Abstract
Background
Current evidence demonstrates that blood glucose fluctuation can be associated with depression and anxiety. The association among blood glucose fluctuation, traditional risk factors and emotional disorders in T2DM should be studied and clarified.
Methods
A total of 182 diabetic patients including 81 patients with depression or anxiety and 101 patients without emotional disorder were enrolled into this study. Data were obtained through medical history and questionnaire survey. Data were analyzed using appropriate statistical methods.
Results
The comparison results of basic information between the two groups showed that the differences of the proportion of female were statistically significant (p = 0.002).
There was no statistical difference in laboratory examination indexes between the two groups, however, standard deviation of blood glucose (SDBG) and postprandial glucose excursion (PPGE) of the comorbidity group were significantly higher than that of control group (p = 0.032 and p = 0.037). The results of questionnaire survey showed that there were statistically significant differences in sleep quality, PSQI and dietary habit between the two groups (p < 0.001, p < 0.001 and p < 0.001). Stratified analysis results according to gender showed that the percentage of cognitive disorder, anxiety and depression in female group was significantly higher than that in male group (p = 0.001, p < 0.001 and p < 0.001). Mini-mental state examination (MMSE), self-rating anxiety scale (SAS) and patient health questionnaire (PHQ-9) score in female group were also higher than male group (p = 0.001, p < 0.001 and p < 0.001). Logistic regression analysis results showed that SDBG and sleep quality were associated with emotional disorders in T2DM (p = 0.040 and p < 0.001) and the OR values of these factors were 7.588 (1.097–52.069) and 4.428 (2.649–7.401).
Conclusions
Blood glucose fluctuation and sleep quality are associated with the increased prevalence of depression and anxiety disorders in T2DM.
Keywords: Anxiety, Depression, Sleep quality, Blood glucose fluctuation, T2DM
Introduction
Despair, depression, anxiety are the common negative emotions in type 2 diabetes. Some studies indicated that the prevalence of anxiety and cognitive impairment were significantly increased in diabetic patients [1–4]. The proportion of depressive symptoms in diabetic patients is increasing gradually, the rate of depression in diabetes is approximate 20%-30%. More serious consequences occur in diabetes accompanied with emotional disorder patients [5–7]. An investigation including 328 T2DM was conducted by Liu's research group and the results showed that the prevalence of anxiety disorders in the patients with complications was significantly higher compared with diabetic patients without complications (48.76% VS. 24.33%) [8]. It showed that emotional disorders were associated with diabetic complications. Bogner et al. have suggested that the higher risk of death was found in diabetic patients combined with depression and anxiety [9]. Endocrinologists suggested that early identification of emotional disorders and multidisciplinary therapy could improve the prognosis of the disease, reduce the incidence of complications and mortality [10, 11]. Recent studies showed that blood glucose fluctuation was closely related to multiple complications, depression and anxiety in T2DM [12, 13]. Standard Deviation of Blood Glucose (SDBG), postprandial glucose excursion (PPGE) and largest amplitude of glycemic excursions (LAGE) were the common indicators of blood sugar fluctuations, which might be associated with depression and anxiety in diabetic patients.
Therefore, to assess the psychological condition of diabetic patients is beneficial for accurate diagnosis, treatment and blood glucose control. Early identification of poor mental state and potential risk factors are helpful to improve the life quality of diabetic patients.
Materials and methods
Study population
A total of 182 T2DM, who were hospitalized in endocrinology department of Jiading District Central Hospital from June 2019 to Apr 2021, became the research subjects of this study. The enrolled patients were distributed into comorbidity group and control group according to the diagnosis of anxiety and depression. After screening, the number of patients was 81 in the comorbidity group and 101 in the control group.
Inclusion and exclusion criteria
Inclusion criteria
i) T2DM was diagnosed according to the WHO diagnostic criteria in 2013.
ii) Anxiety and depression must be clearly diagnosed.
iii) Patients must have sufficient data for the study, including basic information and clinical examination results.
