CONSPECTUS:
Chemiluminescence is a fascinating phenomenon that evolved in nature and has been harnessed by chemists in diverse ways to improve life. This Account tells the story of our research group’s efforts to formulate and manifest spiroadamantane 1,2-dioxetanes with triggerable chemiluminescence for imaging and monitoring important reactive analytes in living cells, animals, and human clinical samples. Analytes like reactive sulfur, oxygen and nitrogen species, as well as pH and hypoxia can be indicators of cellular function or dysfunction and are often implicated in the causes and effects of disease. We begin with a foundation in binding-based and activity-based fluorescence imaging that has provided transformative tools for understanding biological systems. The intense light sources required for fluorescence excitation, however, introduce autofluorescence and light scattering that reduces sensitivity and complicates in vivo imaging. Our work and the work of our collaborators were the first to demonstrate that spiroadamantane 1,2-dioxetanes had sufficient brightness and biological compatibility for in vivo imaging of enzyme activity and reactive analytes like hydrogen sulfide (H2S) inside of living mice. This launched an era of renewed interest in 1,2-dioxetanes that has resulted in a plethora of new chemiluminescence imaging agents developed by groups around the world. Our own research group focused its efforts on reactive sulfur, oxygen, and nitrogen species, pH, and hypoxia, resulting in a large family of bright chemiluminescent 1,2-dioxetanes validated for cell monitoring and in vivo imaging. These chemiluminescent probes feature low background and high sensitivity that have been proven quite useful for studying signaling, for example, the generation of peroxynitrite (ONOO−) in cellular models of immune function and phagocytosis. This high sensitivity has also enabled real-time quantitative reporting of oxygen-dependent enzyme activity and hypoxia in living cells and tumor xenograft models. We reported some of the first ratiometric chemiluminescent 1,2-dioxetane systems for imaging pH and have introduced a powerful kinetics-based approach for quantification of reactive species like azanone (nitroxyl, HNO) and enzyme activity in living cells. These tools have been applied to untangle complex signaling pathways of peroxynitrite production in radiation therapy and as substrates in a split esterase system to provide an enzyme/substrate pair to rival luciferase/luciferin. Furthermore, we have pushed chemiluminescence toward commercialization and clinical translation by demonstrating the ability to monitor airway hydrogen peroxide in the exhaled breath of asthma patients using transiently produced chemiluminescent 1,2-dioxetanedione intermediates. This body of work shows the powerful possibilities that can emerge when working at the interface of light and chemistry, and we hope that it will inspire future scientists to seek out ever brighter and more illuminating ideas.
Graphical Abstract
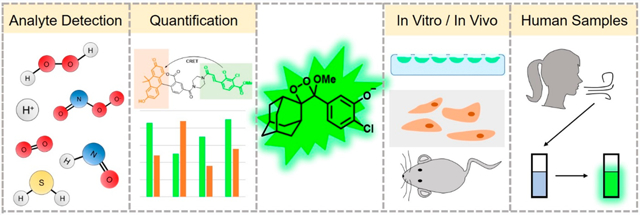
1. INTRODUCTION
Molecular imaging in living cells and animals provides a powerful approach to illuminate complex chemical processes that underlie biological phenomena. Binding-based and activity-based imaging have emerged as two dominant approaches for molecular imaging.5–7 Binding-based imaging relies on linking supramolecular recognition units to fluorophores such that fluorescence emission is modulated, usually based on a photoinduced electron or energy transfer (PeT or PET, respectively) quenching mechanism.8 Activity-based or reaction-based sensing relies on making or breaking covalent bonds to modulate a response, often mediated by a self-immolative linker design.9 These approaches have been widely applied to study cellular signaling of metal ions and cellular parameters including calcium,10 zinc,11,12 magnesium,13 potassium,14 copper,15 iron,16 pH,17 chloride,18,19 and voltage.20 Reactive sulfur, oxygen, and nitrogen (RSON) species are a class of signaling molecules that can rapidly diffuse and whose signaling properties rely on their chemical reactivity. Due to this reactive nature, reaction-based approaches are ideally poised to study these molecules.21,22 These species rapidly interact with each other to form a super family of signaling molecules and aberrant species referred to as the reactive species interactome.23 Fluorescent probe strategies have been implemented for reactive oxygen species like hydrogen peroxide (H2O2) and peroxynitrite (ONOO−),22,24–26 reactive nitrogen species like nitric oxide (NO) and azanone (nitroxyl, HNO),22,27 reactive sulfur species like hydrogen sulfide (H2S) and polysulfides (RSnH/RSnR),21,22,28–30 reactive carbon species like formaldehyde (CH2O) and carbon monoxide (CO),31,32 and oxygen (O2)/hypoxia.33 Given the complexity of their interactions, dual-responsive probes that react with multiple species are being explored as an enticing solution to this problem.34,35 While promising, fluorescence-based methods inherently suffer from light scattering and autofluorescence, which pose some limitations on sensitivity and whole animal imaging.
Chemiluminescence is now emerging as a powerful molecular imaging approach to address these limitations. This area has exploded recently due to demonstrations of in vivo compatibility1,36 and the advent of innovative chemiluminescent 1,2-dioxetane designs by Shabat and co-workers.37,38 Triggered emission from spiroadamantane 1,2-dioxetanes was first developed by Schaap and co-workers,39–41 and these dioxetanes found commercial application for in vitro research purposes. The mechanism of chemiluminescence emission has been extensively studied, but it is still incompletely understood.42 It is thought to proceed via a chemically initiated electron exchange luminescence (CIEEL) mechanism with a solvent-cage back electron transfer step as evidenced by a viscosity dependence on the luminescence emission intensity (Scheme 1).43,44 An odd/even effect on the position of the dioxetane has been observed, although the root cause of this effect remains to be completely elucidated.45,46 We direct the reader to other excellent recent review articles47–49 for expanded current and historical perspectives. This Account will focus on research from our own laboratory on the use of chemiluminescent dioxetanes for imaging RSON species, pH, and hypoxia in living organisms, with an emphasis on quantitative methods and application in mammalian cell culture and in vivo mouse models. This work has resulted in a diverse family of bright chemiluminescent 1,2-dioxetanes (Figure 1).
Scheme 1.
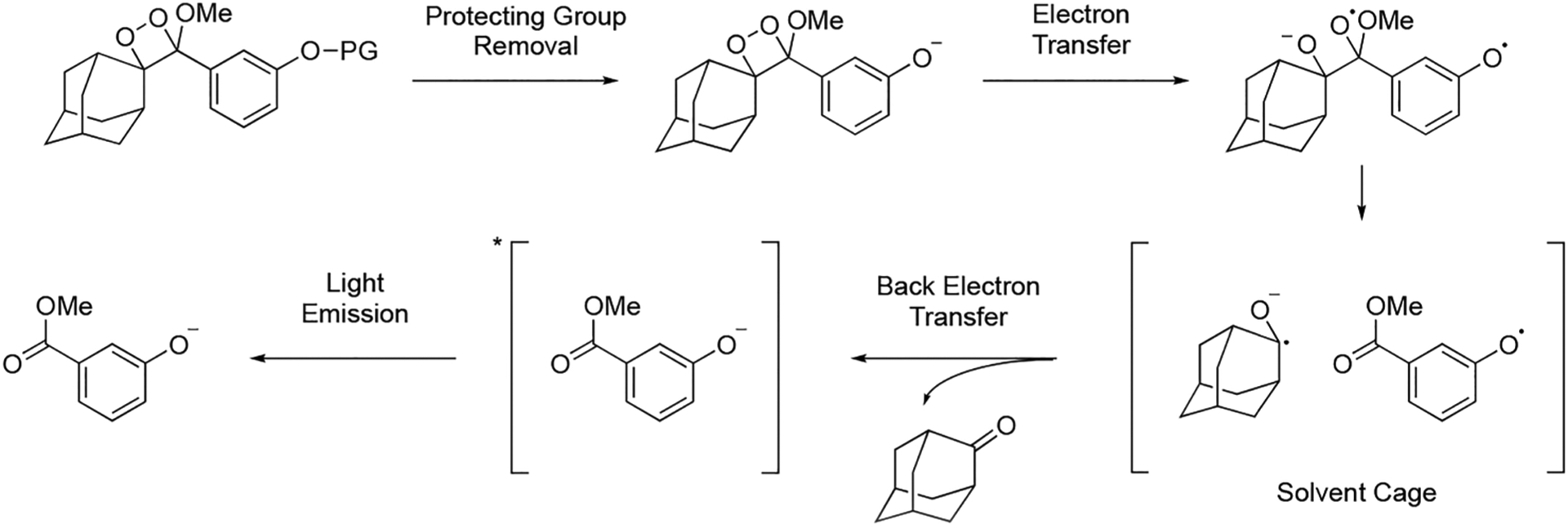
CIEEL Mechanism for Analyte-Triggered Chemiluminescence of 1,2-Dioxetanes
Figure 1.
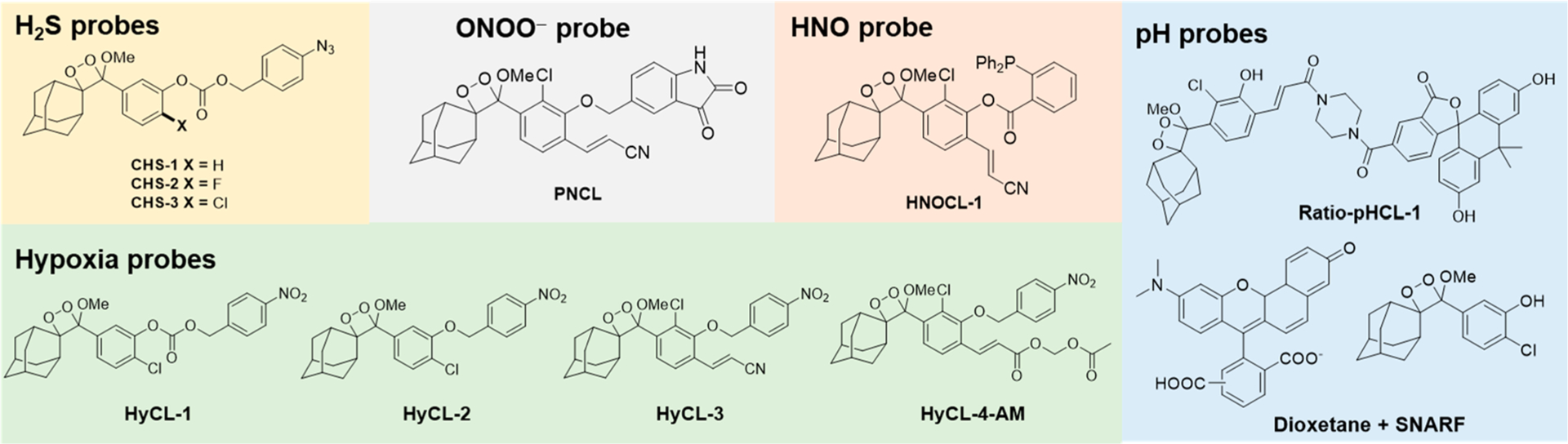
Chemiluminescent 1,2-dioxetane probes developed by our laboratory for the detection of biological analytes.
2. REACTION-BASED APPROACHES FOR STUDYING REACTIVE SULFUR, OXYGEN, AND NITROGEN SPECIES
Reaction-based probes are increasingly being used as sensitive imaging agents for small molecules which are fleeting in nature and similar in shape and size, with reactive species like hydrogen sulfide (H2S), peroxynitrite (ONOO−), and azanone (HNO) being prime examples. Our laboratory has been interested in exploiting the specific reactivity of such biologically active small molecules to develop reactivity-based responsive reporters that do not depend on noncovalent binding of substrates.
One such class of reaction-based fluorescent probes was the azide-based SF series of probes for direct, endogenous, real-time detection of H2S,50 a reactive small molecule with wide-ranging roles in mammalian physiology.51–54 In response to H2S, the fluorophore is unmasked and fluorescence turn-on is observed. SF7-AM,55 the cell-trappable variant in the SF series (Figure 2A), was shown to detect endogenous H2S production in human umbilical vein endothelial cells (HUVECs) when stimulated with vascular endothelial growth factor (VEGF). The study also employed SF7-AM to investigate enzymatic pathways that lead to VEGF-triggered H2S production. Treatment of VEGF-dosed HUVECs with inhibitors for cystathionine γ-lyase (CSE), VEGF Receptor 2 (VEGFR2), and H2O2-generating NADPH-oxidase (Nox), led to decreased SF7-AM turn-on, thus establishing the dependence of VEGF-stimulated H2S generation on CSE, VEGFR2, and Nox-derived H2O2 (Figure 3).
Figure 2.
