Abstract
Antibiotic resistance of bacterial pathogens has become a severe threat to human health. To counteract antibiotic resistance, it is of significance to discover new antibiotics and also improve the efficacy of existing antibiotics. Here we show that 5-methylindole, a derivative of the interspecies signaling molecule indole, is able to directly kill various Gram-positive pathogens (e.g., Staphylococcus aureus and Enterococcus faecalis) and also Gram-negative ones (e.g., Escherichia coli and Pseudomonas aeruginosa), with 2-methylindole being less potent. Particularly, 5-methylindole can kill methicillin-resistant S. aureus, multidrug-resistant Klebsiella pneumoniae, Mycobacterium tuberculosis, and antibiotic-tolerant S. aureus persisters. Furthermore, 5-methylindole significantly potentiates aminoglycoside antibiotics, but not fluoroquinolones, killing of S. aureus. In addition, 5-iodoindole also potentiates aminoglycosides. Our findings open a new avenue to develop indole derivatives like 5-methylindole as antibacterial agents or adjuvants of aminoglycoside.
Keywords: 5-Methylindole, Indole, Aminoglycoside, Antibiotic resistance, Antibiotic tolerance, Persister, Gram-positive bacteria, Staphylococcus aureus, MRSA
Introduction
The discovery and application of antibiotics have dramatically improved the lives of humans and are considered as the greatest medical achievement of last century (Fleming, 1945). However, the antibiotic resistance of bacterial pathogens was immediately observed in laboratory beginning in the 1930s (Fleming, 1945; Abraham & Chain, 1940) and has become a severe threat to global public health and economic development (Shankar, 2016; Lewis, 2010; Antimicrobial Resistance Collaborators, 2022). To counteract this formidable challenge for human society, comprehensive strategies must be adopted. The discovery and development of new antibiotics, including structurally modified existing antibiotics, has played a dominant role in this war (Brown & Wright, 2016; Lewis, 2020). In addition, improving the efficacy of existing antibiotics has also attracted attentions in the recent decade (Allison, Brynildsen & Collins, 2011; Peng et al., 2015; Liu et al., 2019; Wright, 2016; Zhao et al., 2020; Lv et al., 2022a; Lv et al., 2022b), given that these existing antibiotics have been well documented in their toxicity, pharmacokinetics, administration and mechanism of actions.
Indole is an intra-species, interspecies or even interkingdom signaling molecule and plays important biological functions in bacteria and animals (Bansal et al., 2010; Lee & Lee, 2010; Song & Wood, 2020). Cumulative evidence suggests that indole is able to reduce the antibiotic tolerance of Escherichia coli (Hu et al., 2015; Kwan et al., 2015) and Lysobacter enzymogenes (Han et al., 2017; Wang et al., 2019) can be dramatically lowered by indole. In addition, indole also reduces E. coli biofilm formation (Domka, Lee & Wood, 2006; Domka et al., 2007; Lee, Jayaraman & Wood, 2007). Moreover, indole was reported to increase the bacterial uptake of antimicrobials through an interaction with the Mtr permease (Zhang, Yang & Defoirdt, 2022), and indole signaling is a valid target for the development of novel therapeutics in order to control infections caused by Harveyi clade vibrios in aquaculture (Wu et al., 2022). These studies suggest that indole may benefit antibiotic treatment when combined with different antibiotics. Furthermore, halogenated indoles were reported to exhibit antibacterial activity against persister cells and biofilms (Lee et al., 2016), both of which play critical roles in recurrent infections and antibiotic resistance development (Lewis, 2007; Levin-Reisman et al., 2017; de la Fuente-Núñez et al., 2013; Liu et al., 2020; Boya, Lee & Lee, 2022). Recently, a substituted indole was found to kill Pseudomonas aeruginosa persister cells effectively by damaging membranes and causing lysis (Song et al., 2019).
Previously, we found that indole and 5-methylindole could markedly potentiate aminoglycoside antibiotics against bacterial persister cells under hypoionic conditions (i.e., in ion-free solutions) (Sun et al., 2020) after short-term combined treatment. Although such potentiation could be fulfilled within 5-min incubation, the requirement of hypoionic conditions for treatment may limit the potential of its application, as salts and electrolytes are ubiquitously present throughout human and animal bodies. In this work, we attempted to overcome this drawback. We show that 5-methylindole could directly kill various Gram-positive and Gram-negative bacteria and also potentiate aminoglycoside antibiotics against stationary-phase Staphylococcus aureus cells under conventional treatment conditions (i.e., agitation in LB medium at 37 °C for a few hours). Our study may pave the way to develop indole derivatives as antibacterial agents and/or adjuvants of existing antibiotics.
Materials and Methods
Strains, medium and reagents
Various Gram-positive (S. aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus pyogenes, Micrococcus luteus, Streptococcus iniae) and Gram-negative (E. coli, P. aeruginosa and Shigella flexneri) bacterial strains, as well as M. tuberculosis strain (H37Ra) were used in this study and their characteristics are described in Table S1. Briefly, each bacterial strain was cultured over-night and then diluted at 1:500 in Luria-Bertani (LB) medium (Note: the MRS medium was used for E. faecalis) to further agitated in a shaker (37 °C, 220 rpm) for 18–24 h to prepare stationary-phase cells. All chemical reagents are of analytical purity. Stock solutions of indole, 2-methylindole, 5-methylindole and 5-iodoindole were prepared with DMSO and placed in brown, opaque Eppendorf tubes to avoid photo-induced damage.
Cell survival assay
Briefly, bacterial cells in stationary-phase were mixed with indole, 2-methylindole, 5-methylindole at varying concentrations and agitated for 3 h. A total of 100 μL treated cell cultures were centrifuged (13,000 rpm, 1 min) in Eppendorf tubes and washed with PBS (0.27 g/L KH2PO4, 1.42 g/L Na2HPO4, 8 g/L NaCl, 0.2 g/L KCl, pH 7.4). Afterwards, 5 μL of tenfold serially diluted cell suspension were spot plated onto LB agar dishes for cell survival assay. In addition, stationary-phase and exponential-phase S. aureus cell cultures were mixed with aminoglycoside antibiotics in the presence of indole, 2-methylindole or 5-methylindole for varying length of time, and cells were washed with PBS before cell survival assay as described above. Independent experiments were repeated at least three times.
