Abstract
Current guidelines on atrial fibrillation (AF) emphasized that radiofrequency catheter ablation (RFCA) should be decided after fully considering its prognosis. However, a robust prediction model reflecting the complex interactions between the features affecting prognosis remains to be developed. In this paper, we propose a deep learning model for predicting the late recurrence after RFCA in patients with AF. Aiming to predict the late recurrence (LR) of AF within 1 year after pulmonary vein isolation, we designed a multimodal model based on the multilayer perceptron architecture. For quantitative evaluation, we conducted 4-fold cross-validation on data from 177 AF patients including 47 LR patients. The proposed model (area under the receiver operating characteristic curve-AUROC, 0.766) outperformed the acute patient physiologic and laboratory evaluation (APPLE) score (AUROC, 0.605), CHA2DS2-VASc score (AUROC, 0.595), linear regression (AUROC, 0.541), logistic regression (AUROC, 0.546), extreme gradient boosting (AUROC, 0.608), and support vector machine (AUROC, 0.638). The proposed model exhibited better performance than clinical indicators (APPLE and CHA2DS2-VASc score) and machine learning techniques (linear regression, logistic regression, extreme gradient boosting, and support vector machine). The model will support clinical decision-making for selecting good responders to the RFCA intervention.
1. Introduction
Radiofrequency catheter ablation (RFCA) is accepted as the first-line therapy for patients with symptomatic atrial fibrillation (AF) refractory to antiarrhythmic drugs [1], since Haïssaguerre et al. suggested it as a treatment modality [2]. However, the benefits of RFCA in patients with AF are frequently offset by late recurrence (LR) after the procedure [3]. Moreover, various attempts to modify the atrial substrate, including linear ablation lesion set, ablation targeting rotor, or complex fractionated atrial electrogram, do not demonstrate superiority to pulmonary vein isolation [4, 5]. Therefore, the most recent treatment guidelines for AF recommend assessing the benefit to patients for ensuring a high probability of success after RFCA [1].
Although observational studies have suggested the duration of AF, age, left atrium (LA) size, renal function, and other factors as predictors of LR [6–9], no single factor can accurately predict recurrence after AF ablation [1]. To improve prediction, various models providing indicators such as the CHA2DS2-VASc score [10, 11] and acute patient physiologic and laboratory evaluation (APPLE) score [12] have been developed. However, these models have shown modest performance [13], as they are based on simple linear regression where each risk factor is assigned one or two points and the sum represents the final score.
Recently, machine learning (ML) methods have been proposed to analyze high-order interactions between different features [14, 15]. For instance, a predictive model based on a support vector machine (SVM) showed an area under the receiver operating characteristic curve (AUROC) of 0.75 for predicting LR within 1 year after RFCA, by considering the AF type (paroxysmal vs. persistent), previous ablation procedure, LA volume, and epicardial fat volume as inputs [16]. In addition, deep learning methods that automatically extract hierarchical features have outperformed traditional ML methods. A recent study [35] employed convolutional neural networks to predict LR from the N-terminal probrain natriuretic peptide, paroxysmal AF, LA appendage volume, and LA volume.
The multilayer perceptron (MLP) technique which analyzes complex nonlinear relations between input features has demonstrated promising performance in various medical applications [18–20]. Therefore, we proposed an MLP-based model for predicting rhythm outcomes after RFCA in patients with AF and compared our model with conventional prediction models and other ML approaches.
2. Methods
2.1. Study Population and Ethical Statement
We analyzed consecutive patients with AF who underwent RFCA at Chungbuk National University Hospital (CBNUH) from February 2017 to October 2020. All the patients were over 18 years old and underwent their first RFCA. Exclusion criteria included patients with repeated RFCA, with substrate modification lesion sets (e.g., ablation of complex fractionated atrial electrogram or linear ablation), with a follow up period below 1 year, and with missing values in study features. This study was approved by the Institutional Review Board of CBNUH (approval no. 2021-12-009-001). As this was a retrospective observational study, the requirement for informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
2.2. Preprocedural Preparation and Evaluations
Class I or III antiarrhythmic drugs were discontinued at least half-lives of five times before RFCA. Direct oral anticoagulants were not interrupted during the periprocedural period. One day before the procedure, transthoracic echocardiography and transesophageal echocardiography were acquired from the patients. In addition, the following echocardiographic parameters were collected for this study: left ventricular ejection fraction (LVEF), left ventricle mass index, and LA anterior-posterior diameter. The estimated glomerular filtration rate (eGFR) was also evaluated 1 day before the procedure.
