Abstract
Background
In sub-Saharan Africa, malaria is the common diagnosis for febrile illness and related clinical features, resulting in the under-diagnosis of other aetiologies, such as arboviruses and Rickettsia. While these may not be significant causes of mortality in malaria-endemic areas, they affect the daily life and performance of affected individuals. It is, therefore, important to have a clear picture of these other aetiologies to institute correct diagnoses at hospitals and improve patient outcomes.
Methods
Blood samples were collected from patients with fever and other clinical features associated with febrile illness at selected hospitals in the malaria-endemic counties of Busia, Bungoma, and Kakamega, and screened for Crimean-Congo haemorrhagic fever, Sindbis, dengue and chikungunya viruses, Rickettsia africae, and Plasmodium spp. using high-throughput real-time PCR techniques. A logistic regression was performed on the results to explore the effect of demographic and socio-economic independent variables on malaria infection.
Results
A total of 336 blood samples collected from hospital patients between January 2018 and February 2019 were screened, of which 17.6% (59/336) were positive for Plasmodium falciparum and 1.5% (5/336) for Plasmodium malariae. Two patients had dual P. falciparum/P. malariae infections. The most common clinical features reported by the patients who tested positive for malaria were fever and headache. None of the patients were positive for the arboviruses of interest or R. africae. Patients living in Busia (OR 5.2; 95% CI 2.46–11.79; p < 0.001) and Bungoma counties (OR 2.7; 95% CI 1.27–6.16; p = 0.013) had higher odds of being infected with malaria, compared to those living in Kakamega County.
Conclusions
The reported malaria prevalence is in line with previous studies. The absence of arboviral and R. africae cases in this study may have been due to the limited number of samples screened, low-level circulation of arboviruses during inter-epidemic periods, and/or the use of PCR alone as a detection method. Other sero-surveys confirming their circulation in the area indicate that further investigations are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04287-3.
Keywords: Malaria, Fever, Diagnosis, Prevalence, Socio-economic factors
Background
At least a hundred arboviruses, and several Rickettsia spp., are known to cause mild non-pathognomonic febrile illness in humans [1, 2], which in rare cases can lead to a range of severe complications such as encephalitis, haemorrhagic disorders, hepatitis, musculoskeletal impairment, and death [3]. However, some arboviruses, like Crimean-Congo haemorrhagic fever (CCHF), are associated with severe febrile illness and high fatality rates in humans [4]. The impact of the milder form of these diseases on human health is under-appreciated. However, studies show that they have notable impacts on the daily performance of affected individuals in terms of disability-adjusted life years (DALYS) [5]. Traditionally, arboviruses and Rickettsia are not considered significant causes of mortality and morbidity, especially in malaria-endemic resource-poor communities. Thus, funds allocated for their study and surveillance are limited [6], leading to misdiagnosis and poor assessment of their cumulative impact on community health [7, 8].
In the East African region, arboviruses are endemic, with the most important ones affecting humans being CCHF, Rift Valley fever, chikungunya, and dengue viruses [9]. Diverse tick-borne Rickettsia spp., particularly Rickettsia africae, have been widely reported in ticks collected from both the environment and cattle [10–13]; however, there is limited surveillance for these pathogens in the human population [14]. While few acute cases of spotted fever group rickettsiosis (SFGR), including African tick bite fever (caused by R. africae), have been reported in African indigenous people [15, 16], they are widely reported in travellers and expatriates visiting endemic areas in Africa. Indeed, SFGR are only second to malaria as a cause of illness in travellers returning from sub-Saharan Africa [17–19]. In East Africa, previous studies have shown that SFGR and arboviral illnesses contribute to non-malaria febrile illness in patients visiting hospitals [20–22]. Elsewhere in Africa, concurrent malaria and arboviral infections have also been documented [23, 24].
Western Kenya, comprising Busia, Bungoma and Kakamega counties, is situated at the border of Uganda and Kenya within the Lake Victoria basin of East Africa. Studies in this region have reported seropositivity to chikungunya [25], alphaviruses, flaviviruses [26, 27], phleboviruses (RVF) [28], and Rickettsia [14]. A single fatal human case of CCHF was also reported in this region [29]. In terms of malaria endemicity classification, Busia County is defined as a lake-endemic transmission zone (high stable transmission), while Bungoma and Kakamega counties have both lake-endemic and highland epidemic zones [30].
Great strides have been made in Kenya to study arboviral diseases during outbreaks in the known hotspots, such as the northern and coastal areas. However, fewer investigative efforts have been undertaken during inter-epidemic periods in those areas where clinical cases have been detected and have the potential to support circulation and outbreaks. Therefore, this study was undertaken in western Kenya using a subset of blood samples collected from patients with fever and other clinical features associated with febrile illness to determine the occurrence of acute cases of CCHF, Sindbis, dengue, and chikungunya viruses and R. africae in relation to malaria infection.
Methods
Study area
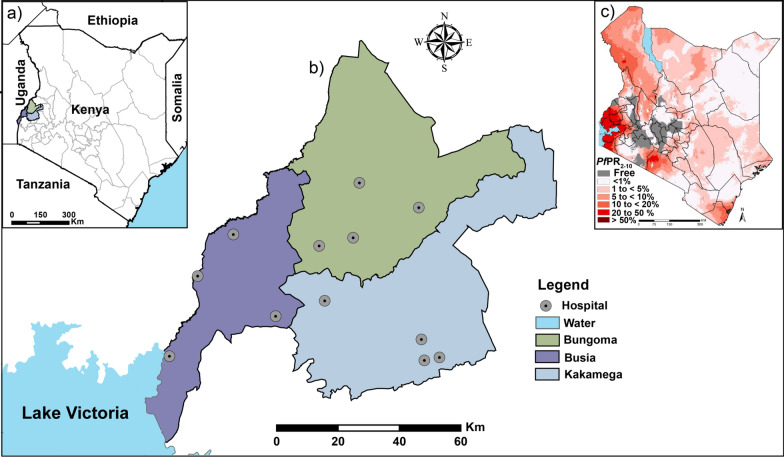
This study was carried out in the three neighbouring counties of Busia, Bungoma, and Kakamega (Fig. 1a). Busia County lies on the shores of Lake Victoria and directly borders Uganda. Mt Elgon in Bungoma County is the highest point in the western region while the Kakamega rainforest is found in Kakamega County. The inhabitants of this region are mostly of the Luhya tribe. The economic activity in the area is centred around sugar cane farming and small holder livestock keeping in Bungoma and Kakamega County, while in Busia, fishing and also small holder livestock keeping is the major economic activity. Common to all the three counties is mixed subsistence farming characterized by intensification, diversification, and close integration of crop and livestock production [31]. The climate in this region is tropical with maximum temperatures ranging from 27 °C to 32 °C and rainfall ranging between 1,350 and 2,400 mm falling over two rainy seasons annually. These conditions favour the breeding of vectors and perennial malaria transmission [32]. The Busia-Uganda border is one of the busiest in East Africa with traffic transiting to Rwanda, Burundi, The Democratic Republic of Congo, and South Sudan. The human population has been estimated at 89,3681 in Busia, 1,671 million in Bungoma, and 1,868 million in Kakamega County [33].
