Abstract
Background
Although mean platelet volume (MPV) has been reported to be associated with poor prognosis of various critical illness, the relationship between MPV and in-hospital mortality among patients undergoing invasive mechanical ventilation (IMV) is unclear.
Methods
A retrospective observational study including patients receiving IMV was conducted from January, 2014 to January, 2019. The patients were divided into two groups by MPV cutoff value. The receiver operating characteristics curve was used to evaluate the predictive ability of MPV for in-hospital mortality. Univariate and multivariate Cox regression analysis were conducted to analyze the value of MPV for predicting in-hospital mortality. Kaplan–Meier cumulative incidence curve was employed to observe the incidence of in-hospital mortality.
Results
A total of 274 patients were enrolled in the study, and 42 patients (15.3%) died in hospital. MPV > 11.4 fl was a valuable predictor for in-hospital mortality (AUC0.848; 95%CI, 0.800–0.889) with sensitivity 66.7%, and specificity = 86.21%. MPV > 11.4 fl was an independent risk factor for in-hospital mortality (adjusted HR 2.640, 95%CI, 1.208–5.767, P = 0.015). Compared to the group of MPV ≤ 11.4 fl, patients with MPV > 11.4 fl had increased mortality (log-rank test = 40.35, HR = 8.723, P < 0.0001). The relationship between MPV and in-hospital mortality was stronger in female patients than in male patients.
Conclusion
MPV > 11.4 fl is a more useful marker for predicting in-hospital mortality among critically ill patients receiving IMV, especially in female patients. Attention to the MPV marker is simple and profitable with immediate applicability in daily clinical practice.
Keywords: Mean platelet volume, Invasive mechanical ventilation, In-hospital mortality
Introduction
Mechanical ventilation is frequently used for life support for patients experiencing respiratory failure or undergoing general anesthesia in critical illness. These patients are at risk for a number of complications related to both their underlying disease state and the mechanical ventilation itself [1]. Some studies evaluating patients requiring mechanical ventilation have reported mortality rates from 12 to 40% [2–9] according to different diseases. Therefore, early predictions of mortality and intervention are of great importance for improving the prognosis of patients. Acute Physiology and Chronic Health Evaluation II (APACHE II) score, solid tumor, severe sepsis/septic shock, acute lung injury/acute respiratory distress syndrome, and acute kidney injury have been reported as predictors of hospital mortality [9]. However, the relationship between inflammation markers and hospital mortality among those adult patients receiving IMV were lacking. Hence, it is critical to explore the predictive ability of inflammation markers for hospital mortality for those patients.
It has been reported that platelet has increasing focus for its role in inflammation and immunity [10]. Mean platelet volume (MPV), a routinely measured marker in clinical practice, is a simple, easy, available, and accurate indicator of platelet size and function [11]. Numerous studies have demonstrated that MPV levels or the change of MPV were correlated with serious compilation or poor prognosis for ICU patients [12, 13], sepsis [14, 15], influenza pneumonia [16], acute pulmonary embolism [17].In addition, MPV also has been shown to be a valuable prognostic marker for patients following acute abdominal surgery [18], chronic obstructive pulmonary disease (COPD) patients [19], coronary artery disease [20] and Type 2 Diabetes Mellitus [21].
Critically ill patients experiencing invasive mechanical ventilation(IMV) are generally in a high state of inflammation, strong stress, and immunity response [22], which may be related to both their underlying disease state, endotracheal intubation and the mechanical ventilation itself [1]. Nonsurvivors may suffer more inflammatory response and stress. However, there were few studies focusing on the inflammation markers and in-hospital mortality among critically ill adult patients receiving IMV.
Accordingly, we assumed that higher MPV could be related with higher in-hospital mortality. And we evaluated the effectiveness of MPV for predicting hospital mortality in patients receiving IMV.