2.3. Radiofrequency Catheter Ablation
RFCA was performed under sinus rhythm at our institution, except when AF recurred immediately after cardioversion. Three-dimensional mapping of the LA was constructed using the EnSite NavX/Velocity system (St. Jude Medical, St. Paul, MN, USA). Circumferential pulmonary vein isolation around the antrum of the ipsilateral pulmonary veins was performed using an irrigated TactiCath Quartz or TactiCath TM Contract Force ablation catheter (St. Jude Medical) with a maximum power of 25–40 W. Radiofrequency energy with contact force above 10–20 g was applied in each ablation lesion point until the force-time integral exceed 400 gs. After verifying the electrical isolation of the four pulmonary veins with a bidirectional block, the existence of a nonpulmonary vein trigger was assessed by cardioversion for AF evoked by rapid atrial pacing under isoproterenol infusion.
2.4. Clinical Follow-Up
Intake of class I or III antiarrhythmic drugs continued until 3 months after catheter ablation. The rhythm status was assessed by surface electrocardiography (EKG) and Holter monitoring at 2 weeks and 1, 2, 3, 6, 9, and 12 months after discharge. In addition, whenever a patient felt symptoms, EKG and Holter monitoring were performed in our institution. Anticoagulants were prescribed to all patients up to 3 months after discharge, and they were selectively prescribed according to the CHA2DS2-VASc score afterward.
2.5. Definitions
The endpoint of this study was the LR of sustained atrial tachyarrhythmia within 1 year after RFCA. Sustained atrial tachyarrhythmia was defined as atrial flutter, atrial tachycardia, or AF lasting for more than 30 s in Holter monitoring or more than 10 seconds in 12-lead EKG. LR was defined as sustained atrial tachyarrhythmia within 1 year of RFCA, but early recurrence during the blanking period of 3 months after ablation was not regarded as late recurrence. The AF duration was defined as the difference between the date of the first AF documented on EKG and the date of index RFCA. The eGFR was calculated using the CKD-EPI (chronic kidney disease–epidemiology collaboration) equation as follows: eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if woman] × 1.159 [if black], where Scr is the serum creatinine level, κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, and min and max denote the minimum and maximum between their arguments, respectively [21].
2.6. Dataset Preparation
For a small dataset, selecting informative features is essential to ensure training stability and convergence. Thus, extreme gradient boosting (XGBoost) was utilized to select informative features while omitting the irrelevant ones according to the weight of each feature during analysis (Figure 1).
Figure 1.

Flowchart of the experimental procedure. Of the 15 factors, 11 were selected using the extreme gradient boosting (XGBoost) algorithm. We used 4-fold cross-validation for model evaluation and applied the synthetic minority oversampling technique to the training set in each fold. ML, machine learning; MLP, multilayer perceptron; PVI, pulmonary vein isolation.
In our dataset, only 47 patients (26.5%) experienced LR. To address the data imbalance that drastically degrades ML performance, we adopted the synthetic minority oversampling technique [22] for data augmentation according to the neighborhood of the minority class. Note that this technique was applied to the training set but not to the test set.
We performed 4-fold cross-validation on our dataset. The dataset was split into 4 equally sized folds. Specifically, in each iteration, one-fold was used for testing (25%) and the others were used for model training (75%).
Figure 2 shows the proposed deep learning model based on the MLP architecture. The model consists of three MLP blocks and one output layer. Each MLP block comprises a dense layer, a batch normalization layer, rectified linear unit (ReLU) activation, and a dropout layer with a rate of 0.2 to avoid overfitting. For the output layer, a dense layer followed by sigmoid activation is used to calculate the LR probability. We use weighted binary cross-entropy as the loss function to handle data imbalance. In this study, the model was trained for 1,000 epochs using the Adam optimizer with a learning rate of 10−4.
Figure 2.
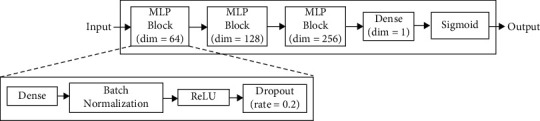
Network architecture based on multilayer perceptron (MLP) to predict the late recurrence probability. Dim, dimension; ReLU, rectified linear unit.