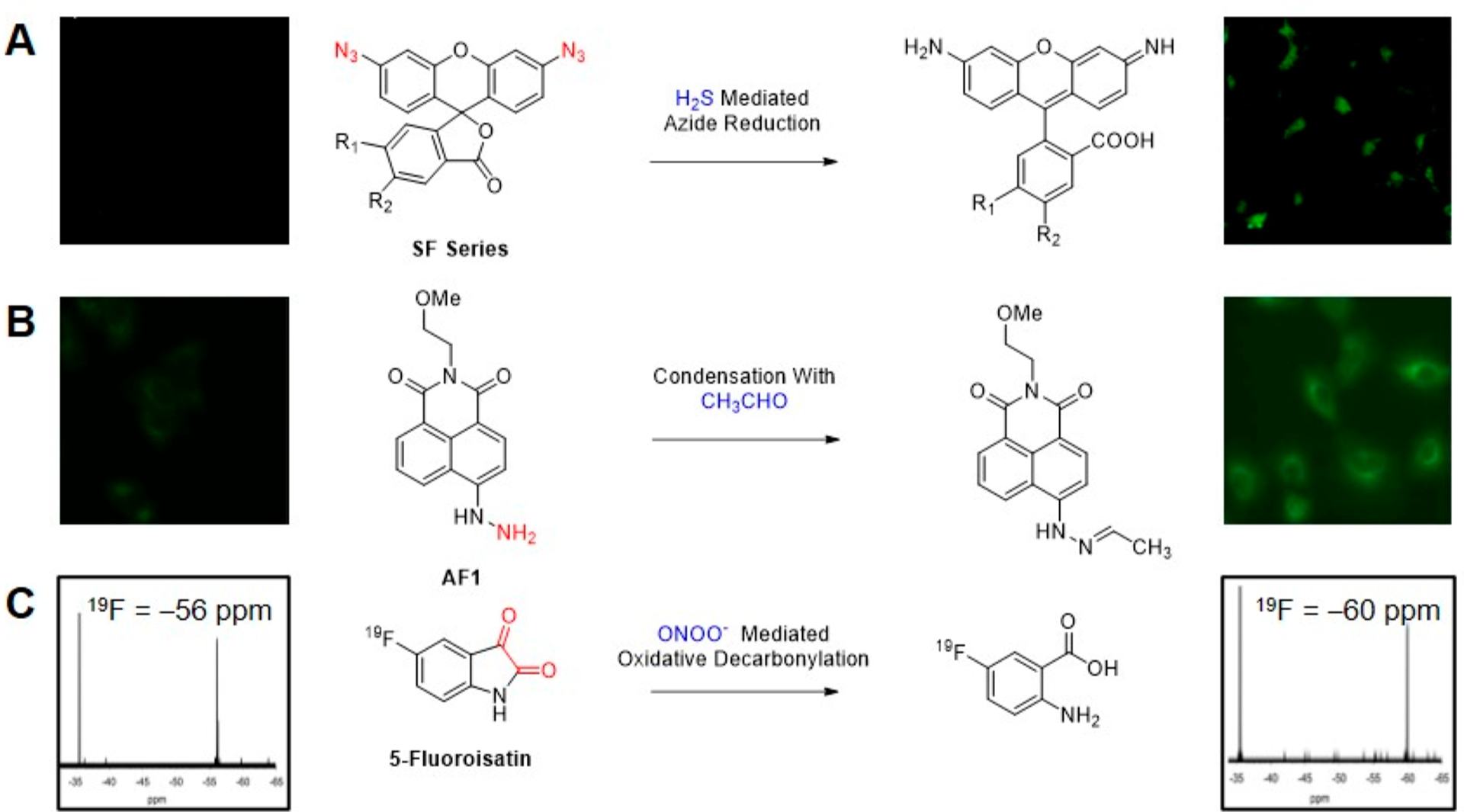
Reaction-based fluorescence and magnetic resonance probes for reactive species. (A) SF Series of probes for fluorescence imaging of H2S. Adapted with permission from ref 50. Copyright 2013 American Chemical Society. (B) AF1 for fluorescence imaging of acetaldehyde. Adapted with permission from ref 57. Copyright 2017 The Royal Society of Chemistry. (C) 5-Fluoroisatin for 19F NMR detection of ONOO−. Adapted with permission from ref 59. Copyright 2014 The Royal Society of Chemistry.
Figure 3.
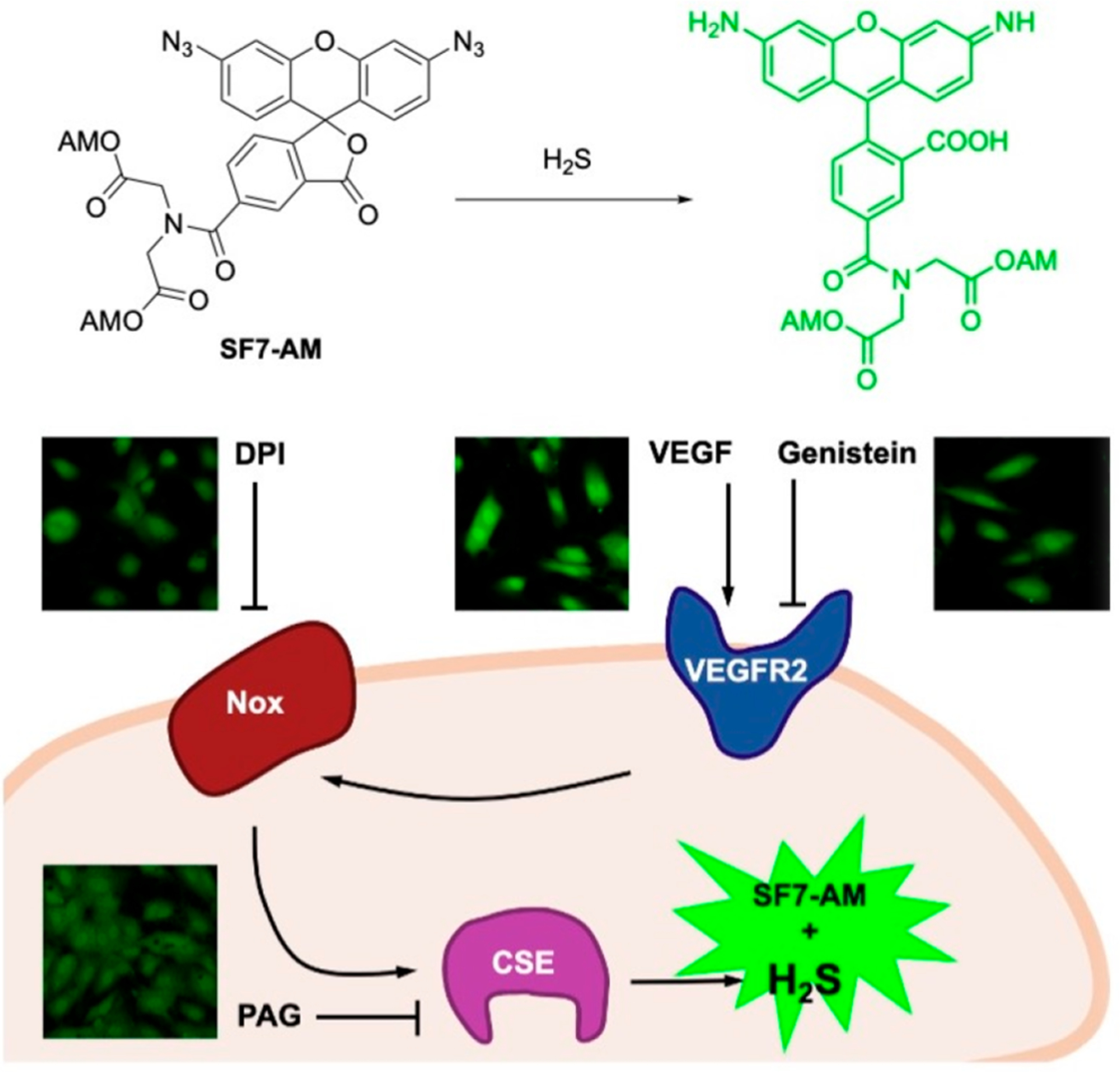
SF7-AM was used to monitor VEGF-induced H2S production in HUVEC cells. Adapted with permission from ref 55. Copyright 2011 United States National Academy of Sciences.
Following this blueprint, our group capitalized on expertise in chemoselective reaction design56 and began its exploration of sensors to detect small molecules, particularly RSON and carbon species. To observe generation of acetaldehyde from ethanol and alcohol dehydrogenase in lung epithelial cells, we designed reaction-based probe acetaldehydefluor-1 (AF1),57 utilizing a condensation reaction between reactive carbonyls and a hydrazinyl naphthalimide based scaffold with quenched fluorescence to induce fluorescence turn-on (Figure 2B). For direct detection of the biological oxidant peroxynitrite (ONOO−)58 we developed a magnetic resonance probe leveraging a newly discovered ONOO− promoted oxidative decarbonylation reaction of fluoroisatin α-ketocarbonyls (Figure 2C).59 Formation of the fluoroanthranilic acid product was monitored using 19F NMR spectroscopy. Though these techniques enabled direct imaging/detection of acetaldehyde and peroxynitrite in cells, we still aspired to develop a more sensitive and dynamic imaging system that could report on analyte fluxes from within tissue and even in whole animals.
3. ESTABLISHING CHEMILUMINESCENT 1,2-DIOXETANES FOR IN VIVO IMAGING
Excited state emitters for chemiluminescence are generated via a chemical reaction as opposed to light excitation for fluorescence.42 Therefore, chemiluminescence imaging is not as impeded by autofluorescence and light scattering, significantly improving sensitivity of this imaging method over fluorescence imaging and affording higher tissue penetration and imaging depth. Since chemiluminescence outputs transient light emission, signal can be detected as it is generated, enabling dynamic imaging and interception of transient species. Despite these obvious advantages, there was great uncertainty about the compatibility of triggerable 1,2-dioxetanes with living systems. Would these compounds be bright enough to be observable through living tissue? Could they generate enough flux upon reaction with small molecule analytes? Would living animals tolerate these compounds physiologically?
In an important study by Liu and Mason in 2010,36 spiroadamantane 1,2-dioxetanes were first shown to be viable as in vivo reporters of enzymatic activity in live mice. A commercially available Galacto-Light Plus microplate-based chemiluminescence assay (Figure 4A) was adapted for in vivo use to image β-galactosidase enzymatic activity in transgenic mice growing LacZ tumors for overexpression of β-galactosidase. The components of this packaged assay, a 1,2-dioxetane with a β-d-galacto pyranoside trigger (Galacton Plus) and Emerald enhancer were administered via intravenous and intratumor injection in live mice growing LacZ tumors. Chemiluminescence light emission was observed within 10 s of injection using an IVIS Spectrum light detection instrument, and up to 10-fold differences in emission intensity were observed between LacZ tumors and wildtype tumors. These studies demonstrated two key points. First, light emission from spiroadamantane 1,2-dioxetanes was sufficient for noninvasive in vivo imaging through animal tissue and second, high-turnover enzymatic reactions could be observed using these probes.
Figure 4.
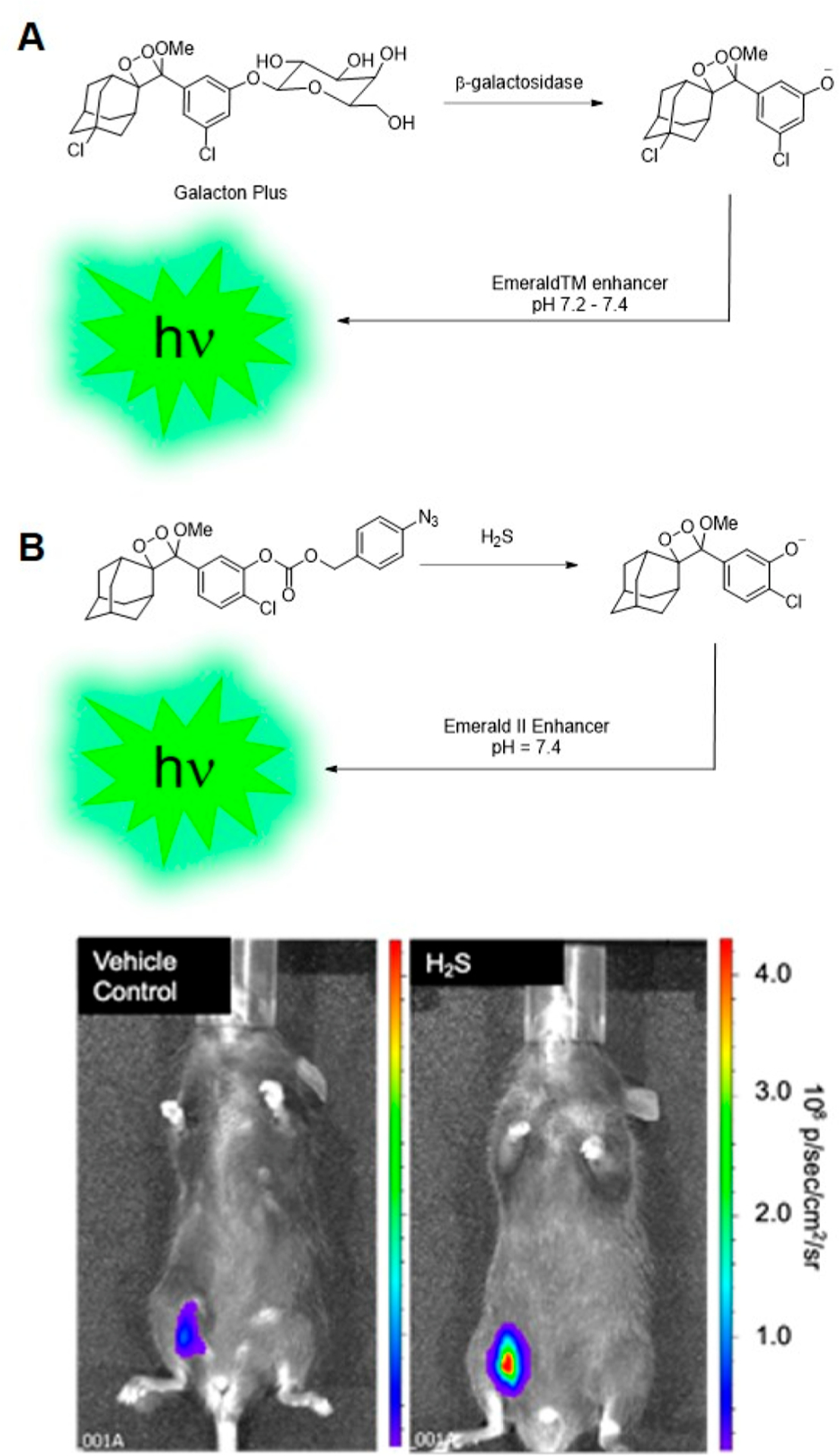
Probes demonstrated as in vivo chemiluminescence imaging agents. (A) Commercially available Galacto-Light Plus chemiluminescence assay adapted for imaging β-galactosidase enzymatic activity in mice. (B) Chemiluminescence H2S imaging in live mice using CHS-3. Adapted with permission from ref 1. Copyright 2015 The Royal Society of Chemistry.
Inspired by this demonstration of sensitive in vivo chemiluminescence imaging, we sought to explore whether spiroadamantane 1,2-dioxetanes could be used with reaction-based approaches for imaging small molecule reactive analytes in living animals. We designed reaction-based first-generation chemiluminescent H2S probes CHS-1, -2, and -3 for in vivo detection of H2S.1 A para-azidobenzyl carbonate trigger attached to a sterically stabilized spiroadamantane 1,2-dioxetane results in light emission upon H2S-mediated azide reduction and consequent self-immolative cleavage to the excited state phenolate. A comparative study of emission response between CHS-1, -2, and -3, differing in structure by the substituent (H, F, or Cl) ortho to the phenolate, showed that emission intensity at pH 7.4 increases with phenol acidity: the chloro-substituted CHS-3 showed highest turn-on response under physiological conditions. CHS-3 with the Emerald II enhancer was able to detect differences in H2S in A549 lung epithelial cells produced in response to addition of homocysteine, a substrate for H2S-generating enzyme cystathionine γ-lyase (CSE), and propargylglycine, a CSE inhibitor. Most significantly, we established that chemiluminescent 1,2-dioxetane based probes could be used for in vivo detection of small molecules such as hydrogen sulfide; CHS-3 exhibited a four times stronger chemiluminescent response when injected into the intraperitoneal cavity of live mice with H2S donor sodium sulfide (Na2S) as compared to a vehicle control (Figure 4B). This was the first demonstration that reactive species could be imaged in vivo using 1,2-dioxetane probes.
