Abstract
Background
The AHNAK2 gene encodes a large nucleoprotein expressed in several tissues, including brain, squamous epithelia, smooth muscle, and neuropil. Its role in calcium signaling has been suggested and to date, clear evidence about its involvement in the pathogenesis of clinical disorders is still lacking.
Methods
Here, we report a female 24‐year‐old patient diagnosed with a cardio‐facio‐cutaneous‐like phenotype (CFC‐like), characterized by epilepsy, psychomotor development delay, atopic dermatitis, congenital heart disease, hypotonia, and facial dysmorphism, who is compound heterozygote for two missense mutations in the AHNAK2 gene detected by exome sequencing.
Results
This patient had no detectable variant in any of the genes known to be associated with the cardio‐facio‐cutaneous syndrome. Moreover, the mode of inheritance does not appear to be autosomal dominant, as it is in typical CFC syndrome. We have performed in silico assessment of mutation severity separately for each missense mutation, but this analysis excludes a severe effect on protein function. Protein structure predictions indicate the mutations are located in flexible regions possibly involved in molecular interactions.
Conclusion
We discuss an alternative interpretation on the potential involvement of the two missense mutations in the AHNAK2 gene on the expression of CFC‐like phenotype in this patient based on inter‐allelic complementation.
Keywords: AHNAK2, borderline intellectual functioning, epilepsy, facio‐cardio‐cutaneous‐like phenotype, NGS exome
The AHNAK2 gene encodes a large nucleoprotein expressed in several tissues, including brain, squamous epithelia, smooth muscle and neuropil. Here, we report a female patient diagnosed with a cardio‐facio‐cutaneous‐like phenotype (CFC‐like), characterized by epilepsy, psychomotor development delay, atopic dermatitis, congenital heart disease, hypotonia, and facial dysmorphism, who is compound heterozygote for two mis‐sense mutations in the AHNAK2 gene detected by exome sequencing.

1. INTRODUCTION
AHNAKs (AHNAK1 NM_001620.3 and AHNAK2 NM_138420.4) are a class of giant propeller‐like proteins associated with L‐type calcium channel activity (Komuro et al., 2004; Matza et al., 2008; Pankonien et al., 2011). The AHNAK nucleoprotein 2 (AHNAK2) gene encodes an intracellular nucleoprotein, which forms PDZ domain‐mediated homodimers in a similar manner to asperiaxin (PRX NM_181882.3) (Han & Kursula, 2014). PRX, a homolog of AHNAK proteins, is involved in myelination of the peripheral nervous system (Gillespie et al., 2000; Maddala et al., 2011; Takashima et al., 2002), and mutations in the PRX gene are associated with human hereditary neuropathies, such as AR‐Charcot‐Marie‐Tooth (CMT) and Dejerine–Sottas diseases. AHNAK1 gene in cardiomyocytes is associated with Ca2+ channels at the plasma membrane (Haase et al., 2005; Komuro et al., 2004). AHNAK2 has recently been identified as a target for CMT mutations (Tey et al., 2019) and in a patient reporting a frameshift AHNAK2 mutation (PMID: 28600779), related to dysmorphic features and skeletal deformities (Monies et al., 2017).
Co‐localization of AHNAK1 and AHNAK2 demonstrates that both proteins are components of the costameric network (Marg et al., 2010). AHNAK2 protein is also expressed in squamous epithelia, respiratory epithelia, smooth muscle, and neuropil (https://www.proteinatlas.org/ENSG00000185567‐AHNAK2/tissue). Calcium ions and their concentration gradient in the epidermis are essential in regulating many skin functions, including keratinocyte differentiation, skin barrier formation, and permeability barrier homeostasis (Lee & Lee, 2018; Marg et al., 2010). AHNAK2 gene (NM_138420.3) localized on chromosome 14 contains at least 7 exons, overall coding for 5795 amino acids. The first 6 exons are relatively small, while exon 7 is nearly18 kb (Komuro et al., 2004).
Cardio‐facio‐cutaneous syndrome is characterized by global developmental delay, hypotonia, characteristic craniofacial dysmorphology, dermatologic abnormalities, intellectual disability, congenital heart disease, especially pulmonary valve stenosis, interatrial septal defects, and hypertrophic cardiomyopathy.
It is a very rare syndrome and only 300 cases are reported in the literature but the real incidence is still unidentified probably because mildly affected individuals may go undiagnosed. All known cases are due to autosomal dominant mutations in four different genes: BRAF (7q34) is present in about 75% of cases, MEK1 (15q22.1‐q22.33) and MEK2 (19p13.3) are both present in about 25% of the cases, and KRAS (12p12.1) is present in less than 2% of the cases, all these genes are implicated in KRAS signaling pathways (Jurcă et al., 2021).
The dysregulation of KRAS signaling pathways strongly influences embryonic development, organogenesis, and synaptic plasticity (Pierpont et al., 2014).