2.7. Evaluation of Model Performance
We compared the performance of the proposed model with that of various ML techniques: linear regression, logistic regression, XGBoost algorithm, and SVM. The proposed model and ML techniques were implemented and evaluated using the Keras and TensorFlow 2 platforms in Python 3.8. The proposed model was trained on the CUDA 11.0.3 toolkit using an NVIDIA GeForce RTX 3090 graphics processor.
For the quantitative evaluation, we determined the AUROC, F1 score, sensitivity, and specificity. The receiver operating characteristic (ROC) curve is a statistical performance measure that depicts the true positive rate according to the false positive rate. The AUROC ranges between 0 and 1, with 0.5 indicating random guessing and 1 indicating perfect classification. The F1 score ranges from 0 to 1 and is the harmonic mean of the precision and recall. The accuracy indicates the similarity between measured and actual values, being an intuitive indicator of model performance. The sensitivity is a measure of the true positive rate, and the specificity is a measure of the true negative rate.
2.8. Statistical Analysis
Categorical features were compared using Pearson's χ2 test or Fisher's exact test when the numbers were below five. The normality of continuous features was evaluated using the Shapiro–Wilk test. The difference for continuous features with normal distribution was compared using Student's t-test, and distributions with skewed features were compared using the Mann–Whitney test. The ROC curves were plotted with the AUROC to evaluate the diagnostic accuracy of the APPLE and CHA2DS2-VASc score for LR after the procedure. All the statistical analyzes were performed using SPSS version 28.0 (IBM, Armonk, NY, USA). We compared the performance of the proposed model with that of the conventional APPLE and CHA2DS2-VASc scores.
3. Results
3.1. Patient Characteristics
Fifteen of the 192 consecutive patients were excluded because of follow-up loss (n = 2) or additional substrate modification adjunct to PVI (n = 13). Of the remaining 177 patients, 47 (26.5%) experienced LR within 1 year after RFCA. LR was identified in 17 (19.1%) of 89 patients with paroxysmal AF and 30 (34.1%) of 88 patients with persistent AF. The baseline characteristics of patients with and without AF following RFCA are summarized in Table 1. Patients with LR had higher APPLE scores (1 (0–2) vs. 1 (1–2), p = 0.026) and proportion of embolism events (8.5% vs. 19%, p = 0.047) than those without LR. A higher proportion of women (12% vs. 23%, p = 0.070) and LVEF below 50% (2.3% vs. 8.5%, p = 0.082), large LA (41 ± 6 vs. 43 ± 7 mm, p = 0.090), and low eGFR (95 ± 21 vs. 88 ± 22, p = 0.055) were observed in patients with LR compared with those without LR.
Table 1.
Baseline characteristics of patients with and without late recurrence following catheter ablation.
| Without late recurrence (n = 130) | With late recurrence (n = 47) | P value | |
|---|---|---|---|
| Age (years) | 59 ± 10 | 60 ± 10 | 0.626 |
| Age > 65 years | 35 (27) | 16 (34) | 0.356 |
| Female sex | 16 (12) | 11 (23) | 0.070 |
| Height (cm) | 167 ± 7 | 166 ± 8 | 0.604 |
| Body weight (kg) | 72 ± 11 | 71 ± 12 | 0.552 |
| BMI (kg/m2) | 26 ± 3 | 26 ± 4 | 0.686 |
| Persistent atrial fibrillation | 58 (45) | 30 (64) | 0.024 |
| AF duration (month) | 23 ± 25 | 36 ± 41 | 0.054 |
| Heart failure | 4 (3) | 7 (15) | 0.009 |
| Hypertension | 49 (38) | 13 (28) | 0.217 |
| Diabetes mellitus | 21 (16) | 9 (19) | 0.639 |
| Prior stroke or TIA or SE | 11 (8.5) | 9 (19) | 0.047 |
| Vascular disease | 8 (6) | 4 (9) | 0.582 |
| TTE findings | |||
| LA diameter (mm) | 41 ± 6 | 43 ± 7 | 0.090 |
| LA diameter ≥ 43 mm | 49 (38) | 23 (49) | 0.179 |
| LVEF (%) | 69 ± 9 | 68 ± 13 | 0.515 |
| LVEF < 50% | 3 (2.3) | 4 (8.5) | 0.082 |
| LV mass index (g/m2) | 87 ± 19 | 90 ± 28 | 0.452 |
| Laboratory findings | |||
| eGFR (ml/min/1.73 m2) | 95 ± 21 | 88 ± 22 | 0.055 |
| eGFR < 60 ml/min/1.73 m2 | 5 (4) | 3 (6) | 0.359 |
| CHA2DS2-VASc score, median (IQR) | 1 (0–2) | 1 (0–2) | 0.299 |
| APPLE score, median (IQR) | 1 (0–2) | 1 (1–2) | 0.026 |
Results are presented as n (%) or means with standard deviation. AF, atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate; LA, left atrium; LV, left ventricle, LVEF, left ventricular ejection fraction; SE, systemic embolism; TIA, transient ischemic attack; TTE, transthoracic echocardiography.