Following successful in vivo chemiluminescence imaging using CHS-3, we focused on designing a chemiluminescent probe that could report on tissue oxygenation in living animals.60 Tumor tissue is characteristically hypoxic owing to its irregular vasculature, and imaging this hypoxia in the tumor microenvironment can provide insight about tumor growth, metastasis, and response to therapy.33 We based our hypoxia chemiluminescent (HyCL) probes on oxygen-dependent enzymatic reduction of a nitroaromatic trigger, with the aim of developing an inexpensive tumor imaging method. Though HyCL-1 and HyCL-2 both showed a sensitive dose-dependent response to nitroreductase, HyCL-2, which incorporates an ether linkage between the 1,2-dioxetane scaffold and the para-nitrobenzyl trigger, was determined to have lower background emission and higher selectivity than the carbonate-linked HyCL-1 (Figure 5A). We conducted studies to establish that HyCL-2 can image different oxygen levels in vitro in aerated, oxygenated, and deoxygenated buffers and further demonstrated its ability to image tumor oxygenation in vivo in transgenic mice grafted with human lung tumors. Intratumoral injection of HyCL-2 with enhancer in mice when inhaling air containing 21% oxygen resulted in higher chemiluminescence emission than when switched to 100% oxygen, showing that HyCL-2 can report on tissue oxygenation levels in vivo (Figure 5B).
Figure 5.
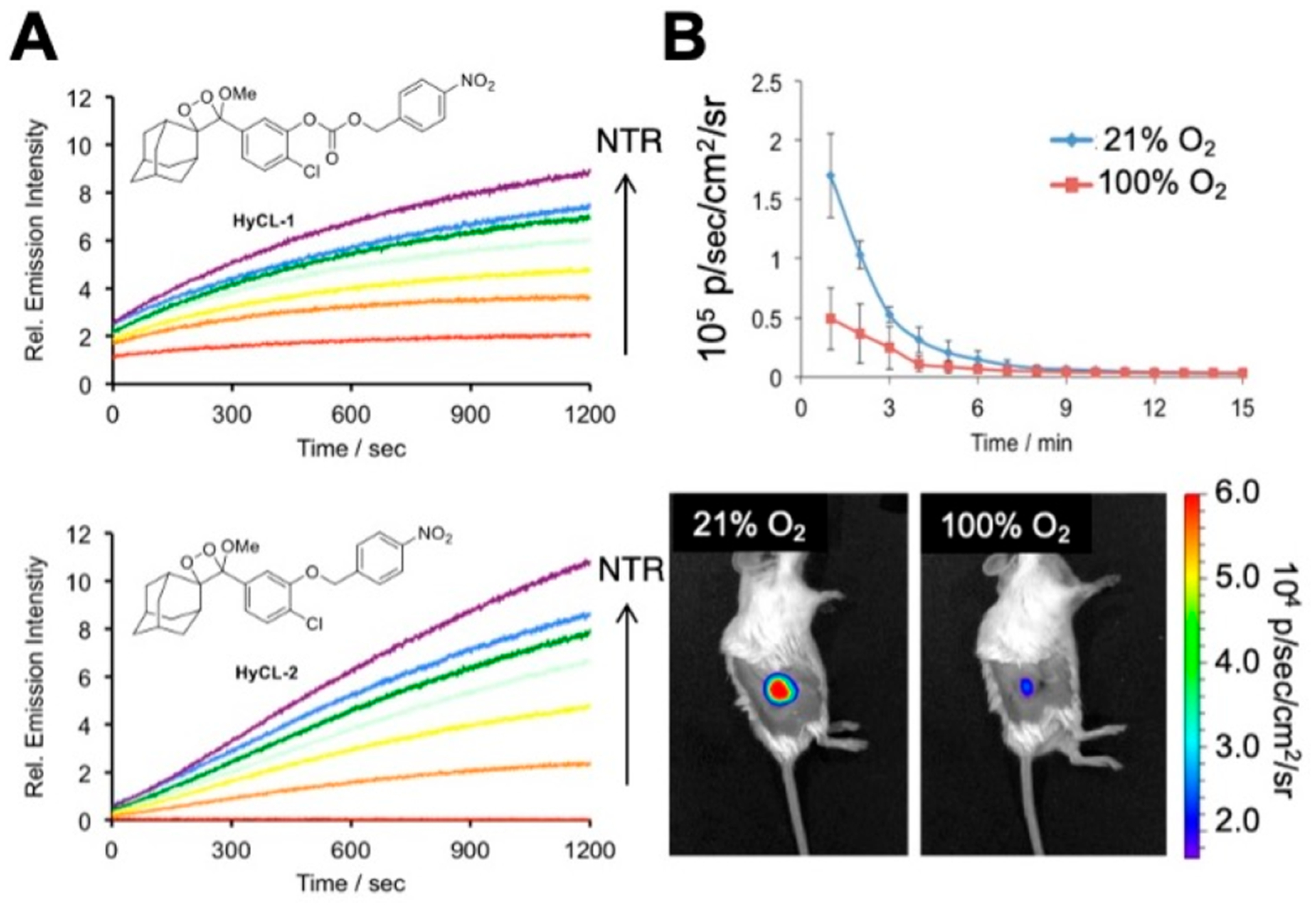
Hypoxia imaging in tumor xenograft models with HyCL-2. (A) Comparison of the carbonate-linked HyCL-1 to the ether linked HyCL-2 reveals significant reduction in background signal. (B) Imaging hypoxia in tumor xenograft models using HyCL-2 while mice breathed 21% or 100% oxygen. Adapted with permission from ref 60. Copyright 2016 American Chemical Society.
4. OPENING A GATEWAY TO ENHANCER FREE IMAGING
Although the 1,2-dioxetane chemiluminescent probes discussed thus far provided us with vital information on their use in biological systems, efficient imaging with these probes relied on the use of polymeric enhancer solutions to prevent luminescence quenching and to enable red-shifted emission. Our laboratory had been exploring energy transfer approaches to overcome this obstacle and had synthesized constructs such as a through-bond energy transfer cassette. During our ongoing work, however, an exciting development in the field of 1,2-dioxetane-based chemiluminescent probes was reported by Green, Eilon, Hananya, Shabat, and co-workers.37,38 They found that a relatively simple modification of the phenol-benzoate ring with an electron-withdrawing methyl acrylate or acrylonitrile group ortho to the phenol led to large increases in chemiluminescence quantum yield under aqueous conditions (Figure 6). Moreover, a red-shifted chemiluminescence emission was attained, making these structures more relevant for biological applications. This work paved the way for further development of analyte-specific direct chemiluminescent probes and certainly enriched the efforts from our own laboratory.
Figure 6.
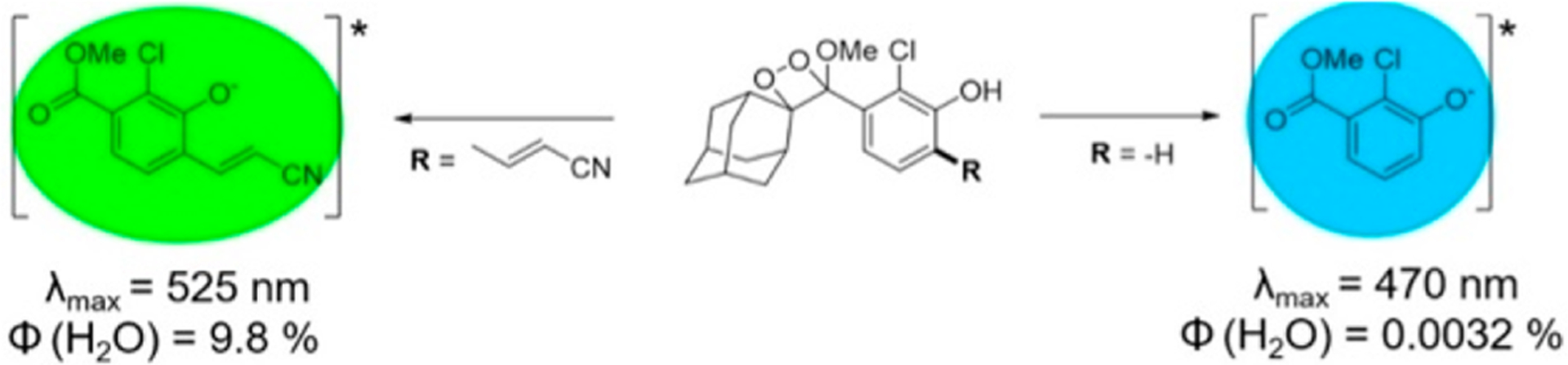
Substitution of an acrylonitrile group dramatically increases the chemiluminescence quantum yield of spiroadamantane 1,2-dioxetanes.
In 2018, our laboratory reported a reaction-based chemiluminescent probe, PNCL, for direct cellular detection of peroxynitrite (ONOO−).2 Armed with knowledge from our earlier work on an 19F magnetic resonance probe for ONOO−, we envisioned attaching an isatin moiety as a reaction handle to a 1,2-dioxetane chemiluminescent scaffold to leverage the peroxynitrite-mediated oxidative decarbonylation reaction and induce chemiluminescence. Studies performed in vitro revealed the ability of PNCL to monitor ONOO− fluxes over time, to demonstrate dose dependence, and to selectively detect ONOO− over a multitude of biological relevant cations, RSON species and radical byproducts. Most importantly, PNCL showed low toxicity in living cells and detected ONOO− generated by donor compounds like SIN-1 across multiple cell lines, as well as ONOO− produced by macrophages stimulated by lipopolysaccharide (LPS) (Figure 7). This study demonstrated that PNCL could serve as a promising tool to identify ONOO− in both chemical and biological systems without addition of signal enhancer.
Figure 7.
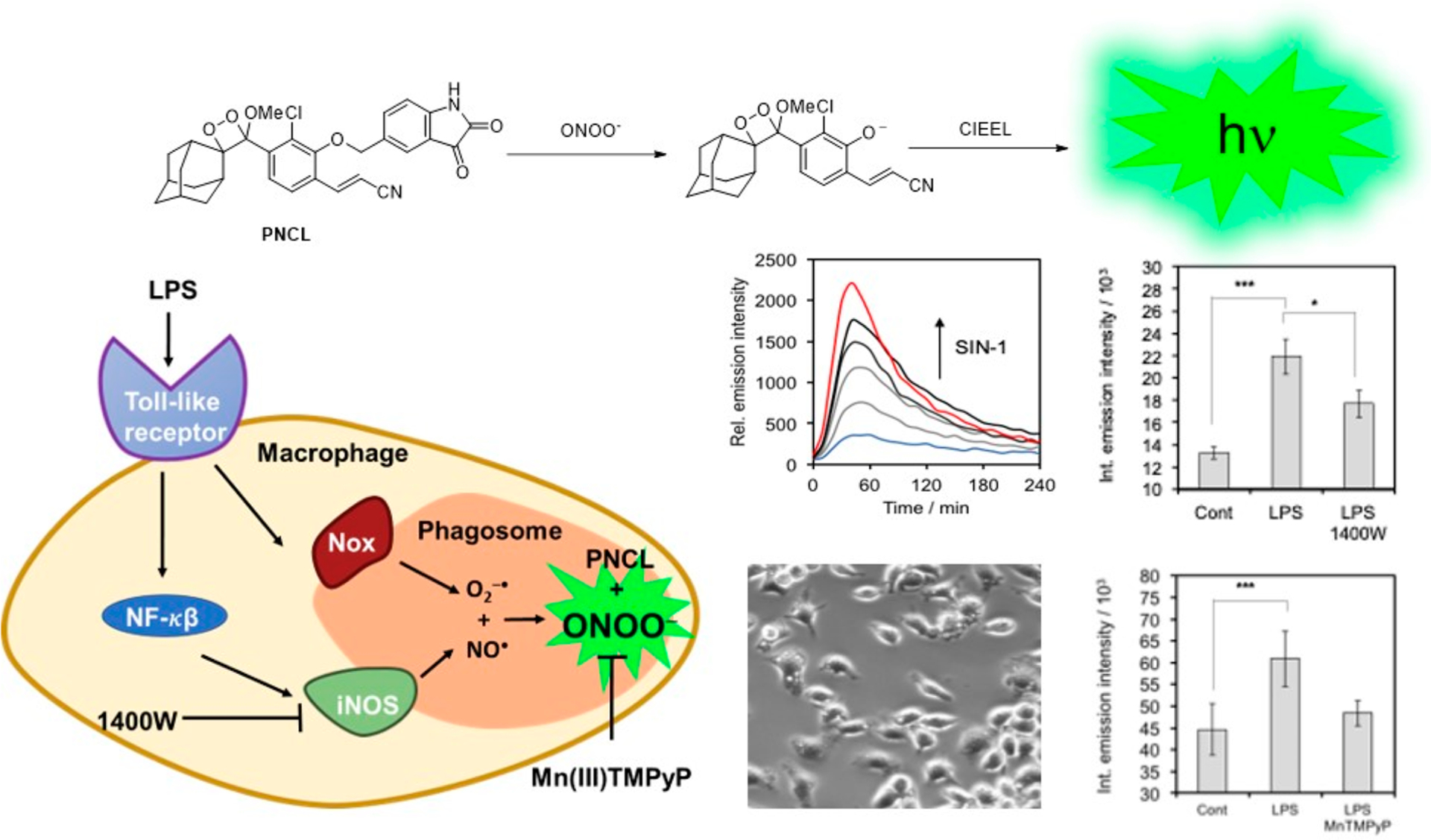
Interrogating cellular immune response with PNCL. Treatment of RAW 264.7 macrophages with lipopolysaccharide (LPS) induces phagosome formation and iNOS-dependent formation of ONOO−. Adapted with permission from ref 2. Copyright 2018 The Royal Society of Chemistry.
