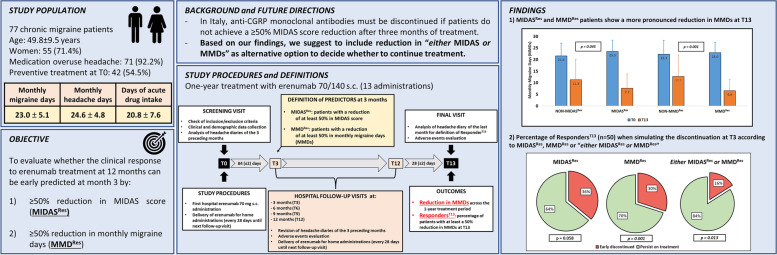
Abstract
Background
In Italy, monoclonal antibodies targeting the CGRP pathway are subsidized for the preventive treatment of high frequency and chronic migraine (CM) in patients with a MIgraine Disability ASsessment (MIDAS) score ≥ 11. Eligibility to treatment continuation requires a ≥ 50% MIDAS score reduction at three months (T3). In this study, we evaluate whether a ≥ 50% MIDAS score reduction at T3 is a reliable predictor of response to one-year erenumab treatment.
Methods
In this prospective, open-label, real-world study, 77 CM patients were treated with erenumab 70–140 mg s.c. every 28 days for one year (T13). We collected the following variables: monthly migraine days (MMDs), monthly headache days (MHDs), days of acute medication intake, MIDAS, HIT-6, anxiety, depression, quality of life and allodynia. Response to erenumab was evaluated as: i) average reduction in MMDs during the 1-year treatment period; and ii) percentage of patients with ≥ 50% reduction in MMDs during the last 4 weeks after the 13th injection (RespondersT13).
Results
Erenumab induced a sustained reduction in MMDs, MHDs and intake of acute medications across the 12-month treatment period, with 64.9% of patients qualifying as RespondersT13. At T3, 55.8% of patients reported a ≥ 50% reduction in MIDAS score (MIDASRes) and 55.4% of patients reported a ≥ 50% reduction in MMDs (MMDRes). MIDASRes and MMDRes patients showed a more pronounced reduction in MMDs during the 1-year treatment as compared to NON-MIDASRes (MIDASRes: T0: 23.5 ± 4.9 vs. T13: 7.7 ± 6.2; NON- MIDASRes: T0: 21.6 ± 5.4 vs. T13: 11.3 ± 8.8, p = 0.045) and NON-MMDRes (MMDRes: T0: 23.0 ± 4.5 vs. T13: 6.6 ± 4.8; NON-MMDRes: T0: 22.3 ± 6.0 vs. T13: 12.7 ± 9.2, p < 0.001) groups. The percentage of RespondersT13 did not differ between MIDASRes (74.4%) and NON-MIDASRes (52.9%) patients (p = 0.058), while the percentage of RespondersT13 was higher in the MMDRes group (83.3%) when compared to NON-MMDRes (42.9%) (p = 0.001). MMDRes predicted the long-term outcome according to a multivariate analysis (Exp(B) = 7.128; p = 0.001), while MIDASRes did not. Treatment discontinuation based on MIDASRes would have early excluded 36.0% of RespondersT13. Discontinuation based on “either MIDASRes or MMDRes” would have excluded a lower percentage (16%) of RespondersT13.
Conclusion
MIDASRes only partly reflects the 12-month outcome of erenumab treatment in CM, as it excludes more than one third of responders. A criterion based on the alternative consideration of ≥ 50% reduction in MIDAS score or MMDs in the first three months of treatment represents a more precise and inclusive option.
Trial registration
The trial was retrospectively registered at www.clinicaltrials.gov (NCT05442008).
Graphical Abstract
CGRP: Calcitonin Gene Related Peptide. MIDAS: MIgraine Disability Assessment. MMDs: monthly migraine days. MIDASRes: Patients with a MIDAS score reduction of at least 50% at T3. MMDRes: Patients with a MMDs reduction of at least 50% at T3. ResponderT13: Patients with a MMDs reduction from baseline of at least 50% in the last 4 weeks of observation period (after 13 erenumab administrations). T0: First erenumab administration. T3, T6, T9, T12: Follow-up visits at three, six, nine, and twelve months after first erenumab administration. T13: Last visit of the protocol.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-022-01480-2.
Keywords: Migraine, Headache, CGRP, Monoclonal antibodies, Pain, Chronic migraine, Disability, Quality of life
Background
Monoclonal antibodies directed against the Calcitonin Gene Related Peptide pathway (CGRP-mAbs) are a silver lining in the setting of migraine treatment, partly overcoming the issues related to poor effectiveness and tolerability of oral preventive treatments [1].
These drugs act on the CGRP pathway, a key vasoactive neuropeptide in migraine pathophysiology involved in activation and sensitization of afferent trigeminal nociceptors of the trigeminovascular system [2, 3]. Galcanezumab, fremanezumab and eptinezumab target the CGRP ligand, while erenumab, a fully human IgG2, targets its receptor [4]. CGRP-mAbs' efficacy and safety have been largely documented in multiple randomized and open-label studies [5, 6]. Effectiveness and good tolerability has also been supported in the real-world setting [7–10].
In 2019, the Italian Medicines Agency (AIFA) approved subsidization of CGRP-mAbs for the preventive treatment of migraine in patients that satisfy the following criteria: i) at least 8 migraine days per month in the last three months, ii) a MIgraine Disability ASsessment (MIDAS) score ≥ 11, and iii) previous failure due to lack of efficacy or tolerability of at least three preventive drugs, among β-blockers, tricyclic antidepressants, antiepileptics, and onabotulinumtoxin-A (this latter only for chronic migraine (CM)) [11].
After an initial period of three months, the treatment can be continued only in those patients reporting a ≥ 50% reduction in MIDAS score. Thus, in Italy MIDAS score became the driving indicator for treatment initiation and the limiting step for treatment continuation. MIDAS is a self-administered five-item questionnaire that quantifies migraine-related limitation in home and workplace performances. MIDAS score ranges from a minimum of 0 (absence of disability) to a maximum of 270 (indicating an extremely severe disability); a score ≥ 11 identifies patients with a moderate migraine-related disability, while a score ≥ 21 suggests a severe disability [12]. The three-month checkpoint adopted by AIFA is in line with the output of the pivotal RCTs and the subsequent observational studies dealing with CGRP-mAbs in migraine prevention demonstrating the onset of efficacy already in the first weeks after starting treatment [13]. It is also in agreement with the initial EHF guidelines for the use of CGRP-mAbs in migraine prevention [14]. It must however be noted that MIDAS score reduction has not been tested as a predictor of long-term outcome of CGRP-mAbs treatment, although MIDAS was considered as a secondary outcome in several RCTs [5, 15–17]. A major concern for using a single numerical parameter as a mandatory criterion for the initiation and continuation of a preventive treatment is the limited ability to capture the multifaced disability that characterizes the migraine spectrum. On the other side, the reduction in monthly migraine days (MMDs), which represented a primary outcome for clinical trials, may not adequately reflect patients’ preferences and perspectives [18, 19].
Furthermore, real life evidence recently brought to light cases of delayed CGRP-mAbs benefit, worth of a subsequent evaluation after six months of treatment, which questions the validity of a check-point at three months [20].
Due to the high cost of long-term course with CGRP-mAbs, the availability of reliable predictors of outcome is crucial for a careful selection of candidates to start and maintain treatment [21, 22]. The primary aim of this study is to evaluate whether the ≥ 50% reduction in MIDAS score at three months of treatment is predictive of response at 12 months or there are other indicators that might be more suitable for the purpose.
Methods
Subjects
We consecutively screened 82 migraine patients (mean age 49.5 ± 9.8 years, 59 females) among those attending the outpatient clinic of the Headache Science & Neurorehabilitation Center of the IRCCS Mondino Foundation (Pavia, Italy) in the period December 2018-January 2020.
The inclusion criteria adopted for selecting patients to be included in the analysis were in agreement with AIFA regulations for CGRP-mAbs prescription and consisted in: age between 18 and 65 years; diagnosis of CM, according to ICHD-3 criteria, for at least 12 months prior to enrollment [23]; prospective completion of a headache diary in the 3 months preceding the enrollment; compliance to complete a daily headache diary for 1 year; previous failure of at least 3 classes of preventive treatments among beta-blockers, antiepileptic drugs, antidepressants, or onabotulinumtoxin-A; a MIDAS score ≥ 11.