3.2. Feature Selection Using Extreme Gradient Boosting
Figure 3 shows the relative importance of risk factors for LR following RFCA. We selected representative features with an f-score higher than 100 as input of the proposed model: age, sex, height, weight, hypertension, AF type, AF duration, LA diameter, left ventricular mass index, LVEF, and eGFR.
Figure 3.

Importance of features obtained from extreme gradient boosting algorithm.AF, atrial fibrillation; LV, left ventricular; LVEF, left ventricular ejection fraction; LA, left atrium; eGFR, estimated glomerular filtration rate; HTN, hypertension; AF type, paroxysmal AF vs. persistent AF; DM, diabetes mellitus; HF, heart failure; CAD, coronary artery disease.
3.3. Performance Evaluation
To evaluate the model performance, we performed 4-fold cross-validation on our dataset. The resulting AUROC, F1 score, sensitivity, specificity, and accuracy per iteration of the proposed model are listed in Table 2. Each fold showed AUROC ≥ 0.73. Figure 4 shows the confusion matrix for every fold, and Figure 5 shows the ROC curves for the 4-fold average and all folds.
Table 2.
Quantitative evaluation results of the proposed model for 4-fold cross-validation.
| AUROC | F1 score | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|
| Fold 1 | 0.760 | 0.615 | 0.727 | 0.781 | 0.767 |
| Fold 2 | 0.803 | 0.640 | 0.727 | 0.812 | 0.791 |
| Fold 3 | 0.766 | 0.640 | 0.727 | 0.812 | 0.791 |
| Fold 4 | 0.735 | 0.629 | 0.786 | 0.706 | 0.729 |
| Mean | 0.766 | 0.633 | 0.745 | 0.777 | 0.768 |
The mean values are indicated in bold.
Figure 4.
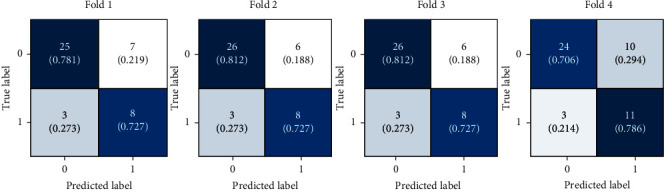
Confusion matrices of the proposed model for 4-fold cross-validation.
Figure 5.
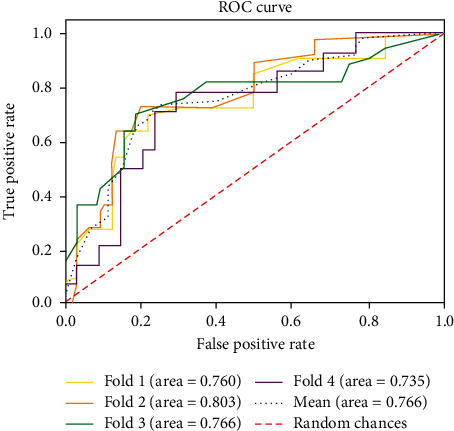
Receiver operating characteristic (ROC) curves for late recurrence of atrial fibrillation after radiofrequency catheter ablation obtained from the proposed model stratified over 4-fold cross-validation.
Table 3 shows the quantitative evaluation results for the evaluated models. The APPLE and CHA2DS2-VASc score underperformed the ML techniques (i.e., linear regression, logistic regression, XGBoost algorithm, and SVM). Figure 6 shows the confusion matrices for all the evaluated models, and Figure 7 shows the ROC curves. Our model achieves an average AUROC of 0.766, F1 score of 0.632, sensitivity of 0.745, and specificity of 0.777. The model showed the highest performance compared to those of the conventional prediction models and ML approaches.