5. QUANTIFICATION USING CHEMILUMINESCENT 1,2-DIOXETANES
Following sustained success with small molecule chemiluminescence imaging in living systems, we turned our attention to precise quantification of these analytes, which can be challenging due to experimental fluctuations. Ratiometric imaging,61 wherein emission intensity at multiple wavelengths is monitored, offered a solution. Ratiometric responses are less sensitive to experimental variables and thus allow a more accurate quantification of analytes important in health and disease such as pH.62,63 While fluorescent ratiometric probes for pH imaging are well-known,64–67 their chemiluminescent 1,2-dioxetane counterparts are quite rare.
Our initial strategy involved inducing an energy transfer cascade between chemiluminescence emission from a spiroadamantane 1,2-dioxetane and a pH sensitive dye, carboxy SNARF-1 via a Sapphire II or Emerald II enhancer (Scheme 2).68 Carboxy SNARF-1 exhibits pH dependent emissions at 585 and 650 nm, the relative intensities being determined by the ratio of the protonated and deprotonated forms, respectively. The peak at 650 nm was found to increase more rapidly with an increase in pH due to increased concentration of the deprotonated form. A ratiometric pH dependent plot could thus be generated by plotting the ratio of emission intensities at 650 and 585 nm, depicting a greater than 5-fold increase in chemiluminescence signal in response to pH over a range of 6–10. We conducted additional studies to show that this ratiometric pH quantification could be achieved in complex biological medium such as fetal bovine serum and established this system to be compatible with IVIS Spectrum imaging when using 580 and 640 nm filters.
Scheme 2.
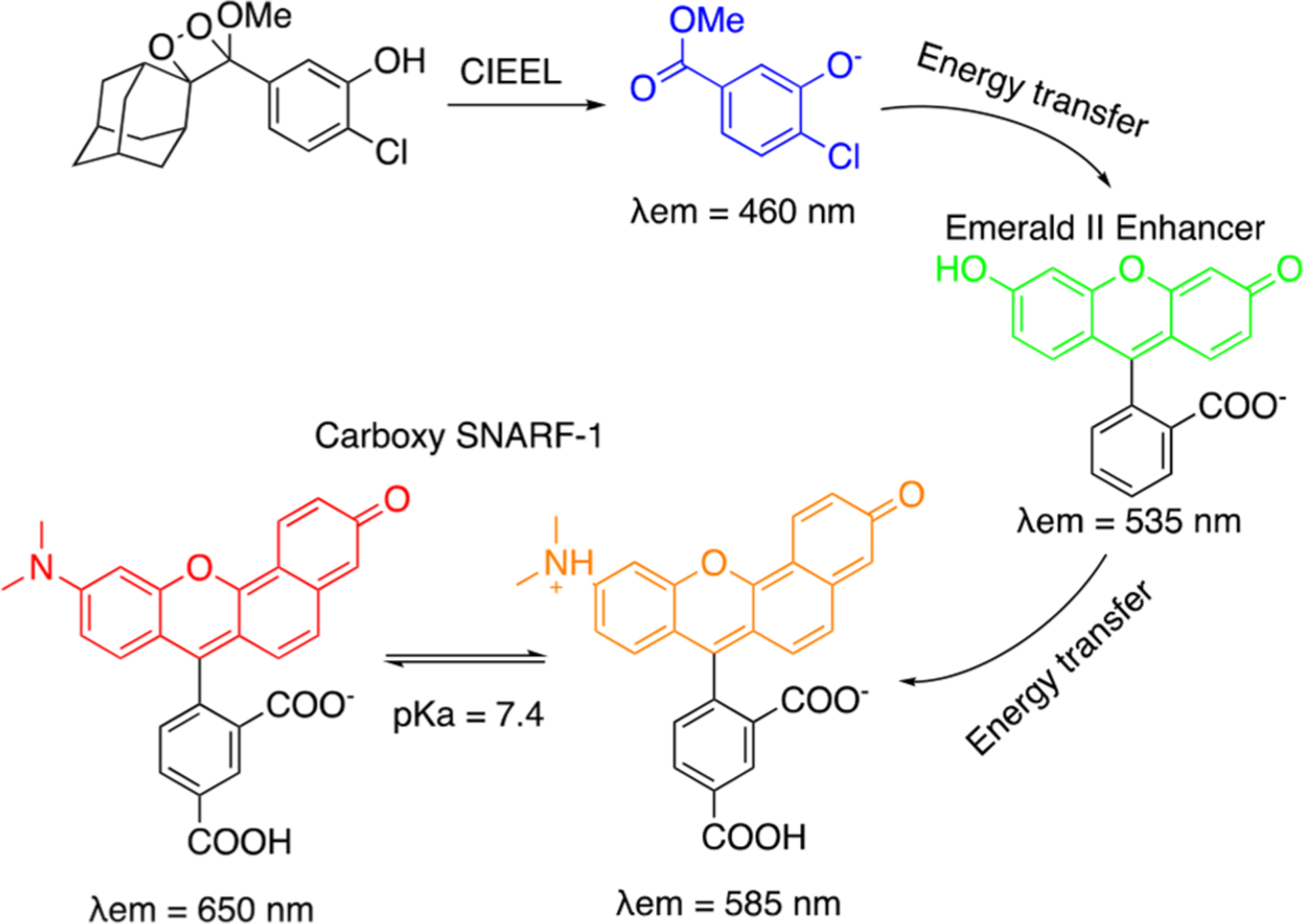
Ratiometric Chemiluminescence pH Imaging Using Chemiluminescence Energy Transfer to Carboxy SNARF-1
Having successfully demonstrated ratiometric pH quantification using the principle of energy transfer between multiple components, we focused on probes that could be more viable for in vivo applications. In 2020, we reported Ratio-pHCL-1, a single molecule ratiometric chemiluminescence resonance energy transfer (CRET) sensor for in vivo pH imaging (Figure 8).4 Here, we conjugated an acrylate modified spiroadamantane 1,2-dioxetane moiety to a pH sensitive carbofluorescein dye via a piperazine linker. Upon being subjected to aqueous pH buffers, energy transfer from the chemiluminescent phenolate (λem = 530 nm) to the carbofluorescein (λem = 580 nm) occurs, with the latter peak increasing more rapidly with pH due to increased concentration of the ring-opened carbofluorescein, allowing for ratiometric measurements (Figure 8A). Ratio-pHCL-1 provided consistent measurements of pH over a biologically relevant range of 6.8–8.4, and the ratiometric response was independent of most common confounding variables. Interestingly, placing a 2.8 mm thick slice of bologna over the reaction well-plate did not hamper the ratiometric response, providing strong evidence of signal tissue penetration. Intraperitoneal injections in live mice with Ratio-pHCL-1 generated flux outputs of more than 108–109 photons/s (Figure 8B), and as with in vitro experiments an increase in flux ratio with increasing pH was observed in vivo (Figure 8C).
Figure 8.
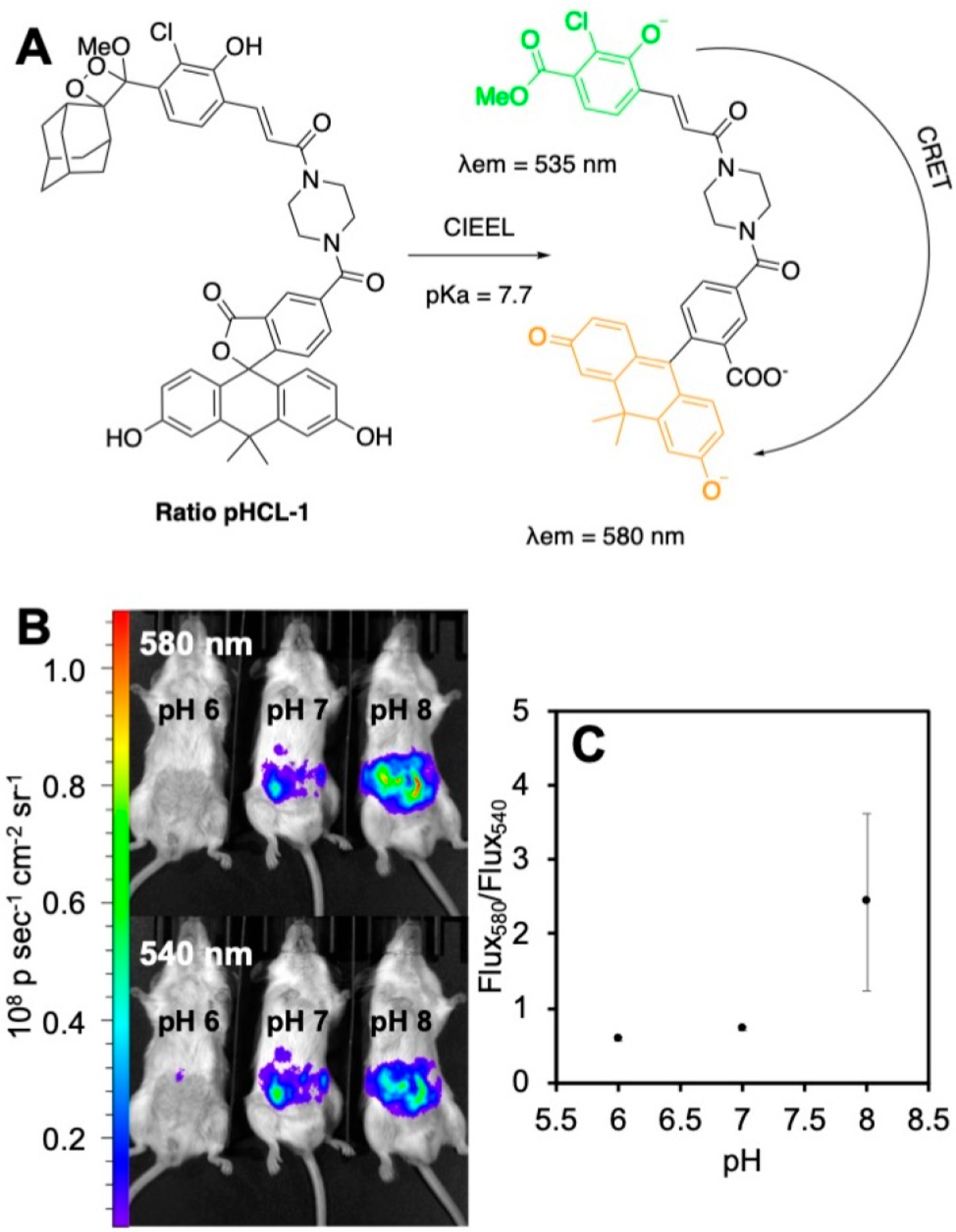
Ratiometric chemiluminescence imaging of pH in live mice using Ratio-pHCL-1. (A) Structure and chemiluminescence sensing mechanism of Ratio-pHCL-1. (B) Chemiluminescence images of Ratio-pHCL-1 after intraperitoneal injection into mice using 580 and 540 nm filters. (C) Ratio of the photon flux of the images in (B). Adapted with permission from ref 4. Copyright 2020 American Chemical Society.
While ratiometric approaches have been well-validated for the quantification of metal ions using probes with rapid rates of binding69,70 or in a reaction-based approach with fast forward and reverse equilibrium reactions,71–73 applying this approach to small molecule reactive analytes and enzymes faces steep obstacles. To be useful for quantification, reaction-based ratiometric approaches must use a fast and reversible reaction to ensure equilibration of the starting material and product. Given these challenges, we have introduced an alternative to ratiometric imaging that we refer to as a kinetics-based approach that capitalizes on the high sensitivity of chemiluminescence to enable quantification by accurate modeling of the probe kinetics. This approach is analogous to how quantification is achieved using analyte-selective electrodes,74 and its application to reaction-based probes is very much underexplored.
To investigate a kinetics-based small molecule quantification approach via chemiluminescence, we devised the probe HNOCL-1 for detection of azanone (nitroxyl, HNO),3 a reactive nitrogen species that has been shown to reduce pain in animal models and can potentially be used for treatment of congestive heart failure.75 HNOCL-1 contains a triarylphosphine trigger which forms an intermediate azaylide and decomposes to generate chemiluminescence in response to HNO (Figure 9). HNOCL-1 showed high selectivity, a dose-dependent response, and up to 833-fold turn-on when interrogated with the HNO donor, Angeli’s salt. With emphasis on quantification, we carried out kinetic modeling and derived a rate expression in terms of the phenol concentration (Figure 9A) with two rate constants: k1, the observed second order rate constant of the probe, and k3, the rate of chemiluminescent decomposition of the dioxetane. These rate constants were measured and the derived equation for concentration was used to successfully convert the raw chemiluminescence data into actual picomolar HNO concentrations produced from the reaction of H2S with nitric oxide (Figure 9B, C). Furthermore, we demonstrated that HNOCL-1 can track real-time HNO production from both Angeli’s salt and the reduction chemistry between the NO donor DEA NONOate and Na2S in live cells, with negligible cellular toxicity up to 100 mM HNOCL-1. Finally, HNO imaging in live mice was achieved via intraperitoneal injection of the probe and Angeli’s salt. We believe that this work, in principle, can be applied to any reactive species if careful considerations of kinetics and calibrations are performed.
Figure 9.