Minimum inhibitory concentration (MIC) assay
The MICs of 5-methylindole for S. aureus ATCC25923, E. faecalis ATCC 29212, S. iniae, E. coli BW25113, P. aeruginosa PAO1, S. flexneri 24T7T, K. pneumoniae KP-D367 were measured by incubating freshly inoculated cultures in LB for 24 h containing varying concentrations of indoles. Results were determined by observing visual turbidity and experiments were performed using at least two independent cultures.
Preparation and eradication of antibiotic-tolerant S. aureus persister cells
Nutrient shift-induced persisters were prepared as previously reported (Chen et al., 2019). In brief, over-night cultures of S. aureus were diluted at 1:100 into LB medium (37 °C, 220 rpm) and cultured to mid-exponential phase at a cell density of OD600 = 0.5–0.6. Cells were centrifuged and washed with M9 medium twice before transferred to M9 medium plus 2 g/L fumarate and agitated for four hours before indole treatment. Starvation-induced persisters were prepared according to an earlier report (Eng et al., 1991). Briefly, S. aureus cells were diluted at 1:500 in Mueller-Hinton broth medium and cultured for 24 h (37 °C, 220 rpm) to a cell density of around 109 CFU/mL. Cells were centrifuged, re-suspended in yeast nitrogen broth medium without amino acids, diluted to a cell density of around 108 CFU/mL and agitated for 5 h before indole treatment.
Hemolysis effect assay
Mouse blood cells were obtained from mice discarded from other animal experiments and sodium citrate at a final concentration of 2.5% was used as anticoagulant. Blood cells were collected by centrifugation (1,000 rpm, 15 min) and washed twice with 0.9% NaCl containing 2.5% sodium citrate. The cell pellets were re-suspended in 0.9% NaCl solution containing 2.5% sodium citrate plus indole, 2-methylindole or 5-methylindole at increasing concentrations, incubated at 37 °C for 3 h and centrifuged, with the supernatant being subjected to absorbance measurement at 545 nm on a 96-well plate reader.
Ethics statement
Animal experiments were performed in accordance with the National Standards of China (GB/T 35892-2018 and GB/T 35823-2018) and after the approval issued by the Animal Ethical and Welfare Committee of Fujian Normal University (approval no. IACUC 20190006).
Results
5-Methylindole kills various Gram-positive bacteria in stationary-phase
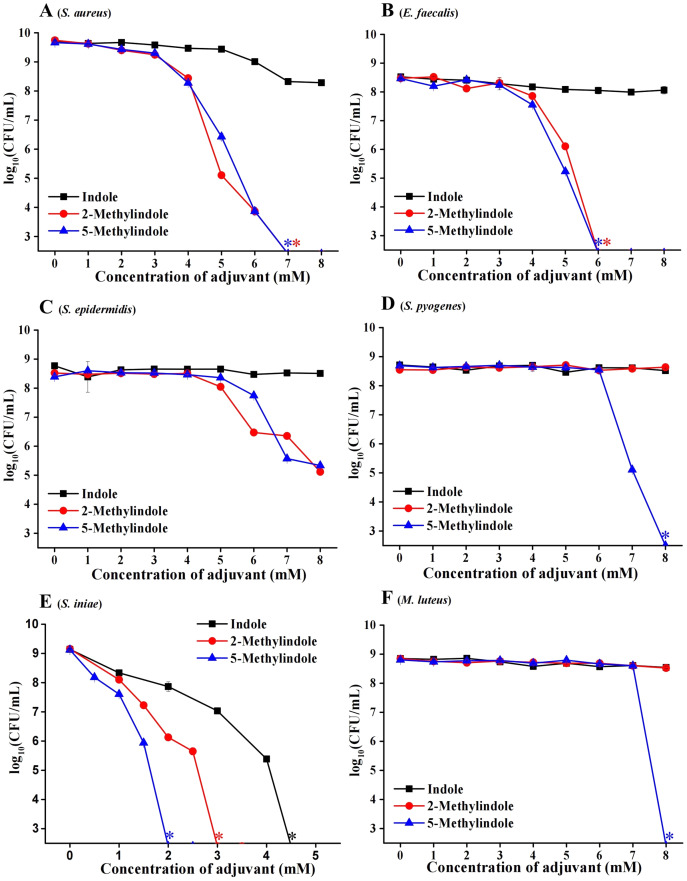
We evaluated the potential bactericidal activity of indole and its two derivatives, i.e., 5-methylindole and 2-methylindole, against typical Gram-positive bacteria, including S. aureus and Staphylococcus epidermidis. We prepared these bacteria in stationary-phase that are generally tolerant to bactericidal antibiotics (Keren et al., 2004) and widely used for antibiotic research (Allison, Brynildsen & Collins, 2011; Peng et al., 2015), and then subjected them to the treatment with indole derivatives at varying concentrations for 3 h. Cell survival assay revealed that (i) while indole hardly exhibited bactericidal activity, 5-methylindole was able to effectively eradicate these pathogens in a concentration-dependent manner (Figs. 1A–1E); (ii) 2-methylindole eradicated S. aureus, S. epidermidis and E. faecalis as effectively as did 5-methylindole but had no killing effect on S. pyogenes and M. luteus. We also examined the aquatic pathogen Streptococcus iniae and found that 5-methylindole could kill S. iniae in a concentration-dependent manner, with 2-methylindole and indole being less effective (Fig. 1F). Furthermore, we determined MICs of 5-methylindole to S. aureus, E. faecalis and S. iniae, which were 4, 16 and 16 mM, respectively (Table 1).
Figure 1. 5-Methylindole exhibited bactericidal activity against Gram-positive bacteria in stationary-phase.
Survival of different Gram-positive bacteria in stationary-phase following a 3-h treatment with indole, 2-methylindole or 5-methylindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM in Panels (A–D and F); 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 4.5 in (Panel E)). Data represent the means ± SD of three replicates of one independent experiment. An asterisk (*) represents no detectable colonies on LB dish when 5 μL treated cells were spot plated.
