Abstract
Objectives
The aim of this study was to evaluate the ability of a modified Nutrition Risk Screening 2002 (modified NRS) compared with other nutrition screening tools such as NRS 2002, Mini Nutrition Assessment Short Form (MNA-SF), and Malnutrition Universal Screening Tool (MUST) on predicting the risk of death in patients with coronavirus disease 2019 (COVID-19).
Methods
We retrospectively collected data of patients who were admitted to the West campus of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology between January 25th, 2020 to April 24th, 2020. The nutritional status of the patients was assessed by modified NRS, NRS 2002, MNA-SF, and MUST. According to the score of modified NRS, patients were divided into malnutrition risk group (score ≥3) and normal nutrition group (score <3). Clinical characteristics were compared between the two groups. Kaplan meier survival curve was used to analyze the difference of compositing survival rate between the two groups. The predictive efficacy of different nutritional scales on the outcome of death was detected by Receiver operating characteristic (ROC) analysis.
Results
The modified NRS, NRS 2002, MNA-SF, and MUST identified malnutrition risk in 71.4%, 57.9%, 73.9%, and 43.4% of the patients, respectively. The patients were divided into malnutrition risk group and normal nutrition group by modified NRS score. Patients in the malnutrition risk group were older (65 y vs. 56 y) and with more severe and critical cases (42.30% vs. 5.20%) and diabetes cases (21.50% vs. 9.80%), worse prognosis (death of 13.80% vs. 0.50%), longer hospital stay (29 days vs. 23 days), lower albumin (31.85 g/L vs. 38.55 g/L) and prealbumin (201.95 mg/L vs. 280.25 mg/L) compared with the normal nutrition group (P were <0.001, respectively). There were more patients with chronic respiratory disease in malnutrition risk group (9.70 vs. 2.10%, P = 0.001). BMI was lower in malnutrition risk group (23.45 kg/m2vs. 24.15 kg/m2, P = 0.017). Kaplan meier survival curve demonstrated that the survival of malnutrition risk group was significantly lower than normal nutrition group (P < 0.001). The area under the ROC curve (AUC) of the modified NRS scale (0.895) outperformed NRS 2002 (0.758), MNA-SF (0.688), and MUST (0.485). The former three scales could predict the risk of death (P were < 0.001), while MUST could not (P = 0.690).
Conclusions
Patients with COVID-19 at risk of malnutrition have a worse prognosis than those with normal nutrition. The modified NRS scale could effectively predict the risk of death among patients with COVID-19.
Keywords: COVID-19, Risk of death, Nutritional risk screening tools, Predict, Prognosis
1. Introduction
COVID-19, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has been an ongoing pandemic all over the world [1]. Malnutrition and risk of malnutrition are prevalent in COVID-19 patients, which is associated with increased mortality and poor long-term outcomes [2]. Patients under malnutrition or malnutrition risk benefit from early and individualized nutritional therapy [3]. Therefore, timely identification of malnutrition or malnutrition risk is particularly important.
At present, many nutritional screening tools were used to evaluate the nutritional status of COVID-19 patients in different countries. Among them, mostly used scales were NRS 2002, MNA-SF and MUST. According to literature reports, nutritional risk was identified in 27.5%–85.1% of participants screened by NRS 2002, MNA-SF, and MUST. NRS 2002, MNA-SF and MUST demonstrated high sensitivity, MUST had better specificity [4,5]. However, it is unclear whether these nutritional assessment scales can predict the prognosis of COVID-19 and the predictive value of various nutritional assessment scales.
Based on NRS 2002, a modified NRS has been developed by the expert team of medical nutrition diagnosis and treatment of Hubei Province for assessing the nutritional status among patients in the first wave of COVID-19 in Wuhan, China [6]. The assessment of disease severity of COVID-19 is added into the scale. This study compared the new modified NRS scale with the classical nutrition screening tools such as NRS 2002, MNA-SF, and MUST to evaluate its efficacy on predicting death of COVID-19 patients.