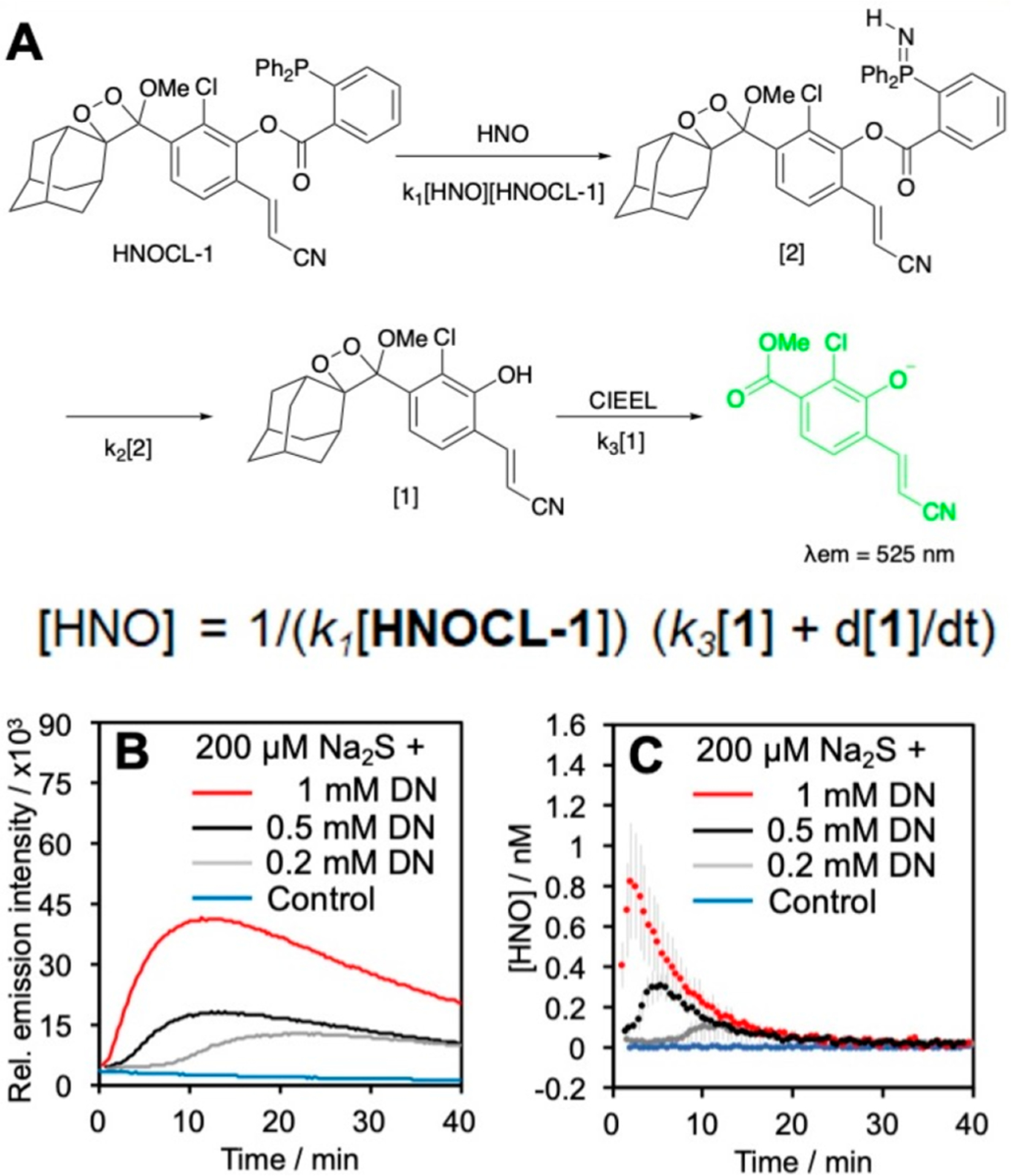
Kinetics-based quantification of HNO. (A) Structure and mechanism of HNO detection with HNOCL-1. The rate equation gives the concentration of HNO in terms of [1], which is proportional to the chemiluminescence emission intensity. (B) Chemiluminescence emission intensity from HNO formed by the reaction of H2S with nitric oxide produced from DEA NONOate (DN). (C) Conversion of the data in (B) into [HNO] using the derived equation. Adapted with permission from ref 3. Copyright 2019 Wiley-VCH GmbH, Weinheim.
Another example of kinetics-based quantification from our laboratory builds on our earlier work with chemiluminescent hypoxia probes. We designed HyCL-3 and HyCL-4-AM with nitrobenzyl triggers attached to a 1,2-dioxetane scaffold via ether linkage, HyCL-4-AM, containing an acetomethoxy ester, and HyCL-3 with an acrylonitrile group (Figure 10).76 While both probes displayed high selectivity for oxygen-dependent enzymatic reduction of the nitroaromatic group, the better cellular uptake of HyCL-4-AM resulted in enhanced sensitivity to nitroreductase (NTR) and in appropriate mammalian hypoxia models. HyCL-4-AM was able to distinguish between hypoxic (1% O2) and normoxic (20% O2) conditions in living A549 cells (Figure 10 B, C) and a kinetic model was devised based on cellular data, which fit well using two rate constants: k1, which determines the cellular uptake and ester cleavage, and k2 which is limited by the nitroaromatic reduction and consequently is sensitive to O2 levels (Figure 10A). After devising this model for kinetic quantification, we used HyCL-4-AM to image and kinetically quantify hypoxia in tumor xenograft models as well as to measure differences between hypoxic tumors and healthy oxygenated tissues in vivo (Figure 10D), demonstrating its applicability for tumor imaging.
Figure 10.
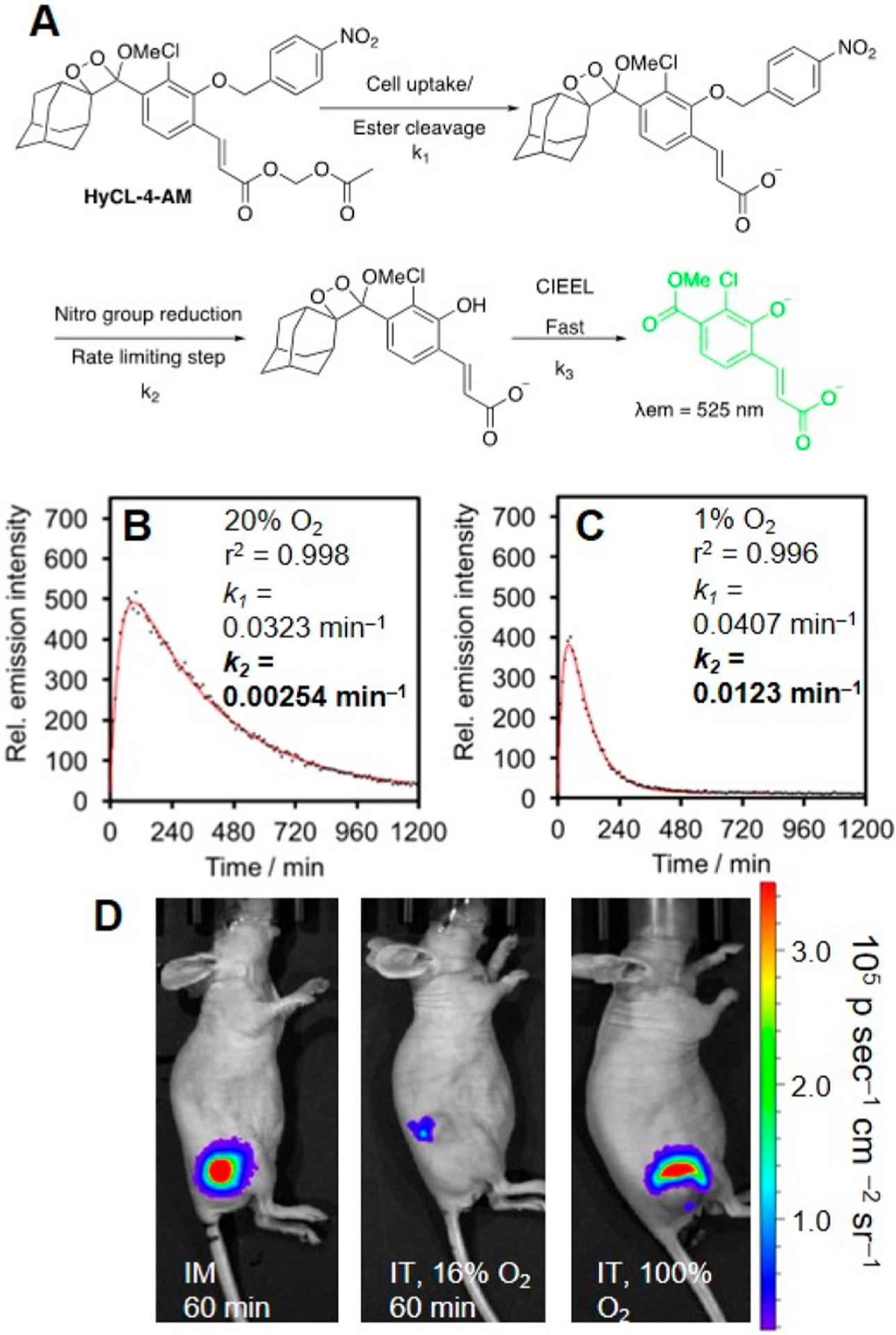
Kinetics-based hypoxia imaging with HyCL-4-AM. (A) Structure of HyCL-4-AM and model for cellular hypoxia sensing. (B, C) Kinetic fitting of the luminescence of HyCL-4-AM in A549 cells under (B) 20% O2 and (C) 1% O2. (D) Comparison of HyCL-4-AM response in mice using an intramuscular (IM) injection, intratumoral (IT) injection at 16% O2, or intratumoral (IT) injection at 100% O2. Adapted with permission from ref 76. Copyright 2019 American Chemical Society.
6. BIOLOGICAL MEASUREMENTS AND APPLICATIONS
In addition to developing chemiluminescent probes for reactive species and analytes, a significant motivation of our laboratory has been to establish the efficacy of both chemiluminescent and fluorescent probes for studying biological interactions and to develop diagnostic procedures and devices for human studies.
In a collaborative study with the Haimotz-Friedman group, we used PNCL to study peroxynitrite levels in cells to elucidate the effects of radiation on oxidative stress levels and the role of phosphodiesterase-5 inhibitor sildenafil, i.e., Viagra, in reducing this oxidative stress (Figure 11).77 Our results shed light on the vascular aspects of postradiotherapy erectile dysfunction and supported previous work which showed that sildenafil helps to preserve erectile function in prostate cancer patients undergoing radiotherapy.78–80
Figure 11.
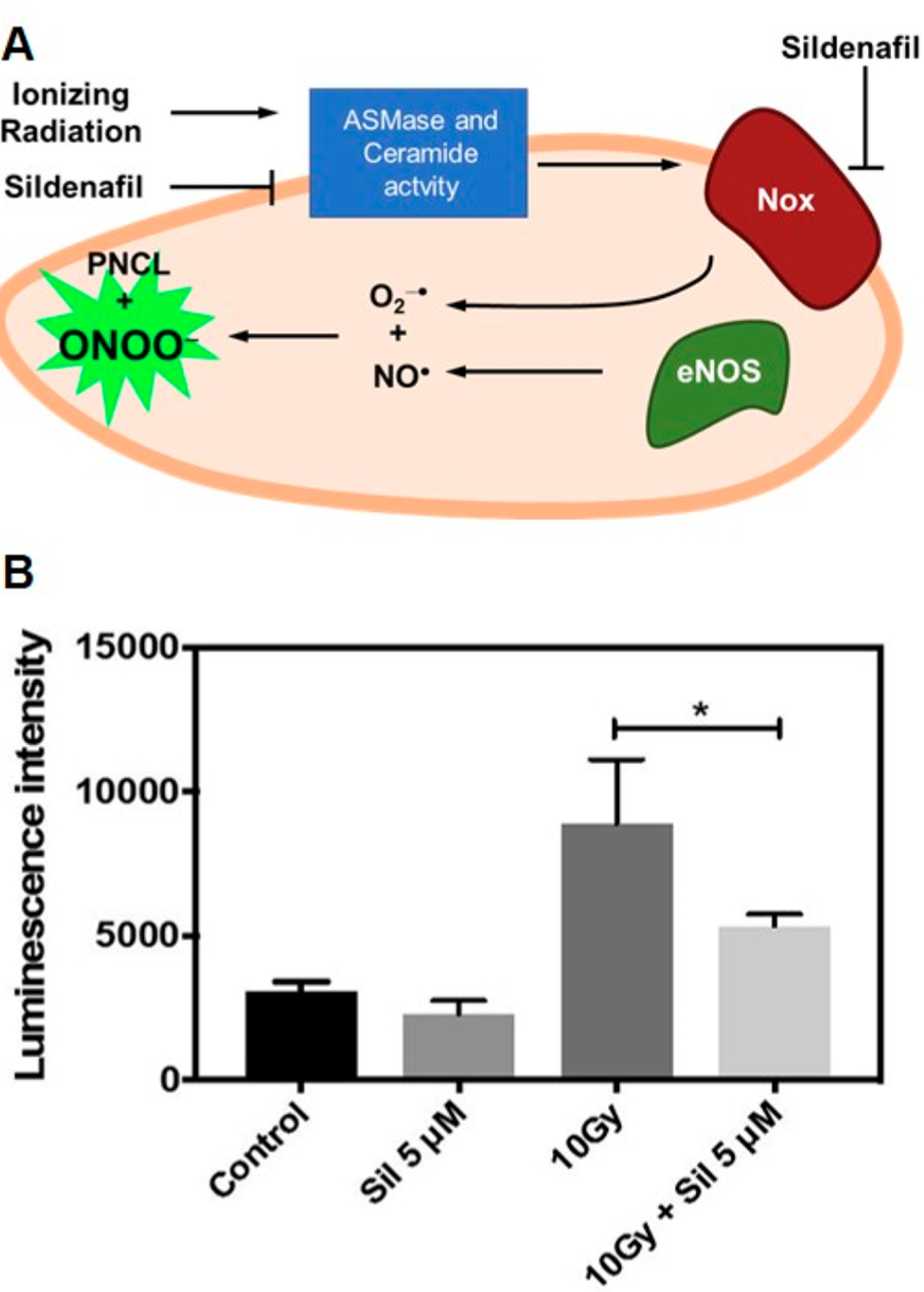
Measuring ONOO− in bovine aortic endothelial cells in response to radiation therapy. (A) Pathway of ONOO− production in radiation therapy and inhibition with sildenafil. (B) Luminescence response from PNCL in cells treated with vehicle control (Control), sildenafil (Sil 5 μM), radiation (10Gy), and radiation and sildenafil (10Gy + Sil 5 μM). Adapted with permission from ref 77. Copyright 2019 Elsevier.
We also developed chemiluminescent reporter Chemilum-CM to complement a BS2 split-esterase system for monitoring cellular protein–protein interactions (Figure 12A).81 The ester-masked probe generated chemiluminescence response upon rapamycin-induced assembly of the esterase within cells, showing increasing chemiluminescence with increasing rapamycin concentration and incubation time (Figure 12B).
Figure 12.
