Abstract
Objective
To identify attributes targeted by rehabilitative treatment within which improvements lead to short- and long-term changes in mobility. Maintaining independence in mobility is important to many older adults and is associated with critical outcomes such as aging in place, morbidity, and mortality.
Design
The Live Long Walk Strong rehabilitation study is a phase 2 single-blind, randomized controlled trial.
Setting
Veterans Affairs Boston Healthcare System, outpatient physical therapy.
Participants
198 community-dwelling middle- and older-aged veterans (aged 50 years and older) will be recruited from primary care practices (N=198).
Interventions
Comparing a moderate-vigorous intensity physical therapy program of 10 sessions with a waitlist control group.
Main Outcome Measure
The primary outcome measure is gait speed. Secondary outcomes include leg strength and power, trunk muscle endurance, gait smoothness, and exercise self-efficacy.
Results
Outcomes will be assessed within 2 weeks of intervention completion, at 8 weeks postintervention, and at 16 weeks postintervention. Two-sample t tests will compare mean change in gait speed and target attributes (leg power, trunk muscle endurance, gait smoothness, and exercise self-efficacy) between treatment and control groups. Paired t tests will examine within-person change at subsequent follow-up visits. Multivariable regression analyses will evaluate relationships between dependent and independent variables and potential mediation adjusting for relevant covariates.
Conclusions
Results of this study are expected to advance and refine the design of Live Long Walk Strong rehabilitative care and demonstrate its proof of concept and efficacy.
KEYWORDS: Gait speed, Middle aged, Older aged, Physical therapy, Rehabilitation, Resistance training, Veterans
Mobility limitations, considered as poor performance on tasks such as walking, rising from a chair, and climbing stairs, affect as many as 1 in 5 community-dwelling older adults.1 For example, slow walking speed among middle- and older-aged adults is a recognized marker for subsequent development of disability, morbidity, and mortality.2, 3, 4 Notably, this association between walking speed and poor outcomes is independent of disease status.3,5 It is estimated that by 2040 the annual health care costs attributed to slow gait speed and associated health conditions will be $42 billion.6 Therefore, it is not surprising that screening for mobility among vulnerable populations is highly advocated.7
One such population is military veterans because they are recognized to demonstrate greater levels of impairment and limitation in mobility when compared to age matched civilians.8 There are 18 million veterans in the United States; within this group the median age is 65 years, with the largest cohort serving during the Vietnam era9; about one-third receive care in the Veterans Health Administration and others receive care in the private sector.10
Though the prescription of physical therapy (PT) care is the established treatment for slow gait speed, no standardized evidence-based treatment approach exists.11, 12, 13 The Live Long Walk Strong (LLWS) rehabilitation program is an innovative model of PT care that has demonstrated preliminary efficacy among civilians, producing robust improvements in functional mobility, including gait speed, in a clinical innovation project.14 There are 3 key features of LLWS care: (1) targeting a subset of physiological impairments linked to mobility decline,15 (2) administration of specific motivation and behavior change strategies, and (3) positioning the physical therapist to function as a case manager assisting with care management postdischarge. The benefits of LLWS have yet to be tested among veterans. Though the presumption that targeting each of the first 2 innovative features of care, targeting impairments associated with mobility decline and directing a behavior change protocol, will mediate both short- and long-term improvements in function, the efficacy of these aspects of LLWS care remains to be seen.
Aims
To advance this line of scientific inquiry and address these knowledge gaps we are conducting a randomized controlled trial of LLWS among middle-aged and older veterans. It will address 3 aims: (1) to examine the efficacy of 8 weeks (10 visits) of LLWS treatment, compared to waitlisted controls, on the changes in gait speed as the primary outcome; (2) to evaluate which attributes (leg power, trunk muscle endurance, walking smoothness or exercise self-efficacy) are associated with improved gait speed after 8 weeks of LLWS treatment; and (3) to identify the attributes that lead to sustained improvements in gait speed 16 weeks after LLWS treatment ends.
