Abstract
We have previously demonstrated nerve growth factor (NGF) regulation of pituitary adenylate cyclase-activating polypeptide (PACAP)/receptors in bladder reflex pathways using a transgenic mouse model of chronic NGF overexpression in the bladder using the urothelial-specific uroplakin II promoter. We have now explored the contribution of target-derived NGF in combination with cyclophosphamide (CYP)-induced cystitis to determine whether additional changes in neuropeptides/receptors are observed in micturition reflex pathways due to the presence of additional inflammatory mediators in the urinary bladder. Quantitative PCR was used to determine PACAP/vasoactive intestinal polypeptide (VIP), substance P, galanin, and receptor transcript expression in the urinary bladder (urothelium, detrusor) in mice with overexpression of NGF in the urothelium (NGF-OE) and wild-type (WT) mice with CYP-induced cystitis (4 h, 48 h, and chronic). With CYP-induced cystitis (4 h), WT and NGF-OE mice exhibited similar changes in galanin transcript expression in the urothelium (30-fold increase) and detrusor (threefold increase). In contrast, PACAP, VIP, and substance P transcripts exhibited differential changes in WT and NGF-OE with CYP-induced cystitis. PAC1, VPAC1, and VPAC2 transcript expression also exhibited differential responses in NGF-OE mice that were tissue (urothelium vs. detrusor) and CYP-induced cystitis duration-dependent. Using conscious cystometry, NGF-OE mice treated with CYP exhibited significant (p≤0.01) increases in voiding frequency above that observed in control NGF-OE mice. In addition, no changes in the electrical properties of the major pelvic ganglia neurons of NGF-OE mice were detected using intracellular recording, suggesting that the urinary bladder phenotype in NGF-OE mice is not influenced by changes in the efferent limb of the micturition reflex. These studies are consistent with target-derived NGF and other inflammatory mediators affecting neurochemical plasticity and the reflex function of micturition pathways.
Keywords: NGF, Neuropeptides, Urothelium, Cystometry, Intracellular recording, MPG
Introduction
Nerve growth factor (NGF) has been suggested to play a role in urinary bladder dysfunction by mediating inflammation as well as morphological and functional changes in sensory and sympathetic neurons innervating the urinary bladder (Dmitrieva et al. 1997; Clemow et al. 1998; Oddiah et al. 1998; Chuang et al. 2001; Hu et al. 2005; Guerios et al. 2006; Zvara and Vizzard 2007). Many previous studies in rodents have demonstrated the importance of NGF in bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation (Jaggar et al. 1999; Zvara and Vizzard 2007; Guerios et al. 2008; Arms et al. 2010). The overall hypothesis for our work is that pain and micturition dysfunction in bladder pain syndrome (BPS)/interstitial cystitis (IC) involves an alteration in urothelium and/or sensory physiology. Neurotrophins have been detected in the urine or in urinary bladders of women with BPS/IC (Lowe et al. 1997; Okragly et al. 1999). During cystitis, the expression of neurotrophins is increased in micturition reflex pathways including the urinary bladder, spinal cord, and peripheral ganglia (Dmitrieva et al. 1997; Clemow et al. 1998; Jaggar et al. 1999; Chuang et al. 2001; Hu et al. 2005; Zvara and Vizzard 2007; Guerios et al. 2008). Increased NGF expression could influence biological function through the activation of signal transduction cascades mediated by the phosphorylation of the tropomyosin-related family of receptor tyrosine kinase receptors (Trk) at the nerve terminals in target tissues or retrograde transport to innervating neurons (Huang and Reichardt 2001; Pezet and McMahon 2006). In the neuronal cell body, the activated neurotrophin/Trk complex can activate signal cascade(s) to induce long-term changes in cells, including mediating neurotransmitter phenotype, synaptic reorganization, increasing synaptic efficacy, and controlling function in target organs (Levi-Montalcini et al. 1996; Huang and Reichardt 2001; Pezet and McMahon 2006).
We recently examined the role of NGF in urinary bladder dysfunction by generating a mouse model of urinary bladder hypersensitivity based on the hypothesis that chronic urothelial NGF overexpression would induce sensory neuronal hypersensitivity and increased urinary bladder reflex function (Schnegelsberg et al. 2010). Chronic overexpression of NGF in the urothelium was achieved through the use of a highly urothelium-specific uroplakin II promoter (Lin et al. 1995; Liang et al. 2005). Our studies (Schnegelsberg et al. 2010) revealed that urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice (1) stimulates neuronal sprouting or proliferation in the urinary bladder; (2) produces local inflammatory changes in the urinary bladder; (3) produces urinary bladder hyperreflexia; and (4) results in increased referred somatic hypersensitivity. Elevated levels of neurotrophins have also been detected in the urine of women (Okragly et al. 1999) and in the urothelium of individuals with BPS/IC or other painful bladder conditions (Lowe et al. 1997). More recently, it was demonstrated that urinary NGF levels are increased in patients with overactive bladder (OAB) symptoms associated with detrusor overactivity, stress urinary incontinence, or bladder outlet obstruction (Okragly et al. 1999; Liu and Kuo 2007, 2008a, b; Liu et al. 2008a, b; Yokoyama et al. 2008). A recent clinical study has provided preliminary support for the use of NGF antibody treatment in reducing urgency episodes and daily pain scores in individuals with moderate to severe BPS/IC (Evans et al. 2011). In addition to NGF, additional NGF-mediated changes might contribute to the urinary bladder hyperreflexia and pelvic hypersensitivity observed in these mice (Schnegelsberg et al. 2010), such as stimulation/recruitment of bladder mast cells, modulation of local neuroinflammatory responses, upregulation of neuropeptide/receptor systems and ion channels, as well as changes in the expression of other neurotrophins and associated receptors (Yoshimura et al. 2002; Ford et al. 2006; Pezet and McMahon 2006; Szallasi et al. 2007).
Our previous studies (Girard et al. 2008, 2010) have demonstrated the expression and regulation of neuropeptides (e.g., pituitary adenylate cyclase-activating polypeptide (PACAP), vasoactive intestinal polypeptide (VIP), substance P (sub P), and galanin) and receptor (e.g., PAC1, VPAC1, and VPAC2) transcripts in the urinary bladder and lumbosacral dorsal root ganglia with cyclophosphamide (CYP)-induced cystitis. Enhanced target-derived NGF availability increases PACAP expression in small nociceptive dorsal root ganglion cells (Jongsma Wallin et al. 2001). Exogenous administration of NGF to the detrusor smooth muscle or CYP-induced cystitis increases sub P and galanin expressions, respectively, in spinal micturition pathways (Zvarova et al. 2004; Zvara and Vizzard 2007). Pharmacological blockade of PACAP/PAC1 interactions reduced urinary frequency in a rodent model of urinary bladder inflammation induced by CYP treatment (Braas et al. 2006). PACAP or VIP null mice exhibit urinary bladder dysfunction (Studeny et al. 2008; May and Vizzard 2010). We have also demonstrated plasticity in PACAP and VIP and associated receptors in a transgenic mouse model of chronic NGF overexpression in the bladder using the urothelial-specific uroplakin II promoter (Girard et al. 2010). In the present study, we have expanded these studies by examining the contribution of target-derived NGF in combination with CYP-induced cystitis to determine whether additional changes in neuropeptides/receptors and/or bladder function would be observed in micturition reflex pathways of NGF-overexpressing (OE) mice.
We have examined neuropeptide (PACAP, VIP, sub P, and galanin) and associated receptor transcript expressions in the urothelium and detrusor smooth muscle in NGF-OE and littermate wild-type mice using real-time quantitative reverse transcription–polymerase chain reaction (Q-PCR) under control conditions (no inflammation) and following the induction of bladder inflammation with CYP treatment of varying durations (Cheppudira et al. 2008; Arms et al. 2010). Open-outlet, conscious cystometry with continuous instillation of saline (Maggi et al. 1986) was performed in wild-type (WT) and NGF-OE mice to determine bladder function under control conditions (no inflammation) and following the induction of acute bladder inflammation with CYP (4 h) of varying concentrations. In addition, the electrical properties of postganglionic neurons in the major pelvic ganglia (MPG) of NGF-OE and WT mice were characterized in whole-mount preparations using intracellular recording techniques to assess potential changes in postganglionic efferent regulation that could underlie urinary bladder hyperreflexia (e.g., increased voiding frequency and non-voiding bladder contractions) observed in NGF-OE mice (Schnegelsberg et al. 2010).
