Summary
The (albumin-bilirubin) ‘ALBI’ score is an index of ‘liver function’ that was recently developed to assess prognosis in patients with hepatocellular carcinoma, irrespective of the degree of underlying liver fibrosis. Other measures of liver function, such as model for end-stage liver disease (MELD) and Child-Pugh score, which were introduced for specific clinical scenarios, have seen their use extended to other areas of hepatology. In the case of ALBI, its application has been increasingly extended to chronic liver disease in general and in some instances to non-liver diseases where it has proven remarkably accurate in terms of prognosis. With respect to chronic liver disease, numerous publications have shown that ALBI is highly prognostic in patients with all types and stages of chronic liver disease. Outside of liver disease, ALBI has been reported as being of prognostic value in conditions ranging from chronic heart failure to brain tumours. Whilst in several of these reports, explanations for the relationship of liver function to a clinical condition have been proposed, it has to be acknowledged that the specificity of ALBI for liver function has not been clearly demonstrated. Nonetheless, and similar to the MELD and Child-Pugh scores, the lack of any mechanistic basis for ALBI’s clinical utility does not preclude it from being clinically useful in certain situations. Why albumin and bilirubin levels, or a combination thereof, are prognostic in so many different diseases should be studied in the future.
Keywords: liver function, liver fibrosis, hepatocellular carcinoma, liver-related diseases, non-liver-related diseases, prognosis
Abbreviations: ALBI, albumin-bilirubin; APRI, aspartate aminotransferase-to-platelet ratio index; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease
Key points.
-
•
ALBI score/grade is a liver function measure which was originally developed as a prognostic factor for patients with HCC, and has since become well established.
-
•
ALBI score is objective and can detect smaller changes in liver dysfunction than the Child-Pugh or MELD scores.
-
•
The ALBI score/grade has been successfully applied to the prediction of survival in patients with non-malignant liver diseases of various aetiologies.
-
•
ALBI is often used as a standard measure of liver function when assessing other putative liver function indicators or assessing the changing degree of liver (dys)function.
-
•
The ALBI score/grade can reportedly be a prognostic factor for several non-liver-related malignant and non-malignant diseases, the mechanism of which should be clarified in future studies.
Introduction
The albumin-bilirubin (ALBI) score/grade was originally developed as a measure of liver function in patients with hepatocellular carcinoma (HCC).1 It was developed by putting all 5 of the original components of the Child-Pugh score into a multivariable model. The clinical features (ascites, encephalopathy and international normalised ratio) were shown to be redundant in that the entire prognostic value of the Child-Pugh score could be explained by just the albumin and bilirubin levels in an appropriate formulation derived from the multivariable model, i.e. the ‘Albumin-Bilirubin or ALBI score’. Furthermore, a review of the literature revealed more than 30 versions of the Child-Pugh score such that consistent scoring was difficult to achieve.2 To this extent, the ALBI score is a simply ‘refined’ version of the Child-Pugh score that excludes redundant features thereby and avoids the inconsistencies inherent in the Child-Pugh grading.
The ALBI ‘grade’, categorised on the basis of the ‘ALBI ‘score’ (from 1-3, 1 being best, 3 being the worst), was shown to be at least comparable with the conventional Child-Pugh score (Child-Pugh score) in terms of its prognostic ability both overall and for different disease stages and treatments. The fact that ALBI is calculated on the basis of just 2 objective laboratory values (albumin and bilirubin), without the requirement for subjective assessment of the extent of ascites or encephalopathy that is necessary for the Child-Pugh score, is also an important advantage. The granularity of the ALBI score permitted the detection of the small changes in liver function in patients with compensated cirrhosis that the Child-Pugh score was not sensitive enough to detect. This is particularly important in an era when the liver function of patients with HCC has been steadily improving,2,3 so that most patients with HCC have no liver (dys)function according to Child-Pugh score.2
The prognostic value of ALBI for all HCC treatments, including hepatic resection,[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20] locoregional ablative therapies,[21], [22], [23], [24], [25], [26] transarterial,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37] and systemic therapies[38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59] has been extensively reported and summarised by Demirtas et al. in this Journal60 and in systematic reviews and a meta-analysis.61,62 As with the Child-Pugh and model for end-stage liver disease (MELD) scores, both of which were introduced for specific clinical situations and then extended to more general applications, the same has happened with ALBI. Increasingly, ALBI is being applied as a preferred measure of liver function due to its objectivity and sensitivity for minor liver function deterioration, across several areas of medical practice and hepatology. In this review, we outline the use of ALBI score/grade across different specialties including general hepatology and beyond.
Beyond HCC: Application of the ALBI score/grade in general hepatological practice
The ALBI score/grade has been successfully applied to the prediction of survival in patients with non-malignant liver diseases of various aetiologies, including chronic viral hepatitis B,[63], [64], [65], [66] C,67 primary biliary cholangitis[68], [69], [70] and autoimmune hepatitis71 (Table 1). This is not surprising as liver function is an important indicator of the progression of liver disease, reflecting how near the patient is towards liver failure and liver-related mortality (Fig. 1).
Table 1.
Studies on ALBI as a prognostic factor in non-HCC patients with liver diseases.
| Authors | Study design | Patients | Aetiology | Subject | Main findings |
|---|---|---|---|---|---|
| Prediction of mortality/survival | |||||
| Chen et al.63 | Retrospective | 806 | HBV | Cirrhosis | ALBI score predicted long-term prognosis more accurately than MELD score or Child-Pugh class. |
| Wang et al.64 | Retrospective | 398 | HBV | Cirrhosis | ALBI score predicted long-term prognosis superior to MELD and MELD-Na score. |
| Qi et al.65 | Retrospective | 81 | HBV | Decompensated cirrhosis | ALBI and MELD scores predicted 1-month mortality. |
| Chen et al.66 | Retrospective | 84 | HBV | ACLF | ALBI and MELD scores were independent predictors of 3-month mortality. |
| Peng et al.77 | Retrospective | 100 | HBV | ACLF | Child-Pugh class, ALBI and MELD scores were ineffective in predicting the in-hospital mortality. |
| Fujita et al.67 | Retrospective | 382 | HCV | All HCV | ALBI score predicted overall survival. |
| Chan et al.68 | Retrospective | 61 | PBC | All PBC | ALBI score was the best for predicting liver-related events among Child-Pugh score, MELD score, Mayo risk score, Yale, European, and Newcastle model. ALBI grade stratified survivals. |
| Fujita et al.69 | Retrospective | 181 | PBC | All PBC | ALBI score differentiated liver transplant-free survival better than APRI. |
| Ito et al.70 | Retrospective | 409 | PBC | All PBC | ALBI score/grade and the Mayo score were superior prognostic tools among other prognostic tools. |
| Song et al.71 | Retrospective | 149 | AIH | Cirrhosis | ALBI predicted 6, 12, 24, and 36-month mortality more accurately than Child-Pugh scores and MELD score. ALBI grade 3 showed lower survival than ALBI grade 1 or 2. |
| Fragaki et al.72 | Retrospective | 195 | Various | Cirrhosis | ALBI score might be a better prognostic indicator of mortality than Child-Pugh score, MELD and MELD-Na scores. |
| Hsieh et al.73 | Retrospective | 242 | Various | Cirrhosis | ALBI score was associated with short-term outcome. ALBI was an independent predictor of survival as well as MELD, HVPG, and serum sodium. |
| Zou et al.74 | Retrospective | 631 | Various | Cirrhosis | The prognostic performance of ALBI score was comparable with that of the Child-Pugh and MELD scores for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. |
| Oikonomou et al.75 | Prospective | 325 | Various | Decompensated cirrhosis | ALBI was associated with survival or complications better than Child-Pugh class and MELD score. |
| Wan et al.76 | Retrospective | 456 | Various | Decompensated cirrhosis | ALBI score provided a reliable prediction of mortality as well as Child-Pugh score, MELD score, MELD-Na score, and iMELD score. |
| Bernardi et al.78 | Retrospective | 301 | Various | Transplantation | ALBI grade 3 was related to lower survival after liver transplantation. |
| Zhang et al.79 | Retrospective | 272 | Various | Living-donor transplantation | ALBI score, Child-Pugh score, and MELD score predicted 30-day mortality with complications. |
| Ma et al.80 | Retrospective | 258 | Various | Cadaveric transplantation | The ALBI score predicted overall survival and postoperative complications after liver transplantation. |
| Prediction of the development of HCC | |||||
| Fujita et al.84 | Retrospective | 91 | HBV | All HBV | ALBI scores < -2.190 correlated with better HCC-free survival. |
| Fujita et al.67 | Retrospective | 382 | HCV | All HCV | Smaller ALBI scores predict better HCC-free survival. |
| Casadei Gardini et al.85 | Retrospective | 514 | HCV | All HCV, after DAAs | ALBI score, platelet count and aspartate aminotransferase-lymphocyte ratio identified patients with higher risk of HCC. |
| Abe et al.86 | Retrospective | 188 | HCV | Cirrhosis, after SVR | ALBI score, platelet count, and diabetes were associated with HCC occurrence after SVR. |
| Tanaka et al.87 | Retrospective | 2,911 | HCV | Cirrhosis, after SVR | ALBI grades 2 or 3 was associated with higher HCC risk as well as higher age and serum AFP levels. |
| Caviglia et al.88 | Retrospective | 575 | HCV | Cirrhosis, after SVR | Only the ALBI score significantly associated with de novo HCC development among Forns index, APRI, FIB-4, and aMAP. |
| Prediction of liver-related complications other than HCC | |||||
| Hsieh et al.73 | Retrospective | 242 | Various | Cirrhosis | ALBI score is best correlated with hepatic venous pressure gradient. |
| Miyamoto et al.91 | Retrospective | 141 | Various | Cirrhosis | ALBI grade may be useful in predicting the presence of gastroesophageal varices and for stratifying bleeding risk. |
| Chen et al.92 | Retrospective | 1,102 | Various | Cirrhosis and HCC | Combination of ALBI grade and platelet counts predicted a presence of high-risk oesophageal varices and variceal haemorrhage. |
| Kawaguchi et al.93 | Retrospective | 883 | Various | Cirrhosis | ALBI score was the most impacted factor associated with severe portopulmonary hypertension. |
ALBI, albumin-bilirubin; APRI, aspartate aminotransferase-to-platelet ratio index; DAA, direct-acting antiviral; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; PBC, primary biliary cholangitis; SVR, sustained virological response.