Methods
Study population
This retrospective, observational, single center study included patients receiving IMV not less than 24 h admitted to the ICU of Beijing Chao-Yang Hospital from January, 2014 to January, 2019. The following patients were excluded: Age < 18 years; pregnancy; tracheotomy or other upper airway disorders; mechanically ventilated less than 24 h; neuromuscular disease; decision to abandon or limit active treatment; incomplete data [23].
This study (NO.2020-KE-94) was approved by the Ethics Committee of the Beijing Chao-Yang Hospital, Capital Medical University. The requirement for informed consent was waived by the Ethics Committee of the Beijing Chao-Yang Hospital, Capital Medical University because of the retrospective nature of the study. All procedures were in accordance with Helsinki Declaration.
Data collection
Patients’ demographic and baseline characteristics were recorded within 24 h after intubation, including age, sex, body mass index (BMI), acute physiology and chronic health evaluation II (APACHE II) score, basic vital signs, arterial blood gas and laboratory data. The following were also recorded: comorbidities and acute causes of IMV, IMV duration, complication, length of stay (LOS) in ICU, LOS in hospital, and in-hospital mortality. Albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood creatinine, complete blood cell counts and CRP within 24 h after intubation were measured. Complete blood cell counts were measured by an automatic blood analyzer. CRP concentrations were measured using immunoscatter turbidimetry by Goldsite Aristo (Goldsite, Ltd., China), and the normal value of CRP ranges from 0 to 5 mg/L [23]. Acute kidney injury(AKI), defined according to established criteria [24]; septic shock, defined according to established criteria [25]. Gastrointestinal bleeding was defined as any clinically suspected or documented of bleeding from the gastrointestinal tract as indicated with a fall in hemoglobin level and the appearance of melena, hematochezia, haematemesis or stool tested positive for occult blood [26]. Acute liver dysfunction was defined as abnormality of the AST and ALT. Acute coronary syndrome included the event of acute ST elevation or nonST elevation myocardial infarction or unstable recurrent angina as indicated by the typical symptom with abnormal ECG or echocardiography or myocardial enzyme elevation. Acute cerebrovascular disease included haemorrhagic stroke, ischaemic stroke and transient ischaemic attack which were diagnosed or suspected by neurologist according to symptoms and computerized tomography of brain.
Clinical outcome
The primary outcome was in-hospital mortality rate, which was defined as deaths occurring during hospitalization. Survivors were discharged to homes or transferred to another hospital.
Statistics
Continuous variables were expressed as mean ± standard deviation (SD) for normality distribution or median (25th-75th percentile) for nonnormally distributed data.
The Kolmogorov–Smirnov test was used for the normality distribution. Student’s t test was employed for normally distributed data. The Mann–Whitney U-test was used for nonnormally distributed data. Categorical data was expressed as frequency and percentages, and Chi square (χ2) test was performed. Spearman correlations were used for correlation analysis between MPV and other variables. A univariate Cox regression analysis was constructed to explore the association between patients’ characteristics and in-hospital mortality. The multivariate Cox regression with the enter method was also employed to examine variables significantly associated with in-hospital mortality in the univariate analysis, including covariates: APACHE II score, MPV, PaO2/FIO2, vasopressor use, CRRT, CKD, AKI, and septic shock, which resulted in adjusted hazard ratios (HR, 95%CI). In Cox models, time at risk was from study entry until death during hospitalization, discharge, or transfer. Furthermore, the receiver operating characteristic (ROC) analysis was used to evaluate the sensitivity and specificity of MPV for predicting in-hospital mortality and to determine the optimum cutoff value. Cumulative survival curves were conducted by Kaplan–Meier method and differences between groups were evaluated by log-rank test. All analyses were two-tailed, and a probability value (p value) less than 0.05 was considered statistically significant. All data were done using SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 22 for Microsoft Windows.