Materials and Methods
Animals
NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School as previously described (Cheppudira et al. 2008; Schnegelsberg et al. 2010). Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F2 to F4 generations maintained through a hemizygous backcross strategy with C57BL/6J wild-type mice. Mice used in this study were bred locally at the University of Vermont College of Medicine. The litters were of normal size and weight, and behaviors (feeding, drinking, activity patterns) appeared normal. All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC no. 08-085). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and the National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
Measurement of Urinary Bladder NGF by ELISA
Determination of NGF content in the urinary bladder of NGF-OE transgenic mice and WT littermate controls was determined using enzyme-linked immunoassays (ELISAs) as previously described (Vizzard 2000b; Cheppudira et al. 2008; Schnegelsberg et al. 2010). Microtiter plates (R&D Systems, Minneapolis, MN) were coated with a mouse anti-rat NGF antibody (R&D Systems). Sample and standard solutions were run in duplicate. A horseradish peroxidase–streptavidin conjugate was used to detect the antibody complex. Tetramethyl benzidine was the substrate and the enzyme activity was measured by the change in optical density. The NGF standard provided with this protocol generated a linear standard curve from 15 to 1000 pg/ml (R200.997, p≤0.0001) for tissue samples. The absorbance values of standards and samples were corrected by the subtraction of the background absorbance due to nonspecific binding. No samples fell below the minimum detection limits of the assay, and no samples were diluted prior to use. Curve fitting of the standards and evaluation of the NGF content of the samples were performed using a least squares fit as previously described (Vizzard 2000b; Schnegelsberg et al. 2010).
Induction of CYP-Induced Cystitis
Mice were anesthetized with isoflurane (2 %) and received intraperitoneal (i.p.) injection(s) of CYP (Sigma Aldrich, St. Louis, MO) to produce urinary bladder inflammation. To induce chronic bladder inflammation, CYP was injected (75 mg/kg, i.p.) every third day for 10 days, with euthanasia occurring on the tenth day (Cheppudira et al. 2008; Arms et al. 2010). To induce acute bladder inflammation, CYP was injected (150 mg/kg, i.p.), with euthanasia occurring 4 or 48 h after injection (Cheppudira et al. 2008; Arms et al. 2010). Control mice received no treatment. For the determination of neuropeptide and receptor transcript expression in the urothelium and detrusor smooth muscle, control (no inflammation) and CYP-treated (4 h, 48 h, and chronic) WT and NGF-OE mice were assessed. For conscious cystometry studies, mice received CYP (50, 100, or 200 mg/kg, i.p.) with bladder function testing occurring 4 h after injection.
Euthanasia and Tissue Harvest
WT and NGE-OE littermate (n=7–9 for each, 12–16 weeks of age) mice were deeply anesthetized with isoflurane (3–4 %) and then euthanized via thoracotomy. The urinary bladder was quickly dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a Sylgard-coated dish and the urothelium removed with the aid of fine forceps and a dissecting microscope; all tissues were snap-frozen on dry ice prior to processing, as previously described (Arms et al. 2010). The urothelium has suburothelial structures associated with it; the term urothelium in this paper refers to both urothelial and suburothelial structures.
Real-Time Q-PCR
Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test ‘B’, Friendswood, TX, USA) as previously described (Girard et al. 2002; Klinger et al. 2008). One to 2 μg of RNA per sample (urothelium and detrusor smooth muscle) was used to synthesize complementary DNA using SuperScript II reverse transcriptase and a mix of random hexamer and oligo-dT primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA, USA) in a 20-μl final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified PAC1, VPAC1, VPAC2, PACAP, VIP, galanin, sub P, NGF, and 18S cDNA products ligated directly into the pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, tenfold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Real-time quantitative PCR was performed using SYBR Green I detection (Girard et al. 2002; Klinger et al. 2008; Arms et al. 2010). Complementary DNA templates, diluted fivefold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using SYBR Green I JumpStart™. Taq ReadyMix™ (Sigma) containing 5 mM MgCl2; 200 mM dATP, dGTP, dCTP, and dTTP; 0.64 U Taq DNA polymerase; and 300 nM of each primer in a final 25-μl reaction volume. Real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA; Girard et al. 2002; Klinger et al. 2008; Arms et al. 2010) using the following standard conditions: (1) serial heating at 50 °C for 2 min and 94 °C for 2 min and (2) amplification over 40 cycles at 94 °C for 15 s and 60–64 °C depending on primers set for 40 s.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60 to 95 °C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating the amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for PACAP (Girard et al. 2002), VIP (Girard et al. 2002), PAC1 (Braas and May 1999), VPAC1 (Girard et al. 2006), VPAC2 (Girard et al. 2006), NGF (Schnegelsberg et al. 2010), galanin (Girard et al. 2002), sub P (Girard et al. 2002), and 18S (Girard et al. 2002; Klinger et al. 2008) used in these studies have been previously described.
For data analyses, a standard curve was constructed by the amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using the Sequence Detection Software version 1.3.1 (Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene 18S. WT samples are set equal to 100 % for urothelium or detrusor.
Open-Voiding Cystometry in Conscious, Unrestrained Mice
Open-voiding cystometry in conscious, unrestrained mice was conducted as previously described (Klinger and Vizzard 2008; Arms et al. 2010; Schnegelsberg et al. 2010) on 12- to 16-week-old NGF-OE (n=7–10 each for control and CYP treatment groups) transgenic mice and WT (n=7–10 each for control and CYP treatment groups) littermate controls of both genders. To determine the effects of CYP treatment in control and NGF-OE mice, cystometry studies were limited to acute CYP treatment (4 h) of varying concentrations (50, 100, or 200 mg/kg CYP, i.p.). The urinary bladder was exposed through a lower midline abdominal incision under general anesthesia (isoflurane 2.5–3.5 %). A saline-filled PE-10 cannula with the end flared by heat was inserted into the dome of the bladder and secured with a 6–0 nylon purse string suture. The distal end of the cannula was sealed, tunneled subcutaneously to the back, and exteriorized. Muscle and skin layers were closed separately using absorbable and non-absorbable sutures, respectively. The exteriorized part of the cannula was placed in the subcutaneous space and the mice returned to normal caging for 72 h to ensure complete recovery. Postoperative analgesics were given for a period of 48 h. Mice were placed conscious and unrestrained in recording cages with a balance and pan for urine collection and measurement placed below the cage. Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates, Inc., St Albans, VT). The cannula was exteriorized and connected to one port of a pressure transducer; the other port of the pressure transducer was connected to a syringe pump. Room temperature saline was continuously infused at a rate of 25 μl/min to elicit repetitive urinary bladder contractions (Maggi et al. 1986). At least four reproducible micturition cycles were recorded after an initial stabilization period of 25–30 min. Voided saline was collected to determine void volume. After each void volume was collected, the infusion was stopped and residual volume was determined by withdrawing the residual saline through the intravesical catheter. Intercontraction interval, maximal voiding pressure, pressure threshold for voiding, and baseline resting pressure were measured (Klinger and Vizzard 2008; Arms et al. 2010; Schnegelsberg et al. 2010). The numbers of non-voiding urinary bladder contractions (NVCs) per voiding cycle were assessed. For these studies, NVCs were defined as rhythmic intravesical pressure rises (>5 cm H2O from baseline pressure) without a release of fluid from the urethra. Mice were excluded from studies when adverse events occurred, such as ≥20 % reduction in body weight post-surgery, a significant postoperative adverse event, lethargy, and pain or distress not relieved by our IACUC-approved regimen of postoperative analgesics. In the present study, one WT and one NGF-OE transgenic mouse were excluded from the study or from analysis due to postsurgical lethargy. Behavioral movements such as grooming, standing, ambulation, and defecation also rendered bladder pressure recordings during these events unusable; these were excluded from analysis (Klinger and Vizzard 2008; Arms et al. 2010; Schnegelsberg et al. 2010). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements (Herrera and Meredith 2011). Mice were euthanized at the conclusion of the study by isoflurane (4 %) and thoracotomy.