Table 3.
Quantitative evaluation results of evaluated models.
| AUROC | F1 score | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|
| APPLE score | 0.605 | 0.357 | 0.426 | 0.654 | 0.593 |
| CHA2DS2-VASc score | 0.595 | 0.397 | 0.489 | 0.646 | 0.605 |
| Linear regression | 0.541 | 0.412 | 0.574 | 0.562 | 0.565 |
| Logistic regression | 0.546 | 0.381 | 0.511 | 0.585 | 0.565 |
| XGBoost | 0.608 | 0.452 | 0.617 | 0.600 | 0.605 |
| SVM | 0.638 | 0.482 | 0.617 | 0.662 | 0.650 |
| Proposed model | 0.766 | 0.632 | 0.745 | 0.777 | 0.768 |
The best and second-best results are shown in boldface and italics, respectively.
Figure 6.
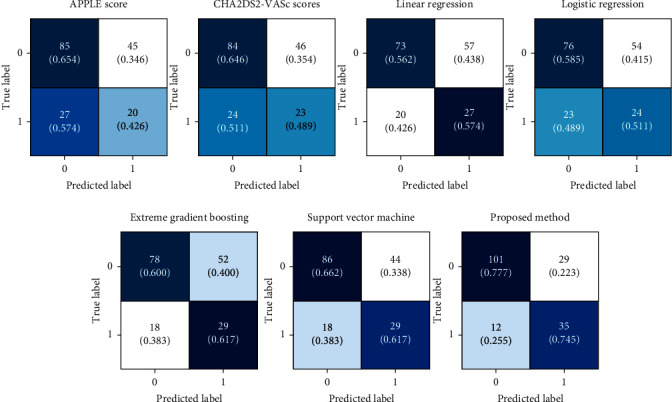
Confusion matrices of conventional prediction models and machine learning approaches. APPLE, acute patient physiologic and laboratory evaluation.
Figure 7.

Receiver operating characteristic (ROC) curves for late recurrence of atrial fibrillation after radiofrequency catheter ablation obtained from conventional prediction models and machine learning approaches. APPLE, acute patient physiologic and laboratory evaluation; XGBoost, extreme gradient boosting; SVM, support vector machine.
4. Discussion
Our results demonstrate that an MLP-based model using easily accessible clinical and echocardiographic features obtained during the preprocedural stage can suitably predict LR after RFCA. Our model outperformed conventional prediction models and ML approaches. This approach may support decision-making for selecting patients with AF considering the LR probability after RFCA.
4.1. Interpretation of the Feature Importance
To observe the impact of each feature on the MLP model training, we utilized Shapley additive planation (SHAP) [23], which is one of the widespread methods to explain model predictions and provide visualization charts. More specifically, the SHAP algorithm calculates the relative importance of the features on the prediction. The magnitude of the SHAP value means the degree of influence on the prediction. The positive SHAP value indicates that the feature contribution to the probability of AF recurrence is higher, and a negative SHAP value indicates that the feature contribution to the probability of AF recurrence is lower.
As illustrated in Figure 8, the LA diameter was the most powerful feature followed by AF duration, weight, eGFR, and LV mass index. In addition, the SHAP results demonstrated that the higher value of LA diameter, the higher value of AF duration, patients with heart failure, patients with hypertension, the higher value of age, the higher value of height, the lower value of sex (0: female, 1: male), patients with diabetes mellitus, patients with stroke were associated with an increased risk of AF recurrence. Interestingly, some features showed unexpected contributions to the AF recurrence in the SHAP results. The AF type gave a lower influence than AF duration, LV mass index, and LA diameter, while the AF type is widely accepted as the primary risk factor for LR after RFCA [24]. This finding can be explained by the fact that the rhythm outcome after RFCA may differ according to the different burdens of AF, especially paroxysmal AF [38].
Figure 8.