2. Methods
2.1. Study design and participants
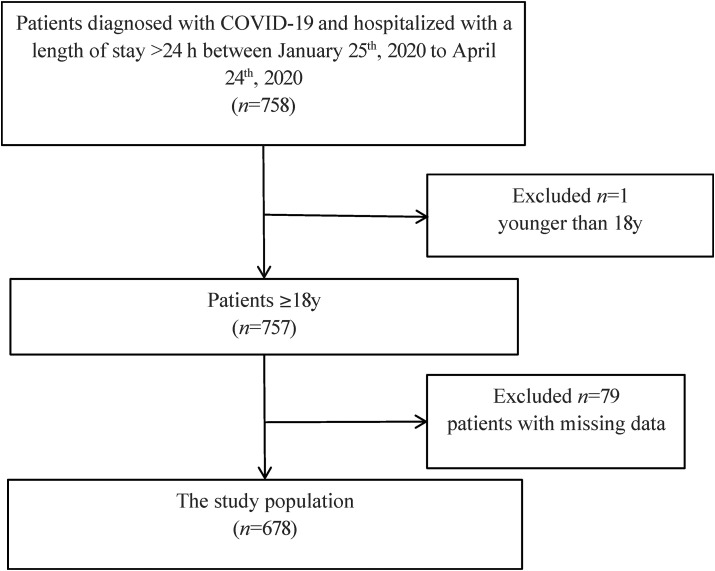
This retrospective cohort analysis consecutively enrolled a series of patients who were admitted to the West campus of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, one of the major hospitals to treat COVID-19 patients in Wuhan between January 25th, 2020 to April 24th, 2020. Patients diagnosed with COVID-19, older than 18 years, and hospitalized with a length of stay of >24 h were enrolled in this study. Patients with missing data for screening of nutrition status were excluded from this study. Finally, 678 participants were included in the analysis (Fig. 1 ). The clinical outcomes were monitored until April 28, 2020, by which date, all the patients either died or were discharged. This study was approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Fig. 1.
Flow diagram of participants inclusion and exclusion.
A diagnosis of COVID-19 required the following [7]: (1) history of epidemiological exposure; (2) clinical symptoms such as a fever (armpit temperature ≥37.3 °C), cough, sputum symptoms, or gastrointestinal symptoms; (3) laboratory test results, indicating either that the total number of white blood cells was normal or decreased in early onset, or that the lymphocyte count decreased; (4) pulmonary imaging changes showing multiple small, patchy shadows or interstitial changes in the early stage, and later findings that extra pulmonary bands were present and had progressed in both lungs, with ground-glass infiltration and infiltration, or pulmonary consolidation. Pleural effusion was rare; and (5) positive detection of SARS-CoV-2 nucleic acid by quantitative real-time reverse-transcription – polymerase chain reaction (qRT-PCR) using pharyngeal swab specimens.
2.2. Data collection
Data on age, gender, comorbidities, length of hospital stay, albumin, prealbumin, body mass index (BMI), and disease severity were retrospectively collected from electronic medical records for each participant. Albumin and prealbumin were the results of the first test after admission. BMI come from the data on admission. Nutrtional status was assessed the next day after admission.
2.3. Nutritional risk screening tools
2.3.1. Nutrition risk screening (NRS) 2002
The NRS 2002 scoring system consists of three parts according to ESPEN guidelines [8]. The first part of NRS 2002 assesses the nutritional status of the patient, which is based on the changes in weight in the recent 3 months, dietary intake one-week before hospitalization, and the BMI. The second part of the NRS 2002 assesses the severity of the disease, which could be scored by its impact on the increased nutritional requirements of patients. The scores for the first two parts of the NRS 2002 vary from 0 to 3. The last part of the NRS 2002 is the age assessment. If the patient is 70 years or older, add 1 score. Therefore, the final score of NRS 2002 can range from 0 to 7. Patients with a total NRS 2002 score of ≥3 indicate a high nutritional risk (Table 1 ).
Table 1.