Fig. 1.
Map of the study site in western Kenya. (a) The highlighted three counties where the study was conducted, (b) the distribution of the hospitals from which patients were recruited and (c) malaria risk grading in Kenya based on the Plasmodium falciparum parasite prevalence data in children aged 2-10 years (PfPR2-10) [30]
Study design and sampling procedure
The blood samples used in this study were collected as part of the Zoonoses in Livestock in Kenya (ZooLinK) project, as described in detail elsewhere [34]. The study aimed to develop and pilot a surveillance system for a number of zoonotic diseases in the area, involving collaborations between the animal and human health sectors for better diagnostics and enhanced awareness.
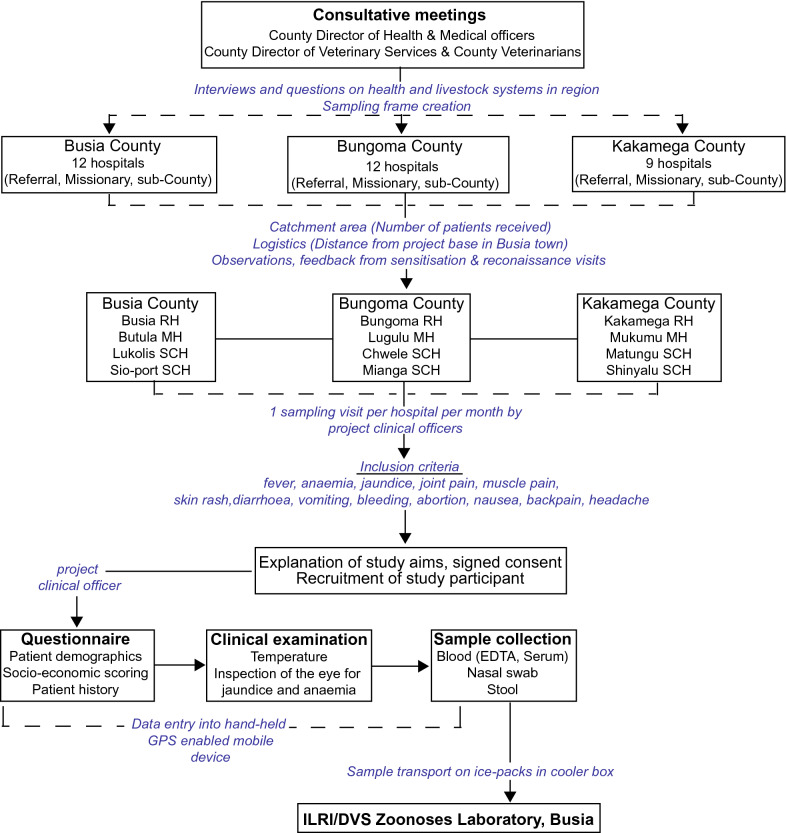
Briefly, a sampling frame of 24 hospitals in the three counties was created, from which a total of 12 (4 per County) were selected for sampling. In each County, a referral hospital, a missionary hospital, and two sub-County hospitals were included in the study. Each of the selected hospitals was visited once every 4 weeks for 20 sampling cycles from 2017 to 2019. At each hospital, two project clinical officers liaised with the hospital staff to ensure smooth recruitment of study participants. Up to 10 participants were recruited at each hospital per visit. The inclusion criterion was patients presenting with one or more clinical features, including fever, suggestive of an arboviral/malaria/bacterial infection (Fig. 2). Fever, defined as a body temperature of ≥ 37.5 °C was determined in patients by measuring axillary temperature with a digital thermometer. Self-reported fever was also inclusive even if the axillary temperature was within normal range.
Fig. 2.
Graphical illustration of the sampling framework at hospitals in western Kenya. Based on Falzon et al. [34]. RH referral hospital; MH missionary hospital; SCH sub-County hospital; ILRI International Livestock Research Institute; DVS Department of Veterinary Services
Presence of jaundice and anaemia was determined by visual inspection of the sclera and palpebral conjunctiva. Muscle, joint, and back pain and other clinical features were captured as reported by the patients during history taking. Following signed consent, a brief questionnaire on current clinical features, current and previous treatments, and demographics, was administered. Blood samples were collected in 5-ml serum tubes and 4-ml EDTA tubes, which were then barcoded to maintain patient anonymity and confidentiality. Samples were transported to the field laboratory in Busia in a cooler box on ice packs and were later shipped on dry ice to the ILRI Nairobi laboratory where they were stored at −80 °C.
For the purpose of this study, 336 of 865 blood samples collected throughout the study, and associated demographic data were selected, based on the following criteria: (i) blood samples collected from patients with fever and other clinical features associated with febrile illness (Fig. 2) between January 2018 and February 2019, corresponding to the period ticks and mosquitoes positive for CCHF and Sindbis viruses, respectively, were collected from the same study site [10, 35], (ii) availability of complete meta-data, and (iii) adequate blood volume to conduct all analyses. Selected samples were thawed, aliquoted and transported on ice (0–5 °C) to the Martin Lüscher Emerging Infectious Disease (ML-EID) laboratory at the International Centre of Insect Physiology and Ecology (icipe) where nucleic acid extraction was performed on arrival and then stored at −80 °C for subsequent molecular analyses. The icipe ML-EID laboratory is located approximately 25 km (30–40 min) from the ILRI Nairobi laboratories.
Nucleic acid extraction
The magnetic-based High Prep Viral DNA/RNA kit (Magbio Genomics Gaithersburg, USA), was used to isolate total nucleic acid from whole blood. Initially 200 µl of blood was added to 528 µl of a lysis master-mix consisting of VDR lysis buffer, isopropanol, and carrier RNA. After vortexing, 10 µl of proteinase K and 10 µl of MAG-S1 magnetic beads were added and mixed into solution by vortexing. The subsequent wash steps to separate protein and cellular debris from nucleic acid bound to magnetic particles were performed according to the manufacturer’s instructions. The High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, USA) was used to synthesize cDNA in 10 µl reactions according to the manufacturer’s instructions, supplementing the random primers with 600 µM non-ribosomal random hexanucleotide primers previously described for maximum yield [36].
Detection of arboviruses and Rickettsia africae
To detect CCHF, Sindbis, dengue and chikungunya viruses in patients, a multiplex touchdown PCR and high-resolution melting analyses (PCR-HRM) were applied as an initial screening test to select positive samples for further identification, as described in detail by Villinger et al. [37] and Ajamma et al. [38] (for more details on the primers used for pathogen detection refer to Additional file 1). To detect the presence of R. africae, patients’ blood samples were screened with PCR-HRM using Rick-F1 and Rick-R2 primers that target the Rickettsia 16S rRNA region [12, 39].