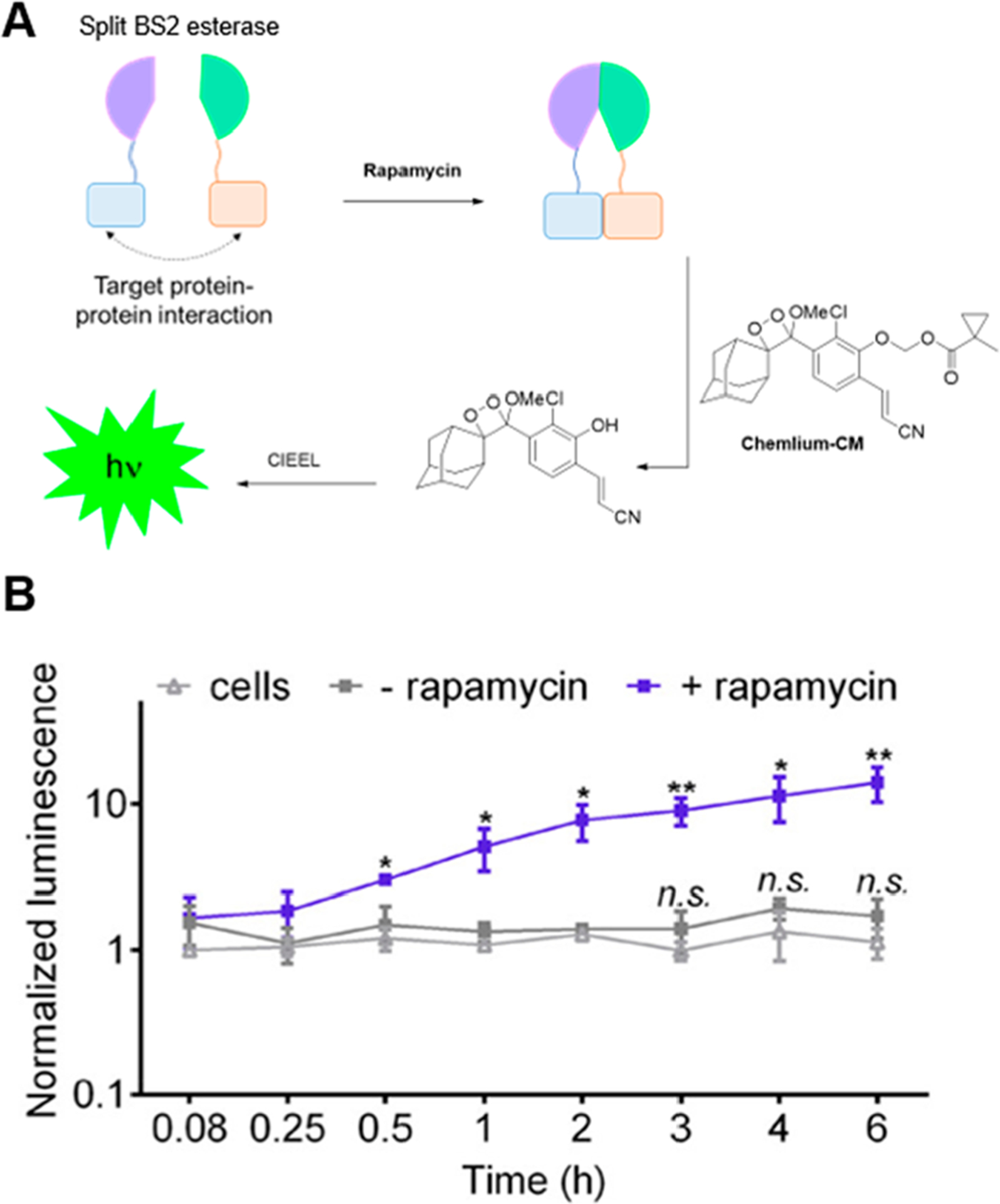
Application of triggered chemiluminescence in a split-esterase system. (A) Chemilum-CM chemiluminescence can be activated by rapamycin-mediated split esterase assembly. (B) Chemiluminescence emission from Chemilum-CM in cells with or without rapamycin. Adapted with permission from ref 81. Copyright 2019 American Chemical Society.
7. IN HUMAN AND CLINICAL STUDIES
An important thrust of our laboratory’s efforts has been toward translating triggered chemiluminescence into clinical and commercial applications. In one such venture, we developed a point-of-care diagnostic chemiluminescent assay for monitoring airway H2O2, consisting of a 1,2-dioxetanedione mediated peroxyoxalate chemiluminescence system, a smartphone camera, and a home-built dark-box (Figure 13A, B).82 H2O2 levels in exhaled breath condensate (EBC) samples from human volunteers determined using this platform were found to be in close agreement with the commercial Amplex Red assay. In collaboration with the start-up company BioLum Sciences LLC, we recently evolved our chemiluminescent airway H2O2 quantification platform into the BioSense 2.0 Laboratory module, a stand-alone photon detection device consisting of a photodiode, a 3D-printed adapter for disposable cuvettes, and a Bluetooth 4.0 data transmission module (Figure 13A, C).83 Using this device, we studied the relationship between airway H2O2 and asthma in EBC samples from 60 adults. Our results agreed with earlier literature showing trends of higher H2O2 in asthma patients as compared to nonasthmatic patients.
Figure 13.
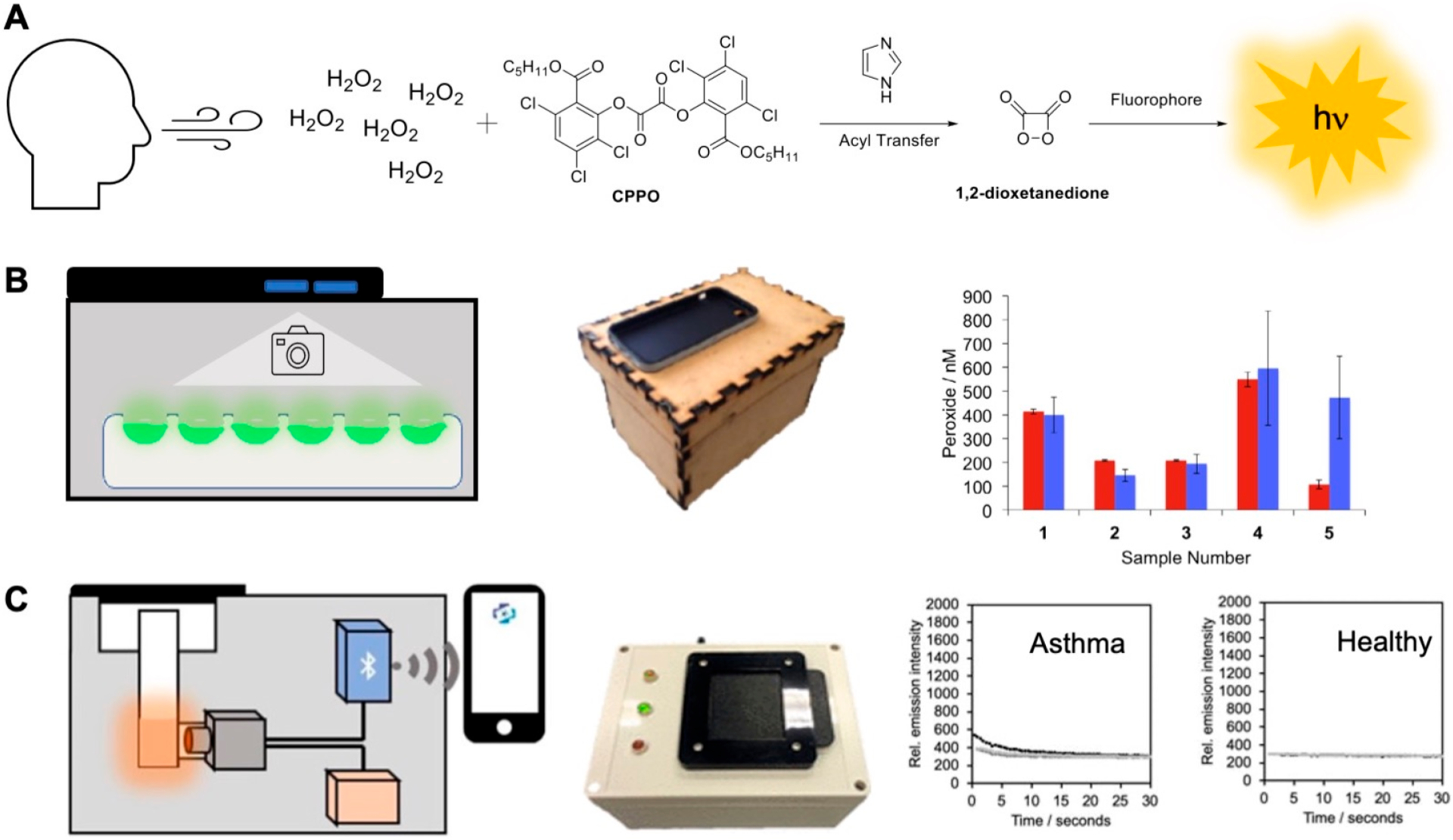
Chemiluminescence measurement of H2O2 in human exhaled breath condensate. (A) Peroxyoxalate chemiluminescence assay for H2O2 detection. (B) A smartphone was integrated to a wooden crafted dark box. Measurements in human exhaled breath condensate were validated versus the fluorescent Amplex Red assay. Adapted with permission from ref 82. Copyright 2016 Elsevier. (C) The BioSense 2.0 Laboratory Module was used to measure H2O2 concentrations in asthma patients and healthy volunteers in the John Peter Smith Hospital.83 Adapted with permission from ref 83. Copyright 2020 American Chemical Society.
We have also used the fluorescent probe Sulfidefluor-4 to quantify H2S levels in saliva samples from college students during exam periods to determine relationships between academic stress and salivary H2S over time.84 Our studies revealed an increase in H2S levels over periods of sustained stress and established a correlation of this increase with NO levels, experience of stress, and negative affect.
8. PHOTOACTIVATABLE CHEMILUMINESCENCE
In a newer direction, our laboratory has recently explored photoactivatable chemiluminescence. We designed UVC454 and UVA454 with ortho-nitrobenzyl protecting groups that are removed upon ultraviolet irradiation, allowing irreversible photochemical uncaging of the chemiluminescent phenolate even in water.85 Spiro-CL, on the other hand, interconverts between its stable spiropyran form and the metastable merocyanine form upon UV or visible light irradiation (Figure 14). The metastable open form can undergo CIEEL and emit light, thus representing the first example of photoswitchable chemiluminescence, which could have applications for targeted in vivo imaging and photoactivable volumetric 3D displays.86,87
Figure 14.
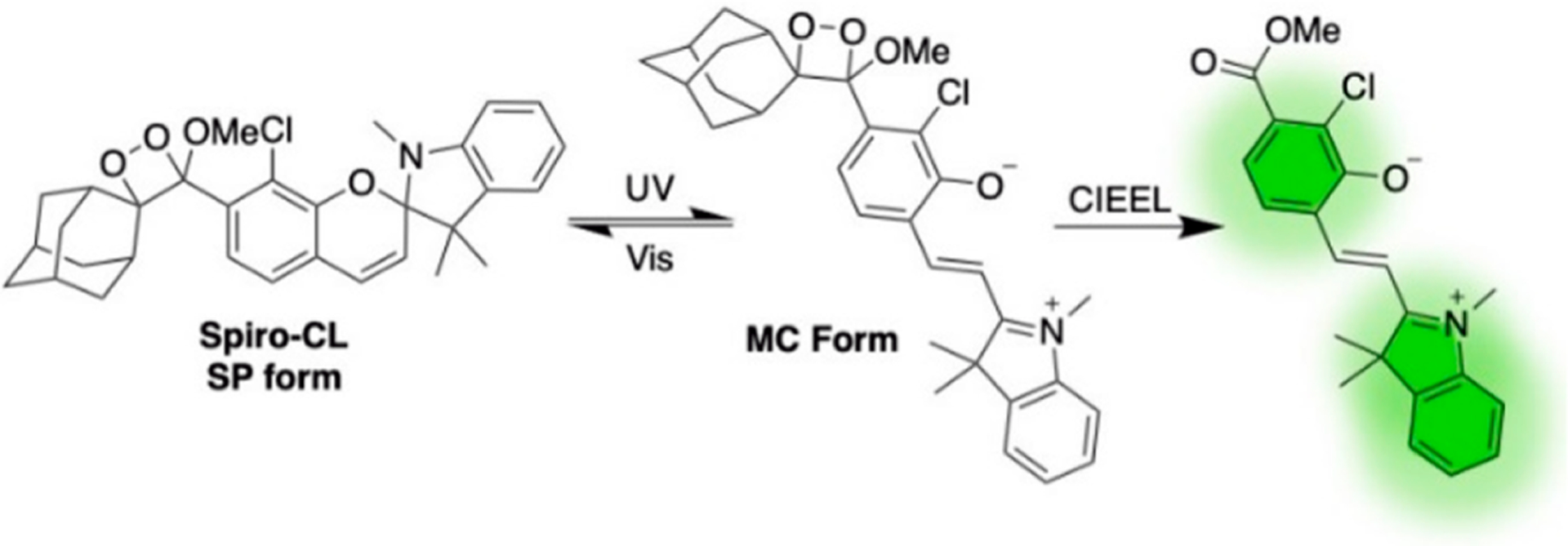
Photoswitchable chemiluminescent 1,2-dioxetane Spiro-CL.
9. CONCLUSIONS
In this Account, we have documented our research group’s development of chemiluminescent 1,2-dioxetanes as triggerable biological imaging probes, specifically for analyzing oxygen, pH, and RSON species (Table 1). Our work helped establish the feasibility of using spiroadamantane 1,2-dioxetanes in vivo and demonstrated their use in investigating biological signaling pathways that involve H2S, hypoxia, and peroxynitrite. To move toward precise quantification, our group showed the first examples of ratiometric imaging using chemiluminescent 1,2-dioxetanes, including a CRET probe for in vivo ratiometric pH imaging. We have also pioneered a new approach for quantitative measurement of reactive species, which we call a kinetics-based approach. Whereas a ratiometric approach achieves quantification by analyses of equilibrium, a kinetics-based approach achieves quantification by careful analysis of reaction rates and mechanisms to deduce analyte concentrations from signal dynamics. This kinetics-based approach was successfully used to quantify HNO concentrations in real-time and provide a quantitative measure of oxygen-dependent enzyme activity in living cells. Finally, we have executed industrial and clinical collaborations to translate chemiluminescence technologies for point-of-care measurement of H2O2 in the exhaled breath condensate of asthma patients.