Exclusion criteria were: severe cardiologic comorbidities; pregnancy and breastfeeding and previous adverse reaction to latex. A previous therapeutic failure was defined as: i) the lack of efficacy after a 6-week treatment course with an adequate dose, or ii) drug interruption due to poor tolerability or adverse events.
Five of the 82 screened patients did not meet inclusion criteria as they presented a baseline MIDAS < 11 and were therefore excluded. The final study population was thus formed by 77 migraine patients (see flowchart in Fig. 1).
Fig. 1.
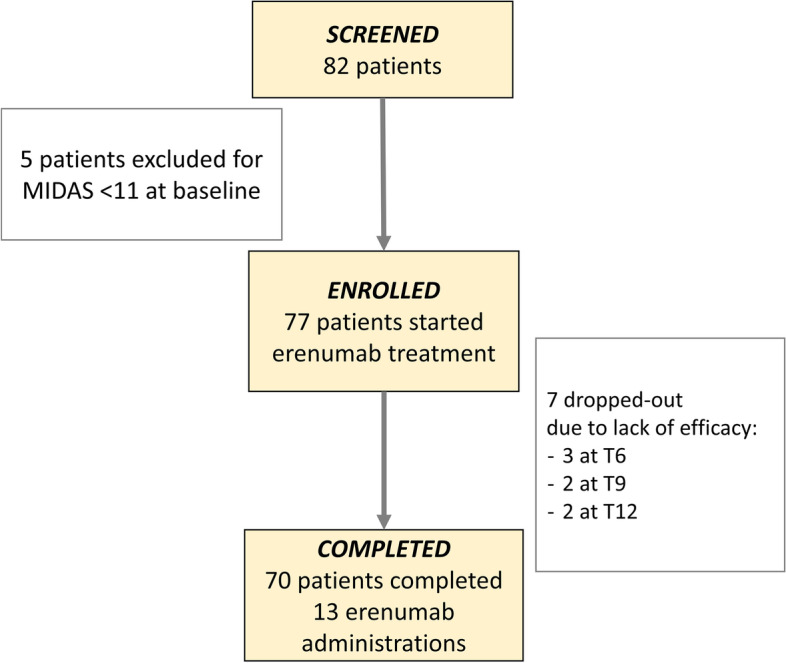
Flowchart of enrolled patients. MIDAS: MIgraine Disability ASsessment. T6, T9, T12: follow-up visits at six, nine, and twelve months after first erenumab administration
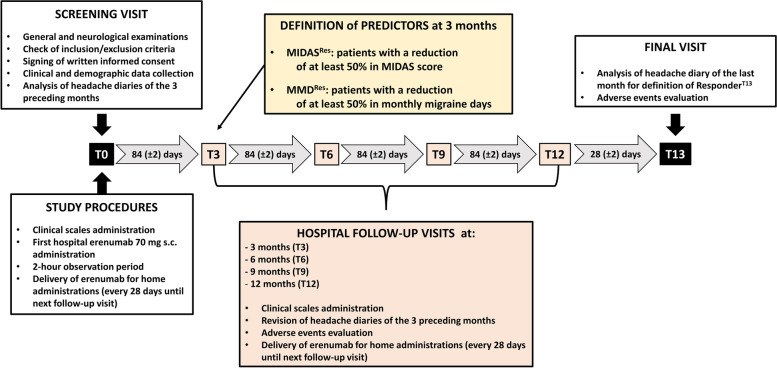
Study procedures
In this prospective, open-label, real-world study, the patients underwent a 1-year treatment with a s.c. erenumab administration every 28 days (13 administrations in total) regardless of MIDAS reduction at three and six months. This was possible due to the temporary dispensation regulations agreed by our Institute with the drug manufacturer, while waiting for AIFA regulations. We used erenumab as it was the first CGRP-mAb that became available in Italy according to this dispensation modality.
At T0, we checked the inclusion/exclusion criteria and the headache diaries of the three preceding months, and we performed a full neurologic and general examination and a thorough anamnestic evaluation.
Patients who agreed to participate in the study signed a written informed consent and completed the baseline procedures, including clinical and demographic data recording and completion of a set of questionnaires to assess migraine related disability/impact (MIDAS and HIT-6), psychological comorbidities (HADS-A and HADS-D), quality of life (MSQ), self-perception of general health (0 to 100 visual analogue scale), and allodynia (ASC-12).
The first erenumab administration (70 mg) was delivered in the hospital setting at T0, where the patients stayed for a 2-h observation to monitor possible acute adverse events. The patients were then instructed to self-administer the subsequent erenumab doses at home, one every 28 days (T1 to T12).
The patients returned to the Center every 12 weeks for the follow-up visits (T3 – T6 – T9 – T12 – Fig. 2), and at T13 for the last visit of the protocol. Monthly headache days (MHDs), monthly migraine days (MMDs), and days and doses of acute drug intake were prospectively recorded in a paper headache diary. At each follow-up visit, the patients completed the same study procedures and clinical scales described for T0. At T3, erenumab dosage was increased to 140 mg in 72 patients based on the clinical response observed in the first three months. More specifically, erenumab dosage was increased to 140 mg in all patients who did not experience a stable reduction in MMDs of at least 75% from baseline. In all of these patients, the dose was then kept stable until the end of the study protocol.
Fig. 2.
Timeline and study procedures. MIDAS: MIgraine Disability Assessment. MIDASRes: Patients with a MIDAS score reduction of at least 50% at T3. MMDRes: Patients with a MMDs reduction of at least 50% at T3. RespondersT13: Patients with a MMDs reduction from baseline of at least 50% in the last 4 weeks of observation period (after 13 erenumab administrations). T3, T6, T9, T12: follow-up visits at three, six, nine, and twelve months after first erenumab administration
All patients were allowed to keep their oral preventive medications with a stable dose across all study duration.
The study was approved by the local Ethics Committee (P-20190105434) and was registered retrospectively on www.clinicaltrials.gov (NCT05442008).
Outcomes
Our primary outcome was to evaluate whether a ≥ 50% reduction in the MIDAS score at T3 (MIDASRes) was predictive of a more pronounced reduction in MMDs during a 1-year erenumab treatment. This was calculated from the diaries as the average reduction from baseline observed in the period month 1—month 12. As co-primary measure, we explored the possibility to use a ≥ 50% reduction in MMDs at T3 (MMDRes) as an alternative indicator.
We also considered the following secondary outcome measures:
- association between MIDASRes or MMDRes and the percentage of patients with a ≥ 50% reduction in MMDs in the last 4 weeks of observation period (T13) when compared to baseline (RespondersT13);
- association between baseline clinical/demographic features and long-term efficacy of erenumab treatment.
Finally, we evaluated the 1-year changes in migraine-related disability, anxiety and depression severity, allodynia, and quality of life as exploratory outcome measures.
Statistical analysis
The sample size was computed with the freeware online platform www.openepi.com. For the co-primary outcomes, we considered as clinically meaningful a difference between MIDASRes and NON-MIDASRes of at least 4 MMDs across the overall study period [15, 24]. Thus, we used the following parameters: confidence interval (2-sided): 95%; power: 80%; ratio of sample size: 2/3 of patients are expected to be in the responder groups; mean difference: 4; standard deviation: 5 for both groups. The minimum suggested sample size was 53 (33 for MIDASRes and 20 for NON-MIDASRes). To compensate for drop-outs during the 1-year study period and for possible variability in the ratio of sample size, we planned to enroll at least 70 patients with a complete follow-up.
Statistical analysis was conducted with the SPSS software, ver. 21 (IBM Corp., USA) and with “R: A language and environment for statistical computing” (R Foundation for Statistical Computing, Vienna, Austria), Version 1.2.5033, for Windows. Categorical data were reported as absolute numbers and percentages, while continuous variables as mean ± standard deviation.
The Kolmogorov–Smirnov test proved a non-normal distribution of a subset of data (for example MMDs, MHDs, and days of acute drug intake), thus non-parametric tests were used.
For continuous variables, differences between groups were analyzed using the Mann–Whitney U test, while for categorical variables, statistical analysis was performed with the chi-square test.
To evaluate the association between the MIDASRes and MMDRes status and the modification of MMDs across the 1 year follow-up, we used non-parametric tests for repeated measures with two factors [25]: factor TIME (within subjects, 14 levels: T0 T13), and factor GROUP (between subjects, 2 levels: MIDASRes vs. NON-MIDASRes, or MMDRes vs. NON-MMDRes).
