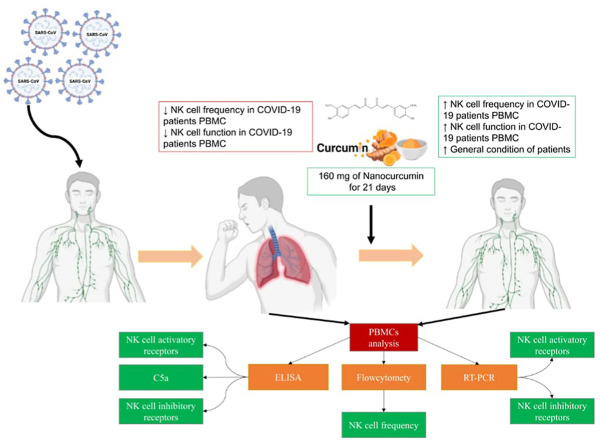
Abstract
The ongoing COVID-19 pandemic is still a challenging problem in the case of infection treatment. The immunomodulatory effect of Nanocurcumin was investigated in the present study in an attempt to counterbalance the immune response and improve the patients' clinical symptoms. 60 confirmed COVID-19 patients and 60 healthy controls enrolled in the study. COVID-19 patients were divided into Nanocurcumin and placebo received groups. Due to the importance of the role of NK cells in this disease, the frequency, cytotoxicity, receptor gene expression of NK cells, and serum secretion levels of inflammatory cytokines IL-1β, IL-6, TNF-α, as well as circulating C5a as a chemotactic factor an inflammatory mediator was evaluated by flow cytometry, real-time PCR and enzyme-linked immunosorbent assay in both experimental groups before and after the intervention. Given the role of measured factors in the progression and pathogenesis of COVID-19 disease, the results can help find appropriate treatments. The results of this study indicated that the Nanocurcumin could significantly increase the frequency and function of NK cells compared to the placebo-treated group. As an immunomodulatory agent, Nanocurcumin may be a helpful choice to improve NK cell function in COVID-19 patients and improve the clinical outcome of patients.
Keywords: COVID-19, SARS-CoV2, Natural killer cell, Cytotoxicity, Nanocurcumin
Abbreviations: Severe acute respiratory coronavirus, SARS-CoV; 2019 novel coronavirus, 2019-nCoV; Chronic mild stress, CMS; Phytohaemagglutinin, PHA; Peripheral blood mononuclear cells, PBMCs; Nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB; Natural killer, NK; Tumour necrosis factor α, TNFα; Regulatory cells, Tregs
Graphical abstract
1. Introduction
An investigation was initiated to uncover the etiology of atypical pneumonia cases reported in Wuhan city, China, in late December 2019 (Guo et al., 2020). As a result, viral particles were extracted from patients' broncho-alveolar lavages, later proving to belong to an emerging type of beta-coronavirus (Wang et al., 2020). Previously, six members of the coronavirus family were known to cause respiratory tract infections. Severe acute respiratory coronavirus (SARS-CoV) and middle eastern respiratory syndrome coronavirus (MERS-CoV) cause severe respiratory diseases, while other members are regarded as low-pathogenic coronaviruses. Further gene sequencing analysis of newly identified coronavirus determined a considerable genetic identity of 79.0% and 51.7% with SARS-CoV and MERS-CoV, respectively (Guo et al., 2020). In terms of statistics, novel coronavirus appeared to be substantially less lethal, with a 4% mortality risk compared to SARS-CoV and MERS-CoV with 9.6 and 36%, respectively (Wu et al., 2020). However, the mysterious viral pneumonia, initially called 2019 novel coronavirus (2019-nCoV) infected pneumonia, soon raised the alarms for health institutions due to its high transmission rate (Guo et al., 2020). Subsequently, local health officials of Wuhan city suspended all measures of public transformation in an attempt to stop the 2019-nCoV from spreading (Yan et al., 2020). On Feb. 11, 2020, the International Committee on Taxonomy of viruses named the novel coronavirus SARS-CoV-2. Considering the imminent threat, World Health Organization announced that the disease caused by SARS-CoV-2 would be called COVID-19 from Feb. 12, 2020 (Wang et al., 2020). Despite the implemented preventive measures, SARS-CoV-2 had spread at such a staggering rate that 89,294 people from 47 countries were reported to be infected within weeks of the Wuhan lockdown (Marofi et al., 2021; Zheng, 2020).
Although not all aspects of COVID-19 pathogenesis are fully explained, the key role of the immune system is among the well-established ones (Ghaebi et al., 2021). Laboratory findings such as a reduction in lymphocyte count, an increase in inflammatory cytokines and D-dimer levels, and hepatic dysfunction are all consistent with immune dysregulation (Sadeghi et al., 2021; Tahmasebi et al., 2020). In an attempt to counterbalance lymphocyte malfunction, pro-inflammatory cytokines are disproportionately produced by macrophages, neutrophils, and monocytes, which contribute to further deterioration in patients' clinical status (Fathi and Rezaei, 2020; Giamarellos-Bourboulis et al., 2020). While there was no completely effective treatment against COVID-19 antivirals, including oseltamivir, ganciclovir, and Kaletra (Lopinavir and ritonavir combination), ribavirin, Tocilizumab, corticosteroids, chloroquine, etc. That were proposed as candidates for monotherapy and combination use in COVID-19 patients (Marofi et al., 2021). These clinical interventions are mainly supported by theoretical knowledge and comparable experiences rather than their proven efficacy (Guo et al., 2020; Yan et al., 2020). With that being said, we focused on a group of substances collectively called curcuminoids. Curcumin (diferuloylmethane), as the prototype, is among the major substances stored in the rhizome of Curcuma longa (turmeric) (Lestari and Indrayanto, 2014). There is evidence that pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) are among the aforementioned curcumin-inflected signaling molecules. Besides, no serious adverse reactions have been reported with its administration (Gupta et al., 2013). Curcumin has a multifaceted role and inhibits COX-2 and the activation factor NF-κB (Khan et al., 2018). Administration of Curcuma longa extracts in CMS model in rats increased the activity of NK cells and augmented the levels of TNF-α and IL-6 (Komal et al., 2019). It also modulates the proliferation and responsiveness of macrophages, NK cells, DCs, and lymphocytes (Khan et al., 2018). Curcumin shows immunosuppressive effects and inhibits mitogen-induced (phorbol-12-myristate-13-acetate, Concanavalin A, Phytohaemagglutinin) proliferation of human spleen-derived lymphocytes and IL-2. In a study in 2005, the immunosuppressive effects of curcumin in human peripheral blood mononuclear cells (PBMCs) were shown, wherein it inhibits IL-2 and expression of NF-κB and PHA-induced proliferation(Yadav et al., 2005).
Interestingly, NK cells can launch direct attacks on virus-infected cells and modulate immune response via cytokine production. Therefore, it is necessary to examine their role in SARS-CoV-2 infection (Pasrija and Naime, 2021). Several studies have reported the considerably lower frequency of NK and CD8+ T cells with dysfunction and exhausted phenotypes along with hyperexpression of NKG2A inhibitory markers as well as PD-1 and Tim3 on CTLs (Zheng et al., 2020). NK cells can be divided into two subsets, dim and bright, according to CD56 surface density expression. Dim (CD3−CD56+dim CD16+) NK cells are cytolytic and comprise more than 90% of CD3−CD56+ NK cells, whereas bright (CD3−CD56+bright CD16−) NK cells are immunoregulatory principally through cytokine production. (Björkström et al., 2016; Lin et al., 2004), which both of them decrease significantly in blood circulation during the SARS-COV2 (Maucourant and Filipovic, 2020).
Moreover, CD56dim NK cells are divided into subtypes, less or more differentiated cells in terms of expressing CD62L, CD57, KIRs, and NKG2A markers. More previously published results have suggested the responsiveness of cytokine-driven NK cells during acute SARS-CoV-2 infection because of the dominance of CD56bright NK cells and less differentiated CD56dim NK cells (Björkström and Ponzetta, 2021; Maucourant and Filipovic, 2020).
Additionally, hyperactivation of NK cells resulting from IL-6, IL-6R, and IL-18, IL-15 induction has been found in the severe stage of COVID-19, leading to dysfunction and suppression of the cytotoxic activity of NK cells during long time (Koutsakos et al., 2021). NK cells are accounted for early responses in acute SARS-CoV-2 infection by recruiting the CD56bright and CD56dim NK cell population from the peripheral blood into the lungs, resulting in NK cell depletion in blood circulation (Maucourant and Filipovic, 2020; Wilk and Rustagi, 2020). The blood circulating NK cells are characterized by increased proliferation with higher expression levels of perforin and granzyme B cycling, and the activated phenotype of NK cells was found in acute SARS-CoV-2 infection with increased expression levels of HLA-DR, CD38, CD69, and Ki67 activating markers as well as TIM3, LAG3 and PD1 inhibitory markers. Moreover, less differentiated NKG2A+CD62L+CD57−KIR− cells in the CD56dim NK cells were the main population during acute SARS-CoV2 infection (Maucourant and Filipovic, 2020; Wilk and Rustagi, 2020). As a result of lymphopenia and leukocyte dysfunction during SARS-COV2 infection, declined count of NK cells and their impaired cytotoxic activity have been reported repeatedly, indicating the importance of these kinds of cells in defense against the virus.
