Abstract
Many case reports have indicated that myocarditis could be a prognostic factor for predicting morbidity and mortality among patients with COVID-19. In this study, using a large database we examined the association between myocarditis among COVID-19 hospitalizations and in-hospital mortality and other adverse hospital outcomes. The present study was a retrospective analysis of data collected in the California State Inpatient Database during 2020. All hospitalizations for COVID-19 were included in the analysis and grouped into those with and without myocarditis. The outcomes were in-hospital mortality, cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome. Propensity score matching, followed by conditional logistic regression, was performed to find the association between myocarditis and outcomes. Among 164,417 COVID-19 hospitalizations, 578 (0.4%) were with myocarditis. After propensity score matching, the rate of in-hospital mortality was significantly higher among COVID-19 hospitalizations with myocarditis (30.0% vs 17.5%, p <0.001). Survival analysis with log-rank test showed that 30-day survival rates were significantly lower among those with myocarditis (39.5% vs 46.3%, p <0.001). Conditional logistic regression analysis showed that the odds of cardiac arrest (odds ratio [OR] 1.90, 95% confidence interval [CI] 1.16 to 3.14), cardiogenic shock (OR 4.13, 95% CI 2.14 to 7.99), mechanical ventilation (OR 3.30, 95% CI 2.47 to 4.41), and acute respiratory distress syndrome (OR 2.49, 95% CI 1.70 to 3.66) were significantly higher among those with myocarditis. Myocarditis was associated with greater rates of in-hospital mortality and adverse hospital outcomes among patients with COVID-19, and early suspicion is important for prompt diagnosis and management.
COVID-19, caused by SARS-CoV-2, has affected millions of people globally and continues to be a significant cause of morbidity and mortality.1 COVID-19 typical presents as interstitial pneumonia and can progress to life-threatening complications such as disseminated intravascular coagulation, diffuse inflammatory syndrome, and multiorgan failure.2 Although initially described in some case reports, it is now established that some patients develop cardiovascular complications, including myocarditis.3, 4, 5 Myocarditis in patients with COVID-19 is caused by both inflammatory response against cardiac myocytes and suppression of protein synthesis within these cells.6 Myocarditis among patients with COVID-19 is often underdiagnosed because of unavailability or restricted use of confirmatory tests such as cardiac magnetic resonance imaging or endomyocardial biopsy, especially during peaks of COVID-19 surges.7 Although many studies have stressed the importance of myocarditis as a prognostic factor for predicting morbidity and mortality among patients with COVID-19, there are few large-scale analyses.8, 9, 10 In this study we examined, using a large database, the association between myocarditis among COVID-19 hospitalizations and in-hospital mortality and other adverse hospital outcomes.
Methods
The present study was a retrospective analysis of the 2020 California State Inpatient Database (SID). Every year, SID collects discharge data from >90% of patients admitted to community hospitals.11 The SID contains inpatient discharge information from participating states; information is transformed into a uniform format for analyses. The SID includes both clinical and nonclinical information on all patients with various insurance coverage and on uninsured patients. The SID contains variables such as primary and secondary discharge diagnoses and procedures, admission and discharge status, demographic characteristics, payment source, hospitalization charges, and length of stay. Intermittent quality assurance procedures are performed to guarantee the validity of the database. We followed the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guideline to ensure the quality of this study.12
All patients ≥18 years of age who were hospitalized with COVID-19 during 2020 in California were included in the analysis. These hospitalizations were subsequently grouped into those with and without myocarditis. We used the International Classification of Diseases, Tenth Revision (ICD-10), Clinical Modification diagnosis and procedure codes to identify hospitalizations and procedures (Supplementary Table 1).
The primary outcome of the study was in-hospital mortality; secondary outcomes were cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome. Other variables included demographic characteristics, clinical risk profile, and Elixhauser co-morbidity index score. We used ICD-10, Clinical Modification diagnosis and procedure codes to identify these variables (Supplementary Table 1).
Descriptive statistics were used to understand the differences in distribution of demographics and clinical characteristics between COVID-19 hospitalizations with and without myocarditis. We conducted propensity score matching to account and control for potential differences in demographics and clinical profiles between hospitalizations with and without myocarditis. We used a 1:1 greedy matching algorithm with a caliper size of 0.25 times the SD of the logit of the propensity score to match the likelihood of having versus not having myocarditis. We set the standardized mean differences in the distribution of covariates at <10% to ensure adequate matching between the 2 groups.