In this study, we report a patient with the cardio‐facio‐cutaneous‐like phenotype (CFC‐like), borderline intellectual functioning, and epilepsy, showing on whole‐exome sequencing (WES) compound heterozygosity for two missense variants in the AHNAK2 gene.
2. MATERIALS AND METHODS
2.1. Editorial policies and ethical considerations
This study was approved by the ethics committee of the Oasi Research Institute‐IRCCS, Troina, Italy (Protocol n. CE/149, May 4, 2020). Written informed consent was obtained from the patient's parents. All investigations were compliant with the principles expressed in the Declaration of Helsinki.
2.2. Genetic detection
Genomic DNA was isolated from peripheral blood leukocytes of a female patient and her parents. Exome analysis was performed using the Ion AmpliSeq™ Exome RDY Kits following the manufacturer's instructions (Thermo Fisher Scientific). The quality of post‐amplification libraries was assessed using DNA 1000 chips on the Tape Station 4200 (Agilent) and Qubit fluorimetric quantitation using Qubit dsDNA BR Assay Kits (Invitrogen). We used pooled libraries to emulsion PCR on the Ion Chef Instrument according to the manufacturer's protocol (Thermo Fisher Scientific). Finally, we sequenced each loaded Ion 550™ chip on the S5plus System (Thermo Fisher Scientific) with the use of recommended reagents. 97% and 95% of regions of interests (ROI) have a minimum coverage of at least 20X and 30X, respectively. Single‐nucleotide variants (SNVs) and short insertions/deletions (INDELs) were identified by a mapping alignment program from Thermo Fisher with germ‐line and low stringency settings. We confirmed pathogenic variants using a conventional Sanger sequencing (Applied Biosystems Prism 3130 DNA Analyzer). Reads were aligned to GrCh37 ‐ h19 human reference sequence using the Torrent Browser 5.10 software (Thermo Fisher Scientific). The resulting alignment BAMs were further processed using Ion Reporter 5.10.5 pipeline (Thermo Fisher Scientific), which incorporated variant calling. The existence of potentially significant variants was reassessed through IGV 2.5 software. The predictions “in silico analyses” (SIFT, Polyphen) were retrieved from the Ion Reporter result files (Thermo Fisher Scientific). We filtered the identified variants according to recessive/de novo/X‐liked pattern of inheritance, gene features, and MAF < 1% using as references dbSNP154, 1000 Genomes, ESP6500, ExAC, gnomAD. Subsequently, variants were evaluated for their phenotypic and biological impact (Vetri et al., 2020).
Predictions of AHNAK2 protein structure and flexibility were carried out using ANCHOR and IUPred2 (Erdős & Dosztányi, 2020; Mészáros et al., 2009). For the region encompassing amino acids 5300–5600, 3D structure prediction was done using AlphaFold2 (Jumper et al., 2021). In addition, secondary structures were predicted using JPred4 (Drozdetskiy et al., 2015).
3. RESULTS
3.1. Patient report
Here, we describe a 24‐year‐old girl. The pregnancy and delivery were uncomplicated, and her APGAR scores were 9 and 10 at 1 and 5 min, respectively. Her birth weight was 3500 g (Z‐score 0.203), length 52 cm (Z‐score 1.072), and head circumference 35 cm (Z‐score 0.834). Psychomotor development was mildly delayed. At 1 year of life, some characteristics of the CFC‐like phenotype were identified, such as atopic dermatitis, patent ductus arteriosus, thin hair, downslanting palpebral fissures, hypertelorism, squint, depressed nasal bridge, and posteriorly rotated ears.
The patient was admitted to our institution for the first time at the age of 40 months with the diagnostic suspicion of psychomotor retardation.
During this hospitalization, the patient showed inflammatory keratotic lesions on the face and on the upper limbs, thin and sparse hair, hypertelorism, epicanthus, ptosis, small nose with low nasal root, posteriorly rotated auricles with bilateral preauricular fistula, long filter, prognathism, broad neck, pectus escavatum, bilateral clinodactyly of the 5th toe, bilateral foot planovalgus deformity, compound hypermetropic astigmatism, and exotropia of right eye (Figure 1).
FIGURE 1.

Patient frontal view and profile displaying facial features at 24 years old.
Regarding cognitive functioning, the patient showed slow processing speed and attentional dysfunction. The Griffiths Mental Developmental scales were administered and scored according to standardized procedures and they provided a developmental age of 30‐month skills.
The echocardiographic examination showed the presence of a patent foramen ovale with a mild left‐to‐right shunt.
In the light of the clinical picture and associated instrumental results, a clinical diagnosis of cardio‐facio‐cutaneous syndrome with borderline intellectual functioning was made.
The patient weight‐for‐height development was normal but she had hypotonia, ligamental hyperlaxity, and bilateral valgus flat foot. From 21 months to 4 years of age, she had three febrile generalized tonic–clonic seizures, and from 16 years of age, she started having afebrile seizures characterized by impaired awareness, head, and eye deviation toward the right side and occasionally associated with bilateral tonic–clonic seizures. Interictal EEG pattern was characterized by numerous sequences of spike‐and‐wave complexes over the frontal‐central‐temporal regions of the left hemisphere. Treatment with levetiracetam was started at 16 years of age, and focal seizures became sporadic, occurring less than once per month. The parents refused to give other antiepileptic drugs to their daughter. Brain MRI was normal at the same age.