Detection of Plasmodium spp
Patients’ blood samples were screened for malaria-causing Plasmodium spp. using two sets of primers. Initially, a primer pair ncMS-F/ncMS-R was used to amplify a non-coding mitochondrial region (large subunit rRNA fragment E) of all Plasmodium spp. [40] using PCR-HRM [41]. All positive samples were selected by analysing melt and normalized profiles on Rotor-Gene Q software 2.1.0. Positive samples were further amplified using cox 1 primers targeting a 540-bp region of the cytochrome oxidase 1 gene of Plasmodium spp. [42]. Plasmodium falciparum DNA amplified and sequenced previously was included as a positive control in all the runs [43] (for more details on the thermocycling conditions refer to Additional file 1). Successful amplification was visualized by gel electrophoresis. Representative amplicons were purified by an Exo 1-rSAP combination (Biolabs, UK) and sent for bidirectional sequencing at Macrogen (Netherlands). Sequences were edited and cleaned using Geneious Prime version 2019.0.4 software (Biomatters, New Zealand). To confirm identity and relationship with previously described Plasmodium spp., nucleotide sequences were queried against the GenBank nr database using BLAST (Basic Local Alignment Search Tool).
Data analyses
Logistic regression to evaluate the determinants of malaria infection was performed in R® version 4.0.3 using the generalized linear model (GLM) for binary responses. The independent variables included baseline socio-economic, demographic, and geographic variables such as county of residence, sex, age, occupation, floor type in house, livestock ownership, use of mosquito nets, and education level (for more details on the management of independent variables for statistical analysis refer to Additional file 2). Malaria prevalence based on a positive PCR test result for Plasmodium spp. was estimated for each of the independent variable categories. Odds ratios, confidence intervals, and P-values were estimated, with a P ≤ 0.05 being considered statistically significant.
Results
Socio-economic and demographic characteristics of study participants
Table 1 shows the socio-economic and demographic profile of the 336 patients whose blood samples were analysed in this study. Of the 529 samples excluded from this study, 338 had inadequate volume for analysis, 162 were collected before January 2018, and 29 had incomplete metadata. Amongst the depleted blood samples (n = 338), 306 were above 13 years of age. Fever as a clinical feature (n = 214) was measured in 70 of the participants and self-reported in the other 144 patients. Overall, 37% (124/336) of the participants were from Bungoma County, 35% (117/336) from Kakamega, and 28% (95/336) from Busia County. Twenty-six percent (87/336) of the participants were males compared to 74% (249/336) females. The age of the participants ranged from 6 to 88 years; most were aged between 10–19 years (27%; 90/336) or 50 years and above (23%; 77/336). Farming was the most common occupation (106/336; 31.5%), while students contributed an equal proportion of the study participants (105/336; 31.3%). Ninety-two percent (309/336) of the participants reported coming from a household that owned livestock. Ninety-six percent (324/336) of the participants reported having at least one mosquito bed-net at home. Only 6% (20/336) of the participants came from a household where the female head/spouse had not received any level of formal education, while 29.5% (99/336) came from a household where the female head/spouse had gone up to form 4 and beyond.
Table 1.
Socio-economic and demographic variables of the patients recruited for this study by county
| Characteristic | Busia county | Bungoma county | Kakamega county | Combined | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total | 95 | 124 | 117 | 336 | ||||
| Sex | ||||||||
| Male | 23 | 24.2 | 35 | 28.2 | 29 | 24.8 | 87 | 25.9 |
| Female | 72 | 75.8 | 89 | 71.8 | 88 | 75.2 | 249 | 74.1 |
| Age (years) | ||||||||
| 0–9 | 5 | 5.3 | 2 | 1.6 | 5 | 4.3 | 12 | 3.6 |
| 10–19 | 33 | 34.7 | 33 | 26.6 | 24 | 20.5 | 90 | 26.8 |
| 20–29 | 18 | 18.9 | 17 | 13.7 | 30 | 25.6 | 65 | 19.3 |
| 30–39 | 10 | 10.5 | 19 | 15.3 | 18 | 15.4 | 47 | 14 |
| 40–49 | 12 | 12.6 | 20 | 16.1 | 13 | 11.1 | 45 | 13.4 |
| 50 + | 17 | 17.9 | 33 | 26.6 | 27 | 23.1 | 77 | 22.9 |
| Occupation | ||||||||
| Farmer | 28 | 29.5 | 44 | 35.5 | 34 | 29.1 | 106 | 31.5 |
| Trader | 13 | 13.7 | 15 | 12.1 | 19 | 16.2 | 47 | 14 |
| Student | 37 | 38.9 | 37 | 29.8 | 31 | 26.5 | 105 | 31.3 |
| Other | 6 | 6.3 | 20 | 16.1 | 16 | 13.7 | 42 | 12.5 |
| Unemployed | 11 | 11.6 | 8 | 6.5 | 17 | 14.5 | 36 | 10.7 |
| Floor type | ||||||||
| Mud/wood | 48 | 50.5 | 75 | 60.5 | 62 | 53 | 185 | 55.1 |
| Cement/tiles | 47 | 49.5 | 49 | 39.5 | 55 | 47 | 151 | 44.9 |
| Livestock ownership | ||||||||
| Yes | 86 | 90.5 | 112 | 90.3 | 111 | 94.9 | 309 | 92 |
| No | 9 | 9.5 | 12 | 9.7 | 6 | 5.1 | 27 | 8 |
| Mosquito nets | ||||||||
| Yes | 92 | 96.8 | 119 | 96 | 113 | 96.6 | 324 | 96.4 |
| No | 3 | 3.2 | 5 | 4 | 4 | 3.4 | 12 | 3.6 |
| Education level | ||||||||
| None | 9 | 9.5 | 3 | 2.4 | 8 | 6.8 | 20 | 5.9 |
| Class 1–7 | 24 | 25.3 | 22 | 17.7 | 27 | 23.1 | 73 | 21.7 |
| Class 8 & Form 1–3 | 39 | 41 | 58 | 46.8 | 47 | 40.2 | 144 | 42.9 |
| Form 4 & above | 23 | 24.2 | 41 | 33.1 | 35 | 29.9 | 99 | 29.5 |
Plasmodium spp., arbovirus, and Rickettsia detection
All patient blood samples tested negative for CCHF, Sindbis, dengue and chikungunya viruses and R. africae. However, by PCR-HRM analysis, P. falciparum (GenBank accessions MT430947-MT430947) and Plasmodium malariae (GenBank accession MT430946) infections were detected in patients presenting to selected hospitals in the counties under study. Two patients had dual P. falciparum/P. malariae infections (Fig. 3).
Fig. 3.