Trial Design
This is a phase II study that will establish the proof of concept for LLWS among veterans and clarify treatment targets that collectively facilitate changes in gait speed.16 This study aims to demonstrate conceptual proof that by targeting the aforementioned body system impairments and behaviors that both short- and long-term gait speed improvements will occur.16 In recognition of these priorities, we decided not to have an active treatment to serve as a control group. It is vital to understand the benefit of LLWS relative to treatment as usual. Because measurement of gait speed is not yet a vital sign within standard care, slow gait speed is not viewed as a standardized indication for rehabilitation care. Currently, “usual care” for most middle-age and older adults who demonstrate slow gait speed is no treatment.
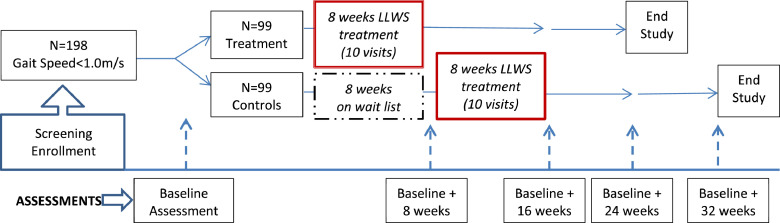
A total of 198 community-dwelling veterans 50 years and older from the Veterans Affairs (VA) Boston Healthcare System who demonstrate gait speed between 0.5 and 1.0 m/s will be randomized to either begin LLWS treatment immediately or serve as waitlist controls for 8 weeks. After 8 weeks, those in the control group will begin LLWS treatment and continue to be followed for 16 weeks after treatment (Figure 1). Therefore, aim 1 is designed to evaluate comparisons between treatment and controls and aims 2 and 3 will use combined data from both groups to identity attributes that mediate changes in gait speed at their corresponding time points. After baseline assessment and randomization, those randomized to begin treatment will start within 2 weeks. After the treatment, all participants will be assessed within 2 weeks of completion and subsequently at 8 and 16 weeks posttreatment. This study has been registered at ClinicalTrials.gov (NCT04026503) and has been approved by the internal review board at VA Boston Healthcare System.
Fig 1.
Study schema: data will be collected at baseline, 8 weeks (when LLWS treatment ends), and then 8 and 16 weeks after treatment ends. The waitlist control will not receive treatment for 8 weeks, after which they will start LLWS and will be treated and assessed for a similar duration frequency as the treatment group.
Methods
Eligibility criteria
The eligibility criteria in this study are aimed at identifying veterans who are at risk for adverse health outcomes by the manifestation of slow gait speed. Eligibility is established through a multistep process involving telephone screening with items that are indicative of mobility limitations and in-person assessments. All participants will complete an informed consent prior to initiating study activities. Please see Table 1 for full details.
Table 1.
Study inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| Age ≥50 years old | Presence of a terminal disease (eg, receiving hospice services) |
| Community dwelling | Major medical problem, unstable chronic disease, or psychiatric disorder interfering with safe and successful testing and training (ie, use of supplemental oxygen, current substance abuse, symptomatic orthostatic hypotension, schizophrenia) |
| Ability to speak and understand English | Myocardial infarction or major surgery in previous 3 months |
| Planned major surgery (eg, joint replacement) within upcoming year | |
| Usual gait speed 0.5-1.0 m/s | Baseline Short Physical Performance Battery Score <4 |
| Use of a walker | |
| Mini-Montreal Cognitive Assessment <10 | |
| Presence of significant disease specific impairment, such as patients who have peripheral neurologic impairment (ie, significant limb spasticity ≥3/5 on modified Ashworth Scale or rigidity ≥2/4 rigidity), orthopedic impairment (significant loss or restricted motion in major joint; ie, amputee, contracture, severe osteoarthritis), visual impairment | |
Temporary exclusion criteria
Participants may receive medical clearance from their primary care physician to participate in the study. Participants would be re-screened for eligibility. Exclusion criteria were myocardial infarction or major surgery within the past 3 months, planned major surgery within the next year, and/or uncontrolled hypertension.