3.2. Whole‐exome sequencing and bioinformatic analyses
Whole‐exome sequencing (WES) performed on DNA from the patient (#03982) and her parents revealed that she is a compound heterozygote for c.16379T>C (p.Ile5460Thr) and c.1198G>A (p.Gly400Ser) variants of the AHNAK2 gene (Figure 2) inherited from the mother and the father, respectively [ClinVar Submission ID: SUB9751031‐SUB9791538]. In addition, we have identified the following variants in CFC‐associated genes: A2Ml1(NM_144670.5): c.4076G>Ap.Arg1359His; GPT(NM_005309.2): c.320G>A p.Arg107Lys; TTN (NM_001256850.1) c.11629A>Tp.Ile3877Phe. These variants are inherited from one of the healthy parents, and in silico analysis performed with bioinformatic tools (Kopanos et al., 2019) predicted that these mutations have no potential damaging effect (Benign). Previously, this patient was negative for mutations in a panel of 14 genes (A2Ml1, BRAF, CBL, HRAS, KRAS, MAP2K1, MAP2K2, NRAS, PTPN11, RAF1, RIT1, SHOC2, SOS1, SPRED1) known to be associated with CFCS. Neither further causative mutation (WES) nor large deletion (CGH‐Array) have been identified in ACTB, ACTG1, CCNK, CDC42, EPHB4, FGD1, KAT6B, LZTR1, MAP3K8, MRAS, NF1, PPP1CB, RASA1, RASA2, RRAS, SASH1, SOS2 genes, previously known as causative to Neuro‐Cardio‐Facio‐Cutaneous (NCFC) syndromes, confirming the WES results.
FIGURE 2.
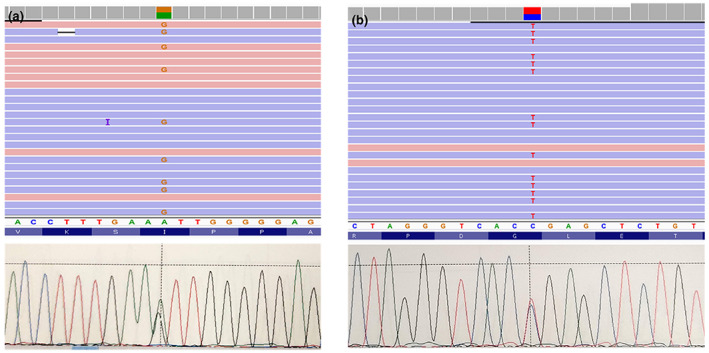
Next‐generation sequencing with the integrative genomics viewer (top) and sanger (down) visualization of the heterozygous missense variants c.16379T>C (p.Ile5460Thr) (a) and c.1198G>A (p.Gly400Ser) (b) in the AHNAK2 gene.
The p.Ile5460Thr variation has not been referenced as a single nucleotide polymorphism (SNP) yet and is not found in the HGMD Professional Database (www.hgmd.cf.ac.uk). On the other hand, the p.Gly400Ser variation has been referenced as an SNP (rs140752229) and is found in GnomAD (with a frequency of 0.001718), ExAC (0.001741), and 1000G (0.0012), not being found in the HGMD Professional Database. Neither variant has been reported in the ClinVar database. The residues at these positions are highly conserved across species, and in silico analysis performed with bioinformatic tools predicted that the two mutations have a potential strong damaging effect on the structure/function of the AHNAK2 protein.c.16379T>C (p.Ile5460Thr): SIFT = 0.0 (Deleterious), PolyPhen = 0.996 (Probably damaging); c.1198G>A (p.Gly400Ser): PolyPhen = 0.975 (Probably damaging). However, standard procedures for assessment of pathogenicity of variants (ACMG criteria‐Varsome) (Kopanos et al., 2019), the variant c.1196G>A (p.Gly400Ser) should be judged as “likely benign.” The variant c.16379T>C (p.Ile5460Thr) should be also classified as “likely benign” or “VUS.”
3.3. In silico protein structure analysis
To further understand the possible effects of the variants at the protein level, we carried out predictions of protein structure and flexibility (Figure 3). Gly400 is in the N‐terminal region of AHNAK2, between the PDZ‐like domain and the repetitive region, in a segment predicted to be disordered and flexible. Ile5460, on the other hand, lies in the C‐terminal region after the repeat domains, and the region comprising residues approximately 5300–5600 is predicted to possibly fold upon intermolecular interactions. AlphaFold2 does not predict a folded globular structure, but short predicted segments of β strand could represent protein–protein interaction sites.
FIGURE 3.