Exclusion criteria
i) Patients with mental disorders who cannot complete questionnaires and assessment scales were excluded.
ii) Diabetic patients with severe acute complications (serious infection, ketoacidosis, hyperosmolar coma, diabetes foot), hepatic or renal insufficiency, heart deficiency, malignant tumor, malignant anemia, had surgery should be ruled out.
iii) Patients receiving hormone therapy or antidepressant drug treatment were excluded.
Research methods and emotional disorders assessment
A questionnaire survey regarding the general information (such as age, gender, body mass index (BMI), smoking status, family history and other medical history materials), behaviors, life style and frequency of food consumption was conducted among the 182 enrolled participants. Meanwhile, the trained physicians assessed the emotional disorders by a variety of scales. The self-rating anxiety scale (SAS) was used to evaluate the state of anxiety. There are 20 items in SAS, the score of each item (range: 1–4) was depending on the severity. The final score was equal to the total score of 20 items multiplied by 1.25 and the standard score was greater than or equal to 50 indicate anxiety. Depressive position was estimated using PHQ-9 scale (0–4: no depression, 5–9: minor depression, 10–14: moderate depression, 15–19: moderately severe major depression, 20–27: severe major depression). Cognitive disorder was recognized by mini mental status examination (MMSE). The total score of the scale was 30 points and the person who score greater than 27 points was thought to be healthy. All patients signed the medical informed consent and agreed to participate in this research. Meanwhile, this study was approved by our local ethics committee.
Biochemical examination
Venipuncture was used to obtain venous blood and the samples were frozen in -70℃ refrigerator. HbA1c, serum cholesterol, hydrocortisone, thyroid function index, blood calcium, blood phosphorus and other indicators were tested in the two groups. Serum biochemicals were measured by automatic biochemical analyzer (Roche D/P/ISE, Switzerland) and HbA1c was measured by high-performance liquid chromatography (HLC-723g7, Japan). C-peptide and cortisol were tested by the method of chemiluminescent immunoassay (Abbott architect i2000 and AutoLumo A2000Plus, USA).
Calculation method of blood glucose fluctuation index
The blood glucose levels of pre-prandial and postprandial 2 h blood glucose of three meals and blood glucose before bedtime were measured, then these glucose values were marked as a, b, c, d, e, f, g, respectively. The blood glucose fluctuation indicators including PPGE, LAGE and SDBG were calculated according to the above glucose levels and the calculation formulas are shown below:
Statistical method
The software STATA version 12.0 (STATA Corp., College Station, Tex) was used to evaluate the collected data. Data consistent with the normal distribution were presented as mean ± standard deviation (Mean ± SD). The numeration data and categorical variables were compared by chi-square analysis or Fisher's exact test. Differences of continuous variables between the two groups were tested by Student’s t-test. Logistic regression analysis was used to identify the associated factors for depression and anxiety disorders in T2DM. The p value less than 0.05 was considered to be statistically significant.
Result
General information and clinical parameters
A total of 182 participants were enrolled into this study. The 81 diabetic patients were distributed into comorbidity group according to the diagnosis of anxiety and depression, the rest of the 101 T2DM were into control group. There were no significant differences in age, BMI, waistline, family history and the ratio of other chronic disease between the two groups. The basic information of the study population and analysis results were shown in Table 1. The results showed that the proportion of female in comorbidity group was significantly higher than the ratio of control group (p = 0.002). The clinical characteristics of all the participants were shown in Table 2. Compared with control group, the biochemical markers including thyroid function parameters and indicators of liver and kidney function were balanced and had no statistical differences. However, SDBG and PPGE in comorbidity group were higher than those in control group, and the differences were statistically significant (p = 0.032 and p = 0.037).
Table 1.