Intervention
The LLWS rehabilitation treatment is composed of 10 one-on-one sessions with a licensed physical therapist focusing on 4 main attributes—timing and coordination of gait, leg power, trunk endurance strength, and behavior change—to encourage exercise adoption. There are 2 sessions in weeks 1 and 2 and then 1 session per week for weeks 3 through 8. The exercise portion of the treatment combines a brief warm-up, stepping and walking patterns, strength and power training with a weighted vest, flexibility exercises, a cooldown, and a behavioral change lesson. The in-person treatment sessions begin with a walking warm-up period at a lighter intensity and self-selected pace. The stepping and walking patterns follow and will be completed continuously over 10-12 minutes at each session. The stepping and walking patterns are progressed first based on speed, amplitude, and accuracy and then progressed based on complexity as the participant's skill improves. The goal with stepping patterns is to have a participant smoothly complete 10 repetitions of a step with little effort before progressing to more complex patterns. Progression of walking patterns should be considered when a participant can maintain a consistent speed throughout the task.
Strength and power training exercises are performed each session for 20-25 minutes at a moderate to vigorous intensity as assessed by a modified rate Borg Rating of Perceived Exertion Scale, which has a range from 0 (no exertion) to 10 (maximal exertion).17 Participants are asked to maintain an intensity of moderate (5-6) to vigorous (7-8) throughout the session. Weight is added to the vest when the participant rates the intensity less than 5 or rates intensity less than 7 for 2 consecutive sessions. Participants are discouraged from exercising at levels that are considered light (<5) or higher than vigorous (9-10). The strengthening exercises will be performed in sets of 8-12 repetitions progressing from 1-2 sets as tolerated, without increase in pain to the participant, to achieve the desired training effort of moderate to vigorous intensity. Static trunk endurance exercises are held for 10 to 40 seconds and are progressed in increments of 10 seconds. The sets are progressed on participant tolerance. Table 2 provides the exercise menu options. The physical therapist selects exercises based on the participant's capabilities, tolerance, and preferences with the goal of completing 6-7 exercises focusing on leg power and trunk endurance strength. The goal of the strength training portion of the treatment session is to maintain a moderate to vigorous level of training with repetitions, sets, and weight progressed to maintain this level. Participants are encouraged to perform their exercises from their treatment sessions prescribed by the physical therapist at home to achieve a goal of exercising 3 days per week.
Table 2.
Exercise menu
| Walking Patterns | |
|---|---|
| Wide to narrow ovals | |
| Spirals | |
| Serpentine | |
| Nine cone sequences | |
| Stepping Patterns | |
| Weight shifting | |
| Forward stepping | |
| Backward stepping | |
| Out-out-in-in | |
| Across forward | |
| Backward to forward stepping | |
| Leg Strength and Power | |
| Two-legged squat | |
| Chair rise | |
| Step ups | |
| Stair climb | |
| Side lunges | |
| Two-legged heel raise | |
| Trunk Endurance | |
| Planks | |
| Modified bird-dog | |
| Triceps press with bridging | |
| Hip flexion-abduction-extension |
The intervention treatment includes a component of behavior change led by the physical therapist. Participants undergo 10 sessions in conjunction with their exercise training that incorporate cognitive and behavioral education, address barriers and facilitators to exercise, and engage the participant in goal setting using the SMART (specific, measurable, actionable, realistic and time-oriented) goal framework.18 An interactive protocol was developed that expanded on each feature of SMART goal setting and reviewed previously covered information to build upon the goal setting structure.19 In addition to goal setting, multiple sessions addressed barriers to exercise, which included motivational interviewing techniques to aid the participant in ideas on how to address the challenge. The lessons were written at a sixth-grade reading level and are meant to be tailored to each participant. There is flexibility to spend time addressing appropriate and realistic barriers to make the treatment patient centered and not content centered.
Adherence
Treatment sessions will be scheduled at mutually agreeable days and times between the participant and physical therapist. For interruptions to the treatment schedule due to illness or inability to keep their scheduled appointment, participants may make up 2 sessions within a 2-week period. Treatment may be suspended or terminated early due to hospitalization, injury, or other serious health event. Restarting the treatment depends on the effect of the illness or event at the discretion of the participant's medical team and the study physicians. Attendance rate will be calculated (number of sessions attended/number of sessions offered × 100) for each participant and used as an indicator of adherence.