Table 1. MICs of 5-methylindole examined in this study.
| Strain | 5-Methylindole (mM) |
|---|---|
| S. aureus ATCC 25923 | 4 |
| E. faecalis ATCC 29212 | 16 |
| S. iniae | 16 |
| E. coli BW25113 | 8 |
| P. aeruginosa PAO1 | 16 |
| S. flexneri 24T7T | 2 |
| K. pneumoniae KP-D367 | 4 |
5-Methylindole kills various Gram-negative bacteria in stationary-phase
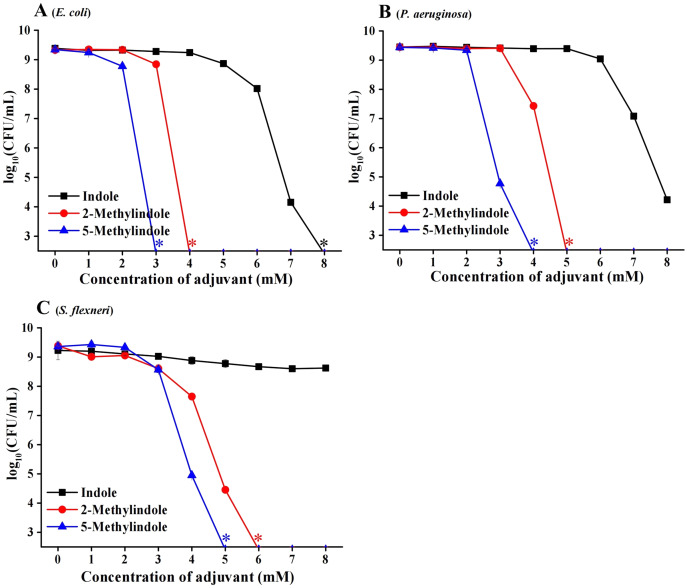
We then examined the potential bactericidal activity of 5-methylindole against typical Gram-negative bacteria in stationary-phase, including E. coli, P. aeruginosa and Shigella flexneri. The cell survival assay showed that 5-methylindole was able to effectively kill all these pathogens in a concentration-dependent manner (Figs. 2A–2C). Similarly, 2-methylindole and indole were also able to kill these pathogens in a concentration-dependent manner, albeit at lower efficacy than 5-methylindole. Notably, Gram-negative bacteria overall seemed more sensitive to 5-methylindole than Gram-positive bacteria (Fig. 2 vs Fig. 1). In addition, we determined MICs of 5-methylindole to E. coli, P. aeruginosa and Shigella flexneri, which were 8, 16 and 2 mM, respectively (Table 1).
Figure 2. 5-Methylindole exhibited bactericidal activity against Gram-negative bacteria in stationary-phase.
Survival of different Gram-negative bacteria in stationary-phase following a 3-h treatment with indole, 2-methylindole or 5-methylindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM). Data represent the means ± SD of three replicates of one independent experiment. An asterisk (*) represents no detectable colonies on LB dish when 5 μL treated cells were spot plated.
5-Methylindole kills antibiotic-resistant/tolerant bacteria and Mycobacterium tuberculosis
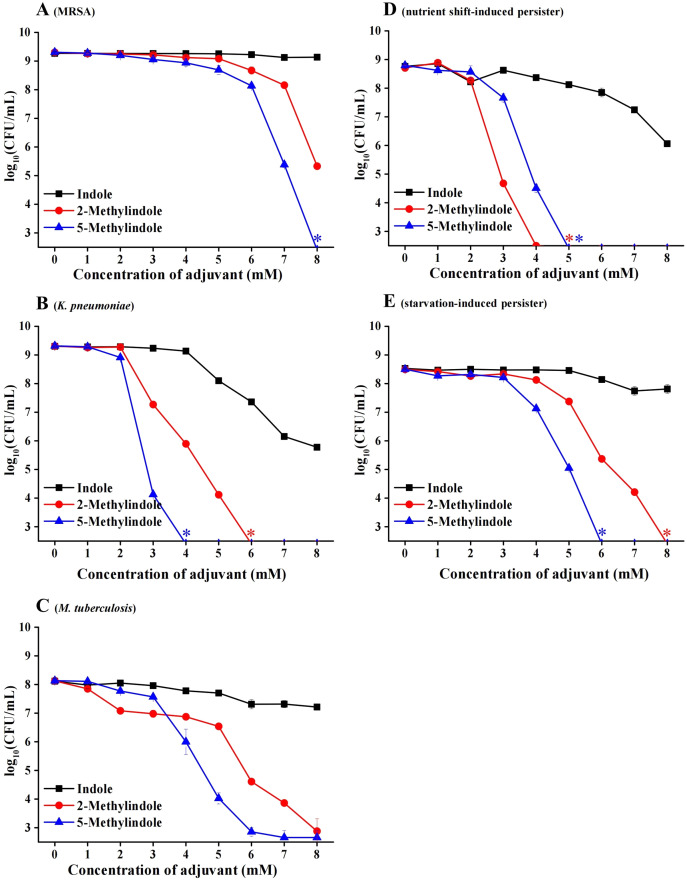
Considerable bactericidal activity of 5-methylindole against both Gram-positive and Gram-negative bacterial pathogens, as described in Figs. 1 and 2, prompted us to further examine whether it could eradicate those antibiotic-resistant/tolerant pathogens that give rise to the failure of antibiotic therapy. For this purpose, we first examined methicillin-resistant Staphylococcus aureus (MRSA) that has been designated on the WHO priority pathogens list (WHO, 2017), as well as multidrug-resistant pathogen Klebsiella pneumoniae that has been reported as one of the most critical antibiotic-resistant pathogens in China (China Antimicrobial Resistance Surveillance System, 2020). The cell survival assay showed that 5-methylindole was able to eradicate these two antibiotic-resistant pathogens in a concentration-dependent manner (Figs. 3A and 3B) while 2-methylindole was less effective. Notably, we found that 5-methylindole and 2-methylindole were also able to kill Mycobacterium tuberculosis H37Ra (an avirulent strain) in a concentration-dependent manner (Fig. 3C). In addition, MIC of 5-methylindole to K. pneumoniae was determined to be 4 mM (Table 1).
Figure 3. 5-Methylindole exhibited bactericidal activity against antibiotic-resistant/tolerant bacteria.
(A–C) Survival of MRSA (panel A), K. pneumoniae (panel B) and M. tuberculosis H37Ra (an avirulent strain, panel C) in stationary-phase following a 3-h treatment with indole, 2-methylindole or 5-methylindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM). (D, E) Survival of S. aureus nutrient shift-induced (panel D) and starvation-induced persister cells (panel E) following a 3-h treatment with indole, 2-methylindole or 5-methylindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM). Data represent the means ± SD of three replicates of one independent experiment. An asterisk (*) represents no detectable colonies on LB dish when 5 μL treated cells were spot plated.