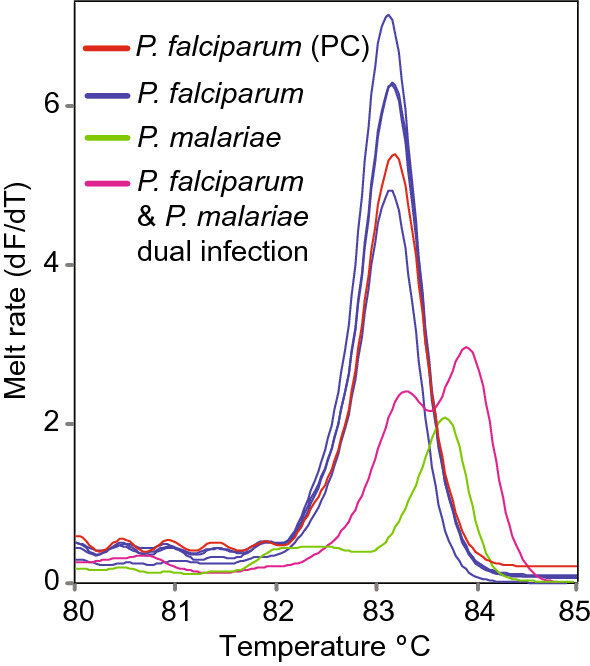
Melt rate profiles of positive representative samples. Plasmodium falciparum, P. malariae and P. falciparum/P. malariae mixed infections based on amplification of the non-coding mitochondrial gene (nc-MS) of Plasmodium spp. are shown. PC positive control
Malaria prevalence, univariable and multivariable logistic regression analyses
The overall prevalence of malaria in the patients that were recruited in this study at hospitals in the three neighbouring counties was 19.6% (66/336), as determined by a positive PCR result for P. falciparum or P. malariae. Specifically, the prevalence was 17.6% for P. falciparum and 1.5% for P. malariae. The prevalence of malaria in the different independent variable categories is shown in Table 2. Busia County had the highest prevalence (32.6%) followed by Bungoma County (20.2%), while Kakamega County had the lowest (8.5%). Overall, more females were recruited into the study, compared to male participants; nonetheless, the malaria prevalence was higher in males. The prevalence of malaria was highest in those patients who were between 0 and 9 years of age. Most of the participants came from farming households followed by those who reported as being students; however, the malaria prevalence was higher in the latter group, possibly confounded by age. The participants were equally divided into those who reported living in a house with a mud/wood floor and those living in a house with a cement/tile floor, with a higher prevalence of malaria in the latter group. The prevalence of malaria was higher in patients that reported having no mosquito net, compared to those that had one at home. Similarly, the prevalence was higher in patients who came from households without livestock, compared to those who had livestock at home. Malaria prevalence was highest in patients who came from a household where the female head or spouse did not have any formal education, compared to the other education categories.
Table 2.
Univariable logistic regression of malaria infection in patients at hospitals in western Kenya
| Variable | Categories | Prevalence % | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| County | ||||
| Busia | 31/95 (32.6) | 5.2 (2.46–11.79) | < 0.001 | |
| Bungoma | 25/124 (20.2) | 2.7 (1.27- 6.16) | 0.013 | |
| Kakamega | 10/117 (8.5) | Ref. | Overall: < 0.001 | |
| Sex | ||||
| Male | 21/87 (24) | 1.4 (0.79–2.57) | 0.222 | |
| Female | 45/249 (18.1) | Ref. | ||
| Age (years) | ||||
| 0–9 | 5/12 (41.7) | 3.2 (0.85–11.63) | 0.075 | |
| 10–19 | 24/90 (26.7) | 1.6 (0.79–3.51) | 0.195 | |
| 20–29 | 11/65 (16.9) | 0.92 (0.38–2.18) | 0.844 | |
| 30–39 | 5/47 (10.6) | 0.5 (0.16–1.52) | 0.263 | |
| 40–49 | 7/45 (15.6) | 0.82 (0.29–2.18) | 0.711 | |
| 50 + | 14/77 (18.2) | Ref. | Overall: 0.096 | |
| Occupation | ||||
| Unemployed | 9/36 (25) | 1.6 (0.63–3.98) | 0.292 | |
| Trader | 5/47 (10.6) | 0.6 (0.18–1.57) | 0.315 | |
| Student | 27/105 (25.7) | 1.69 (0.87–3.35) | 0.124 | |
| Other | 7/42 (16.7) | 1 (0.35–2.46) | 0.963 | |
| Farmer | 18/106 (17) | Ref. | Overall: 0.169 | |
| Floor type | ||||
| Mud/wood | 33/185 (17.8) | 0.8 (0.45–1.33) | 0.357 | |
| Cement/tiles | 33/151 (21.9) | Ref. | ||
| Livestock ownership | ||||
| Yes | 60/309 (19.4) | 0.8 (0.34–2.38) | 0.725 | |
| No | 6/27 (22.2) | Ref. | ||
| Mosquito nets | ||||
| No | 5/12 (41.7) | 3.1 (0.89–9.97) | 0.062 | |
| Yes | 61/324 (18.8) | Ref. | ||
| Education level | ||||
| None | 8/20 (0.4) | 4 (1.38–11.69) | 0.01 | |
| Class 1–7 | 15/73 (20.5) | 1.57 (0.7–3.53) | 0.27 | |
| Class 8 & Form 1–3 | 29/144 (20.1) | 1.53 (0.77–3.15) | 0.231 | |
| Form 4 & above | 14/99 (14.1) | Ref. | Overall: 0.09 | |
Statistically significant p-values are shown in italics
The most common clinical features in the patients who tested positive for malaria were fever and headache, followed by abdominal cramps, joint pain, and back pain in decreasing order. There were fewer occurrences of the other clinical features as reported by the patients.
On univariable logistic regression analysis, county of residence, age, “mosquito nets” and education level were marginally associated with the occurrence of malaria, while the other independent variables were not (Table 2). Therefore, multivariable logistic regression analysis was carried out on the association of these four variables and malaria infection.
The multivariable model contained only ‘county of residence’ as the variable significantly associated with malaria infection; therefore, no final model is presented as this was the same as that presented for the univariable analysis (Table 2). Age, “mosquito nets”, and education level were sequentially dropped as their association with malaria infection in the multivariable analysis was not significant. At each stage the resultant model was compared to the preceding model using the likelihood ratio test. Malaria prevalence was higher in Busia (OR 5.2; 95% CI 2.46–11.79; p < 0.001) and Bungoma (OR 2.7; 95% CI 1.27–6.16; p = 0.013) counties, compared to Kakamega County (Table 2). These estimated odds ratios indicate 5- and 3-times higher odds of having malaria in the patients residing in the counties of Busia and Bungoma counties, respectively, than in those from Kakamega County.
Discussion
Co-circulation of malaria, arboviruses and/Rickettsia infections in malaria endemic areas can result in misdiagnoses, as they share the same clinical syndromes and diagnostic testing for the latter two pathogens is not routinely available. This study, reports on the prevalence of malaria in patients that presented to hospitals in western Kenya with fever and other symptoms suggestive of an arboviral or Rickettsia infection. However, CCHF, Sindbis, dengue and chikungunya viruses and R. africae were not detected. The study clearly shows that, while malaria is an important cause of febrile illness and other associated clinical features in this region, overall, it was present in only 17% of the patients. This means that, besides the pathogens investigated in this study, further work is still required to understand other causes of febrile illness and associated clinical features.