Rationale for the intervention components
There are several investigations that link the attributes targeted within LLWS to mobility skills in general and to gait speed specifically. Also, there are clinical trials that provide evidence showing that improvements in these attributes can lead to both short-term and long-term improvements in mobility. These collective studies listed in Table 3 provide a rationale for the components of LLWS care. Empiric evidence that LLWS improves all of these attributes has yet to be established and is a focus of this study's aims.
Table 3.
Rationale for LLWS treatment
| Targeted Attribute | Outcomes of Study | Associative Studies | Treatment Studies |
|---|---|---|---|
| Leg power | Mobility measures | Kuo et al36 | Bean et al38 |
| Bean et al37 | Bean et al28 | ||
| Ward et al15 | |||
| Trunk muscle endurance | Mobility measures | Makris et al39 | Suri et al41 |
| Ward et al15 | |||
| Jacob et al40 | |||
| Timing and Coordination of gait | Gait speed | Brach et al42 | Brach et al29 |
| Brach et al29 | Collins et al43 | ||
| Self-Efficacy | Mobility measures | McAuley et al44 Bean et al45 | Chang et al35 |
Outcome measures
All study measures will be obtained by trained staff blinded to randomization assignment. A brief description of the main study outcomes is listed below.
Primary
Four-m Gait Speed
The primary outcome in LLWS is change in usual gait speed. At each assessment visit, time to complete a 4-m walking course is measured. Participants will walk the course from a standing start. The faster of 2 trials will be recorded. This test has demonstrated reliability and validity among older adults.20
Secondary
Leg power, strength, and velocity
Each characteristic will be measured separately for each leg on a leg press machine.a Leg strength will be measured by determining a 1 repetition maximum (1-RM). Leg power will be performed at 40% and 70% of 1-RM. The highest value will be recorded. Peak leg velocity will be derived from power recorded at 40% of 1-RM (velocity=power/0.4 (1-RM)). 1-RM is a reliable and valid measurement of strength.21
Trunk extensor muscle endurance
The measure of trunk extensor muscle endurance is done while the participant is lying prone on a specialized plinth positioned at 45 degrees from vertical. The participant's feet are supported in a fixed position on a footplate. A portion of the table is hinged and positioned at waist level. The participant is asked to maintain their unsupported position for up to 150 seconds. The test is terminated when the participant can no longer maintain their initial position or after 150 seconds. Trunk muscle endurance measures have been previously validated by McGill et al and have shown excellent reliability.22
Gait smoothness
This measure is assessed by capturing walking passes on a gait mat.b Gait is quantified using established measures of temporal and spatial gait characteristics including stance time, step length, and step width. Variability is calculated as the standard deviation of the set of steps recorded on the instrumented walkway. Assessment of gait variability has been shown to be valid and reliable in older adults.23
Changes in exercise self-efficacy
The Self-Efficacy Scale for Exercise is a reliable and valid scale to measure an individual's confidence to engage in exercise.24
Tertiary
Self-reported mobility
This measure is assessed by using the Activity Measure for Post-Acute Care Basic Mobility Outpatient short form and has achieved acceptable psychometric properties.25
Functional power
The modified stair climb test is an important clinical measure of lower extremity power and has shown excellent reliability.26,27
Falls
Falls are being assessed through monthly calendars for 16 weeks of follow-up postintervention. Participants are asked to return monthly calendars via prepaid postage stamped envelopes. If a calendar is not received within 10 days after the start of a new month, phone call follow-up is completed by study staff.
Health care utilization
Monthly follow-up to assess emergency department visits and hospitalizations will be completed for 16 weeks postintervention utilizing the same self-report format as for fall assessment.