As exploratory outcomes, we analyzed association between MIDASRes or MMDRes and HIT-6, HADS-A and HADS-D, ASC-12, MSQ, and self-perceived quality of life; in this case the non-parametric tests for repeated measures included five timepoints (T0, T3, T6, T9, T12). HIT-6, HADS-A and HADS-D, MSQ were available for 43 patients.
Finally, we performed a logistic regression with “RespondersT13 vs. NON-RespondersT13” as dependent variable, MIDASRes, MMDRes, age, sex as well as other clinical and demographic variables of interest according to the univariate analysis. For all the previously described analyses, the levels of significance were corrected with a Bonferroni method to account for multiple comparison, when necessary.
For all the performed tests, the level of significance was set at α = 0.050.
Results
Study population
The final study population included 77 migraine patients (71.4% females, 49.8 ± 9.5 years) with headache onset at 14.5 ± 6.9 years, and a history of CM of 13.1 ± 10.3 years. Of these 77 patients, 7 (9.1%) withdrew from the study between T6 and T12 (due to lack of efficacy) and were considered as drop-outs; according to the planned intention-to-treat analysis, these patients were still included as NON-RespondersT13.
At baseline, patients reported 23.0 ± 5.1 MMDs and 24.6 ± 4.8 MHDs. Seventy-one out of 77 patients (92.2%) had a concomitant diagnosis of medication overuse headache (MOH). Sixty-nine (89.6%) patients previously underwent at least one in-hospital detoxification procedure. The patients had previously failed 5.1 ± 1.5 preventive medications, and 42 (54.5%) were still taking a preventive treatment at baseline.
The most prevalent comorbidities were anxiety (28.6%), depression (5.2%), or both (23.4%), insomnia (42.9%), or controlled hypertension (24.7%). Clinical and demographic features are detailed in Table 1.
Table 1.
Clinical and demographic features of study population, and comparison between MIDASRes / NON-MIDASRes and MMDRes / NON-MMDRes groups
| Total | MIDASRes | NON-MIDASRes | p-value | MMDRes | NON-MMDRes | p-value | |
|---|---|---|---|---|---|---|---|
| n | 77 | 43 | 34 | - | 42 | 35 | - |
| Age, (years, m ± sd) | 49.8 ± 9.5 | 50.3 ± 9.1 | 49.2 ± 10.0 | 0.597 | 50.0 ± 9.1 | 49.2 ± 10.0 | 0.967 |
| Females, n (%) | 55 (71.4) | 32 (74.4) | 23 (67.6) | 0.614 | 31 (73.8) | 24 (68.6) | 0.623 |
| Age at headache onset (years, m ± sd) | 14.5 ± 6.9 | 13.2 ± 5.5 | 16.2 ± 8.1 | 0.104 | 15.2 ± 7.3 | 16.2 ± 8.1 | 0.300 |
| Years lived with migraine (years, m ± sd) | 35.6 ± 10.8 | 37.1 ± 9.6 | 33.6 ± 11.9 | 0.177 | 35.3 ± 11.0 | 33.6 ± 11.9 | 0.701 |
| Years lived with chronic migraine (years, m ± sd) | 13.1 ± 10.3 | 13.7 ± 10.3 | 12.4 ± 10.3 | 0.495 | 12.0 ± 9.3 | 12.4 ± 10.3 | 0.424 |
| Migraine with aura, n (%) | 13 (16.9) | 6 (14.0) | 7 (20.6) | 0.545 | 8 (19.0) | 5 (14.3) | 0.762 |
| MOH, n (%) | 71 (92.2) | 41 (95.3) | 30 (88.2) | 0.397 | 39 (92.9) | 32 (91.4) | 0.654 |
| Previous detoxification for MOH, n (%) | 69 (89.6) | 40 (93.0) | 29 (85.3) | 38 (90.5) | 32 (91.4) | 0.652 | |
| Preventive treatment | |||||||
| Patients on preventive treatment at T0, n (%) | 42 (54.5) | 18 (41.9) | 24 (70.6) | 0.021 | 24 (57.1) | 18 (51.4) | 0.652 |
| Number of previously failed preventive treatments (m ± sd) | 3.8 ± 1.2 | 4.0 ± 1.3 | 3.6 ± 1.5 | 0.199 | 3.8 ± 1.6 | 4.1 ± 0.98 | 0.439 |
| Previous BoNT-A treatment, n (%) | 47 (61.0) | 27 (62.8) | 20 (58.8) | 0.815 | 24 (57.1) | 23 (65.7) | 0.488 |
| Classes of acute drugs | |||||||
| NSAIDs, n (%) | 12 (15.6) | 8 (18.6) | 4 (11.8) | 0.809 | 7 (16.7) | 5 (14.3) | 0.457 |
| Triptans, n (%) | 20 (26.0) | 9 (20.9) | 11 (32.4) | 9 (21.4) | 11 (31.4) | ||
| Combination, n (%) | 4 (5.2) | 3 (7.0) | 1 (2.9) | 1 (2.4) | 16 (45.7) | ||
| Multiple drug classes, n (%) | 41 (53.2) | 23 (53.5) | 18 (52.9) | 25 (59.5) | 3 (8.57) | ||
| Comorbidities | |||||||
| Hypertension, n (%) | 19 (24.7) | 9 (20.9) | 10 (29.4) | 0.434 | 11 (26.2) | 8 (22.9) | 0.795 |
| Anxiety, n (%) | 22 (28.6) | 13 (30.2) | 9 (26.5) | 0.916 | 12 (28.6) | 10 (28.6) | 0.999 |
| Depression, n (%) | 4 (5.2) | 3 (7.0) | 1 (2.9) | 2 (4.8) | 2 (5.7) | ||
| Anxiety and depression, n (%) | 18 (23.4) | 9 (20.9) | 9 (26.5) | 10 (23.8) | 8 (22.9) | ||
| Insomnia, n (%) | 33 (42.9) | 18 (41.9) | 15 (44.1) | 0.842 | 18 (42.9) | 15 (42.9) | 1.000 |
| Migraine features at baseline | |||||||
| Monthly headache days (m ± sd) | 24.6 ± 4.8 | 25.3 ± 4.2 | 23.7 ± 5.4 | 0.281 | 24.5 ± 4.4 | 23.7 ± 5.4 | 0.545 |
| Monthly migraine days (m ± sd) | 23.0 ± 5.1 | 23.7 ± 4.8 | 22.0 ± 5.3 | 0.172 | 23.2 ± 4.5 | 22.0 ± 5.3 | 0.861 |
| Monthly days of acute drugs intake (m ± sd) | 20.8 ± 7.6 | 22.8 ± 7.1 | 18.1 ± 7.5 | 0.005 | 21.5 ± 6.9 | 18.1 ± 7.5 | 0.534 |
| Monthly doses of acute drugs intake (m ± sd) | 34.7 ± 29.9 | 39.9 ± 33.5 | 28.7 ± 23.5 | 0.045 | 33.4 ± 29.9 | 36.4 ± 31.6 | 0.662 |
| Questionnaires at baseline | |||||||
| MIDAS (m ± sd) | 77.5 ± 69.0 | 80.9 ± 74.1 | 73.2 ± 62.6 | 0.275 | 72.1 ± 71.8 | 73.2 ± 62.8 | 0.452 |
| Mean headache intensity (m ± sd) | 8.0 ± 6.4 | 7.3 ± 1.3 | 9.0 ± 9.4 | 0.461 | 7.3 ± 1.2 | 9.0 ± 9.4 | 0.349 |
| HIT-6 (m ± sd) | 66.8 ± 5.8 | 67.1 ± 5.3 | 66.5 ± 6.4 | 0.651 | 65.8 ± 5.7 | 66.5 ± 6.5 | 0.113 |
| ASC-12 (allodynia) (m ± sd) | 6.4 ± 5.1 | 5.9 ± 5.4 | 6.9 ± 4.8 | 0.264 | 6.2 ± 5.2 | 6.9 ± 4.8 | 0.707 |
| MSQ (m ± sd) | 34.4 ± 18.1 | 38.1 ± 15.4 | 30.0 ± 20.3 | 0.068 | 37.4 ± 14.3 | 29.6 ± 20.3 | 0.190 |
| General Health (0–100) (m ± sd) | 54.4 ± 22.3 | 54.2 ± 22.8 | 54.7 ± 21.9 | 0.869 | 61.5 ± 16.6 | 54.7 ± 22.0 | 0.002 |
| HADS-A (m ± sd) | 6.51 ± 4.03 | 6.1 ± 3.7 | 7.0 ± 4.4 | 0.437 | 5.7 ± 3.6 | 7.0 ± 4.4 | 0.079 |
| HADS-D (m ± sd) | 6.39 ± 4.40 | 6.0 ± 5.0 | 6.9 ± 4.3 | 0.255 | 5.4 ± 3.7 | 6.9 ± 4.3 | 0.035 |
Legend: MIDASRes: Patients with a MIDAS score reduction of at least 50% at T3. NON-MIDASRes: Patients with a MIDAS score reduction < 50% at T3. MMDRes: Patients with a MMDs reduction of at least 50% at T3. NON-MMDRes: Patients with a MMDs reduction < 50% at T3. T3: follow-up visit at three months after first erenumab administration.