Comparison of the modified NRS and NRS 2002 scoring criteria.
| NRS 2002 | Modified NRS | Score in the present study |
|---|---|---|
| Normal nutritional status | ||
| Impaired nutritional status: | Normal nutritional status: Score 0 | |
| Mild: Weight loss >5% in 3 months or food intake below 50–75% of normal requirement in preceding week | Unchanged | Mild: Score 1 |
| Moderate: Weight loss >5% in 2 months or BMI 18.5–20.5 plus impaired general condition or food intake 25–50% of normal requirement in preceding week | Moderate: Score 2 | |
| Severe: Weight loss >5% in 1 month (or 15% in 3 months) or BMI <18.5 plus impaired general condition or food intake 0–25% of normal requirement in preceding week | Severe: Score 3 | |
| Severity of disease: | ||
| Normal nutritional requirements | Normal nutritional requirements: Score 0 | |
| Mild: Hip fracture, chronic patients in particular with acute complications, cirrhosis, COPD, chronic hemodialysis, diabetes, oncology | Mild: Add mild and moderate COVID-19 patients | Mild: Score 1 |
| Moderate: Major abdominal surgery, stroke, severe pneumonia, hematologic malignancy | Moderate: Add severe COVID-19 patients | Moderate: Score 2 |
| Severe: Head injury, bone marrow transplantation, intensive care patients (APACHE II > 10) | Severe: Add critical COVID-19 patients | Severe: Score 3 |
| Patients with age ≥ 70 years | Unchanged | Age < 70 years: score 0 |
| Age ≥ 70 years: score 1 | ||
| Presence of nutritional risk | Total score ≥3 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
2.3.2. Modified nutrition risk screening (NRS) 2002
The modified NRS adds the assessment of disease severity of COVID-19 to the second part of NRS 2002 [5]. The mild and moderate cases of COVID-19 was scored 1. The severe cases of COVID-19 was scored 2. The critical cases of COVID-19 was scored 3. The scale is scored out of a total of 7 points. A score of <3 points indicating normal nutrition and a score of ≥3 points indicating a risk of malnutrition (Table 1).
2.3.3. Mini nutrition assessment short-form (MNA-SF)
The MNA-SF consists of six items: decline in food intake, weight loss, mobility, psychological stress or acute disease, neuropsychological problems, and the BMI. The scale is scored out of a total of 14 points and values below a threshold of 12 were used to identify at-risk patients [9].
2.3.4. Malnutrition universal screening tool (MUST)
The MUST includes three components, such as BMI, unplanned weight loss in past 3–6 months, and absence or inadequacy of dietary intake for >5 day due to the presence of acute disease [10]. The score for each component varies from 0 to 2. Overall risk of malnutrition according to MUST is rated as low (score = 0), medium (score = 1), or high (score ≥ 2). Patients with a score of 0 was assigned in normal nutrition group and a score ≥1 was assigned in malnutrition risk group in this study.
2.4. Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR) and categorical variables as a number and percentage (%). Patients were classified into malnutrition risk and normal nutrition groups based on the four nutritional risk screening tools and Mann–Whitney test was used to test the frequency of malnutrition risk. Kaplan meier analysis was performed for survival curves. Features of the malnutrition risk group and normal nutrition group were compared using the Mann–Whitney or chi-squared tests. Receiver operating characteristic (ROC) curves were used for assessing the performance of the modified NRS, NRS 2002, MNA-SF, and MUST in predicting mortality risk of patients with COVID-19. The SPSS13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Statistical significance was considered at P value of <0.05.
3. Results
In the study cohort, 68 (10.00%) of the 678 COVID-19 patients died during hospitalization, and 610 (90.00%) were discharged. First, we analyzed the characteristics of 678 COVID-19 patients (Table 2 ). In the participants, 49.3% were male. Most of the participants were moderate cases (60.3%). The median age of the patients were 60.3 years. The median albumin concentration 33.9 g/L was lower than the lower limit of range of normal (35–55 g/L).
Table 2.