Predictions of AHNAK2 protein structure and flexibility. (a) A schematic view of AHNAK2 with known or predicted interactions. The PDZ‐like domain is involved in homodimerization, and the C‐terminal domain in AHNAK1 binds to the p11/annexin A2 complex – it is not known if this interaction occurs with AHNAK2. The N‐ and C‐terminal segments of AHNAK1 interact with the ⍺1 and β subunits of the VGCC, respectively (Jin et al., 2020; Sundararaj et al., 2021). Some of these interactions may differ between AHNAK1 and AHNAK2. (b) Protein flexibility prediction using IUPred (black) and ANCHOR (red). A score close to 1 reflects a disordered structure not likely to fold upon ligand binding. I5460T is located in a segment predicted to possibly fold upon protein interactions. (c) AlphaFold2 prediction for the segment corresponding to residues 5300–5600 indicates a lack of globular fold. Ile5460 is shown as spheres.
4. DISCUSSION
Although our patient presents a CFC‐like phenotype, all the genes causing CFC syndrome screened by WES resulted in negative. Moreover, if we assume a causal link between the AHNAK2 gene and CFC in the patient phenotype in the patient, the detected heterozygous missense variants should be in opposition with the autosomal dominant inheritance typical of CFC syndrome.
We performed an extensive bibliomic search to look for evidence confirming the involvement of the AHNAK2 function in the expression of several endophenotypes (psychomotor development delay, epilepsy, atopic dermatitis, congenital heart disease, hypotonia) observed in the patient. This search revealed that both AHNAK1 and AHNAK2 are co‐expressed and associated with L‐type voltage‐regulated calcium channels (Gentil et al., 2001; Komuro et al., 2004) in the tissues related to the above endophenotype. The implication of calcium channels in epilepsy is well documented (Rajakulendran & Hanna, 2016; Zamponi et al., 2010), and recently they represent a promising therapeutic target. Anticonvulsant AMPA glutamate antagonists are already used to treat seizures and they work by preventing the entry of calcium and sodium into the brain cell reducing or abolishing epileptiform activity (Hanada, 2020).
Moreover, calcium signaling has been implicated in modulating various aspects of cellular functions in the skin: epidermal homeostasis, keratinocyte differentiation, skin barrier formation, and permeability barrier homeostasis, being consistent with atopic dermatitis seen in the patient (Lee & Lee, 2018; Rzepnikowska et al., 2020). A dysfunction of AHNAK proteins has been shown to be critical for cardiac calcium channel function and its beta‐adrenergic regulation (Haase et al., 2005; Monies et al., 2017), suggesting that a similar mechanism could be present in our patient, who has congenital cardiomyopathy. AHNAK2 is a nucleoprotein forms homodimers similarly to periaxin, in turn, involved in myelination of the peripheral nervous system (Gillespie et al., 2000; Maddala et al., 2011), and many studies have shown the significance of myelination in relation to psychomotor development (Kato et al., 2020; Van der Knaap et al., 1991). Impaired regulation of myelination is frequently associated with learning deficits and cognitive dysfunction (Bennett & Madden, 2014; Makinodan et al., 2012; McKenzie et al., 2014; Xiao et al., 2016). Altogether, these data are consistent with the delay of psychomotor development and borderline intellectual functioning of the patient. We did not observe a delayed myelination in our patient, but this could be explained by the timing of brain MRI, at 16 years old, when this feature is usually no more evident. Mutations in both AHNAK2 and PRX genes have been described as causing Charcot–Marie–Tooth disease (Han & Kursula, 2014; Precenzano et al., 2020; Tey et al., 2019). Altogether, the literature strongly suggests pleiotropic effects caused by mutations in the AHNAK2 gene. In other words, a single genetic locus harboring variants that are associated with multiple, sometimes distinct, traits. For instance, pleiotropy has been challenged by the remarkably diverse neurodegenerative syndromes (Pang et al., 2017).
Our in silico assessment of mutation severity performed separately for each missense mutation did not uncover severe predicted effects on protein function. On the other hand, protein structure predictions indicate that both variants are located in regions with high flexibility but the possibility for protein–protein interactions. However, we need to point out here that such bioinformatic tools do not allow to simultaneously assess the combined effect of the two mutations, nor are they “quantitative” tests providing a measure of the extent of protein dysfunction. Thus, the ideal situation, in which these tools are useful, is when we are dealing with patients homozygous for the same mutation. In contrast, because our patient is a compound heterozygote, it is possible that a dysfunctioning AHNAK2 protein may arise, when the two mutant polypeptides interact, even if each mutation does not score “severe” by the specific algorithm used. The described phenomenon is known as inter‐allelic complementation (IC), and it has been described to occur in several cases (Elsas et al., 1995; Ribeiro et al., 2020; Wang et al., 2011). Formally, IC refers to the change in the properties of a multimeric protein as a consequence of the interaction of subunits coded by two different mutant alleles (in contrast to the protein consisting of subunits derived from a single mutant allele). The mixed protein (heteromultimer) may exhibit more activity (positive complementation) or less activity (negative complementation, q.v.) than the homomultimer (cited from Oxford Reference: https://www.oxfordreference.com/view/10.1093/doi/authority.20110810105137382).