CM Chronic migraine, CM + MOH Chronic migraine and medication overuse headache, BoNT-A Onabotulinumtoxin-A, MIDAS MIgraine Disability Assessment, HIT-6 Headache Impact Test-6, ASC-12 Allodynia Symptoms Checlist, MSQ Migraine-Specific Quality of Life Questionnaire, HADS Hospital Anxiety and Depression Scale. Data are presented as means ± standard deviations (m ± sd) or absolute values (percentages)
Clinical modification with erenumab administration
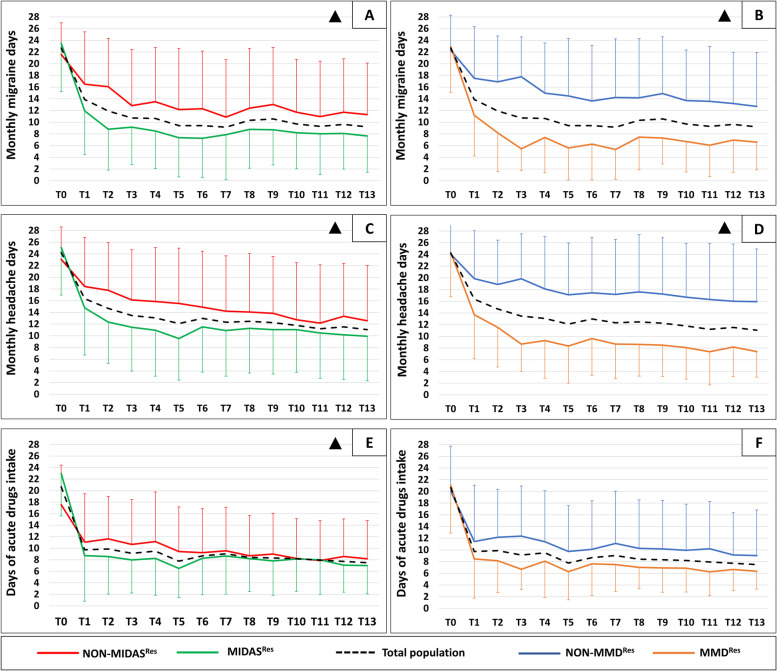
Erenumab induced a sustained reduction in MMDs (T0: 22.7 ± 5.2 days, T1-T13: 10.3 ± 8.2; TIME: F13,56 27.847, p < 0.001), and MHDs (T0: 24.5 ± 4.8, T1-T13: 12.7 ± 8.5; TIME: F13,56 37.622, p < 0.001) (Fig. 3). In line with these results, the days of acute anti-migraine drug intake decreased significantly from T0 to T13 (TIME: F13,56 26.096; p < 0.001). At T13, 50 out of 77 patients (64.9%) qualified as RespondersT13.
Fig. 3.
Changes in monthly migraine days, monthly headache days, and days of acute drugs intake in MIDASRes and MMDRes groups. MIDAS: MIgraine Disability Assessment. MIDASRes (green lines): Patients with a MIDAS score reduction of at least 50% at T3. NON-MIDASRes (red lines): Patients with a MIDAS score reduction < 50% at T3. MMDRes (orange lines): Patients with a MMDs reduction of at least 50% at T3. NON-MMDRes (blu lines): Patients with a MMDs reduction < 50% at T3. Black dotted lines represent changes in the overall study population. Δ: TIMExGROUP interaction < 0.050. Statistical analysis was performed with a non-parametric test for repeated measures, with the following factors: TIME: changes in the overall study population across the 1-year treatment period; GROUP: overall difference between study groups; TIMExGROUP interaction: different behavior of study groups across the 1-year treatment period. A: difference in monthly migraine days between MIDASRes and NON-MIDASRes groups. TIME = p < 0.001, GROUP = p = 0.034, TIMExGROUP = p = 0.045. B: difference in monthly migraine days between MMDRes and NON-MMDRes groups. TIME = p < 0.001, GROUP = p < 0.001, TIMExGROUP = p < 0.001. C: difference in monthly headache days between MIDASRes and NON-MIDASRes groups. TIME = p < 0.001, GROUP = p = 0.099, TIMExGROUP = p = 0.005. D: difference in monthly headache days between MMDRes and NON-MMDRes groups. TIME = p < 0.001, GROUP = p < 0.001, TIMExGROUP = p < 0.001. E: difference in days of acute drugs intake between MIDASRes and NON-MIDASRes groups. TIME = p < 0.001, GROUP = p = 0.424, TIMExGROUP = p = 0.045. F: difference in days of acute drugs intake between MMDRes and NON-MMDRes groups. TIME = p < 0.001, GROUP = p = 0.078, TIMExGROUP = p = 0.323
RespondersT13 were characterized by higher baseline self-perceived general health score (RespondersT13 59.2 ± 19.4 vs. NON-RespondersT13 45.6 ± 24.9, p = 0.022) and lower baseline HADS-D score (RespondersT13 5.6 ± 4.3 vs. NON-RespondersT13 7.8 ± 4.3, p = 0.031). No further associations were found with other comorbidities or clinical/demographic features and long-term outcome. Baseline features of RespondersT13 and NON-RespondersT13 are detailed in Table 2.
Table 2.
Clinical and demographic features of RespondersT13 and NON-RespondersT13
| Total | RespondersT13 | NON-RespondersT13 | p-value | |
|---|---|---|---|---|
| n | 77 | 50 | 27 | - |
| Age, (years, m ± sd) | 49.8 ± 9.5 | 49.4 ± 9.4 | 50.6 ± 9.7 | 0.967 |
| Females, n (%) | 55 (71.4) | 33 (66.0) | 22 (81.5) | 0.191 |
| Age at headache onset (years, m ± sd) | 14.5 ± 6.9 | 15.0 ± 7.3 | 13.6 ± 6.2 | 0.300 |
| Years lived with migraine (years, m ± sd) | 35.6 ± 10.8 | 34.8 ± 10.5 | 36.9 ± 11.2 | 0.701 |
| Years lived with chronic migraine (years, m ± sd) | 13.1 ± 10.3 | 12.6 ± 10.0 | 14.2 ± 12.6 | 0.424 |
| Migraine with aura, n (%) | 13 (16.9) | 10 (20.0) | 3 (11.1) | 0.525 |
| MOH, n (%) | 71 (92.2) | 40 (95.2) | 31 (88.6) | 0.402 |
| Previous detoxification for MOH, n (%) | 69 (89.6) | 45 (90.0) | 24 (88.9) | 0.879 |
| Preventive treatment | ||||
| Patients on preventive treatment at T0, n (%) | 42 (54.5) | 28 (56.0) | 14 (51.9) | 0.812 |
| Number of previously failed preventive treatments (m ± sd) | 3.8 ± 1.2 | 3.7 ± 1.3 | 4.0 ± 1.0 | 0.439 |
| Previous BoNT-A treatment, n (%) | 47 (61.0) | 27 (54.0) | 20 (74.1) | 0.094 |
| Classes of acute drugs | ||||
| NSAIDs, n (%) | 12 (15.6) | 7 (16.7) | 5 (14.3) | 0.408 |
| Triptans, n (%) | 20 (26.0) | 9 (21.4) | 11 (31.4) | |
| Combination, n (%) | 4 (5.2) | 1 (2.4) | 3 (8.6) | |
| Multiple drug classes, n (%) | 41 (53.2) | 25 (59.5) | 16 (45.7) | |
| Comorbidities | ||||
| Hypertension, n (%) | 19 (24.7) | 11 (26.2) | 8 (22.9) | 0.795 |
| Anxiety, n (%) | 22 (28.6) | 12 (28.6) | 10 (28.6) | 0.999 |
| Depression, n (%) | 4 (5.2) | 2 (4.8) | 2 (5.7) | |
| Anxiety and depression, n (%) | 18 (23.4) | 10 (12.9) | 8 (10.3) | |
| vInsomnia, n (%) | 33 (42.9) | 18 (42.9) | 15 (42.9) | 1.000 |
| Migraine features at baseline | ||||
| Monthly headache days (m ± sd) | 24.6 ± 4.8 | 24.3 ± 4.9 | 25.2 ± 4.6 | 0.545 |
| Monthly migraine days (m ± sd) | 23.0 ± 5.1 | 22.9 ± 4.9 | 23.1 ± 5.4 | 0.861 |
| Monthly days of acute drugs intake (m ± sd) | 20.8 ± 7.6 | 21.0 ± 7.3 | 20.3 ± 8.2 | 0.534 |
| Monthly doses of acute drugs intake (m ± sd) | 34.7 ± 29.9 | 33.8 ± 28.6 | 36.3 ± 32.6 | 0.898 |
| Questionnaires at baseline | ||||
| MIDAS (m ± sd) | 77.5 ± 69.0 | 74.0 ± 70.7 | 84.0 ± 66.5 | 0.452 |
| Mean headache intensity (m ± sd) | 8.0 ± 6.4 | 8.3 ± 7.9 | 7.5 ± 1.1 | 0.349 |
| HIT-6 (m ± sd) | 66.8 ± 5.8 | 66.0 ± 5.7 | 68.3 ± 5.6 | 0.113 |
| ASC-12 (allodynia) (m ± sd) | 6.4 ± 5.1 | 6.1 ± 5.5 | 6.8 ± 4.4 | 0.707 |
| MSQ (m ± sd) | 34.4 ± 18.1 | 36.8 ± 16.0 | 29.7 ± 21.0 | 0.190 |
| General Health (0–100) (m ± sd) | 54.4 ± 22.3 | 59.2 ± 19.4 | 45.6 ± 2 4.9 | 0.022 |
| HADS-A (m ± sd) | 6.51 ± 4.03 | 6.0 ± 3.9 | 7.4 ± 4.1 | 0.079 |
| HADS-D (m ± sd) | 6.39 ± 4.40 | 5.6 ± 4.3 | 7.8 ± 4.3 | 0.031 |
Legend: RespondersT13: Patients with a MMDs reduction from baseline of at least 50% in the last 4 weeks of observation period (after 13 erenumab administrations). NON-RespondersT13: Patients with a MMDs reduction from baseline < 50% in the last 4 weeks of observation period (after 13 erenumab administrations).