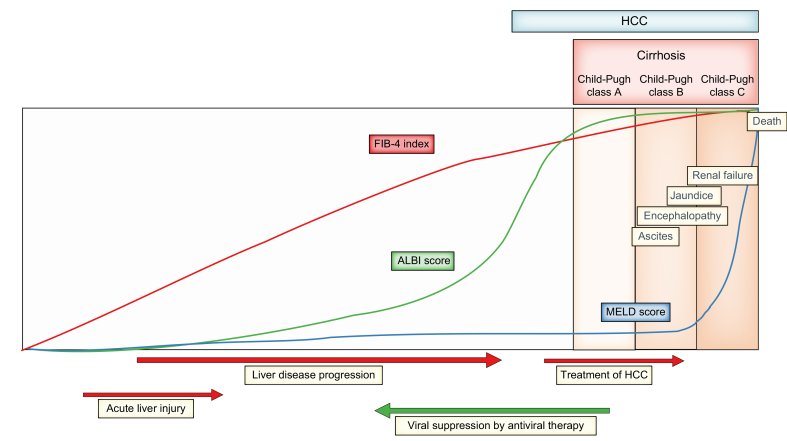
Fig. 1.
Schematic representation of the changes of ALBI score in comparison to FIB-4 index and MELD score, in association with the progression of liver diseases to end-stage liver disease.
ALBI score increases earlier than MELD score and before the development of cirrhosis, revealing slight deterioration in liver function. Liver function deteriorates with the treatment of HCC and may be restored by the eradication or suppression of viral hepatitis. ALBI, albumin-bilirubin; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease.
In comparison to the MELD score which, by definition, relates to ‘end-stage liver disease’, ALBI is sufficiently sensitive to detect the early deterioration of liver function (Fig. 1). Thus, ALBI might be expected to predict longer-term mortality in patients with cirrhosis[72], [73], [74] better than the MELD score, which would be expected to predict shorter-term mortality in patients with decompensated cirrhosis or liver failure. However, several, but not all, studies reported that ALBI is comparable to MELD for predicting short-term mortality in patients with decompensated cirrhosis.66,[75], [76], [77] Perhaps surprisingly, it has been reported that ALBI predicts outcomes for patients undergoing liver transplantation.[78], [79], [80] The initial ALBI report showed no difference in survival according to pre-transplant ALBI, implying that prognosis was attributable to the function of the new liver. It is possible that, in those studies citing an association between ALBI and post-transplant survival, ALBI was acting as a measure of general peri-operative ill health. The ALBI score is strongly associated with the degree of hepatic fibrosis, as assessed by the FIB-4 (fibrosis-4) index81,82 or aspartate aminotransferase-to-platelet ratio index (APRI).83 This observation presumably explains several reports that the ALBI score is an important risk factor for HCC development,67,[74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88] particularly after sustained virologic response.[86], [87], [88] The best documented risk score for HCC in patients with chronic liver disease is the aMAP score89,90 which combines ALBI with age, male sex and platelet count and may form the basis of a realistic risk stratification strategy.
In addition to HCC, ALBI has been reported to predict various complications associated with liver diseases, including portal hypertension,73 oesophagogastric varices,91,92 or portopulmonary hypertension.93 These can be understood in the context that ALBI reflects liver function and liver disease progression.
ALBI is often used as a standard measure of liver function when assessing other putative liver function indicators or assessing the changing degree of liver (dys)function (Table 2). In the area of radiology, the enhancement effects of liver images (i.e. uptake of contrast materials by the liver) on contrast-enhanced CT or MRI, are reportedly influenced by liver function as measured by ALBI.[94], [95], [96] Other studies have reported the effect of liver function, as assessed by ALBI, on serum levels of tumour markers, such as CA-125,97 and the frequency of muscle cramps in patients with cirrhosis.98 Several pharmacokinetic studies have assessed the role of liver dysfunction in relation to drug-induced adverse events.[99], [100], [101]
Table 2.
ALBI used as a measure of liver function.
| Authors | Study design | Patients | Subject | Main findings |
|---|---|---|---|---|
| Used as a standard measure of liver function | ||||
| Ozaki et al.94 | Retrospective | 303 | Dual-energy CT images | Comparison of hepatic extracellular volume fractions between liver segments based on ALBI grade. |
| Takatsu et al.95 | Retrospective | 220 | Hepatobiliary phase enhancement by EOB-MRI | Quantitative liver-spleen contrast ratio was correlated better by ALBI grade than by Child-Pugh score. |
| Takatsu et al.96 | Retrospective | 212 | Hepatobiliary phase enhancement by EOB-MRI | Quantitative liver-spleen contrast ratio and ALBI grade could predict the liver contrast enhancement effect in hepatobiliary phase images of EOB-MRI. |
| Edula et al.97 | Retrospective | 172 | Tumour marker | CA-125 concentration in cirrhotic patients based on liver function assessed by ALBI score, MELD score, and Child-Pugh class. |
| Shimada et al.98 |
Retrospective |
70 |
Muscle cramp |
Muscle cramp was observed more frequently in association of the deterioration of liver function assessed by ALBI score or Child-Pugh class. |
|
Liver function by ALBI and pharmacokinetics | ||||
| Shimizu et al.99 | Retrospective | 183 | Serum drug concentrations | ALBI score could be used to assess variations in the serum concentration of methadone. |
| Kokubun et al.100 | Retrospective | 25 | Drug-induced adverse event | ALBI score was significantly correlated with the incidence of ifosfamide-related neuropsychiatric symptoms. |
| Asai et al.101 |
Retrospective |
109 |
Drug-induced liver injury |
Low liver function assessed by ALBI was predictor for micafungin-induced liver injury. |
|
Serial changes in liver function assessed by ALBI | ||||
| Guha et al.102 | Retrospective | 379 | Liver disease progression | A scoring system, based on a combination of ALBI score and FIB-4 index, that identifies patients at risk for liver decompensation. |
| Sakamaki et al.103 | Retrospective and prospective | 159 | Liver disease progression | A longitudinal increase in the ALBI score is closely associated with non-malignancy-related mortality and quality of life. |
| Johnson et al.108 | Retrospective | 2,394 | Liver function after SVR in HCV | ALBI score decreased in SVR and increased in non-SVR in both IFN- and DAAs-treated patients. |
| Nakajima et al.109 | Retrospective | 403 | Liver function after SVR in HCV | ALBI grade decreased by SVR by DAA even in elderly patients. |
| Ogawa et al.110 | Retrospective | 392 | Liver function after SVR in HCV cirrhosis | FIB-4 index and ALBI score significantly decreased after SVR. |
| Tada et al.111 | Prospective | 65 | Liver function after SVR in HCV decompensated cirrhosis | ALBI scores decreased during and after treatment in patients who achieved SVR. |
| Waguri et al.112 | Retrospective | 57 | Liver function after B-RTO | ALBI scores and Child-Pugh class significantly decreased 3 years after B-RTO. |
| Ishikawa et al.113 | Retrospective | 21 | Liver function after treatment for encephalopathy | ALBI scores and CONUT score significantly decreased after rifaximin administration. |
| Kudo et al.104 | Retrospective | 534 | Liver function by ramucirumab for HCC | Ramucirumab for HCC did not negatively impact liver function assessed by ALBI grade. |
| Vogel et al.105 | Retrospective | 413 | Liver function by pembrolizumab for HCC | Pembrolizumab did not adversely impact liver function assessed by ALBI grade. |
| Muto et al.106 | Retrospective | 237 | Liver function by molecular-targeted therapy for HCC | Transient deterioration of liver function assessed by ALBI associated with sorafenib or lenvatinib. |
| Hiraoka et al.107 | Retrospective | 123 | Liver function by lenvatinib for HCC | Decline in hepatic function assessed by ALBI was common in the early stage after introducing lenvatinib. |
ALBI, albumin-bilirubin; B-RTO, balloon-occluded retrograde transvenous obliteration; DAA, direct-acting antiviral; EOB-MRI, ethoxybenzyl-MRI; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; IFN, interferon; SVR, sustained virological response.