Results
Patients’ characteristics and outcomes
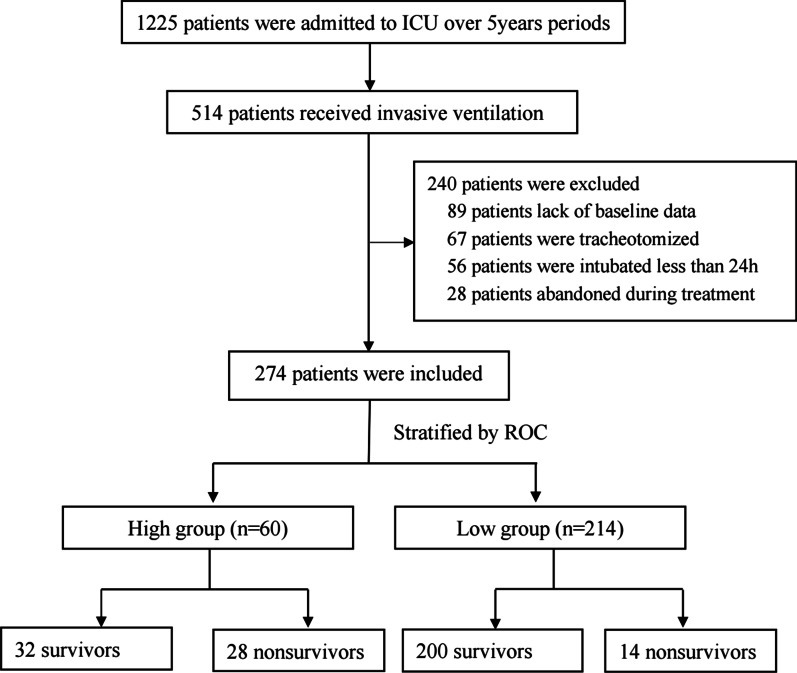
A total of 274 patients were enrolled in the study, as shown in Fig. 1. Of these, 42 patients (15.3%) died in hospital. According to the optimal cutoff value of MPV predicting in-hospital mortality, baseline characteristics and outcomes were summarized in Table 1. Compared with patients with MPV ≤ 11.4 fl, patients with MPV > 11.4 fl had higher APACHE II score, increased frequency of patients with congestive heart failure, and immunosuppression, longer ICU LOS, much more complications including septic shock, AKI, acute liver dysfunction, gastrointestinal bleeding, and acute coronary syndrome, higher frequency treatment of vasopressor use and CRRT. Moreover, patients with MPV > 11.4 fl showed lower PaO2/FIO2, lower platelet count, higher lactate, higher PDW, higher larger platelet ratio, and higher CRP level. The hospital mortality rate was higher in patients with MPV > 11.4 fl than those with MPV ≤ 11.4 fl (46.67% vs 6.54%, P = 0.000).
Fig. 1.
Flow chart of patient selection and outcome. Note: high group: MPV > 11.4 fl; low group, MPV ≤ 11.4 fl; ROC, receiver operating characteristic curve; ICU, intensive care unit
Table 1.
Baseline characteristics based on the MPV cutoff value
| Characteristics | MPV > 11.4 fl (n = 60) | MPV ≤ 11.4 fl (n = 214) | P |
|---|---|---|---|
| Age, year (mean ± SD) | 72.75 ± 11.24 | 70.77 ± 13.52 | 0.300 |
| Male, n (%) | 37 (61.67) | 123 (53.02) | 0.561 |
| BMI, kg/m2 (mean ± SD) | 24.02 ± 3.42 | 24.31 ± 4.71 | 0.597 |
| APACHE II score, (mean ± SD) | 21.02 ± 6.67 | 18.44 ± 6.96 | 0.007 |
| IMV duration, days (median, 25th–75th percentiles) | 7 (4–16) | 4 (1–9.25) | 0.053 |
| ICU LOS, days (median, 25th–75th percentiles) | 17 (10–32) | 10 (3–20) | 0.000 |
| H LOS, days (median, 25th–75th percentiles) | 21 (15.25–40.75) | 19 (13–28) | 0.069 |
| Cause of mechanical ventilation, n (%) | |||
| Exacerbation of chronic respiratory disorders | 15 (25) | 37 (17.29) | 0.178 |
| Pneumonia | 18 (30) | 62 (28.97) | 0.887 |
| Sepsis | 12 (20) | 43 (20.09) | 0.987 |
| Postoperation | 8 (13.33) | 52 (24.30) | 0.070 |