This form of IC is also known as intragenic complementation and has been described in various species from plants to mammals and humans. Hereafter, we provide few examples. Ribeiro et al. (2020) suggested potential IC, in heterozygous Glutaric Aciduria Type I (GA‐I) patients, based on the fact that in the GCDH enzyme, the low active p.Arg227Pro variant contributes to stabilize the tetramer while the structurally unstable p.Val400Met variant compensates for enzyme activity. Wang et al. (2011) proposed that in patients with Retinitis Pigmentosa (RP10), due to defects in inosine monophosphate dehydrogenase 1 (IMPDH1), hybrid tetramers of IMPDH1, mutant subunits, impose their faulty conformation on normal partners. The interallelic complementation at the Mitf locus in mice was characterized in detail by Konyukhov and Osipov (1968) who crossed the MitfMi‐Wh mutation to the Mitfmi mutation, which exhibits severe microphthalmia in homozygous condition. Surprisingly, the resulting compound heterozygote (Mitfmi/MitfMi‐Wh) had normal eye size; pigment was still lacking from eyes and coat. Thus, the combination of the two alleles resulted in normal eye development, which is in stark contrast to the microphthalmia seen when each mutation is in homozygous condition (Steingrímsson, 2010; Steingrímsson et al., 2003).
Obviously, this speculative model needs experimental confirmation. For example, one could develop an in vitro system (cells expressing the two mutant polypeptides) to test the ability of mutant AHNAK2 polypeptides to perform a specific function, such as interaction with AHNAK1 or calcium channels (see Introduction).
To explore further the potential involvement of AHNAK2 in CFC, we have looked at its protein partners as well as its participation in signaling pathways. By querying the STRING database, we have found that ANHAK2 interacts directly with 10 proteins (see Figure 4, Table S1). However, although some of these proteins play a role in cellular functions affected in CFC (i.e., ANXA2 and MYOF for calcium metabolism; MYOF and S100A10 for neuronal differentiation), none of these 10 proteins have been so far implicated in CFC syndrome. On the other hand, several studies have implicated AHNAK2 in various signaling pathways, although these findings concern exclusively tumor cells. Interestingly, one of such studies (Wang et al., 2020) performed in lung adenocarcinoma cells it was shown that a reduction of AHNAK2 could hinder cell proliferation, migration, invasion, and apoptosis via inactivating the RasMAPK signaling pathway. In particular, Wang et al., in their study, in order to verify, in an empirical way, whether AHNAK2 affects the MAPK pathway measured the key markers of the MAPK pathway (MEK/ERK/P90RSK) after si‐AHNAK2 transfection. The authors found that the down‐regulation of AHNAK2 induced a decreased level of p‐MEK, p‐ERK, and p‐P90RSK, and concluded that AHNAK2 plays a role in activating the MAPK signaling pathway. This finding is especially important for our present study in light of the established association of CFC with dysfunction of the Ras pathway (for this reason CFC is classified as a “Rasopathy”) (Rauen, 2013).
FIGURE 4.

Ten potential direct interactors of ANKAK2 detected by the STRING database (https://string‐db.org/).
5. CONCLUSIONS
Although the literature is suggestive of the involvement of the AHNAK2 protein in the expression of multiple phenotypes displayed by the patient it remains unclear what are the detailed cellular and molecular mechanisms affected by the lack of function of this protein.
Indeed, database searches, such as KEGG, Gene Ontology, or STRING, did not reveal detailed information on biological processes or pathways, which may account for the pleiotropic effects of the two missense variants. On the other hand, AHNAK2 proteins expressed in developing brain, squamous epithelia, as well as in cardiomyocytes. In this context, and in the absence of in vitro (inter‐allelic complementation) or in vivo functional studies, the pathogenic significance of mutations in the AHNAK2 gene remains uncertain, and at the present time, the contribution of this gene to the pathogenesis of the cardio‐facio‐cutaneous‐like syndrome can only be proposed on a provisional basis, waiting for further evidence. Finally, more patients need to be analyzed by exome sequencing in order to expand the number of cases with mutated AHNAK2 protein.
AUTHOR CONTRIBUTIONS
Conceptualization, F.C.; M.V.; M.E.; Methodology, F.C.; M.V.; V.C.; S.D.; P.K.; Data curation, F.C.; M.V.; Writing‐original draft preparation, F.C.; V.R.; M.V.; M.E.; Writing‐review and editing, F.C.; M.V.; V.R.; M.E.; C.S.; D.G.; L.V.; P.K.; supervision, M.E.; F.C.; M.C.; M.R.; All authors have read and agreed to the published version of the manuscript.
FUNDING
This work was partially supported by the Italian Ministry of Health ‐ Ricerca Corrente ‐ and ‘5 per mille’ funding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of the Oasi Research Institute‐IRCCS, Troina, Italy (Protocol code CE/149; May 04, 2020).