Although ALBI is widely used according to grade, the score on which ‘grade’ is based is, in fact, a continuous variable and, as such, is particularly appropriate for assessment of changes in liver function over time. Thus, changes in ALBI score have been reported during the progression of liver disease to cirrhosis and to decompensation,99,100 during the course of systemic therapy for HCC, and in response to viral suppression by antiviral therapy or other therapies for liver diseases. In patients with HCC who had undergone systemic therapy, the adverse effect of molecular-targeted drugs or immune checkpoint inhibitors on liver function was often evaluated based on changes in ALBI score.[102], [103], [104], [105], [106], [107] Conversely, the restoration of liver function in association with viral eradication or suppression – by nucleot(s)ide analogues for HBV or interferon- or direct-acting antiviral-based therapy for HCV – has been characterised by a decrease in ALBI score.[108], [109], [110], [111] Further, the improvement of liver function associated with balloon-occluded retrograde transvenous obliteration for gastric varices or administration of rifaximin to control hyperammonaemia have been analysed with the ALBI score.112,113
Beyond liver diseases – ALBI in non-hepatic tumours
Since the original publication, there have been reports of a strong association between ALBI and mortality in many non-hepatological conditions (Table 3). For example, ALBI has been shown to be prognostic in cancers other than HCC, including pancreatic, colon and gastric cancer, intrahepatic cholangiocarcinoma, extrahepatic bile duct cancer, and even brain cancer (glioma and medulloblastoma).[114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136] ALBI is likely to be reflective of liver dysfunction in patients undergoing hepatic resection for intrahepatic tumours, such as intrahepatic cholangiocarcinoma or metastatic liver tumours, and tolerance for those undergoing systemic therapy, including chemotherapies, molecular-targeted drugs and immune checkpoint inhibitors.118,123,[126], [127], [128],131,133,134 Although most authors have attempted to link the association of ALBI with a particular cancer to liver function, we should consider whether its association with mortality is actually through some aspect of liver dysfunction or whether it simply reflects the prognostic power of serum albumin and bilirubin levels, independent of liver function. The mechanism underlying the prognostic impact of albumin and bilirubin remains unknown, but authors of the aforementioned studies have discussed the role of albumin as an indicator of malnutrition, and the immunomodulatory roles of both albumin and bilirubin. Further supporting the relative non-specificity of ALBI is its role in predicting very short-term mortality of patients with all malignancies in terminal care.137
Table 3.
Studies on ALBI as a prognostic factor in patients with non-liver-related diseases.
| Authors | Study design | Patients | Subject | Main findings |
|---|---|---|---|---|
|
Cancer other than HCC | ||||
| Li et al.114 | Retrospective | 535 | Intrahepatic cholangiocarcinoma | ALBI grade with prognostic nutritional index is a predictor for overall survival and progression-free survival after radical resection. |
| Tsilimigras et al.115 | Retrospective | 706 | Intrahepatic cholangiocarcinoma | ALBI score was associated with both short- and long-term mortalities following resection. |
| Yang et al.116 | Retrospective | 52 | Intrahepatic cholangiocarcinoma | ALBI grade was a significant biomarker for predicting survival in patients within the Milan criteria who underwent microwave ablation. |
| Ni et al.117 | Retrospective | 78 | Intrahepatic cholangiocarcinoma | ALBI grade was effective to predict long-term survivals of patients treated with CT-guided microwave ablation. |
| Deng et al.118 | Retrospective | 42 | Intrahepatic cholangiocarcinoma | The median overall survival of patients with ALBI grade 1 was longer than that of patients with ALBI grade 2 treated with PD-1-targeted immunotherapy. |
| Wang et al.119 | Retrospective | 109 | Extrahepatic cholangiocarcinoma | ALBI grade could be used as a predictor of survival in patients who underwent biliary stenting combined with iodine-125 seed implantation. |
| Fernandez-Placencia et al.120 | Retrospective | 101 | Ampullary of Vater cancer | ALBI grade and eGFR were predictors of mortality after pancreaticoduodenectomy. |
| Imamura et al.121 | Retrospective | 877 | Pancreatic cancer | ALBI grade was a predictor for overall survival in patients who underwent pancreatectomy. |
| Yagyu et al.122 | Retrospective | 100 | Pancreatic cancer | The combination of ALBI grade and CA19-9 concentration predicted overall survival. |
| Sakin et al.123 | Retrospective | 273 | Pancreatic cancer with liver metastasis | ALBI grade was related to overall survival and progression-free survival in patients with liver metastasis treated with a first-line chemotherapy. |
| Zhang et al.124 | Retrospective | 269 | Advanced pancreatic cancer | ALBI score was correlated with overall survival in patients with liver metastasis but not in patients without liver metastasis. |
| Koh et al.125 | Retrospective | 1,015 | Colorectal cancer | ALBI score was an independent factor associated with overall survival and further discriminated survival in combination with myosteatosis. |
| Watanabe et al.126 | Retrospective | 60 | Colorectal cancer with metastasis | ALBI score was significantly correlated with overall survival in patients receiving later-line chemotherapy with regorafenib. |
| Pereyra et al.127 | Retrospective | 339 | Colorectal cancer with liver metastasis | ALBI with APRI predicted liver dysfunction associated with neoadjuvant chemotherapy and postoperative mortality. |
| Abdel-Rahman et al.128 | Retrospective | 1,434 | Colorectal cancer with liver metastasis | Higher baseline ALBI score is associated with worse overall and progression-free survival in patients treated with first-line systemic therapy (panitumumab). |
| Zhu et al.129 | Retrospective | 243 | Gastric cancer | ALBI grade could predict postoperative complications and overall survival, especially those with TNM stages II-III. |
| Kanda et al.130 | Retrospective | 283 | Gastric cancer | ALBI grade was a predictive factor for disease-free and disease-specific survival in patients with pT2-4 cancer after radical gastrectomy. |
| Miwa et al.131 | Retrospective | 98 | Gastric cancer | ALBI was associated with the tolerability of postoperative adjuvant S-1 monotherapy in patients with pStage II/III cancer. |
| Kinoshita et al.132 | Retrospective | 947 | Non-small cell lung cancer | ALBI grade 2/3 was an independent predictor of worse cancer-specific survival in patients who underwent resection. |
| Matsukane et al.133 | Retrospective | 140 | Non-small cell lung cancer | ALBI grade was an independent prognostic factor for both progression-free survival and overall survival who received immune checkpoint inhibitors. |
| Takada et al.134 | Retrospective | 452 | Non-small cell lung cancer | The ALBI grade was an independent prognostic factor for survival in patients with advanced or recurrent cancer who receive anti-PD-1-based therapy. |
| Zhang et al.135 | Retrospective | 324 | High-grade glioma | ALBI score was independent predictor for both progression-free survival and overall survival in patients who received resection. |
| Zhu et al.136 | Retrospective | 111 | Medulloblastoma | ALBI score was a prognostic biomarker for overall survival in patients undergoing surgical resection as well as systemic immune-inflammation index and prognostic nutritional index. |
| Ieda et al.137 |
Retrospective |
483 |
Terminal cancer |
ALBI as well as CRP/albumin, prognostic nutritional index, FIB-4 and their combinations helped identify cancer patients who have a life expectancy less than 2 weeks. |
|
Non-malignant diseases | ||||
| Luo et al.138 | Retrospective | 3,381 | Heart failure | The ALBI score was useful at predicting the mortality of patients with heart failure requiring ICU admission. |
| Yamada et al.139 | Retrospective | 180 | Heart failure | ALBI score had a predictive value for death from heart failure in patients who underwent cardiac resynchronisation therapy. |
| Han et al.140 | Retrospective | 9,749 | Heart failure | ALBI score was an independent prognosticator of in-hospital mortality. |
| Saito et al.141 | Retrospective | 274 | Heart failure | Higher ALBI score was associated with higher all-cause mortality in cardiac resynchronisation therapy recipients. |
| Matsue et al.142 | Retrospective | 1,190 | Acute heart failure | ALBI score, but not the MELD score excluding prothrombin time was associated with fluid overload and was associated with 1-year mortality. |
| Kawata et al.143 | Retrospective | 262 | Acute heart failure | ALBI score was independently associated with in-hospital mortality in patients hospitalised for acute heart failure. |
| Shi et al.144 | Retrospective | 284 | Acute pancreatitis | ALBI score could be a useful marker of in-hospital mortality better than SOFA, SAPS II, APACHE II, Ranson and Glasgow scores. |
| Liu et al.145 | Retrospective | 812 | Aortic dissection | ALBI score as well as MELD score and APRI was associated with in-hospital and follow-up mortality in patients with type B aortic dissection treated with thoracic endovascular aortic repair. |
ALBI, albumin-bilirubin; APACHE, acute physiology and chronic health evaluation; APRI, aspartate aminotransferase-to-platelet ratio index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; MELD, model for end-stage liver disease; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment.
