Key Points
Question
What contributes to the success of improvement programs designed to reduce time to surgery (TTS) for adult patients with hip fractures?
Findings
In this systematic review of 69 studies, 49 programs were associated with significant decreases in TTS, and 20 programs were not. Common themes among successful improvement strategies (eg, identifying barriers and facilitators to program implementation) were cataloged according to Expert Recommendations for Implementing Change, and contextual factors contributing to failed experimental results were evaluated.
Meaning
These findings suggest that many of the assessed improvement programs show promise for reducing hip fracture TTS across different clinical settings in the near future.
This systematic review catalogs improvement programs designed to decrease time to surgery for hip fractures, identifies their results, and categorizes program strategies according to the Expert Recommendations for Implementing Change.
Abstract
Importance
Longer time to surgery (TTS) for hip fractures has been associated with higher rates of postoperative complications and mortality. Given that more than 300 000 adults are hospitalized for hip fractures in the United States each year, various improvement programs have been implemented to reduce TTS with variable results, attributed to contextual patient- and system-level factors.
Objective
To catalog TTS improvement programs, identify their results, and categorize program strategies according to Expert Recommendations for Implementing Change (ERIC), highlighting components of successful improvement programs within their associated contexts and seeking to guide health care systems in implementing programs designed to reduce TTS.
Evidence Review
A systematic review was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline. Three databases (MEDLINE/PubMed, EMBASE, and Cochrane Trials) were searched for studies published between 2000 and 2021 that reported on improvement programs for hip fracture TTS. Observational studies in high-income country settings, including patients with surgical, low-impact, nonpathological hip fractures aged 50 years or older, were considered for review. Improvement programs were assessed for their association with decreased TTS, and ERIC strategies were matched to improvement program components.
Findings
Preliminary literature searches yielded 1683 articles, of which 69 articles were included for final analysis. Among the 69 improvement programs, 49 were associated with significantly decreased TTS, and 20 programs did not report significant decreases in TTS. Among 49 successful improvement programs, the 5 most common ERIC strategies were (1) assess for readiness and identify barriers and facilitators, (2) develop a formal implementation blueprint, (3) identify and prepare champions, (4) promote network weaving, and (5) develop resource-sharing agreements.
Conclusions and Relevance
In this systematic review, certain components (eg, identifying barriers and facilitators to program implementation, developing a formal implementation blueprint, preparing intervention champions) are common among improvement programs that were associated with reducing TTS and may inform the approach of hospital systems developing similar programs. Other strategies had mixed results, suggesting local contextual factors (eg, operating room availability) may affect their success. To contextualize the success of a given improvement program across different clinical settings, subsequent investigation must elucidate the association between interventional success and facility-level factors influencing TTS, such as hospital census and type, teaching status, annual surgical volume, and other factors.
Introduction
Annually, more than 300 000 patients are diagnosed with new hip fractures in the United States, and more than 1.6 million patients present with hip fractures worldwide.1,2 The incidence of hip fractures is projected to more than double by 2050,1,3 and, given that the geriatric population in the United States is the fastest growing segment of the population, the most rapid growth in annual fracture rates and cost are expected for patients aged between 65 to 74 years.4 Hip fractures confer substantial economic burdens to patients and the health care system,4,5,6,7 with total annual direct medical costs associated with hip fractures approaching $5.96 billion in the United States.8,9 Given that hip fractures are also associated with significant rates of morbidity and mortality, optimizing hip fracture outcomes has, therefore, become a public health priority worldwide.
Among older patients with hip fracture, mortality in the first year following fracture surgery ranges from 15% to 36%, approximately 4 times higher than that of younger patients with hip fractures. The risk for surgical complications and mortality among patients with hip fracture is dependent on multiple factors, including scope and severity of injury, course of treatment, and patient characteristics.10,11,12 In particular, a longer time to surgery (TTS) has been identified as an independent risk factor for mortality and surgical complication.13,14,15 For example, an operative delay of more than 48 hours has been associated with a 41% increase in the odds of 30-day mortality and as much as 32% increased 1-year mortality.16 The impacts of delayed TTS on hip fracture surgical outcomes has therefore motivated work to identify drivers of TTS.17,18,19,20,21,22 These drivers include patient-level factors, including socioeconomic status and medication use (eg, anticoagulants), as well as facility-level factors, including hospital payment systems and operating room hours. In response, hospitals have implemented programs dedicated to modifying these factors, particularly at the facility-level scale, with the goal of optimizing TTS and thereby improving patient outcomes.
Across other sectors of medicine, the Expert Recommendations for Implementing Change (ERIC) framework has been used to address barriers to achieving a desired health services outcomes (eg, optimizing response to time-sensitive conditions, such as pulmonary embolism23 or acute coronary syndrome24) by mapping improvement program strategies. A Cochrane review25 found that such tailored improvement can address implementation barriers and improve professional practice and health care outcomes more effectively than untailored strategies. This systematic approach to classifying improvement strategies has not yet been applied to the management of hip fractures. The aims of our study were to (1) catalog improvement programs aimed at reducing TTS and (2) use the ERIC taxonomy to characterize the improvement strategies most commonly used in promising programs. This approach highlights successful improvement program components to provide strategic recommendations for future TTS improvement implementation.
Methods
Literature Search Strategy
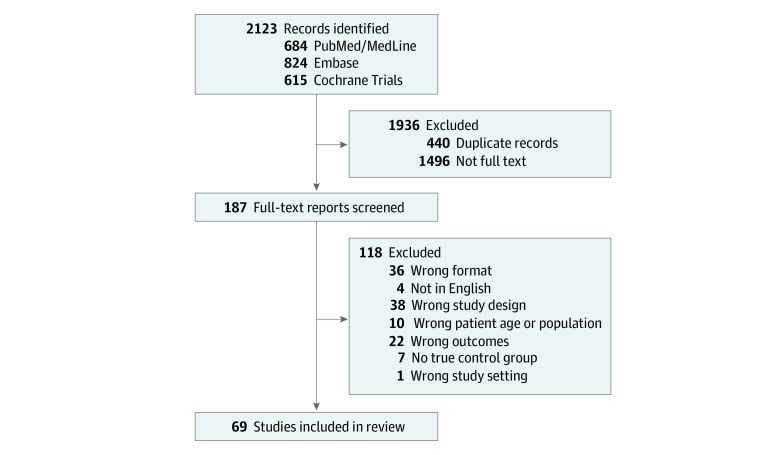
Our review used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methods and was registered with Prospero (CRD42021267021). Two authors (P.T. and J.L.L) conducted literature searches from peer-reviewed journals in 3 medical databases (MEDLINE/PubMed, EMBASE, and Cochrane Trials) to identify improvement programs for which TTS was an experimental outcome. Search strategies included terms such as hip fracture, time to treatment, surgery, and intervention (eAppendix 1 in the Supplement). Searches were performed from July 16 to August 13, 2021. Articles published before January 1, 2000, were excluded from screening. Then, search results were filtered according to predetermined inclusion and exclusion criteria (Figure). All eligible publications were uploaded to Covidence, a Cochrane-sanctioned collaborative tool for systematic review screening and data extraction. Duplicate studies were removed.
