell surface receptors with greater affinity than isolated monomers.43 In this study, we were able to answer the question of whether homotrimers or monomer of TNF-α could bind to single-stranded microRNAs. We constructed a TNF-α-A84V mutant that could not oligomerize into a trimer and that remain in a monomeric form, which resulted in the loss of biological activity.24 It was found that there were no significant changes in binding affinities after mutation, indicating that binding was dependent on monomers. Moreover, the TNF-α-A84V mutant proteins could only bind to microRNAs rather than transfer them into recipient cells. These results led us to the conclusion that the binding of TNF-α to microRNAs was mainly dependent on the N-terminal motif, while the delivery process was not related to homotrimer conformation.
To test whether other TNF superfamily members have similar function in binding to microRNAs, we chose five TNF superfamily members and verified their binding ability to miR-146a by BLI. It was found that the binding of TNF superfamily members (TNFSF10, TNFSF11, TNFSF12, TNFSF13, and TNFSF14) to miR-146a was selective. We found that only TNFSF12 can bind to miR-146a robustly (Kd = 85.7 nM; Figure S1K). According to the PDB database, these five TNF superfamily members are all in trimeric form, which is the same as TNF-α. However, when using HDOCK for the interaction docking simulation, it was found that the binding of TNF superfamily members to miR-146a is different from that of TNF-α binding to miR-146a. We could not find a universal binding site like that of TNF-α binding to miR-146a (the N-terminal of TNF-α or the 3′ tail of miR-146a). The reason why the binding of TNF superfamily members to miR-146a has selectivity needs further investigation.
Human TNF-α signaling is mainly mediated by two different receptors, TNFR1 and TNFR2. TNF-α binds strongly to TNFR1 and TNFR2 with a disassociation constant Kd of around 0.5 and 0.1 nM, respectively, and the diverse biological effects of TNF-α are mainly mediated by binding to the TNFR-1 receptor.44, 45, 46 In this paper, we confirmed that the entrance of the TNF-α-microRNA complex into recipient cells was through endocytosis mediated by TNFR. To block the recognition of TNF-α by TNFRs or decrease the expression level of TNFRs could abolish the delivery of the TNF-α-microRNA complex. Based on our findings, the concept of “non-conjugated ligand-mediated RNA delivery (ncLMRD)” by TNF-α was proposed, which is expected to become a promising strategy for RNAi therapeutic delivery in the future (Figure 5). The research on aptamer-RNA delivery or RNA-ligand display for exosomes are currently hot topics in the field of nucleic acid delivery because of their incomparable advantages. Taking aptamer-RNA delivery as an example, there are several differences between aptamer-RNA delivery and ncLMRD. First, both delivery strategies can deliver small non-coding RNAs. Aptamers can be used to deliver a variety of RNAs, including siRNAs and miRNAs, but TNF-α protein can mainly deliver the single-stranded unmodified microRNAs. Second, aptamer-RNA delivery is target/organ specific and depends on the characteristics of aptamers. The aptamer-RNA complex is through hepatic aggregation and metabolized mainly in the kidney. TNF-α-RNA delivery relies on TNFR receptors existing on the surface of targeting cells. Finally, aptamer-RNA delivery relies on chemical conjugation or specific modification. The aptamer-RNA complex itself has difficulty in entering cells, and it needs other carriers, such as LNP, that have high toxicity and off-target effects. The delivery strategy in this study relies on the natural TNF-α protein, which is secreted by human cells under pathological conditions with lower or no immunogenicity. TNF-α can directly transport microRNA into TNFR-expressed cells without any modification. The delivery strategy in this study is also different from the classical ligand-conjugated receptor delivery system that transports chemical conjugated ligand-molecules to cells through specific receptors expressed on the cell surface, such as folate receptor or transferrin receptor.47, 48, 49, 50 There is no need for chemical conjugation of the receptor ligand with microRNAs for ncLMRD. The carriers of microRNAs for ncLMRD are natural RNA binding proteins, which can avoid immune recognition and extracellular toxicity. The functional motif of ligands can be deleted while retaining binding activity to microRNAs, making it possible to develop a universal non-functional ncLMRD carrier. As ligand receptors commonly exists in cells, more natural protein ligands with RNA binding activity need to be found in the future for ncLMRD.