Beyond liver diseases – ALBI in benign non-liver disease
There have been several reports that the ALBI score is prognostic in certain non-hepatological conditions including acute or chronic heart failure (short-term and long-term prognosis),[138], [139], [140], [141], [142], [143] acute pancreatitis144 and aortic dissection. In some conditions the prognostic value is remarkably high. For example, in acute pancreatitis145 ALBI proved superior to well-established scores such as SOFA (sequential organ failure assessment), SAPS (simplified acute physiology score)-II, APACHE (acute physiology and chronic health evaluation)-II, Ranson and Glasgow. In each of these instances authors have attempted to explain their results in terms of liver dysfunction associated with the particular condition described. For example, in the case of cardiac failure, several authors have noted that elevated bilirubin, hypoalbuminaemia, and hepatic fibrosis often accompany advanced heart failure due to severe passive congestion.138 Whilst such explanations may be valid, it is important to acknowledge, as in the case of non-hepatological cancer, that these observations may cast doubt on the specificity of ALBI for liver function. However, such an observation does not decrease the value of ALBI in routine hepatological practice. The Child-Pugh score, of which ALBI is just a ‘refinement’, has never been shown to be specific for liver disease and yet it has been widely used, with benefit, for more than 40 years. However, there is considerable literature on the association of disturbances in both albumin and bilirubin metabolism with many chronic conditions. Such issues are worthy of further research and it is likely that, in different clinical conditions, different combinations of albumin and bilirubin, and perhaps other parameters, might prove to be optimal.
Conclusion and future perspectives
Severe deterioration of liver function causes serious morbidity irrespective of its origin. It is therefore not surprising that ALBI, a measure of liver function, can be a prognostic factor for diseases other than those of the liver. The Child-Pugh score has been used for a long time as a measure of liver (dys)function in patients with cirrhosis, predominantly by hepatologists to assess the severity of cirrhosis. In contrast, shortly after its original description, ALBI has been being widely used by healthcare specialists, this is perhaps due to its objectivity and simplicity. The severe liver dysfunction in patients with cirrhosis (i.e., right part of Fig. 1) is the area mainly dealt with by hepatologists. It is likely therefore that the Child-Pugh or MELD scores will continue to be used by hepatologists. In contrast, in many patients who do not have primary liver disease but may have modest liver dysfunction (i.e., middle or left parts of Fig. 1), the ALBI score can be used by non-hepatologists. Studies on the specificity of ALBI for liver function will be an interesting research area in the future.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Concept: Hidenori Toyoda and Philip J. Johnson. Data acquisition: Hidenori Toyoda. Drafting the manuscript: Hidenori Toyoda. Manuscript editing: Philip J. Johnson. Manuscript approval: Hidenori Toyoda and Philip J. Johnson.
Conflict of interest
The authors declare that there is no conflict of interest on this review.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100557.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L., et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson P., Pinato D.J., Kalyuzhnyy A., Toyoda H. Breaking the Child-Pugh dogma in hepatocellular carcinoma. J Clin Oncol. 2022;40:2078–2082. doi: 10.1200/JCO.21.02373. [DOI] [PubMed] [Google Scholar]
- 3.Kumada T., Toyoda H., Tada T., Yasuda S., Tanaka J. Changes in background liver function in patients with hepatocellular carcinoma over 30 years –comparison of Child-Pugh classification and albumin bilirubin grade–. Liver Cancer. 2020;9:518–528. doi: 10.1159/000507933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.Y., Zhong J.H., Su Z.Y., Huang J.F., Lu S.D., Xiang B.D., et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:725–734. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]
- 5.Li M.X., Zhao H., Bi X.Y., Li Z.Y., Huang Z., Han Y., et al. Prognostic value of the albumin- bilirubin grade in patients with hepatocellular carcinoma: validation in a Chinese cohort. Hepatol Res. 2017;47:731–741. doi: 10.1111/hepr.12796. [DOI] [PubMed] [Google Scholar]
- 6.Ho S.Y., Liu P.H., Hsu C.Y., Tan K., Zheng D., Du X., et al. Comparison of twelve liver functional reserve models for outcome prediction in patients with hepatocellular carcinoma undergoing surgical resection. Sci Rep. 2018;8:4773. doi: 10.1038/s41598-018-22923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao S., Wang M., Yang Z., Tan K., Zheng D., Du X., et al. Comparison between Child-Pugh score and Albumin-Bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC. Ann Transl Med. 2020;8:539. doi: 10.21037/atm.2020.02.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X.L., Zhou J.Y., Gao X.H., Tian L., Wu J., Zhang C.Y., et al. Application of the albumin- bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta. 2016;462:15–22. doi: 10.1016/j.cca.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z.R., Zou J., Sun D., Shi G.M., Ke A.W., Cai J.B., et al. Preoperative albumin-bilirubin score for postoperative solitary hepatocellular carcinoma within the Milan criteria and Child-Pugh A cirrhosis. J Cancer. 2017;8:3862–3867. doi: 10.7150/jca.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y.H., Koh Y.S., Hur Y.H., Cho C.K., Kim H.J., Park E.K. Effectiveness of the albumin-bilirubin score as a prognostic factor for early recurrence after curative hepatic resection for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg. 2018;22:335–343. doi: 10.14701/ahbps.2018.22.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho S.Y., Hsu C.Y., Liu P.H., Hsia C.Y., Su C.W., Huang Y.H., et al. Albumin-bilirubin (ALBI) grade-based nomogram to predict tumor recurrence in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2019;45:776–781. doi: 10.1016/j.ejso.2018.10.541. [DOI] [PubMed] [Google Scholar]
- 12.Xu W., Li R., Liu F. Novel Prognostic Nomograms for predicting early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Cancer Manag Res. 2020;12:1693–1712. doi: 10.2147/CMAR.S241959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M.Y., Qiao Q., Wang K., Ji G.W., Cai B., Li X.C. Development and validation of pre- and post-operative models to predict recurrence after resection of solitary hepatocellular carcinoma: a multi-institutional study. Cancer Manag Res. 2020;12:3503–3512. doi: 10.2147/CMAR.S251413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C.Y., Lin C.C., Wang C.C., Chen C.L., Hu T.H., Hung C.H., et al. The ALBI grade is a good predictive model for very late recurrence in patients with hepatocellular carcinoma undergoing primary resection. World J Surg. 2020;44:247–257. doi: 10.1007/s00268-019-05197-3. [DOI] [PubMed] [Google Scholar]
- 15.Cho W.R., Hung C.H., Chen C.H., Lin C.C., Wang C.C., Liu Y.W., et al. Ability of the post-operative ALBI grade to predict the outcomes of hepatocellular carcinoma after curative surgery. Sci Rep. 2020;10:7290. doi: 10.1038/s41598-020-64354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L., Liang R., Zhang J., Chen C., Chen X., Zhang Y., et al. Postoperative albumin-bilirubin grade and albumin-bilirubin change predict the outcomes of hepatocellular carcinoma after hepatectomy. Ann Transl Med. 2019;7:367. doi: 10.21037/atm.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J.Y., Sun L.Y., Quan B., Xing H., Li C., Liang L., et al. A novel online calculator based on noninvasive markers (ALBI and APRI) for predicting post-hepatectomy liver failure in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2020 doi: 10.1016/j.clinre.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Amisaki M., Uchinaka E., Morimoto M., Tokuyasu N., Sakamoto T., Honjo S., et al. Post- operative albumin-bilirubin grade predicts long-term outcomes among Child-Pugh grade A patients with hepatocellular carcinoma after curative resection. Hepatobiliary Pancreat Dis Int. 2018;17:502–509. doi: 10.1016/j.hbpd.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Zou H., Wen Y., Yuan K., Miao X.Y., Xiong L., Liu K.J. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018;38:494–502. doi: 10.1111/liv.13514. [DOI] [PubMed] [Google Scholar]