| Congestive heart failure | 4 (6.67) | 3 (1.40) | 0.040 |
| Neurological disease | 2 (3.33) | 12 (5.61) | 0.741 |
| others | 1 (1.67) | 5 (2.34) | 1.000 |
| Comorbidity disease, n (%) | |||
| Hypertension | 36 (60) | 114 (53.27) | 0.335 |
| Diabetes Mellitus | 23 (38.33) | 54 (25.23) | 0.050 |
| Chronic heart disorders | 21 (35) | 64 (29.9) | 0.451 |
| Chronic respiratory disorders | 19 (31.67) | 61 (28.5) | 0.634 |
| Malignancy | 14 (23.33) | 49 (22.90) | 0.943 |
| Chronic kidney disease | 11 (18.33) | 22 (10.28) | 0.090 |
| Cerebrovascular disease | 10 (16.67) | 33 (15.42) | 0.815 |
| Immunosuppression | 3 (5) | 1 (0.46) | 0.034 |
| Clinical outcome, n (%) | |||
| Septic shock | 25 (41.67) | 41 (19.16) | 0.000 |
| Acute renal injury | 29 (48.33) | 41 (19.16) | 0.000 |
| Gastrointestinal bleeding | 18 (30) | 36 (16.82) | 0.023 |
| Acute liver dysfunction | 26 (43.33) | 61 (28.50) | 0.029 |
| Acute coronary syndrome | 8 (13.33) | 12 (5.61) | 0.042 |
| Acute cerebrovascular disease | 3 (5) | 8 (3.74) | 0.710 |
| Vasopressor use | 19 (31.67) | 39 (18.22) | 0.024 |
| CRRT | 3 (5) | 11 (5.14) | 1.000 |
| Sedation | 46 (76.67) | 146 (68.22) | 0.207 |
| Hospital mortality | 28 (46.67) | 14 (6.54) | 0.000 |
| Laboratory findings | |||
| RR, breaths/min | 21.10 ± 5.252 | 19.83 ± 4.712 | 0.074 |
| SpO2, % | 98 (96.25–100) | 99 (97.75–100) | 0.057 |
| HR, beats/min | 87.70 ± 15.358 | 87.44 ± 14.794 | 0.907 |
| SPB, mmHg | 130.38 ± 18.94 | 129.50 ± 21.98 | 0.778 |
| PH | 7.45 (7.42–7.48) | 7.45 (7.42–7.48) | 0.718 |
| PaCO2, mmHg | 54.5 (45.95–71) | 50 (44.3–60.7) | 0.054 |
| PaO2, mmHg | 89 (78.65–95) | 89 (80–101.5) | 0.148 |
| PaO2/FiO2, mmHg | 153.87 (130.26–172.86) | 161.82 (138.33–187.38) | 0.035 |
| Lactate, mmol/L | 1.6 (1.2–2.5) | 1.3 (1.00–1.7) | 0.002 |
| Hemoglobin, g/L | 93.13 ± 24.90 | 99.10 ± 21.73 | 0.070 |
| Albumin, g/L | 26.08 ± 6.12 | 27.53 ± 6.65 | 0.130 |
| Creatinine | 88.85 (55.43–125.30) | 74 (57.4–117.3) | 0.363 |
| ALT, IU/L | 23 (16.63–32.5) | 19.15 (13–34.65) | 0.100 |
| AST, IU/L | 32.55 (21.18–53.75) | 26.9 (18.5–43.05) | 0.095 |