Characteristics of study participants.
| Variable | All subjects (n = 678) |
|---|---|
| Age (years) | 62.00 (53.00, 70.00) |
| Male | 343.0 (49.30) |
| BMI (kg/m2) | 23.56 (21.68, 25.64) |
| Length of hospital stay (days) | 27.00 (19.75, 39.00) |
| Chronic respiratory disease | 51.00 (7.500) |
| Hypertension | 241.0 (35.50) |
| Malignancy | 41.00 (6.000) |
| Diabetes | 123.0 (18.10) |
| Renal inadequacy | 22.00 (3.200) |
| Coronary heart disease | 83.00 (12.20) |
| Albumin (g/L) | 34.20 (29.90, 37.70) |
| Prealbumin (mg/L) | 230.5 (148.4, 292.0) |
| Severity of disease | |
| Mild cases | 54.00 (7.900) |
| Moderate cases | 409.0 (60.30) |
| Severe cases | 104.0 (15.40) |
| Critical cases | 111.0 (16.40) |
Values are median (IQR) or n (%), respectively.
BMI, body mass index.
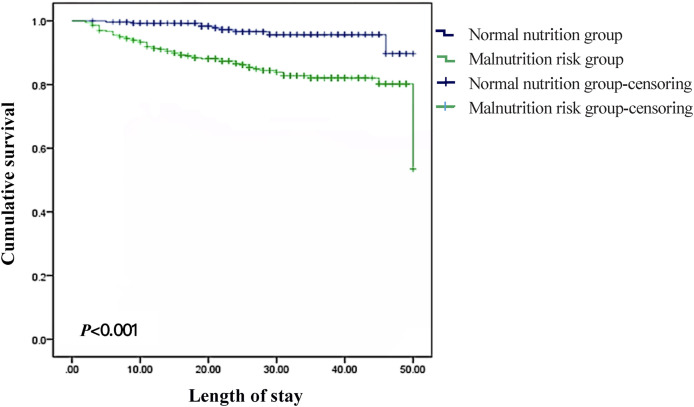
Secondly, we performed the nutritional assessment of the patients using the four nutrition screening scales (Table 3 ). We found that 43.4%–73.9% of the patients were at risk of malnutrition according to the scales. MNA-SF detected the highest prevalence of risk of malnutrition (73.9%). The second was modified NRS (71.4%). NRS-2002 followed with 57.9%. MUST had the lowest probability of detecting the risk of malnutrition (43.4%). Patients were divided into malnutrition risk group and normal nutrition group by modified NRS. Differences in characteristics of participants between malnutrition risk group and normal nutrition group were compared (Table 4 ). Patients in the malnutrition risk group were older (65 y vs. 56 y) and with more severe and critical cases (42.30% vs. 5.20%), more diabetes cases (21.50% vs. 9.80%), worse prognosis (death of 13.80% vs. 0.50%), longer hospital stay (29 days vs. 23d ays), lower albumin (31.85 g/L vs. 38.55 g/L) and prealbumin (201.95 mg/L vs. 280.25 mg/L) compared with the normal nutrition group (P were <0.001, respectively). There were more patients with chronic respiratory disease in malnutrition risk group (9.70% vs. 2.10%, P = 0.001). BMI was lower in malnutrition risk group (23.45 kg/m2 vs. 24.15 kg/m2, P = 0.017). Most patients in malnutrition risk group had malignant tumor (7.4% vs. 2.6%, P = 0.016) and coronary heart disease (13.80% vs. 8.20%, P = 0.045). Survival rate of patients at risk of malnutrition was much lower than those with normal nutrition (P < 0.001) (Fig. 2 ).
Table 3.
Nutritional status of 678 patients classified by modified NRS, NRS-2002, MNA-SF and MUST.
| Malnutrition risk group, n (%) | Normal nutrition group, n (%) | Z | P | |
|---|---|---|---|---|
| Modified NRS | 484.0 (71.40) | 194.0 (28.60) | 3.316 | <0.001 |
| NRS 2002 | 393.0 (57.90) | 285.0 (42.10) | 4.390 | <0.001 |
| MNA-SF | 501.0 (73.90) | 177.0 (26.10) | 6.007 | <0.001 |
| MUST | 294.0 (43.40) | 384.0 (56.60) | 8.977 | <0.001 |
Table 4.