As a pro-inflammatory cytokine, TNF-α plays important, dual but paradoxical roles in inflammation-associated diseases and cancers depending on its concentration.51,52 TNF-α can induce tumor regression at a high dosage, whereas endogenous low-dose TNF-α is associated with cancer development and progression, which was validated in our results (colony formation and migration assays). We found the opposite effect of TNF-α itself on colony formation and migration of HCT116 cells, which is consistent with a previous study.53 In our study, high-dose TNF-α was found to deliver extracellular microRNAs into recipient cells. It is worth considering that TNF-α plus the single-stranded anti-tumor microRNA, such as let-7c, may be a new formula for cancer treatment. As the concentration of TNF-α is very high in some tissue microenvironments, such as lung tissue with a cytokine storm during infections, such as SARS-CoV-2, there is reason to speculate that the possible synergistic effects of TNF-α and microRNAs, such as miR-146a/b, may exist in the process of cytokine storm-induced tissue damages and cell-to-cell communication at a distance, which was observed for TNF-α alone.54,55 These phenomena need to be investigated in the future.
Materials and methods
Cell lines and cell culture
The human colorectal cancer HCT116 cell line was obtained from Procell, and A549, HUVEC, HEK293T, and HT-29 cell lines were obtained from the Cell Resource Center of Peking Union Medical College. THP-1 and U937 cells were kindly provided by Prof. Gencheng Han in our institute. HCT116 cells were routinely cultured in McCoy’s 5A media (HyClone). U937, THP-1, and A549 cells were maintained in RPMI 1640 medium (Gibco). HUVEC, HEK293T, and HT-29 were maintained in DMEM (HyClone), DMEM with high glucose (HyClone), and IMDM media (HyClone), respectively. All the cells were cultured in medium supplemented with 10% fetal bovine serum (FBS) (Gibco, 10270) and 1% penicillin/streptomycin (Thermo Scientific, 15140163) under a 5% CO2 atmosphere. When cells reached 80%–90% of confluence, cell passages were conducted, and cells in logarithmic growth phase were collected for use.
rhTNF-α, rhTNF-α mutants, and TNF superfamily members
Recombinant human TNF-α (rhTNF-α) was purchased from Peprotech (AF-300-01A). nrhTNF (NH2-1-7Del-P8R-S9K-D10R-L157F) was kindly provided by Professor Yingqi Zhang from Air Force Medical University. TNF-mut 1 (NH2-A84V), TNF-mut 2 (NH2-V91G), TNF-mut 3 (nrhTNF-A84V), and TNF-mut 4 (NH2-R2G-R6G) were customized by GenScript (Nanjing, China). All the recombinant proteins were expressed in E.coli expression hosts. Recombinant human TNF superfamily members were purchased from Origene (TNFSF13, cat. no. TP761373), Sino Biological (TNFSF10, cat. no. 10409-HNAE; TNFSF11, cat. no. 11682-HNCH; TNFSF14, cat. no. 10386-H15H3), and Proteintech (TNFSF13, cat. no. Ag29054).
EMSA
Biotinylated microRNAs synthesized by Bsyntech (Suzhou, China), were first heated to 100°C for 5 min to denature the secondary structure. Then the denatured microRNAs were incubated with rhTNF-α at 37°C for 30 min. The reaction products were separated by electrophoresis on 6% non-denaturing poly-acrylamide gel, transferred to nylon membrane, and crosslinked inside the UV crosslinker at 1.2 × 105 μJ/cm2 for 2 min. The protein was digested by 50 μg/mL protease K (Solarbio, Beijing, China, P1120) at 37°C overnight in the digestion buffer (10 mM CaCl2, Tris-HCl [pH 7.5]). The membrane was detected by Chemiluminescent Nucleic Acid Detection Module Kit (Thermo Scientific, 89880) according to the manufacturer’s instruction. For competition assay, non-biotinylated microRNAs were added to the reaction in 20-, 100-, or 200-fold excess.