Figure. Flow Diagram for Study Selection.
Selection Criteria
This review considered studies that reported on systemwide improvement programs designed to reduce TTS for patients aged 50 years or older, sustaining nonpathological, low-energy hip fractures. Studies that included patients younger than 50 years were excluded. Eligible full-length texts were required to be published or fully translated in English and based in high-income country settings, as defined by the World Bank.26 In acknowledgment of wide discrepancies in global health care system resources, we sought to mitigate for confounding variables by selecting improvement programs with comparable high-income country health care settings to the United States. Furthermore, included studies were required to include control and/or baseline data for results on TTS to further elucidate the success of a given improvement program.
Through Covidence, the research team (P.T., B.F.S., and J.L.L.) conducted several stages of text review, consisting of abstract screening, full-text review, and data extraction prior to final article selection. Two researchers independently reviewed eligible texts, according to the predetermined selection criteria, and voted to include or exclude a given text. In the event of a voting conflict, a third reviewer independently resolved disagreements.
Data Extraction
For each eligible study, data on study design, years of data collection, sample size, improvement program components, results of analysis and variable-outcome associations were extracted onto an Excel spreadsheet and cross-checked for accuracy.
Quality Appraisal
The 1998 Downs and Black Quality Assessment Checklist27 was used to assess the methodological quality of both randomized and nonrandomized studies. This checklist comprises 27 questions across 5 domains: (1) reporting; (2) external validity; (3) internal validity, bias; (4) internal validity, confounding (selection bias); and (5) power. It assigns points to each study accordingly. Studies earning 20 or more points are considered having good individual methodological quality, while studies assigned 14 points or fewer are considered having poor methodological quality and high risk of bias.28
Improvement Program Classification
The ERIC project, introduced by Powell et al29 in 2012, maps implementation strategies across studies to mitigate contextual barriers to implementation.30 This method, used across other sectors of medicine, creates a standardized and potentially generalizable approach to improvement efforts.25 For example, Ebben et al23 used ERIC strategies to match improvement program components across studies and optimize emergency department response to time-sensitive conditions such as asthma, pulmonary embolism, triage, acute gastroenteritis, and foot and ankle issues. Similarly, Doucette et al24 used ERIC strategies to effectively match improvement program components and improve outcomes for acute coronary syndrome and myocardial injury.
To develop the taxonomy of strategies, extracted data were classified according to 73 ERIC strategies, organized by hierarchical clustering into 9 domain groupings.31 The improvement programs identified for our review were partitioned into program components and were independently classified by 2 coauthors (B.F.S. and P.T.) according to concise definitions obtained in literature for each of the 73 ERIC strategies.32 Any discrepancies between researchers’ ERIC classifications were discussed and resolved. Descriptive statistics were used to calculate the rate of specific ERIC strategies used among improvement programs that were associated with reduced TTS.
Results
Included Studies
A total of 1683 studies were eligible for abstract screening based on literature searches. 190 studies underwent full-text review, and 69 studies were selected for final analysis (Figure and eAppendix 3 in the Supplement).33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103 Among the final 69 improvement programs, 49 programs significantly decreased TTS33,35,38,41,43,44,45,46,47,48,49,51,52,53,54,56,57,59,60,61,62,65,66,67,68,69,70,71,72,74,75,76,79,80,81,82,85,86,87,88,91,94,96,97,99,101,103 and 20 programs39,40,42,50,55,58,63,64,73,77,78,83,89,90,92,93,95,98,100,102 did not. Delayed TTS cutoffs were most often defined dichotomously as more than 24 hours, more than 36 hours, more than 48 hours, or more than 72 hours, although some studies collected continuous data.
Quality Appraisal
Among the 69 total studies scored, no study earned a checklist score of lower than 20 points, indicating a relatively high-quality body of studies and low risk of bias.27 Each study collected and used patient medical records. TTS was also calculated according to hospital medical records and/or databases.
Determinants of Improvement Program Success
Among the 49 improvement programs associated with significant decreases in TTS, each program had a mean (SD) of 9 (4) strategies defined under ERIC. The 20 improvement programs that did not significantly decrease TTS had a mean (SD) of 8 (2) strategies, from which the 5 improvement programs that were associated with increases in TTS had a mean (SD) of 7 (2) strategies. Successful improvement programs most often had program components corresponding to 3 ERIC domains: (1) use of evaluative and iterative strategies (domain 1; 49/49 studies [100%]); (2) development of stakeholder interrelationships (domain 4; 35/49 studies [71%]); and (3) support of clinicians (domain 6; 33/49 studies [67%]) (Table 1). Specifically, the 5 most common ERIC strategies among improvement programs associated with statistically significant decreases in TTS included assessing for readiness and identifying barriers and facilitators (49 of 49 [100%]), developing a formal implementation blueprint (49 of 49 [100%]), identifying and preparing champions (35 of 49 [71%]), promoting network weaving (35 of 49 [71%]), and developing resource sharing agreements (33 of 49 [67%]) (Table 2). Table 3 lists the recurring ERIC strategy components among unsuccessful programs, and Table 4 presents limitations to those programs.
Table 1. Number of ERIC Domains Among Significantly Successful Improvement Programs.
| Domain title | ERIC domain No. | Occurrence, No. (%) (N = 49) | Example of strategy from literature (source) |
|---|---|---|---|
| Use of evaluative and iterative strategies | 1 | 49 (100) | Develop an implementation blueprint that summarizes the intervention purpose; scope of change; timeframe and intervention milestones; and defines measures of performance and success (Anderson et al,40 2017) |
| Provision of interactive assistance | 2 | 6 (12.2) | Implement electronic order sets (Anderson et al,40 2017) and e-pathways (Talevski et al,45 2020) |
| Adapt and tailor to context | 3 | 17 (34.7) | Prioritize surgery of older patients with hip fractures and tailor timing of procedures to within the first hours after admission (Sánchez-Hernández et al,47 2016) |
| Development of stakeholder interrelationships | 4 | 35 (71.4) | Implement combined multidisciplinary and comanagement systems (VanTienderen et al,75 2021) |
| Training and education of stakeholders | 5 | 3 (6.1) | Recruit integrated care managers to improve care path compliance and coordination of care (Heyzer et al,60 2021) |
| Support of clinicians | 6 | 33 (67.3) | Hire a designated lean manager in the orthopaedic department to assess processes involved with quality improvement project, including tracking new hip fracture patients (Sayeed et al,76 2018) |
| Engagement with consumers | 7 | 1 (2) | In the event that a patient was unable to give consent, delay surgery only when efforts to contact the immediate family failed (Kosy et al,65 2013) |
| Use of financial strategies | 8 | 6 (12.2) | Incentivize meeting a 24-hour, 48-hour, or other target time for surgical fixation of hip fractures via a reimbursement system (Uri et al,46 2020) |
| Change of infrastructure | 9 | 10 (20.4) | Designate a dedicated “out of hours” trauma room (Keren et al,59 2017) |
Abbreviation: ERIC, Expert Recommendations for Implementing Change.