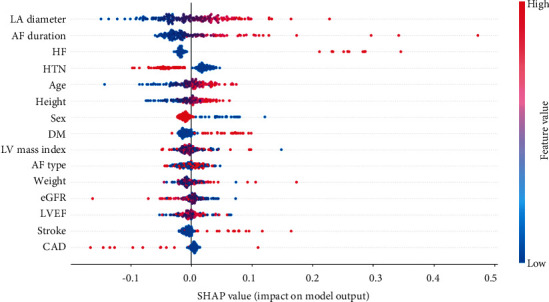
The Shapley additive explanation (SHAP) summary plot of the proposed model. It represents the feature importance of the model output. The color of the dots indicates the attribution value of the feature. For categorical features (CAD {0, 1}; AF type {0: paroxysmal, 1: persistent}; DM {0, 1}; HTN {0, 1}; sex {0: female, 1: male}; stroke {0, 1}; HF {0, 1}), the red and blue dots represent 1 and 0, respectively. AF, atrial fibrillation; LV, left ventricular; LVEF, left ventricular ejection fraction; LA, left atrium; eGFR, estimated glomerular filtration rate; HTN, hypertension; AF type, paroxysmal AF vs persistent AF; DM, diabetes mellitus; HF, heart failure; CAD, coronary artery disease.
4.2. Conventional Prediction Models for Radiofrequency Catheter Ablation Prognosis in Patients with Atrial Fibrillation
Clinical risk factors including the AF type and duration [16, 17, 25], obesity [26], sleep apnea [27], and hypertension [28] are associated with the development of abnormal atrial substrate that leads to AF recurrence after RFCA. Moreover, the LA diameter and volume [29], the volume of epicardial fat [30], and the severity of atrial tissue fibrosis [31] are structural predictors of the RFCA outcome in patients with AF. However, no single factor has shown superiority over others in predicting the outcome.
To overcome this limitation, various prediction models combining well-known risk factors have been developed, with models including ALARMEc, HATCH, CHA2DS2-VASc, and APPLE scores showing a moderate performance with AUROC ranging from 0.44 to 0.74 [32]. However, these scoring models may not reflect high-order interactions between various features because they consider simple linear equations, in which one or two points are arbitrarily assigned to the corresponding risk factors. Although the concordance statistics of the BASE-AF2 score have shown good to excellent discrimination ability of 0.61–0.94 [33], postprocedural features such as early recurrence after RFCA should be included to show such performance. Similarly, the MB-LATER score with AUROC ranging from 0.57 to 0.83 should include early recurrence after RFCA to calculate the scoring system to provide an excellent performance [34].
4.3. Machine Learning Models for Radiofrequency Catheter Ablation Prognosis in Patients with Atrial Fibrillation
A deep learning model has shown a good prediction performance (C-index of 0.76) by simply using four features: N-terminal pro brain natriuretic peptide, AF type, LA appendage volume, and LA volume [35]. The easily obtainable input data used in this model may enable practical application. However, this model excludes accepted clinical features including AF duration and comorbidities related to LR after RFCA. More recently, Baalman et al. [36] proposed an ML prediction model using input data selected from 166 clinical features. Although the model suitably predicts LR (AUROC of 0.73, 95% confidence interval of 0.68–0.77), the continuous input features, such as age, LA volume index, and CHA2DS2-VASc score, are expressed as discrete values, leading to information loss.
Unlike existing ML models, the proposed MLP-based deep learning prediction model uses continuous and multiple features without information loss. Moreover, it can achieve a superior discriminative ability compared with established prediction models such as the APPLE and CHA2DS2-VASc score. The promising performance of our model may be attributed to the ability of MLP to learn high-order interactions between accepted risk factors and the rhythm outcomes after RFCA.
4.4. Limitations of the Study
Various limitations of this study should be noted. First, LR may have been underestimated because the rhythm outcome after RFCA was evaluated by intermittent EKG and Holter monitoring. Second, the study population included in this study was limited. Nevertheless, to prevent overfitting due to the small sample size and evaluate the test model robustness, we employed 4-fold cross-validation, consistently achieving an AUROC above 0.73 in each fold. Third, deviations of the patient's characteristics may have occurred owing to relatively short AF duration, a smaller proportion of heart failure with reduced ejection fraction, and pulmonary vein isolation lesion set at index procedure. Therefore, the model performance should be confirmed by considering an external validation cohort in future work. Finally, we did not consider a recent deep learning model that uses LA fibrosis findings in magnetic resonance images as an input feature [37].
5. Conclusion
We proposed an MLP-based model that outperforms conventional prediction models and state-of-the-art ML methods in predicting rhythm outcomes after RFCA in patients with AF. The model may support clinical decision-making for selecting good responders to the RFCA intervention. In future work, we will further to improve the proposed model by considering imaging data related to the atrial substrate.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI21C1074070021).
Data Availability
The data underlying this article cannot be shared publicly due to ethical issues.