CM Chronic migraine, CM + MOH Chronic migraine and medication overuse headache, BoNT-A Onabotulinumtoxin-A, MIDAS MIgraine Disability Assessment, HIT-6 Headache Impact Test-6, ASC-12 Allodynia Symptoms Checlist, MSQ Migraine-Specific Quality of Life Questionnaire, HADS Hospital Anxiety and Depression Scale. Data are presented as means ± standard deviations (m ± sd) or absolute values (percentages).
Fourteen (33%) out of 42 patients taking oral preventive therapies at T0 reduced or discontinued these medications during the 1-year erenumab treatment. Fifty out of 77 patients (64.9%) reported at least one adverse event during the study period. The most prevalent adverse events were constipation (46.8%), injection site reactions (10.4%), and fatigue (23.4%). No patient discontinued the treatment due to adverse events (Supplementary Table 1).
Percentage of MIDASRes and MMDRes and association with clinical and demographic features
Forty-three out of 77 patients (55.8%) were MIDASRes. MIDASRes patients were characterized by higher baseline doses of monthly acute drugs (MIDASRes 39.9 ± 33.5 vs. NON-MIDASRes 28.7 ± 23.5, p = 0.045) and days of intake (MIDASRes 22.8 ± 7.1 vs. NON-MIDASRes 18.1 ± 7.5, p = 0.005) of monthly acute drugs. They were also less likely to take a preventive treatment at baseline (41.9% of MIDASRes vs. 70.6% of NON-MIDASRes, p = 0.021) (Table 1).
Forty-two out of 77 patients (54.5%) qualified as MMDRes. MMDRes patients were characterized by a lower baseline HADS-D score (MMDRes 5.4 ± 3.7 vs. NON-MMDRes 7.5 ± 4.9, p = 0.035), and higher baseline self-perceived general health score (MMDRes 61.5 ± 16.6 vs. non-MMDRes 45.9 ± 25.3, p = 0.002) (Table 1).
Twenty-eight patients (36.4%) qualified as MIDASRes and MMDRes, 15 patients (19.5%) were only MIDASRes, 14 patients (18.2%) were only MMDRes, and 57 patients (74.0%) were “either MIDASRes or MMDRes”.
Predictors of long-term clinical outcome
MIDASRes patients showed a more pronounced reduction in MMDs during the 1-year treatment as compared to NON- MIDASRes (GROUP: F1,68 4.503, p = 0.034; and TIMExGROUP: F13,56 1.935, p = 0.045) (Fig. 3). MIDASRes patients also presented a greater reduction in MHDs and days of acute drug intake during the 1-year treatment (MHDs: GROUP: F1,68 2.705, p = 0.099; and TIMExGROUP: F13,56 2.857, p = 0.005; days of acute drug intake: GROUP: F1,68 0.640, p = 0.424; and TIMExGROUP: F13,56 1.986, p = 0.045) (Supplementary Table 2).
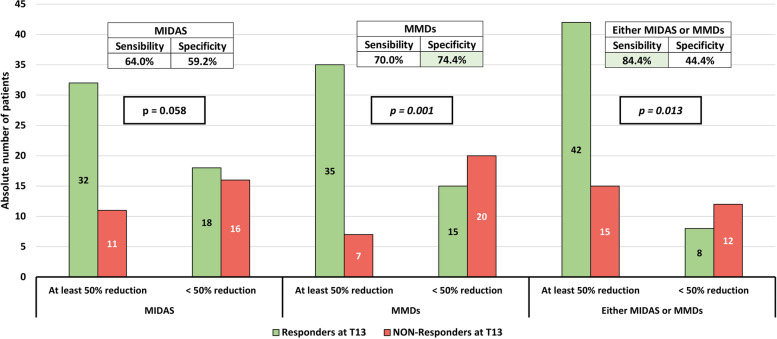
The percentage of RespondersT13 did not differ between MIDASRes (74.4%, 32/43) and NON-MIDASRes (52.9%, 18/34) patients (p = 0.058) (Fig. 4).
Fig. 4.
Distribution of RespondersT13 according to MIDAS and MMDs reductions after 3 months of erenumab administration. MIDAS: MIgraine Disability Assessment. MMDs: monthly migraine days. RespondersT13: patients with a MMDs reduction from baseline of at least 50% in the last 4 weeks of observation period (after 13 erenumab administrations). The p-value in the box was calculated with a χ2 test. The tables in the top of the figure show the sensibility and specificity of being RespondersT13 according to reduction of at least 50% in MIDAS, MMDs, or "either MIDAS or MMDs". The green shadows in the tables highlight the parameters with the best accuracy in sensibility or specificity
MMDRes patients showed a more pronounced reduction in MMDs during the 1-year treatment as compared to NON-MMDRes (GROUP: F1,68 26.611, p < 0.001; and TIMExGROUP: F13,56 5.476, p < 0.001) (Fig. 3). MMDRes patients also presented a greater reduction in MHDs, but not of days of acute drug intake, during the 1-year treatment (MHDs: GROUP: F1,68 23.881, p < 0.001; and TIMExGROUP: F13,56 6.166, p < 0.001; days of acute drug intake: GROUP: F1,68 3.112, p = 0.078; and TIMExGROUP: F13,56 1.159, p = 0.323) (Supplementary Table 2).
In addition, a higher percentage of MMDRes (83.3%, 35/42) qualified as RespondersT13 when compared to NON-MMDRes (42.9%, 15/35) (p = 0.001) (Fig. 4).
The percentage of RespondersT13 who did not qualify as MIDASRes or MMDRes was 36.0% and 30.0%, respectively. If we analyze those patients who were “either MIDASRes or MMDRes” the percentage of early excluded RespondersT13 markedly decreased to 16% (8/50, p = 0.013) (Fig. 4).
The sensibility and specificity in predicting 1-year erenumab response were: 64% and 59.2% when considering MIDASRes, 70% and 74% when considering MMDRes, and 84% and 44.4% when considering “either MIDASRes or MMDRes” (Fig. 4).