The prevalence of malaria in Busia County was less than that reported previously in the same area around the Lake Victoria shores (37%) [44], in Kisumu (38.3%) [32] and in Homa bay County (45.8%) [45]. These studies however, mostly focused on and reported malaria infection only in children ranging between 6 months and 15 years, rather than in patients of all ages. In malaria endemic regions with stable transmission, malaria infection is higher in children compared to adults due to a gradual age-dependent build-up of immunity [46]. This is also evident in this study where the prevalence of malaria was 41.7% in children under 9 years but comparatively lower in the older age groups. Comparable malaria prevalences were reported in other studies in patients on Mfangano and neighbouring islands on Lake Victoria in Homa Bay County [47, 48]. Higher prevalence of malaria in Busia and Bungoma, compared to Kakamega County, concurs with past reports of heterogeneity in transmission patterns in western Kenya, with higher transmission around the Lake Victoria shores in Busia being reported compared to other areas [44]. In all these previous studies, the reported malaria prevalence determined by PCR was consistently higher than that by microscopy or rapid diagnostic test (RDT), affirming the higher sensitivity of PCR in comparison to the latter tests. The complications that can arise because of this misdiagnosis, which include improper treatment options, drug wastage and confounding of malaria prevalence estimates, have been extensively reviewed before [48, 49].
Overall, the counties of Kisumu, Siaya, Migori, Homa Bay, Kakamega, Busia, Bungoma, and Vihiga are classified as having lake endemic malaria transmission [44]. However, the western Kenyan region where this study was carried out has both stable and epidemic malaria transmission. This is because among these eight counties, Bungoma, Kakamega and Vihiga do not share a border with Lake Victoria hence they have pockets of epidemic malaria transmission resulting in lower malaria prevalence. Stable transmission is characterized by hyper endemic transmission rates, with an average of one infective bite/person/week throughout the year, while epidemic transmission occurs in less prevalent zones where the population has less immunity [30].
The most frequent species in this study was P. falciparum as reported by other studies with less occurrence of P. malariae and Plasmodium ovale [32, 47, 48]. Most of the clinical features that were reported by the patients who tested positive for malaria are generally shared between rickettsial/arboviral illnesses, highlighting the difficulty of deriving a correct diagnosis for these infections based on clinical signs and symptoms alone without further laboratory testing.
The other variables in this study were not significantly associated with malaria infection. Bed net possession was high among the participants; however, its correct and consistent use was not assessed. The association between bed nets and malaria prevalence may be masked in some studies, as some patients get infective bites before retiring to bed or early morning due to the changing ecology and behaviour of the mosquito vector [50], improper use of the bed nets [51], and aging of the nets themselves [52].
Patient age is usually an important predictor in higher transmission settings, with infants under the age of 5 years being at a higher risk [53]. However, in low transmission settings, the risk will also extend into adulthood [32, 54]. The effect of sex has not been fully elucidated, with previous studies showing conflicting findings. Women were more likely to be infected than males in Kisumu [55]. Socio-cultural activities expose both women and men to infective mosquito bites, albeit in different ways [56].
The effect of livestock at households on malaria transmission is dynamic, with the consensus that livestock can zoo-potentiate transmission when close to or inside human dwellings [57–59], but can also act as zoo-prophylaxis when placed a distance away from households [60]. In this study, the proximity of livestock to the homesteads was not established. Floor-type was used as a proxy to determine the socio-economic status of the households in which the patients lived. Higher socio-economic status is usually associated with improvement in the housing conditions and consequently less predisposition to malaria [61–63]. This association may be confounded by the fact that most of the proxies for socio-economic status are in essence more directly related to malaria exposure and risk [64].
This study attempted to determine acute arboviral and rickettsial infections in relation to malaria infection, as has been reported before in Kisumu County [22], Homa Bay County [65], and Asembo [14, 66] in western Kenya, and in Tanzania [21]. However, outside outbreak phases, circulation of arboviruses is very low and it is therefore challenging to detect clinical/acute cases [22, 67]. This is also reflected in the low infection rates of the vector mosquito species from the same region, where only one pool of Culex mosquitoes was positive for Sindbis virus, in combination with a comparatively high infection rate with insect-specific flaviviruses [35]; the latter are thought to block superinfection of vectors with pathogenic viruses [68]. On the other hand, Plasmodium spp.-chikungunya dual infection has been shown to cause interferon gamma induced suppression of viraemia and joint pathology caused by arthralgic alphaviruses [69]. This is plausible in this study given that this is a malaria endemic region where most people are likely to be asymptomatic carriers [70], and the most sero-prevalent arbovirus detected previously in this area was chikungunya [25].
Due to the low detection of arboviral infections by PCR, mostly because of a brief viraemic period and low viral load, previous studies in the same region have tried to highlight the circulation of arboviruses in human populations and/or determine their contribution to febrile illness by serology [25, 27, 71]. However, serological tests are time consuming due to the need to measure different antibodies (IgA/IgM/IgG) and their titers at several stages of illness to distinguish between an active infection and past exposure [27]. Additionally, these tests have low specificity as they are prone to cross-reactivity in populations where several alphaviruses and flaviviruses are endemic [25, 72]. The problem of cross-reactivity can be alleviated by performing plaque reduction neutralization tests, which usually require at least BSL-2 facilities. Therefore, the standard recommendations for the detection of both arboviruses and Rickettsia involve a combination of direct pathogen detection (culture, antigen, or nucleic acid) and detection of a fourfold rise in antibody titers between acute- and convalescent-phase sera [73, 74]. In this study, some cases may have been missed due to the afore-mentioned limitations, that are associated with the use of PCR alone. Future studies combining the use of serology and PCR may be required to generate more informative conclusions on the occurrence of arboviral disease and rickettsioses. In this case, serology could not be performed due to serum sample constraints.
While previous studies have described high infection rates of Amblyomma variegatum ticks with R. africae [10], the findings in this study suggest a lack of significant widespread rickettsaemia in humans and livestock. If actively circulating in humans, the prevalence is likely to be extremely low, requiring very large sample sizes to be accurately quantified. The use of archived blood samples for this study may have affected the recovery and detection of pathogens of interest by PCR in the blood due to several freeze–thaw cycles. To counter the occurrence of false negatives, internal PCR controls that amplify host messenger RNA can be developed and used in future viral analysis. The other limitation of the study was that the subjects were recruited as part of a larger surveillance study to determine the occurrence of zoonotic diseases (including arboviral) in hospital patients. Therefore, subjects were selected on the basis of inclusion criteria that did not solely consider fever, hence the absence of standard non-malarial febrile illness definition and assessment of anaemia for some of the subjects. Additionally, some of the hospitals that were included had a separate children’s clinic, which could not be sampled simultaneously, resulting in the enrolment of comparatively fewer children. Further, the malaria infection described in this study was based on PCR, which can detect even asymptomatic malaria carriers. This means that their clinical signs and symptoms could have been caused by other concurrent infecting pathogens that were not detected. It is, therefore, important in future studies to compare PCR based diagnosis versus microscopy and RDT as it highlights the advantages and limitations of each method.