- 20.Fagenson A.M., Gleeson E.M., Pitt H.A., Lau K.N. Albumin-bilirubin score vs model for end- stage liver disease in predicting post-hepatectomy outcomes. J Am Coll Surg. 2020;230:637–645. doi: 10.1016/j.jamcollsurg.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg A., Witt U., Schernhammer M., Kornberg J., Muller K., Friess H., et al. The role of preoperative albumin-bilirubin grade for oncological risk stratification in liver transplant patients with hepatocellular carcinoma. J Surg Oncol. 2019;120:1126–1136. doi: 10.1002/jso.25721. [DOI] [PubMed] [Google Scholar]
- 22.Tai K., Kuramitsu K., Kido M., Tanaka M., Komatsu S., Awazu M., et al. Impact of albumin-bilirubin score on short- and long-term survival after living-donor liver transplantation: a retrospective study. Transpl Proc. 2020;52:910–919. doi: 10.1016/j.transproceed.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Oh I.S., Sinn D.H., Kang T.W., Lee M.W., Kang W., Gwak G.Y., et al. Liver function assessment using albumin-bilirubin grade for patients with very early-stage hepatocellular carcinoma treated with radiofrequency ablation. Dig Dis Sci. 2017;62:3235–3242. doi: 10.1007/s10620-017-4775-8. [DOI] [PubMed] [Google Scholar]
- 24.An C., Li X., Yu X., Cheng Z., Han Z., Liu F., et al. Nomogram based on albumin-bilirubin grade to predict outcome of the patients with hepatitis C virus-related hepatocellular carcinoma after microwave ablation. Cancer Biol Med. 2019;16:797–810. doi: 10.20892/j.issn.2095-3941.2018.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P.C., Chiu N.C., Su C.W., Huang Y.H., Hou M.C., Lin H.C., et al. Albumin-bilirubin grade may determine the outcomes of patients with very early stage hepatocellular carcinoma after radiofrequency ablation therapy. J Chin Med Assoc. 2019;82:2–10. doi: 10.1097/JCMA.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 26.Kao W.Y., Su C.W., Chiou Y.Y., Chiu N.C., Liu C.A., Fang K.C., et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology. 2017;285:670–680. doi: 10.1148/radiol.2017162382. [DOI] [PubMed] [Google Scholar]
- 27.Ho S.Y., Liu P.H., Hsu C.Y., Hsia C.Y., Lee Y.H., Lee R.C., et al. Prognostic role of noninvasive liver reserve markers in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waked I., Berhane S., Toyoda H., Chan S.L., Stern N., Palmer D., et al. Transarterial chemoembolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer. 2017;116:448–454. doi: 10.1038/bjc.2016.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I.C., Hung Y.W., Liu C.A., Lee R.C., Su C.W., Huo T.I., et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int. 2019;39:1704–1712. doi: 10.1111/liv.14194. [DOI] [PubMed] [Google Scholar]
- 30.Zhong B.Y., Ni C.F., Ji J.S., Yin G.W., Chen L., Zhu H.D., et al. Nomogram and artificial neural network for prognostic performance on the albumin-bilirubin grade for hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2019;30:330–338. doi: 10.1016/j.jvir.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Ni J.Y., Fang Z.T., Sun H.L., An C., Huang Z.M., Zhang T.Q., et al. A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol. 2020;30:2377–2390. doi: 10.1007/s00330-019-06438-8. [DOI] [PubMed] [Google Scholar]
- 32.Nam J.Y., Choe A.R., Sinn D.H., Lee J.H., Kim H.Y., Yu S.J., et al. A differential risk assessment and decision model for transarterial chemoembolization in hepatocellular carcinoma based on hepatic function. BMC Cancer. 2020;20:504. doi: 10.1186/s12885-020-06975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao S., Zhang T., Li H., Wang M., Xu K., Zheng D., et al. Comparison of albumin-bilirubin grade versus Child-Pugh score in predicting the outcome of transarterial chemoembolization for hepatocellular carcinoma using time-dependent ROC. Ann Transl Med. 2020;8:538. doi: 10.21037/atm.2020.02.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho S.Y., Hsu C.Y., Liu P.H., Lee R.C., Ko C.C., Huang Y.H., et al. Albumin-bilirubin (ALBI) grade-based nomogram for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Dig Dis Sci. 2021;66:1730–1738. doi: 10.1007/s10620-020-06384-2. [DOI] [PubMed] [Google Scholar]
- 35.Mohammadi H., Abuodeh Y., Jin W., Frakes J., Friedman M., Biebel B., et al. Using the albumin-bilirubin (ALBI) grade as a prognostic marker for radioembolization of hepatocellular carcinoma. J Gastrointest Oncol. 2018;9:840–846. doi: 10.21037/jgo.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gui B., Weiner A.A., Nosher J., Lu S.E., Foltz G.M., Hasan O., et al. Assessment of the albumin-bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol. 2018;41:861–866. doi: 10.1097/COC.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antkowiak M., Gabr A., Das A., Ali R., Kulik L., Ganger D., et al. Prognostic role of albumin, bilirubin, and ALBI scores: analysis of 1000 patients with hepatocellular carcinoma undergoing radioembolization. Cancers (Basel) 2019;11 doi: 10.3390/cancers11060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinato D.J., Yen C., Bettinger D., Ramaswami R., Arizumi T., Ward C., et al. The albumin- bilirubin grade improves hepatic reserve estimation post-sorafenib failure: implications for drug development. Aliment Pharmacol Ther. 2017;45:714–722. doi: 10.1111/apt.13904. [DOI] [PubMed] [Google Scholar]
- 39.King J., Palmer D.H., Johnson P., Ross P., Hubner R.A., Sumpter K., et al. Sorafenib for the treatment of advanced hepatocellular cancer -a UK audit. Clin Oncol (R Coll Radiol) 2017;29:256–262. doi: 10.1016/j.clon.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Lee P.C., Chen Y.T., Chao Y., Huo T.I., Li C.P., Su C.W., et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int. 2018;38:321–330. doi: 10.1111/liv.13527. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Rahman O. Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol. 2018;144:901–908. doi: 10.1007/s00432-018-2610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tada T., Kumada T., Toyoda H., Tsuji K., Hiraoka A., Michitaka K., et al. Impact of albumin- bilirubin grade on survival in patients with hepatocellular carcinoma who received sorafenib: an analysis using time-dependent receiver operating characteristic. J Gastroenterol Hepatol. 2019;34:1066–1073. doi: 10.1111/jgh.14564. [DOI] [PubMed] [Google Scholar]
- 43.Rovesti G., Orsi G., Kalliopi A., Vivaldi C., Marisi G., Faloppi L., et al. Impact of baseline characteristics on the overall survival of HCC patients treated with sorafenib: ten years of experience. Gastrointest Tumors. 2019;6:92–107. doi: 10.1159/000502714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo Y.H., Wang J.H., Hung C.H., Rau K.M., Wu I.P., Chen C.H., et al. Albumin-bilirubin grade predicts prognosis of HCC patients with sorafenib use. J Gastroenterol Hepatol. 2017;32:1975–1981. doi: 10.1111/jgh.13783. [DOI] [PubMed] [Google Scholar]
- 45.Takada H., Kurosaki M., Tsuchiya K., Komiyama Y., Itakura J., Takahashi Y., et al. Baseline and early predictors of good patient candidates for second-line after sorafenib treatment in unresectable hepatocellular carcinoma. Cancers (Basel) 2019;11:1256. doi: 10.3390/cancers11091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edeline J., Blanc J.F., Johnson P., Campillo-Gimenez B., Ross P., Ma Y.T., et al. A multicentre comparison between Child Pugh and albumin-bilirubin scores in patients treated with sorafenib for hepatocellular carcinoma. Liver Int. 2016;36:1821–1828. doi: 10.1111/liv.13170. [DOI] [PubMed] [Google Scholar]
- 47.Vogel A., Frenette C., Sung M.W., Daniele B., Baron A.D., Chan S.L., et al. Baseline liver function and outcomes in the phase III REFLECT study in patients with unresectable hepatocellular carcinoma (uHCC) J Clin Oncol. 2020;38 doi: 10.1159/000516490. 524-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueshima K., Nishida N., Hagiwara S., Aoki T., Minami T., Chishina H., et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: a multicenter study. Cancers (Basel) 2019;11:952. doi: 10.3390/cancers11070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimose S., Iwamoto H., Niizeki T., Shirono T., Noda Y., Kamachi N., et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel) 2020;12:1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimose S., Kawaguchi T., Iwamoto H., Tanaka M., Miyazaki K., Ono M., et al. Controlling nutritional status (CONUT) score is associated with overall survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter cohort study. Nutrients. 2020;12:1076. doi: 10.3390/nu12041076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatanaka T., Kakizaki S., Nagashima T., Namikawa M., Tojima H., Shimada Y., et al. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: a multicenter retrospective study. Hepatol Res. 2020;50:382–395. doi: 10.1111/hepr.13460. [DOI] [PubMed] [Google Scholar]