Multivariate analysis
We conducted a logistic regression analysis using the 1-year clinical outcome (RespondersT13 vs. non-RespondersT13) as the dependent variable, and MIDASRes and MMDRes as covariates, with age and sex included as correction factors. According to the results of the univariate analysis, a stepwise analysis was performed to test the role of baseline general health score, HADS-D score, days of acute drug intake, and use of preventive treatment as possible confounding factors (Table 3).
Table 3.
Multivariate regression analysis of independent determinants for being RespondersT13
| aOR = Exp(B) | SE | Wald χ2 | 95% confidence interval | p value | |
|---|---|---|---|---|---|
| Age (years) | 0.97 | 0.03 | 0.84 | 0.92–1.0 | 0.360 |
| Sex | 3.6 | 0.68 | 3.6 | 0.96–13.6 | 0.058 |
| MIDASRes | 2.1 | 0.56 | 1.8 | 0.70–6.3 | 0.185 |
| MMDRes | 7.1 | 0.58 | 11.3 | 2.3–22.4 | 0.001 |
Legend: aOR: adjusted Odds Ratio; MIDASRes: Patients with a MIDAS reduction of at least 50% at T3. MMDRes: Patients with MMDs reduction of at least 50% at T3. +T3: follow-up visit at three months after first erenumab administration. Variables tested but not included in the final model according to a stepwise analysis: baseline general health score, baseline HADS-D score, days of acute drug intake at baseline, and use of preventive treatment at baseline
Using this model, MMDRes was the only predictor of long-term outcome. Specifically, MMDRes patients had 7-time higher odds of being RespondersT13 (Exp (B) = 7.128; p = 0.001) for the same age, sex, and T3 MIDAS score reduction. The Hosmer–Lemeshow test showed a proper good-of-fit (p = 0.687). This model explained up to 32% (Nagelkerke R2) of ResponseT13 variance, classifying our patients correctly in 76.6% of cases.
Exploratory outcomes
Erenumab induced a sustained improvement in migraine related disability (MIDAS: TIME: F4,65 13.408, p < 0.001; HIT-6: TIME: F4,65 36.665, p < 0.001), severity of anxiety (HADS-A: TIME: F4,38 6.078, p = 0.001) and depression (HADS-D: TIME: F4,38 3.603, p < 0.014), allodynia (ASC-12: TIME: F4,65 6.400, p < 0.001), and quality of life (MSQ: TIME: F4,38 2.996, p < 0.030; self-perceived quality of life: TIME: F4,38 8.581, p < 0.001) (Supplementary Table 3).
When compared to NON-MIDASRes group, MIDASRes patients showed a more pronounced improvement in migraine related disability (HIT-6: GROUP: F1,68 2.628, p = 0.105; TIMExGROUP: F4,65 4.824, p = 0.002) and quality of life (MSQ: GROUP: F1,41 10.346, p = 0.003; TIMExGROUP: F4,38 5.340, p < 0.001).
When compared to NON-MMDRes group, MMDRes patients showed a greater improvement in quality of life (MSQ: GROUP: F1,41 6.665, p = 0.014; TIMExGROUP: F4,38 3.073, p = 0.027).
Discussion
In this real-world, open-label, clinical trial, 77 patients with resistant CM were treated with erenumab every 28 days for 1 year. The main objective was to evaluate the performance of MIDAS and MMDs ≥ 50% reduction after 3 months of treatment in predicting the long-term (1-year) clinical effectiveness.
Our findings can be summarized as follows: i) after 3 months of treatment, a similar percentage (about 55%) of patients achieved a ≥ 50% reduction either in MIDAS score (MIDASRes) or MMDs (MMDRes); ii) 36% of patients qualified as MIDASRes and MMDRes; iii) both MIDASRes and MMDRes patients showed a greater reduction in MMDs during the 1-year erenumab treatment; and iv) only MMDRes group was associated to the 50% responder outcome at month 13 and this clinical parameter was the only predictor that survived a logistic regression analysis.
In addition, we further confirmed the well-established clinical effectiveness of erenumab. Indeed, we found a sustained reduction in MMDs and days and doses of acute anti-migraine drugs over the 12-month treatment period, together with an improvement in several patients’ reported outcomes. The overall percentage of RespondersT13 was 65%.
The rate of RespondersT13 between MIDASRes and NON-MIDASRes patients was not statistically significant, although we detected a trend towards a difference. At present, we cannot exclude that this observation may be related to an insufficient statistical power for this secondary outcome and larger cohort studies are needed to confirm this result.
The cost of CGRP-mAbs has required payors to put in place specific regulations [26]. We are however aware that migraine is a social disease that affects people during the most productive years of their life, leading to a huge economic burden due to health care resources exploitation, presenteeism and absenteeism [27, 28]. Thus, a clinical improvement directly translates in a reduction in direct and indirect costs. This is corroborated by a recent study that demonstrated a reduction in healthcare costs in patients with a long-term oral preventive treatment adherence [29]. We feel it is important to provide evidence-based data on novel drugs to guide local as well as global regulations in the decision-making process.
Based on the robust body of evidence, the European Headache Federation (EHF) has recently updated its guidelines for the use of CGRP-mAbs in the prevention of migraine, based on the GRADE system. These evidence-based recommendations confirmed the moderate-to-high quality evidence on the efficacy and safety of CGRP-mAbs in migraine prevention and proposed to include these drugs among first line preventive therapies [20].
A pivotal finding in our cohort, is that up to 36% of patients qualifying as responders at month 13 would have been discontinued early with the application of the present ≥ 50% MIDAS reduction criterion. The same would also be the case if we considered the MMDRes parameter. By contrast, the combination of these two parameters provides an improved accuracy. Indeed, if we applied the “either MIDASRes or MMDRes” criterion, only 16% of RespondersT13 would have been discontinued after only 3 months of erenumab treatment.
A recent study by Iannone et al. suggested that the ≥ 50% MIDAS reduction may represent an advantageous outcome measure, as it allows the highest proportion of difficult-to-treat migraine patients to persist on CGRP-mAbs treatment [15]. In their study, the percentage of patients achieving a ≥ 50% MIDAS reduction ranged between 63.5% and 96.1%, which is higher than our experience (55%). The study by Iannone et al. differs from our trial in several aspects: i) first of all, their patients had different follow-up periods, and discontinued treatment at month 3 according to the present AIFA criteria; ii) the study included patients treated with the three available CGRP-mAbs; iii) out of 203 patients enrolled, only 52 completed a 12-month follow-up, and a lower number (n = 33) completed a 12-month treatment period, thus analysis of predictors of response could not be projected beyond the 6-month horizon; and iv) the effectiveness of MIDAS score reduction as predictor of clinical outcome was not the primary outcome of the study. In our cohort, we planned to treat all patients for 12 consecutive months, independently of MIDAS score reduction. Only 7 patients were discontinued (9.1%) because they withdrew consent to participate due to lack of efficacy; these patients withdrew after T3, so we were able to record MIDAS modification at this time-point, and all of them were included in the analysis as NON-RespondersT13.
The overall clinical improvement observed in the present study is in line, or even better, when compared with previous double blind clinical trials, open-label extension trials and real-world evidence. Tepper et al. demonstrated a reduction of 6.6 MMDs after 3 months of erenumab treatment, with a 50% responder rate of 40% [30]. In the subsequent 52-week open-label, MMDs decreased by 9.3 from baseline, and the percentage of patients qualifying as 50% responders was 59% [31]. In the real-world setting, the percentage of 50% responders for CM ranged between 34 and 75%, while the reduction in MMDs varied from 9.3 to 12.8, a quite ample heterogeneity that can be explained by differences in study design, duration of follow-up, and inclusion/exclusion criteria [9, 10, 32, 33].
Identification of clinical, biological or instrumental predictors of outcome represents a top priority in medicine.
Precision and tailored medicine will improve patients’ management as well as resources allocation, but solid predictors of outcome for CGRP-mAbs treatment are lacking so far, although several parameters have been proposed. Some studies suggest that a lower baseline MIDAS score may predict the short- and long-term erenumab response [34–36]. Lower baseline MMDs, short history of chronification, comorbidity with medication overuse headache (MOH), good response to triptans, unilateral pain, cutaneous allodynia, dopaminergic symptoms and spinal central sensitization were proposed as long-term predictors of CGRP-mAbs response [9, 15, 35–39]. Also higher basal headache frequency was associated to a better erenumab response in CM [9]. At variance, psychological and psychiatric comorbidities as well as previously failed preventive therapies seem to play an important role as negative predictors of outcome [7–10, 34–38]. No concordance was found on gender and erenumab responsiveness in CM, as both an association to male sex and no difference among gender were detected [10, 40].