We conducted survival analysis on unmatched and matched data using Kaplan-Meier estimator to compare differences in 30-day mortality between COVID-19 hospitalizations with and without myocarditis. After adjusting for covariates, multivariate conditional logistic regression analyses were used to find the associations between myocarditis and cardiac arrest, cardiogenic shock, use of mechanical ventilation, and acute respiratory distress syndrome.
To identify the potential confounding effects of coronary artery disease, diabetes, and hypertension on the association between myocarditis and in-hospital mortality, we conducted a subgroup analysis that included hospitalizations with these potential confounding variables. For this group, we compared the differences in 30-day mortality between hospitalizations with and without myocarditis using propensity-score-matched data. Because missing data was <5% for any variable, imputation was not conducted. To account for misclassification, we considered the uncertainty in the true values of bias parameters and simulated the effects of adjusting for a range of sensitivity and specificity values. Statistical significance was set at p <0.05, and all tests were 2-sided. All statistical analyses were conducted using SAS, version 9.4 (SAS Inc., Cary, North Carolina).
Results
A total of 164,417 COVID-19 hospitalizations were included in the analysis. Among these, 578 hospitalizations (0.4%) had myocarditis. The majority of these hospitalizations occurred in patients aged ≥65 years, and most were in men (Table 1 ). Hispanics constituted the majority of these hospitalizations, followed by Whites, Asians, Pacific Islanders, and Native Americans and Blacks. Medicare was the most common insurance coverage, followed by Medicaid and private insurance; some patients were uninsured. The most common comorbidities were hypertension, hyperlipidemia, and congestive heart failure (CHF). Nearly 3/4 of these hospitalizations had Elixhauser co-morbidity index score ≥3. There were significant differences in gender and in Elixhauser comorbidity index scores between COVID-19 hospitalizations with and without myocarditis. The prevalence of comorbidities such as atrial fibrillation, coagulation disorder, liver disease, chronic renal failure, CHF, previous myocardial infarction, and alcohol abuse was significantly higher among COVID-19 hospitalizations with myocarditis. Hospital length of stay was significantly higher among COVID-19 hospitalizations with myocarditis (Table 1).
Table 1.
Demographic and clinical characteristics of COVID-19 hospitalizations with and without myocarditis
| Characteristic | Myocarditis |
P value | |
|---|---|---|---|
| No n = 163,893 | Yes n = 578 | ||
| Age (years) | 0.415 | ||
| 18-44 | 33616 (20.5%) | 120 (20.8%) | |