General information of the study population
| Comorbidity group(n = 81) | Control group (n = 101) |
F/χ2 | P | |
|---|---|---|---|---|
| Gendera | ||||
| Male | 34 (41.98%) | 66 (65.35%) | 9.92 | 0.002 |
| Female | 47 (58.02%) | 35 (34.65%) | ||
| Age | 56.11 ± 16.20 | 53.72 ± 13.61 | 1.17 | 0.281 |
| BMI | 25.30 ± 6.73 | 25.31 ± 3.69 | 0.00 | 0.990 |
| Waistline | 88.46 ± 13.98 | 90.24 ± 9.87 | 0.96 | 0.328 |
| Family history of diabetes | 31 38.27%) | 46 (45.54%) | 0.97 | 0.324 |
| Hypertension history | 45 55.56%) | 56 (55.45%) | 0.00 | 0.988 |
| Coronary heart disease history | 8 (9.88%) | 13 (12.87%) | 0.40 | 0.530 |
| Fatty liver | 34 (41.98%) | 48(47.52%) | 0.58 | 0.448 |
| Diabetic peripheral neuropathy | 41 (50.62%) | 44 (43.56%) | 0.90 | 0.343 |
| Diabetic retinopathy | 29 (35.80%) | 27(26.73%) | 1.74 | 0.188 |
| Atherosclerosis or plaque | 54 (66.67%) | 68 (67.33%) | 0.01 | 0.925 |
aThe difference was statistically significant
Table 2.
Clinical characteristics of the two groups
| Comorbidity group (n = 81) |
Control group (n = 101) |
F/χ2 | P | |
|---|---|---|---|---|
| Hba1c | 9.62 ± 2.45 | 9.90 ± 2.58 | 0.55 | 0.457 |
| Triglycerides | 1.70 ± 0.85 | 2.07 ± 1.59 | 3.46 | 0.065 |
| Total cholesterol | 4.44 ± 1.13 | 4.40 ± 1.23 | 0.06 | 0.811 |
| ALT | 27.04 ± 28.75 | 35.83 ± 36.18 | 3.18 | 0.076 |
| AST | 22.00 ± 17.52 | 25.13 ± 23.32 | -0.59 | 0.122 |
| Creatinine, μmol/L | 65.55 ± 25.37 | 69.81 ± 25.18 | 1.26 | 0.264 |
| 25-dihydroxyvitamin-D | 14.98 ± 7.06 | 16.37 ± 6.80 | 1.78 | 0.184 |
| Serum calcium | 2.37 ± 0.16 | 2.39 ± 0.13 | 0.70 | 0.404 |
| Serum phosphate | 1.16 ± 0.21 | 1.18 ± 0.18 | 0.38 | 0.539 |
| UA | 318.5 ± 112.65 | 347.76 ± 126.66 | 2.63 | 0.107 |
| FT3 | 4.20 ± 2.37 | 4.06 ± 1.56 | 0.24 | 0.626 |
| FT4 | 13.91 ± 3.57 | 14.26 ± 3.59 | 0.41 | 0.524 |
| TSH | 1.97 ± 1.46 | 1.89 ± 1.21 | 0.15 | 0.699 |
| TPO-Ab | 40.63 ± 152.11 | 21.57 ± 69.73 | 1.19 | 0.277 |
| TG-Ab | 20.24 ± 82.29 | 25.08 ± 79.97 | 0.15 | 0.695 |
| Cortisol (8:00 AM) | 344.42 ± 98.11 | 335.37 ± 127.53 | 0.23 | 0.636 |
| Cortisol (16:00 PM) | 195.59 ± 86.33 | 183.56 ± 82.97 | 0.72 | 0.399 |
| Cortisol (24:00 PM) | 126.53 ± 93.73 | 124.73 ± 97.32 | 0.01 | 0.912 |
| C-peptide level | 1.47 ± 0.96 | 1.61 ± 0.91 | 0.89 | 0.346 |
| C-peptide level(1 h) | 2.73 ± 2.43 | 3.60 ± 7.13 | 0.82 | 0.366 |
| C-peptide level(2 h) | 4.11 ± 3.19 | 5.17 ± 6.62 | 1.62 | 0.205 |
| FBG | 8.72 ± 2.67 | 8.41 ± 2.73 | 0.55 | 0.460 |
| PBG(1 h) | 13.88 ± 4.58 | 13.39 ± 3.47 | 0.52 | 0.474 |
| PBG(2 h) | 16.45 ± 5.33 | 15.93 ± 4.92 | 0.45 | 0.506 |
| SDBGa | 1.29 ± 1.29 | 0.98 ± 0.34 | 4.68 | 0.032 |
| PPGEa | 2.61 ± 3.11 | 1.83 ± 1.36 | 4.43 | 0.037 |