We next examined the effect of 5-methylindole on bacterial persisters, which are transiently tolerant to the attack of bactericidal antibiotics and play an important role in chronic infections and the evolution of antibiotic resistance (Lewis, 2010; Levin-Reisman et al., 2017; Liu et al., 2020). To this end, nutrient shift-induced S. aureus persisters were prepared by switching the carbon source of S. aureus cells in exponential-phase to fumarate (Radzikowski et al., 2016), and starvation-induced S. aureus persisters were made by transferring S. aureus cells in stationary-phase to medium without any nutrients (Chen et al., 2019). These nutrient shift-induced and starvation-induced S. aureus persister cells were kept in non-replicating status and highly tolerant to the attack by tobramycin under conventional treatment condition (Fig. S1; or refer to our earlier reports (Sun et al., 2020; Chen et al., 2019)). However, both of them were killed by 5-methylindole and 2-methylindole in a concentration-dependent manner, with indole being much less effective (Figs. 3D and 3E).
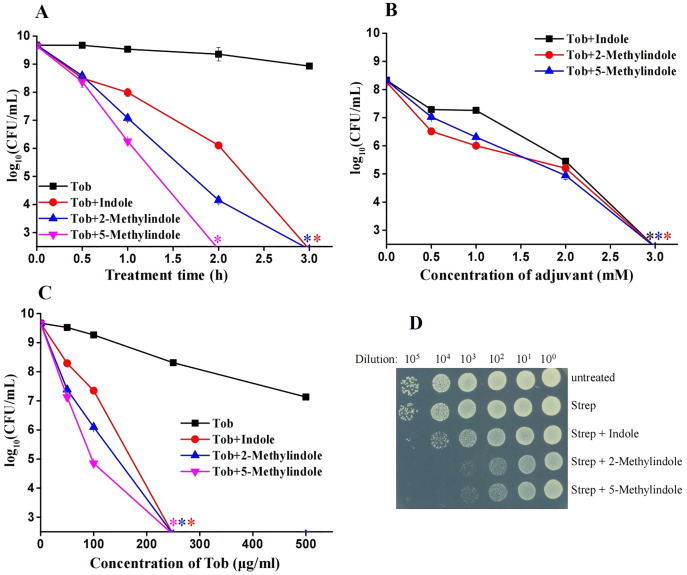
5-Methylindole potentiates aminoglycosides killing of conventional S. aureus and MRSA cells in stationary-phase but not the cells in exponential-phase
We previously reported that 5-methylindole could potentiate aminoglycoside antibiotics against conventional S. aureus and MRSA cells under hypoionic conditions (i.e., in ion-free solutions) and that such potentiation could be achieved within a couple of minutes (Sun et al., 2020). Here we examined whether 5-methylindole could potentiate aminoglycosides under conventional treatment conditions (i.e., agitation in LB medium for a couple of hours). The cell survival assay showed that 5-methylindole could significantly enhance the bactericidal actions of tobramycin against conventional S. aureus cells in stationary-phase, in both time-dependent (Fig. 4A) and concentration-dependent manners (Fig. 4B). In addition, tobramycin alone at 100 μg/mL only exhibited marginal bactericidal activity, but eradicated S. aureus cells by more than four orders of magnitude when combined with 5-methylindole (Fig. 4C). More importantly, we found that both 5-methylindole and 2-methylindole significantly increased the killing efficacy of streptomycin against MRSA cells, with streptomycin alone hardly killing the bacteria (Fig. 4D). We also observed that 5-methylindole, 2-methylindole and indole could dramatically potentiate gentamicin, streptomycin and kanamycin against S. aureus cells in stationary-phase (Figs. S2B–S2D). However, they had little effects on fluoroquinolone antibiotics ofloxacin and ciprofloxacin (Figs. S2E and S2F), in contrast to earlier reports showing that ofloxacin was potentiated by ascorbic acid against Staphylococcus aureus (Dey & Bishayi, 2018) and certain oils could reduce the MIC of ofloxacin against E. coli and S. aureus (Pereira et al., 2020). In addition, we found that none of 5-methylindole, 2-methylindole and indole could potentiate tobramycin and other types of aminoglycosides against S. aureus cells in exponential-phase (Figs. S3). Together, these results suggest that 5-methylindole could potentiate aminoglycoside antibiotics against S. aureus cells in stationary-phase.
Figure 4. 5-Methylindole potentiates aminoglycosides to kill conventional S. aureus and MRSA cells in stationary-phase.
(A) Survival of S. aureus cells in stationary-phase following a combined treatment with 250 μg/mL tobramycin plus 4 mM indole, 2-methylindole or 5-methylindole for varying length of time (0.5, 1, 2 and 3 h). (B) Survival of S. aureus cells in stationary-phase following a 3-h combined treatment with 250 μg/mL tobramycin plus indole, 2-methylindole or 5-methylindole at increasing concentrations. (C) Survival of S. aureus cells in stationary-phase following a 3-h combined treatment with increasing concentrations of tobramycin (0, 50, 100, 250 and 500 μg/mL) plus 4 mM indole, 2-methylindole or 5-methylindole. (D) Survival of MRSA cells in stationary-phase following a 3-h combined treatment with 500 μg/ml streptomycin plus 4 mM indole, 2-methylindole or 5-methylindole. Data in panels A, B and C represent the means ± SD of three replicates of one independent experiment and an asterisk (*) represents no detectable colonies on LB dish when 5 μL treated cells were spot plated.