In our analysis, baseline self-perceived general health and lower HADS-D scores were positively associated with RespondersT13 status. In a logistic regression analysis, only MMDRes group had 7-time higher odds of being RespondersT13, even when corrected for age, sex, and MIDASRes status. It is also true that an extensive evaluation of predictors of outcome was beyond the aims of our study. Thus, further analyses are needed to implement an accurate characterization of RespondersT13 and NON-RespondersT13 groups as it represents a key-point in the decision-making process and, by far, little concordance is demonstrated among studies.
AIFA selection of the ≥ 50% reduction in MIDAS score at 3 months may be considered a reasonable approach to the issue of CGRP-mAbs subsidization but it lacks scientific evidence [15]. The inclusion/exclusion criteria of the RCTs where CGRP-mAbs proved effective did not consider baseline MIDAS score [24]. The criteria adopted by AIFA for identifying the migraine patients who qualify for CGRP-mAbs subsidization include multiple previous failure of preventive treatments, in analogy to the EHF guidelines for resistant migraine, but these latter did not take into consideration MIDAS score as a driver of treatment duration [41]. MIDAS score was also not considered in the first and second edition of the EHF expert opinion based guidelines for the use of CGRP-mAbs in clinical practice [14, 20], while it is included in the guidelines of the American Headache Society but among a large number of other indicators, which ensures that the physician can use clinical judgement, as expected from our profession [42]. Two last observations are worth consideration. Firstly, the reduction in MIDAS score has never been tested as a predictor of outcome before CGRP-mAbs approval in Italy, and secondly, MIDAS score reduction is not a mandatory decision-making step in other European countries. The first point is crucial, as it prevents testing the effectiveness of CGRP-mAbs in the cohort of patients who do not meet the MIDASRes status. The second one is also important because it creates disparity in the access to care across Europe and beyond. We believe that the present findings are amenable to a wider application outside of the Italian boundaries to foster the creation of a European or globally shared approach that overcomes differences in local regulations to provide the best care solutions to migraine patients.
Ideally, the best clinical approach for the physicians is not to be forced by a single parameter in the decision to prolong or interrupt CGRP-mAbs therapy, in line with the conventional oral preventive drugs. An isolated parameter cannot reflect the clinical improvement in its complexity, as it depends on several features, namely: reduction in headache frequency, acute drugs consumption, severity of pain levels and most bothersome symptoms as well as improvement in patients’ reported outcomes such as disability, quality of life, working productivity and so on. Pain is a subjective experience that is difficult to assess thoroughly. It is well known that there are patients who may be satisfied by preventive treatments just by experiencing a reduction in pain and associated symptoms intensity [19]. If a check-point step is required to reduce costs associated to CGRP-mAbs, this should be built so as to guarantee treatment to all patients who can benefit.
We acknowledge that our study has some limitations. First of all, we explored the role of MIDAS and MMD reduction as predictors of outcome. As such, we cannot exclude that other clinical parameters, or their combination, might yield a better sensibility and/or specificity. In addition, we did not explore predictors at later points of treatment, as we wanted to focus on the earliest time-point for CGRP-mAbs discontinuation. Although based on a sample size calculation, the result obtained in a population of 77 patients may require replication studies on larger cohorts for confirmation and wider transferability. Our findings are limited to a CM treated with erenumab, which prevents generalization to high frequency episodic migraine or to other anti-CGRP drugs. Other measures, namely reduction in moderate to severe headache days or 30% MMD reduction, may also be considered as clinical outcomes, but we decided to stick to the most recent guidelines for clinical trials in CM [18].
Conclusions
The present data questions the suitability of a ≥ 50% MIDAS score reduction at 3 months as a requirement for the continuation of CGRP-mAbs treatment in patients with CM based on the following evidence: i) the indicator is poorly associated with the long-term responder rate, ii) it has low sensibility and specificity, and iii) it was outperformed by MMDsRes, which showed better sensibility, with acceptable specificity.
However, when considering that CM is one of the most debilitating diseases and that the patients qualifying for the CGRP-mAbs treatment must have endured multiple previous failures of preventive treatments, we believe that a more inclusive and conservative indicator should be adopted to guide treatment continuation: our findings suggest that the “either MIDASRes or MMDRes” indicator is a valid alternative, as it showed the highest degree of sensibility in identifying responders at month 12.
Supplementary Information
Additional file 1: Supplementary Table 1. Drop-outs and adverse event reporting. Supplementary Table 2. Monthly headache days, monthly migraine days and days of acute drug intake according to MIDAS and monthly migraine days (MMDs) response at T3. Supplementary Table 3. Patients’ reported outcomes according to MIDAS and monthly migraine days (MMDs) response at T3.
Acknowledgements
The authors thank the Research Nurse Team of the Headache Science & Neurorehabilitation Center of the IRCCS Mondino Foundation for their precious assistance in all of the activities.
Abbreviations
- CGRP
Calcitonin Gene Related Peptide
- CGRP-mAbs
Monoclonal antibodies targeting the CGRP pathway
- CM
Chronic Migraine
- MIDAS
MIgraine Disability ASsessment
- MIDASRes
Patients with a MIDAS score reduction of at least 50% at T3
- NON-MIDASRes
Patients with a MIDAS score reduction < 50% at T3
- MMDs
Monthly Migraine Days
- MMDRes
Patients with a MMDs reduction of at least 50% at T3
- NON-MMDRes
Patients with a MMDs reduction < 50% at T3
- MHDs
Monthly Headache Days
- MOH
Medication Overuse Headache
- RespondersT13
Patients with a MMDs reduction from baseline of at least 50% in the last 4 weeks of observation period (after 13 erenumab administrations)
- NON-RespondersT13
Patients with a MMDs reduction from baseline < 50% in the last 4 weeks of observation period (after 13 erenumab administrations)
Authors’ contributions
RDI and GV: Study concept and design; Patients enrollment; Statistical analysis; Interpretation of data; Writing of the first draft. MA, NG, EG, DM: Patients enrollment; Interpretation of data; Drafting/revision of the manuscript for content. LA, MC, FB, FT, SB, FC: Acquisition of data; Interpretation of data; Drafting/revision of the manuscript for content. GS, CT: Study concept and design; Interpretation of data; Drafting/revision of the manuscript for content. All authors read and approved the final manuscript.
Funding
This study was supported by a Research Grant from the Italian Ministry of Health to IRCCS Mondino Foundation (Ricerca Corrente 2017–2019) and by the Era-Net Biomiga project.
Availability of data and materials
The dataset generated and/or analysed during the current study is available in the Zenodo repository, with the 10.1186/s10194-022-01480-2. The dataset is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local Ethics Committee of Pavia, Italy (P-20190105434). All patients signed a written informed consent before enrollment. The study procedures were carried out following the guidelines for proper human research conduct in accordance with the Helsinki Declaration of the World Medical Association and its revisions.
Consent for publication
Not applicable.
Competing interests
RDI: honoraria for scientific presentations from Eli-Lilly, and Teva. MA: honoraria for scientific presentations from Eli-Lilly. CT: personal fees for scientific presentations or participation in advisory boards from Allergan, Abbvie, Eli Lilly, Lundbeck, Novartis and Teva; PI or collaborator in clinical trials sponsored by Abbvie, Alder, Amgen, Eli-Lilly, IBSA, Lundbeck, Novartis and Teva; grants from the European Commission, the Italian Ministry of Health and the Italian Ministry of University and Research, Migraine Research Foundation, Abbvie; GS: honoraria for the participation in advisory boards or for oral presentations from: Eli-Lilly, Novartis and Teva. GV, NG, EG, DM, LA, MC, FB, FT, SB, FC: nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roberto De Icco and Gloria Vaghi contributed equally to this work.