Serum degradation assay
After TNF-α (3 μg) and biotin-labeled miR-146a (100 fmol) were incubated for 30 min, different concentration of serum (0%, 0.1%, 1%, and 5%) was added in 10 μL of PBS solution. The mixture then continued to incubate at 37°C for 5 h. Then the samples were separated in 20% native polyacrylamide gels and visualized using EMSA according to a previous study with little modification.56
TNF-α-microRNA complex binding kinetic characterization assay
The binding kinetic characteristics of TNF-α-microRNA complex were monitored by using the BLI biosensor (Gator Bio.). In brief, biotin-labeled microRNAs (100 nM) were loaded on to the streptavidin sensors (Gator Bio., 160002) and then were exposed to 25–30 μg/mL TNF-α protein for 120 s of association and 180 s of dissociation with PBST buffer supplied with 0.2% BSA. Association and dissociation steps were divided by the dotted line. The value of wavelength shift (nm) assessed during association procedures was used to measure the binding affinity, which was shown in the results as the binding ratio to compare the changes of binding affinities between different groups. The relative control under the same conditions was used to normalize the binding ability. All the binding assays were repeated at least twice. The association constant Kd value of each binding pair was listed in Table S1.
Flow cytometry
For FACS analysis, HCT116 cells were planted into 12-well plates. The TNF-α-microRNA complex (pre-incubated with 5 μg/mL of TNF-α labeled by AF-488 fluorescence and 10 nM microRNA labeled by Cy3 fluorescence for 30 min at 37°C) was added to the cells the next day. After being cultured for another 15 h, cells were separated into single-cell suspension and then washed by glycine-HCl (0.1 M [pH 2.5]) acid buffer followed by PBS solution at least twice to eliminate the fluorescence signal adhered to the cell surface. The average intensity of fluorescence in the cells of interest and the MFI of Alexa Fluor 488 or Cy3 signals was measured by flow cytometry with BD FACS Calibur and analyzed by FlowJo software.
Confocal laser scanning microscopy
HCT116 cells were seeded into 35 mm confocal dishes for 24 h. And then the complete culture media were changed into McCoy’s 5A medium supplemented with 1% FBS. The TNF-α-microRNA complex (pre-incubated with 5 μg/mL of TNF-α labeled by AF-488 fluorescence and 10 nM microRNA labeled by Cy3 fluorescence for 30 min at 37°C) was added to the cells for incubation of 5 h. Then the media were discharged, and the cells were washed with PBS four to five times. Before scanning, the nuclei were stained with Hoechst 33324 for 30 min and then the cells were washed with PBS three times. Lysosomes were labeled with LysoTracker Green (Thermo Scientific, L7526). Cells were immediately visualized under a laser scanning confocal microscope (LSM Zeiss 880, Carl Zeiss) with 63× oil objectives. For antibody blocking assay, 50 μg/mL of Adalimumab (Selleck, Houston, TX, USA, A2010) was added with Fluo-labeled TNF-α or TNF-α-microRNA complex into HCT116 cells and visualized under a confocal microscope same as above. Pearson’s correlation coefficient analysis was used to quantify the degree of co-localization between TNF-α or lysosome and microRNA by using the built-in Carl Zeiss co-localization analysis module from ZEN (blue edition, ZEISS) software combined with ImageJ software.
RNA isolation and quantitative real-time PCR
Total RNA derived from cells or supernatants was extracted by using TRIzol Reagent (Invitrogen, 15596026) according to the manufacturer’s instructions. For microRNA quantification, a poly(A) tail was added to the 3ʹ end of each mature miRNA by the poly(A) polymerase (NEB, M02765). Poly(A) tailed miRNAs were converted into cDNA along with mRNAs in a reverse transcription reaction by M-MLV reverse transcriptase (Thermo Scientific, 28025013) and a unique oligo (dT) adaptor primer. PCR reactions were carried out in 10 μL reactions of THUNDERBIRD SYBR qPCR Mix (Toyobo, QPS-201) containing 0.5 mM specific primers with a Roche LightCycler 96 Real-Time PCR system. U6 was used as an internal control. All samples were normalized to internal controls, and fold changes were calculated through relative quantification as follows: 2–((Ct of gene)–(Ct of internal control)). Primer sequences are listed in supplemental information (Table S3).
U937 cell activation assay
U937 cell viability was estimated regularly by trypan blue dye exclusion. To induce differentiation of U937 cells into monocytes or adherent macrophages, the cells were seeded at an initial density of 2 × 105 cells/mL and cultured in the presence of 200 ng/mL PMA (Sigma, P1585) for 24 h at 37°C. For stimulation, differentiated cells were washed with PBS and then stimulated by adding 1 μg/mL of LPS (Sigma, L2880) for another 10–24 h. Then the cells or culture media were collected for use.