| Leukocyte count, × 109/L | 10.25 (8.25–12.05) | 9.7 (6.9–12.65) | 0.546 |
| Neutrophils count, × 109/L | 7.52 (5.82–9.84) | 8.29 (5.8–11.74) | 0.380 |
| Lymphocyte count, × 109/L | 0.73 (0.5–1.07) | 0.79 (0.53–1.29) | 0.303 |
| Platelet count, × 109/L | 148 (107.5–202.75) | 184 (134.75–254.5) | 0.003 |
| PDW, % | 14.45 (11.53–16.48) | 12 (10.5–12.925) | 0.000 |
| Platelet large cell ratio, % | 34.02 ± 9.90 | 27.44 ± 7.74 | 0.000 |
| CRP, mg/L | 70.5 (34.5–109.5) | 44.5 (18.75–84.0) | 0.002 |
Continuous variables were presented as median (25th-75th percentile) or (mean ± SD). Categorical variables were presented as numbers (n) and percentages (%)
BMI body mass index; APACHE II acute physiology and chronic health evaluation II; IMV invasive mechanical ventilation; LOS length of stay; RR respiratory rate; HR heart rate; SBP systolic blood pressure; SPO2 peripheral oxygen saturation; PaCO2 arterial carbon dioxide tension; PaO2 arterial oxygen tension; FiO2 fraction of inspired oxygen; MPV mean platelet volume; PDW platelet distribution width; CRP C-reactive protein; CRRT continuous renal replacement therapy; ALT alanine aminotransferase; AST aspartate aminotransferase
Relationship between MPV and other variables
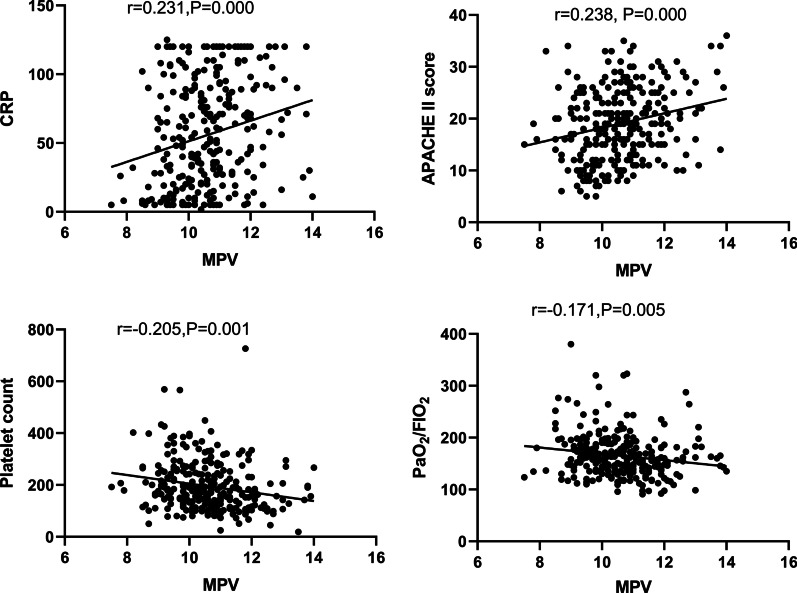
By the correlation analysis as shown in Fig. 2, MPV was positively related with CRP (r = 0.231, P = 0.000), APACHE II score (r = 0.238, P = 0.000), while it was negatively correlated with platelet count (r = − 0.205, P = 0.001) and PaO2/FIO2 (r = − 0.171, P = 0.005).
Fig. 2.
Correlations between MPV and other variables. Abbreviate: MPV, mean platelet volume; APACHE II, acute physiology and chronic health evaluation II; PaO2, arterial oxygen tension; FiO2, fraction of inspired oxygen; CRP, C-reactive protein
Risk factors for in-hospital mortality
The possible risk factors for higher in-hospital mortality were shown in Table 2. After adjusting for covariates (P < 0.05 in univariate Cox regression), the multivariate Cox regression suggested that MPV, PaO2/FIO2, CRP and CRRT were associated with in-hospital mortality (P = 0.001, P = 0.015, P = 0.008 and P = 0.005 respectively).
Table 2.