Characteristics of patients in malnutrition risk group and normal nutrition group according to the modified NRS.
| Malnutrition risk group (n = 194) | Normal nutrition group (n = 484) | Total (n = 678) | Z/X2 | P | |
|---|---|---|---|---|---|
| Age (years) | 65.00 (56.00, 72.00) | 56.00 (44.00, 64.00) | 62.00 (53.00, 70.00) | −8.074 | <0.001 |
| Male, n (%) | 263.0 (54.30%) | 80.00 (41.20%) | 343.0 (50.60%) | 9.510 | 0.002 |
| Death n (%) | 67.00 (13.80%) | 1.000 (0.5000%) | 68.00 (10.00%) | 27.26 | <0.001 |
| BMI (kg/m2) | 23.45 (21.63, 25.46) | 24.15 (22.07, 26.18) | 23.56 (21.68, 25.64) | −2.395 | 0.017 |
| Length of hospital stay (days) | 29.00 (21.00, 40.00) | 23.00 (17.00, 32.00) | 27.00 (19.75, 39.00) | −4.968 | <0.001 |
| Chronic respiratory disease, n (%) | 47.00 (9.700%) | 4.000 (2.100%) | 51.00 (7.500%) | 11.64 | 0.001 |
| Hypertension, n (%) | 182.0 (37.60%) | 59.00 (30.40%) | 241.0 (35.50%) | 3.126 | 0.077 |
| Malignancy, n (%) | 36.00 (7.400%) | 5.000 (2.600%) | 41.00 (6.000%) | 5.759 | 0.016 |
| Diabetes, n (%) | 104.0 (21.50%) | 19.00 (9.800%) | 123.0 (18.10%) | 12.75 | <0.001 |
| Renal inadequacy, n (%) | 18.00 (3.700%) | 4.000 (2.100%) | 22.00 (3.200%) | 1.211 | 0.271 |
| Coronary heart disease, n (%) | 67.00 (13.80%) | 16.00 (8.200%) | 83.00 (12.20%) | 4.036 | 0.045 |
| Albumin (g/L) | 31.85 (28.40, 34.70) | 38.55 (36.80, 40.92) | 34.20 (29.90, 37.72) | −16.30 | <0.001 |
| Prealbumin (mg/L) | 201.9 (127.0, 269.7) | 280.2 (230.3, 331.7) | 230.5 (148.4, 292.0) | −9.641 | <0.001 |
| Severity of disease, n (%) | 95.32 | <0.001 | |||
| Mild cases | 26.00 (5.400%) | 28.00 (14.40%) | 54.00 (8.000%) | ||
| Moderate cases | 253.0 (52.30%) | 156.0 (80.40%) | 409.0 (60.30%) | ||
| Severe cases | 94.00 (19.40%) | 10.00 (5.20%) | 104.0 (15.30%) | ||
| Critical cases | 111.0 (22.90%) | 0 (0.0%) | 111.0 (16.40%) |
Values are median (IQR) unless otherwise indicated.
BMI, body mass index.
Fig. 2.
Cumulative survival in normal nutrition group and malnutrition risk group. Survival rate of patients at risk of malnutrition was much lower than those with normal nutrition (P < 0.001). Kaplan meier analysis was performed for survival curves.