INFORMED CONSENT STATEMENT
Written informed consent has been obtained from the patient to publish this paper.
Supporting information
Table S1
ACKNOWLEDGMENTS
We would like to thank Alda Ragalmuto, Angelo Gloria, Antonino Musumeci, and Rosanna Galati for their technical contribution.
Vinci, M. , Kursula, P. , Greco, D. , Elia, M. , Vetri, L. , Schepis, C. , Chiavetta, V. , Donadio, S. , Roccella, M. , Carotenuto, M. , Romano, V. , & Calì, F. (2022). Exome sequencing in a child with neurodevelopmental disorder and epilepsy: Variant analysis of the AHNAK2 gene. Molecular Genetics & Genomic Medicine, 10, e2012. 10.1002/mgg3.2012
Mirella Vinci and Petri Kursula contributed equally.
Funding informationThis work was partially supported by the Italian Ministry of Health – Ricerca Corrente ‐ and ‘5 per mille’ funding
Contributor Information
Luigi Vetri, Email: lvetri@oasi.en.it.
Francesco Calì, Email: fcali@oasi.en.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Bennett, I. J. , & Madden, D. J. (2014). Disconnected aging: Cerebral white matter integrity and age‐related differences in cognition. Neuroscience, 276, 187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdetskiy, A. , Cole, C. , Procter, J. , & Barton, G. J. (2015). JPred4: A protein secondary structure prediction server. Nucleic Acids Research, 43(W1), W389–W394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsas, L. J. , Langley, S. , Steele, E. , Evinger, J. , Fridovich‐Keil, J. L. , Brown, A. , Singh, R. , Fernhoff, P. , Hjelm, L. N. , & Dembure, P. P. (1995). Galactosemia: A strategy to identify new biochemical phenotypes and molecular genotypes. American Journal of Human Genetics, 56(3), 630–639. [PMC free article] [PubMed] [Google Scholar]
- Erdős, G. , & Dosztányi, Z. (2020). Analyzing protein disorder with IUPred2A. Current Protocols in Bioinformatics, 70(1), e99. [DOI] [PubMed] [Google Scholar]
- Gentil, B. J. , Delphin, C. , Mbele, G. O. , Deloulme, J. C. , Ferro, M. , Garin, J. , & Baudier, J. (2001). The giant protein AHNAK is a specific target for the calcium‐and zinc‐binding S100B protein: Potential implications for Ca2+ homeostasis regulation by S100B. Journal of Biological Chemistry, 276(26), 23253–23261. [DOI] [PubMed] [Google Scholar]
- Gillespie, C. S. , Sherman, D. L. , Fleetwood‐Walker, S. M. , Cottrell, D. F. , Tait, S. , Garry, E. M. , Wallace, V. C. , Ure, J. , Griffiths, I. R. , Smith, A. , & Brophy, P. J. (2000). Peripheral demyelination and neuropathic pain behavior in periaxin‐deficient mice. Neuron, 26(2), 523–531. [DOI] [PubMed] [Google Scholar]
- Haase, H. , Alvarez, J. , Petzhold, D. , Doller, A. , Behlke, J. , Erdmann, J. , Hetzer, R. , Regitz‐Zagrosek, V. , Vassort, G. , & Morano, I. (2005). Ahnak is critical for cardiac ca (v) 1.2 calcium channel function and its β‐adrenergic regulation. The FASEB Journal, 19(14), 1969–1977. [DOI] [PubMed] [Google Scholar]
- Han, H. , & Kursula, P. (2014). Periaxin and AHNAK nucleoprotein 2 form intertwined homodimers through domain swapping. Journal of Biological Chemistry, 289(20), 14121–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada, T. (2020). Ionotropic glutamate receptors in epilepsy: A review focusing on AMPA and NMDA receptors. Biomolecules, 10(3), 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J. , Bhatti, D. L. , Lee, K.‐W. , Medrihan, L. , Cheng, J. , Wei, J. , Zhong, P. , Yan, Z. , Kooiker, C. , Song, C. , Ahn, J. H. , Obermair, G. J. , Lee, A. , Gresack, J. , Greengard, P. , & Kim, Y. (2020). Ahnak scaffolds p11/Anxa2 complex and L‐type voltage‐gated calcium channel and modulates depressive behavior. Molecular Psychiatry, 25(5), 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper, J. , Evans, R. , Pritzel, A. , Green, T. , Figurnov, M. , Ronneberger, O. , Tunyasuvunakool, K. , Bates, R. , Žídek, A. , Potapenko, A. , Bridgland, A. , Meyer, C. , Kohl, S. A. A. , Ballard, A. J. , Cowie, A. , Romera‐Paredes, B. , Nikolov, S. , Jain, R. , Adler, J. , … Hassabis, D. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, 596(7873), 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcă, M. C. , Iuhas, O. A. , Puiu, M. , Chiriţă‐Emandi, A. , Andreescu, N. I. , Petcheşi, C. D. , Jurcă, A. D. , Magyar, I. , Jurcă, S. I. , Kozma, K. , Severin, E. M. , & Bembea, M. (2021). Cardiofaciocutaneous syndrome–a longitudinal study of a case over 33 years: Case report and review of the literature. Romanian Journal of Morphology and Embryology = Revue Roumaine de Morphologie et Embryologie, 62(2), 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, D. , Wake, H. , Lee, P. R. , Tachibana, Y. , Ono, R. , Sugio, S. , Tsuji, Y. , Tanaka, Y. H. , Tanaka, Y. R. , Masamizu, Y. , Hira, R. , Moorhouse, A. J. , Tamamaki, N. , Ikenaka, K. , Matsukawa, N. , Fields, R. D. , Nabekura, J. , & Matsuzaki, M. (2020). Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia, 68(1), 193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro, A. , Masuda, Y. , Kobayashi, K. , Babbitt, R. , Gunel, M. , Flavell, R. A. , & Marchesi, V. T. (2004). The AHNAKs are a class of giant propeller‐like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proceedings of the National Academy of Sciences, 101(12), 4053–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konyukhov, B. V. , & Osipov, V. V. (1968). Interallelic complementation of microphthalmia and white genes in mice. Genetika, 4, 65–76. [Google Scholar]
- Kopanos, C. , Tsiolkas, V. , Kouris, A. , Chapple, C. E. , Aguilera, M. A. , Meyer, R. , & Massouras, A. (2019). VarSome: The human genomic variant search engine. Bioinformatics, 35(11), 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. E. , & Lee, S. H. (2018). Skin barrier and calcium. Annals of Dermatology, 30(3), 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala, R. , Skiba, N. P. , Lalane Iii, R. , Sherman, D. L. , Brophy, P. J. , & Rao, P. V. (2011). Periaxin is required for hexagonal geometry and membrane organization of mature lens fibers. Developmental Biology, 357(1), 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan, M. , Rosen, K. M. , Ito, S. , & Corfas, G. (2012). A critical period for social experience–dependent oligodendrocyte maturation and myelination. Science, 337(6100), 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marg, A. , Haase, H. , Neumann, T. , Kouno, M. , & Morano, I. (2010). AHNAK1 and AHNAK2 are costameric proteins: AHNAK1 affects transverse skeletal muscle fiber stiffness. Biochemical and Biophysical Research Communications, 401(1), 143–148. [DOI] [PubMed] [Google Scholar]
- Matza, D. , Badou, A. , Kobayashi, K. S. , Goldsmith‐Pestana, K. , Masuda, Y. , Komuro, A. , McMahon‐Pratt, D. , Marchesi, V. T. , & Flavell, R. A. (2008). A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity, 28(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, I. A. , Ohayon, D. , Li, H. , De Faria, J. P. , Emery, B. , Tohyama, K. , & Richardson, W. D. (2014). Motor skill learning requires active central myelination. Science, 346(6207), 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros, B. , Simon, I. , & Dosztányi, Z. (2009). Prediction of protein binding regions in disordered proteins. PLoS Computational Biology, 5(5), e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monies, D. , Abouelhoda, M. , AlSayed, M. , Alhassnan, Z. , Alotaibi, M. , Kayyali, H. , al‐Owain, M. , Shah, A. , Rahbeeni, Z. , al‐Muhaizea, M. A. , Alzaidan, H. I. , Cupler, E. , Bohlega, S. , Faqeih, E. , Faden, M. , Alyounes, B. , Jaroudi, D. , Goljan, E. , Elbardisy, H. , … Alkuraya, F. S. (2017). The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Human Genetics, 136(8), 921–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, S. Y.‐Y. , Teo, K.‐C. , Hsu, J. S. , Chang, R. S.‐K. , Li, M. , Sham, P.‐C. , & Ho, S.‐L. (2017). The role of gene variants in the pathogenesis of neurodegenerative disorders as revealed by next generation sequencing studies: A review. Translational Neurodegeneration, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankonien, I. , Alvarez, J. L. , Doller, A. , Köhncke, C. , Rotte, D. , Regitz‐Zagrosek, V. , Morano, I. , & Haase, H. (2011). Ahnak1 is a tuneable modulator of cardiac ca (v) 1.2 calcium channel activity. Journal of Muscle Research and Cell Motility, 32(4–5), 281–290. [DOI] [PubMed] [Google Scholar]