On the other hand, NK cell absence leads to ARDS derived from severe hyper inflammation in the severe stage of the COVID-19 disease. Additionally, it is essential to highlight the importance of the complement system's role in SARS-COV2 infection. It is worth noting that C5a, an inflammatory factor, stimulates the release of cytokines and chemokines from various immune cells by linking to its receptor, C5aR (Yan and Gao, 2012). Elevated expression of C5aR receptor on NK cells and NKT cells and higher production of inflammatory cytokines were found after exposure of these kinds of cells to a sepsis-like immune environment such as SARS-COV2 infection (Carvelli et al., 2020; Fusakio et al., 2011). Altogether, the production of proinflammatory cytokines and C5a mediators' engagement with NK and NKT cells would contribute to hyperinflammation and subsequent occurrence of cytokine storm in COVID-19.
We hypothesize that Nanocurcumin could adjust immune dysregulation using NK cell activity as the biomarker and mitigate the inflammation by targeting inflammatory mediators. Therefore, we conducted this randomized double-blinded clinical trial to evaluate curcumin's clinical efficacy on NK cells, mentioned pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), and C5a in COVID-19 patients compared to placebo.
2. Methods and materials
2.1. Study design and patient selection
This is a placebo-controlled study that received the approval of the Research Ethics Committee of Tabriz University of Medical Sciences. Patients were selected among those admitted to Imam Reza hospital, affiliated with Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.1314). Moreover, the trial was registered in the Iranian Registry of Clinical Trials (IRCTID: IRCT20200324046851N1). After applying inclusion criteria, a total number of 60 confirmed COVID-19 patients and 60 healthy control subjects were considered eligible. The COVID-19 diagnosis was made by a pulmonologist based on laboratory data and clinical symptoms with respect to protocols. Then, a pre-designed informed consent form was obtained from all participants either in the patient or control group. COVID-19 patients group was later separated into placebo and Nanocurcumin groups. The inclusion criteria were: age between 18 and 70, eagerness to participate, and COVID-19 confirmed by RT-PCR. 10% (3 out of 30) of patients in the Nanocurcumin group and 6% (2 out of 30) of patients in the placebo group were on ventilators, with no significant difference between both groups.
In the case of a compromising immune condition (like immunosuppressive agent usage or human immunodeficiency virus (HIV) infection), pregnancy, and breastfeeding, subjects were excluded. The subject's information, including clinical symptoms and laboratory findings, is summarized in Table 1 .
Table 1.
Subjects clinical symptoms and laboratory finding.
| Nano-curcumin group (n = 30) | Placebo group (n = 30) | Healthy Control (n = 60) | ||
|---|---|---|---|---|
| Age, Years | 18-67 (52.7 ± 8.6) | 20-68 (52.4 ± 7.6) | 21-69 (50.3 ± 8.4) | |
| Sex | Men | 21 (%70) | 23 (%76.6) | 48 (%80) |
| Women | 9 (%30) | 7 (%23.3) | 12 (%20) | |
| Current smoking | 5 (%16.6) | 6 (%20) | 10 (%16.6) | |
| Diabetes | 1 (%3.3) | 2 (%6.6) | 0 | |
| Hypertension | 2 (%6.6) | 1 (%3.3) | 0 | |
| Cardiovascular disease | 2 (%6.6) | 2 (%6.6) | 0 | |
| Chronic kidney disease | 0 | 2 (%6.6) | 0 | |
| Fever | <37.3 °C | 3 (%10) | 1 (%3.3) | 0 |
| 37.3–38.0 °C | 11 (%36.6) | 14 (%46.6) | ||
| 38.1–39.0 °C | 9 (%30) | 9 (%30) | ||
| >39.0 °C | 7 (%23.3) | 6 (%20) | ||
| Cough | 18 (%60) | 20 (%66.6) | 0 | |
| Headache | 2 (%6.6) | 3 (%10) | 0 | |
| Dyspnea | 8 (%26.6) | 7 (%23.3) | 0 | |
|
White blood cell count, × 109/L |
<4 | 8 (%26.6) | 7 (%23.3) | 0 |
| 4–10 | 12 (%40) | 14 (%46.6) | 53(88.3%) | |
| >10 | 10 (%33.3) | 9 (%30) | 7 (11.7%) | |
|
Lymphocyte count, × 109/L |
<1·0 | 20 (%66.6) | 18 (%60) | 2 (3.3%) |
| ≥1·0 | 10 (%33.3) | 12 (%40) | 58 (96.7%) | |
| Platelet count, × 109/L | <100 | 19 (%63.3) | 21 (%70) | 0 |
| ≥100 | 11 (%36.6) | 9 (%30) | 60 (100%) | |
| Creatinine, μmol/L | ≤133 | 25 (%83.3) | 27 (%90) | 0 |
| >133 | 5 (%16.6) | 3 (%10) | ||
| Lactate dehydrogenase, U/L | ≤245 | 20 (%66.6) | 19 (%63.3) | 0 |
| >245 | 10 (%33.3) | 11 (%36.6) | ||
| Bilateral involvement of chest radiographs | 28 (%93.3) | 28 (%93.3) | 0 | |
| mechanical ventilation | 3 (10%) | 2 (6%) | 0 |
2.2. Therapeutic intervention
The Nanocurcumin group received 160 mg of nanocurcumin as two 80 mg dosages for 21 consecutive days, and on the other hand, the placebo group received placebo capsules. The oral curcumin capsules used in this study were “SinaCurcumin®”, a registered product from curcuminoids in Iran (IRC:1228225765), which were developed in the nanotechnology research center of Mashhad University of Medical Sciences and marketed by Exir nano Sina company (Hatamipour et al., 2019). Nanocurcumin oral capsules were administered to patients, known as improved oral bioavailability formulation of curcumin. SinaCurcumin is a soft gelatin capsule containing 40 mg of curcuminoids, the dietary polyphenols extracted from the dried rhizomes of Curcuma longa L. (turmeric), which is introduced as C3 complex [curcumin, bisdemethoxycurcumin (BDMC), and desmethoxycurcumin (DMC)] (Hatamipour et al., 2019; Rahimi et al., 2016). Differences that make Nanocurcumin more applicable and beneficial than curcumin for therapeutic applications include Particle size, hydrophobicity, as well as charge, and area of the surface that increases effectiveness, solubility, oral bioavailability, active targeting, and pharmacokinetic profile (Karthikeyan et al., 2020). Small particle size (mostly 10–100 nm size nanoparticles) improve the permeability, accessibility, and effectiveness of Nanocurcumin in different medicinal applications compared to curcumin. Nanocurcumin possesses a higher absorption capability into cells and can enter body organs more feasible than normal curcumin, especially in infections, by targeting intracellular pathogens (Flora et al., 2013). Thereby, Nanocurcumin could be considered as a useful drug choice compared to normal curcumin. Increased systemic bioavailability and higher distribution of Nanocurcumin in tissues than normal curcumin with 60 folds of enhancement in the biological half-life could be mentioned as another advantage (Ma et al., 2007). Curcumin nanoformulation has also been reported to augment its mean residence- retention- and circulation time inside the body (Mythri et al., 2007).
On the other hand, a larger surface area of Nanocurcumin elevates its solubility, improves pharmacological activity, fast drug-releasing, and specific responsiveness to target molecules (Mohanty et al., 2012). Interestingly, surface charges defined as zeta potential, established in Nanocurcumin formulation, avoid the aggregation of nanomaterials and increases the solubility and stability in suspension. At the same time, free curcumin is susceptible to aggregations and opsonization due to its lower solution in water (Muller and Keck, 2004).
In addition to Nanocurcumin, atorvastatin, bromhexine, azithromycin, protease inhibitors, broad-spectrum antibiotics, and corticosteroids were also the routine therapeutics used in both Nanocurcumin- and placebo-treated COVID-19 patients; therefore, did not affect the study findings, and the differences between groups were only related to the placebo and Nanocurcumin.
2.3. Blood sampling and sample preparation
Peripheral blood samples of healthy control and COVID-19 groups were obtained before treatment, and NK cell frequency, cytotoxicity, inflammatory cytokines, C5a serum level, and NK cell receptor gene expression were evaluated and compared between the two groups. Sampling was also done for Nanocurcumin and placebo group after treatment to assess the mentioned parameters. The PBMCs isolation was done using Ficoll (lymphosep) 1.077 g/ml (Biosera, UK) density gradient centrifugation method. The isolated PBMCs were used for flow cytometry analysis and mRNA expression assessment.