| 45-64 | 57654 (35.2%) | 196 (33.9%) | |
| ≥65 | 72623 (44.3%) | 262 (45.3%) | |
| Sex | <0.001 | ||
| Male | 88470 (54.0%) | 356 (61.6%) | |
| Female | 75417 (46.0%) | 222 (38.4%) | |
| Race/ethnicity | 0.264 | ||
| White | 41057 (25.4%) | 135 (24.3%) | |
| Black | 10626 (6.6%) | 38 (6.8%) | |
| Hispanic | 86668 (53.7%) | 285 (51.4%) | |
| Asian or Pacific Islander and Native American | 14782 (9.2%) | 65 (11.7%) | |
| Other | 8313 (5.1%) | 32 (5.8%) | |
| Insurance | 0.893 | ||
| Medicare | 69287 (42.3%) | 250 (43.3%) | |
| Medicaid | 50285 (30.7%) | 166 (28.7%) | |
| Private insurance | 36167 (22.1%) | 132 (22.8%) | |
| Uninsured | 3060 (1.9%) | 11 (1.9%) | |
| Other | 5019 (3.1%) | 19 (3.3%) | |
| Risk profile | |||
| Hypertension | 96338 (58.8%) | 344 (59.5%) | 0.720 |
| Diabetes mellitus | 25734 (15.7%) | 87 (15.1%) | 0.668 |
| Hyperlipidemia | 60198 (36.7%) | 215 (37.2%) | 0.816 |
| Obesity | 42590 (26.0%) | 155 (26.8%) | 0.649 |
| Atrial fibrillation | 17965 (11.0%) | 87 (15.1%) | 0.001 |
| Coagulation disorder | 21584 (13.2%) | 139 (24.0%) | <0.001 |
| Peripheral vascular disease | 10764 (6.6%) | 32 (5.5%) | 0.317 |
| Liver disease | 11052 (6.7%) | 80 (13.8%) | <0.001 |
| Chronic renal failure | 33004 (20.1%) | 140 (24.2%) | 0.014 |
| Stroke | 8494 (5.2%) | 41 (7.1%) | 0.058 |
| Congestive heart failure | 23029 (14.1%) | 188 (32.5%) | <0.001 |
| Prior MI | 5333 (3.3%) | 39 (6.7%) | <0.001 |
| Prior PCI | 4166 (2.5%) | 18 (3.1%) | 0.383 |
| Prior CABG | 3721 (2.3%) | 15 (2.6%) | 0.600 |
| Tobacco use | 8545 (5.2%) | 26 (4.5%) | 0.439 |
| Alcohol abuse | 5095 (3.1%) | 29 (5.0%) | 0.007 |
| Drug abuse | 6450 (3.9%) | 29 (5.0%) | 0.182 |
| Elixhauser comorbidity index | <0.001 | ||
| 0 | 13770 (8.4%) | 18 (3.1%) | |
| 1 or 2 | 51636 (31.5%) | 130 (22.5%) | |
| ≥3 | 98487 (60.1%) | 430 (74.4%) | |
| Length of stay (days), median (IQR) | 5.0 (3.0-10.0) | 8.0 (4.0-16.0) | <0.001 |
| CABG = coronary artery bypass grafting; IQR = interquartile range; MI = myocardial infarction; PCI = percutaneous coronary intervention. | |||
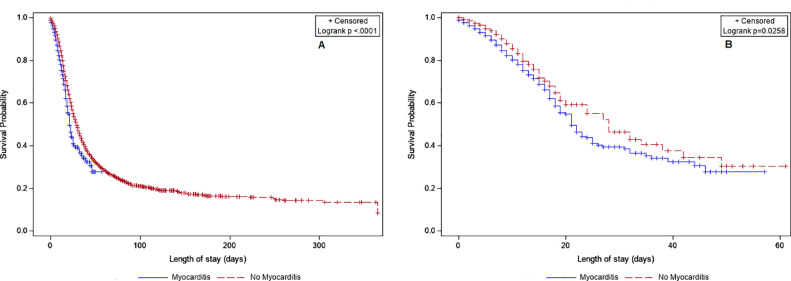
Before propensity score matching, the rate of in-hospital mortality was significantly higher among COVID-19 hospitalizations with myocarditis (Figure 1 ); it remained significant even after propensity score matching (30.0% vs 17.5%, p <0.001). Matching was successful in achieving covariate balance between the groups with and without myocarditis, as shown by a standardized difference of <10% for all covariates after matching (Supplementary Figure 1). Supporting this finding, survival analysis with log-rank test also showed that 30-day survival rates were significantly lower among those with myocarditis (39.5% vs 46.3%, p <0.001) (Figure 2 ).
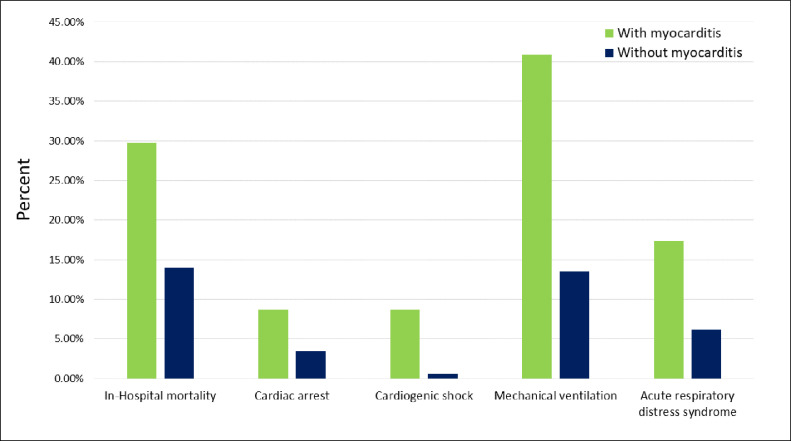
Figure 1.