Conclusions
This study reports on the occurrence of malaria and associated risk factors in western Kenya in patients wherein it also sought to determine other causes of fever and related clinical features such as arboviruses and Rickettsia. While arboviruses or Rickettsia were not detected, they may be contributing a proportion of the febrile illness, at levels too low to be detected without very large studies. This presumption is on the backdrop of the reported circulation of arboviruses and Rickettsia by several serological studies in the same study region. The presence of livestock in the participants’ households is a variable of interest as reports on zoo-potentiation and zoo-prophylaxis are still conflicting. It is also an important factor in the control of arbovirus-transmitting mosquitoes, which are more zoophilic compared to malaria vectors. Distinguishing the proximity of the livestock to households is, therefore, of paramount importance in future studies. Only the ‘county of origin’ was a significant determinant of malaria infection in this study, highlighting the regions/counties of priority in malaria prevention programmes. This information is important in the implementation of targeted interventions.
Supplementary Information
Additional file 1. Detailed description of primers and thermocycling conditions used for the detection of Rickettsia africae, arboviruses and Plasmodium spp.
Additional file 2. Detailed description of the management of independent variables for statistical analysis.
Acknowledgements
We acknowledge the Zoonoses in Livestock in Kenya (ZooLinK) team for the collection of blood samples used in this analysis. We also acknowledge Dr Bester T. Mudereri of icipe’s Data Management, Modelling and Geo-information unit for assistance in producing the study site map and Dickens O. Ondifu for technical assistance during the laboratory analyses.
Abbreviations
- OR
Odds ratio
- CCHF
Crimean-Congo haemorrhagic fever
- DALYS
Disability adjusted life years
- IREC
Institutional Research Ethics Committee
- ZooLinK
Zoonoses in livestock in Kenya
- PfPR2-10
Plasmodium falciparum parasite prevalence data in children aged 2–10 years
- ILRI
International Livestock Research Institute
- NACOSTI
National Commission for Science, Technology and Innovation
- EDTA
Ethylene diamine tetra acetic acid
- ML-EID
Martin lüscher emerging infectious disease
- icipe
International centre of insect physiology and ecology
- DVS
Department of veterinary services
- MH
Missionary hospital
- SCH
Sub-County hospital
- RH
Referral hospital
- DNA
De-oxy ribonucleic acid
- RNA
Ribonucleic acid
- cDNA
Copy DNA
- PCR-HRM
Polymerase chain reaction-high resolution melting
- rRNA
Ribosomal RNA
- GLM
Generalized linear model
- RDT
Rapid diagnostic test
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- IgM
Immunoglobulin M
- SFGR
Spotted fever group rickettsioses
- CI
Confidence interval
Author contributions
LCF, LA, FA, TC, JV, DKM, and EMF designed the study and sampling. TC performed the laboratory work. TC and JV analysed the results. TC wrote the original manuscript while JV, DKM, LCF, EMF, LA, FA and ADSB edited and reviewed the manuscript. All the authors read and approved the final manuscript.
Funding
The study received support from the European Union’s Integrated Biological Control Applied Research Program (EU-IBCARP) (Grant Number: DCI-FOOD/2014/346–739) and icipe institutional funding from UK’s Foreign, Commonwealth & Development Office (FCDO); Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); Federal Democratic Republic of Ethiopia; and the Kenyan Government. Tatenda Chiuya was supported by a German Academic Exchange Service (DAAD) through the icipe ARPPIS-DAAD scholarship and through a University of Pretoria postgraduate bursary. The study also received funding from the ZooLinK project which was supported by the Biotechnology and Biological Sciences Research Council, the Department for International Development, the Economic & Social Research Council, the Medical Research Council, the Natural Environment Research Council and the Defence Science & Technology Laboratory, under the Zoonoses and Emerging Livestock Systems (ZELS) programme, grant reference BB/L019019/1. It also received support from the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). We also acknowledge the CGIAR Fund Donors (http://www.cgiar.org/funders/). The funders had no role in the design, data collection, interpretation, or decision to submit this publication.
Availability of data and materials
The dataset generated and analysed in this study can be made available from the corresponding authors on reasonable request. All the nucleotide sequences generated from this study have been deposited and are available in the GenBank database under the accession numbers indicated in text.
Declarations
Ethics approval and consent to participate
Samples and data from patients were collected after approval by the International Livestock Research Institute (ILRI) Institutional Research Ethics Committee (ref ILRI-IREC2017-08/2) licensed by the National Commission for Science, Technology and Innovation (NACOSTI) in Kenya. Permission to carry out sampling was also obtained from each County Director of Health and on site at each participating hospital. Signed and informed consent was sought from patients before they were enrolled into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatenda Chiuya, Email: tchiuya@icipe.org, Email: tatendachiuya@gmail.com.
Daniel K. Masiga, Email: dmasiga@icipe.org
References
- 1.Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vectors. 2010;3:35. doi: 10.1186/1756-3305-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards AL, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, et al. Human infection with Rickettsia felis. Kenya Emerg Infect Dis. 2010;16:1081–1086. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollidge BS, González-Scarano F, Soldan SS. Arboviral encephalitides: transmission, emergence, and pathogenesis. J Neuroimmune Pharmacol. 2010;5:428–442. doi: 10.1007/s11481-010-9234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ergonul O. Crimean—Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. 2012;2:215–220. doi: 10.1016/j.coviro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.LaBeaud AD, Sutherland LJ, Muiruri S, Muchiri EM, Gray LR, Zimmerman PA, et al. Arbovirus prevalence in mosquitoes Kenya. Emerg Infect Dis. 2011;17:233–241. doi: 10.3201/eid1702.091666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 7.Hooft AM, Ripp K, Ndenga B, Mutuku F, Vu D, Baltzell K, et al. Principles, practices and knowledge of clinicians when assessing febrile children: a qualitative study in Kenya. Malar J. 2017;16:381. doi: 10.1186/s12936-017-2021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyaoke BA, Mureithi MW, Beynon C. Factors associated with treatment type of non-malarial febrile illnesses in under-fives at Kenyatta National Hospital in Nairobi Kenya. PLoS One. 2019;14:e0217980. doi: 10.1371/journal.pone.0217980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyaruaba R, Mwaliko C, Mwau M, Mousa S, Wei H. Arboviruses in the East African community partner states: a review of medically important mosquito-borne arboviruses. Pathog Glob Health. 2019;113:209–228. doi: 10.1080/20477724.2019.1678939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiuya T, Masiga DK, Falzon LC, Bastos ADS, Fèvre EM, Villinger J. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound Emerg Dis. 2021;68:2429–2445. doi: 10.1111/tbed.