2.4. NK cells frequency assessment
To evaluate NK cell proportion, immunostaining and triple-color immunofluorescence analyses were done. Immunostaining was carried out by fluorescein isothiocyanate (FITC; BD Biosciences) labeled anti-CD3, phycoerythrin (PE; BD Biosciences) labeled Anti-CD16, and allophycocyanin (APC; BD Biosciences) labeled anti-CD56 antibodies to distinguish NK cells. Finally, the NK cell population was determined as CD3− CD56+ CD16+ cells. FITC, PE, and APC mouse IgG2a were used as the isotype controls. The gating strategy was based on the cells' forward and side scatter profiles. The FACSCalibur flow cytometer (BD Biosciences) and the Cell Quest Pro software (BD Biosciences, SanJose, CA) were used to measure the stained cells and data analysis, respectively.
2.5. NK cells cytotoxicity assessment
The relative proportion of killed K562 target cells by NK cells accounted for NK cells cytotoxicity and was evaluated by flow cytometry. After co-incubation of K562 target cells with patient mononuclear cells, including NK cells, propidium-iodide uptake was quantified. After 2 h of co-incubation at 37 °C with 5% CO2, the permeability of Killed K562 cells by NK cells allowed DNA staining by propidium iodide. Subsequently, the percentage of killed target cells was measured using flow cytometry. Beyond 15% of target cell killing is determined as increased cytotoxicity in an effector to target ratio of 50:1.
2.6. mRNA expression assessment of NK cells inhibitory and activating receptors
The expression of NK cells receptors, including KIR and C-type lectin inhibitory receptors (KIR2DL1, KIR2DL2, KIR2DL3, and NKG2A) and KIR2DS1, KIR2DS4, and NKG2C, activating receptors were evaluated by qRT-PCR based on SYBR-Green; therefore, total RNA of isolated PBMCs were extracted by RNX-PLUS Solution (SinaClon, Tehran, Iran). Subsequently, cDNA was synthesized by reverse transcription of extracted mRNA using the RevertAid Reverse Transcriptase kit (Thermo Fisher, Waltham, Massachusetts). An SYBR GREEN qRT-PCR Master Mix was used for amplification, and data analysis was carried out by a Light Cycler 2.0 Real-Time PCR System machine (Roche Applied Science, Germany). Beta-2 microglobulin (β2M) was selected as an endogenous control gene. The sequence of primers has been presented in Table 2 .
Table 2.
The primers sequence.
| Gene | Forward (5′-3′) /Reverse (3′-5′) |
Sequence | |
|---|---|---|---|
| Activating receptors | KIR2DS1 | F | ACGATGCACCTGTACGATCA |
| R | TCTTTCAACACGCAGGACAG | ||
| KIR2DS4 | F | AGAGAAACTAAAGAACTGGAC | |
| R | AGAGATATGTGTTTCATGTGCC | ||
| NKG2C | F | GCATAAAGACATACTCCAAACC | |
| R | ACTTCTCCACAACCCTCTG | ||
| Inhibitory receptors | KIR2DL1 | F | GTTGGTCAGATGTCATGTTTGAA |
| R | CCTGCCAGGTCTTGCG | ||
| KIR2DL2 | F | AAACCTTCTCTCTCAGCCCA | |
| R | GCCCTGCAGAGAACCTACA | ||
| KIR2DL3 | F | AGACCCTCAGGAGGTGA | |
| R | CAGGAGACAACTTTGGATCA | ||
| NKG2A | F | GTGATGATGAAGGAATCGTACC | |
| R | TAAATCCTCTGAAACTGCCG | ||
2.7. Measurements of IL-1β, IL-6, TNF-α, and C5a secretion levels by ELISA
Serum secretion levels of IL-1β, IL-6, TNF-α cytokines, as well as circulating C5a were assessed in the serum samples of COVID-19 patients (before and after treatment) and controlled by enzyme-linked immunosorbent assay (ELISA) technique using ELISA Kit (MyBioSource) for cytokines and HK349 ELISA Kit (Hycult Biotech) for C5a, according to the Manufacturer's protocols. HRP-conjugated secondary antibodies and tetramethylbenzidine, a peroxidase substrate, were applied to detect the abovementioned mediators. After stopping the reaction with acidification (H2SO4), the absorbance values were read by the Medgenix ELISA reader (BP-800; Biohit) at 450 nm. Standard curves were utilized to calculate the concentrations.
2.8. Statistical analysis
Statistical analysis was done by SPSS PC Statistics (version 19.0; SPSS Inc). An unpaired T-test was utilized to compare the differences in immunologic parameters between COVID-19 and the control group. To compare the parameters before and after treatment, paired T-test was used. The descriptive data were reported as the mean ± SD, and p values < 0.05 were considered to be statistically significant. The GraphPad Prism (version 7.00 for Windows) (GraphPad Software, La Jolla, California, www.graphpad.com) was used for graph drawing.
3. Results
3.1. Population and function of NK cells
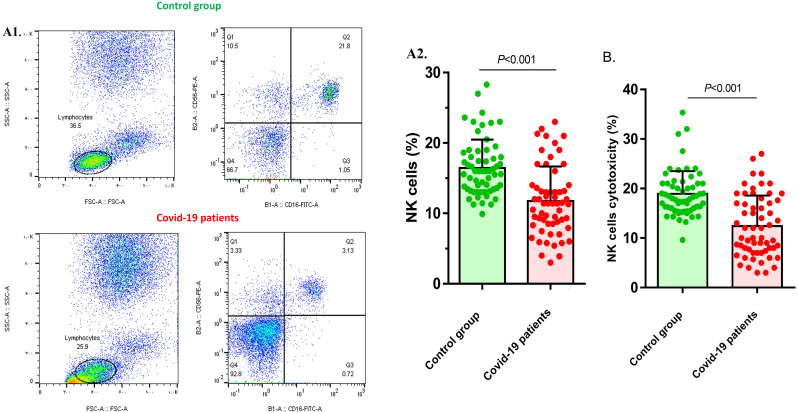
NK cell population was evaluated by flow cytometry to compare the frequency of NK cells in COVID-19 patients and the control group. The results showed that NK cell frequency was 16.45 ± 4.02 percent in each ml of whole blood in the control group, while it was decreased in COVID-19 patients (11.77 ± 4.87), and the difference was statistically significant (P < 0.001) (Fig. 1 A). The results of cytotoxicity evaluation also showed that NK cell cytotoxicity was significantly reduced in COVID-19 patients (12.42 ± 6.15) compared with the control group (18.92 ± 4.6) (P < 0.001) (Fig. 1B).
Fig. 1.
NK cells frequency and function in COVID-19 patients and control group. A1) The results pertaining to the flow cytometry analysis of the control group, and COVID-19 patients. A2) Flowcytometry analysis of NK cells frequency showed that NK cells population was significantly lower in patients in comparison with control group (p < 0.001). B) NK cells cytotoxicity was also reduced in COVID-19 patients when compared to control group (p < 0.001). COVID-19 patient group, n = 60. Control group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; NK cells, Natural killer cells.
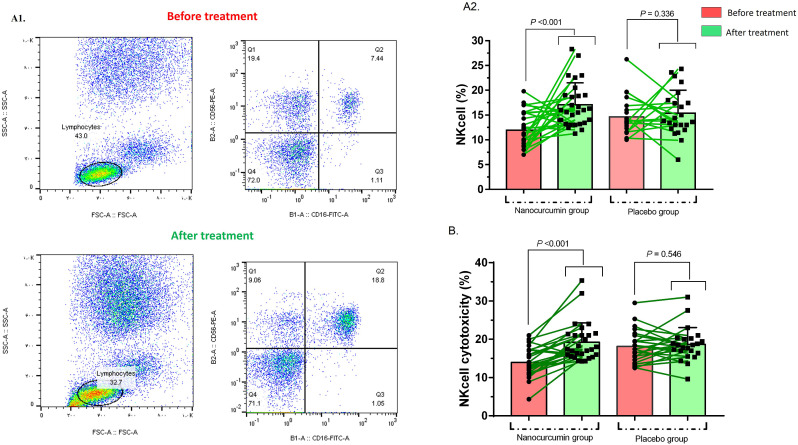
NK cell population was also compared in Nanocurcumin and placebo groups before and after the treatment. Before the treatment, the frequency of NK cells in the Nanocurcumin group was 11.94 ± 3.5%, while after the treatment, the frequency was dramatically elevated to 17.05 ± 4.3% (p < 0.001) (Fig. 2 A).
Fig. 2.
NK cells frequency and function in Nanocurcumin and placebo group. A1 & A2) Flowcytometry analysis of NK cells frequency showed that NK cells population was significantly increased in Nanocurcumin group after treatment (p = 0.001). B) NK cells cytotoxicity was also elevated after Nanocurcumin therapy (p < 0.001). COVID-19 patient group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; NK cells, Natural killer cells.
The comparison of NK cells' cytotoxicity pre-treatment (13.94 ± 3.7%) and post-treatment (19.21 ± 5.0%) demonstrated that Nanocurcumin was able to increase the cytotoxicity in the Nanocurcumin group notably (p < 0.001) (Fig. 2B).