Sample size justification
All power estimates were based on N=160 (n=80 per treatment group). We estimate that a total study size of 198 (n=99 per treatment group) will allow for an expected attrition rate of ∼20% and provide adequate power to detect the expected effects sizes based upon our previously reported work.28,29
Recruitment
The goal of the study is to randomly assign 198 veterans to treatment or control over a 2.5-year time frame. An equal number of participants will be recruited into 3 age groups, 50-65 years, 65-75 years, and over 75 years of age, with at least 12% of the total being female veterans. (Twelve percent was chosen as an effort to oversample and overrepresent women in these age groups receiving VA care, so that we were adequately powered to for subsample analyses to explore the influence of sex within secondary analyses.) Recruitment is also designed to ensure a broad distribution of physical functioning with ≥40% of participants manifesting a gait speed less <0.8 m/s.
Assignment of intervention
Participants will be randomly assigned either to receive LLWS for 8 weeks and followed for 16 subsequent weeks or to an 8-week waitlist control group that will subsequently initiate 8 weeks of LLWS treatment after completing their time on the waitlist. For these participants, long-term follow-up will be sequenced identically to the non-waitlist group. Using a computer-generated block randomization scheme, based on sex, age, and gait speed status, enrolled participants will be blindly assigned to treatment groups. Group allocation will be provided to participants in sealed envelopes by the study biostatistician at completion of the baseline assessment. The protocol and resultant participant education are provided to help ensure blinding of the assessors to group assignment.
Data collection methods
Staff performing screening and assessments will attend trainings and demonstrate proficiency for all assessments. Screening and assessment visit spot checks will be conducted to ensure and review protocol adherence and overall study adherence. Cognitive assessments will be audio-recorded to review adherence to consistent assessment instruction and reliable assessment scoring. Additional training for staff will be conducted as required from these reviews as signs of drift from test directions and performance are noted.
Treatment of study participants will be monitored monthly by the research coordinator for the first quarter year and then every 3-6 months thereafter. If retraining is required, it will be provided and more frequent monitoring will occur. The research physical therapist will track the content of each treatment session and variation will be monitored weekly to ensure appropriate tolerance, engagement, intensity, and progression. Participation in home exercise activities will also be tracked and recorded weekly by the LLWS physical therapist by characterizing exercise type, duration, frequency, intensity, and any complications that may have occurred. These training logs will be reviewed during the weekly staff meetings to foster treatment fidelity.
Data Analysis
All primary analyses will be based on an intention-to-treat approach. Two-sample t tests will compare mean values in changes in gait speed as the primary outcome and target attributes (leg power, trunk muscle endurance, smoothness of gait, and exercise self-efficacy) as the secondary outcomes between treatment and control groups. Paired t tests will examine within-person change at subsequent follow-up visits. Multiple regression models will assess relationships between dependent and independent variables and causal mediation models will be used for mediation analysis. Mixed effects regression models will be used to analyze repeated measures. In regression and mediation models, we will examine and adjust for potential confounding factors (by design or as covariates), including baseline scores (as appropriate), age, sex, body mass index, comorbidity score, and so on. Additional analysis will examine factors associated with non-adherence to protocol and consider per protocol analyses (ie, only those subjects with strict protocol adherence). Hypotheses tests will be performed at α=.05. Analyses will be performed using SAS software v9.3.c
Safety/Harms
During the screening phase, potential participants will be/are monitored closely during study assessments and those at high risk for complications of exercise training, based on medical history, are reviewed by the study physician and safety officer. During the treatment sessions, participants are monitored and supervised by the physical therapist and coached on safety during their home exercise sessions. The study monitors adverse events and assesses their potential relationship to the intervention. All adverse events are reported to the institutional review board.