Table 2. Most Common ERIC Strategy Components Among Significantly Successful Improvement Programs.
| ERIC strategy title | ERIC domain and strategy No. | Occurrence, No. (%) (N = 49) |
|---|---|---|
| Assess for readiness and identify barriers and facilitators | 1.4 | 49 (100) |
| Develop a formal implementation blueprint | 1.23 | 49 (100) |
| Identify and prepare champions | 4.35 | 35 (71.4) |
| Promote network weaving | 4.52 | 35 (71.4) |
| Develop resource sharing agreements | 6.30 | 33 (67.3) |
| Organize clinician implementation team meetings | 4.48 | 26 (53.1) |
| Create new clinical teams | 6.21 | 26 (53.1) |
| Facilitate relay of clinical data to providers | 6.32 | 25 (51.0) |
| Capture and share local knowledge | 4.7 | 24 (49.0) |
| Conduct local consensus discussions | 4.17 | 22 (44.9) |
| Promote adaptability | 3.51 | 17 (34.7) |
| Conduct local need assessment | 1.18 | 13 (26.5) |
| Develop an implementation glossary | 4.25 | 11 (22.4) |
| Change physical structure and equipment | 9.11 | 10 (20.4) |
| Recruit, designate, and train for leadership | 4.57 | 8 (16.3) |
| Develop and organize quality monitoring systems | 1.27 | 7 (14.3) |
| Centralize technical assistance | 2.8 | 6 (12.2) |
| Provide local technical assistance | 2.54 | 6 (12.2) |
| Alter incentive and/or allowance structures | 8.2 | 6 (12.2) |
| Revise professional roles | 6.59 | 6 (12.2) |
| Use advisory boards and workgroups | 4.64 | 5 (10.2) |
| Develop and implement tools for quality monitoring | 1.26 | 4 (8.2) |
| Facilitation | 2.33 | 4 (8.2) |
| Involve executive boards | 4.40 | 3 (6.1) |
| Audit and provide feedback | 1.5 | 3 (6.1) |
| Conduct educational meetings | 5.15 | 3 (6.1) |
| Inform local opinion leaders | 4.38 | 2 (4.1) |
| Purposefully reexamine the implementation | 1.56 | 2 (4.1) |
| Create a learning collaborative | 5.20 | 2 (4.1) |
| Conduct cyclical small tests of change | 1.14 | 1 (2.0) |
| Tailor strategies | 3.63 | 1 (2.0) |
| Obtain formal commitments | 4.47 | 1 (2.0) |
| Conduct ongoing training | 5.19 | 1 (2.0) |
| Develop educational materials | 5.29 | 1 (2.0) |
| Distribute educational materials | 5.31 | 1 (2.0) |
| Involve patients, consumers, and family members | 7.41 | 1 (2.0) |
| Conduct educational outreach visits | 5.16 | 1 (2.0) |
| Model and simulate change | 4.45 | 1 (2.0) |
| Use an implementation advisor | 4.65 | 1 (2.0) |
Abbreviation: ERIC, Expert Recommendations for Implementing Change.
Table 3. Recurring ERIC Strategy Components Among Unsuccessful Improvement Programs.
| ERIC strategy title | ERIC domain and strategy No. | Occurrence, No. (%) (N = 20) |
|---|---|---|
| Assess for readiness and identify barriers and facilitators | 1.4 | 20 (100) |
| Develop a formal implementation blueprint | 1.23 | 17 (85.0) |
| Develop resource sharing agreements | 6.30 | 16 (80.0) |
| Identify and prepare champions | 4.35 | 15 (75.0) |
| Promote network weaving | 4.52 | 14 (70.0) |
| Organize clinician implementation team meetings | 4.48 | 12 (60.0) |
| Create new clinical teams | 6.21 | 11 (55.0) |
| Facilitate relay of clinical data to providers | 6.32 | 11 (55.0) |
Abbreviation: ERIC, Expert Recommendations for Implementing Change.
Table 4. Limitations Among Unsuccessful TTS Improvement Programs.
| Top limitations of study design | Top limitations of improvement programs |
|---|---|
| Participant selection bias (eg, comorbidity rates, relative patient health status, exclusion criteria, small sample sizes) | Multiple improvement programs implemented concurrently (eg, integrated orthogeriatric care with anticoagulant use, comanaged care with multidisciplinary pathways, electronic order sets), making it difficult to discern outcomes of individual improvement strategies |
| Insufficient system resources (eg, lack of sufficient trauma theaters/beds/staff, lack of complication rates data, among other comprehensive patient data collection insufficiencies, seasonal increases in patient demand by hospital region) | Inadequate specificity of program design (eg, lack of clear documentation for fast-track programs, poor process performance measures, liberal medical recommendations vs conservative delaying TTS rates) |
| Insufficient leadership (eg, lack of trauma-trained orthopaedic surgeon, lack of geriatrician) | Hesitancy to designate leaders for new clinical teams and follow-up for adherence (eg, hiring staff to oversee program implementation, clearly revising roles and responsibilities within clinical teams) |
Abbreviation: TTS, time to surgery.
Discussion
As a result of the strong body of evidence supporting the association of TTS with morbidity and mortality, various organizations (eg, the American Academy of Orthopaedic Surgeons) have recommended surgical treatment within 48 hours of presentation for patients sustaining hip fractures.104 Novel improvement programs have demonstrated potential in reducing in-hospital and 30-day mortality rates as well as decreasing the length of hospital stay.34,35,36 Recent studies have further explored the results of improvement programs designed to reduce TTS for hip fracture patients. However, poor consistency in implementation methods has impeded efforts for generalizable strategies for improvement efforts to address hip fracture TTS.31 In this systematic review, we cataloged and identified program results using the ERIC classification system. These results highlighted commonalities across studies and provided the foundation for guiding the implementation of future TTS optimization programs in health systems. While designing TTS improvement programs, health care systems may utilize the findings of this review to reference the individual programs we reviewed that bear similarities to the needs of their clinical settings. Additionally, common ERIC strategies and domains of successful programs described in this review can provide nuanced insight into the strategy and design of future TTS improvement programs.