| LAGE | 7.45 ± 8.34 | 6.23 ± 2.40 | 1.72 | 0.192 |
aThe difference was statistically significant
Comparison of sleep status and life behavior
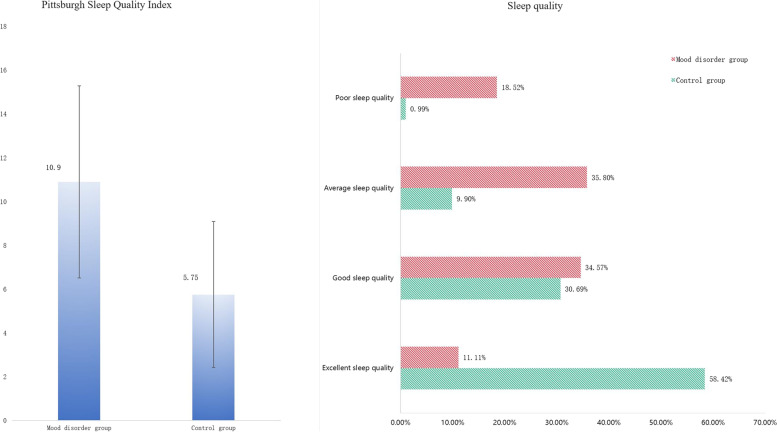
In the comorbidity group, people who had the habit of napping account for 66.67%, which was higher than 55.45% in control group, but the difference was not statistically significant (p = 0.124). Significant differences of sleep quality and PSQI were existed in the two groups (p < 0.001 and p < 0.001, Fig. 1). The results of behavioral questionnaires survey (Table 3) showed the habits including alcohol, tea, and smoking habit of the two group had no statistically significant difference (p = 0.083, p = 0.65 and p = 0.095). The difference of dietary structure between comorbidity and control groups was statistically significant (p < 0.001).
Fig. 1.
Contrastive analysis of Pittsburgh sleep quality index and sleep quality
Table 3.
The comparison of sleep quality and behavioral style
| Comorbidity group (n = 81) | Control group (n = 101) |
F/χ2 | P | |
|---|---|---|---|---|
| Siesta habit (yes/no) | 54/27 | 56/45 | 2.37 | 0.124 |
| Pittsburgh Sleep Qualitya Index | 10.90 ± 4.39 | 5.75 ± 3.33 | 81.03 | 0.000 |
| Sleep qualitya | ||||
| Excellent | 9 (11.11%) | 59 (58.42%) | 56.91 | 0.000 |
| Good | 28 (34.57%) | 31 (30.69%) | ||
| Average | 29 (35.80%) | 10 (9.90%) | ||
| Poor | 15 (18.52%) | 1 (0.99%) | ||
| Smoke | 24(29.63%) | 42 (41.58%) | 2.779 | 0.095 |
| Alcohol | 11 (13.58%) | 24(23.76%) | 3.00 | 0.083 |
| Tea | 31(38.27%) | 42 (41.58%) | 0.21 | 0.650 |
| Dietary habita | ||||
| Meat-based meal | 13(16.05%) | 81 (80.20%) | 78.86 | 0.000 |
| Meat pigment mix | 54 (66.67%) | 10 (9.90%) | ||
| Plant-based diet | 14 (17.28%) | 10 (9.90%) | ||
aThe difference was statistically significant
Stratification analysis
In this study, there were statistical differences in the gender ratio between the two groups. So the status of anxiety, depression and cognitive disorder in both male and female group were analyzed in this study. The analysis results showed that ratios of cognitive disorder, anxiety and depression in female patients were all significantly higher than that in male diabetes patients (p = 0.001, p < 0.001 and p < 0.001). The differences of MMSE score, SAS score and PHQ9 score were also statistically significant between female T2DM group and male patient group (p = 0.001, p < 0.001 and p < 0.001). The specific analysis results were shown in Table 4. In this research, there was a significant association between gender and depression, thus it was a confounding factor needing to be controlled.
Table 4.