Table 1.
Chemiluminescent 1,2-Dioxetane Probes for Detection of Biological Analytes
| probe | analyte | enhancer | λem (nm) | expt studies | quantification |
|---|---|---|---|---|---|
| CHS-1 | H2S | Emerald II | 545 | in vitro | |
| CHS-2 | H2S | Emerald II | 545 | in vitro | |
| CHS-3 | H2S | Emerald II | 545 | in vitro, in cells, in vivo | |
| dioxetane + SNARF | pH | Sapphire II, Emerald II | 460, 585, 650 | in vitro | ratiometric |
| HNOCL-1 | HNO | 525 | in vitro, in cells, in vivo | kinetics-based | |
| HyCL-1 | O2 | Emerald II | 455, 545 | in vitro | |
| HyCL-2 | O2 | Emerald II | 455, 545 | in vitro, in vivo | |
| HyCL-3 | O2 | 525 | in vitro | ||
| HyCL-4-AM | O2 | 516 | in vitro, in cells, in vivo | kinetics-based | |
| PNCL | ONOO− | 525 | in cells | ||
| Ratio-pHCL-1 | pH | 530, 580 | in vitro, in vivo | ratiometric |
We also note the excellent work in this area being performed by other groups and provide a brief comparison to our own work. Sun and co-workers88 developed an acrylic acid 1,2-dioxetane probe for hypoxia based on a nitroaromatic trigger similar to our HyCL series. The acrylic acid group improves aqueous solubility, but HyCL-4-AM features improved cellular uptake. Huang and co-workers89,90 have developed a series of near-infrared 1,2-dioxetane probes using a formyl ester trigger for peroxynitrite detection. The near-infrared emission can image deeper in tissue and the different trigger is likely to have a disparate selectivity from the isatin-based PNCL probe. In comparison to the CHS probes series, Simsek Turan and Somzen,91 and Levinn and Pluth92 have reported probes for hydrogen sulfide using dinitrophenyl and azide triggers, although these studies were limited to in vitro experiments. More recently, Gholap and co-workers93 reported a series of hydrogen sulfide 1,2-dioxetane probes with various triggers and used these to study hydrogen sulfide production from β-Lactam antibiotic degradation. Amongst these studies, only the CHS series has been used for in vivo imaging of hydrogen sulfide.
The future is bright and open for further development and applications of chemiluminescent 1,2-dioxetanes. Ultimately, more advanced designs may have potential for clinical translation and real-time monitoring of analytes and clinical parameters. The ability to deliver light to deep tissues using chemiluminescent 1,2-dioxetanes has intriguing potential for photopharmacology, radiation therapy, and photodynamic therapy, and these molecules could also be creatively applied in photocatalysis for unique mechanisms of targeted drug release. While we have focused on cellular, animal, and clinical imaging, optimized chemiluminescent agents could be quickly applied to environmental testing for contaminants in water or air. We have recently disclosed a series of photocaged and photoswitchable 1,2-dioxetanes that enable light-mediated spatiotemporal control of chemiluminescence emission. This offers exciting new opportunities for targeted biological imaging and advanced materials like volumetric 3D chemiluminescent displays and formal energy upconversion photonic materials.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM114792-02 and the National Science Foundation under CHE1653474. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Uroob Haris received her B.A. in Chemistry and B.B.A in Management from Southern Methodist University in 2018. She is currently a Ph.D. student in Prof. Alex Lippert’s group. Her research interests involve light-driven chemistry, including luminescence imaging and systems for light-based lithography.
Husain N. Kagalwala obtained his B.S. in Chemistry (2006) and M.S. in Inorganic Chemistry (2008) from the University of Pune, India. He then pursued graduate studies with Prof. Stefan Bernhard at Carnegie Mellon University, Pittsburgh and received his Ph.D. in 2015. This was followed by postdoctoral research in the laboratories of Prof. Randolph Thummel at University of Houston and Prof. Thomas B. Rauchfuss at University of Illinois, Urbana–Champaign. He is currently working as a research scientist in Prof. Alex Lippert’s group, where he is developing near-IR chemiluminescent oxygen sensors.
Yujin Lisa Kim is currently a research assistant in Prof. Alex Lippert’s research group. She received her B.S. in Biochemistry from Southern Methodist University in 2021. Her research interests include development of assays for quantification of biological molecules in human clinical samples.
Alexander R. Lippert received his B.S. degree in 2003 from Caltech, followed by Ph.D. research with Prof. Jeffrey W. Bode where he received his degree from the University of Pennsylvania in 2008. After postdoctoral research with Prof. Christopher J. Chang at the University of California, Berkeley, he started his independent career at Southern Methodist University in 2012 where he is currently an Associate Professor. Prof. Lippert is cofounder and Chief Science Officer (CSO) of BioLum Sciences, LLC, which works toward commercialization of chemiluminescence technologies.
Footnotes
Notes
The authors declare the following competing financial interest(s): A.R.L. declares a financial stake in BioLum Sciences.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.1c00185
Contributor Information
Uroob Haris, Department of Chemistry, Southern Methodist University, Dallas, Texas 75275-0314, United States.
Husain N. Kagalwala, Department of Chemistry, Southern Methodist University, Dallas, Texas 75275-0314, United States.
Yujin Lisa Kim, Department of Chemistry, Southern Methodist University, Dallas, Texas 75275-0314, United States.
Alexander R. Lippert, Department of Chemistry, Southern Methodist University, Dallas, Texas 75275-0314, United States.
REFERENCES
- (1).Cao J; Lopez R; Thacker JM; Moon JY; Jiang C; Morris SNS; Bauer JH; Tao P; Mason RP; Lippert AR Chemiluminescent Probes for Imaging H2S in Living Animals. Chem. Sci 2015, 6, 1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]; A spiroadamantane 1,2-dioxetane-based chemiluminescent probe is demonstrated to be able to image small molecule analytes in vivo in live mice for the first time.
- (2).Cao J; An W; Reeves AG; Lippert AR A Chemiluminescent Probe for Cellular Peroxynitrite Using a Self-Immolative Oxidative Decarbonylation Reaction. Chem. Sci 2018, 9, 2552–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]; An enhancer-free 1,2-dioxetane-based chemiluminescent probe is used for direct detection of peroxynitrite generated in cells.
- (3).An W; Ryan LS; Reeves AG; Bruemmer KJ; Mouhaffel M; Gerberich JL; Winters A; Mason RP; Lippert AR A Chemiluminescent Probe for HNO Quantification and Real-time Monitoring in Living Cells. Angew. Chem., Int. Ed 2019, 58, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]; A kinetics-based method is formulated and implemented for quantification of small molecules based on kinetic response of a triggerable chemiluminescent probe.
- (4).Ryan LS; Gerberich J; Haris U; Nguyen D; Mason RP; Lippert AR Ratiometric pH Imaging Using a 1,2-Dioxetane Chemiluminescence Resonance Energy Transfer Sensor in Live Animals. ACS Sens. 2020, 5, 2925–2932. [DOI] [PubMed] [Google Scholar]; A single-molecule energy transfer chemiluminescent probe is investigated and used for ratiometric quantification of pH with demonstrated viability in living mice.
- (5).Bruemmer KJ; Crossley SWM; Chang CJ Activity-Based Sensing: A Synthetic Methods Approach for Selective Molecular Imaging and Beyond. Angew. Chem., Int. Ed 2020, 59, 13734–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Xie D; Yu M; Kadakia RT; Que EL 19F Magentic Resonance Activity-Based Sensing Using Paramagnetic Metals. Acc. Chem. Res 2020, 53, 2–10. [DOI] [PubMed] [Google Scholar]
- (7).Chan J; Dodani SC; Chang CJ Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem 2012, 4, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Carter KP; Young AM; Palmer AE Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev 2014, 114, 4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gnaim S; Shabat D Activity-Based Optical Sensing Enabled by Self-Immolative Scaffolds: Monitoring of Release Events by Fluorescence and Chemiluminescence Output. Acc. Chem. Res 2019, 52, 2806–2817. [DOI] [PubMed] [Google Scholar]
- (10).Grynkiewicz G; Poenie M; Tsien RY A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J. Biol. Chem 1985, 260, 3440–3450. [PubMed] [Google Scholar]
- (11).Nolan EM; Lippard SJ Small-Molecule Fluorescent Sensors for Investigating Zinc Metalloneurochemistry. Acc. Chem. Res 2009, 42, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Que EL; Bleher R; Duncan FE; Kong BY; Gleber SC; Vogt S; Chen S; Garwin SA; Bayer AR; Dravid VP; Woodruff TK; O’Halloran TV Quantitative Mapping of Zinc Fluxes in the Mammalian Egg Reveals the Origin of Fertilization-Induced Zinc Sparks. Nat. Chem 2015, 7, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lazarou TS; Buccella D Advances in Imaging of Understudied Ions in Signaling: A Focus on Magnesium. Curr. Opin. Chem. Biol 2020, 57, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wang Z; Detomasi TC; Chang CJ A Dual-Fluorophore Sensor Approach for Ratiometric Fluorescence Imaging of Potassium in Living Cells. Chem. Sci 2021, 12, 1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cotruvo JA Jr.; Aron AT; Ramos-Torres KM; Chang CJ Synthetic Fluorescent Probes for Studying Copper in Biological Systems. Chem. Soc. Rev 2015, 44, 4400–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Aron AT; Reeves AG; Chang CJ Activity-based Sensing Fluorescent Probes for Iron in Biological Systems. Curr. Opin. Chem. Biol 2018, 43, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Han J; Burgess K Fluorescent Indicators for Intracellular pH. Chem. Rev 2010, 110, 2709–2728. [DOI] [PubMed] [Google Scholar]
- (18).Tutol JN; Kam HC; Dodani SC Identification of mNeonGreen as a pH-dependent, Turn-On Fluorescent Protein Sensor for Chloride. ChemBioChem 2019, 20, 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Prakash V; Saha S; Chakraborty K; Krishnan Y Rational Design of a Quantitative, pH-insensitive, Nucleic Acid Based Fluorescent Chloride Reporter. Chem. Sci 2016, 7, 1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liu P; Miller EW Electrophysiology, Unplugged: Imaging Membrane Potential with Fluorescent Indicators. Acc. Chem. Res 2020, 53, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lippert AR Designing Reaction-Based Fluorescent Probes for Selective Hydrogen Sulfide Detection. J. Inorg. Biochem 2014, 133, 136–142. [DOI] [PubMed] [Google Scholar]
- (22).Bezner BJ; Ryan LS; Lippert AR Reaction-Based Luminescent Probes for Reactive Sulfur, Oxygen, and Nitrogen Species: Analytical Techniques and Recent Progress. Anal. Chem 2020, 92, 309–326. [DOI] [PubMed] [Google Scholar]
- (23).Cortese-Krott MM; Koning A; Kuhnle GGC; Nagy P; Bianco CL; Pasch A; Wink DA; Fukuto JM; Jackson AA; van Goor H; Olson KR; Feelisch M The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signaling 2017, 27, 684–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kaur A; New EJ Bioinspired Small-Molecule Tools for the Imaging of Redox Biology. Acc. Chem. Res 2019, 52, 623–632. [DOI] [PubMed] [Google Scholar]
- (25).Lippert AR; Van de Bittner GC; Chang CJ Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res 2011, 44, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bai X; Ng KKH; Hu JJ; Ye S; Yang D Small-Molecule-Based Fluorescent Sensors for Selective Detection of Reactive Oxygen Species in Biological Systems. Annu. Rev. Biochem 2019, 88, 605–633. [DOI] [PubMed] [Google Scholar]
- (27).Kojima H; Urano Y; Kikuchi K; Higuchi T; Hirata Y; Nagano T Fluorescent Indicators for Imaging Nitric Oxide Production. Angew. Chem., Int. Ed 1999, 38, 3209–3212. [DOI] [PubMed] [Google Scholar]
- (28).Lau N; Pluth MD Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems. Curr. Opin. Chem. Biol 2019, 49, 1–8. [DOI] [PubMed] [Google Scholar]
- (29).Lin VS; Chen W; Xian M; Chang CJ Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev 2015, 44, 4596–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Liu H; Radford MN; Yang CT; Chen W; Xian M Inorganic hydrogen polysulfides: chemistry, chemical biology and detection. Br. J. Pharmacol 2019, 176, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bruemmer KJ; Brewer TF; Chang CJ Fluorescent Probes for Imaging Formaldehyde in Biological Systems. Curr. Opin. Chem. Biol 2017, 39, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ohata J; Bruemmer KJ; Chang CJ Activity-Based Sensing Methods for Monitoring the Reactive Carbon Species Carbon Monoxide and Formaldehyde in Living Systems. Acc. Chem. Res 2019, 52, 2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Krohn KA; Link JM; Mason RP Molecular Imaging of Hypoxia. J. Nucl. Med 2008, 49, 129S–148S. [DOI] [PubMed] [Google Scholar]
- (34).Kolanowski JL; Liu F; New EJ Fluorescent Probes for the Simultaneous Detection of Multiple Analytes in Biology. Chem. Soc. Rev 2018, 47, 195–208. [DOI] [PubMed] [Google Scholar]