3.2. Expression of NK cells receptors
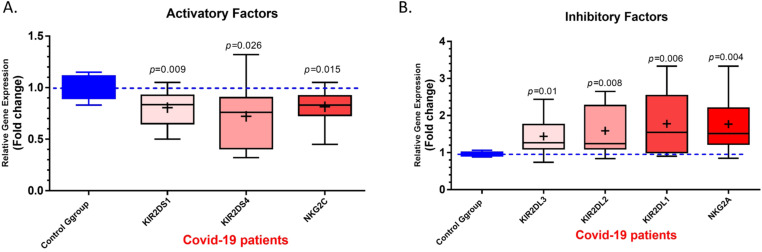
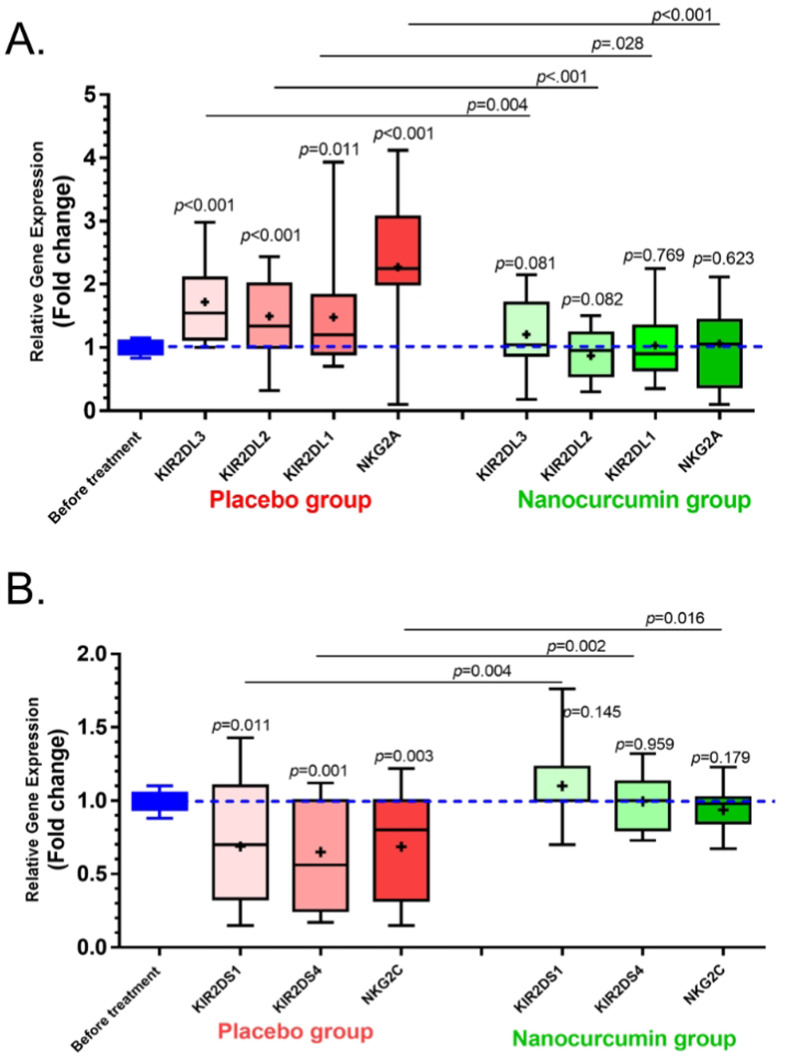
QRT-PCR assessment was done to evaluate the mRNA expression level of NK cells activating and inhibitory receptors. The expression levels of KIR2DS1, KIR2DS4, and NKG2C activating receptors of NK cells in the COVID-19 group were significantly lower than in the control group (p = 0.009, p = 0.026, and p = 0.015, respectively) (Fig. 3 A). In the case of inhibitory receptors, the expression levels of KIR2DL3, KIR2DL2, KIR2DL1, and NKG2A were increased in COVID-19 patients compared with the control group (p = 0.01, p = 0.008, p = 0.006, and p = 0.004, respectively) (Fig. 3B).
Fig. 3.
The mRNA expression level of Activating and inhibitory receptors in COVID-19 patients and control group. A) The results of qRT-PCR assessment for NK cells activating receptors demonstrated that the expression levels of KIR2DS1, KIR2DS4 and NKG2C in COVID-19 group were significantly lower than control group (p = 0.009, p = 0.026 and p = 0.015, respectively). B) The expression levels of KIR2DL1, KIR2DL2, KIR2DL3, and NKG2A inhibitory receptors were increased in COVID-19 patients in comparison with control group (p = 0.006, p = 0.008, p = 0.01and p = 0.004, respectively). COVID-19 patient group, n = 60. Control group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; NK cells, Natural killer cells; KIR, Killer-cell immunoglobulin-like receptor.
Activating and inhibitory receptor expression was also assessed in Nanocurcumin and placebo group after treatment. Initially, a comparison was made between the expression of receptors in the control group with Nanocurcumin and the placebo group. The expression levels of KIR2DL3, KIR2DL2, KIR2DL1, and NKG2A inhibitory receptors were higher in the placebo group in comparison with before treatment (P < 0.001, P < 0.001, P = 0.011, and P < 0.001, respectively). However, the expression level of mentioned receptors was decreased after Nanocurcumin therapy when compared to the placebo group (P = 0.028, P < 0.001, p = 0.004, and P < 0.001, respectively) (Fig. 4 A). The same analysis was done about activating receptors. The mRNA expression levels of KIR2DS1, KIR2DS4, and NKG2C activating receptors were significantly lower in the placebo group pre-treatment (P = 0.011, p = 0.001, and P = 0.003, respectively). The post-treatment results showed that Nanocurcumin therapy was able to upregulate the expression levels of activating receptors when compared to the placebo group (p = 0.004, p = 0.002, and p = 0.016, respectively) (Fig. 4B). All the results are also summarized in Table 3 .
Fig. 4.
The mRNA expression level of Activating and inhibitory receptors in Nanocurcumin and placebo group. A) The results of the qRT-PCR assessment for NK cells' inhibitory receptors demonstrated that the expression levels of KIR2DL1, KIR2DL2, KIR2DL3, and NKG2A of NK cells were significantly higher in the placebo group (p = 0.011, p < 0.001, p < 0.001 and p < 0.001, respectively). The expression levels of these receptors were also downregulated after Nanocurcumin therapy in comparison with the placebo group (p = 0.028, p < 0.001, p = 0.004, and p < 0.001, respectively). B) The mRNA expression levels of KIR2DS1, KIR2DS4 and NKG2C activating receptors were significantly lower in placebo group (p = 0.011, p = 0.001 and p = 0.003, respectively) and the post treatment results showed that Nanocurcumin therapy was able to upregulate the expression levels of these receptors when compared to placebo group (p = 0.004, p = 0.002 and p = 0.016, respectively). COVID-19 patient group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; NK cells, Natural killer cells; KIR, Killer-cell immunoglobulin-like receptor.
Table 3.
Real-time PCR (mRNA expression of activating and inhibitory receptors of NK cells) results.