Anxiety, depression and cognitive disorder status in male and female group
| Male (n = 100) |
Female (n = 82) |
F/χ2 | P | |
|---|---|---|---|---|
| Ratio of cognitive disordera | 12(12.00%) | 27 (32.93%) | 11.72 | 0.001 |
| Ratio of anxietya | 1(1.00%) | 12 (14.63%) | 12.63 | 0.000 |
| Ratio of depressiona | 32 (32.00%) | 50 (60.98%) | 15.28 | 0.000 |
| MMSE scorea | 28.39 ± 2.61 | 26.78 ± 3.50 | 12.58 | 0.001 |
| SAS scorea | 32.92 ± 7.40 | 38.11 ± 9.83 | 16.48 | 0.000 |
| PHQ-9 scorea | 2.80 ± 3.35 | 4.59 ± 3.79 | 11.35 | 0.000 |
aThe difference was statistically significant
Analysis of the influence factors associated with anxiety and depression in T2DM
Logistic regression analysis was used to identify the influence factors for anxiety and depression disorders in T2DM. According to the comparative analysis results between the two groups, it was found that sex ratio, the blood glucose fluctuation index, sleeping status and dietary habit were statistically significant. Therefore, sex ratio and other traditional risk factors such as smoking and alcohol consumption were considered as potential confounders. After controlling the confounders, the results showed that SDBG and sleep quality were associated with depression and anxiety disorders in T2DM (p = 0.040 and p < 0.001) and the OR values of these factors were 7.588 (1.097–52.069) and 4.428 (2.649–7.401), respectively. The male–female ratio, age, BMI, smoke, alcohol and dietary habit were not associated with depression and anxiety in T2DM (p = 0.801, p = 0.393, p = 0.337, p = 0.652, p = 0.489 and p = 0.828, separately). PPGE, LAGE and MBG had no effects on depression and anxiety in T2DM (p = 0.437, p = 0.180 and p = 0.836). The analysis results were shown in Table 5.
Table 5.
Analysis of factors related to anxiety and depression in T2DM
| OR | 95% CI | P | |
|---|---|---|---|
| Male–female ratio | 0.860 | (0.266, 2.778) | 0.801 |
| Age | 0.987 | (0.958, 1.017) | 0.393 |
| BMI | 1.040 | (0.960, 1.126) | 0.337 |
| Smoke | 0.769 | (0.245, 2.408) | 0.652 |
| Alcohol | 0.651 | (0.193, 2.193) | 0.489 |
| Sleep qualitya | 4.428 | (2.649, 7.401) | 0.000 |
| Dietary habit | 1.076 | (0.555, 2.084) | 0.828 |
| SDBGa | 7.558 | (1.097, 52.069) | 0.040 |
| PPGE | 0.880 | (0.637, 1.215) | 0.437 |
| LAGE | 0.829 | (0.631, 1.090) | 0.180 |
| MBG | 0.984 | (0.848, 1.142) | 0.836 |
aThe differences were statistically significant
Discussion
In the current study we found that SDBG was significantly associated with depression and anxiety in T2DM (p = 0.032) and blood sugar that fluctuated widely was associated with depression and anxiety (OR = 7.558, p = 0.040). The T2DM patients having poor self-regulating ability might lead to a wide range of blood glucose fluctuations, multiple complications were emerging including common complications, psychological and emotional diseases [14–16]. The reason of SDBG fluctuation associated with the prevalence of depression and anxiety might be that long-term blood glucose disorder and increased complications could cause the proportion of anxiety and depression increasing.
Our results comported with several prior studies that good sleep quality, health of dietary patterns and regular behaviors were considered as the advantage factors, which could improve the depressive symptoms [17–19]. Logistic analysis results showed that sleep disorder was the risk factor for depression and anxiety in T2DM patients (OR = 4.428, 95%CI: 2.649–7.401, p < 0.001). The traditional risk factors such as smoking and alcohol, were not statistically associated with depression and anxiety in this study (p = 0.652 and p = 0.489). The cause might be that gender imbalance between the two groups and insufficient sample size.