Electrophysiological Recording from the MPG
MPG were isolated from male NGF-OE and WT mice (6–8 weeks). MPG were pinned to the Sylgard (Dow Corning, Midland, MI) floor of a custom recording chamber and superfused continuously (2–3 ml/min; Tompkins et al. 2009) with a HEPES-modified Krebs–Henseleit solution of the following composition (in millimolars): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 8 glucose, and 10 HEPES. The pH was maintained at 7.4 by aeration with 95 % 02–5 % CO2. Intracellular recordings were obtained from visually identified pelvic neurons with high-impedance (75–125 MΩ) borosilicate glass microelectrodes (2 M KCl-filled). Active and passive membrane properties were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Molecular Devices, Sunnyvale, CA; Tompkins et al. 2009). Neurons were characterized as “phasic” or “multiple firing” based on the membrane responsiveness to long-duration (1 s) suprathreshold depolarizing current pulses of increasing intensity (0.1–0.6 nA; Tompkins et al. 2009). Cells firing four or fewer action potentials were classified “phasic” and those with 5 or more action potentials classified as “multiple firing” (Tompkins et al. 2009). Multiple firing neurons included those exhibiting a burst of action potentials at the beginning of the depolarization stimulus or tonic firing throughout the stimulation period (Tompkins et al. 2009). Neuronal input resistance was determined from the change in membrane potential in response to long-duration hyperpolarizing current pulses. The afterhyperpolarization (AHP) duration and the amplitude were measured following brief (5 ms) suprathreshold depolarizations. AHP duration was measured at two thirds the peak AHP amplitude.
Statistical Analyses
One-way analysis of variance was used to evaluate differences among groups for Q-PCR. Percentage data from image analysis were arcsin-transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (p≤ 0.05), the Newman–Keul’s post hoc test was used to compare the experimental means. Membrane properties were compared within strains (phasic vs. multiple firing) and between strains (phasic vs. phasic, multiple firing vs. multiple firing) using an unpaired t test. Differences were considered statistically significant if p≤0.05.
Results
NGF Expression in the Urothelium and Detrusor of WT and NGF-OE Mice
Consistent with our previous studies (Cheppudira et al. 2008; Schnegelsberg et al. 2010), NGF transcript and protein expression was significantly (p≤0.001) increased in the urothelium of NGF-OE mice; no changes were observed in the detrusor smooth muscle between NGF-OE and littermate WT mice (data not shown).
PACAP, VIP, Sub P, and Galanin Transcript Expression in the Urothelium and Detrusor of WT and NGF-OE Mice: Control and CYP
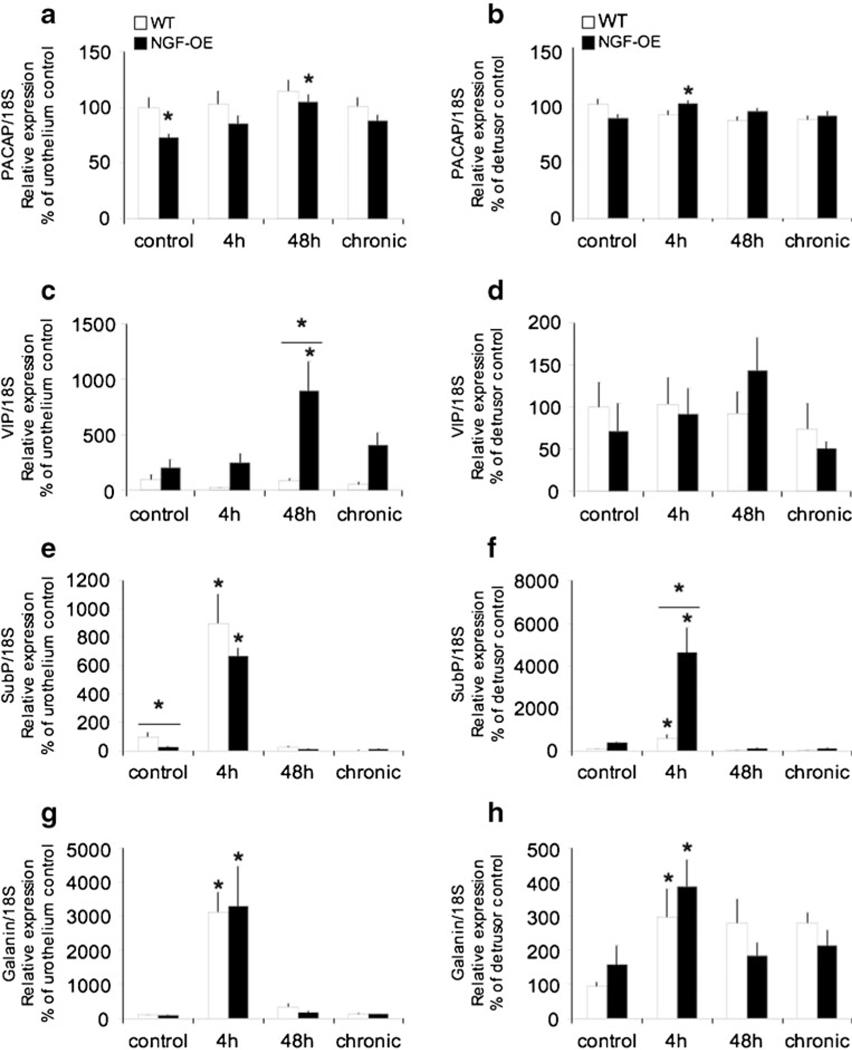
Consistent with previous studies in rats (Braas et al. 2006; Girard et al. 2008), PACAP, VIP, as well as sub P and galanin transcripts were expressed in the urothelium and detrusor smooth muscle of mouse urinary bladder (Fig. 1a–h). PACAP transcript expression was significantly (p≤0.01) decreased in the urothelium of control (no inflammation) NGF-OE mice, whereas PACAP transcript expression was similar in the detrusor smooth muscle in both control WT and NGF-OE mice (Fig. 1a, b). In contrast, VIP and galanin transcript expressions were similar in the urothelium and detrusor smooth muscle of control (no inflammation) WT and NGF-OE mice (Fig. 1c, d, g, h). Sub P transcript expression was significantly (p≤0.01) decreased in the urothelium of control NGF-OE mice compared to WT (Fig. 1e, f). With CYP-induced cystitis, PACAP transcript expression significantly increased in the urothelium (48 h) and detrusor (4 h) of NGF-OE mice (Fig. 1a, b). Similarly, VIP transcript expression significantly increased in the urothelium (48 h) of NGF-OE mice, and this increase was also significantly greater than that observed in WT mice with CYP-induced cystitis (48 h; Fig. 1c, d). Sub P transcript expression was significantly increased in the urothelium and detrusor of WT and NGF-OE mice with CYP-induced cystitis (4 h; Fig. 1e, f). In the detrusor, sub P transcript expression was significantly increased in NGF-OE mice with 4 h of CYP treatment compared to WT mice with 4 h of CYP treatment (Fig. 1e, f). Galanin transcript expression was significantly increased in the urothelium and detrusor of WT and NGF-OE mice with CYP-induced cystitis (4 h), with no differences being observed between WT and NGF-OE mice (Fig. 1g, h).
Fig. 1.