| Prior to treatment |
Post treatment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 patients (Mean ± SD) | Healthy control (Mean ± SD) | p-value | Control | Placebo group (Mean ± SD) | P value | Control | Nano-curcumin group (Mean ± SD) | P value | P value (Nano-curcumin vs placebo) | ||
| mRNA expression level of activating and inhibitory receptors | KIR2DL3 | 1.437 ± 0.50 | 0.975 ± 0.06 | 0.01 | 0.998 ± 0.09 | 1.717 ± 0.63 | <0.001 | 0.998 ± 0.09 | 1.205 ± 0.59 | 0.081 | 0.028 |
| KIR2DL2 | 1.587 ± 0.65 | 0.008 | 1.495 ± 0.66 | <0.001 | 0.870 ± 0.38 | 0.082 | <0.001 | ||||
| KIR2DL1 | 1.778 ± 0.83 | 0.006 | 1.478 ± 0.85 | 0.011 | 1.030 ± 0.54 | 0.769 | 0.004 | ||||
| NKG2A | 1.769 ± 0.77 | 0.004 | 2.273 ± 0.94 | <0.001 | 1.060 ± 0.63 | 0.623 | <0.001 | ||||
| KIR2DS1 | 0.805 ± 0.16 | 1.011 ± 0.11 | 0.009 | 0.994 ± 0.06 | 0.688 ± 0.41 | 0.011 | 0.994 ± 0.06 | 1.100 ± 0.29 | 0.145 | 0.004 | |
| KIR2DS4 | 0.722 ± 0.30 | 0.026 | 0.649 ± 0.34 | 0.001 | 0.996 ± 0.19 | 0.959 | 0.002 | ||||
| NKG2C | 0.814 ± 0.17 | 0.015 | 0.685 ± 0.35 | 0.003 | 0.936 ± 0.15 | 0.179 | 0.016 | ||||
3.3. Secretion levels IL-1β, IL-6, TNF-α, and C5a
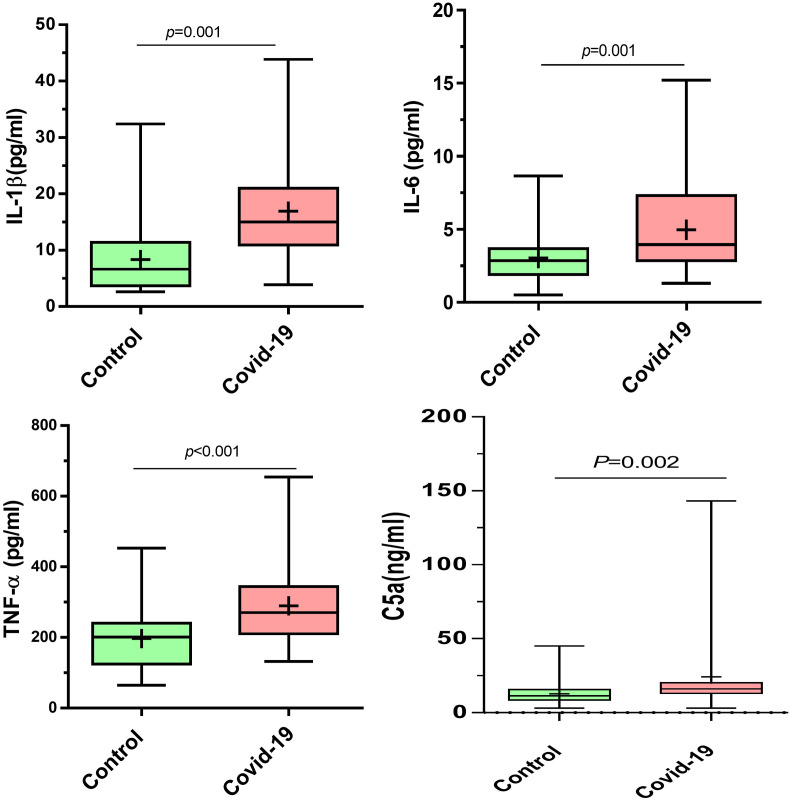
Serum levels of IL-1β, IL-6, TNF-α, and C5a were measured by ELISA in COVID-19 patients before and after treatment with Nanocurcumin and healthy control individuals. Based on obtained results, serum secretion levels of all IL-1β (p = 0.001), IL-6 (p = 0.001), TNF-α (p < 0.001), and C5a (p = 0.002) were significantly higher in COVID-19 patients compared to control group (Fig. 5 ). In the Nanocurcumin group, findings represented that the Nanocurcumin could meaningfully decrease the serum secretion levels of all IL-1β (p = 0.003), IL-6 (p = 0.008), TNF-α (p = 0.002), and C5a (p = 0.002) after treatment when compared to pre-treatment condition (Fig. 6 ). All the results are also summarized in Table 4 .
Fig. 5.
Serum levels of IL-1β, IL-6, TNF-α and C5a in COVID-19 patient and healthy control groups. The results of ELISA measurement revealed the significantly higher Serum levels of IL-1β (p < 0.001), IL-6 (p = 0.001), TNF-α (p < 0.001) and C5a (p = 0.002) in COVID-19 patients compared to the healthy control individuals. COVID-19 patient group, n = 60. Control group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; IL, interleukin; TNF-α, tumor necrosis factor alpha.
Fig. 6.
Serum levels of IL-1β, IL-6, TNF-α and C5a in Nanocurcumin- and placebo-treated groups. After treatment, findings indicated that the Nanocurcumin could considerably decreased the serum secretion levels of IL-1β (p < 0.001), IL-6 (p = 0.008), (p = 0.002), and C5a (p = 0.002) in Nanocurcumin-treated group than in placebo-treated group. COVID-19 patient group, n = 60. Results were presented as mean ± SD. P < 0.05 was described as statistically significant. COVID-19, coronavirus disease 2019; IL, interleukin; TNF-α, tumor necrosis factor alpha.
Table 4.
ELISA results.
| Prior to treatment (n = 60) |
Post treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 patients (Mean ± SD) | Healthy control (Mean ± SD) | p-value | Nano-curcumin group (Mean ± SD) (n = 28) |
Placebo group (Mean ± SD) (n = 23) |
|||||
| Before | After | p-value | Before | After | p-value | ||||
| IL-1β | 16.93 ± 9.76 | 8.34 ± 6.07 | 0.001 | 15.82 ± 7.43 | 11.48 ± 6.81 | 0.003 | 15.99 ± 10.08 | ؟15.38 ± 8.63 | 0.738 |
| IL-6 | 4.97 ± 3.2 | 3.04 ± 1.77 | 0.001 | 4.95 ± 3.06 | 3.82 ± 2.13 | 0.008 | 5.42 ± 4 | 4.95 ± 3.44 | 0.564 |
| TNF-α | 289.5 ± 122.3 | 197.3 ± 88.27 | <0.001 | 293.7 ± 120.1 | 234.8 ± 89.88 | 0.002 | 285.4 ± 127.5 | 279.5 ± 166.2 | 0.762 |
| C5a | 24.25 ± 28.07 | 12.76 ± 6.94 | 0.002 | 28.57 ± 29.07 | 17.96 ± 16.18 | 0.002 | 24.67 ± 31.88 | 20.24 ± 14.01 | 0.533 |
3.4. Clinical outcomes
A subject evaluation was done prior to the treatment in the case of clinical manifestation and laboratory tests, which are summarized in Table 1. Clinical symptoms such as fever, cough, headache, or chronic diseases like diabetes and hypertension were evaluated besides the laboratory tests, including white blood cells (WBCs) and platelet count. There were also chest radiographs to monitor the lung situation. No statistically significant difference was observed between the Nanocurcumin and placebo group before the treatment. However, there was a noticeable improvement in the clinical outcome of patients after the Nanocurcumin therapy; the associated data is summarized in Table 5 . The studied COVID-19 patients had 6% (2 out of 30) and 23% (7 out of 30) mortality rates in the Nanocurcumin- and placebo-treated groups, respectively. As a result, the mortality rate was found to be higher in the placebo-treated group than in the Nanocurcumin-treated group (p < 0.001).
Table 5.
Subjects clinical symptoms and laboratory finding before and after the treatment.
| Nano-curcumin group (n = 28) |
Placebo group (n = 23) |
||||||
|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 28) | P value | Before (n = 30) | After (n = 23) | P value | ||
| Fever | <37.3 °C | 3 (%10) | 21 (75%) | <0.001 | 1 (%3.3) | 2 (7%) | 0.546 |
| 37.3–38.0 °C | 11 (%36.6) | 7 (25%) | 14 (%46.6) | 10 (43%) | |||
| 38.1–39.0 °C | 9 (%30) | 0 (0%) | 9 (%30) | 8 (34%) | |||
| >39.0 °C | 7 (%23.3) | 0 (0%) | 6 (%20) | 4 (13%) | |||
| Cough | 18 (%60) | 3 (10%) | <0.001 | 20 (%66.6) | 6 (26%) | 0.038 | |
| Headache | 2 (%6.6) | 0 (0%) | – | 3 (%10) | 2 (8%) | – | |
| Dyspnea | 8 (%26.6) | 1 (3%) | <0.001 | 7 (%23.3) | 5 (21%) | 0.124 | |
| White blood cell count, × 109/L | <4 | 8 (%26.6) | 3 (10%) | <0.001 | 7 (%23.3) | 4 (17%) | 0.223 |
| 4–10 | 12 (%40) | 20 (71%) | 14 (%46.6) | 13 (56%) | |||
| >10 | 10 (%33.3) | 5 (17%) | 9 (%30) | 6 (26%) | |||
| Lymphocyte count, × 109/L | <1·0 | 20 (%66.6) | 7 (25%) | <0.001 | 18 (%60) | 9 (39%) | 0.043 |
| ≥1·0 | 10 (%33.3) | 21 (75%) | 12 (%40) | 14 (60%) | |||
| Platelet count, × 109/L | <100 | 19 (%63.3) | 6 (21%) | <0.001 | 21 (%70) | 15 (65%) | 0.367 |
| ≥100 | 11 (%36.6) | 22 (78%) | 9 (%30) | 8 (34%) | |||
| Creatinine, μmol/L | ≤133 | 25 (%83.3) | 27 (96%) | <0.0001 | 27 (%90) | 19 (82%) | |
| >133 | 5 (%16.6) | 1 (3%) | 3 (%10) | 4 (17%) | 0.381 | ||
| Lactate dehydrogenase, U/L | ≤245 | 20 (%66.6) | 26 (92%) | <0.0001 | 19 (%63.3) | 16 (69%) | |
| >245 | 10 (%33.3) | 2 (56%) | 11 (%36.6) | 7 (30%) | 0.184 | ||
| Bilateral involvement of chest radiographs | 28 (%93.3) | 9 (32%) | <0.0001 | 28 (%93.3) | 12 (52%) | 0.011 | |
| mechanical ventilation | 3 (10%) | 0 (0%) | – | 2 (6%) | 0 (0%) | – | |
4. Discussion
The COVID-19 pandemic has so far claimed over Four million lives globally. Moreover, the ongoing pandemic threatens to become a global humanitarian crisis since no aspect of human life has remained untouched by it (Ćurković et al., 2020). Various studies have concentrated on lightning up the shadowy transition of SARS-CoV-2 through a possible intermediate host(s) to humans (Yan et al., 2020). These efforts may provide invaluable data on effectively controlling or preventing future pandemics. Nonetheless, we believe that a decent understanding of COVID-19 immunopathology is pivotal for better managing the ongoing pandemic (Giamarellos-Bourboulis et al., 2020).