5-Methylindole at sub-toxic concentrations potentiates aminoglycosides killing of S. aureus in stationary-phase
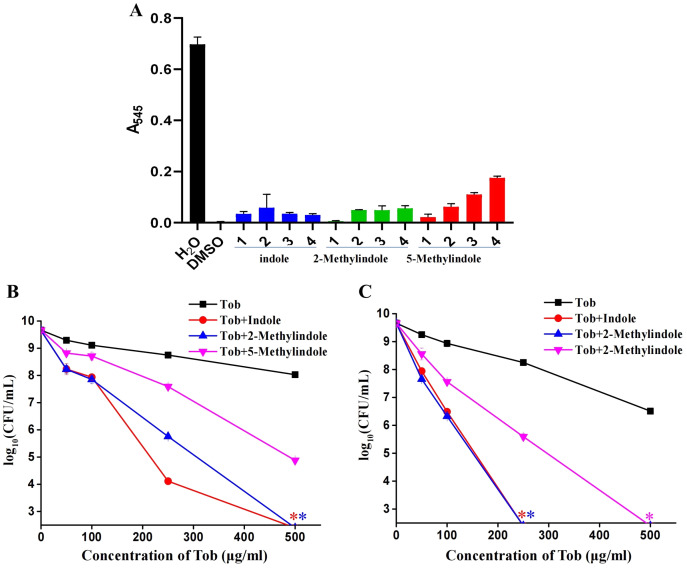
Because 5-methylindole alone could kill bacteria, we thus evaluated its cytotoxicity towards mammalian cells by examining its hemolysis effect. Cell lysis assay revealed that 5-methylindole could disrupt mouse blood cells in a concentration-dependent manner (Fig. 5A, Fig. S4), with the hemolysis effect of 1 mM 5-methylindole being minimal. In comparison, the hemolysis effects of 2-methylindole and indole were less potent than that of 5-methylindole. Based on this, we then evaluated the potentiation effects of 5-methylindole, 2-methylindole and indole at sub-toxic concentrations (1, 2 and 4 mM, respectively) on tobramycin. The cell survival assay revealed that all of them could effectively enhance the killing efficacy of tobramycin against S. aureus cells in stationary-phase after agitation for 3 h (Fig. 5B) or 5 h (Fig. 5C).
Figure 5. 5-Methylindole at sub-toxic concentration potentiates tobramycin killing of S. aureus in stationary-phase.
(A) Absorbance at 545 nm of the supernatant of mouse blood cell suspension following a 3-h treatment with indole, 2-methylindole or 5-methylindole at increasing concentrations. (B, C) Survival of S. aureus cells in stationary-phase following a 3-h (panel B) or 5-h (panel C) combined treatment with increasing concentrations of tobramycin (0, 50, 100, 250 and 500 μg/mL) plus 4 mM indole, 2 mM 2-methylindole or 1 mM 5-methylindole. Data represent the means ± SD of three replicates of one independent experiment. An asterisk (*) represents no detectable colonies on LB dish when 5 μL treated cells were spot plated.
Discussion
In comparison with our earlier report (Sun et al., 2020), several novel findings have been made in the current work. First, we observed that 5-methylindole itself exhibited substantial antibacterial activities against a number of Gram-positive and Gram-negative bacterial pathogens, including antibiotic-tolerant S. aureus persisters, MRSA and M. tuberculosis. Second, we observed the potentiation effect of 5-methylindole on aminoglycoside antibiotics against S. aureus cells under conventional treatment conditions (i.e., agitation in LB medium for several hours). In contrast, the potentiation effect of 5-methylindole on aminoglycosides was detected only under hypoionic conditions (i.e., without the presence of ions and electrolytes) in our earlier report (Sun et al., 2020), apparently being distinct from physiological conditions. In addition, this unique treatment lasts only a few minutes whereas the treatment in the current study lasts several hours, which is more similar to antibiotic chemotherapy. Therefore, the current work further illustrates the potential of 5-methylindole for the development of new antibiotics and/or antibiotic adjuvants.
Indole exerts diverse roles in multiple signaling pathways of bacteria as an endogenous metabolite, such as spore formation, plasmid stability, drug resistance, biofilm formation, and virulence in indole-producing bacteria (Lee & Lee, 2010). In a rich medium (Lee, Jayaraman & Wood, 2007), E. coli can generate endogenous indole up to 0.6 mM, which exhibits no toxicity to E. coli (Fig. 2A) but can induce persister formation (Vega et al., 2012). Moreover, indole can mediate the signaling cross-talk between enteric bacteria and their host (Bansal et al., 2010). Notably, indole and some of indole-based derivatives have been reported to exhibit antibacterial, antibiofilm and/or antivirulence activities (Song & Wood, 2020; Lee et al., 2016; Chimerel et al., 2012; Qin et al., 2020; Sethupathy et al., 2020). In retrospect, 2-methylindoe, but not 5-methylindole, was found to inhibit biofilm formation of an opportunistic fungal pathogen Candida albicans (Lee et al., 2018); 7-methylindole was reported to significantly suppress biofilm formation of the Gram-negative pathogen Serratia marcescens (Sethupathy et al., 2020). In particular, 5-iodoindole and 5-chloroindole were reported to exhibit antibiofilm and antibacterial activities (Lee et al., 2016; Boya, Lee & Lee, 2022). Prompted by these reports, we examined 5-iodoindole and found that this indole derivative could also potentiate tobramycin killing of stationary-phase S. aureus cells (Fig. S5A). Notably, the potentiation effect of 5-iodoindole on tobramycin appears to be higher than that of 5-methylindole, presumably due to its stronger antibacterial effect than the latter (Fig. S5B). Together, these observations illustrate a high potential of methylindole derivatives in the discovery and development of new antibiotics.
Besides antibacterial activity, indole and its derivatives have also been shown to be able to change the efficacy of existing antibiotics in killing of bacterial pathogens. For instance, indole has been reported to increase the antibiotic tolerance of E. coli cells (Lee et al., 2010; Vega et al., 2013; Han et al., 2011). Such suppression effect might be achieved by elevating the expression of efflux pumps that could transport the intracellular antibiotics out of the bacterial cells (Hirakawa et al., 2005) and/or activating stress response-related pathways that could counteract the lethality of the antibiotics (Vega et al., 2012). Nevertheless, several studies revealed that indole can reduce the antibiotic tolerance of E. coli (Hu et al., 2015; Kwan et al., 2015) and Lysobacter enzymogenes (Han et al., 2017; Wang et al., 2019), likely by activating or upregulating antibiotic importers. These contradictory observations might result from the difference in cell growth/metabolic status, as we observed that 5-methylindole potentiates aminoglycoside antibiotics under hypoionic conditions against S. aureus cells in stationary-phase but suppress the antibiotics against the cells in exponential-phase (Sun et al., 2020). In support of this, here we show that 5-methylindole potentiates tobramycin against S. aureus cells in stationary-phase but had no effects on the antibiotics against the cells in exponential-phase.