Regulation of PACAP, VIP, substance P (sub P), and galanin transcript levels in littermate wild-type (WT) and NGF-overexpressing (NGF-OE) mice in the urothelium and detrusor smooth muscle with or without cyclophosphamide (CYP) treatment of varying durations (4 h, 48 h, and chronic). Relative expressions of the urothelium (a, c, e, g) and detrusor (b, d, f, h) receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. a, b PACAP mRNA expression. c, d VIP mRNA expression. e, f Sub P mRNA expression. g, h Galanin mRNA expression. Samples size are n=7–9. *p≤0.01 vs. control; asterisk plus solid line, p≤0.01 between WT and NGF-OE
PAC1, VPAC1, and VPAC2 Receptor Transcript Expression in the Urothelium and Detrusor of WT and NGF-OE Mice: Control and CYP
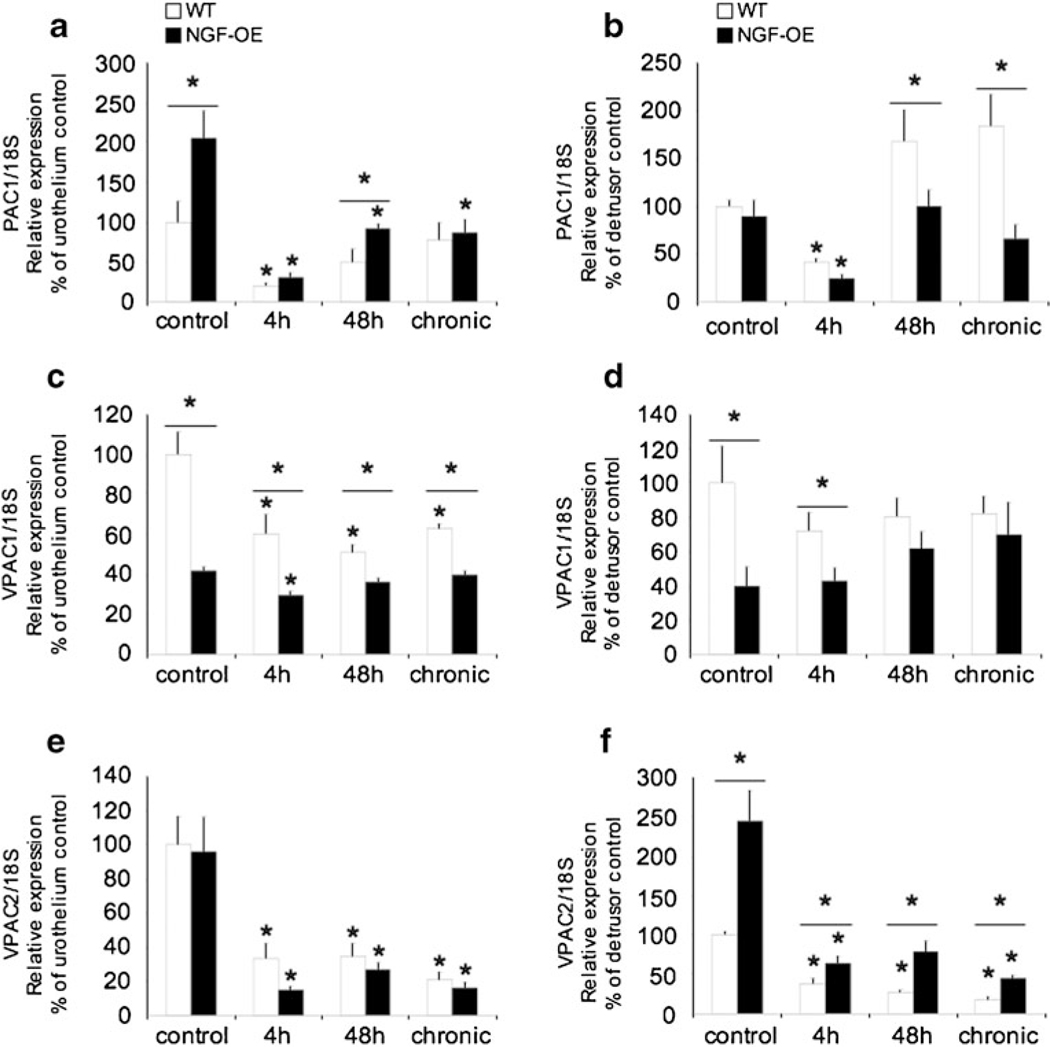
Consistent with previous studies (Braas et al. 2006; Girard et al. 2008), PAC1, VPAC1, and VPAC2 receptor transcripts were expressed in the urothelium and detrusor smooth muscle of mouse urinary bladder in control (no inflammation) WT and NGF-OE mice (Fig. 2a–f). In the urothelium of control NGF-OE mice, PAC1 receptor transcript exhibited a significant (p≤ 0.05) increase in expression, whereas no changes were exhibited in the detrusor smooth muscle between WT and NGF-OE mice (Fig. 2a, b). With 4-h CYP-induced cystitis, PAC1 transcript expression was significantly (p≤0.01) decreased in WT and NGF-OE mice in both the urothelium and detrusor (Fig. 2a, b). With 48-h CYP-induced cystitis, PAC1 receptor transcript expression was still significantly (p≤0.01) decreased in NGF-OE mice—the expression was significantly (p≤ 0.01) greater in NGF-OE mice compared to WT—but this difference in PAC1 receptor transcript expression was absent with chronic CYP-induced cystitis (Fig. 2a). PAC1 transcript expression in the detrusor smooth muscle was significantly (p≤0.01) increased in WT compared to NGF-OE mice following 48 h and chronic CYP treatment (Fig. 2b). CYP-induced cystitis significantly (p≤0.01) decreased VPAC1 receptor transcript expression in the urothelium of WT (4 h, 48 h, and chronic) and NGF-OE mice (4 h; Fig. 2c). In the detrusor smooth muscle of control NGF-OE mice, VPAC2 receptor transcript expression was significantly (p≤0.01) increased compared to control WT, whereas no changes were observed in the urothelium (Fig. 2e, f). CYP-induced cystitis (4 h, 48 h, and chronic) significantly decreased VPAC2 receptor expression in the urothelium and detrusor of both WT and NGF-OE mice (Fig. 2e, f). Reductions in VPAC2 receptor expression in the detrusor smooth muscle were significantly (p≤ 0.05) greater in WT mice compared to NGF-OE mice treated with CYP (4 h, 48 h, and chronic; Fig. 2e, f). In contrast, reductions in VPAC2 receptor expression in the urothelium with CYP treatment (4 h, 48 h, and chronic) were comparable between WT and NGF-OE mice (Fig. 2e, f).
Fig. 2.