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koka H, Sang R, Kutima HL, Musila L, Macaluso K. The detection of spotted fever group rickettsia DNA in tick samples from pastoral communities in Kenya. J Med Entomol. 2017;54:774–780. doi: 10.1093/jme/tjw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mwamuye MM, Kariuki E, Omondi D, Kabii J, Odongo D, Masiga D, et al. Novel Rickettsia and emergent tick-borne pathogens : a molecular survey of ticks and tick-borne pathogens in Shimba Hills National. Ticks Tick Borne Dis. 2017;8:208–218. doi: 10.1016/j.ttbdis.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Oundo JW, Villinger J, Jeneby M, Ongamo G, Otiende MY, Makhulu EE, et al. Pathogens, endosymbionts, and blood-meal sources of host-seeking ticks in the fast-changing Maasai Mara wildlife ecosystem. PLoS ONE. 2020;15:e0228366. doi: 10.1371/journal.pone.0228366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, et al. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. 2012;18:328–331. doi: 10.3201/eid1802.111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly P. African tick-bite fever: a new spotted fever group rickettsiosis under an old name. Lancet. 1992;340:982–983. doi: 10.1016/0140-6736(92)92878-J. [DOI] [PubMed] [Google Scholar]
- 16.Ndip LM, Bouyer DH, Travassos Da Rosa AP, Titanji VP, Tesh RB, Walker DH. Acute spotted fever rickettsiosis among febrile patients Cameroon. Emerg Infect Dis. 2004;10:432–7. doi: 10.3201/eid1003.020713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Althaus F, Greub G, Raoult D, Genton B. African tick-bite fever : a new entity in the differential diagnosis of multiple eschars in travelers. Description of five cases imported from South Africa to Switzerland. Int J Infect Dis. 2010;14:e274–6. doi: 10.1016/j.ijid.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Jensenius M, Davis X, Von Sonnenburg F, Schwartz E, Keystone JS, Leder K, et al. Multicenter geosentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis. 2009;15:1791–1798. doi: 10.3201/eid1511.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, Ngatunga SD, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital Tanzania. PLoS Negl Trop Dis. 2014;8:e3335. doi: 10.1371/journal.pntd.0003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waggoner J, Brichard J, Mutuku F, Ndenga B, Heath CJ, Mohamed-Hadley A, et al. Malaria and chikungunya detected using molecular diagnostics among febrile Kenyan children. Open Forum Infect Dis. 2017;4:ofx110. doi: 10.1093/ofid/ofx110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayorinde AF, Oyeyiga AM, Nosegbe NO, Folarin OA. A survey of malaria and some arboviral infections among suspected febrile patients visiting a health centre in Simawa, Ogun State. Nigeria J Infect Public Health. 2016;9:52–59. doi: 10.1016/j.jiph.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, Senghor CS, et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J. 2016;15:47. doi: 10.1186/s12936-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mease LE, Coldren RL, Musila LA, Prosser T, Ogolla F, Ofula VO, et al. Seroprevalence and distribution of arboviral infections among rural Kenyan adults: a cross-sectional study. Virol J. 2011;8:371. doi: 10.1186/1743-422X-8-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossi-soyster EN, Cook EAJ, De GWA, Thomas F, Krystosik AR, Lee J, et al. Serological and spatial analysis of alphavirus and flavivirus prevalence and risk factors in a rural community in western Kenya. PLoS Negl Trop Dis. 2017;17(11):e0005998. doi: 10.1371/journal.pntd.0005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inziani M, Adungo F, Awando J, Kihoro R, Inoue S, Morita K, et al. Seroprevalence of yellow fever, dengue, West Nile and chikungunya viruses in children in Teso South Sub-County Western Kenya. Int J Infect Dis. 2020;91:104–110. doi: 10.1016/j.ijid.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Cook EAJ, Grossi-Soyster EN, de Glanville WA, Thomas LF, Kariuki S, Bronsvoort BMC, et al. The sero-epidemiology of Rift Valley fever in people in the Lake Victoria Basin of western Kenya. PLoS Negl Trop Dis. 2017;11:e0005731. doi: 10.1371/journal.pntd.0005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunster L, Dunster M, Ofula V, Beti D, Kazooba-Voskamp F, Burt F, et al. First documentation of human Crimean-Congo hemorrhagic fever Kenya. Emerg Infect Dis. 2002;8:1005–1006. doi: 10.3201/eid0809.010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health . The epidemiology and control profile of malaria in Kenya: reviewing the evidence to guide the future vector control. Nairobi: National Malaria Control Programme; 2016. [Google Scholar]
- 31.Fèvre EM, de Glanville WA, Thomas LF, Cook EAJ, Kariuki S, Wamae CN. An integrated study of human and animal infectious disease in the Lake Victoria crescent small-holder crop-livestock production system Kenya. BMC Infect Dis. 2017;17:457. doi: 10.1186/s12879-017-2559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapesa A, Kweka EJ, Atieli H, Afrane YA, Kamugisha E, Lee M, et al. The current malaria morbidity and mortality in different transmission settings in Western Kenya. PLoS ONE. 2018;13:e0202031. doi: 10.1371/journal.pone.0202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenya Population and Housing Census. Distribution of Population by Administrative Units. Vol. 2, 2019.
- 34.Falzon LC, Alumasa L, Amanya F, Kang'ethe E, Kariuki S, Momanyi K, et al. One health in action: operational aspects of an integrated surveillance system for zoonoses in Western Kenya. Front Vet Sci. 2019;6:252. doi: 10.3389/fvets.2019.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiuya T, Masiga DK, Falzon LC, Bastos ADS, Fèvre EM, Villinger J. A survey of mosquito-borne and insect-specific viruses in hospitals and livestock markets in western Kenya. PLoS ONE. 2021;16:e0252369. doi: 10.1371/journal.pone.0252369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endoh D, Mizutani T, Kirisawa R, Maki Y, Saito H, Kon Y, et al. Species-independent detection of RNA virus by representational difference analysis using non-ribosomal hexanucleotides for reverse transcription. Nucleic Acids Res. 2005;33:e65. doi: 10.1093/nar/gni064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villinger J, Mbaya MK, Ouso D, Kipanga PN, Lutomiah J, Masiga DK. Arbovirus and insect-specific virus discovery in Kenya by novel six genera multiplex high-resolution melting analysis. Mol Ecol Resour. 2017;17:466–480. doi: 10.1111/1755-0998.12584. [DOI] [PubMed] [Google Scholar]
- 38.Ajamma YU, Onchuru TO, Ouso DO, Omondi D, Masiga DK, Villinger J. Vertical transmission of naturally occurring bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo in Kenya. PLoS Negl Trop Dis. 2018;12:e0006949. doi: 10.1371/journal.pntd.0006949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijhof ARDM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007;7:585–595. doi: 10.1089/vbz.2007.0130. [DOI] [PubMed] [Google Scholar]
- 40.Bourgeois N, Boutet A, Bousquet P, Basset D, Charachon S, Lachaud L, et al. Comparison of three real-time PCR methods with blood smears and rapid diagnostic test in Plasmodium sp. infection. Eur Soc Clin Infect Dis. 2009;16:1305–11. doi: 10.1111/j.1469-0691.2009.02933.x. [DOI] [PubMed] [Google Scholar]