According to Hussain's systematic review analysis, the prevalence of depression was 26.67% ~ 29% in diabetes [20]. However, the prevalence of emotional distress in our study was 45.05%, the increased prevalence of anxiety and depression might be probably associated with diagnostic mistakes, delayed therapy. Peyrot team's findings confirmed that gender was the independent risk factors for emotional disorders in diabetic patients [21]. Our results were consistent with those research findings, the female patients were more likely to suffer from anxiety, depression and cognitive disorder (p = 0.001, p < 0.001 and p < 0.001). As we all know that menopause can lead to endocrine disorders and emotional fluctuation in women, this might account for the difference in gender.
This research was a rigorous retrospective study and focused on the correlation of blood glucose fluctuation, sleep quality and the prevalence of depression and anxiety in T2DM. However, it should be noted that there were some limitations in this research. The research data were collected from the electronic medical record and questionnaires, it was impossible to eliminate information bias, selection bias and confounding bias completely. We can only minimize the effects of these biases by collecting data objectively and reasonable statistical analysis. Large-sample and multicenter studies were needed to clarify the causal relationship between blood glucose fluctuation and emotional disorders in T2DM.
Conclusion
To conclude, this retrospective analysis indicated that blood glucose fluctuation and sleep quality were associated with the increased prevalence of depression and anxiety in T2DM. It is known that early identification of poor mental state and potential risk factors are helpful to improve the life quality of diabetic patients.
Acknowledgements
We are very grateful to the clinical doctors, nurses and laboratory administrators
Abbreviations
- T2DM
Type 2 diabetes mellitus
- BMI
Body Mass Index
- HbA1c
Glycosylated hemoglobin
- UA
Uric Acid
- FBG
Fasting Blood Glucose
- PBG
Postprandial blood glucose
- TSH
Thyroid Stimulating Hormone
- FT3
Free Triiodothyronine
- FT4
Free Thyroxine
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- TG-Ab
Thyroglobulin Antibody
- TPO-Ab
Thyroid Peroxidase Antibody
- LAGE
Largest amplitude of glycemic excursions
- PPGE
Postprandial glucose excursion
- SDBG
Standard Deviation of Blood Glucose
- PSQI
Pittsburgh sleep quality index
- MMSE
Mini-mental state examination
- SAS
Self-Rating Anxiety Scale
- PHQ-9
Patient health questionnaire
Authors’ contributions
W. Yang and M. Liu were responsible for the conception and design. M. Liu and Y. Chen drafted the initial manuscript and revising it critically for important intellectual content. M. Liu and Y. Chen analyzed the data. Y. Tian, Q.W. Zhang, J.H. Zhang, Q.Y. Chen and L.X. Suo were responsible for data collection. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Shanghai Municipal Jiading District Health Commission Foundation(No. 2017-KY-05 and No. 2020-QN-07).
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to personal data protection legislation but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences (Approval number: No. 2017-B-14). All participants signed the informed consent and all methods were carried out according to the 1995 Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Liu, Email: xiaoshiliu20609@sina.com.
Yang Chen, Email: tonychen112@hotmail.com.
References
- 1.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: Time for an appraisal. Lancet Diabetes Endocrinol. 2015;3(6):450–460. doi: 10.1016/S2213-8587(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 2.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress-a modifiable risk factor. Nat Rev Endocrinol. 2017;13(9):547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 3.AlBekairy A, AbuRuz S, Alsabani B, Alshehri A, Aldebasi T, Alkatheri A, Almodaimegh H. Exploring factors associated with depression and anxiety among hospitalized patients with type 2 diabetes mellitus. Med Princ Pract. 2017;26(6):547–553. doi: 10.1159/000484929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy M, Sengupta N, Sahana PK, Das C, Talukdar P, Baidya A, Goswami S. Type 2 Diabetes and Influence of Diabetes-Specific Distress on Depression. Diabetes Res Clin Pract. 2018;143:194–198. doi: 10.1016/j.diabres.