RIP
U937 cells were seeded into T75 cm2 flasks at 1 × 108 cells and incubated with 200 ng/mL PMA for 24 h. Then the cells were washed with PBS and cultured in fresh medium with 1 μg/mL LPS for 20 h. For cell lysate preparation, activated U937 cells were cross-linked with 1% formaldehyde solution in PBS for 15 min. Then 250 mM glycine was added to the medium and incubated for 5 min. The cells were washed with PBS and lysed in RIPA buffer with protease inhibitor cocktail and RNase inhibitor (Thermo Scientific, EO0381). For the cell supernatant preparation, conditioned medium of activated U937 cells was collected, and then concentrated with 3K closure ultrafiltration tubes. The cell lysates or ultra-filtrated supernatants of activated U937 cells were then precleared by 40 μg/mL normal rabbit IgG and 40 μL protein A Sepharose beads. Anti-TNF-α antibodies (Proteintech, 60291, 40 μg/mL) were added to the pre-treated supernatants and subsequently incubated with 20 μL protein A beads, with normal IgG as the negative control. After being washed with PBS four times, the beads were resuspended by PBS. The protein bound by the antibody-coated beads was subjected to western blot assay. Meanwhile, the bound RNAs were extracted from the beads using TRIzol and subjected to quantitative real-time PCR analysis.
Cell membrane leakage assay
HCT116 cells were seeded into 96-well plates and treated with different concentrations of TNF-α (0, 50, 200, 1,000 μg/mL) the following day. Cell viability was monitored for a prolonged period (24 h) using trypan blue dye (0.4%).
Immunofluorescence staining
HCT116 cells were seeded into 35 mm confocal dishes for 24 h and then fixed in 4% paraformaldehyde for 1 h at 4°C. Fixed cells were permeabilized and blocked with PBS containing 1% BSA and 1% Triton X-100 for 1 h. Cells were incubated with the primary ZO-1 Polyclonal antibody (Proteintech, 21773-1-AP) overnight at 4°C followed by staining with the secondary antibody Alexa Fluor 488 goat anti-rabbit IgG (H + L) (Abcam, ab150077) for 2 h at room temperature.
Western blot
Whole-cell lysates were obtained by using the M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, 78501) containing 5–10 μL protease inhibitor cocktail (Thermo Scientific, 87786). Proteins (20–50 μg) were separated by 10%–15% SDS-PAGE and transferred to nitrocellulose membranes. The membrane was blocked with 5% non-fat dried milk in TBST at room temperature for 1 h then incubated with antibodies in the blocking solution overnight at 4°C. After washing three times with TBST, the membrane was incubated with the HRP-conjugated secondary antibody at room temperature for 1 h and washed three times again with TBST. Antigen-antibody complex was detected using SuperSignal West Pico PLUS (Thermo Scientific, 4577). Antibodies against TNFR1 (60192) and TNFR2 (19272) were purchased from Proteintech. Antibodies against TNF-α (6945) and LIN28B (4196) were purchased from Cell Signaling Technology. Antibodies against NUMB (A9352), TRAF6 (A16991), and Actin (AC026) were purchased from ABclonal (Wuhan, China).
RNAi and cell transfection
siRNA sequence targeting TNFR1 as well as one negative control were obtained from Ribo Tech (no. 1, GAACCTACTTGTACAATGA; no. 2, CCGGCATTATTGGAGTGAA; no. 3, AGTCCAAGCTCTACTCCAT, Guangzhou, China). In brief, 100 nM of each siRNA and negative control was introduced into cells by using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, 13778-075) according to the manufacturer’s instructions.
Cell migration assay
The cell migratory ability was detected with an 8 μm pore, 6.5 mm polycarbonate transwell filter (Coring, 3422). TNF-α (5 μg/mL), microRNA (10 nM), TNF-α-microRNA complexes, and the corresponding blank control were pre-incubated in fresh medium without sera at 37°C for 30 min and then added to HCT116 cells, which were planted into 12-well plates 12 h before. The following day, a total of 2–5 million cells was counted and planted into the top chamber of a transwell filter in a volume of 0.2 mL of McCoy’s 5A medium without FBS. The lower chamber was added with McCoy’s 5A medium (0.8 mL) supplemented with 10% FBS. After incubation for 36–48 h, the cells were fixed with paraformaldehyde and stained with 0.1% crystal violet (Beyotime, Shanghai, China, C0121) for 0.5 h. Cells were counted under a microscope. A total of five fields were counted for each transwell filter. The experiment was repeated three times.