Comparison of adverse hospital outcomes among COVID-19 hospitalizations with and without myocarditis.
Figure 2.
Kaplan–Meier curves comparing 30-day in-hospital mortality among COVID-19 hospitalizations with and without myocarditis. (A) Unmatched hospitalizations; (B) propensity-score-matched hospitalizations.
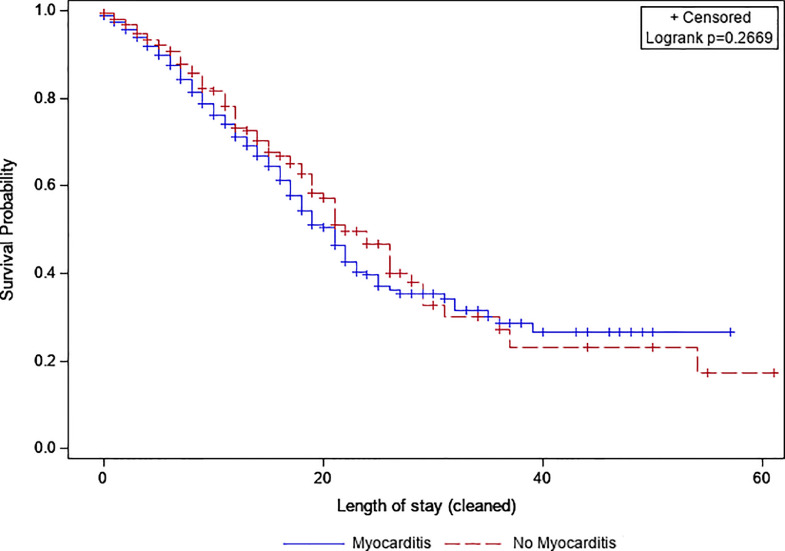
Subsequently, we conducted a subgroup analysis that included all COVID-19 hospitalizations with coronary artery disease, diabetes, and hypertension. In this subset, survival analysis with log-rank test in a propensity-score-matched sample of 449 hospitalizations with myocarditis and 449 without myocarditis showed that 30-day survival rates were not significantly different between the 2 groups (32.8% vs 35.4%, p = 0.267) (Figure 3 ).
Figure 3.
Kaplan–Meier curves comparing 30-day in-hospital mortality among COVID-19 hospitalizations with and without myocarditis in the subgroup with coronary artery disease, diabetes, and hypertension.
Before propensity score matching, adverse hospital outcomes such as cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome were significantly higher among COVID-19 hospitalizations with myocarditis (Figure 1). Conditional logistic regression analysis showed that the odds of cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome were significantly higher among those with myocarditis (Table 2 ).
Table 2.
Association between myocarditis and hospital outcomes among COVID-19 hospitalizations
| Characteristic | Odds ratio | P value |
|---|---|---|
| Cardiac arrest | 1.90 (1.16-3.14) | 0.011 |
| Cardiogenic shock | 4.13 (2.14-7.99) | <0.001 |
| Mechanical ventilation | 3.30 (2.47-4.41) | <0.001 |
| Acute respiratory distress syndrome | 2.49 (1.70-3.66) | <0.001 |
Odds ratios were calculated using conditional logistic regression after adjusting for age, sex race, insurance, hypertension, diabetes mellitus, hyperlipidemia, obesity, atrial fibrillation, coagulation disorder, peripheral vascular disease, liver disease, chronic renal failure, tobacco use, alcohol abuse, drug abuse, stroke, congestive heart failure, prior MI, prior PCI, and prior CABG. Complete model results are available from the authors upon request.
Trends in in-hospital mortality, cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome among patients with myocarditis did not change significantly across quarters of the year 2020 (Supplementary Figure 2).
Discussion
California ranks highest in the United States with respect to total number of COVID-19 cases and COVID-19 deaths. We found that in-hospital mortality, cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome were significantly higher among COVID-19 hospitalizations with myocarditis.