Ethical Approval
This study was approved by the Institutional Review Board of Chungbuk National University Hospital (approval no. 2021-12-009-001). This study was conducted in accordance with the Declaration of Helsinki.
Consent
This was a retrospective observational study, so the requirement for informed consent was waived by the institutional review board.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
SP and DL designed this study. Data were collected and annotated by DL and JC. MP implemented the prediction models for AF recurrence and conducted the performance evaluation. DL conducted the statistical analysis via SPSS software. SP, DL, and MP contributed to the interpretation of the results. MP and DL wrote the manuscript and SP supervised the project. All authors discussed the results and contributed to the final manuscript. Dae-In Lee and Mi-Jung Park authors contributed equally to this work as the first author.
References
- 1.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. European Heart Journal . 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M., Jaïs P., Shah D. C., et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine . 1998;339(10):659–666. doi: 10.1056/nejm199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Ghanbari H., Başer K., Jongnarangsin K., et al. Mortality and cerebrovascular events after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm . 2014;11(9):1503–1511. doi: 10.1016/j.hrthm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Narayan S. M., Baykaner T., Clopton P., et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone. Journal of the American College of Cardiology . 2014;63(17):1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostock T., Salukhe T. V., Steven D., et al. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm . 2011;8(9):1391–1397. doi: 10.1016/j.hrthm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Costa F. M., Ferreira A. M., Oliveira S., et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. International Journal of Cardiology . 2015;184:56–61. doi: 10.1016/j.ijcard.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 7.D’Ascenzo F., Corleto A., Biondi-Zoccai G., et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. International Journal of Cardiology . 2013;167(5):1984–1989. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y. G., Choi J.-I., Boo K. Y., et al. Clinical and echocardiographic risk factors predict late recurrence after radiofrequency catheter ablation of atrial fibrillation. Scientific Reports . 2019;9(1):6890–6899. doi: 10.1038/s41598-019-43283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teh A. W., Kistler P. M., Lee G., et al. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. Journal of Cardiovascular Electrophysiology . 2012;23(3):232–238. doi: 10.1111/j.1540-8167.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- 10.Letsas K. P., Efremidis M., Giannopoulos G., et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace . 2014;16(2):202–207. doi: 10.1093/europace/eut210. [DOI] [PubMed] [Google Scholar]
- 11.Sciacqua A., Perticone M., Tripepi G., et al. CHADS2 and CHA2DS2-VASc scores are independently associated with incident atrial fibrillation: the Catanzaro Atrial Fibrillation Project. Internal and Emergency Medicine . 2015;10(7):815–821. doi: 10.1007/s11739-015-1243-3. [DOI] [PubMed] [Google Scholar]
- 12.Kornej J., Hindricks G., Arya A., Sommer P., Husser D., Bollmann A. The APPLE score - a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One . 2017;12(1) doi: 10.1371/journal.pone.0169933.e0169933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornej J., Hindricks G., Shoemaker M. B., et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clinical Research in Cardiology . 2015;104(10):871–876. doi: 10.1007/s00392-015-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caballé-Cervigón N., Castillo-Sequera J. L., Gómez-Pulido J. A., Gómez-Pulido J. M., Polo-Luque M. L. Machine learning applied to diagnosis of human diseases: a systematic review. Applied Sciences . 2020;10(15):p. 5135. doi: 10.3390/app10155135. [DOI] [Google Scholar]
- 15.Saberi-Karimian M., Khorasanchi Z., Ghazizadeh H., et al. Potential value and impact of data mining and machine learning in clinical diagnostics. Critical Reviews in Clinical Laboratory Sciences . 2021;58(4):275–296. doi: 10.1080/10408363.2020.1857681. [DOI] [PubMed] [Google Scholar]
- 16.Mont L., Bisbal F., Hernández-Madrid A., et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study) European Heart Journal . 2014;35(8):501–507. doi: 10.1093/eurheartj/eht457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilber D. J., Pappone C., Neuzil P., et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation. JAMA . 2010;303(4):333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 18.Jo Y.-Y., Han J., Park H. W., et al. Prediction of prolonged length of hospital stay after cancer surgery using machine learning on electronic Health records: retrospective cross-sectional study. JMIR Medical Informatics . 2021;9(2) doi: 10.2196/23147.e23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazlan N., Yazid H., Arof H., Mohd Isa H. Automated microaneurysms detection and classification using multilevel thresholding and multilayer perceptron. Journal of Medical and Biological Engineering . 2020;40(2):292–306. doi: 10.1007/s40846-020-00509-8. [DOI] [Google Scholar]