Colony formation assays
A total of 1,000 cells were counted and planted into 6-well plates in 1 mL medium. The following day, the cells were changed with fresh medium without sera. TNF-α (5 μg/mL), microRNA (10 nM), TNF-α-microRNA complexes, and the corresponding blank control were pre-incubated in fresh medium without sera at 37°C for 30 min and then added to HCT116 cells for the two subsequent days. Then the cells were changed with fresh medium supplemented with 10% sera for a week. Then, the cells were fixed with paraformaldehyde and stained with 0.1% crystal violet for 0.5 h. Cells were captured under a microscope. The experiment was repeated three times.
Proliferation assays
Rates of cell proliferation were assessed by CCK8 as per the manufacturer’s instructions (Dojindo, CK04). A total of 2,000–5,000 cells were counted and planted into 96-well plates at 100 μL medium per well. Next day, the cells were changed with fresh medium without sera. Different concentrations of TNF-α or TNF-mutants were added to HCT116 cells. Absorbance was measured using a spectrophotometer at a dual wavelength of 450/550 nm every day.
In vivo tumorigenesis
Animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Laboratory Animal Center, Beijing Institute of Basic Medical Sciences. The accreditation number of this study was AF/SC-08/02.25. Ten million HCT116 cells were resuspended in 0.2 mL PBS buffer and implanted into the nude mouse in the posterior dorsal flank region (male BALB/c nude mice, aged 4–6 weeks, Vital River, Beijing, China). Mice were maintained under standard conditions according to the institutional guidelines for animal care. When the tumor volumes reached about 80 ± 30 mm3, the tumor-bearing mice were randomized into TNF-α, microRNA, TNF-α-microRNA complexes, and the corresponding blank control group (n = 5 per group) and the intertumoral injection treatment was conducted as following: (1) TNF-α group, intratumoral injection with TNF-α (10 μg/mL); (2) microRNA group, intratumoral injection with miR-146a, miR-146b, or let-7c (10 nM); (3) TNF-α-microRNA group, intratumoral injection with TNF-α-microRNA complexes (10 μg/mL TNF-α and 10 nM microRNAs, preincubated for 30 min); (4) the corresponding blank control. All the injections were in a volume of 0.1 mL PBS buffer. Considering the stability of microRNAs and the tolerance of mice, the treatment was conducted for two subsequent days and repeated two times every 6 days. Tumor length and width were measured every day using a caliper. From these parameters, tumor volumes were calculated using the formula: tumor volume = (length × width2)/2.
Statistical analysis
Data are presented as mean ± SD and analyzed by GraphPad Prism software. Unpaired Student’s t test or one-way ANOVA with Dunnett’s multiple comparisons test were used for testing significance. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (8200104590 to Y.Z.). We thank Professor Xiansheng Lu for his help in linguistic revision.
Author contributions
Y.Z., T.Z., and X. Shen contributed equally to this work. N.S. and J.D. designed and supervised the project. Y.Z., T.Z., X. Shen, A.H., H.L., X.L., and J.D. performed the experiments. Y.Z., T.Z., X. Shen, A.H., H.L., L.W., X.L., J.D., X.W., X. Song, S.W., and N.S. interpreted and/or reviewed the data. X.W., X. Song, and S.W. performed the binding simulation of TNF-α and microRNA. Y.Z., J.D., and N.S. wrote or edited the manuscript. All the coauthors reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.06.017.
Contributor Information
Jie Dong, Email: dongjie@bmi.ac.cn.
Ningsheng Shao, Email: shaoningsheng@bmi.ac.cn.