Acute lung injury (ALI) that could lead to acute respiratory distress syndrome (ARDS) is COVID-19 patients' significant morbidity and mortality cause (Li et al., 2020). It is hypothesized that lung inflammation triggers a series of events underlying acute lung injury (ALI). The unconstrained inflammatory response to SARS-CoV-2 infection may be partly due to the increased production of inflammatory cytokines (including IL-6 and TNF-α) (Market et al., 2020). These cytokines were even suggested to be used as predicting parameters for disease prognosis since numerous studies reported their increased concentration in severe COVID-19 cases (Fathi and Rezaei, 2020). The results of our previous study also confirmed that the expression and secretion level of inflammatory cytokines, including IL-1β, IL-6, TNF-α, and IL-18, were significantly increased in COVID-19 patients when compared to healthy controls (Valizadeh et al., 2020a).
As more evidence regarding the immunopathology of COVID-19 was accumulating, administration of immune-modulatory agents as a potential treatment came under consideration (Esmaeilzadeh et al., 2021a, 2021b). Anti-cytokines (i.e. Tocilizumab), corticosteroids (i.e. dexamethasone), and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) were among the evaluated medications for alleviating immunopathology COVID-19 trials. However, various studies concentrated on natural health substances with well-established immunomodulatory effects (Market et al., 2020). Curcumin (diferuloylmethane) is a natural pharmacologically active compound with a safe adverse reaction profile. As a consequence of extensive investigations, curcumin's capability to modulate diverse molecular signaling pathways has been directly linked to its different biological effects, such as anti-inflammatory, antioxidant, and antimicrobial (Aggarwal et al., 2003).
The cytokine storm is associated with severe COVID-19 cases mainly characterized by acute respiratory distress syndrome (ARDS), multi-organ failure, and death (Coperchini et al., 2020; Li et al., 2020). In this regard, we found dramatically higher levels of IL-1β, IL-6, and TNF-α in blood samples of COVID-19 patients than healthy controls. Interestingly, as we expected, Nanocurcumin could strikingly decline the secretion levels of the cytokines mentioned above after treatment.
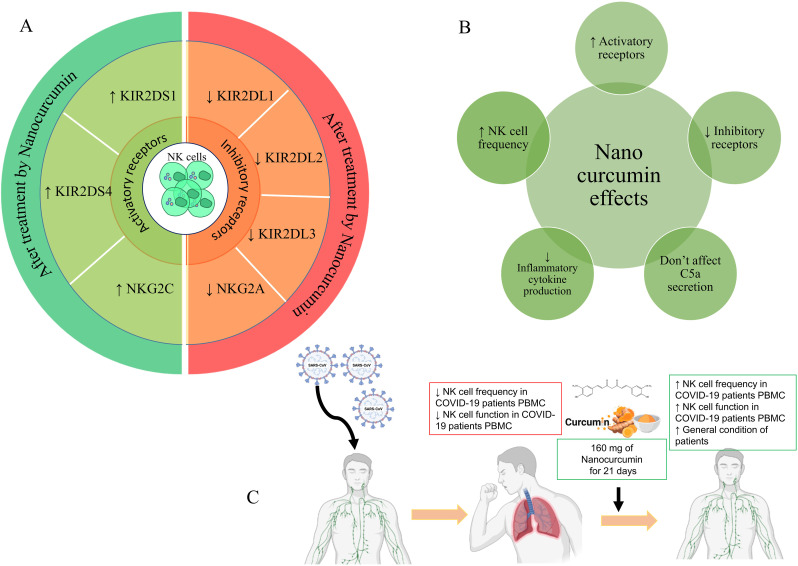
In line with our study, the therapeutic effect of Nanocurcumin in 2019-nCoV patients was recently proven in similar studies. The findings of recent studies documented the immunomodulatory and anti-inflammatory role of Nanocurcumin in reducing the proinflammatory cytokines (IL-1β and IL-6) in mRNA and protein levels (Valizadeh et al., 2020b), mitigating inflammatory Th17 cell responses (Tahmasebi et al., 2020) and increasing T regulatory (Treg) cell responses (Tahmasebi et al., 2021b) in COVID-19-treated patients, which was suggested as a potent natural-based immunomodulatory agent for improving the patients' condition. Moreover, in this line, the immunomodulatory role of Nanocurcumin has been proven in various inflammatory and autoimmune diseases such as ankylosing spondylitis (AS), multiple sclerosis (MS), inflammatory bowel disease (IBD), diabetes, and neurologic disorders, which increasingly emphasizes the therapeutic role of Nanocurcumin in inflammatory conditions and confirms our obtained results (Dolati et al., 2018a, 2018b; Ghosh et al., 2015; Hajialilo et al., 2019). It seems that nanocurcumin exerts a part of its anti-inflammatory function by inhibiting the expression and activation of different transcription factor, including NF-Kβ, inflammatory cytokines including TNF-α, IL-1β, and IL-6 and various chemokines including MIP-1α (Trivedi et al., 2017). However, all aspects of nanocurcumin's immunomodulation have not been discovered, and more studies are needed for this purpose (Fig. 7 ).
Fig. 7.
Different functions of nanocurcumin in modulating immune system responses. (A) Inhibitory functions that cause reductions in immune responses (B) Activating functions that lead to increased immune responses. (C) The use of Nanocurcumin by improving the function of NK cells leads to the improvement of patients' conditions.
Therefore, we decided to investigate the theory that curcumin application in COVID-19 patients could regulate the defect in the immune system. Imbalance of the immune system responses, which is characterized by an increase in inflammatory cells (Th1 and Th17), increased production of inflammatory mediators, as well as a decrease and dysfunction of regulatory cells (Tregs), is the leading cause of inflammation in SARS-CoV2 infected patients (Sadeghi et al., 2021). Following this fact, the disturbance of the immune system responses has been studied and proved in many autoimmune and inflammatory diseases, including Behçet's disease (Ahmadi and Yousefi, 2019), preeclampsia (Eghbal-Fard and Yousefi, 2019), MS (Izadi et al., 2020; Tahmasebi et al., 2021a), AS (Ahmadi et al., 2020), and recurrent implantation failure (RIF) (Ahmadi et al., 2019), which emphasize the immune system role in the pathogenesis of these type of disorders.
The profound lymphopenia seen in COVID-19 patients is due to depletion of T-cells, B-cells, and natural killer (NK) cells, probably caused by rapid virus replication. This lymphocyte exhaustion phenomenon was also reported in patients infected with SARS-CoV (Market et al., 2020). NK-cells, as innate responders, hold quite an important role in fighting viral infections and immune modulation (van Eeden et al., 2020). There is a lack of data on how NK-cells may directly contribute to failed control of SARS-CoV-2. However, evidence supports the hypothesis that NK-cell diminished function is linked to COVID-19 severity. Interestingly, obesity, old age, and malignancy are described by CDC (centers for disease control and prevention) as predisposing conditions against COVID-19 and are also associated with decreased NK-cell activity (Market et al., 2020).
Although the mechanism of NK cell depletion during SARS-COV2 infection is not clearly understood, it is probably due to the absence or decreased expression of activating receptors (KIR2DS and NKG2C) along with increased expression of inhibitory receptors (KIR2DL and NKG2A) on NK cells (Maucourant and Filipovic, 2020; Osman et al., 2020).
Our study results indicated the significantly lower counts of circulating NK cells in COVID-19 patients, which is consistent with previously published results reporting the notably declined number of NK cells in peripheral blood mononuclear cells (PBMCs) of COVID-19 patients (Taghiloo et al., 2021).