- 20.Morbidoni C., Principi L., Mascia G., et al. Mediterranean Conference on Medical and Biological Engineering and Computing . USA: Springer; 2019. Gait phase classification from surface EMG signals using neural networks; pp. 75–82. [DOI] [Google Scholar]
- 21.Levey A. S., Stevens L. A., Schmid C. H., et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine . 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla N. V., Bowyer K. W., Hall L. O., Kegelmeyer W. P. SMOTE: synthetic minority over-sampling technique. Journal of Artificial Intelligence Research . 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 23.Lundberg S. M., Lee S.-I. A unified approach to interpreting model predictions. Proceedings of the International Conference in Neural Information Processing Systems; December 2017; pp. 4765–4774. [Google Scholar]
- 24.Epicoco G., Sorgente A. Predictors of atrial fibrillation recurrence after catheter. Ablation . 2014;6(5):p. 1016. doi: 10.4022/jafib.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oral H., Pappone C., Chugh A., et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. New England Journal of Medicine . 2006;354(9):934–941. doi: 10.1056/nejmoa050955. [DOI] [PubMed] [Google Scholar]
- 26.Naruse Y., Tada H., Satoh M., et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm . 2013;10(3):331–337. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Patel D., Mohanty P., Di Biase L., et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea. Circulation: Arrhythmia and Electrophysiology . 2010;3(5):445–451. doi: 10.1161/circep.109.858381. [DOI] [PubMed] [Google Scholar]
- 28.Trines S. A., Stabile G., Arbelo E., et al. Influence of risk factors in the ESC‐EHRA EORP atrial fibrillation ablation long‐term registry. Pacing and Clinical Electrophysiology . 2019;42(10):1365–1373. doi: 10.1111/pace.13763. [DOI] [PubMed] [Google Scholar]
- 29.Njoku A., Kannabhiran M., Arora R., et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. EP Europace . 2018;20(1):33–42. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 30.Masuda M., Mizuno H., Enchi Y., et al. Abundant epicardial adipose tissue surrounding the left atrium predicts early rather than late recurrence of atrial fibrillation after catheter ablation. Journal of Interventional Cardiac Electrophysiology . 2015;44(1):31–37. doi: 10.1007/s10840-015-0031-3. [DOI] [PubMed] [Google Scholar]
- 31.Marrouche N. F., Wilber D., Hindricks G., et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation. JAMA . 2014;311(5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 32.Deng H., Bai Y., Shantsila A., Fauchier L., Potpara T. S., Lip G. Y. H. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clinical Research in Cardiology . 2017;106(10):813–823. doi: 10.1007/s00392-017-1123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canpolat U., Aytemir K., Yorgun H., Şahiner L., Kaya E. B., Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. International Journal of Cardiology . 2013;169(3):201–206. doi: 10.1016/j.ijcard.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 34.Dretzke J., Chuchu N., Agarwal R., et al. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. EP Europace . 2020;22(5):748–760. doi: 10.1093/europace/euaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X., Nakamura K., Sahara N., et al. Deep learning-based recurrence prediction of atrial fibrillation after catheter ablation. Circulation Journal . 2022;86(2):299–308. doi: 10.1253/circj.cj-21-0622. [DOI] [PubMed] [Google Scholar]
- 36.Baalman S. W. E., Lopes R. R., Ramos L. A., et al. Prediction of atrial fibrillation recurrence after thoracoscopic surgical ablation using machine learning techniques. Diagnostics . 2021;11(10):p. 1787. doi: 10.3390/diagnostics11101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shade J. K., Ali R. L., Basile D., et al. Preprocedure application of machine learning and mechanistic simulations predicts likelihood of paroxysmal atrial fibrillation recurrence following pulmonary vein isolation. Circulation: Arrhythmia and Electrophysiology . 2020;13(7) doi: 10.1161/circep.119.008213.e008213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strisciuglio T., El Haddad M., Debonnaire P., et al. Paroxysmal atrial fibrillation with high vs. low arrhythmia burden: atrial remodelling and ablation outcome. EP Europace . 2020;22(8):1189–1196. doi: 10.1093/europace/euaa071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical issues.