Regulation of PAC1, VPAC1, and VPAC2 receptor transcript levels in littermate wild-type (WT) and NGF-overexpressing (NGF-OE) mice in the urothelium and detrusor smooth muscle with or without cyclophosphamide (CYP) treatment of varying durations (4 h, 48 h, and chronic). Relative expressions of the urothelium (a, c, e) and detrusor (b, d, f) receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. a, b PAC1 mRNA expression. c, d VPAC1 mRNA expression. e, f VPAC2 mRNA expression. Samples size are n=7–9. *p≤ 0.01 vs. control; asterisk plus solid line, p≤0.01 between WT and NGF-OE
Altered Urinary Bladder Function in NGF-OE Transgenic Mice: Control and CYP-Treated
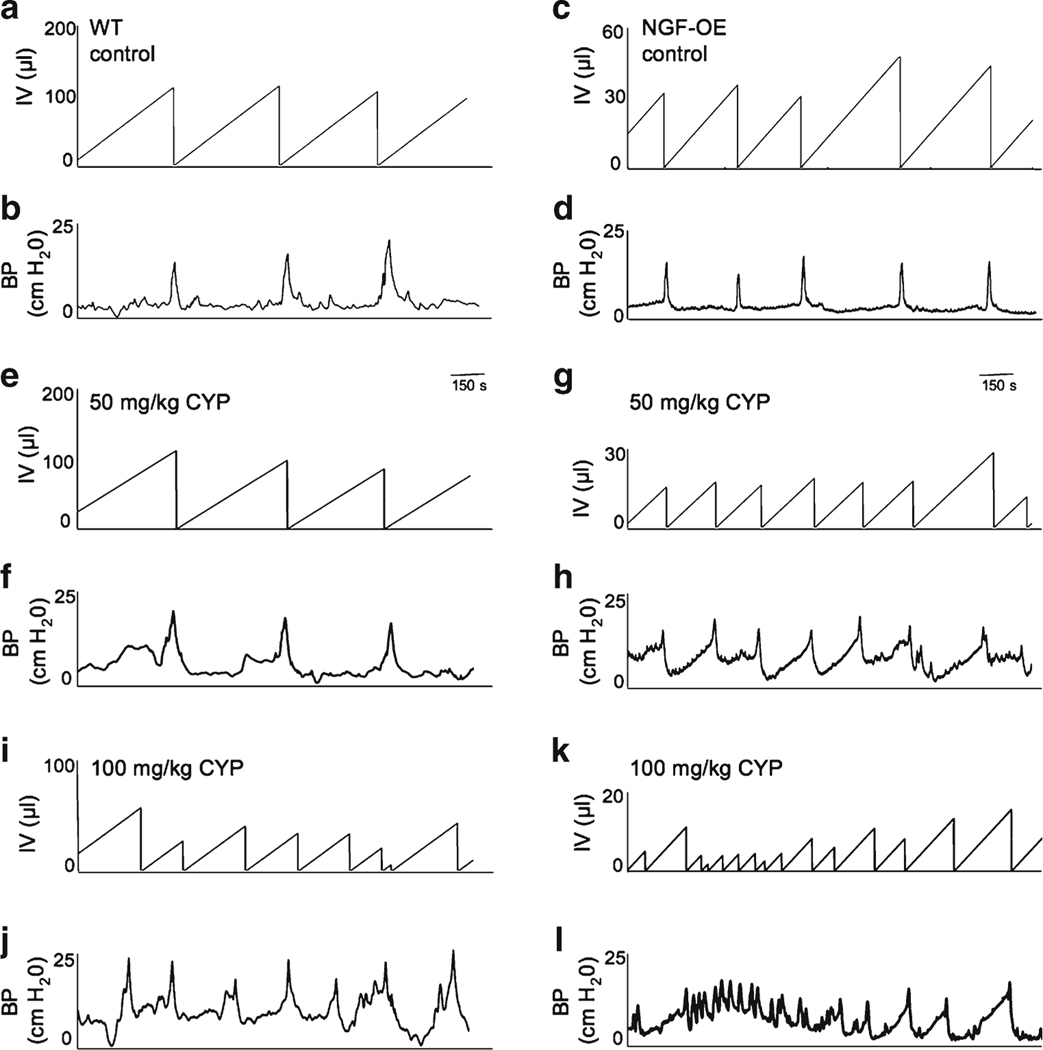
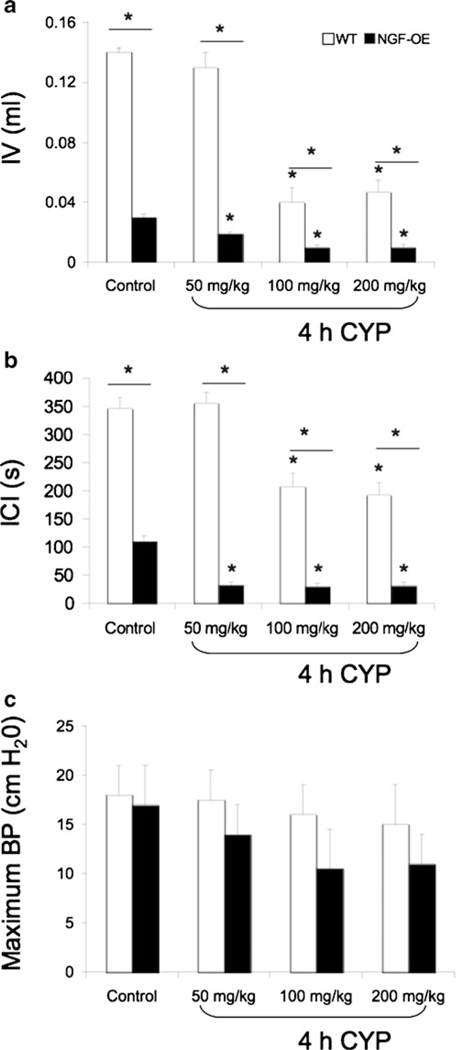
Consistent with previous studies (Schnegelsberg et al. 2010), NGF-OE mice exhibited increased voiding frequency (Fig. 3a, c) with significantly (p≤0.001) reduced void volumes and intercontraction intervals compared to WT mice (Fig. 4a, b). Figure 3 shows representative open-voiding cystometrograms in conscious, unrestrained mice in which the voiding reflexes were measured in response to a continuous infusion of saline. The reductions in void volume and intercontraction interval were present in both male and female transgenic mice with a similar magnitude of change; thus, data from both genders were pooled and analyzed together. No changes in baseline resting pressure, micturition threshold pressure, or maximum voiding pressure were observed between WT and NGF-OE mice (Fig. 4c and data not shown). There were also no differences in residual volume between WT (6±2 μl) and NGF-OE mice (5±2.5 μl). NVCs were present in WT and NGF-OE mice under conscious cystometry conditions, but were not detected with each voiding cycle for either group and were not analyzed further. To determine whether CYP-induced cystitis could induce an additional change in voiding frequency in NGF-OE mice as shown in control rats and mice, NGF-OE mice and littermate WT mice were treated acutely (4 h) with different concentrations of CYP (50, 100, or 200 mg/kg). CYP treatment (100 or 200 mg/kg) in WT mice increased voiding frequency with significantly (p≤0.01) reduced void volumes and intercontraction intervals; however, no changes in bladder function were observed with the lowest concentration (50 mg/kg) of CYP evaluated in WT mice (Fig. 3a, b, e, f, i, j and Fig. 4a, b). In contrast, each concentration of CYP evaluated in NGF-OE mice increased voiding frequency with significantly (p≤0.01) reduced void volumes and intercontraction intervals compared to control (no inflammation) NGF-OE mice (Fig. 3c, d, g, h, k, l and Fig. 4a, b). Changes in micturition parameters evaluated for NGF-OE were comparable among all CYP concentrations tested (Fig. 4). No changes in baseline resting pressure, micturition threshold pressure, or maximum voiding pressure were observed between WT and NGF-OE mice treated with different concentrations of CYP (Fig. 4c and data not shown).
Fig. 3.
Open cystometry in conscious WT and NGF-OE transgenic mice. Representative cystometrogram traces from conscious, unrestrained WT (a, b) without inflammation (no cyclophosphamide (CYP) treatment) and NGF-OE (c, d) mouse without CYP treatment during a continuous intravesical infusion (25 μl/min) of room temperature saline. e, f Effects of CYP (50 mg/kg, i.p.) on WT bladder function. g, h Effects of CYP (50 mg/kg, i.p.) on NGF-OE bladder function. i, j Effects of CYP (100 mg/kg, i.p.) on WT bladder function. k, l Effects of CYP (100 mg/kg, i.p.) on NGF-OE bladder function. Infused volume (IV, in microliters) and bladder pressure (BP, in centimeter H2O) are shown
Fig. 4.
Summary bar graphs from open cystometry. Bar graphs of infused volume (IV, in milliliters) (a), intercontraction interval (ICI, in seconds) (b), and maximum bladder pressure (BP, in centimeter H2O) (c) from open cystometry in conscious, unrestrained WT and NGF-OE transgenic mice with and without CYP treatment. IV (a) and ICI (b) were significantly (asterisk with solid line, p≤0.001) reduced in transgenic mice compared to WT mice under control conditions (no CYP). CYP treatment (50 mg/kg) significantly (*p≤0.01) reduced IV (a) and ICI (b) in NGF-OE mice, but was without effect in WT mice. CYP treatment (100 or 200 mg/kg) significantly (*p≤0.01) reduced IV (a) and ICI (b) in both WT and NGF-OE mice. Differences between WT and NGF-OE IV (a) and ICI (b) were maintained with CYP treatment (asterisk with solid line, p≤0.001). No changes in baseline, threshold (data not shown) or maximum BP (c) were observed between WT and transgenic mice using conscious cystometry with or without CYP treatment. Data represent the mean±SEM of n=7–10 mice per group
Electrophysiological Properties of MPG Neurons in WT and NGF-OE Mice
Neurons of the MPG in WT and NGF-OE mice were classified into two groups, “phasic” and “multiple firing” (Table 1), based on the membrane response to multiple long-duration (1 s) depolarizing current pulses of increasing amplitudes (0.1–0.6 nA). Cells termed “phasic” fired four or fewer action potentials, and the frequency did not increase in response to increasing stimulus intensity. “Multiple firing” cells fired five or more spikes and increased action potential frequency with increasing stimulus intensity. Sixty percent of the MPG neurons of WT mice sampled were phasic (15/25) and 40 % were multiple firing (10/25; Table 1). Seventy-two percent of the MPG neurons of NGF-OE mice sampled were phasic (18/25) and 28 % were multiple firing (7/25) (Table 1).