- 52.Hatanaka T., Kakizaki S., Nagashima T., Namikawa M., Ueno T., Tojima H., et al. Liver function changes in patients with hepatocellular carcinoma treated with lenvatinib: predictive factors of progression to Child-Pugh class B, the formation of ascites and the candidates for the post-progression treatment. Cancers (Basel) 2020;12:2906. doi: 10.3390/cancers12102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiraoka A., Kumada T., Tada T., Fukunishi S., Atsukawa M., Hirooka M., et al. Nutritional index as prognostic indicator in patients receiving lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology. 2020;98:295–302. doi: 10.1159/000506293. [DOI] [PubMed] [Google Scholar]
- 54.Chan S.L., Miksad R., Cicin I., Chen Y., Klumpen H.J., Kim S., et al. Outcomes based on albumin-bilirubin (ALBI) grade in the phase III CELESTIAL trial of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma (HCC) Ann Oncol. 2019;30:ix45–ix46. [Google Scholar]
- 55.Kudo M., Galle P.R., Brandi G., Kang Y.K., Yen C.J., Finn R.S., et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: results from REACH and REACH-2. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2020.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H.D., Bang Y., Lee M.A., Kim J.W., Kim J.H., Chon H.J., et al. Regorafenib in patients with advanced Child-Pugh B hepatocellular carcinoma: a multicentre retrospective study. Liver Int. 2020;40:2544–2552. doi: 10.1111/liv.14573. [DOI] [PubMed] [Google Scholar]
- 57.Sung P.S., Jang J.W., Lee J., Lee S.K., Lee H.L., Yang H., et al. Real-world outcomes of nivolumab in patients with unresectable hepatocellular carcinoma in an endemic area of hepatitis B virus infection. Front Oncol. 2020;10:1043. doi: 10.3389/fonc.2020.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong J.S.L., Kwok G.G.W., Tang V., Li B.C.W., Leung R., Chiu J., et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinato D.J., Kaneko T., Saeed A., Pressiani T., Kaseb A., Wang Y., et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: adjunctive role of the ALBI grade. Cancers (Basel) 2020;12:1862. doi: 10.3390/cancers12071862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demirtas C.O., D’Alessio A., Rimassa L., Sharma R., Pinato D.J. The ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng Y., Wei Q., He Y., Xie Q., Liang Y., Zhang L., et al. ALBI versus child-pugh in predicting outcome of patients with HCC: a systematic review. Expert Rev Gastroenterol Hepatol. 2020;14:383–400. doi: 10.1080/17474124.2020.1748010. [DOI] [PubMed] [Google Scholar]
- 62.Mishra G., Majeed A., Dev A., Eslick G.D., Pinato D.J., Izumoto H., et al. Clinical utility of albumin bilirubin grade as a prognostic marker in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a systematic review and meta-analysis. J Gastrointest Cancer. 2022 doi: 10.1007/s12029-022-00832-0. (in press) [DOI] [PubMed] [Google Scholar]
- 63.Chen R.C., Cai Y.J., Wu J.M., Wang X.D., Song M., Wang Y.Q., et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24:238–245. doi: 10.1111/jvh.12638. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Zhang Z., Yan X., Li M., Xia J., Liu Y., et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51:1172–1178. doi: 10.1016/j.dld.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Qi X.T. Albumin-bilirubin score predicts short-term mortality in patients with hepatitis B virus-related decompensated cirrhosis. Clin Lab. 2018;64:777–783. doi: 10.7754/Clin.Lab.2017.171134. [DOI] [PubMed] [Google Scholar]
- 66.Chen B., Lin S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujita K., Oura K., Yoneyama H., Shi T., Takuma K., Nakahara M., et al. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol Res. 2019;49:731–742. doi: 10.1111/hepr.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan A.W., Chan R.C.K., Wong G.L.H., Wong V.W.S., Choi P.C.L., Chan H.L.Y., et al. New simple prognostic score for primary biliary cirrhosis: albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391–1396. doi: 10.1111/jgh.12938. [DOI] [PubMed] [Google Scholar]
- 69.Fujita K., Nomura T., Morishita A., Shi T., Oura K., Tani J., et al. Prediction of transplant-free survival through albumin-bilirubin score in primary biliary cholangitis. J Clin Med. 2019;8:1258. doi: 10.3390/jcm8081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito T., Ishigami M., Morooka H., Yamamoto K., Imai N., Ishizu Y., et al. The albumin–bilirubin score as a predictor of outcomes in Japanese patients with PBC: an analysis using time-dependent ROC. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Y., Yang H., Lin L., Jiang K., Liu W.T., Wang B.M., et al. Albumin-to-bilirubin scores for assessing the prognosis in autoimmune hepatitis-related cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2019;27:772–776. doi: 10.3760/cma.j.issn.1007-3418.2019.10.007. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 72.Fragaki M., Sifaki-Pistolla D., Orfanoudaki E., Kouroumalis E. Comparative evaluation of ALBI, MELD, and Child-Pugh scores in prognosis of cirrhosis: is ALBI the new alternative? Ann Gastroenterol. 2019;32:626–632. doi: 10.20524/aog.2019.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh Y.C., Lee K.C., Wang Y.W., Yang Y.Y., Hou M.C., Huo T.I., et al. Correlation and prognostic accuracy between noninvasive liver fibrosis markers and portal pressure in cirrhosis: role of ALBI score. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou D., Qi X., Zhu C., Ning Z., Hou F., Zhao J., et al. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: a retrospective study. Turk J Gastroenterol. 2016;27:180–186. doi: 10.5152/tjg.2016.15502. [DOI] [PubMed] [Google Scholar]
- 75.Oikonomou T., Goulis L., Doumtsis P., Tzoumari T., Akriviadis E., Cholongitas E. ALBI and PALBI grades are associated with the outcome of patients with stable decompensated cirrhosis. Ann Hepatol. 2019;18:126–136. doi: 10.5604/01.3001.0012.7904. [DOI] [PubMed] [Google Scholar]
- 76.Wan S.Z., Nie Y., Zhang Y., Liu C., Zhu X. Assessing the prognostic performance of the Child-Pugh, model for end-stage liver disease, and albumin-bilirubin scores in patients with decompensated cirrhosis: a large Asian cohort from gastroenterology department. Dis Markers. 2020;2020 doi: 10.1155/2020/5193028. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Peng Y., Qi X., Tang S., Deng H., Li J., Ning Z., et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10:971–980. doi: 10.1080/17474124.2016.1177788. [DOI] [PubMed] [Google Scholar]
- 78.Bernardi N., Chedid M.F., Grezzana-Filho T.J.M., Chedid A.D., Pinto M.A., Leipnitz I., et al. Pre-transplant ALBI grade 3 is associated with increased mortality after liver transplantation. Dig Dis Sci. 2019;64:1695–1704. doi: 10.1007/s10620-019-5456-6. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W., Liu C., Tan Y., Tan L., Jiang L., Yang J., et al. Albumin-bilirubin score for predicting post-transplant complications following adult-to-adult living donor liver transplantation. Ann Transpl. 2018;23:639–646. doi: 10.12659/AOT.910824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma T., Li Q.S., Wang Y., Wang B., Wu Z., Lv Y., et al. Value of pretransplant albumin-bilirubin score in predicting outcomes after liver transplantation. World J Gastroenterol. 2019;25:1879–1889. doi: 10.3748/wjg.v25.i15.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 82.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 83.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 84.Fujita K., Nomura T., Morishita A., Oura K., Yoneyama H., Kobara H., et al. Albumin-bilirubin score differentiates liver fibrosis stage and hepatocellular carcinoma incidence in chronic hepatitis B virus infection: a retrospective cohort study. Am J Trop Med Hyg. 2019;101:220–225. doi: 10.4269/ajtmh.19-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casadei Gardini A., Foschi F.G., Conti F., Petracci E., Vukotic R., Marisi G., et al. Immune inflammation indicators and ALBI score to predict liver cancer in HCV-patients treated with direct-acting antivirals. Dig Liver Dis. 2019;51:681–688. doi: 10.1016/j.dld.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Abe K., Wakabayashi H., Nakayama H., Suzuki T., Kuroda M., Yoshida N., et al. Factors associated with hepatocellular carcinoma occurrence after HCV eradication in patients without cirrhosis or with compensated cirrhosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka Y., Ogawa E., Huang C.F., Toyoda H., Jun D.W., Tseng C.H., et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int. 2020;14:1023–1033. doi: 10.1007/s12072-020-10105-2. [DOI] [PubMed] [Google Scholar]