Supplemental information
References
- 1.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 2.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;23:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Bumcrot D., Manoharan M., Koteliansky V., Sah D.W. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X. The growth of siRNA-based therapeutics: updated clinical studies. Biochem. Pharmachol. 2021;189:114432. doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Wang Z., Gemeinhart R.A. Progress in microRNA delivery. J. Control. Release. 2013;28:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng Y., Xiao H., Zhang J., Liang X.J., Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019;37:801–825. doi: 10.1016/j.biotechadv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crooke S.T., Witztum J.L., Bennett C.F., Baker B.F. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol. Ther. Nucleic Acids. 2017;17:116–132. doi: 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo J.D., Chevillet J.R., Kroh E.M., Ruf I.K., Pritchard C.C., Gibson D.F., Mitchell P.S., Bennett C.F., Pogosova-Agadjanyan E.L., Stirewalt D.L., et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Old L.J. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 13.Sethi G., Sung B., Aggarwal B.B. TNF: a master switch for inflammation to cancer. Front. Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 14.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 15.Cao G., Yang G., Liu Z., Liu X., Zhang J., Zhang D., Liu N., Ding H., Fan M., Shen B., et al. Identification of the RNA chaperone activity of recombinant human tumor necrosis factor alpha in vitro. Biochem. Biophys. Res. Commun. 2005;328:573–579. doi: 10.1016/j.bbrc.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Shen X., Li J., Huang A., Wang C., Sun L., Li S., Ding H., Li H., Guo X., Zheng W., et al. Novel biological characteristics of hTNF-α as miR-146b binding protein. Lett. Biotech. (In Chinese) 2006;27:183–187. [Google Scholar]
- 17.Sheedy F.J., O'Neill L.A. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann. Rheum. Dis. 2008;67:iii50–iii55. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 18.Gaudet A.D., Fonken L.K., Watkins L.R., Nelson R.J., Popovich P.G. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist. 2018;24:221–245. doi: 10.1177/1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Testa U., Pelosi E., Castelli G., Labbaye C. miR-146 and miR-155: two key modulators of immune response and tumor development. Noncoding RNA. 2017;26:22. doi: 10.3390/ncrna3030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S. Recent findings regarding let-7 in immunity. Cancer Lett. 2018;10:130–131. doi: 10.1016/j.canlet.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Huang S.Y., Zou X. A knowledge-based scoring function for protein-RNA interactions derived from a statistical mechanics-based iterative method. Nucleic Acids Res. 2014;42:e55. doi: 10.1093/nar/gku077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehnal D., Bittrich S., Deshpande M., Svobodová R., Berka K., Bazgier V., Velankar S., Burley S.K., Koča J., Rose A.S. Mol∗ Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021;49:W431–W437. doi: 10.1093/nar/gkab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q., Hou M., Li L., Ren L., Qiu M., Yang Y., Huang W., Chen Z., Meng Z., Song M., et al. A multicenter randomized phase II trial of domestic product of nrhTNF in the treatment of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi (in Chinese) 2003;20:42–45. doi: 10.3779/j.issn.1009-3419.2003.01.11. [DOI] [PubMed] [Google Scholar]
- 24.Van Ostade X., Tavernier J., Prangé T., Fiers W. Localization of the active site of human tumour necrosis factor (hTNF) by mutational analysis. EMBO J. 1991;10:827–836. doi: 10.1002/j.1460-2075.1991.tb08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnell G., Loo Y.M., Marcotrigiano J., Gale M., Jr. Uridine composition of the poly-U/UC tract of HCV RNA defines non-self recognition by RIG-I. PLoS Pathog. 2012;8:e1002839. doi: 10.1371/journal.ppat.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer R., Maier O., Naumer M., Krippner-Heidenreich A., Scheurich P., Pfizenmaier K. Ligand-induced internalization of TNF receptor 2 mediated by a di-leucin motif is dispensable for activation of the NFκB pathway. Cell Signal. 2011;23:161–170. doi: 10.1016/j.cellsig.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Yu B., Zhao X., Lee L.J., Lee R.J. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986;320:584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 29.Mease P.J. Adalimumab: an anti-TNF agent for the treatment of psoriatic arthritis. Expert Opin. Biol. Ther. 2005;5:1491–1504. doi: 10.1517/14712598.5.11.1491. [DOI] [PubMed] [Google Scholar]
- 30.Hwang W.L., Jiang J.K., Yang S.H., Huang T.S., Lan H.Y., Teng H.W., Yang C.Y., Tsai Y.P., Lin C.H., Wang H.W., et al. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat. Cell Biol. 2014;16:268–280. doi: 10.1038/ncb2910. [DOI] [PubMed] [Google Scholar]