The results of our review indicated that, of the studies included, successful TTS improvement programs had a mean (SD) of 9 (4) strategies defined under ERIC. These studies suggest that a comprehensive strategic approach, using multiple, diverse implementation strategies across different domains, can be beneficial in reducing TTS. Given the studies that did not significantly improve or failed to improve TTS used a mean (SD) of 8 (2) and 7 (2) strategies, respectively, it appears that more implementation strategies were associated with improved TTS. The most prevalent ERIC domains among successful improvement programs were the use of evaluative and iterative strategies (100% rate), development of stakeholder interrelationships (71.4% rate), and support of clinicians (67.3% rate). This pattern suggests the need for increased efficient preoperative communication and partnerships between medical and surgical teams to reduce TTS. Furthermore, the 100% rate of the ERIC strategies assessing for readiness and identify barriers and facilitators and developing a formal implementation blueprint among the 49 successful improvement programs indicates that preemptive strategic planning appears to be positively associated with the success of a given improvement strategy.
The 5 most common ERIC strategies found among improvement programs includes assessing for readiness and identifying barriers and facilitators, describing a tailored, 3-pronged approach to (1) assess the readiness of a given study site to implement an improvement program; (2) assess barriers that could impede implementation efforts; and (3) assess strengths for implementation31 (eAppendix 4 in the Supplement). Other common strategies included developing a formal implementation blueprint, which entails an outline of improvement program goals and strategies, such as program aims, scope of organizations affected, timeframe and milestones, and measures of progress and performance. Identifying and preparing champions describes the process of delegating implementation leadership to champions of improvement programs. These champions were often already leaders in the care pathway of the patient and responsible for implementing improvement strategies. Promoting network weaving involves identifying existing networks to (1) promote information sharing and (2) collaborate on solving problems relevant to the improvement program. For example, this strategy often described improvement programs geared toward promoting multidisciplinary and comanaged clinical care. Lastly, developing resource sharing agreements entails developing partnerships with organizations to share resources needed for improvement program implementation. This strategy was also common among improvement programs promoting multidisciplinary and comanaged clinical care.
ERIC strategies common to successful programs were also found among the 20 studies that did not report significant decreases in TTS (Table 3). However, the 5 studies that resulted in increased TTS offered additional, nuanced explanations for the barriers impeding the performance of their improvement programs, including the influence of patient-level factors (eg, anticoagulant use,42 confounding comorbidities63) (Table 4 and eAppendix 2 in the Supplement). These studies often cited numerous limitations inherent to study design, and relatively sparse limitations to improvement programs, themselves. Nijmeijer et al42 cited the introduction of direct oral anticoagulants, which were to be omitted 48 hours prior to surgery, as a potential limitation of its study design and noted that its improvement program defined a lower threshold for delaying surgery to reduce perioperative complications and limited operating room capacity. Lemos et al89 supported the potential of dedicated orthopaedic trauma theaters as an improvement program but suggested that a full 7-day theater may optimally decrease TTS, rather than the 4-day theater implemented. This suggestion is consistent with the findings of several other improvement program studies,35,77,105 where a consistently designated trauma room achieved lower TTS. Bellas et al93 supported the potential of preoperative specialty consultations on hospitalist comanagement as an improvement program but suggested that consultations should be strategically used on a needs-only basis, rather than as standard practice. Finally, the clinical pathway improvement program implemented by Choong et al55 included an additional information checklist step in the emergency department, which resulted in a slight increase in TTS that did not yield statistical significance. These 5 studies noted continued barriers to success, including patient-level characteristics or insufficient context-dependent tailoring of strategies to suit the individual needs of each clinical setting. These improvement strategies, including implementing documented protocols for the preoperative care pathway and dedicated operating room times as well as changing surgical prioritization, have demonstrated the potential to be successful at decreasing TTS in some hospital settings, while unsuccessful in others.74,82 These conflicting data suggest that both patient-level factors and facility-level factors (eg, hospital census, type, teaching status, annual surgical volume) may affect the success of improvement programs and should be a consideration of future TTS improvement initiatives.
In this review, studies did not commonly include information on facility-level factors when describing the environment of hospital and health care settings. For hospital and health system settings that are already advanced prior to implementation of a given improvement program, smaller margins of program success will be reflected in study results regardless of a given program’s true potential. Therefore, the environment of a health system provides essential context for the performance of a given improvement program. This lack of comprehensive facility-level factor data impeded our ability to provide context for the success of a given improvement program based upon its environment.
Another consideration for our review is the inclusion of studies that only focused on TTS as a main outcome. Several systemwide improvement programs have instead focused on mortality and length of hospital stay as primary outcomes, without also considering changes in TTS. It may be beneficial to consider the replication of these successful programs, with a focus on TTS improvement.
Limitations
This study has limitations. The ability to gauge the potential of improvement programs was limited by inconsistencies in defining delayed TTS cutoffs between studies. For example, the relative success of an improvement program that reduced TTS to less than 48 hours may depend on the delayed TTS cutoff decided for that study. Similarly, an improvement program aiming to increase surgical intervention within the first 24 hours of patient admission may report smaller margins of program success than an improvement program aiming promote surgical intervention within the first 3 days of patient admission.
It is important to note the heterogeneity of the study age cutoff as a limitation to this review. While geriatric age is typically defined as those 65 years and older, this study included all reports with a patient population aged 50 years and older. This allowed for a more robust analysis of systematic improvements while adding a level of heterogeneity to the study population. Future studies may wish to investigate improvement programs of geriatric populations specifically to identify targeted systematic improvements for this population.
Furthermore, our review was limited by a lack of data regarding program failure, as only 5 included studies described increased TTS results. No failed improvement program provided reasoning for why a program should not be pursued for further implementation efforts in the clinical setting. Thus, the available data informing future improvement program strategies may be limited by publication bias. This publication bias may further occlude research teams from providing reasoning as to why a given improvement program fails and, instead, encourage researchers to focus on cases for future program promise.