References
- 1.Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2014;0:1–11. doi: 10.1177/0333102414547138. [DOI] [PubMed] [Google Scholar]
- 2.Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40:99–105. doi: 10.1007/s10072-019-03769-8. [DOI] [PubMed] [Google Scholar]
- 3.Edvinsson L, Haanes KA, Warfvinge K, DiN K. CGRP as the target of new migraine therapies - Successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–350. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 4.Scuteri D, Adornetto A, Rombolà L, et al. New trends in migraine pharmacology: Targeting calcitonin gene–related peptide (CGRP) with monoclonal antibodies. Front Pharmacol. 2019;10:363. doi: 10.3389/fphar.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28:1716–1725. doi: 10.1111/ene.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394:1765–1774. doi: 10.1016/S0140-6736(19)32504-8. [DOI] [PubMed] [Google Scholar]
- 7.Vernieri F, Altamura C, Brunelli N, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study) J Headache Pain. 2021;22:35. doi: 10.1186/s10194-021-01247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottiroli S, De Icco R, Vaghi G, et al. Psychological predictors of negative treatment outcome with Erenumab in chronic migraine : data from an open label long-term prospective study. J. Headache Pain. 2021;4:1–10. doi: 10.1186/s10194-021-01333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbanti P, Aurilia C, Egeo G, et al. Erenumab in the prevention of high-frequency episodic and chronic migraine: Erenumab in Real Life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache. 2021;61:363–372. doi: 10.1111/head.14032. [DOI] [PubMed] [Google Scholar]
- 10.Barbanti P, Aurilia C, Cevoli S, et al (2021) Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: Results of the EARLY 2 study. Headache 61:1351–1363. 10.1111/head.14194 [DOI] [PubMed]
- 11.Italian Medicines Agency (2019) Attivazione web e pubblicazione schede di monitoraggio. https://www.aifa.gov.it/en/-/attivazione-web-e-pubblicazione-schede-di-monitoraggio-registro-aimovig. Accessed 23 May 2022
- 12.Stewart WF, Lipton RB, Kolodner KB, et al. Validity of the Migraine Disability Assessment ( MIDAS ) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88:41–52. doi: 10.1016/S0304-3959(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 13.Gottschalk C, Buse DC, Marmura MJ, et al. The importance of an early onset of migraine prevention: an evidence-based, hypothesis-driven scoping literature review. Ther Adv Neurol Disord. 2022;15:175628642210959. doi: 10.1177/17562864221095902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:1–33. doi: 10.1186/s10194-018-0955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannone LF, Fattori D, Benemei S, et al. Long-Term Effectiveness of Three Anti-CGRP Monoclonal Antibodies in Resistant Chronic Migraine Patients Based on the MIDAS score. CNS Drugs. 2022;36:191–202. doi: 10.1007/s40263-021-00893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton RB, Tepper SJ, Reuter U, et al (2019) Erenumab in chronic migraine: Patient-reported outcomes in a randomized double-blind study. Neurology 92:E2250–E2260. 10.1212/WNL.0000000000007452 [DOI] [PMC free article] [PubMed]
- 17.Goadsby PJ, Reuter U, Lanteri-minet M, Paiva G. Long-term Efficacy and Safety of Erenumab. Neurology. 2021;96:2724–2735. doi: 10.1212/WNL.0000000000012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassorelli C, Diener H-C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815–832. doi: 10.1177/0333102418758283. [DOI] [PubMed] [Google Scholar]
- 19.Alpuente A, Gallardo VJ, Caronna E, et al. In search of a gold standard patient-reported outcome measure to use in the evaluation and treatment-decision making in migraine prevention A real-world evidence study. J Headache Pain. 2021;22(1):151. doi: 10.1186/S10194-021-01366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco S, Amin FM, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention – 2022 update. J Headache Pain. 2022;23:1–19. doi: 10.1186/s10194-022-01431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannone LF, Fattori D, Benemei S, et al. Predictors of sustained response and effects of the discontinuation of anti-calcitonin gene related peptide antibodies and reinitiation in resistant chronic migraine. Eur J Neurol. 2022;29:1505–1513. doi: 10.1111/ene.15260. [DOI] [PubMed] [Google Scholar]
- 22.Vernieri F, Brunelli N, Messina R, et al (2021) Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain 22:1–10. 10.1186/s10194-021-01363-y [DOI] [PMC free article] [PubMed]
- 23.Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38:1–211. 10.1177/0333102417738202 [DOI] [PubMed]
- 24.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–434. doi: 10.1016/S1474-4422(17)30083-2. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD : An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J Stat Softw. 2012;50:1–23. doi: 10.18637/jss.v050.i12. [DOI] [Google Scholar]
- 26.Giannouchos TV, Mitsikostas DD, Ohsfeldt RL, et al. Cost-Effectiveness Analysis of Erenumab Versus OnabotulinumtoxinA for Patients with Chronic Migraine Attacks in Greece. Clin Drug Investig. 2019;39:979–990. doi: 10.1007/s40261-019-00827-z. [DOI] [PubMed] [Google Scholar]
- 27.Burch RC, Buse DC, Lipton RB. Migraine. Neurol Clin. 2019;37:631–649. doi: 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Berra E, Sances G, De Icco R, et al. Cost of Chronic and Episodic Migraine: a pilot study from a tertiary headache centre in northern Italy. J Headache Pain. 2015;16:1. doi: 10.1186/s10194-015-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irimia P, García-Azorín D, Núñez M, et al. Persistence, use of resources and costs in patients who start preventive medication for the treatment of migraine in Spain: The persec study. J Neurol Sci. 2021;429:119343. doi: 10.1016/j.jns.2021.119343. [DOI] [Google Scholar]
- 30.Tepper S, Reuter U, Brandes JL, Dolezil D, Silberstein S, Winner P, Leonardi D, Mikol D, Lenz RAM, Tepper S, Ashina M, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–434. doi: 10.1016/S1474-4422(17)30083-2. [DOI] [PubMed] [Google Scholar]
- 31.Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study. Cephalalgia. 2020;40:543–553. doi: 10.1177/0333102420912726. [DOI] [PubMed] [Google Scholar]
- 32.Becker WJ, Spacey S, Leroux E, et al. A real-world, observational study of erenumab for migraine prevention in Canadian patients. Headache J Head Face Pain. 2022;62:522–529. doi: 10.1111/head.14291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambru G, Hill B, Murphy M, et al. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21:1. doi: 10.1186/s10194-020-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zecca C, Cargnin S, Schankin C, et al (2022) Clinic and genetic predictors in response to erenumab. Eur J Neurol 29:1209–1217. 10.1111/ene.15236 [DOI] [PMC free article] [PubMed]
- 35.Baraldi C, Castro F Lo, Cainazzo MM, et al (2021) Predictors of response to erenumab after 12 months of treatment. Brain Behav 11:1–8. 10.1002/brb3.2260 [DOI] [PMC free article] [PubMed]
- 36.Silvestro M, Tessitore A, Scotto F, et al. Refractory migraine profile in CGRP- monoclonal antibodies scenario. Acta Neurol Scand. 2021;144:325–333. doi: 10.1111/ane.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoenen J, Timmermans G, Nonis R, et al. Erenumab for Migraine Prevention in a 1-Year Compassionate Use Program : Efficacy Tolerability, and Differences Between Clinical Phenotypes. Front Neurol. 2021;12:1–12. doi: 10.3389/fneur.2021.805334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernieri F, Altamura C, Brunelli N, et al (2022) Rapid response to galcanezumab and predictive factors in chronic migraine patients: A 3-month observational, longitudinal, cohort, multicenter, Italian real-life study. Eur J Neurol 29:1198–1208. 10.1111/ene.15197 [DOI] [PubMed]
- 39.De Icco R, Fiamingo G, Greco R, et al. Neurophysiological and biomolecular effects of erenumab in chronic migraine: An open label study. Cephalalgia. 2020;40:1336–1345. doi: 10.1177/0333102420942230. [DOI] [PubMed] [Google Scholar]
- 40.Ornello R, Baraldi C, Guerzoni S, et al (2021) Gender Differences in 3-Month Outcomes of Erenumab Treatment—Study on Efficacy and Safety of Treatment With Erenumab in Men. Front Neurol 12:1–8. 10.3389/fneur.2021.774341 [DOI] [PMC free article] [PubMed]
- 41.Sacco S, Braschinsky M, Ducros A, et al. European headache federation consensus on the definition of resistant and refractory migraine. J Headache Pain. 2020;21:76. doi: 10.1186/s10194-020-01130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ailani J, Burch RC, Robbins MS. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache J Head Face Pain. 2021;61(7):1021–1039. doi: 10.1111/head.14153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Drop-outs and adverse event reporting. Supplementary Table 2. Monthly headache days, monthly migraine days and days of acute drug intake according to MIDAS and monthly migraine days (MMDs) response at T3. Supplementary Table 3. Patients’ reported outcomes according to MIDAS and monthly migraine days (MMDs) response at T3.
Data Availability Statement
The dataset generated and/or analysed during the current study is available in the Zenodo repository, with the 10.1186/s10194-022-01480-2. The dataset is available from the corresponding author on reasonable request.