Table 1.
Electrophysical characteristics of MPG neurons in WT and NGF-OE mice
| WT (25) |
NGF-OE (25) |
|||
|---|---|---|---|---|
| Phasic | Multiple firing | Phasic | Multiple firing | |
|
| ||||
| Cell type | 60 % (15/25) | 40 % (10/25) | 72 % (18/25) | 28 % (7/25) |
| RMP (mV) | −60±3 (8) | −58±3 (7) | −58±1 (10) | −56±1 (4) |
| Input R (MΩ) | 116±20 (15) | 201±31 (10)* | 142±18 (18) | 161±41 (7) |
| AHP amplitude (mV) | 10±1 (15) | 6±1 (9) | 8±1 (17) | 7±1 (7) |
| AHP duration (ms) | 202±60 (15) | 26±10 (9)* | 213±51 (17) | 65±26 (7) |
MPG major pelvic ganglion, WT wild-type, NGF-OE nerve growth factor overexpressing, RMP resting membrane potential, R resistance, AHP. afterhyperpolarization
p≤0.05 (compared to phasic-firing MPG neurons in WT mice)
The amplitude and duration of the AHP, input resistance, and resting membrane potential of MPG neurons in WT and NGF-OE mice were also characterized (Table 1). Peak AHP amplitude was the difference between the maximum hyperpolarized membrane potential value recorded after the AP and the resting membrane potential. AHP duration was measured from the time the AHP crossed the resting membrane potential until it returned to two thirds of the peak AHP amplitude. For cells in which no holding current was used, the resting membrane potentials (RMP) values of phasic MPG neurons of WT mice were −60±3 mV (n=8) and −58±3 mV (n=7) for tonic MPG neurons of WT mice. RMP of the phasic and tonic MPG neurons of NGF-OE mice were similar (Table 1). The input resistance in multiple firing MPG neurons of WT mice was significantly (p≤0.05) greater than that in phasic-firing MPG neurons of WT mice (201±31 MΩ (n=10) vs. 116±20 MΩ (n=15)). An AHP followed the action potential in all cells, from which recordings were made. AHP durations in multiple firing MPG neurons of WT mice were significantly (p≤0.05) shorter than the AHP duration in phasic-firing MPG neurons of WT mice (Table 1). A similar difference in AHP duration in phasic-firing MPG neurons compared to multiple firing MPG neurons was observed in NGF-OE mice, but the difference was not significant (p=0.08). No differences in electrophysiological properties in MPG neurons (phasic or multiple firing) were observed between WT and NGF-OE mice (Table 1).
Discussion
We previously examined PACAP, VIP, and associated receptors in the urothelium and detrusor of littermate WT and transgenic mice with chronic overexpression of NGF in the urothelium (NGF-OE; Girard et al. 2010), but in these studies, we questioned whether additional plasticity in neuropeptide and receptor expression in the urinary bladder as well as bladder function would be observed in NGF-OE mice treated with cyclophosphamide (CYP) to induce urinary bladder inflammation (Cheppudira et al. 2008; Arms et al. 2010). These studies demonstrate additional changes in neuropeptide and receptor transcript expression in the urothelium and detrusor as well as additional bladder functional changes when NGF-OE mice are treated with CYP to develop urinary bladder inflammation. The electrical properties of postganglionic neurons in the MPG from littermate WT and NGF-OE mice were not different, as demonstrated with intracellular recording techniques. These findings suggest that urinary bladder hyperreflexia observed in the NGF-OE mice is mediated by changes in the afferent limb of the micturition reflex involving the dorsal root ganglia and/or spinal cord.
Increased urinary bladder NGF content may underlie many of the sensory changes that occur in patients with OAB symptoms or IC/BPS, including irritative voiding symptoms and pain in the case of IC/BPS (Kim et al. 2005, 2006). Altered NGF content is associated with urinary bladder inflammation and dysfunction in rodents (Vizzard 2001; Zvarova et al. 2004; Braas et al. 2006; Klinger and Vizzard 2008). IC/BPS is a chronic inflammatory bladder disease of unknown etiology characterized by urinary frequency, urgency, and suprapubic/pelvic pain (Driscoll and Teichman 2001). Pain and altered bladder/visceral hypersensitivity in IC/BPS patients may involve organizational or functional changes in peripheral bladder afferents and central pathways such that bladder afferent neurons become sensitized and hyper-responsive to normally innocuous stimuli such as bladder filling (Driscoll and Teichman 2001; Evans et al. 2011; Mantyh et al. 2011). A few studies have demonstrated increased innervation of the bladder suburothelial and detrusor layers in IC/BPS patients (Christmas et al. 1990; Lundeberg et al. 1993; Hoyle et al. 1998; Peeker et al. 2000). In NGF-OE mice, we demonstrated an increased density of CGRP- and sub P-positive C-fiber sensory afferents, neurofilament 200 myelinated sensory afferents, and tyrosine hydroxylase-positive sympathetic nerve fibers within the suburothelial nerve plexus of the urinary bladder (Schnegelsberg et al. 2010).
In this study, we have begun to determine whether chronic NGF-OE in the urothelium combined with CYP-induced urinary bladder inflammation causes additional neurochemical plasticity of PACAP, VIP, and associated receptors (PAC1, VPAC1, VPAC2) and sub P and galanin expressions in the urinary bladder that might contribute to the bladder hyperreflexia and pelvic hypersensitivity in NGF-OE mice with CYP-induced cystitis (Schnegelsberg et al. 2010). We have previously demonstrated (Schnegelsberg et al. 2010) that NGF in the urinary bladder of transgenic mice (1) stimulates neuronal sprouting or proliferation in the urinary bladder; (2) produces local inflammatory changes in the urinary bladder; (3) produces urinary bladder hyperreflexia; and (4) results in increased referred somatic hypersensitivity. Chronic NGF overexpression in the urothelium may cause additional phenotypic or excitability changes in bladder sensory afferents that might contribute to the bladder hyperreflexia and pelvic hypersensitivity in NGF-OE mice. Growth factors, such as BDNF; ion channels, such as TRPV1, P2X3, P2X2/3, or Nav1.8; and neuropeptides, such as PACAP, sub P, and calcitonin gene-related peptide, can directly modulate pain and bladder/visceral sensory function and NGF-mediated changes in these mediators, either peripherally or centrally, and could contribute to altered urinary bladder functions in NGF-OE mice (Yoshimura et al. 2002; Allen and Dawbarn 2006; Ford et al. 2006; Pezet and McMahon 2006; Szallasi et al. 2007; Schnegelsberg et al. 2010).