Moreover, several studies included TTS data during the improvement program’s implementation as well as after implementation, while other studies merged these data into an overall postimplementation category, limiting an assessment of rates of reduced TTS. Additionally, this review focused on timely surgical management of femoral neck, intertrochanteric, and subtrochanteric fractures only. This was done to minimize confounding considerations for surgical management and to simplify comparisons of improvement program data. Nevertheless, future studies should aim to replicate improvement program results across other hip fracture types to confirm TTS program generalizability. Also, while this systematic review included studies with levels of evidence III and IV, ie controlled trials without randomization and cohort studies, it excluded case reports and case studies to reduce confounding sample sizes; varying sample sizes among our included studies may skew the success rates presented for any given improvement program. Additionally, while each improvement program was independently read and cataloged according to ERIC strategies by 2 authors, and any conflicts were independently reviewed and resolved by a third author, study classifications may be subject to, albeit minimal, manual human error.
Conclusions
In this review of improvement programs for TTS for hip fracture, we summarized the results of novel programs. Generally, the 69 improvement programs possessed the capability to decrease TTS. Particular promise for replicable success was demonstrated by the 49 improvement programs that were associated with significant TTS decreases. Among these successful programs, general themes were ascertained regarding common ERIC strategies and domains. Future study designs may incorporate these common implementation strategies from previously successful programs to inform and structure novel improvement programs. Finally, this review suggests a need for understanding the facility-level and patient-level factors of potential implementation settings to provide necessary context associated with the success of a given improvement program. By highlighting promising strategies and bodies of work, this review may therefore inform the direction of future TTS improvement programs to promote the development of both generalizable and context-specific strategies for improvement programs in the future.
eAppendix 1. Database Search Criteria
eAppendix 2. Limitations Among Unsuccessful Improvement Programs
eAppendix 3. Included Studies, Improvement Programs, Quality Scores, and Results
eAppendix 4. ERIC Domains and Strategies
References
- 1.Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med. 1997;103(2A):20S-25S. doi: 10.1016/S0002-9343(97)90023-1 [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States: numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;(252):163-166. [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407-413. doi: 10.1007/PL00004148 [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 5.Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031. doi: 10.1093/aje/kwg081 [DOI] [PubMed] [Google Scholar]
- 6.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(suppl 6):S164-S169. [PubMed] [Google Scholar]
- 7.Williamson S, Landeiro F, McConnell T, et al. Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporos Int. 2017;28(10):2791-2800. doi: 10.1007/s00198-017-4153-6 [DOI] [PubMed] [Google Scholar]
- 8.Adeyemi A, Delhougne G. Incidence and economic burden of intertrochanteric fracture: a Medicare claims database analysis. JB JS Open Access. 2019;4(1):e0045. doi: 10.2106/JBJS.OA.18.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgström F, Karlsson L, Ortsäter G, et al. ; International Osteoporosis Foundation . Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15(1):59. doi: 10.1007/s11657-020-0706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morri M, Ambrosi E, Chiari P, et al. One-year mortality after hip fracture surgery and prognostic factors: a prospective cohort study. Sci Rep. 2019;9(1):18718. doi: 10.1038/s41598-019-55196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpintero P, Caeiro JR, Carpintero R, Morales A, Silva S, Mesa M. Complications of hip fractures: a review. World J Orthop. 2014;5(4):402-411. doi: 10.5312/wjo.v5.i4.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzon-Illescas O, Perez Fernandez E, Crespí Villarias N, et al. Mortality after osteoporotic hip fracture: incidence, trends, and associated factors. J Orthop Surg Res. 2019;14(1):203. doi: 10.1186/s13018-019-1226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi: 10.1001/jama.291.14.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moja L, Piatti A, Pecoraro V, et al. Timing matters in hip fracture surgery: patients operated within 48 hours have better outcomes: a meta-analysis and meta-regression of over 190,000 patients. PLoS One. 2012;7(10):e46175. doi: 10.1371/journal.pone.0046175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? systematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088 [DOI] [PubMed] [Google Scholar]
- 17.Sheehan KJ, Sobolev B, Villán YF, Guy P. Patient and system factors of time to surgery after hip fracture: a scoping review. BMJ Open. 2017;7(8):e016939. doi: 10.1136/bmjopen-2017-016939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone AP, Fusco D, Colais P, et al. Effects of socioeconomic position on 30-day mortality and wait for surgery after hip fracture. Int J Qual Health Care. 2009;21(6):379-386. doi: 10.1093/intqhc/mzp046 [DOI] [PubMed] [Google Scholar]
- 19.Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29(3):e109-e114. doi: 10.1097/BOT.0000000000000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantini MP, Fabbri G, Laus M, et al. Determinants of surgical delay for hip fracture. Surgeon. 2011;9(3):130-134. doi: 10.1016/j.surge.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 21.Ryan DJ, Yoshihara H, Yoneoka D, Egol KA, Zuckerman JD. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29(8):343-348. doi: 10.1097/BOT.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 22.Zeltzer J, Mitchell RJ, Toson B, Harris IA, Close J. Determinants of time to surgery for patients with hip fracture. ANZ J Surg. 2014;84(9):633-638. doi: 10.1111/ans.12671 [DOI] [PubMed] [Google Scholar]
- 23.Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi: 10.1136/bmjopen-2017-017572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doucette RS, Dibble E, Arora NS, et al. Emergency department clinician perceptions of implementing high-sensitivity troponin T assay in an academic hospital emergency department. Am J Med. 2020;133(9):e483-e494. doi: 10.1016/j.amjmed.2020.01.039 [DOI] [PubMed] [Google Scholar]