Further, our findings represented the meaningfully higher expression levels of inhibitory receptors on NK cells that may lead to NK cell dysfunction (Fig. 7). In this context, the upregulated expression level of inhibitory receptors on NK cells has been documented as one of the main reasons for NK cell depletion and dysregulated function in SARS-COV2 infection. Cytotoxicity of NK cells is prevented by NKG2A binding to the HLA-E molecule (Zheng et al., 2020). It was found that the upregulation of NKG2A on NK cell surface results in limited production of cytokines and Granzyme B during SAS-COV2 infection. Furthermore, COVID-19 infection elicits the exhausted phenotype of NK cells by inducing the expression of PDCD1, HAVCR, and LAG3 on their surface (Wilk and Rustagi, 2020). Some other evidence documented that downregulated expression of NKG2C, an activating receptor of NK cells, leads to a severe condition in COVID-19 (Vietzen et al., 2021). As a result, due to the reduction in the number of cells and their activity in patients, maybe increasing the reserves, activity, and restoration of these cells can help improve the condition of COVID-19 patients (Giamarellos-Bourboulis et al., 2020). The results of this study showed that Nanocurcumin, as a natural treatment with its immunomodulatory function, could increase the frequency of NK cells and their function in SARS-COV2 infected patients by upregulating the expression of activating receptors and downregulating inhibitory receptors on these cells. In our study, NK-cell number was used as a biomarker to determine Nanocurcumin efficacy against COVID-19 immune pathology. As stated in the results, Nanocurcumine could increase the lethal activity of cells in patients receiving Nanocurcumine compared with placebo. Consistent with these findings, previously, it was demonstrated that curcumin could intensify the NK cell cytotoxicity and killing activity (Trivedi et al., 2017). In a study, it was reported that Nanocurcumin could boosted the function of NK cells by increasing the expression of CD16+ and CD56dim on NK cells and activation of STAT4 and STAT5 in NK cells (Lee and Cho, 2018). Besides, another study has shown that curcuminoids could elevate the NK cells' cytotoxicity and killing function by stimulating the NK cells to secrete IFN-γ (Fiala et al., 2015). Considering the importance of the C5a complement fragment in the COVID-19 immunopathogenesis, serum levels of this mediator were evaluated in the current study. We found significantly increased serum levels of C5a in COVID-19 patients than in controls, which were significantly decreased after treatment with Nanocurcumin compared to placebo. It was demonstrated that C5a-C5aR involved in inflammatory responses and development of coagulopathy in SARS-COV2 infected patients (Risitano and Mastellos, 2020). Monocytes, macrophages, neutrophils, NK and NKT cells expressing C5aR were recruited to the lungs by binding to C5, which leads to the production of IL-1β, IL-6, and TNF-α pro-inflammatory cytokines and subsequently cytokine storm (Bosmann and Ward, 2012; Mastellos et al., 2020). Using anti-C5a antibody as a treatment choice in patients supports the abovementioned facts by elevating the lung oxygenation, mitigating the systemic inflammation, and mediating clinical improvement (Gao et al., 2020). In line with our obtained results, the increased serum levels of C5a have been reported recently in COVID-19 patients which was correlated with disease severity and were significantly higher in patients suffering from lung damage and ARDS. Also, it was suggested that the elevated level of C5a in the patients with the most-severe conditions has an important role in developing the inflammation mostly in ARDS patients (Carvelli et al., 2020; Gao et al., 2020).
5. Conclusion
The present study confirmed the presence of a dysregulated immune response, including diminished frequency and function of NK cells in addition to higher expression of inhibitory receptors and lower expression of stimulatory receptors in COVID-19 patients. The results of Nanocurcumin therapy as an immunomodulatory agent demonstrated that controlling the exaggerated immune response may improve NK cells function and infected patients' situation. Also, as a hopeful outcome, Nanocurcumin could considerably decrease the mortality rate in treated patients compared to the placebo-treated group. Therefore, Nanocurcumin may be a helpful choice in modulating immune system dysregulated responses like increased NK cells in COVID-19 patients.
CRediT authorship contribution statement
Sanaz Abbaspour-Aghdam: Investigation, Writing – original draft, Writing – review & editing. Ali Hazrati: Investigation, Writing – original draft, Writing – review & editing. Samaneh Abdolmohammadi-Vahid: Validation, Visualization. Safa Tahmasebi: Data curation, Formal analysis. Jafar Mohseni: Validation, Visualization. Hamed Valizadeh: Conceptualization, Supervision, Writing – review & editing. Mehdi Nadiri: Data curation, Formal analysis. Haleh Mikaeili: Methodology, Software. Armin Sadeghi: Validation, Visualization. Mehdi Yousefi: Conceptualization, Resources. Leila Roshangar: Conceptualization, Resources. Behzad Nikzad: Data curation, Formal analysis. Farhad Jadidi-Niaragh: Methodology, Project administration. Hossein Samadi Kafil: Methodology, Project administration. Kosar Malekpour: Validation, Visualization. Majid Ahmadi: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Acknowledgment
The authors gratefully acknowledge the financial support of this project by the Stem Cell Research Center (SCRC) at Tabriz University of Medical Sciences, Tabriz, Iran (grant No. 66469 and 66470).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2022.175267.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Ahmadi M., Abdolmohamadi-Vahid S., Ghaebi M., Dolati S., Abbaspour-Aghdam S., Danaii S., Berjis K., Madadi-Javid R., Nouri Z., Siahmansouri H. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: a double-blind, phase II randomized clinical trial. Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105730. [DOI] [PubMed] [Google Scholar]
- Ahmadi M., Hajialilo M., Dolati S., Eghbal‐Fard S., Heydarlou H., Ghaebi M., Ghassembaglou A., Aghebati‐Maleki L., Samadi Kafil H., Kamrani A. The effects of nanocurcumin on Treg cell responses and treatment of ankylosing spondylitis patients: a randomized, double‐blind, placebo‐controlled clinical trial. J. Cell. Biochem. 2020;121:103–110. doi: 10.1002/jcb.28901. [DOI] [PubMed] [Google Scholar]
- Ahmadi M., Yousefi M. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet's disease. J. Cell. Physiol. 2019;234:3985–3994. doi: 10.1002/jcp.27207. [DOI] [PubMed] [Google Scholar]
- Björkström N.K., Ljunggren H.G., Michaëlsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016;16:310–320. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- Björkström N.K., Ponzetta A. Natural killer cells and unconventional T cells in COVID-19. Curr. Opin. Virol. 2021;49:176–182. doi: 10.1016/j.coviro.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M., Ward P.A. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv. Exp. Med. Biol. 2012;946:147–159. doi: 10.1007/978-1-4614-0106-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli J., Demaria O., Vély F., Batista L., Chouaki Benmansour N., Fares J., Carpentier S., Thibult M.L., Morel A., Remark R., André P., Represa A., Piperoglou C., Cordier P.Y., Le Dault E., Guervilly C., Simeone P. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Regular and young investigator award abstracts. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćurković M., Košec A., Ćurković D. Math and aftermath of COVID-19 pandemic and its interrelationship from the resilience perspective. J. Infect. 2020;81(2):173–174. doi: 10.1016/j.jinf.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolati S., Aghebati‐Maleki L., Ahmadi M., Marofi F., Babaloo Z., Ayramloo H., Jafarisavari Z., Oskouei H., Afkham A., Younesi V. Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double‐blind, placebo‐controlled trial. J. Cell. Physiol. 2018;233:5222–5230. doi: 10.1002/jcp.26301. [DOI] [PubMed] [Google Scholar]
- Dolati S., Ahmadi M., Aghebti-Maleki L., Nikmaram A., Marofi F., Rikhtegar R., Ayromlou H., Yousefi M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018;70:1158–1167. doi: 10.1016/j.pharep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Eghbal-Fard S., Yousefi M. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell. Physiol. 2019;234:5106–5116. doi: 10.1002/jcp.27315. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh A., Jafari D., Tahmasebi S., Elahi R., Khosh E. Immune-based therapy for COVID-19. Adv. Exp. Med. Biol. 2021;1318:449–468. doi: 10.1007/978-3-030-63761-3_26. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh A., Rostami S., Yeganeh P.M., Tahmasebi S. Recent advances in antibody-based immunotherapy strategies for COVID-19. J. Cell. Biochem. 2021 doi: 10.1002/jcb.30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi N., Rezaei N. Lymphopenia in COVID‐19: therapeutic opportunities. Cell Biol. Int. 2020;44:1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M., Halder R., Almasi A., Sagong B., Leung J., Jewett A. Curcuminoids and ω-3 fatty acids with anti-oxidants potentiate cytotoxicity of natural killer cells against pancreatic ductal adenocarcinoma cells and inhibit interferon γ production. Front. Physiol. 2015;6:129. doi: 10.3389/fphys.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G., Gupta D., Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013;30:331–368. doi: 10.1615/critrevtherdrugcarriersyst.2013007236. [DOI] [PubMed] [Google Scholar]
- Fusakio M.E., Mohammed J.P., Laumonnier Y., Hoebe K., Köhl J., Mattner J. C5a regulates NKT and NK cell functions in sepsis. J. Immunol. 2011;187:5805–5812. doi: 10.4049/jimmunol.1100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., Dong Q., Zhang Z., Wang Z., Hu Y. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 [Google Scholar]