Independent predictors of in-hospital mortality by univariate and multivariate Cox regression analysis
| Variables | HR (95%CI) | P |
|---|---|---|
| Univariate Cox analysis | ||
| APACHE II score | 1.046 (1.001–1.092) | 0.043 |
| Albumin | 1.016 (0.963–1.072) | 0.561 |
| Creatinine | 1.001 (1.000–1.002) | 0.214 |
| MPV | 1.712 (1.358–2.159) | 0.000 |
| Leukocyte | 0.982 (0.908–0.993) | 0.657 |
| CRP | 1.011 (1.003–1.020) | 0.011 |
| Vasopressor use | 2.087 (1.115–3.908) | 0.021 |
| CRRT | 3.134 (1.193–8.232) | 0.003 |
| CKD | 2.052 (1.017–4.140) | 0.045 |
| Septic shock | 2.538 (1.371–4.697) | 0.003 |
| AKI | 1.949 (1.051–3.612) | 0.034 |
| PaO2/FIO2 | 0.982 (0.971–0.993) | 0.002 |
| Age | 1.028 (1.000–1.057) | 0.052 |
| Lactate | 1.080 (0.953–1.223) | 0.228 |
| sex | 0.849 (0.447–1.613) | 0.849 |
| Multivariate Cox* analysis | ||
| MPV | 1.741 (1.267–2.393) | 0.001 |
| PaO2/FIO2 | 0.984 (0.972–0.997) | 0.015 |
| CRP | 1.013 (1.003–1.022) | 0.008 |
| CRRT | 5.455 (1.676–17.759) | 0.005 |
*Covariates included in multivariate analysis: APACHE II score, MPV, CRP, PaO2/FIO2, vasopressor use, CRRT, CKD, AKI, septic shock
APACHE II acute physiology and chronic health evaluation II; MPV mean platelet volume; CRP C-reactive protein; CRRT continuous renal replacement therapy; CKD chronic kidney disease; AKI acute kidney injury; PaO2 arterial oxygen tension; FiO2 fraction of inspired oxygen;
Predicting ability of MPV for in-hospital mortality
As shown by ROC curves (Fig. 3), the cutoff value of MPV for predicting in-hospital mortality was 11.4 fl with a sensitivity of 66.67%, a specificity of 86.21% and diagnostic accuracy of 83.21%, and had a moderate power for predicting in-hospital mortality (AUC 0.848; 95%CI, 0.800–0.889, P < 0.0001).
Fig. 3.
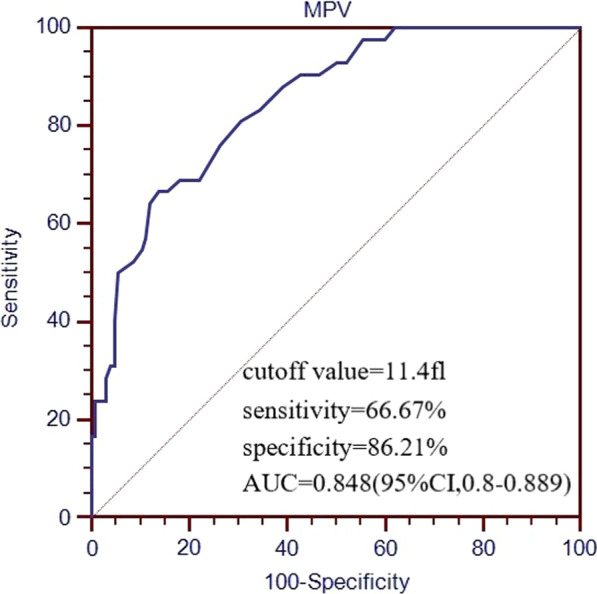
ROC curves of MVP for predicting in-hospital mortality. Abbreviate: MPV, mean platelet volume; ROC, receiver operating characteristic; AUC, area under the curve
MPV associated with in-hospital mortality
According to Kaplan–Meier survival curves for the MPV level, patients with MPV > 11.4 fl had clearly higher mortality than those with MPV ≤ 11.4 fl (log-rank chi square test = 40.35, HR = 8.723, P < 0.0001) (Fig. 4).