- 25.Baker R, Camosso-Stefinovic J, Gillies C, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010;(3):CD005470. doi: 10.1002/14651858.CD005470.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The World Bank. High income. Accessed July 9, 2022. https://data.worldbank.org/country/XD
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43(2):180-187. doi: 10.3129/i08-001 [DOI] [PubMed] [Google Scholar]
- 29.Powell BJ, McMillen JC, Proctor EK, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev. 2012;69(2):123-157. doi: 10.1177/1077558711430690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell BJ, Waltz T, Chinman M, Damschroder L. A refining a compilation of discrete implementation strategies and determining their importance and feasibility. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waltz TJ, Powell BJ, Matthieu MM, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10:109. doi: 10.1186/s13012-015-0295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White MC, Peven K, Clancy O, et al. Implementation strategies and the uptake of the World Health Organization surgical safety checklist in low and middle income countries: a systematic review and meta-analysis. Ann Surg. 2021;273(6):e196-e205. doi: 10.1097/SLA.0000000000003944 [DOI] [PubMed] [Google Scholar]
- 33.Noticewala MS, Swart E, Shah RP, Macaulay W, Geller JA. First Place Award Multidisciplinary care of the hip fracture patient: a case control analysis of differing treatment protocols. Curr Orthop Pract. 2016;27(4):346-350. doi: 10.1097/BCO.0000000000000394 [DOI] [Google Scholar]
- 34.Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. doi: 10.1097/BOT.0b013e3182a5a045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleton M, Wan B, da Assunçao R. Improving hip fracture outcomes with integrated orthogeriatric care: a comparison between two accepted orthogeriatric models. Age Ageing. 2017;46(3):465-470. doi: 10.1093/ageing/afw232 [DOI] [PubMed] [Google Scholar]
- 36.Arshi A, Rezzadeh K, Stavrakis AI, Bukata SV, Zeegen EN. Standardized hospital-based care programs improve geriatric hip fracture outcomes: an analysis of the ACS NSQIP targeted hip fracture series. J Orthop Trauma. 2019;33(6):e223-e228. doi: 10.1097/BOT.0000000000001443 [DOI] [PubMed] [Google Scholar]
- 37.Jones LK, Tilberry S, Gregor C, et al. Implementation strategies to improve statin utilization in individuals with hypercholesterolemia: a systematic review and meta-analysis. Implement Sci. 2021;16(1):40. doi: 10.1186/s13012-021-01108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haugan K, Johnsen LG, Basso T, Foss OA. Mortality and readmission following hip fracture surgery: a retrospective study comparing conventional and fast-track care. BMJ Open. 2017;7(8):e015574. doi: 10.1136/bmjopen-2016-015574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson G, Strömberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47(4):881-886. doi: 10.1016/j.injury.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 40.Anderson ME, Mcdevitt K, Cumbler E, et al. Geriatric hip fracture care: fixing a fragmented system. Perm J. 2017;21:16-104. doi: 10.7812/TPP/16-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc. 2009;57(1):159-167. doi: 10.1111/j.1532-5415.2008.02069.x [DOI] [PubMed] [Google Scholar]
- 42.Nijmeijer WS, Folbert EC, Vermeer M, Vollenbroek-Hutten MMR, Hegeman JH. The consistency of care for older patients with a hip fracture: are the results of the integrated orthogeriatric treatment model of the Centre of Geriatric Traumatology consistent 10 years after implementation? Arch Osteoporos. 2018;13(1):131. doi: 10.1007/s11657-018-0550-5 [DOI] [PubMed] [Google Scholar]
- 43.O’Mara-Gardner K, Redfern RE, Bair JM. Establishing a geriatric hip fracture program at a level 1 community trauma center. Orthop Nurs. 2020;39(3):171-179. doi: 10.1097/NOR.0000000000000655 [DOI] [PubMed] [Google Scholar]
- 44.Pablos-Hernández C, González-Ramírez A, da Casa C, et al. Time to surgery reduction in hip fracture patients on an integrated orthogeriatric unit: a comparative study of three healthcare models. Orthop Surg. 2020;12(2):457-462. doi: 10.1111/os.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talevski J, Guerrero-Cedeño V, Demontiero O, et al. Implementation of an electronic care pathway for hip fracture patients: a pilot before and after study. BMC Musculoskelet Disord. 2020;21(1):837. doi: 10.1186/s12891-020-03834-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uri O, Folman Y, Laufer G, Behrbalk E. A Reimbursement system based on a 48-hour target time for surgery shortens the waiting time for hip fracture fixation in elderly patients. J Orthop Trauma. 2020;34(5):248-251. doi: 10.1097/BOT.0000000000001681 [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Hernández N, Sáez-López P, Paniagua-Tejo S, Valverde-García JA. Results following the implementation of a clinical pathway in the process of care to elderly patients with osteoporotic hip fracture in a second level hospital. [Article in Spanish] Rev Esp Cir Ortop Traumatol. 2016;60(1):1-11. doi: 10.1016/j.recote.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Kusen J, van der Vet P, Wijdicks FJ, et al. Different approaches towards geriatric trauma care for hip fracture patients: an inter-hospital comparison. Eur J Trauma Emerg Surg. 2021;47(2):557-564. doi: 10.1007/s00068-019-01129-x [DOI] [PubMed] [Google Scholar]
- 49.Chechik O, Amar E, Khashan M, Kadar A, Rosenblatt Y, Maman E. In support of early surgery for hip fractures sustained by elderly patients taking clopidogrel: a retrospective study. Drugs Aging. 2012;29(1):63-68. doi: 10.2165/11598490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 50.Anighoro K, Bridges C, Graf A, et al. From ER to OR: results after implementation of multidisciplinary pathway for fragility hip fractures at a level I trauma center. Geriatr Orthop Surg Rehabil. Published online May 22, 2020. doi: 10.1177/2151459320927383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biber R, Singler K, Curschmann-Horter M, Wicklein S, Sieber C, Bail HJ. Implementation of a co-managed geriatric fracture center reduces hospital stay and time-to-operation in elderly femoral neck fracture patients. Arch Orthop Trauma Surg. 2013;133(11):1527-1531. doi: 10.1007/s00402-013-1845-z [DOI] [PubMed] [Google Scholar]
- 52.Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. doi: 10.4065/81.1.28 [DOI] [PubMed] [Google Scholar]
- 53.Metcalfe D, Zogg CK, Judge A, et al. Pay for performance and hip fracture outcomes: an interrupted time series and difference-in-differences analysis in England and Scotland. Bone Joint J. 2019;101-B(8):1015-1023. doi: 10.1302/0301-620X.101B8.BJJ-2019-0173.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Divecha HM, Smith RD, Cairns C, Bayer J. Improving patient flow: the impact of consultant work pattern on trauma ward efficiency. Surgeon. 2011;9(4):175-178. doi: 10.1016/j.surge.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 55.Choong PF, Langford AK, Dowsey MM, Santamaria NM. Clinical pathway for fractured neck of femur: a prospective, controlled study. Med J Aust. 2000;172(9):423-426. doi: 10.5694/j.1326-5377.2000.tb124038.x [DOI] [PubMed] [Google Scholar]