The prevalence of myocarditis among COVID-19 hospitalizations in our study was 0.4%. Studies have shown that the prevalence of myocarditis among patients with COVID-19 ranges from 0% to 15%.13, 14, 15, 16 In a large-scale study including 1,452,773 patients with COVID-19, the prevalence of myocarditis was 0.3%.17 Several other studies that have reported higher mortality rates among patients with COVID-19 with myocarditis also support our findings. For example, a retrospective analysis of a large registry database showed that 30-day all-cause mortality was significantly higher among those with myocarditis (13.4% vs 4.2%, p <0.001).18 Similarly, fulminant myocarditis was associated with greater mortality rates among patients with COVID-19.19, 20, 21
It has been hypothesized that inflammatory cytokine storm and direct action on myocytes through suppression of protein synthesis could be responsible for myocardial injury. Interleukin-6 constitutes the primary mediator of cytokine storm and triggers proinflammatory responses among immune cells such as T lymphocytes.6 This leads to further activation of and cytokine release by immune cells, leading to a negative spiral of immune-mediated myocardial injury. The special affinity of activated T lymphocytes for myocardial cells is regulated through the interaction between cardiac-synthesized hepatocyte growth factor and hepatocyte growth factor receptor (c-Met), which is present on naive T lymphocytes.22 Although many case studies have associated COVID-19 with clinically suspected myocarditis,23, 24, 25 there have been very few cases in which it has been confirmed histologically.26 , 27 Therefore, a general consensus on the prognostic potential of myocarditis in models predicting mortality among patients with COVID-19 is yet to be established.
We found that adverse outcomes such as cardiac arrest, cardiogenic shock, mechanical ventilation, and acute respiratory distress syndrome were higher in the myocarditis group. These findings are not surprising because complications such as CHF, ventricular arrhythmias, and cardiogenic shock are common after acute myocarditis.28 However, it is concerning that these complications occur even among healthy patients without a history of underlying cardiac disease.29 It is also troubling that among patients experiencing cardiac arrest, very few survive the transition time to the hospital, and even fewer survive hospitalization and management.30 Cardiogenic shock and cardiac arrest among patients with COVID-19 are characterized by transient and global left ventricular dysfunction, even in the absence of any pathology of the coronary vasculature.31 In addition, these complications are precipitated by disruption of the conduction system caused by altered intracellular signaling and ensuing interstitial edema and fibrotic changes.32 Higher rates of mechanical ventilation and acute respiratory distress syndrome in this population are also obviously because of the common mechanisms of tissue injury, such as cytokine storm and T-cell activation.33 Given these implications, it is important to be watchful for emerging myocarditis to successfully identify and manage the complications during COVID-19 hospitalization.
Several new models have been developed for predicting mortality among patients with COVID-19, and cardiac injury is one of the important prognostic factors that indicate poor outcomes.34 , 35 However, some new models do not include cardiac injury as a prognostic factor for predicting mortality in this population.36 , 37 In addition, conventional risk scores such as Confusion, Urea, Respiratory rate, Blood pressure, and age ≥65 years; National Early Warning Score 2; and Quick Sequential Organ Failure Assessment do not accurately estimate the risk of adverse COVID-19 outcomes.38 These conventional risk scores do not include cardiac injury as a prognostic factor. Similar to many other investigators who have emphasized the importance of including cardiac injury as a prognostic indicator for predicting mortality among patients with COVID-19, we emphasize that inclusion of myocarditis would significantly improve the accuracy of existing prognostic tools.8, 9, 10
Our study had some limitations. We used ICD-10 codes for identifying hospitalizations, and there could be some coding errors leading to misclassification bias. SID is an administrative database, and therefore we could not ensure whether these diagnoses were based on clinical findings or on confirmatory tests such as cardiac magnetic resonance imaging or endomyocardial biopsy. In addition, SID does not have data on troponin levels or on echocardiographic evidence of left ventricular dysfunction to ensure that the selected population indeed had clinical myocarditis. This could have affected the accuracy of our estimates. SID does not include laboratory results and medications. Availability of such information could have significantly improved the accuracy of our findings. Because vaccinations against SARS-CoV-2 started to become available only during the terminal stages of the study period, we did not have adequate information on vaccination status. The findings of our study are not generalizable to the entire United States population because we used data from California, where there were relatively very few patients with COVID-19 with myocarditis.
Our study using a large administrative database found that myocarditis was associated with greater rates of in-hospital mortality and adverse hospital outcomes among patients with COVID-19. Hence, care providers should be vigilant for emerging signs of myocarditis and attempt to aggressively manage the condition. For better accuracy, future prognostic models for estimating mortality and adverse outcomes among patients with COVID-19 should include myocarditis. Only through these efforts can we successfully manage myocarditis among patients with COVID-19 and avert some of the fatal complications.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Funding: None.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.08.009.