- 88.Caviglia G.P., Troshina G., Santaniello U., Rosati G., Bombaci F., Birolo G., et al. Long-term hepatocellular carcinoma development and predictive ability of non-invasive scoring systems in patients with HCV-related cirrhosis treated with direct-acting antivirals. Cancers (Basel) 2022;14:828. doi: 10.3390/cancers14030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan R., Papatheodoridis G., Sun J., Innes H., Toyoda H., Xie Q., et al. aMAP risk score predicts hepatocellular carcinoma development in chronic hepatitis patients: an international cohort collaboration. J Hepatol. 2020;73:1368–1378. doi: 10.1016/j.jhep.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 90.Johnson P.J., Innes H., Hughes D.M., Kalyuzhnyy A., Kumada T., Toyoda H. Evaluation of the aMAP score for hepatocellular carcinoma surveillance: a realistic opportunity to risk stratify. Br J Cancer. 2022 doi: 10.1038/s41416-022-01851-1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyamoto Y., Enomoto H., Nishikawa H., Nishimura T., Iwata Y., Nishiguchi S., et al. Association of the modified ALBI grade with endoscopic findings of gastroesophageal varices. In Vivo. 2021;35:1163–1168. doi: 10.21873/invivo.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen P.H., Hsieh W.Y., Su C.W., Hou M.C., Wang Y.P., Hsin I.F., et al. Combination of albumin-bilirubin grade and platelets to predict a compensated patient with hepatocellular carcinoma who does not require endoscopic screening for esophageal varices. Gastrointest Endosc. 2018;88:230–239. doi: 10.1016/j.gie.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 93.Kawaguchi T., Honda A., Sugiyama Y., Nakano D., Tsutsumi T., Tahara N., et al. Association between the albumin-bilirubin (ALBI) score and severity of portopulmonary hypertension (PoPH): a data-mining analysis. Hepatol Res. 2021;51:1207–1218. doi: 10.1111/hepr.13714. [DOI] [PubMed] [Google Scholar]
- 94.Ozaki K., Ishida T., Ohtani T., Shimada M., Kimura H., Gabata T. Assessing the progression of segmental fibrosis in chronic liver disease using extracellular volume fractions. Eur J Radiol. 2021;145 doi: 10.1016/j.ejrad.2021.110033. [DOI] [PubMed] [Google Scholar]
- 95.Takatsu Y., Kobayashi S., Miyati T., Shiozaki T. Hepatobiliary phase images using gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI as an imaging surrogate for the albumin-bilirubin grading system. Eur J Radiol. 2016;85:2206–2210. doi: 10.1016/j.ejrad.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Takatsu Y., Nakamura M., Kobayashi S., Miyati T. Prediction of sufficient liver enhancement on the gadoxetate disodium-enhanced hepatobiliary phase imaging using transitional phase images and albumin-bilirubin grade. Magn Reson Med Sci. 2021;20:152–159. doi: 10.2463/mrms.mp.2020-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edula R.G., Muthukuru S., Moroianu S., Wang Y., Lingiah V., Fung P., et al. CA-125 significance in cirrhosis and correlation with disease severity and portal hypertension: a retrospective study. J Clin Transl Hepatol. 2018;6:241–246. doi: 10.14218/JCTH.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimada M., Hirashima N., Iwase H., Saito M., Kondo H., Urata N., et al. Evaluation of muscle cramp associated with liver cirrhosis with a focus on the liver function and nutritional status. Intern Med. 2021;60:1343–1348. doi: 10.2169/internalmedicine.6231-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimizu S., Hayashi Y., Nishida S., Fujii H., Nakamura M., Yoshikura N., et al. Albumin-bilirubin score for predicting neuropsychiatric symptoms in patients receiving ifosfamide-based chemotherapy. J Clin Pharm Ther. 2021;46:794–799. doi: 10.1111/jcpt.13355. [DOI] [PubMed] [Google Scholar]
- 100.Kokubun H., Takigawa C., Chihara S., Hara S., Uezono Y. Population pharmacokinetics of methadone after oral administration in Japanese patients with cancer-related pain. J Pain Palliat Care Pharmacother. 2020;34:203–210. doi: 10.1080/15360288.2020.1785070. [DOI] [PubMed] [Google Scholar]
- 101.Asai Y., Yamamoto T., Sato Y. Risk assessment of micafungin-induced liver injury using spontaneous reporting system data and electronic medical records. J Infect Chemother. 2022;28:690–695. doi: 10.1016/j.jiac.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 102.Guha I.N., Harris R., Berhane S., Dillon A., Coffey L., James M.W., et al. Validation of a model for identification of patients with compensated cirrhosis at high risk of decompensation. Clin Gastroenterol Hepatol. 2019;17:2330–2338. doi: 10.1016/j.cgh.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 103.Sakamaki A., Takamura M., Sakai N., Watanabe Y., Arao Y., Kimura N., et al. Longitudinal increase in albumin-bilirubin score is associated with non-malignancy-related mortality and quality of life in patients with liver cirrhosis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kudo M., Galle P.R., Brandi G., Kang Y.K., Yen C.J., Finn R.S., et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: results from REACH and REACH-2. JHEP Rep. 2020;3 doi: 10.1016/j.jhepr.2020.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vogel A., Merle P., Verslype C., Finn R.S., Zhu A.X., Cheng A.L., et al. ALBI score and outcomes in patients with hepatocellular carcinoma: post hoc analysis of the randomized controlled trial KEYNOTE-240. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211039928. 17588359211039928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muto H., Kuzuya T., Ito T., Ishizu Y., Honda T., Ishikawa T., et al. Transient deterioration of albumin-bilirubin scores in early post-dose period of molecular targeted therapies in advanced hepatocellular carcinoma with 50% or higher liver occupation: a STROBE-compliant retrospective observational study. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000026820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hiraoka A., Kumada T., Atsukawa M., Hirooka M., Tsuji K., Ishikawa T., et al. Early relative change in hepatic function with lenvatinib for unresectable hepatocellular carcinoma. Oncology. 2019;97:334–340. doi: 10.1159/000502095. [DOI] [PubMed] [Google Scholar]
- 108.Johnson P.J., Berhane S., Walker A.J., Gordon F.H., Ryder S.D., McPherson S., et al. Impact of direct-acting antiviral agents on liver function in patients with chronic hepatitis C virus infection. J Viral Hepat. 2021;28:168–176. doi: 10.1111/jvh.13408. [DOI] [PubMed] [Google Scholar]
- 109.Nakajima T., Karino Y., Hige S., Suii H., Tatsumi R., Yamaguchi M., et al. Factors affecting the recovery of hepatic reserve after sustained virologic response by direct-acting antiviral agents in chronic hepatitis C virus-infected patients. J Gastroenterol Hepatol. 2021;36:367–375. doi: 10.1111/jgh.15280. [DOI] [PubMed] [Google Scholar]
- 110.Ogawa E., Kawano A., Ooho A., Furusyo N., Satoh T., Takahashi K., et al. Long-term hepatic function of patients with compensated cirrhosis following successful direct-acting antiviral treatment for hepatitis C virus infection. J Gastroenterol Hepatol. 2022;37:371–377. doi: 10.1111/jgh.15703. [DOI] [PubMed] [Google Scholar]
- 111.Tada T., Kurosaki M., Nakamura S., Hasebe C., Kojima Y., Furuta K., et al. Real-world clinical outcomes of sofosbuvir and velpatasvir treatment in HCV genotype 1- and 2-infected patients with decompensated cirrhosis: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Med Virol. 2021;93:6247–6256. doi: 10.1002/jmv.27157. [DOI] [PubMed] [Google Scholar]
- 112.Waguri N., Osaki A., Watanabe Y., Matsubara T., Yamazaki S., Yokoyama H., et al. Balloon-occluded retrograde transvenous obliteration for gastric varices improves hepatic functional reserve in long-term follow-up. JGH Open. 2021;5:1328–1334. doi: 10.1002/jgh3.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishikawa T., Endo S., Imai M., Azumi M., Nozawa Y., Sano T., et al. Changes in the body composition and nutritional status after long-term rifaximin therapy for hyperammonemia in Japanese patients with hepatic encephalopathy. Intern Med. 2020;59:2465–2469. doi: 10.2169/internalmedicine.5094-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Q., Chen C., Zhang J., Wu H., Qiu Y., Song T., et al. Prediction efficacy of prognostic nutritional index and albumin-bilirubin grade in patients with intrahepatic cholangiocarcinoma after radical resection: a multi-institutional analysis of 535 patients. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.769696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsilimigras D.I., Hyer J.M., Moris D., Sahara K., Bagante F., Guglielmi A., et al. Prognostic utility of albumin-bilirubin grade for short- and long-term outcomes following hepatic resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 706 patients. J Surg Oncol. 2019;120:206–213. doi: 10.1002/jso.25486. [DOI] [PubMed] [Google Scholar]