- 56.Elder GM, Harvey EJ, Vaidya R, Guy P, Meek RN, Aebi M. The effectiveness of orthopaedic trauma theatres in decreasing morbidity and mortality: a study of 701 displaced subcapital hip fractures in two trauma centres. Injury. 2005;36(9):1060-1066. doi: 10.1016/j.injury.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 57.Bohm E, Loucks L, Wittmeier K, Lix LM, Oppenheimer L. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58(4):257-263. doi: 10.1503/cjs.017714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. doi: 10.1177/2151458516661383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keren Y, Sailofsky S, Keshet D, Barak M. The effect of ‘out of hours surgery service’ in Israel on hip fracture fixation outcomes: a retrospective analysis. Isr J Health Policy Res. 2017;6(1):27. doi: 10.1186/s13584-017-0150-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heyzer L, Ramason R, Molina JAC, Chan WWL, Loong CY, Kwek EBK. Integrated hip fracture care pathway (IHFCP): reducing complications and improving outcomes. Singapore Med J. Published online April 19, 2021. doi: 10.11622/smedj.2021041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalmet PHS, Koc BB, Hemmes B, et al. Effectiveness of a multidisciplinary clinical pathway for elderly patients with hip fracture: a multicenter comparative cohort study. Geriatr Orthop Surg Rehabil. 2016;7(2):81-85. doi: 10.1177/2151458516645633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoriati AA, Dandachli W, Deol R, de Roeck N. Does a dedicated unit for the treatment of hip fractures improve acute outcomes? Int Sch Res Notices. 2014;2014:385701. doi: 10.1155/2014/385701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi: 10.1016/j.injury.2008.01.048 [DOI] [PubMed] [Google Scholar]
- 64.Gholve PA, Kosygan KP, Sturdee SW, Faraj AA. Multidisciplinary integrated care pathway for fractured neck of femur: a prospective trial with improved outcome. Injury. 2005;36(1):93-98. doi: 10.1016/j.injury.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 65.Kosy JD, Blackshaw R, Swart M, Fordyce A, Lofthouse RA. Fractured neck of femur patient care improved by simulated fast-track system. J Orthop Traumatol. 2013;14(3):165-170. doi: 10.1007/s10195-013-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilchrist N, Dalzell K, Pearson S, et al. Enhanced hip fracture management: use of statistical methods and dataset to evaluate a fractured neck of femur fast track pathway-pilot study. N Z Med J. 2017;130(1455):91-101. [PubMed] [Google Scholar]
- 67.Kalmet PHS, de Joode SGCJ, Fiddelers AAA, Ten Broeke RHM, Poeze M, Blokhuis T. Long-term patient-reported quality of life and pain after a multidisciplinary clinical pathway for elderly patients with hip fracture: a retrospective comparative cohort study. Geriatr Orthop Surg Rehabil. Published online June 6, 2019. doi: 10.1177/2151459319841743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baroni M, Serra R, Boccardi V, et al. The orthogeriatric comanagement improves clinical outcomes of hip fracture in older adults. Osteoporos Int. 2019;30(4):907-916. doi: 10.1007/s00198-019-04858-2 [DOI] [PubMed] [Google Scholar]
- 69.Collinge CA, McWilliam-Ross K, Beltran MJ, Weaver T. Measures of clinical outcome before, during, and after implementation of a comprehensive geriatric hip fracture program: is there a learning curve? J Orthop Trauma. 2013;27(12):672-676. doi: 10.1097/BOT.0b013e318291f0e5 [DOI] [PubMed] [Google Scholar]
- 70.Colais P, Pinnarelli L, Fusco D, Davoli M, Braga M, Perucci CA. The impact of a pay-for-performance system on timing to hip fracture surgery: experience from the Lazio region (Italy). BMC Health Serv Res. 2013;13:393. doi: 10.1186/1472-6963-13-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan W, Kwek EBK. Management of elderly hip fractures by an orthopaedic trauma surgeon reduces surgical delays but does not improve outcomes compared to non-trauma surgeons. Arch Orthop Trauma Surg. 2019;139(1):35-41. doi: 10.1007/s00402-018-3047-1 [DOI] [PubMed] [Google Scholar]
- 72.Diament M, MacLeod K, O’Hare J, Tate A, Eardley W. “Early trigger” intravenous vitamin K: optimizing target-driven care in warfarinised patients with hip fracture. Geriatr Orthop Surg Rehabil. 2015;6(4):263-268. doi: 10.1177/2151458515595669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kristensen PK, Thillemann TM, Søballe K, Johnsen SP. Can improved quality of care explain the success of orthogeriatric units? a population-based cohort study. Age Ageing. 2016;45(1):66-71. doi: 10.1093/ageing/afv155 [DOI] [PubMed] [Google Scholar]
- 74.Reguant F, Arnau A, Lorente JV, Maestro L, Bosch J. Efficacy of a multidisciplinary approach on postoperative morbidity and mortality of elderly patients with hip fracture. J Clin Anesth. 2019;53:11-19. doi: 10.1016/j.jclinane.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 75.VanTienderen RJ, Bockelman K, Khalifa R, Reich MS, Adler A, Nguyen MP. Implementation of a multidisciplinary “code hip” protocol is associated with decreased time to surgery and improved patient outcomes. Geriatr Orthop Surg Rehabil. Published online March 25, 2021. doi: 10.1177/21514593211004904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sayeed Z, Anoushiravani A, El-Othmani M, et al. Implementation of a hip fracture care pathway using Lean Six Sigma methodology in a level I trauma center. J Am Acad Orthop Surg. 2018;26(24):881-893. doi: 10.5435/JAAOS-D-16-00947 [DOI] [PubMed] [Google Scholar]
- 77.McDonald M, Ward L, Wortham H, Sorenson B, Jarski R, El-Yussif E. Effect of a 6 am-9 am dedicated orthopaedic trauma room on hip fracture outcomes in a community level II trauma center. J Orthop Trauma. 2021;35(5):245-251. doi: 10.1097/BOT.0000000000001966 [DOI] [PubMed] [Google Scholar]