Appendix. Supplementary materials
References
- 1.Singh R, Kang A, Luo X, Jeyanathan M, Gillgrass A, Afkhami S, Xing Z. COVID-19: current knowledge in clinical features, immunological responses, and vaccine development. FASEB J. 2021;35:e21409. doi: 10.1096/fj.202002662R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori H, Ohkawara H, Togawa R, Rikimaru M, Shibata Y, Ikezoe T. Diagnosis and treatment of disseminated intravascular coagulation in COVID-19 patients: a scoping review. Int J Hematol. 2021;113:320–329. doi: 10.1007/s12185-021-03084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoo P, Fonseca EKUN, Sasdelli Neto R, Ishikawa WY, Silva MMA, Yanata E, Chate RC, Nunes Filho ACB, Bettega M, Fernandes JRC, Tarasoutchi F, Szarf G. COVID-19 myocarditis: a case report. Einstein (São Paulo) 2020;18:eRC5876. doi: 10.31744/einstein_journal/2020RC5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal-Torres W, Herrera-Escandón Á, Hurtado-Rivera M, Plata-Mosquera CA. COVID-19 fulminant myocarditis: a case report. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawalha K, Abozenah M, Kadado AJ, Battisha A, Al-Akchar M, Salerno C, Hernandez-Montfort J, Islam AM. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: what do we know so far? CJC Open. 2020;2:278–285. doi: 10.1016/j.cjco.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:567–571. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 9.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agency for Healthcare Research and Quality. Overview of the State Inpatient Databases (SID). Available at: https://www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed on April 8, 2022.
- 12.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, initiative STROBE. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 13.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging. 2021;14:1279–1281. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, Purtell CS, Schiebler ML, Reeder SB. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf H, Romano SD, Gundlapalli AV, Oster ME, Harris AM. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annie FH, Alkhaimy H, Nanjundappa A, Elashery A. Association between myocarditis and mortality in COVID-19 patients in a large registry. Mayo Clin Proc Innov Qual Outcomes. 2022;6:114–119. doi: 10.1016/j.mayocpiqo.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Lane DA, Lip GYH. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Invest. 2021;51:e13679. doi: 10.1111/eci.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss HP. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7:2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicol M, Cacoub L, Baudet M, Nahmani Y, Cacoub P, Cohen-Solal A, Henry P, Adle-Biassette H, Logeart D. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail. 2020;7:4371–4376. doi: 10.1002/ehf2.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 29.Purdy A, Ido F, Sterner S, Tesoriero E, Matthews T, Singh A. Myocarditis in COVID-19 presenting with cardiogenic shock: a case series. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab028. ytab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins GD, Couper K. COVID-19: long-term effects on the community response to cardiac arrest? Lancet Public Health. 2020;5:e415–e416. doi: 10.1016/S2468-2667(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaha KB, Manandhar DN, Cho JR, Adhikari A, K C MB. COVID-19 and the heart: what we have learnt so far. Postgrad Med J. 2021;97:655–666. doi: 10.1136/postgradmedj-2020-138284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali-Ahmed F, Dalgaard F, Al-Khatib SM. Sudden cardiac death in patients with myocarditis: evaluation, risk stratification, and management. Am Heart J. 2020;220:29–40. doi: 10.1016/j.ahj.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang Y, Liu T, Wei Y, Li J, Shao L, Liu M, Zhang Y, Zhao Z, Xu H, Peng Z, Zhou F, Wang X. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.BMJ Best Practice. Coronavirus disease 2019 (COVID-19). Available at: https://bestpractice.bmj.com/topics/en-us/3000168/prognosis. Accessed on April 2, 2022.
- 36.Altschul DJ, Unda SR, Benton J, de la Garza Ramos R, Cezayirli P, Mehler M, Eskandar EN. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci Rep. 2020;10:16726. doi: 10.1038/s41598-020-73962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gue YX, Tennyson M, Gao J, Ren S, Kanji R, Gorog DA. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10:21379. doi: 10.1038/s41598-020-78505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley P, Frost F, Tharmaratnam K, Wootton DG, NW Collaborative Organisation for Respiratory Research Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.