Conceptual underpinnings of this study's design
This study's design is informed by 2 important conceptual models guiding rehabilitation research: (1) the International Classification of Function and Disability (ICF)30 and (2) advocated approaches to rehabilitation research as defined by treatment theory.16 The relevance of these conceptual models to this study requires some brief elaboration. The ICF, which is advocated by the World Health Organization, defines 3 hierarchical levels of functioning around which disability is defined. It defines body systems that when deficient are impaired, activities such as walking speed that when deficient are limited, and participation in life roles that when deficient are restricted. Impairments, limitations, and restrictions are all forms of disability according to the ICF framework. The interrelationships between these 3 levels of functioning are influenced by the effect of disease (ie, comorbidity), personal factors (ie, self-efficacy), and environmental factors. Applying the ICF model can help address how the relative contributions on one level (body systems) enable changes on higher level, which for this study corresponds to walking activity defined as walking speed. These interrelationships have been characterized by the investigation of enablement theory. In contrast, treatment theory focuses on the degree to which a specific treatment improves the desired target at a specific level of the ICF. Thus, our study will evaluate whether LLWS improves body system impairments in leg power, trunk muscle endurance, timing, and coordination of gait and a personal factor (self-efficacy). This corresponds to elements of our design based on treatment theory. Our study will also evaluate the degree to which improvement in these attributes enable both immediate- and long-term improvements in walking speed. This corresponds to elements of our design based upon enablement theory.
The effect of the COVID-19 pandemic
Originally, we intended to begin recruitment for this clinical trial during the first quarter of 2020. However, all clinical research activities at our medical center were stopped in March 2020 in response to the pandemic. Reopening of clinical research activities was done in a staged process. As of December 7, 2020, we were able to reinitiate recruitment of participants for a modified version of our study methods. This included face-to-face assessments but with the treatment being delivered virtually via the VA telehealth platform known as VA Video Connect.d A total of n=12 participants were enrolled in the study using this hybrid approach to our methods. As of June 21, 2021, recruitment resumed with enrolled participants undergoing face-to-face assessments and treatment sessions. However, as of December 27, 2021, due to the Omicron variant surge, all clinical research activities were again put on a temporary hold, resulting in a loss of participants actively enrolled in the study. Strategies for dealing with these challenges are discussed below.
As highlighted in the Methods, the COVID-19 pandemic has posed significant methodological challenges to the successful conduct of our study. It is our intention to resume study operations going forward as originally planned, utilizing only face-to-face treatment sessions and assessments to optimize the integrity of our methods. Because we will have participants who received treatment via a telehealth platform, we will perform sensitivity analyses both excluding and including those who underwent hybrid treatment. Also, we anticipate that we may experience a higher dropout rate than originally proposed due to the pandemic. We will continue to monitor dropouts and may expand recruitment to ensure that we have sufficient statistical power to test our hypotheses. All final decisions regarding our analysis and recruitment plans will be done in consultation with our study biostatistician. We know that there are many clinical trials that are facing similar challenges, and we intend to employ the most scientifically appropriate approaches to ensuring the integrity of our study's findings.7,31
Discussion
Several prior studies have demonstrated the beneficial effect of designed exercise programs on functional outcomes in older adults but have not been implemented in clinical settings because of challenges with feasibility and reimbursement.32,33 LLWS care is unique because it is grounded within a context of a of care (outpatient physical therapy) that is both feasible and reimbursable.
This study is both innovative and significant for several reasons. Firstly, it aims to identify whether LLWS successfully targets both physiological impairments and behavioral factors linked to mobility decline. Though specific treatments are known to be efficacious at improving these attributes individually, LLWS is the first treatment designed to target them collectively as a means of improving gait speed. Secondly, in standard outpatient mobility rehabilitation for older patients, short-term gains are commonly not sustained because patients have difficulty adopting new exercise behaviors. The LLWS program incorporates a cognitive behavioral strategy within PT care that is based on Bandura's social cognitive theory targeting the promotion of self-efficacy.34,35 LLWS is unique in its efforts to combine these different evidence-based elements into a single treatment. Lastly, an important aspect of our design is the stratification of participants based on important factors such as age, sex, and baseline level of function. Not only does this stratification allow for a more representative cohort but it will also allow for exploratory analyses observing differences in responsiveness based upon baseline differences.
Limitations
The COVID-19 pandemic has posed significant methodological challenges to the successful conduct of our clinical trial, as well as many others. Because we will have participants who received treatment via a telehealth platform, sensitivity analyses both excluding and including those who underwent hybrid treatment will be considered. Also, we anticipate that we may experience a higher dropout rate than originally proposed due to the pandemic. We will work with our study team and utilize the best scientific approach to handle these challenges when investigating our findings.