- (35).Wu L; Sedgwick AC; Sun X; Bull SD; He XP; James TD Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res 2019, 52, 2582–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Liu L; Mason RP Imaging beta-galactosidase Activity in Human Tumor Xenografts and Transgenic Mice Using a Chemiluminescent Substrate. PLoS One 2010, 5, e12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Green O; Eilon T; Hananya N; Gutkin S; Bauer CR; Shabat D Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent. Sci 2017, 3, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lippert AR Unlocking the Potential of Chemiluminescence Imaging. ACS Cent. Sci 2017, 3, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Schaap AP; Handley RS; Giri BP Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 1: Aryl Esterase-catalyzed Chemiluminescence from a Naphthyl Acetate-Substituted Dioxetane. Tetrahedron Lett. 1987, 28, 935–938. [Google Scholar]
- (40).Schaap AP; Chen TS; Handley RS; DeSilva R; Giri BP Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 2: Fluoride-induced Chemiluminescence from tert-Butyldimethylsilyloxy-substituted Dioxetanes. Tetrahedron Lett. 1987, 28, 1155–1158. [Google Scholar]
- (41).Schaap AP; Sandison MD; Handley RS Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 3: Alkaline Phosphatasecatalyzed Chemiluminescence from an Aryl Phosphate-substituted Dioxetane. Tetrahedron Lett. 1987, 28, 1159–1162. [Google Scholar]
- (42).Vacher M; Fdez. Galván I; Ding BW; Schramm S; Berraud-Pache R; Naumov P; Ferré N; Liu Y-J; Navizet I; Roca-Sanjuán D; Baader WJ; Lindh R Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev 2018, 118, 6927–6974. [DOI] [PubMed] [Google Scholar]
- (43).Adam W; Bronstein I; Trofimov AV; Vasil’ev RF Solvent-Cage Effect (Viscosity Dependence) as a Diagnostic Probe for the Mechanism of the Intramolecular Chemically Initiated Electron-Exchange Luminescence (CIEEL) Triggered from a Spiroadmantyl-Substituted Dioxetane. J. Am. Chem. Soc 1999, 121, 958–961. [Google Scholar]
- (44).Augusto FA; De Souza GA; de Souza Júnior SP; Khalid M; Baader WJ Efficiency of Electron Transfer Initiated Chemiluminescence. Photochem. Photobiol 2013, 89, 1299–1317. [DOI] [PubMed] [Google Scholar]
- (45).Hoshiya N; Fukuda N; Maeda H; Watanabe N; Matsumoto M Synthesis and Fluoride-induced Chemiluminescent Decomposition of Bicyclic Dioxetanes Substituted with a 2-Hydroxynaphthyl Group. Tetrahedron 2006, 62, 5808–5820. [Google Scholar]
- (46).Yue L; Liu YJ Mechanism of AMPPD Chemiluminescence in a Different Voice. J. Chem. Theory Comput 2013, 9, 2300–2312. [DOI] [PubMed] [Google Scholar]
- (47).Hananya N; Shabat D Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent. Sci 2019, 5, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gnaim S; Green O; Shabat D The Emergence of Aqueous Chemiluminescence: New Promising Class of Phenoxy 1,2-Dioxetane Luminophores. Chem. Commun 2018, 54, 2073–2085. [DOI] [PubMed] [Google Scholar]
- (49).Hananya N; Shabat D A Glowing Trajectory between Bio- and Chemiluminescence: From Luciferin-Based Probes to Triggerable Dioxetanes. Angew. Chem., Int. Ed 2017, 56, 16454–16463. [DOI] [PubMed] [Google Scholar]
- (50).Lippert AR; New EJ; Chang CJ Reaction-Based Fluorescent Probes for the Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc 2011, 133, 10078–10080. [DOI] [PubMed] [Google Scholar]
- (51).Yang G; Wu L; Jiang B; Yang W; Qi J; Cao K; Meng Q; Mustafa AK; Mu W; Zhang S; Snyder SH; Wang R H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Peng YJ; Nanduri J; Raghuraman G; Souvannakitti D; Gadalla MM; Kumar GK; Snyder SH; Prabhakar NR H2S Mediates O2 Sensing in the Carotid Body. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Papapetropoulos A; Pyriochou A; Altaany Z; Yang G; Marazioti A; Zhou Z; Jeschke MG; Branski LK; Herndon DN; Wang R; Szabó C Hydrogen Sulfide is an Endogenous Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Paul BD; Sbodio JI; Xu R; Vandiver MS; Cha JY; Snowman AM; Snyder SH Cystathionine γ-Lyase Deficiency Mediates Neurodegeneration in Huntington’s Disease. Nature 2014, 509, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Lin VS; Lippert AR; Chang CJ Cell-trappable Fluorescent Probes for Endogenous Hydrogen Sulfide Signaling and Imaging H2O2-dependent H2S Production. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 7131–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ju L; Lippert AR; Bode JW Stereoretentive Synthesis and Chemoselective Amide-Forming Ligations of C-Terminal Peptide alpha-Ketoacids. J. Am. Chem. Soc 2008, 130, 4253–4255. [DOI] [PubMed] [Google Scholar]
- (57).Reeves AG; Subbarao M; Lippert AR Imaging Acetaldehyde Formation During Ethanol Metabolism in Living Cells Using a Hydrazine Naphthalimide Fluorescent Probe. Anal. Methods 2017, 9, 3418–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Pacher P; Beckman JS; Liaudet L Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev 2007, 87, 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Bruemmer KJ; Merrikhihaghi S; Lollar CT; Morris SNS; Bauer JH; Lippert AR 19F Magnetic Resonance Probes for Detecting Peroxynitrite in Living Cells Using an Oxidative Decarbonylation Reaction. Chem. Commun 2014, 50, 12311–12314. [DOI] [PubMed] [Google Scholar]
- (60).Cao J; Campbell J; Liu L; Mason RP; Lippert AR In Vivo Chemiluminescent Imaging Agents for Nitroreductase and Tissue Oxygenation. Anal. Chem 2016, 88, 4995–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Huang X; Song J; Yung BC; Huang X; Xiong Y; Chen X Ratiometric Optical Nanoprobes Enable Accurate Molecular Detection and Imaging. Chem. Soc. Rev 2018, 47, 2873–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Aoi W; Marunaka Y Importance of pH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. BioMed Res. Int 2014, 2014, 598986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Boedtkjer E; Pedersen SF The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol 2020, 82, 103–126. [DOI] [PubMed] [Google Scholar]
- (64).Ma T; Hou Y; Zeng J; Liu C; Zhang P; Jing L; Shangguan D; Gao M Dual-Ratiometric Target-Triggered Fluorescent Probe for Simultaneous Quantitative Visualization of Tumor Microenvironment Protease Activity and pH in Vivo. J. Am. Chem. Soc 2018, 140, 211–218. [DOI] [PubMed] [Google Scholar]
- (65).Pratiwi FW; Hsia CH; Kuo CW; Yang SM; Hwu YK; Chen P Construction of Single Fluorophore Ratiometric pH Sensors Using Dual-Emission Mn2+-Doped Quantum Dots. Biosens. Bioelectron 2016, 84, 133–140. [DOI] [PubMed] [Google Scholar]
- (66).Richardson DS; Gregor C; Winter FR; Urban NT; Sahl SJ; Willig KI; Hell SW SRpHi Ratiometric pH Biosensors for Super-Resolution Microscopy. Nat. Commun 2017, 8, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Hanson GT; McAnaney TB; Park ES; Rendell MEP; Yarbrough DK; Chu S; Xi L; Boxer SG; Montrose MH; Remington SJ Green Fluorescent Protein Variants as Ratiometric Dual Emission pH Sensors. 1. Structural Characterization and Preliminary Application. Biochemistry 2002, 41, 15477–15488. [DOI] [PubMed] [Google Scholar]
- (68).An W; Mason RP; Lippert AR Energy Transfer Chemiluminescence for Ratiometric pH Imaging. Org. Biomol. Chem 2018, 16, 4176–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zhang G; Jacquemin D; Buccella D Tuning the Spectroscopic Properties of Ratiometric Fluorescent Metal Indicators: Experimental and Computational Studies on Mag-fura-2 and Analogues. J. Phys. Chem. B 2017, 121, 696–705. [DOI] [PubMed] [Google Scholar]
- (70).Egawa T; Hanaoka K; Koide Y; Ujita S; Takahashi N; Ikegaya Y; Matsuki N; Terai T; Ueno T; Komatsu T; Nagano T Development of a Far-Red to Near-Infrared Fluorescence Probe for Calcium Ion and its Application to Multicolor Neuronal Imaging. J. Am. Chem. Soc 2011, 133, 14157–14159. [DOI] [PubMed] [Google Scholar]
- (71).Chen J; Jiang X; Zhang C; MacKenzie KR; Stossi F; Palzkill T; Wang MC; Wang J Reversible Reaction-Based Fluorescent Probe for Real-Time Imaging of Glutathione Dynamics in Mitochondria. ACS Sens 2017, 2, 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Jiang X; Yu Y; Chen J; Zhao M; Chen H; Song X; Matzuk AJ; Caroll SL; Tan X; Sizovs A; Cheng N; Wang MC; Wang J Quantitative Imaging of Glutathione in Live Cells Using a Reversible Reaction-Based Ratiometric Fluorescent Probe. ACS Chem. Biol 2015, 10, 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Myochin T; Kiyose K; Hanaoka K; Kojima H; Terai T; Nagano T Rational Design of Ratiometric Near-Infrared Fluorescent pH Probes with Various pKa Values, Based on Aminocyanine. J. Am. Chem. Soc 2011, 133, 3401–3409. [DOI] [PubMed] [Google Scholar]
- (74).Suarez SA; Bikiel DA; Wetzler DE; Marti MA; Doctorovich F Time-Resolved Electrochemical Quantification of Azanone (HNO) at Low Nanomolar Level. Anal. Chem 2013, 85, 10262–10269. [DOI] [PubMed] [Google Scholar]
- (75).Cowart D; Venuti R; Guptill J; Noveck R; Foo S A Phase 1 Study of the Safety and Pharmacokinetics of the Intravenous Nitroxyl Prodrug, CXL-1427. J. Am. Coll. Cardiol 2015, 65, A876. [Google Scholar]
- (76).Ryan LS; Gerberich J; Cao J; An W; Jenkins BA; Mason RP; Lippert AR Kinetics-Based Measurement of Hypoxia in Living Cells and Animals Using an Acetoxymethyl Ester Chemiluminescent Probe. ACS Sens. 2019, 4, 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Wortel RC; Mizrachi A; Li H; Markovsky E; Enyedi B; Jacobi J; Brodsky O; Cao J; Lippert AR; Incrocci L; Mulhall JP; Haimovitz-Friedman A Sildenafil Protects Endothelial Cells from Radiation-Induced Oxidative Stress. J. Sex. Med 2019, 16, 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Zelefsky MJ; Cowen D; Fuks Z; Shike M; Burman C; Jackson A; Venkatramen ES; Leibel SA Long Term Tolerance of High Dose Three-dimensional Conformal Radiotherapy in Patients with Localized Prostate Carcinoma. Cancer 1999, 85, 2460–2468. [DOI] [PubMed] [Google Scholar]
- (79).Zelefsky MJ; Shasha D; Branco RD; Kollmeier M; Baser RE; Pei X; Ennis R; Stock R; Bar-Chama N; Mulhall JP Prophylactic Sildenafil Citrate Improves Select Aspects of Sexual Function in Men Treated with Radiotherapy for Prostate Cancer. J. Urol 2014, 192, 868–874. [DOI] [PubMed] [Google Scholar]
- (80).Gebska MA; Stevenson BK; Hemnes AR; Bivalacqua TJ; Haile A; Hesketh GG; Murray CI; Zaiman AL; Halushka MK; Krongkaew N; Strong TD; Cooke CA; El-Haddad H; Tuder RM; Berkowitz DE; Champion HC Phosphodiesterase-5A (PDE5A) is Localized to the Endothelial Caveolae and Modulates NOS3 Activity. Cardiovasc. Res 2011, 90, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Jones KA; Kentala K; Beck MW; An W; Lippert AR; Lewis JC; Dickinson BC Development of a Split Esterase for Protein-Protein Interaction-Dependent Small-Molecule Activation. ACS Cent. Sci 2019, 5, 1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Quimbar ME; Krenek KM; Lippert AR A Chemiluminescent Platform for Smartphone Monitoring of H2O2 in Human Exhaled Breath Condensates. Methods 2016, 109, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Quimbar ME; Davis SQ; Al-Farra ST; Hayes A; Jovic V; Masuda M; Lippert AR Chemiluminescent Measurement of Hydrogen Peroxide in Exhaled Breath Condensate of Healthy and Asthmatic Adults. Anal. Chem 2020, 92, 14594–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kroll JL; Werchan CA; Reeves AG; Bruemmer KJ; Lippert AR; Ritz T Sensitivity of Salivary Hydrogen Sulfide to Psychological Stress and its Association with Exhaled Nitric Oxide and Affect. Physiol. Behav 2017, 179, 99–104. [DOI] [PubMed] [Google Scholar]
- (85).Ryan LS; Nakatsuka A; Lippert AR Photoactivatable 1,2 Dioxetane Chemiluminophores. Results in Chemistry 2021, 3, 100106. [Google Scholar]
- (86).Patel SK; Cao J; Lippert AR A Volumetric Three-Dimensional Digital Light Photoactivatable Dye Display. Nat. Commun 2017, 8, 15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Li B; Haris U; Aljowni M; Nakatsuka A; Patel SK; Lippert AR Tuning the Photophysical Properties of Spirolactam Rhodamine Photoswitches. Isr. J. Chem 2021, 61, 244–252. [Google Scholar]
- (88).Sun J; Hu Z; Wang R; Zhang S; Zhang X A Highly Sensitive Chemiluminescent Probe for Detecting Nitroreductase and Imaging in Living Animals. Anal. Chem 2019, 91, 1384–1390. [DOI] [PubMed] [Google Scholar]
- (89).Huang J; Huang J; Cheng P; Jiang Y; Pu K Near-Infrared Chemiluminescent Reporters for In Vivo Imaging of Reactive Oxygen and Nitrogen Species in Kidneys. Adv. Funct. Mater 2020, 30, 2003628. [Google Scholar]
- (90).Huang J; Jiang Y; Li J; Huang J; Pu K Molecular Chemiluminescent Probes with a Very Long Near-Infrared Emission Wavelength for in Vivo Imaging. Angew. Chem 2021, 133, 4045–4049. [DOI] [PubMed] [Google Scholar]
- (91).Simsek Turan I; Sozmen F A Chromogenic Dioxetane Chemosensor for Hydrogen Sulfide and pH Dependent Off–On Chemiluminescence Property. Sens. Actuators, B 2014, 201, 13–18. [Google Scholar]
- (92).Levinn CM; Pluth MD Direct Comparison of Triggering Motifs on Chemiluminescent Probes for Hydrogen Sulfide Detection in Water. Sens. Actuators, B 2021, 329, 129235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Gholap SP; Yao C; Green O; Babjak M; Jakubec P; Malatinsky T; Ihssen J; Wick L; Spitz U; Shabat D Chemiluminescence Detection of Hydrogen Sulfide Release by β-Lactamase-Catalyzed β-Lactam Biodegradation: Unprecedented Pathway for Monitoring β-Lactam Antibiotic Bacterial Resistance. Bioconjugate Chem. 2021, 32, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]