PACAP peptides have diverse functions in the endocrine, nervous, gastrointestinal, and cardiovascular systems (Braas and May 1996; Arimura 1998) and differential effects on nociception (Sandor et al. 2009) through PAC1, VPAC1, and VPAC2 G protein-coupled receptors. High levels of PACAP and VIP expression have been identified in many central nervous system neurons and in sensory and autonomic ganglia (Arimura et al. 1991; Sundler et al. 1996; Moller et al. 1997a, b; Arimura 1998; Braas et al. 1998). Both PACAP and VIP immunoreactivity has been identified in the urinary bladder (Fahrenkrug and Hannibal 1998; Mohammed et al. 2002). PACAP and VIP peptides regulate smooth muscle function, either directly or by facilitating cholinergic and nitric oxide mechanisms, in a tissue- and species-specific manner (Mizumoto et al. 1992; Onaga et al. 1998; Fox-Threlkeld et al. 1999; Seebeck et al. 2002; Zizzo et al. 2004). A number of studies have implicated PACAP in lower urinary tract function (Vizzard 2000a; Zvarova et al. 2005; Braas et al. 2006; Herrera et al. 2006; Zvara et al. 2006). Each of these neuropeptides (e.g., PACAP, VIP, sub P, and galanin) and/or receptors (PAC1, VPAC1, and VPAC2) is expressed in micturition reflex pathways and exhibits altered expressions in micturition pathways with CYP-induced cystitis or in NGF-OE mice (Vizzard 2000a, 2001; Braas et al. 2006; Zvarova and Vizzard 2006; Zvara and Vizzard 2007). Pharmacological blockade of PACAP/PAC1 interactions with PACAP6–38 reduces bladder hyperreflexia in rats treated with CYP (Braas et al. 2006), and PACAP null or VIP null mice exhibit altered bladder function and morphological changes in the urinary bladder (Studeny et al. 2008; May and Vizzard 2010).
Additional pleiotropic changes in transcript expression of other growth factors and associated receptors in NGF-OE mice, including BDNF, TrkB, TrkA, and p75NTR in the urothelium and detrusor smooth muscle of NGF-OE mice, were recently evaluated (Girard et al. 2011). In the urothelium of NGF-OE mice, significant decreases (e.g., BDNF, TrkA, and TrkB) or no change (e.g., p75NTR) in expression was detected in mRNA and protein expression (Girard et al. 2011). We hypothesized that reductions in BDNF, TrkA, and TrkB expressions in NGF-OE mice were an ineffective compensatory mechanism to reduce urinary bladder hyperreflexia (Girard et al. 2011). Similarly, in the present study, decreases (e.g., PACAP and sub P) or no changes (VIP and galanin) in transcript expression were observed in the urothelium, and no changes were observed in the detrusor smooth muscle of NGF-OE mice in the absence of CYP-induced cystitis. In general, the induction of CYP-induced cystitis in NGF-OE mice did produce increases in PACAP, VIP, sub P, and galanin transcript expression, and in some instances, expression (VIP and sub P) in NGF-OE mice was significantly greater in the urothelium or detrusor than that observed in littermate WT mice. More significant changes in PAC1, VPAC1, and VPAC2 receptor expressions were observed in the urothelium and detrusor of NGF-OE mice with CYP-induced cystitis. Significant differences in receptor expression between NGF-OE and littermate WT mice treated with CYP were observed for PAC1, VPAC1, and VPAC2, but this was tissue (urothelium vs. detrusor) and duration (4 h, 48 h, and chronic) of CYP treatment-dependent. Thus, chronic NGF overexpression in the urothelium does not prevent additional neurochemical plasticity in neuropeptide and receptor expression in the urinary bladder of NGF-OE mice with CYP-induced cystitis. Future immunohistochemical and in situ hybridization studies are essential to unequivocally differentiate the localization of these neuropeptides and their receptors in urothelial cells, suburothelial nerve plexus, and/or detrusor smooth muscle of the urinary bladder. Once the tissue distribution of these neuropeptides and receptors is clear, the functional contribution of altered PACAP, VIP, and associated receptor expressions in the urinary bladder of NGF-OE mice with CYP-induced cystitis will require future pharmacological studies to block PACAP/receptor signaling.
These results suggest that chemical mediators upregulated with CYP-induced bladder inflammation other than or in addition to NGF (e.g., neurotrophins: Vizzard 2000b; cytokines: Malley and Vizzard 2002; and/or chemokines: Yuridullah et al. 2006; Arms et al. 2010) contribute to neuropeptide/receptor transcript and protein expression in micturition pathways following CYP-induced cystitis in NGF-OE mice. A number of recent studies have evaluated the utility of NGF antagonism for the treatment of symptoms associated with IC/BPS as well as other chronic pain conditions (Evans et al. 2011; Mantyh et al. 2011). Such approaches depend on the contribution and the duration or stage of the NGF contribution to the pain condition being evaluated (Mantyh et al. 2011). It has been suggested that it is important to determine which types of pain conditions are driven by and maintained by NGF as well as understanding which mediators induced by NGF signaling can contribute to the activation and sensitization of nociceptors (Mantyh et al. 2011). The present studies begin to define the contribution of NGF as well as neuropeptides/receptors induced by NGF/signaling to neurochemical and functional plasticity in micturition reflex pathways.
NGF-OE mice exhibit urinary bladder hyperreflexia associated with increased voiding frequency, as previously determined with conscious, open-outlet, cystometry with continuous instillation of saline (Schnegelsberg et al. 2010) and confirmed in this study. It was uncertain whether urinary bladder inflammation induced by CYP could further increase voiding frequency in NGF-OE mice or whether the change in voiding frequency was saturated and no additional increases in voiding frequency would be observed. NGF-OE mice treated with different concentrations (50, 100, or 200 mg/kg) of CYP for 4 h demonstrated an additional increase in voiding frequency with each concentration tested above, which was observed in control NGF-OE mice without CYP-induced cystitis. In contrast, WT mice treated with different concentrations (50, 100, or 200 mg/kg) of CYP for 4 h demonstrated an increase in voiding frequency following treatment with 100 or 200 mg/kg CYP, but the lowest concentration evaluated (50 mg/kg) was without effect in WT mice. TRPA1, a member of the transient receptor potential (TRP) family of ion channels, is expressed by dorsal root ganglion neurons, urothelial cells, and by cells of the inner ear, where it has proposed roles in sensing sound, painful cold, and irritating chemicals including acrolein, the metabolite of CYP that induces urinary bladder inflammation (Bautista et al. 2006; Kwan et al. 2006). Studies from our laboratory demonstrate that several TRP channels, including TRPA1, TRPV1, and TRPV4, are increased in the urinary bladder of NGF-OE mice (Vizzard et al., unpublished observations). Differences in the CYP sensitivity to increase urinary bladder frequency may involve the increased expression of TRPA1 in the urinary bladder of NGF-OE mice. Ongoing studies are evaluating the role (s) of TRPA1 in urinary bladder hyperreflexia in NGF-OE mice with and without CYP-induced cystitis.
The analysis of the electrophysiological properties of MPG neurons using intracellular recording techniques demonstrated no differences between NGF-OE and WT mice. We acknowledge that these data are from randomly sampled MPG neurons; however, in a subset of dye-labeled (Fastblue) bladder postganglionic neurons in NGF-OE and WT mice, no differences in the electrophysiological properties were observed (Tompkins et al., unpublished observations). The absence of changes in the electrical properties of pelvic ganglion neurons in NGF-OE mice suggests that changes in urinary bladder hyperreflexia in NGF-OE mice are not likely to be driven by changes in the postganglionic efferent limb of the micturition reflex. Previous studies (Schnegelsberg et al. 2010) also demonstrate no changes in urinary bladder contractility in NGF-OE mice using myograph recording techniques. As previously suggested (Schnegelsberg et al. 2010), the urinary bladder phenotype observed in the NGF-OE mice likely reflects a change in the afferent limb of the micturition reflex. These data are consistent with studies (Lin et al. 2010; Stewart et al. 2008) demonstrating that NGF is not as effective as other growth factors (e.g., BDNF, GDNF, VEGF, NT-3, neurturin, and artemin) in eliciting MPG neurite outgrowth and cell migration in cultured MPG explants from embryonic and newborn mice or postnatal rats. It is possible that growth factors (e.g., BDNF, GDNF, VEGF, NT-3, neurturin, and artemin) that elicit neurite outgrowth and cell migration (Lin et al. 2010; Stewart et al. 2008) could also influence the functional properties of MPG neurons; such studies can be pursued in the future. Future studies will also examine the properties of bladder afferent cells in the lumbosacral dorsal root ganglia in NGF-OE mice.