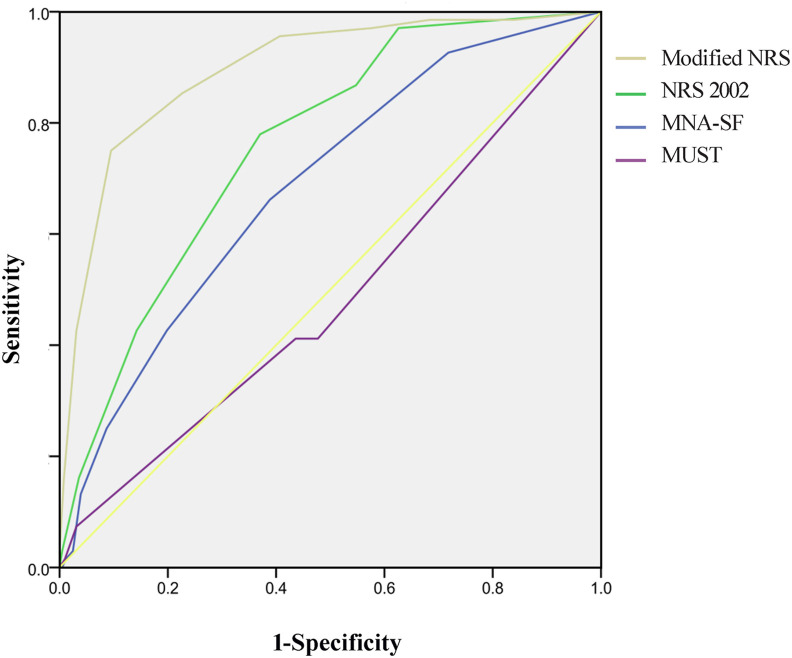
Finally, the efficacy to predict the risk of death of modified NRS, NRS 2002, MNA-SF, and MUST were compared in the 678 patient using ROC analysis. Their AUCs for predicting the risk of death were 0.895 (95% CI, 0.854–0.935), 0.758 (95% CI, 0.705–0.812), 0.685 (95% CI, 0.621–0.749), and 0.485 (95% CI, 0.410–0.561), respectively. The former three scales could predict the risk of death (P were <0.001), while MUST could not (P = 0.690). The corresponding sensitivities were 90.50%, 63.00%, and 61.10%, and the specificities were 75.00%, 77.90%, and 66.20%, respectively (Table 5 , Fig. 3 ).
Table 5.
ROC curve analysis of modified NRS, NRS-2002, MNA-SF and MUST in predicting risk of death.
| AUC | Standard error | P | 95% CI | Optimal boundary value problem | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| Modified NRS | 0.895 | 0.021 | <0.001 | 0.854–0.935 | 0.655 | 0.905 | 0.750 |
| NRS 2002 | 0.758 | 0.027 | <0.001 | 0.705–0.812 | 0.409 | 0.630 | 0.779 |
| MNA-SF | 0.685 | 0.031 | <0.001 | 0.621–0.749 | 0.273 | 0.611 | 0.662 |
| MUST | 0.485 | 0.039 | 0.690 | 0.410–0.561 | 0.043 | 0.969 | 0.074 |
Fig. 3.
ROC analysis of sensitivity and specificity of predicted probabilities for risk of death among modified NRS, NRS-2002, MNA-SF, and MUST. The efficacy to predict the risk of death of modified NRS, NRS 2002, MNA-SF, and MUST were compared in the 678 patient using ROC analysis. Modified NRS (AUC = 0.898, 95% CI [0.854–0.935]); NRS 2002 (AUC = 0.758, 95% CI [0.705–0.812]); MNA-SF (AUC = 0.685, 95% CI [0.621–0.749]); MUST (AUC = 0.485, 95% CI [0.410–0.561]). ROC, receiver operating characteristic; AUC, area under the curve.
4. Discussion
In this study, there were 43.4%–73.9% patients with COVID-19 were at risk of malnutrition revealed by four nutrition screening tools: the modified NRS, NRS 2002, MNA-SF, and MUST. According to the screening of modified NRS, patients were divided into risk of malnutrition group and normal nutrition group. Patients in the malnutrition risk group were older, with more severe and critical cases, more diabetes cases, longer hospital stay, lower albumin and prealbumin compared with the normal nutrition group. The probability of survival of patients with risk of malnutrition was much lower than those with normal nutrition. In ROC analysis, the modified NRS showed the best performance in predicting the risk of death among the four malnutrition risk screening tools in patients with COVID-19.
Malnutrition risk were prevalent in patients with COVID-19. Patients with COVID-19 frequently presents with a broad clinical spectrum of symptoms and complications such as fever, coughing, nasal cold, shortness of breath, pain during breathing, sore throat, general malaise, fatigue, general pain symptoms, headache, muscle ache, stomachache, decreased appetite, ageusia, changed taste, anosmia, nausea, vomiting and diarrhea [11]. Several of these symptoms are associated with reduced nutrient intake, increased energy expenditure or decreased nutrient absorption [12]. In addition, psychological stress from COVID-19 increases the risk of depression and anxiety, further affecting food intake [13]. As a consequence, nutritional requirements of COVID-19 patients are often not being met, which resulting in weight loss and risk of malnutrition.