The molecular mechanism by which indole and 5-methylindole kills bacterial pathogens and potentiates aminoglycosides remains unknown. One possibility is that indole and 5-methylindole may reduce the electrochemical potential across the cytoplasmic membrane of bacterial cells, as indole was reported to act as a proton ionophore and thereby prevent formation of the FtsZ ring that is a prerequisite for cell division (Chimerel et al., 2012). Another possibility is that 5-methylindole may activate certain membrane channels for enhancing aminoglycoside uptake, as indole was reported to increase the antibiotic susceptibility of Lysobacter enzymogenes (Lewis, 2020; Wang et al., 2019) by up-regulating antibiotic importers. Aminoglycosides have been widely applied to cure various infections in humans and animals (van Duijkeren et al., 2019) and their applications in clinics are largely limited due to the toxicity and the wide spread of aminoglycoside resistance (Mingeot-Leclercq & Tulkens, 1999; Mingeot-Leclercq, Glupczynski & Tulkens, 1999). In this regard, 5-methylindole-induced aminoglycoside potentiation against conventional S. aureus and MRSA cells, under both hypoionic conditions as reported earlier by us (Sun et al., 2020) and conventional treatment conditions as seen here, is of clinical importance. Dissection of the underlying mechanism may help to develop indole derivatives as aminoglycoside adjuvants, which would extend the lifetime of this important class of bactericidal antibiotics.
Supplemental Information
(A) Survival of S. aureus cells after nutrient shift followed by a 2-h treatment with indicated antibiotics (100 μg/ml ampicillin, 5 μg/ml ofloxacin, and 50 μg/ml tobramycin). (B) Survival of S. aureus cells after starvation followed by 50 μg/ml tobramycin treatment for varying length of time (0.5, 1, or 2 h).
(A, B, C, D, E, F) Survival of stationary-phase S. aureus cells following a 3-h treatment with 500 μg/ml tobramycin (panel A), 2,000 μg/ml streptomycin (panel B), 500 μg/ml gentamicin (panel C), 1,000 μg/ml kanamycin (panel D), 100 μg/ml ofloxacin (panel E) or 100 μg/ml ciproxacin (panel F) in the absence or presence of 4 mM indole, 2-Methylindole or 5-Methylindole.
(A) Survival of exponential-phase S. aureus cells following a 3-h treatment with 1 mM indole, 2-Methylindole or 5-Methylindole. (B) Survival of exponential-phase S. aureus cells following treatments with 25 μg/ml tobramycin plus 1 mM indole, 2-Methylindole or 5-Methylindole for 1, 2 or 3 h. (C) Survival of exponential-phase S. aureus cells following treatments with 100 μg/ml streptomycin, 25 μg/ml gentamicin or 50 μg/ml kanamycin plus 1 mM indole, 2-Methylindole or 5-Methylindole for 1 or 2 h.
Upper part: Photos for mouse blood cells dissolved in 0.9% NaCl containing increasing concentrations of DMSO (0, 1, 2, 3 and 4 mM). Cells dissolved in pure water were used as a positive control, i.e., red blood cells were largely lysed in such hypoosmotic solution. Lower part: Photos for mouse blood cells dissolved in 0.9% NaCl solution containing increasing concentration of indole, 2-methylindole or 5-methylindole (0, 1, 2, 3 and 4 mM).
(A) Survival of stationary-phase S. aureus cells following a 3-h treatment with 250 μg/ml tobramycin plus 5-methylindole or 5-iodoindole (0, 1, 2, 3 and 4 mM). (B) Survival of stationary-phase S. aureus cells following a 3-h treatment with 5-methylindole or 5-iodoindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM).
Acknowledgments
We thank Profs. Luhua Lai, Xiaoyun Liu, Zengyi Chang and Dr. Xiaoyun Liu (all from Peking University), Dr. Qingeng Huang (Fujian Normal University) and Prof. Xuanxian Peng (SUN YAT-SEN University) for their kindness in providing bacterial strains as described in Table S1.
Funding Statement
This work was supported by research grants from the Natural Science Foundation of Fujian Province (2019J01278 to Yajuan Chen), the National Natural Science Foundation of China (No. 31972918 to Xinmiao Fu) and the Natural Science Foundation of Fujian Province (No. 2021J02029 to Xinmiao Fu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Fengqi Sun, Email: 972094096@qq.com.
Yajuan Chen, Email: cyj288@fjnu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Zhongyan Li performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Fengqi Sun conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Xinmiao Fu conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Yajuan Chen conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Animal Ethical and Welfare Committee of Fujian Normal University (IACUC 20190006).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Abraham & Chain (1940).Abraham EP, Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146(3713):837. doi: 10.1038/146837a0. [DOI] [PubMed] [Google Scholar]
- Allison, Brynildsen & Collins (2011).Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antimicrobial Resistance Collaborators (2022).Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal et al. (2010).Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proceedings of the National Academy of Sciences. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya, Lee & Lee (2022).Boya BR, Lee JH, Lee J. Antibiofilm and antimicrobial activities of chloroindoles against uropathogenic Escherichia coli. Frontiers in Microbiology. 2022;13:872943. doi: 10.3389/fmicb.2022.872943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown & Wright (2016).Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529(7586):336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen Z, Gao Y, Lv B, Sun F, Yao W, Wang Y, Fu X. Hypoionic shock facilitates aminoglycoside killing of both nutrient shift- and starvation-induced bacterial persister cells by rapidly enhancing aminoglycoside uptake. Frontiers in Microbiology. 2019;10:2028. doi: 10.3389/fmicb.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimerel et al. (2012).Chimerel C, Field CM, Pinero-Fernandez S, Keyser UF, Summers DK. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012;1818(7):1590–1594. doi: 10.1016/j.bbamem.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Antimicrobial Resistance Surveillance System (2020).China Antimicrobial Resistance Surveillance System 2019 National bacterial resistance surveillance report of China (in Chinese) 2020. http://www.carss.cn/Sys/res/file/202102/20210224140246_2949_2947ca16e15a46e5a4f063f9f529b895_CARSS2019report.pdf http://www.carss.cn/Sys/res/file/202102/20210224140246_2949_2947ca16e15a46e5a4f063f9f529b895_CARSS2019report.pdf