Acknowledgments
The authors thank Dr. Debra Cockayne, Roche Palo Alto, for the generous gift of NGF-OE mouse breeders used in the present study. The authors acknowledge the technical support of Abbey Peterson in the conduct of these studies. The authors gratefully acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility. This work was funded by National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV), DK065989 (MAV), and DK081444 (JDT). This publication was also supported by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
Contributor Information
Beatrice M. Girard, Department of Anatomy and Neurobiology, University of Vermont, Burlington, VT 05405, USA
John D. Tompkins, Department of Anatomy and Neurobiology, University of Vermont, Burlington, VT 05405, USA
Rodney L. Parsons, Department of Anatomy and Neurobiology, University of Vermont, Burlington, VT 05405, USA
Victor May, Department of Anatomy and Neurobiology, University of Vermont, Burlington, VT 05405, USA.
Margaret A. Vizzard, Department of Anatomy and Neurobiology, University of Vermont, Burlington, VT 05405, USA Department of Neurology, University of Vermont College of Medicine, D415A Given Research Building, Burlington, VT 05405, USA.
References
- Allen SJ, Dawbarn D (2006) Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 110:175–191 [DOI] [PubMed] [Google Scholar]
- Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48:301–331 [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C (1991) Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129:2787–2789 [DOI] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA (2010) Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298:F589–F600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J et al. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282 [DOI] [PubMed] [Google Scholar]
- Braas KM, May V (1996) Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci 805:204–216, discussion 217–208 [DOI] [PubMed] [Google Scholar]
- Braas KM, May V (1999) Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274:27702–27710 [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL (1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18:9766–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD et al. (2006) Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290:R951–R962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA (2008) Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295:F826–F836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT (1990) Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol 416:447–451 [DOI] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu YB, Chancellor MB, deGroat WC, Yoshimura N (2001) The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165:975–979 [PubMed] [Google Scholar]
- Clemow DB, Steers WD, McCarty R, Tuttle JB (1998) Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 44:R1279–R1286 [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Shelton D, Rice ASC, McMahon SB (1997) The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78:449–459 [DOI] [PubMed] [Google Scholar]
- Driscoll A, Teichman JMH (2001) How do patients with interstitial cystitis present? J Urol 166:2118–2120 [PubMed] [Google Scholar]
- Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D (2011) Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 185:1716–1721 [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J (1998) Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience 83:1261–1272 [DOI] [PubMed] [Google Scholar]
- Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP et al. (2006) Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147(Suppl 2):S132–S143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox-Threlkeld JA, McDonald TJ, Woskowska Z, Iesaki K, Daniel EE (1999) Pituitary adenylate cyclase-activating peptide as a neurotransmitter in the canine ileal circular muscle. J Pharmacol Exp Ther 290:66–75 [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM (2002) Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109:89–101 [DOI] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y et al. (2006) Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem 99:499–513 [DOI] [PubMed] [Google Scholar]
- Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA (2008) PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36:310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Braas KM, May V, Vizzard MA (2010) PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci 42:378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Vizzard MA (2011) Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol 300:F345–F355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Bjorling DE (2006) Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392:193–197 [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE (2008) Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295:R111–R122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Meredith AL (2011) Diurnal variation in urodynamics of rat. PLoS One 5:e12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Braas KM, May V, Vizzard MA (2006) PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann N Y Acad Sci 1070:330–336 [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M (1998) Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18:149–157 [DOI] [PubMed] [Google Scholar]
- Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D et al. (2005) Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173:1016–1021 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci 24:677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar SI, Scott HCF, Rice ASC (1999) Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83:442–448 [DOI] [PubMed] [Google Scholar]
- Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VMK (2001) Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci 14:267–282 [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK (2005) Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol 12:875–880 [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Seo SI, Park YH, Hwang TK (2006) Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175:1773–1776, discussion 1776 [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA (2008) Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295:F1778–F1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA (2008) p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507:1379–1392 [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ et al. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Skaper SD, Dal TR, Petrelli L, Leon A (1996) Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 19:514–520 [DOI] [PubMed] [Google Scholar]
- Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM et al. (2005) Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Zhao H, Sun TT (1995) A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci U S A 92:679–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, Lue TF (2010) Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int 105:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Kuo HC (2007) Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 70:463–468 [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC (2008a) Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol 179:2270–2274 [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC (2008b) Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72:104–108 [DOI] [PubMed] [Google Scholar]
- Liu HT, Chancellor MB, Kuo HC (2008a) Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56:700–706 [DOI] [PubMed] [Google Scholar]
- Liu HT, Chancellor MB, Kuo HC (2008b) Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int 102:1440–1444 [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL (1997) Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79:572–577 [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Liedberg H, Nordling L, Theodorsson E, Owzarski A, Ekman P (1993) Interstitial cystitis: correlation with nerve fibres, mast cells and histamine. Br J Urol 71:427–429 [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A (1986) The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15:157–167 [DOI] [PubMed] [Google Scholar]
- Malley SE, Vizzard MA (2002) Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9:5–13 [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL (2011) Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 115:189–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Vizzard MA (2010) Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183:772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto A, Fujimura M, Ohtawa M, Ueki S, Hayashi N, Itoh Z et al. (1992) Pituitary adenylate cyclase activating polypeptide stimulates gallbladder motility in conscious dogs. Regul Pept 42:39–50 [DOI] [PubMed] [Google Scholar]
- Mohammed H, Hannibal J, Fahrenkrug J, Santer R (2002) Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res 30:248–255 [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M (1997a) Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res 775:156–165 [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M et al. (1997b) The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res 775:166–182 [DOI] [PubMed] [Google Scholar]
- Oddiah D, Anand P, McMahon SB, Rattray M (1998) Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9:1455–1458 [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF et al. (1999) Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161:438–442 [PubMed] [Google Scholar]
- Onaga T, Harada Y, Okamoto K (1998) Pituitary adenylate cyclase-activating polypeptide (PACAP) induces duodenal phasic contractions via the vagal cholinergic nerves in sheep. Regul Pept 77:69–76 [DOI] [PubMed] [Google Scholar]
- Peeker R, Enerback L, Fall M, Aldenborg F (2000) Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol 163:1009–1015 [PubMed] [Google Scholar]
- Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507–538 [DOI] [PubMed] [Google Scholar]
- Sandor K, Bolcskei K, McDougall JJ, Schuelert N, Reglodi D, Elekes K et al. (2009) Divergent peripheral effects of pituitary adenylate cyclase-activating polypeptide-38 on nociception in rats and mice. Pain 141:143–150 [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP et al. (2010) Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298:R534–R547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebeck J, Lowe M, Kruse ML, Schmidt WE, Mehdorn HM, Ziegler A et al. (2002) The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul Pept 107:115–123 [DOI] [PubMed] [Google Scholar]
- Stewart AL, Anderson RB, Kobayashi K, Young HM (2008) Effects of NGF, NT-3 and GDNF family members on neurite outgrowth and migration from pelvic ganglia from embryonic and newborn mice. BMC Dev Biol 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA et al. (2008) Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H et al. (1996) Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci 805:410–426 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6:357–372 [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Lawrence YT, Parsons RL (2009) Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol 297: R52–R59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA (2000a) Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420:335–348 [PubMed] [Google Scholar]
- Vizzard MA (2000b) Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161:273–284 [DOI] [PubMed] [Google Scholar]
- Vizzard MA (2001) Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21:125–138 [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Kumon H, Nagai A (2008) Correlation of urinary nerve growth factor level with pathogenesis of overactive bladder. Neurourol Urodyn 27:417–420 [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T (2002) Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 59:61–67 [DOI] [PubMed] [Google Scholar]
- Yuridullah R, Corrow KA, Malley SE, Vizzard MA (2006) Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci 126–127:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R (2004) Interplay between PACAP and NO in mouse ileum. Neuropharmacology 46:449–455 [DOI] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA (2007) Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Braas KM, May V, Vizzard MA (2006) A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI). Ann N Y Acad Sci 1070:622–628 [DOI] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA (2006) Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell Tissue Res 324:213–224 [DOI] [PubMed] [Google Scholar]
- Zvarova K, Murray E, Vizzard MA (2004) Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol 475:590–603 [DOI] [PubMed] [Google Scholar]
- Zvarova K, Dunleavy JD, Vizzard MA (2005) Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol 192:46–59 [DOI] [PubMed] [Google Scholar]