The risk of malnutrition increases with age, accompanied by functional limitations, increased morbidity and mortality [14,15]. Age-related changes in swallowing physiology and dysphagia reduced food intake in aged patients, which contributed to increase malnutrition risk [16,17]. In addition, increased age may cause a decline in cognitive functioning and particularly memory. Memory relates to many aspects of everyday functioning, such as eating, and in turn may lead to an increased risk of malnutrition [18].
Disease-related risk of malnutrition are common [19]. Diabetes leads to changes in body composition, with reduced fat and fat-free body mass that often lead to diminished physical and mental function [20]. Nutritional abnormalities are frequent in different chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD), bronchiectasis, and interstitial fibrosis. The nutritional abnormalities result from the interaction of several factors, including tobacco smoking, low physical activity, systemic inflammation and the imbalance between energy supply and requirements, which essentially lead to a negative balance between protein breakdown and synthesis [21]. Cancer-associated malnutrition occurs through a variety of mechanisms such as the host response to the tumor and its treatment. These include the various treatment modalities and systemic and local effects of the cancer itself, resulting in loss of appetite, alterations in absorption and metabolism of nutrients, and impaired organ function [22].
In our study, higher mortality was found in patients with malnutrition risk than with normal nutrition. The immune system is highly affected by nutrition. Malnutrition leads to decreased immune responses with consequent augmented risk of infection and disease severity. Malnutrition has been observed increasing the susceptibility and severity of SARS-CoV-2 infection [23]. At the same time, severe and critical patients with COVID-19 have a high risk of malnutrition [24]. Malnutrition risk is a negative prognostic factor in terms of mortality, hospitalization length and clinical status of the patient at discharge in patients with COVID-19 [25].
The rate of malnutrition risk is different with screening by different scales because some score items assessing the risk of malnutrition are different in these scales. Weight loss in recent 3–6 months and low BMI are common evaluation items of these scales. But there are other different evaluation items in different scales. Fasting or insufficient intake for more than 5 days due to the impact of acute diseases is included in MUST [10]. This item usually failed to be scored during admission evaluation, resulting in low score and low detection rate of malnutrition risk among the patients who were admitted to hospital within 5 days after onset. NRS 2002 takes the severity of diseases as one of the evaluation items. According to this scale, severe pneumonia with COVID-19 gets 2 points, critical cases of COVID-19 with mechanical ventilation gets 3 points [8]. However, patients with mild symptoms are not scored. In the modified NRS, patients with mild or moderate COVID-19 were assigned a score of 1 [6], which may increase the identification of patients at risk of malnutrition. Due to the different disease severity of COVID-19, different scores are assigned in the modified NRS, which increases its predictive value of risk of death. Compared with the classical malnutrition risk assessment tools such as NRS 2002, MNA-SF, and MUST, it showed better predicting efficacy on the risk of death in patients with COVID-19.
This study has several limitations. First, it is failure to perform sampling from multicenters. Second, we did not conduct dynamic nutritional screening and were unable to find the changing characteristics of nutritional status in the acute phase and recovery phase of the participants. Moreover, we did not obtain data on body composition. Patients did not have their waist-to-hip ratio measured, and data concerning central obesity based on waist circumference were not reported either.
In conclusion, risk of malnutrition is common in hospitalized patients with COVID-19. Patients with risk of malnutrition have worse prognosis of death than patients with normal nutrition compared with NRS 2002, MNA-SF and MUST, the modified NRS demonstrated better predicting efficacy on the risk of death among patients with COVID-19. Therefore, the modified NRS could be used to evaluate the nutritional status and predict disease prognosis in hospitalized COVID-19 patients.
Funding sources
The study has no funding sources.
Author contribution
PH designed study. KMZ and PH wrote manuscript. KMZ performed statistical analysis. KMZ, HHG and JJC collected data. All authors have approved the final version of this paper.
The sources of support
None.
Declaration of competing interest
All authors report no conflicts of interest.