Conclusions
The findings from this trial will advance and refine the design of LLWS rehabilitative care and demonstrate its proof of concept and efficacy. This will support the rationale for dissemination and future development of a larger scale multicenter trial evaluating the comparative effectiveness of LLWS on other important health outcomes such as fall injury, disability, and health care costs.
Suppliers
a. Keiser Pneumatic Leg Press, Keiser Corporation. b. Zeno Walkway Gait Analysis System, ProtoKinetics LLC. c. SAS Software v9.3, SAS Institute. d. VA Video Connect, VA Mobile Health VHA Office of Connected Care.
Footnotes
List of abbreviations: ICF, International Classification of Function and Disability; LLWS, Live Long Walk Strong; 1-RM, 1 repetition maximum; PT, physical therapy; SMART, specific, measurable, actionable, realistic, time-oriented; VA, Veterans Affairs.
This work was supported in part by the following grant from the US Department of Veterans Affairs Rehabilitation Research and Development Service: Merit Award # 5I01RX003095-03 (J.B.). Research reported in this publication was also supported by the National Institute On Aging of the National Institutes of Health under Award Number K24AG069176 (JB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial registration No.: NCT04026503.
Disclosures: None.
References
- 1.Chen Y, Sloan FA. Explaining disability trends in the US elderly and near-elderly population. Health Serv Res. 2015;50:1528–1549. doi: 10.1111/1475-6773.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande N, Metter EJ, Guralnik J, Bandinelli S, Ferrucci L. Predicting 3-year incident mobility disability in middle-aged and older adults using physical performance tests. Arch Phys Med Rehabil. 2013;94:994–997. doi: 10.1016/j.apmr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility-giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311:2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–546. doi: 10.1682/jrrd.2004.07.0087. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135–2136. doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 8.Selim AJ, Berlowitz D, Kazis LE, et al. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Serv Res. 2010;45:376–396. doi: 10.1111/j.1475-6773.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vespa JE. US Census Bureau; Washington DC: 2020. Those who served: America's veterans from World War II to the War on Terror. American Community Survey reports. [Google Scholar]
- 10.National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Available at: https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf. Accessed January 17, 2022.
- 11.Bean JF, Vora A, Frontera WR. The benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 13.Avin KG, Hanke TA, Kirk-Sanchez N, et al. Management of falls in community-dwelling older adults: clinical guidance statement from the Academy of Geriatric Physical Therapy of the American Physical Therapy Association. Phys Ther. 2015;95:815–834. doi: 10.2522/ptj.20140415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown LG, Ni M, Schmidt CT, Bean JF. Evaluation of an outpatient rehabilitative program to address mobility limitations among older adults. Am J Phys Med Rehab. 2017;96:600–606. doi: 10.1097/PHM.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward RE, Beauchamp MK, Latham NK, et al. Neuromuscular impairments contributing to persistently poor and declining lower-extremity mobility among older adults: new findings informing geriatric rehabilitation. Arch Phys Med Rehabil. 2016;97:1316–1322. doi: 10.1016/j.apmr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyte J, Barrett AM. Advancing the evidence base of rehabilitation treatments: a developmental approach. Arch Phys Med Rehabil. 2012;93(Suppl):S101–S110. doi: 10.1016/j.apmr.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath EM. Borg's Perceived Exertion and Pain Scales. Med Sci Sports Exerc. 1998;30:1461. [Google Scholar]
- 18.US Department of Veterans Affairs. How to set a SMART goal. Available at: https://www.va.gov/WHOLEHEALTHLIBRARY/tools/how-to-set-a-smart-goal.asp. Accessed August 1, 2019.