- de la Fuente-Núñez et al. (2013).de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Current Opinion in Microbiology. 2013;16(5):580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Dey & Bishayi (2018).Dey S, Bishayi B. Killing of S. aureus in murine peritoneal macrophages by Ascorbic acid along with antibiotics Chloramphenicol or Ofloxacin: correlation with inflammation. Microbial Pathogenesis. 2018;115:239–250. doi: 10.1016/j.micpath.2017.12.048. [DOI] [PubMed] [Google Scholar]
- Domka et al. (2007).Domka J, Lee J, Bansal T, Wood TK. Temporal gene-expression in Escherichia coli K-12 biofilms. Environmental Microbiology. 2007;9(2):332–346. doi: 10.1111/j.1462-2920.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- Domka, Lee & Wood (2006).Domka J, Lee J, Wood TK. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Applied and Environmental Microbiology. 2006;72(4):2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng et al. (1991).Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrobial Agents and Chemotherapy. 1991;35(9):1824–1828. doi: 10.1128/AAC.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming (1945).Fleming A. Penicillin Nobel Lecture. 1945. https://todayinsci.com/F/Fleming_Alexander/FlemingAlexander-Quotations.htm https://todayinsci.com/F/Fleming_Alexander/FlemingAlexander-Quotations.htm
- Han et al. (2011).Han TH, Lee JH, Cho MH, Wood TK, Lee J. Environmental factors affecting indole production in Escherichia coli. Research in Microbiology. 2011;162(2):108–116. doi: 10.1016/j.resmic.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al. (2017).Han Y, Wang Y, Yu Y, Chen H, Shen Y, Du L. Indole-induced reversion of intrinsic multiantibiotic resistance in Lysobacter enzymogenes. Applied and Environmental Microbiology. 2017;83(17):427. doi: 10.1128/AEM.00995-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa et al. (2005).Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A. Indole induces the expression of multidrug exporter genes in Escherichia coli. Molecular Microbiology. 2005;55(4):1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2015).Hu Y, Kwan BW, Osbourne DO, Benedik MJ, Wood TK. Toxin YafQ increases persister cell formation by reducing indole signalling. Environmental Microbiology. 2015;17(4):1275–1285. doi: 10.1111/1462-2920.12567. [DOI] [PubMed] [Google Scholar]
- Keren et al. (2004).Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiology Letters. 2004;230(1):13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- Kwan et al. (2015).Kwan BW, Osbourne DO, Hu Y, Benedik MJ, Wood TK. Phosphodiesterase DosP increases persistence by reducing cAMP which reduces the signal indole. Biotechnology and Bioengineering. 2015;112(3):588–600. doi: 10.1002/bit.25456. [DOI] [PubMed] [Google Scholar]
- Lee, Jayaraman & Wood (2007).Lee J, Jayaraman A, Wood TK. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiology. 2007;7(1):42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2018).Lee JH, Kim YG, Gupta VK, Manoharan RK, Lee J. Suppression of fluconazole resistant candida albicans biofilm formation and filamentation by methylindole derivatives. Frontiers in Microbiology. 2018;9:2641. doi: 10.3389/fmicb.2018.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2016).Lee JH, Kim YG, Gwon G, Wood TK, Lee J. Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express. 2016;6(1):123. doi: 10.1186/s13568-016-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Lee (2010).Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiology Reviews. 2010;34(4):426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2010).Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467(7311):82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Reisman et al. (2017).Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. Antibiotic tolerance facilitates the evolution of resistance. Science. 2017;355(6327):826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- Lewis (2007).Lewis K. Persister cells, dormancy and infectious disease. Nature Reviews Microbiology. 2007;5(1):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Lewis (2010).Lewis K. Persister cells. Annual Review of Microbiology. 2010;64(1):357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- Lewis (2020).Lewis K. The science of antibiotic discovery. Cell. 2020;181(1):29–45. doi: 10.1016/j.cell.2020.02.056. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2020).Liu J, Gefen O, Ronin I, Bar-Meir M, Balaban NQ. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science. 2020;367(6474):200–204. doi: 10.1126/science.aay3041. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2019).Liu Y, Li R, Xiao X, Wang Z. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant Gram-negative bacteria. Critical Reviews in Microbiology. 2019;45(3):301–314. doi: 10.1080/1040841X.2019.1599813. [DOI] [PubMed] [Google Scholar]
- Lv et al. (2022a).Lv B, Bian M, Huang X, Sun F, Gao Y, Wang Y, Fu Y, Yang B, Fu X. n-Butanol potentiates subinhibitory aminoglycosides against bacterial persisters and multidrug-resistant MRSA by rapidly enhancing antibiotic uptake. ACS Infectious Diseases. 2022a;8(2):373–386. doi: 10.1021/acsinfecdis.1c00559. [DOI] [PubMed] [Google Scholar]
- Lv et al. (2022b).Lv B, Zeng Y, Zhang H, Li Z, Xu Z, Wang Y, Gao Y, Chen Y, Fu X. Mechanosensitive channels mediate hypoionic shock-induced aminoglycoside potentiation against bacterial persisters by enhancing antibiotic uptake. Antimicrobial Agents and Chemotherapy. 2022b;66(2):e0112521. doi: 10.1128/aac.01125-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingeot-Leclercq, Glupczynski & Tulkens (1999).Mingeot-Leclercq M-P, Glupczynski Y, Tulkens PM. Aminoglycosides activity and resistance. Antimicrobial Agents and Chemotherapy. 1999;43(4):727–737. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingeot-Leclercq & Tulkens (1999).Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrobial Agents and Chemotherapy. 1999;43(5):1003–1012. doi: 10.1128/AAC.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng et al. (2015).Peng B, Su YB, Li H, Han Y, Guo C, Tian YM, Peng XXJCM. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metabolism. 2015;21(2):249–261. doi: 10.1016/j.cmet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Pereira et al. (2020).Pereira RLS, Freitas TS, Freitas PR, Araujo ACJ, Campina FF, Fidelis KR, Vale J, Barreto ACH, Coutinho HDM, Bandeira PN, Teixeira AMR, Santos HSD. Seasonality effects on antibacterial and antibiotic potentiating activity against multidrug-resistant strains of Escherichia coli and Staphylococcus aureus and ATR-FTIR spectra of essential oils from Vitex gardneriana leaves. Current Microbiology. 2020;77(12):3969–3977. doi: 10.1007/s00284-020-02226-6. [DOI] [PubMed] [Google Scholar]