References
- 1.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4) doi: 10.1128/CMR.00028-20. e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva D.F.O., Lima S., Sena-Evangelista K.C.M., Marchioni D.M., Cobucci R.N., Andrade F.B. Nutritional risk screening tools for older adults with COVID-19: a systematic review. Nutrients. 2020;12(10):2956. doi: 10.3390/nu12102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alencar E.S., Muniz L., Holanda J.L.G., Oliveira B.D.D., Carvalho M.C.F., Leitão A.M.M., et al. Enteral nutritional support for patients hospitalized with COVID-19: results from the first wave in a public hospital. Nutrition. 2022;94:111512. doi: 10.1016/j.nut.2021.111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74(6):876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubei Provincial Hospital Association Clinical Nutrition Management Professional Committee, Hubei Clinical Nutrition Quality Control Center, Nutrition Society of Hubei Province. Consensus on medical nutrition diagnosis and treatment of novel coronavirus pneumonia in Hubei Province (trial version)[s]. (2020-02-17) [2020-02-19]. hb.pepple.com.cn/BIG5/n2/2020/0219/c192237-33810419.html.
- 7.National Health Commission of China New coronavirus pneumonia prevention and control program (6th edn) J Infect Contr China. 2020;2:192–195. [Google Scholar]
- 8.Kondrup J. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein L.Z., Harker J.O., Salva A., Guigoz Y., Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56(6) doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 10.Stratton R.J., Hackston A., Longmore D., Dixon R., Price S., Stroud M., et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92(5):799–808. doi: 10.1079/bjn20041258. [DOI] [PubMed] [Google Scholar]
- 11.Borges Do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4):941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wierdsma N.J., Kruizenga H.M., Konings L.A., Krebbers D., Jorissen J.R., Joosten M.I., et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021;43:369–376. doi: 10.1016/j.clnesp.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen H.C., Nguyen M.H., Do B.N., Tran C.Q., Nguyen T.T.P., Pham K.M., et al. People with suspected COVID-19 symptoms were more likely depressed and had lower health-related quality of life: the potential benefit of health literacy. J Clin Med. 2020;9(4):965. doi: 10.3390/jcm9040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal E., Miller M., Yaxley A., Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76(4):296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Sieber C.C. Malnutrition and sarcopenia. Aging Clin Exp Res. 2019;31(6):793–798. doi: 10.1007/s40520-019-01170-1. [DOI] [PubMed] [Google Scholar]
- 16.Sura L., Madhavan A., Carnaby G., Crary M.A. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287–298. doi: 10.2147/CIA.S23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley N.C., Martin R.E., Salter K.L., Teasell R.W. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. 2009;41(9):707–713. doi: 10.2340/16501977-0415. [DOI] [PubMed] [Google Scholar]
- 18.Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer F., Valentini L. Disease-related malnutrition and sarcopenia as determinants of clinical outcome. Vis Med. 2019;35(5):282–291. doi: 10.1159/000502867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G.X., Chen Y., Yang Y.X., Yang K., Liang J., Wang S., et al. Pilot study of the Mini Nutritional Assessment on predicting outcomes in older adults with type 2 diabetes. Geriatr Gerontol Int. 2017;17(12):2485–2492. doi: 10.1111/ggi.13110. [DOI] [PubMed] [Google Scholar]
- 21.Gea J., Sancho-Munoz A., Chalela R. Nutritional status and muscle dysfunction in chronic respiratory diseases: stable phase versus acute exacerbations. J Thorac Dis. 2018;10(Suppl 12):S1332–S1354. doi: 10.21037/jtd.2018.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Cutsem E., Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S51–S63. doi: 10.1016/j.ejon.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Quilliot D., Gerard M., Bonsack O., Malgras A., Vaillant M.F., Di Patrizio P., et al. Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open. 2021;11(7) doi: 10.1136/bmjopen-2021-048948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G., Zhou C.L., Ba Y.M., Wang Y.M., Song B., Cheng X.B., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefano M., Andrea B., Daniela C., Emanuela M., Lorena P., Daniela D., et al. Malnutrition risk as a negative prognostic factor in COVID-19 patients. Clin Nutr ESPEN. 2021;45:369–373. doi: 10.1016/j.clnesp.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]