- 31.Wu H., Fan H., Shou Z., Xu M., Chen Q., Ai C., Dong Y., Liu Y., Nan Z., et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Han H.B., Gu J., Zuo H.J., Chen Z.G., Zhao W., Li M., Ji D.B., Lu Y.Y., Zhang Z.Q. Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J. Pathol. 2012;226:544–555. doi: 10.1002/path.3014. [DOI] [PubMed] [Google Scholar]
- 33.Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E.E., Nitsch R., Wulczyn F.G. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 34.King C.E., Wang L., Winograd R., Madison B.B., Mongroo P.S., Johnstone C.N., Rustgi A.K. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and-independent mechanisms. Oncogene. 2011;30:4185–4193. doi: 10.1038/onc.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boon R.A., Vickers K.C. Intercellular transport of microRNAs. Arterioscler Thromb. Vasc. Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson A.L., Bartz S.R., Schelter J., Kobayashi S.V., Burchard J., Mao M., Li B., Cavet G., Linsley P.S. Expression of profiling reveals off-target gene regulation by RNAi. Nat. Biotech. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 37.Koller E., Vincent T.M., Chappell A., De S., Manoharan M., Bennett C.F. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hypocytes. Nucleic Acids Res. 2011;39:4795–4807. doi: 10.1093/nar/gkr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts J.K., Corey D.R. Silencing disease genes in the laborarory and the clinic. J. Pahto. 2012;226:365–379. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu D., Pendergraff H., Liu J., Kordasiewicz H.B., Cleveland D.W., Swayze E.E., Lima W.F., Crooke S.T., Prakash T.P., Corey D.R. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant huntingtin expression. Cell. 2012;150:895–908. doi: 10.1016/j.cell.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima W.F., Prakash T.P., Murray H.M., Kinberger G.A., Li W., Chappell A.E., Li C.S., Murray S.F., Gaus H., Seth P.P., et al. Single-stranded siRNAs activate RNAi in animals. Cell. 2012;150:883–894. doi: 10.1016/j.cell.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Matsui M., Prakash T.P., Corey D.R. Argonaute-2 dependent regulation of gene expression by single-stranded miRNA mimics. Mol. Ther. 2016;24:946–955. doi: 10.1038/mt.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chorn G., Klein-McDowell M., Zhao L., Saunders M.A., Flanagan W.M., Willingham A.T., Lim L.P. Single-stranded microRNA RNA mimics. RNA. 2012;18:1796–1804. doi: 10.1261/rna.031278.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith R.A., Baglioni C. The active form of tumor necrosis factor is a trimer. J. Biol. Chem. 1987;262:6951–6954. [PubMed] [Google Scholar]
- 44.Beyaert R., Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity. What we do understand and what we do not. FEBS Lett. 1994;28:9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- 45.Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;22:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 46.Vilcek J., Lee T.H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991;25:7313–7316. [PubMed] [Google Scholar]
- 47.Abdelaal A.M., Kasinski A.L. Ligand-mediated delivery of RNAi-based therapeutics for the treatment of oncological diseases. NAR Cancer. 2021;3:zcab030. doi: 10.1093/narcan/zcab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orellana E.A., Abdelaal A.M., Rangasamy L., Tenneti S., Myoung S., Low P.S., Kasinski A.L. Enhancing MicroRNA activity through increased endosomal release mediated by Nigericin. Mol. Ther. -Nucleic Acids. 2019;16:505–518. doi: 10.1016/j.omtn.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugo T., Terada M., Oikawa T., Miyata K., Nishimura S., Kenjo E., Ogasawara-Shimizu M., Makita Y., Imaichi S., Murata S., et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J. Control. Release. 2016;237:1–13. doi: 10.1016/j.jconrel.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 50.Gangopadhyay S., Nikam R.R., Gore K.R. Folate receptor-mediated siRNA delivery: recent developments and future directions for RNAi therapeutics. Nucleic Acid Ther. 2021;31:245–270. doi: 10.1089/nat.2020.0882. [DOI] [PubMed] [Google Scholar]
- 51.Mocellin S., Nitti D. TNF and cancer: the two sides of the coin. Front. Biosci. 2008;13:2774–2783. doi: 10.2741/2884. [DOI] [PubMed] [Google Scholar]
- 52.Bertazza L., Mocellin S. The dual role of tumor necrosis factor (TNF) in cancer biology. Curr. Med. Chem. 2010;17:3337–3352. doi: 10.2174/092986710793176339. [DOI] [PubMed] [Google Scholar]
- 53.Zhao P., Zhang Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018;15:3820–3827. doi: 10.3892/ol.2018.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Creemers E.E., Tijsen A.J., Pinto Y.M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 55.Redis R.S., Calin S., Yang Y., You M.J., Calin G.A. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol. Ther. 2012;136:169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoerter J.A., Walter N.G. Chemical modification resolves the asymmetry of siRNA strand degradation in human blood serum. RNA. 2007;13:1887–1893. doi: 10.1261/rna.602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.