- 19.Bamonti PM, Moye J, Harris R, et al. The development of a coaching protocol to enhance self-efficacy within outpatient physical therapy. Arch Rehabil Res Clin Transl. 2022;4 doi: 10.1016/j.arrct.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rydwik E, Bergland A, Forsén L, Frändin K. Investigation into the reliability and validity of the measurement of elderly people's clinical walking speed: a systematic review. Physiother Theory Pract. 2012;28:238–256. doi: 10.3109/09593985.2011.601804. [DOI] [PubMed] [Google Scholar]
- 21.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 22.McGill SM, Childs A, Liebenson C. Endurance times for low back stabilization exercises: clinical targets for testing and training from a normal database. Arch Phys Med Rehabil. 1999;80:941–944. doi: 10.1016/s0003-9993(99)90087-4. [DOI] [PubMed] [Google Scholar]
- 23.Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise Scale. Nurs Res. 2000;49:154–159. doi: 10.1097/00006199-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Jette AM, Haley SM, Ni P, Moed R. Adaptive short forms for outpatient rehabilitation outcome assessment. Am J Phys Med Rehabil. 2008;87:842–852. doi: 10.1097/PHM.0b013e318186b7ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni M, Brown LG, Lawler D, Bean JF. Reliability, validity, and minimal detectable change of Four-Step Stair Climb Power Test in community-dwelling older adults. Phys Ther. 2017;97:767–773. doi: 10.1093/ptj/pzx039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Wilson R, Pryymachenko Y, et al. Reliability, validity, responsiveness, and minimum important change of the Stair Climb Test in adults with hip and knee osteoarthritis. Arthritis Care Res (Hoboken) 2021 doi: 10.1002/acr.24821. Nov 21. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Bean JF, Kiely DK, LaRose S, O'Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging's strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi: 10.1093/gerona/glp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brach JS, Lowry K, Perera S, et al. Improving motor control in walking: a randomized clinical trial in older adults with subclinical walking difficulty. Arch Phys Med Rehabil. 2015;96:388–394. doi: 10.1016/j.apmr.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. International classification of function, disability and health (ICF). Available at: https://www.who.int/classifications/international-classification-of-functioning-disability-and-health. Accessed June 15, 2019.
- 31.Sathian B, Asim M, Banerjee I, et al. Impact of COVID-19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol. 2020;10:878–887. doi: 10.3126/nje.v10i3.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 34.Bandura A. Worth Publishers; New York: 1997. Self-efficacy: the exercise of control. [Google Scholar]
- 35.Chang FH, Latham NK, Ni P, Jette AM. Does self-efficacy mediate functional change in older adults participating in an exercise program after hip fracture? A randomized controlled trial. Arch Phys Med Rehabil. 2015;96:1014–1020. doi: 10.1016/j.apmr.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo HK, Leveille SG, Yen CJ, et al. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am J Phys Med Rehabil. 2006;85:650–658. doi: 10.1097/01.phm.0000228527.34158.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bean JF, Leveille SG, Kiely DK, et al. Comparison of Leg Power and Leg Strength Within the InCHIANTI Study: Which Influences Mobility More? J Gerontol A. 2003;58:M728–M733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 38.Bean JF, Kiely DK, LaRose S, et al. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? Am Geriatr Soc. 2010;58:2362–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makris UE, Paul TM, Holt NE, et al. The Relationship Among Neuromuscular Impairments, Chronic Back Pain, and Mobility in Older Adults. PM&R. 2016;8:738–747. doi: 10.1016/j.pmrj.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob ME, Travison TG, Ward RE, et al. Neuromuscular Attributes Associated With Lower Extremity Mobility Among Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2019;74:544–549. doi: 10.1093/gerona/gly102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suri P, Kiely DK, Leveille SG, et al. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM&R. 2009;1:916–924. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brach JS, Studenski S, Perera S, et al. Stance time and step width variability have unique contributing impairments in older persons. Gait and Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins KJ, Schrak JA, VanSwearingen JM, et al. Randomized Controlled Trial of Exercise to Improve Walking Energetics in Older Adults. Innov Aging. 2018;2 doi: 10.1093/geroni/igy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAuley E, Jerome JG, Elavsky S, et al. Predicting long-term maintenance of physical activity in older adults. Prev Med. 2003;37:110–118. doi: 10.1016/s0091-7435(03)00089-6. [DOI] [PubMed] [Google Scholar]
- 45.Bean JF, Olveczky DD, Kiely DK, et al. Performance-based versus patient-reported physical function: what are the underlying predictors? Phys Ther. 2011;91:1804–1811. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]