- 41.Chaumeau V, Andolina C, Fustec B, Ndam NT, Brengues C, Herder S, et al. Comparison of the performances of five primer sets for the detection and quantification of Plasmodium in anopheline vectors by Real-Time PCR. PLoS ONE. 2016;11:e0159160. doi: 10.1371/journal.pone.0159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Echeverry DF, Deason NA, Makuru V, Davidson J, Xiao H, Niedbalski J, et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar J. 2017;16:230. doi: 10.1186/s12936-017-1881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogola EO, Fillinger U, Ondiba IM, Villinger J, Masiga DK, Torto B, et al. Insights into malaria transmission among Anopheles funestus mosquitoes Kenya. Parasit Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bashir IM, Nyakoe N, Van Der Sande M. Targeting remaining pockets of malaria transmission in Kenya to hasten progress towards national elimination goals: an assessment of prevalence and risk factors in children from the Lake endemic region. Malar J. 2019;18:233. doi: 10.1186/s12936-019-2876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onchiri FM, Pavlinac P, Singa BO, Mulongo JN, Farquhar C, Walson J. Frequency and correlates of malaria overdiagnosis and treatment in western Kenya. Lancet Glob Health. 2014;2:S45. doi: 10.1016/S2214-109X(15)70067-7. [DOI] [Google Scholar]
- 46.Mawili-Mboumba DP, Akotet MKB, Kendjo E, Nzamba J, Medang MO, Mbina JRM, et al. Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malar J. 2013;12:3. doi: 10.1186/1475-2875-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Idris ZM, Chan CW, Kongere J, Gitaka J, Logedi J, Omar A, et al. High and heterogeneous prevalence of ssymptomatic and sub-microscopic malaria infections on islands in Lake Victoria. Kenya Sci Rep. 2016;6:36958. doi: 10.1038/srep36958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kipanga PN, Omondi D, Mireji PO, Sawa P, Masiga DK, Villinger J. High-resolution melting analysis reveals low Plasmodium parasitaemia infections among microscopically negative febrile patients in western Kenya. Malar J. 2014;13:429. doi: 10.1186/1475-2875-13-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 50.Van Bortel W, Trung HD, Hoi LX, Van Ham N, Van Chut N, Luu ND, et al. Malaria transmission and vector behaviour in a forested malaria focus in central Vietnam and the implications for vector control. Malar J. 2010;9:373. doi: 10.1186/1475-2875-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerra M, De Sousa B, Ndong-Mabale N, Berzosa P, Arez AP. Malaria determining risk factors at the household level in two rural villages of mainland Equatorial Guinea. Malar J. 2018;17:203. doi: 10.1186/s12936-018-2354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andronescu LR, Buchwald AG, Coalson JE, Cohee L, Bauleni A, Walldorf JA, et al. Net age, but not integrity, may be associated with decreased protection against Plasmodium falciparum infection in southern Malawi. Malar J. 2019;18:329. doi: 10.1186/s12936-019-2930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization. 2020. https://www.who.int/publications/i/item/9789240015791. Accessed 16 Sept 2021.
- 54.O'Meara WP, Mwangi TW, Williams TN, McKenzie FE, Snow RW, Marsh K. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg. 2008;79:185–191. doi: 10.4269/ajtmh.2008.79.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenkins R, Omollo R, Ongecha M, Sifuna P, Othieno C, Ongeri L, et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County. Kenya Malar J. 2015;14:263. doi: 10.1186/s12936-015-0781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UNDP. Discussion Paper Gender and Malaria. 2015. 1–14. https://www1.undp.org. Accessed 14 Sept 2021.
- 57.Hasyim H, Dhimal M, Bauer J, Montag D, Groneberg DA, Kuch U, et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17:302. doi: 10.1186/s12936-018-2447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwashita H, Dida GO, Sonye GO, Sunahara T, Futami K, Njenga SM, et al. Push by a net, pull by a cow : can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control ? Parasit Vectors. 2014;7:52. doi: 10.1186/1756-3305-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H, Ngonyani H, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17. doi: 10.1186/s12936-014-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franco AO, Gomes MG, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: a one health approach. PLoS ONE. 2014;9:e101699. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayele DG, Zewotir TT, Mwambi HG. Prevalence and risk factors of malaria in Ethiopia. Malar J. 2012;11:195. doi: 10.1186/1475-2875-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Glanville WA, Thomas LF, Cook EAJ, Bronsvoort BMC, Wamae NC, Kariuki S, et al. Household socio-economic position and individual infectious disease risk in rural Kenya. Sci Rep. 2019;9:2972. doi: 10.1038/s41598-019-39375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kateera F, Mens PF, Hakizimana E, Ingabire CM, Muragijemariya L, Karinda P, et al. Malaria parasite carriage and risk determinants in a rural population: a malariometric survey in Rwanda. Malar J. 2015;14:16. doi: 10.1186/s12936-014-0534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? A review of the literature. Trop Med Int Health. 2005;10:1047–1059. doi: 10.1111/j.1365-3156.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 65.Omondi D. Bionomics of vector-borne diseases in sites adjacent to Lakes Victoria and Baringo in Kenya. PhD thesis, University of Western Cape. 2016.
- 66.Maina AN, Farris CM, Odhiambo A, Jiang J, Laktabai J, Armstrong J, et al. Q fever, scrub typhus, and rickettsial diseases in children, Kenya, 2011–2012. Emerg Infect Dis. 2016;22:883–886. doi: 10.3201/eid2205.150953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu W, Lampman R, Novak RJ. Problems in estimating mosquito infection rates using minimum infection rate. J Med Entomol. 2009;40:595–596. doi: 10.1603/0022-2585-40.5.595. [DOI] [PubMed] [Google Scholar]
- 68.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–97. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teo TH, Lum FM, Ghaffar K, Chan YH, Amrun SN, Tan JJL, et al. Plasmodium co-infection protects against chikungunya virus-induced pathologies. Nat Commun. 2018;9:3905. doi: 10.1038/s41467-018-06227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Touray AO, Mobegi VA, Wamunyokoli F, Herren JK. Diversity and Multiplicity of P. falciparum infections among asymptomatic school children in Mbita Western Kenya. Sci Rep. 2020;10:5924. doi: 10.1038/s41598-020-62819-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwallah AO, Inoue S, Thairu-Muigai AW, Kuttoh N, Morita K, Mwau M. Seroprevalence of yellow fever virus in selected health facilities in Western Kenya from 2010 to 2012. Jpn J Infect Dis. 2015;68:230–234. doi: 10.7883/yoken.JJID.2014.288. [DOI] [PubMed] [Google Scholar]
- 72.Musso D, Despres P. Serological diagnosis of Flavivirus-associated human infections. Diagnostics (Basel) 2020;10:302. doi: 10.3390/diagnostics10050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paris DH, Dumler JS. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curr Opin Infect Dis. 2016;29:433–439. doi: 10.1097/QCO.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piantadosi A, Kanjilal S. Diagnostic approach for arboviral infections in the United States. J Clin Microbiol. 2020;58:e01926–e2019. doi: 10.1128/JCM.01926-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed description of primers and thermocycling conditions used for the detection of Rickettsia africae, arboviruses and Plasmodium spp.
Additional file 2. Detailed description of the management of independent variables for statistical analysis.
Data Availability Statement
The dataset generated and analysed in this study can be made available from the corresponding authors on reasonable request. All the nucleotide sequences generated from this study have been deposited and are available in the GenBank database under the accession numbers indicated in text.