- Pierpont, M. E. M. , Magoulas, P. L. , Adi, S. , Kavamura, M. I. , Neri, G. , Noonan, J. , Pierpont, E. I. , Reinker, K. , Roberts, A. E. , Shankar, S. , Sullivan, J. , Wolford, M. , Conger, B. , Santa Cruz, M. , & Rauen, K. A. (2014). Cardio‐facio‐cutaneous syndrome: Clinical features, diagnosis, and management guidelines. Pediatrics, 134(4), e1149–e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precenzano, F. , Parisi, L. , Lanzara, V. , Vetri, L. , Operto, F. F. , Pastorino, G. M. G. , Ruberto, M. , Messina, G. , Risoleo, M. C. , & Santoro, C. (2020). Electroencephalographic abnormalities in autism spectrum disorder: Characteristics and therapeutic implications. Medicina, 56(9), 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran, S. , & Hanna, M. G. (2016). The role of calcium channels in epilepsy. Cold Spring Harbor Perspectives in Medicine, 6(1), a022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen, K. A. (2013). The rasopathies. Annual Review of Genomics and Human Genetics, 14, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, J. V. , Lucas, T. G. , Bross, P. , Gomes, C. M. , & Henriques, B. J. (2020). Potential complementation effects of two disease‐associated mutations in tetrameric glutaryl‐CoA dehydrogenase is due to inter subunit stability‐activity counterbalance. Biochimica et Biophysica Acta (BBA)‐Proteins and Proteomics, 1868(1), 140269. [DOI] [PubMed] [Google Scholar]
- Rzepnikowska, W. , Kaminska, J. , Kabzińska, D. , Binięda, K. , & Kochański, A. (2020). A yeast‐based model for hereditary motor and sensory neuropathies: A simple system for complex, heterogeneous diseases. International Journal of Molecular Sciences, 21(12), 4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson, E. (2010). Interpretation of complex phenotypes: Lessons from the Mitf gene. Pigment Cell & Melanoma Research, 23(6), 736–740. [DOI] [PubMed] [Google Scholar]
- Steingrímsson, E. , Arnheiter, H. , Hallsson, J. H. , Lamoreux, M. L. , Copeland, N. G. , & Jenkins, N. A. (2003). Interallelic complementation at the mouse Mitf locus. Genetics, 163(1), 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararaj, S. , Ravindran, A. , & Casarotto, M. G. (2021). AHNAK: The quiet giant in calcium homeostasis. Cell Calcium, 96, 102403. [DOI] [PubMed] [Google Scholar]
- Takashima, H. , Boerkoel, C. F. , De Jonghe, P. , Ceuterick, C. , Martin, J. J. , Voit, T. , Schröder, J. M. , Williams, A. , Brophy, P. J. , Timmerman, V. , & Lupski, J. R. (2002). Periaxin mutations cause a broad spectrum of demyelinating neuropathies. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 51(6), 709–715. [DOI] [PubMed] [Google Scholar]
- Tey, S. , Shahrizaila, N. , Drew, A. P. , Samulong, S. , Goh, K.‐J. , Battaloglu, E. , Atkinson, D. , Parman, Y. , Jordanova, A. , Chung, K. W. , Choi, B. O. , Li, Y. C. , Auer‐Grumbach, M. , Nicholson, G. A. , Kennerson, M. L. , & Ahmad‐Annuar, A. (2019). Linkage analysis and whole exome sequencing reveals AHNAK2 as a novel genetic cause for autosomal recessive CMT in a Malaysian family. Neurogenetics, 20(3), 117–127. [DOI] [PubMed] [Google Scholar]
- Van der Knaap, M. S. , Valk, J. , Bakker, C. J. , Schooneveld, M. , Faber, J. A. J. , Willemse, J. , & Gooskens, R. (1991). Myelination as an expression of the functional maturity of the brain. Developmental Medicine & Child Neurology, 33(10), 849–857. [DOI] [PubMed] [Google Scholar]
- Vetri, L. , Calì, F. , Vinci, M. , Amato, C. , Roccella, M. , Granata, T. , Freri, E. , Solazzi, R. , Romano, V. , & Elia, M. (2020). A de novo heterozygous mutation in KCNC2 gene implicated in severe developmental and epileptic encephalopathy. European Journal of Medical Genetics, 63(4), 103848. [DOI] [PubMed] [Google Scholar]
- Wang, D.‐W. , Zheng, H.‐Z. , Cha, N. , Zhang, X.‐J. , Zheng, M. , Chen, M.‐M. , & Tian, L.‐X. (2020). Down‐regulation of AHNAK2 inhibits cell proliferation, migration and invasion through inactivating the MAPK pathway in lung adenocarcinoma. Technology in Cancer Research & Treatment, 19, 1533033820957006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.‐t. , Mion, B. , Aherne, A. , & Engel, P. C. (2011). Molecular recruitment as a basis for negative dominant inheritance? Propagation of misfolding in oligomers of IMPDH1, the mutated enzyme in the RP10 form of retinitis pigmentosa. Biochimica et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1812(11), 1472–1476. [DOI] [PubMed] [Google Scholar]
- Xiao, J. , Huang, Y. , Li, X. , Li, L. , Yang, T. , Huang, L. , Yang, L. , Jiang, H. , Li, H. , & Li, F. (2016). TNP‐ATP is beneficial for treatment of neonatal hypoxia‐induced hypomyelination and cognitive decline. Neuroscience Bulletin, 32(1), 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi, G. W. , Lory, P. , & Perez‐Reyes, E. (2010). Role of voltage‐gated calcium channels in epilepsy. Pflügers Archiv‐European Journal of Physiology, 460(2), 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