- Ghaebi M., Tahmasebi S., Jozghorbani M., Sadeghi A., Thangavelu L., Zekiy A.O., Esmaeilzadeh A. Risk factors for adverse outcomes of COVID-19 patients: possible basis for diverse responses to the novel coronavirus SARS-CoV-2. Life Sci. 2021;277 doi: 10.1016/j.lfs.2021.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Banerjee S., Sil P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem. Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajialilo M., Dolati S., Abdolmohammadi‐Vahid S., Ahmadi M., Kamrani A., Eghbal‐Fard S., Ghassembaglou A., Valizadeh A., Shenas M.H.M., Aghebati‐Maleki L. Nanocurcumin: a novel strategy in treating ankylosing spondylitis by modulating Th17 cells frequency and function. J. Cell. Biochem. 2019;120:12027–12038. doi: 10.1002/jcb.28488. [DOI] [PubMed] [Google Scholar]
- Hatamipour M., Sahebkar A., Alavizadeh S.H., Dorri M., Jaafari M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic Med. Sci. 2019;22:282–289. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi M., Tahmasebi S., Pustokhina I., Yumashev A.V., Lakzaei T., Alvanegh A.G., Roshangar L., Dadashpour M., Yousefi M., Ahmadi M. Changes in Th17 cells frequency and function after ozone therapy used to treat multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102466. [DOI] [PubMed] [Google Scholar]
- Karthikeyan A., Senthil N., Min T. Nanocurcumin: a promising candidate for therapeutic applications. Front. Pharmacol. 2020;11:487. doi: 10.3389/fphar.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S.A., Ahmad I., Chattopadhyay D. Academic Press; 2018. New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads. [Google Scholar]
- Komal K., Chaudhary S., Yadav P., Parmanik R., Singh M. The therapeutic and preventive efficacy of curcumin and its derivatives in esophageal cancer. Asian Pac. J. Cancer Prev. APJCP. 2019;20:1329. doi: 10.31557/APJCP.2019.20.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos M., Rowntree L.C., Hensen L., Chua B.Y., van de Sandt C.E., Habel J.R., Zhang W., Jia X., Kedzierski L., Ashhurst T.M., Putri G.H., Marsh-Wakefield F., Read M.N., Edwards D.N., Clemens E.B., Wong C.Y., Mordant F.L., Juno J.A., Amanat F., Audsley J., Holmes N.E., Gordon C.L., Smibert O.C., Trubiano J.A., Hughes C.M., Catton M., Denholm J.T., Tong S.Y.C., Doolan D.L., Kotsimbos T.C., Jackson D.C., Krammer F., Godfrey D.I., Chung A.W., King N.J.C., Lewin S.R., Wheatley A.K., Kent S.J., Subbarao K., McMahon J., Thevarajan I., Nguyen T.H.O., Cheng A.C., Kedzierska K. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell reports. Medicine. 2021;2 doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.H., Cho H. Improved anti-cancer effect of curcumin on breast cancer cells by increasing the activity of natural killer cells. J. Microbiol. Biotechnol. 2018;28:874–882. doi: 10.4014/jmb.1801.01074. [DOI] [PubMed] [Google Scholar]
- Lestari M.L., Indrayanto G. Curcumin. Profiles Drug Subst. Excipients Relat. Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- Li L., Huang Q., Wang D.C., Ingbar D.H., Wang X. Acute lung injury in patients with COVID-19 infection. Clin. Transl. Med. 2020;10:20–27. doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Gonzalez S., Cunningham-Rundles S., Dorante G., Marshall S., Tignor A., Ha C., Jacobson I., Talal A. CD56+ dim and CD56+ bright cell activation and apoptosis in hepatitis C virus infection. Clin. Exp. Immunol. 2004;137:408–416. doi: 10.1111/j.1365-2249.2004.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Shayeganpour A., Brocks D.R., Lavasanifar A., Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed. Chromatogr. : BMC (Biomed. Chromatogr.) 2007;21:546–552. doi: 10.1002/bmc.795. [DOI] [PubMed] [Google Scholar]
- Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., Boucher D.M., Ng J., Ardolino M., Auer R.C. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marofi F., Azizi R., Motavalli R., Vahedi G., Nasimi M., Yousefi M., Motavalli Y., Tahmasebi S., Gharibi T., Mohammed R.N., Etemadi J., Khiavi F.M. COVID-19: our current knowledge of epidemiology, pathology, therapeutic approaches, and diagnostic methods. Anti Cancer Agents Med. Chem. 2021 doi: 10.2174/1871520621666210201101245. [DOI] [PubMed] [Google Scholar]
- Mastellos D.C., Pires da Silva B.G.P., Fonseca B.A.L., Fonseca N.P., Auxiliadora-Martins M., Mastaglio S., Ruggeri A., Sironi M., Radermacher P., Chrysanthopoulou A., Skendros P., Ritis K., Manfra I., Iacobelli S., Huber-Lang M., Nilsson B., Yancopoulou D., Connolly E.S., Garlanda C., Ciceri F., Risitano A.M., Calado R.T., Lambris J.D. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucourant C., Filipovic I. vol. 5. 2020. (Natural Killer Cell Immunotypes Related to COVID-19 Disease Severity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty C., Das M., Sahoo S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012;9:2801–2811. doi: 10.1021/mp300075u. [DOI] [PubMed] [Google Scholar]
- Muller R.H., Keck C.M. Challenges and solutions for the delivery of biotech drugs--a review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004;113:151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mythri R.B., Jagatha B., Pradhan N., Andersen J., Bharath M.M. Mitochondrial complex I inhibition in Parkinson's disease: how can curcumin protect mitochondria? Antioxidants Redox Signal. 2007;9:399–408. doi: 10.1089/ars.2006.1479. [DOI] [PubMed] [Google Scholar]
- Osman M., Faridi R.M., Sligl W., Shabani-Rad M.T., Dharmani-Khan P., Parker A., Kalra A., Tripathi M.B., Storek J., Cohen Tervaert J.W., Khan F.M. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020;4:5035–5039. doi: 10.1182/bloodadvances.2020002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasrija R., Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int. Immunopharm. 2021;90 doi: 10.1016/j.intimp.2020.107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi H.R., Mohammadpour A.H., Dastani M., Jaafari M.R., Abnous K., Mobarhan M.G., Oskuee R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J. Phytomed. 2016;6:567. [PMC free article] [PubMed] [Google Scholar]
- Risitano A.M., Mastellos D.C. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., Nazemiyeh M., Aghebati‐Maleki L., Jadidi‐Niaragh F., Abbaspour‐Aghdam S. Th17 and Treg cells function in SARS‐CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236:2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- Taghiloo S., Aliyali M., Abedi S., Mehravaran H., Sharifpour A., Zaboli E., Eslami‐Jouybari M., Ghasemian R., Vahedi‐Larijani L., Hossein‐Nattaj H. Apoptosis and immunophenotyping of peripheral blood lymphocytes in Iranian COVID‐19 patients: clinical and laboratory characteristics. J. Med. Virol. 2021;93:1589–1598. doi: 10.1002/jmv.26505. [DOI] [PubMed] [Google Scholar]
- Tahmasebi S., Khosh E., Esmaeilzadeh A. The outlook for diagnostic purposes of the 2019-novel coronavirus disease. J. Cell. Physiol. 2020;235:9211–9229. doi: 10.1002/jcp.29804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S., Qasim M.T., Krivenkova M.V., Zekiy A.O., Thangavelu L., Aravindhan S., Izadi M., Jadidi‐Niaragh F., Ghaebi M., Aslani S. The effects of Oxygen‐Ozone therapy on regulatory T‐cell responses in multiple sclerosis patients. Cell Biol. Int. 2021 doi: 10.1002/cbin.11589. [DOI] [PubMed] [Google Scholar]
- Tahmasebi S., Saeed B.Q., Temirgalieva E., Yumashev A.V., El-Esawi M.A., Navashenaq J.G., Valizadeh H., Sadeghi A., Aslani S., Yousefi M., Jadidi-Niaragh F., Adigozalou J., Ahmadi M., Roshangar L. Life sciences; 2021. Nanocurcumin Improves Treg Cell Responses in Patients with Mild and Severe SARS-CoV2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi M.K., Mondal S.C., Gangwar M., Jana S. Immunomodulatory potential of nanocurcumin-based formulation. Inflammopharmacology. 2017;25:609–619. doi: 10.1007/s10787-017-0395-3. [DOI] [PubMed] [Google Scholar]
- Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Gencer M.Z., Ammari A., Sadeghi A., Roshangar L., Aslani S., Esmaeilzadeh A., Ghaebi M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharm. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Ziya Gencer M., Ammari A., Sadeghi A., Roshangar L., Aslani S., Esmaeilzadeh A., Ghaebi M., Valizadeh S., Ahmadi M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharm. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden C., Khan L., Osman M.S., Cohen Tervaert J.W. Natural killer cell dysfunction and its role in COVID-19. Int. J. Mol. Sci. 2020;21:6351. doi: 10.3390/ijms21176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietzen H., Zoufaly A., Traugott M., Aberle J., Aberle S.W., Puchhammer-Stöckl E. Deletion of the NKG2C receptor encoding KLRC2 gene and HLA-E variants are risk factors for severe COVID-19. Genet. Med. 2021;23:963–967. doi: 10.1038/s41436-020-01077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk A.J., Rustagi A. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V., Mishra K., Singh D., Mehrotra S., Singh V. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005;27:485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- Yan C., Gao H. New insights for C5a and C5a receptors in sepsis. Front. Immunol. 2012;3:368. doi: 10.3389/fimmu.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Shin W.I., Pang Y.X., Meng Y., Lai J. The first 75 Days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17072323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Tahmasebi S., El‐Esawi M.A., Mahmoud Z.H., Timoshin A., Valizadeh H., Roshangar L., Varshoch M., Vaez A., Aslani S., Navashenaq J.G., Aghebati‐Maleki L. Immunomodulatory effects of Nanocurcumin on Th17 cell responses in mild and severe COVID‐19 patients. J. Cell. Physiol. 2021 Jul;236(7):5325–5338. doi: 10.1002/jcp.30233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.