- 78.Rincón Gómez M, Hernández Quiles C, García Gutiérrez M, et al. Hip fracture co-management in the elderly in a tertiary referral hospital: a cohorts study. [Article in Spanish] Rev Clin Esp (Barc). 2020;220(1):1-7. doi: 10.1016/j.rceng.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 79.Kommer M, Gokaraju K, Singh S. Changing the consultant on calls from a daily to weekly rotation system reduces time to theater for patients with hip fracture to improve quality of care: a retrospective study of 2 cohorts of patients presenting with hip fracture. Geriatr Orthop Surg Rehabil. 2014;5(2):69-72. doi: 10.1177/2151458514527762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mittal C, Lee HCD, Goh KS, et al. ValuedCare program: a population health model for the delivery of evidence-based care across care continuum for hip fracture patients in Eastern Singapore. J Orthop Surg Res. 2018;13(1):129. doi: 10.1186/s13018-018-0819-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rostagno C, Buzzi R, Campanacci D, et al. In hospital and 3-month mortality and functional recovery rate in patients treated for hip fracture by a multidisciplinary team. PLoS One. 2016;11(7):e0158607. doi: 10.1371/journal.pone.0158607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts HJ, Rogers SE, Ward DT, Kandemir U. Protocol-based interdisciplinary co-management for hip fracture care: 3 years of experience at an academic medical center. Arch Orthop Trauma Surg. 2022;142(7):1491-1497. doi: 10.1007/s00402-020-03699-7 [DOI] [PubMed] [Google Scholar]
- 83.Marcheix PS, Collin C, Hardy J, Mabit C, Tchalla A, Charissoux JL. Impact of orthogeriatric management on the average length of stay of patients aged over seventy five years admitted to hospital after hip fractures. Int Orthop. 2021;45(6):1431-1438. doi: 10.1007/s00264-020-04908-z [DOI] [PubMed] [Google Scholar]
- 84.Tittel S, Burkhardt J, Roll C, Kinner B. Clinical pathways for geriatric patients with proximal femoral fracture improve process and outcome. Orthop Traumatol Surg Res. 2020;106(1):141-147. doi: 10.1016/j.otsr.2019.07.029 [DOI] [PubMed] [Google Scholar]
- 85.Kohli S, Bawa A, Crooks S, Nagarajakumar A, Brooker J, Doddi S. A hip fracture nurse specialist has a positive outcome on the length of stay for patients with hip fractures. G Chir. 2019;40(6):551-555. [PubMed] [Google Scholar]
- 86.Jackson K, Bachhuber M, Bowden D, Etter K, Tong C. Comprehensive hip fracture care program: successive implementation in 3 hospitals. Geriatr Orthop Surg Rehabil. Published online May 15, 2019. doi: 10.1177/2151459319846057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sermon A, Rochus I, Smeets B, et al. The implementation of a clinical pathway enhancing early surgery for geriatric hip fractures: how to maintain a success story? Eur J Trauma Emerg Surg. 2019;45(2):199-205. doi: 10.1007/s00068-018-1034-4 [DOI] [PubMed] [Google Scholar]
- 88.Phy MP, Vanness DJ, Melton LJ III, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. doi: 10.1001/archinte.165.7.796 [DOI] [PubMed] [Google Scholar]
- 89.Lemos D, Nilssen E, Khatiwada B, et al. Dedicated orthopedic trauma theatres: effect on morbidity and mortality in a single trauma centre. Can J Surg. 2009;52(2):87-91. [PMC free article] [PubMed] [Google Scholar]
- 90.Shenouda M, Silk Z, Radha S, Bouanem E, Radford W. The introduction of a multidisciplinary hip fracture pathway to optimise patient care and reduce mortality: a prospective audit of 161 patients. Open Orthop J. 2017;11:309-315. doi: 10.2174/1874325001711010309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burton A, Davis CM, Boateng H, et al. A multidisciplinary approach to expedite surgical hip fracture care. Geriatr Orthop Surg Rehabil. Published online January 22, 2020. doi: 10.1177/2151459319898646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuijt HJ, Kusen J, van Hernen JJ, et al. Orthogeriatric trauma unit improves patient outcomes in geriatric hip fracture patients. Geriatr Orthop Surg Rehabil. Published online August 14, 2020. doi: 10.1177/2151459320949476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellas N, Stohler S, Staff I, et al. Impact of preoperative specialty consults on hospitalist comanagement of hip fracture patients. J Hosp Med. 2020;15(1):16-21. doi: 10.12788/jhm.3264 [DOI] [PubMed] [Google Scholar]
- 94.Blauth M, Joeris A, Rometsch E, et al. Geriatric fracture centre vs usual care after proximal femur fracture in older patients: what are the benefits? results of a large international prospective multicentre study. BMJ Open. 2021;11(5):e039960. doi: 10.1136/bmjopen-2020-039960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Godin J, Brown C, Mardam-Bey S, et al. Two admission pathways for elderly patients with hip fracture: clinical outcomes at a single institution. Curr Orthop Pract. 2015;26(4):8. doi: 10.1097/BCO.0000000000000255 [DOI] [Google Scholar]
- 96.Hormaechea Bolado L, Ortiz Gómez JR, Fornet Ruiz I, et al. Development and implementation of a perioperative management guide for patients with hip fracture: health management and clinical impact. Rev Esp Cir Ortop Traumatol. 2021;65(4):294-304. doi: 10.1016/j.recote.2021.04.011 [DOI] [Google Scholar]
- 97.McNamara R, Butler A, Baker C, et al. Use of lean principals to improve flow of patients with fractured neck of femur: the HOPE study. Ir Med J. 2014;107(3):70-72. [PubMed] [Google Scholar]
- 98.Pajulammi HM, Pihlajamäki HK, Luukkaala TH, Jousmäki JJ, Nuotio MS. Association of comprehensive geriatric assessment with quality-related care practices during implementation and development of an orthogeriatric hip fracture program. Eur Geriatr Med. 2017;8(5-6):424-429. doi: 10.1016/j.eurger.2017.06.002 [DOI] [Google Scholar]
- 99.Pinnarelli L, Nuti S, Sorge C, et al. What drives hospital performance? the impact of comparative outcome evaluation of patients admitted for hip fracture in two Italian regions. BMJ Qual Saf. 2012;21(2):127-134. doi: 10.1136/bmjqs-2011-000218 [DOI] [PubMed] [Google Scholar]
- 100.Valsamis EM, Husband H, Burchette D, Milošević M, Bakota B. Modelling the effect of a dedicated hip fracture unit on patient outcomes using segmented robust linear regression techniques. Injury. 2021;52(suppl 5):S3-S6. doi: 10.1016/j.injury.2020.03.056 [DOI] [PubMed] [Google Scholar]
- 101.Wu X, Tian M, Zhang J, et al. The effect of a multidisciplinary co-management program for the older hip fracture patients in Beijing: a “pre- and post-” retrospective study. Arch Osteoporos. 2019;14(1):43. doi: 10.1007/s11657-019-0594-1 [DOI] [PubMed] [Google Scholar]
- 102.Shigemoto K, Sawaguchi T, Goshima K, Iwai S, Nakanishi A, Ueoka K. The effect of a multidisciplinary approach on geriatric hip fractures in Japan. J Orthop Sci. 2019;24(2):280-285. doi: 10.1016/j.jos.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 103.Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31. [PubMed] [Google Scholar]
- 104.American Academy of Orthopaedic Surgeons. OrthoGuidelines. Accessed July 9, 2022. https://www.orthoguidelines.org/
- 105.Kusen JQ, Schafroth B, Poblete B, et al. The implementation of a Geriatric Fracture Centre for hip fractures to reduce mortality and morbidity: an observational study. Arch Orthop Trauma Surg. 2019;139(12):1705-1712. doi: 10.1007/s00402-019-03229-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Database Search Criteria
eAppendix 2. Limitations Among Unsuccessful Improvement Programs
eAppendix 3. Included Studies, Improvement Programs, Quality Scores, and Results
eAppendix 4. ERIC Domains and Strategies