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga S, Tanaka S, Fujihara K, Horikawa C, Iimuro S, Kitaoka M, Sato A, Nakamura J, Haneda M, Shimano H, Akanuma Y, Ohashi Y, Sone H. Association between all-cause mortality and severity of depressive symptoms in patients with type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS) J Psychosom Res. 2017;99:34–39. doi: 10.1016/j.jpsychores.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Limongi F, Noale M, Crepaldi G, Maggi S, ILSA Working Group Prevalence of Diabetes and Depressive Symptomatology and Their Effect on Mortality Risk in Elderly Italians: The Italian Longitudinal Study on Aging. Diabetes Metab. 2014;40(5):373–378. doi: 10.1016/j.diabet.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Holt RI, Groot M, Golden SH. Diabetes and Depression. Curr Diab Rep. 2014;14(6):491. doi: 10.1007/s11892-014-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Feng H, Niu J, Du L, Dang X, Wu S. Investigation and analysis of anxiety in patients with type 2 diabetes mellitus. Chinese J Clin Rational Drug Use. 2016;5:94–95. [Google Scholar]
- 9.Bogner HR, Morales KH, Post EP, Bruce ML. Diabetes, depression, and death, a randomized controlled trial of a depression treatment program for older adults based in primary care(PROSPECT) Diab Care. 2007;30(12):3005–3010. doi: 10.2337/dc07-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bădescu SV, Tătaru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Walders-Abramson N. Depression and quality of life in youth-onset type 2 diabetes mellitus. Curr Diab Rep. 2014;14(1):449. doi: 10.1007/s11892-013-0449-x. [DOI] [PubMed] [Google Scholar]
- 12.Yang XJ, He H, Lü XF, Wen XR, Wang C, Chen DW, Li XJ, Ran XW. Association of glycaemic variability and carotid intima-media thickness in patients with type 2 diabetes mellitus. Sichuan Da Xue Bao Yi Xue Ban. 2012;43(5):734–8. [PubMed] [Google Scholar]
- 13.Alfieri V, Myasoedova VA, Vinci MC, Rondinelli M, Songia P, Massaiu I, Cosentino N, Moschetta D, Valerio V, Ciccarelli M, Marenzi G, Genovese S, Poggio P. The Role of Glycemic Variability in Cardiovascular Disorders. Int J Mol Sci. 2021;22(16):8393. doi: 10.3390/ijms22168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eto F, Washida K, Matsubara M, Makino H, Takahashi A, Noda K, Hattori Y, Nakaoku Y, Nishimura K, Hosoda K, Ihara M. Glucose fluctuation and severe internal carotid artery siphon stenosis in type 2 diabetes Patients. Nutrients. 2021;13(7):2379. doi: 10.3390/nu13072379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monnier L, Colette C, Owens D. Glucose variability and diabetes complications: risk factor or biomarker? Can we disentangle the "Gordian Knot"? Diabetes Metab. 2021;47(3):101225. doi: 10.1016/j.diabet.2021.101225. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Ao L, Hu X, Ma J, Bao K, Gu Y, Zhao J, Huang W. Influence of blood glucose fluctuation, C-peptide level and conventional risk factors on carotid artery intima-media thickness in Chinese Han patients with type 2 diabetes mellitus. Eur J Med Res. 2019;24(1):13. doi: 10.1186/s40001-019-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farooque R, Herekar F, Iftikhar S, Patel MJ. The Frequency of Poor Sleep Quality in Patients With Diabetes Mellitus and Its Association With Glycemic Control. Cureus. 2020;12(11):e11608. doi: 10.7759/cureus.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Şahin S, Haliloğlu Ö, Polat Korkmaz Ö, Durcan E, Rekalı Şahin H, Yumuk VD, Damcı T, İlkova HM, Oşar SZ. Does treatment with sodium-glucose co-transporter-2 inhibitors have an effect on sleep quality, quality of life, and anxiety levels in people with Type 2 diabetes mellitus? Turk J Med Sci. 2020;51(2):735–742. doi: 10.3906/sag-2008-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang UE, Beglinger C, et al. Nutritional aspects of depression. Cell Physiol Biochem. 2015;37(3):1029–1043. doi: 10.1159/000430229. [DOI] [PubMed] [Google Scholar]
- 20.Hussain S, Habib A, Singh A, Akhtar M, Najmi AK. Prevalence of depression among type 2 diabetes mellitus patients in India: a meta-analysis. Psychiatry Res. 2018;270:264–273. doi: 10.1016/j.psychres.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20(4):585. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to personal data protection legislation but are available from the corresponding author on reasonable request.