- Qin et al. (2020).Qin HL, Liu J, Fang WY, Ravindar L, Rakesh KP. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA) European Journal of Medicinal Chemistry. 2020;194(1–15):112245. doi: 10.1016/j.ejmech.2020.112245. [DOI] [PubMed] [Google Scholar]
- Radzikowski et al. (2016).Radzikowski JL, Vedelaar S, Siegel D, Ortega AD, Schmidt A, Heinemann M. Bacterial persistence is an active sigmaS stress response to metabolic flux limitation. Molecular Systems Biology. 2016;12(9):882. doi: 10.15252/msb.20166998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy et al. (2020).Sethupathy S, Sathiyamoorthi E, Kim YG, Lee JH, Lee J. Antibiofilm and antivirulence properties of indoles against Serratia marcescens. Frontiers in Microbiology. 2020;11:584812. doi: 10.3389/fmicb.2020.584812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar (2016).Shankar P. Book review: tackling drug-resistant infections globally. Archives of Pharmacy Practice. 2016;7(3):110. doi: 10.4103/2045-080X.186181. [DOI] [Google Scholar]
- Song et al. (2019).Song S, Gong T, Yamasaki R, Kim JS, Wood TK. Identification of a potent indigoid persister antimicrobial by screening dormant cells. Biotechnology and Bioengineering. 2019;116(9):2263–2274. doi: 10.1002/bit.27078. [DOI] [PubMed] [Google Scholar]
- Song & Wood (2020).Song S, Wood TK. Combatting persister cells with substituted indoles. Frontiers in Microbiology. 2020;11:1565. doi: 10.3389/fmicb.2020.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2020).Sun F, Bian M, Li Z, Lv B, Gao Y, Wang Y, Fu X. 5-methylindole potentiates aminoglycoside against gram-positive bacteria including staphylococcus aureus persisters under hypoionic conditions. Frontiers in Cellular and Infection Microbiology. 2020;10:84. doi: 10.3389/fcimb.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijkeren et al. (2019).van Duijkeren E, Schwarz C, Bouchard D, Catry B, Pomba C, Baptiste KE, Moreno MA, Rantala M, Ružauskas M, Sanders P, Teale C, Wester AL, Ignate K, Kunsagi Z, Jukes H. The use of aminoglycosides in animals within the EU: development of resistance in animals and possible impact on human and animal health: a review. Journal of Antimicrobial Chemotherapy. 2019;74(9):2480–2496. doi: 10.1093/jac/dkz161. [DOI] [PubMed] [Google Scholar]
- Vega et al. (2012).Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nature Chemical Biology. 2012;8(5):431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega et al. (2013).Vega NM, Allison KR, Samuels AN, Klempner MS, Collins JJ. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proceedings of the National Academy of Sciences. 2013;110(35):14420–14425. doi: 10.1073/pnas.1308085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang Y, Tian T, Zhang J, Jin X, Yue H, Zhang XH, Du L, Bai F. Indole reverses intrinsic antibiotic resistance by activating a novel dual-function importer. mBio. 2019;10(3):e00676. doi: 10.1128/mBio.00676-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017).WHO Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- Wright (2016).Wright GD. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends in Microbiology. 2016;24(11):928. doi: 10.1016/j.tim.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2022).Wu T, Wilhelm MJ, Li Y, Ma J, Dai HL. Indole facilitates antimicrobial uptake in bacteria. ACS Infectious Diseases. 2022;8(6):1124–1133. doi: 10.1021/acsinfecdis.1c00618. [DOI] [PubMed] [Google Scholar]
- Zhang, Yang & Defoirdt (2022).Zhang S, Yang Q, Defoirdt T. Indole decreases the virulence of pathogenic vibrios belonging to the Harveyi clade. Journal of Applied Microbiology. 2022;132(1):167–176. doi: 10.1111/jam.15227. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2020).Zhao Y, Lv B, Sun F, Liu J, Wang Y, Gao Y, Qi F, Chang Z, Fu X. Rapid freezing enables aminoglycosides to eradicate bacterial persisters via enhancing mechanosensitive channel MscL-mediated antibiotic uptake. mBio. 2020;11(1):2365. doi: 10.1128/mBio.03239-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Survival of S. aureus cells after nutrient shift followed by a 2-h treatment with indicated antibiotics (100 μg/ml ampicillin, 5 μg/ml ofloxacin, and 50 μg/ml tobramycin). (B) Survival of S. aureus cells after starvation followed by 50 μg/ml tobramycin treatment for varying length of time (0.5, 1, or 2 h).
(A, B, C, D, E, F) Survival of stationary-phase S. aureus cells following a 3-h treatment with 500 μg/ml tobramycin (panel A), 2,000 μg/ml streptomycin (panel B), 500 μg/ml gentamicin (panel C), 1,000 μg/ml kanamycin (panel D), 100 μg/ml ofloxacin (panel E) or 100 μg/ml ciproxacin (panel F) in the absence or presence of 4 mM indole, 2-Methylindole or 5-Methylindole.
(A) Survival of exponential-phase S. aureus cells following a 3-h treatment with 1 mM indole, 2-Methylindole or 5-Methylindole. (B) Survival of exponential-phase S. aureus cells following treatments with 25 μg/ml tobramycin plus 1 mM indole, 2-Methylindole or 5-Methylindole for 1, 2 or 3 h. (C) Survival of exponential-phase S. aureus cells following treatments with 100 μg/ml streptomycin, 25 μg/ml gentamicin or 50 μg/ml kanamycin plus 1 mM indole, 2-Methylindole or 5-Methylindole for 1 or 2 h.
Upper part: Photos for mouse blood cells dissolved in 0.9% NaCl containing increasing concentrations of DMSO (0, 1, 2, 3 and 4 mM). Cells dissolved in pure water were used as a positive control, i.e., red blood cells were largely lysed in such hypoosmotic solution. Lower part: Photos for mouse blood cells dissolved in 0.9% NaCl solution containing increasing concentration of indole, 2-methylindole or 5-methylindole (0, 1, 2, 3 and 4 mM).
(A) Survival of stationary-phase S. aureus cells following a 3-h treatment with 250 μg/ml tobramycin plus 5-methylindole or 5-iodoindole (0, 1, 2, 3 and 4 mM). (B) Survival of stationary-phase S. aureus cells following a 3-h treatment with 5-methylindole or 5-iodoindole at increasing concentrations (0, 1, 2, 3, 4, 5, 6, 7 and 8 mM).
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.