- 116.Yang H., Cheng Z., Han Z., Liu F., Yu X., Yu J., et al. Assessment of the outcomes of intrahepatic cholangiocarcinoma after ultrasound-guided percutaneous microwave ablation based on albumin-bilirubin grade. Cardiovasc Intervent Radiol. 2021;44:261–270. doi: 10.1007/s00270-020-02637-9. [DOI] [PubMed] [Google Scholar]
- 117.Ni J.Y., An C., Zhang T.Q., Huang Z.M., Jiang X.Y., Huang J.H. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int J Hyperthermia. 2019;36:328–336. doi: 10.1080/02656736.2019.1567834. [DOI] [PubMed] [Google Scholar]
- 118.Deng M., Li S., Wang Q., Zhao R., Zou J., Lin W., et al. Real-world outcomes of patients with advanced intrahepatic cholangiocarcinoma treated with programmed cell death protein-1-targeted immunotherapy. Ann Med. 2022;54:803–811. doi: 10.1080/07853890.2022.2048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y., Pang Q., Jin H., Zhou L., Hu X., Qian Z., et al. Albumin-bilirubin grade as a novel predictor of survival in advanced extrahepatic cholangiocarcinoma. Gastroenterol Res Pract. 2018;2018 doi: 10.1155/2018/8902146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fernandez-Placencia R., Berrospi-Espinoza F., Uribe-Rivera K., Medina-Cana J., Chavez-Passiuri I., Sanchez-Bartra N., et al. Preoperative predictors for 90-day mortality after pancreaticoduodenectomy in patients with adenocarcinoma of the ampulla of Vater: a single-centre retrospective cohort study. Surg Res Pract. 2021;2021 doi: 10.1155/2021/6682935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imamura T., Okamura Y., Sugiura T., Ito T., Yamamoto Y., Ashida R., et al. Clinical significance of preoperative albumin-bilirubin grade in pancreatic cancer. Ann Surg Oncol. 2021;28:6223–6235. doi: 10.1245/s10434-021-09593-9. [DOI] [PubMed] [Google Scholar]
- 122.Yagyu T., Saito H., Sakamoto T., Uchinaka E.I., Morimoto M., Amisaki M., et al. Preoperative albumin-bilirubin grade as a useful prognostic indicator in patients with pancreatic cancer. Anticancer Res. 2019;39:1441–1446. doi: 10.21873/anticanres.13260. [DOI] [PubMed] [Google Scholar]
- 123.Sakin A., Sahin S., Sakin A., Atci M.M., Yasar N., Arici S., et al. Assessment of pretreatment albumin-bilirubin grade in pancreatic cancer patients with liver metastasis. J BUON. 2020;25:1941–1946. [PubMed] [Google Scholar]
- 124.Zhang T.N., Yin R.H., Wang L.W. The prognostic and predictive value of the albumin-bilirubin score in advanced pancreatic cancer. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koh H.H., Cho E.S., Lee J.H., Shin S.J., Lee H.S., Park E.J., et al. Association of albumin-bilirubin grade and myosteatosis with its prognostic significance for patients with colorectal cancer. Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11445-z. (in press) [DOI] [PubMed] [Google Scholar]
- 126.Watanabe D., Fujii H., Yamada Y., Matsuhashi N., Makiyama A., Iihara H., et al. Association of albumin-bilirubin score in patients with colorectal cancer receiving later-line chemotherapy with regorafenib. Int J Clin Oncol. 2021;26:1257–1263. doi: 10.1007/s10147-021-01910-2. [DOI] [PubMed] [Google Scholar]
- 127.Pereyra D., Rumpf B., Ammann M., Perrodin S.F., Tamandl D., Haselmann C., et al. The combination of APRI and ALBI facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26:791–799. doi: 10.1245/s10434-018-07125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abdel-Rahman O. Prognostic value of baseline ALBI score among patients with colorectal liver metastases: a pooled analysis of two randomized trials. Clin Colorectal Cancer. 2019;18:e61–e68. doi: 10.1016/j.clcc.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 129.Zhu C., Wang X., Chen S., Yang X., Sun J., Pan B., et al. Efficacy of the preoperative albumin-bilirubin grade for predicting survival and outcomes of postoperative chemotherapy for advanced gastric cancer. Cancer Manag Res. 2020;12:11921–11932. doi: 10.2147/CMAR.S279782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kanda M., Tanaka C., Kobayashi D., Uda H., Inaoka K., Tanaka Y., et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pT2-4 gastric cancer. World J Surg. 2018;42:773–781. doi: 10.1007/s00268-017-4234-x. [DOI] [PubMed] [Google Scholar]
- 131.Miwa T., Kanda M., Tanaka C., Kobayashi D., Hayashi M., Yamada S., et al. Albumin-bilirubin score predicts tolerability to adjuvant S-1 monotherapy after curative gastrectomy. J Gastric Cancer. 2019;19:183–192. doi: 10.5230/jgc.2019.19.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kinoshita F., Yamashita T., Oku Y., Kosai K., Ono Y., Wakasu S., et al. Prognostic impact of albumin-bilirubin (ALBI) grade on non-small lung cell carcinoma: a propensity-score matched analysis. Anticancer Res. 2021;41:1621–1628. doi: 10.21873/anticanres.14924. [DOI] [PubMed] [Google Scholar]
- 133.Matsukane R., Watanabe H., Hata K., Suetsugu K., Tsuji T., Egashira N., et al. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takada K., Takamori S., Shimokawa M., Toyokawa G., Shimamatsu S., Hirai F., et al. Assessment of the albumin-bilirubin grade as a prognostic factor in patients with non-small-cell lung cancer receiving anti-PD-1-based therapy. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J., Xu Q., Zhang H., Zhang Y., Yang Y., Luo H., et al. High preoperative albumin-bilirubin score predicts poor survival in patients with newly diagnosed high-grade gliomas. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu S., Cheng Z., Hu Y., Chen Z., Zhang J., Ke C., et al. Prognostic value of the systemic immune-inflammation index and prognostic nutritional index in patients with medulloblastoma undergoing surgical resection. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.754958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ieda S., Miyamoto T., Hosomi K., Takegami M., Kawabata A. Identification of remaining life expectancy less than two weeks by C-reactive protein/albumin ratio, prognostic nutritional index, Fibrosis-4 index, and albumin-bilirubin score in terminal cancer patients. J Palliat Med. 2022 doi: 10.1089/jpm.2021.0243. (in press) [DOI] [PubMed] [Google Scholar]
- 138.Luo Y., Li Z., Liu J., Chong Y., Wu B. Prognostic value of the albumin-bilirubin score in critically ill patients with heart failure. Ann Palliat Med. 2021;10:12727–12741. doi: 10.21037/apm-21-3424. [DOI] [PubMed] [Google Scholar]
- 139.Yamada S., Kaneshiro T., Yoshihisa A., Nodera M., Amami K., Nehashi T., et al. Albumin-bilirubin score for prediction of outcomes in heart failure patients treated with cardiac resynchronization therapy. J Clin Med. 2021;10:5378. doi: 10.3390/jcm10225378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Han S., Wang C., Tong F., Li Y., Li Z., Sun Z., et al. Prognostic impact of albumin-bilirubin score on the prediction of in-hospital mortality in patients with heart failure: a retrospective cohort study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-049325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saito Y., Nakai T., Ikeya Y., Kogawa R., Otsuka N., Wakamatsu Y., et al. Clinical significance of the albumin-bilirubin score in patients with heart failure undergoing cardiac resynchronization therapy. Heart Vessels. 2022 doi: 10.1007/s00380-021-02008-5. (in press) [DOI] [PubMed] [Google Scholar]
- 142.Matsue Y., Kagiyama N., Yamaguchi T., Kuroda S., Okumura T., Kida K., et al. Clinical and prognostic values of ALBI score in patients with acute heart failure. Heart Lung Circ. 2020;29:1328–1337. doi: 10.1016/j.hlc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Kawata T., Ikeda A., Masuda H., Komatsu S. Association between albumin-bilirubin score at admission and in-hospital mortality in patients with acute heart failure. Int Heart J. 2021;62:829–836. doi: 10.1536/ihj.21-080. [DOI] [PubMed] [Google Scholar]
- 144.Shi L., Zhang D., Zhang J. Albumin-bilirubin score is associated with in-hospital mortality in critically ill patients with acute pancreatitis. Eur J Gastroenterol Hepatol. 2020;32:963–970. doi: 10.1097/MEG.0000000000001753. [DOI] [PubMed] [Google Scholar]
- 145.Liu J., Wu M., Xie E., Chen L., Su S., Zeng H., et al. Assessment of liver function for evaluation of short- and long-term outcomes in type B aortic dissection patients undergoing thoracic endovascular aortic repair. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.643127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.