Fig. 4.
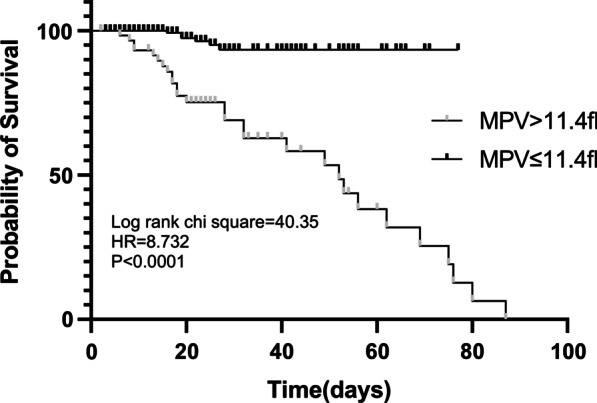
Kaplan–Meier survival curve according to the cutoff value of MPV for in-hospital mortality. MPV, mean platelet volume
Associations of MPV with in-hospital mortality were shown in Table 3. After adjusting for covariates, Cox regression analysis indicated that patients with MPV > 11.4 fl was significantly related to higher in-hospital mortality. In model 3 with the maximum covariates including age, sex, APACHE II score, PaO2/FIO2,vasopressor use, CRRT, CKD, AKI, septic shock, albumin, creatinine, leukocyte, and CRP, adjusted HR for in-hospital mortality was 2.640 (95%CI, 1.208–5.767,P = 0.015).
Table 3.
Relationship between MPV level and in-hospital mortality
| In-hospital mortality | MPV > 11.4 fl group | |
|---|---|---|
| HR (95%CI) | P | |
| Unadjusted | 4.30 (2.223–8.317) | 0.000 |
| Model 1 | 4.245 (2.18–8.266) | 0.000 |
| Model 2 | 3.996 (1.862–8.578) | 0.000 |
| Model 3 | 2.640 (1.208–5.767) | 0.015 |
Reference group is MPV ≤ 11.4 fl. Model 1: age and sex; Model 2: model 1 plus APACHE II score, PaO2/FIO2, vasopressor use, CRRT, CKD, AKI, septic shock; Model 3: model 2 plus albumin, creatinine, leukocyte, and CRP
MPV mean platelet volume; APACHE II acute physiology and chronic health evaluation II; CRP C-reactive protein; CRRT continuous renal replacement therapy; CKD chronic kidney disease; AKI acute kidney injury; PaO2 arterial oxygen tension; FiO2 fraction of inspired oxygen; HR hazard ratio; CI confidence interval
Relationship between mortality and MPV in the sex subgroup
A subgroup analysis by sex was conducted using Cox model after adjusting covariates including APACHE II score, CRP, PaO2/FIO2, vasopressor use, CRRT, CKD, AKI, septic shock. In the subgroup analysis, the relationship between MPV > 11.4 fl and in-hospital mortality seemed to be stronger in female subgroup (HR, 14.265;95%CI: 2.323–87.579; P = 0.004) than that in male subgroup (HR, 2.905;95%CI:1.042–8.098; P = 0.042) (Table 4). The Kaplan–Meier curve also suggested that the MPV > 11.4 fl had a greater impact on in-hospital mortality in female patients than in male patients (Fig. 5).
Table 4.
Relationship between in-hospital mortality and MPV cutoff by sex
| In-hospital mortality | Female | Male | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| MPV > 11.4 fl | 14.265 (2.323–87.579) | 0.004 | 2.905 (1.042–8.098) | 0.042 |
Covariates included in Cox model: APACHE II score, CRP, PaO2/FIO2, vasopressor use, CRRT, CKD, AKI, septic shock
MPV mean platelet volume; APACHE II acute physiology and chronic health evaluation II; CRP C-reactive protein; CRRT continuous renal replacement therapy; CKD chronic kidney disease; AKI acute kidney injury; PaO2 arterial oxygen tension; FiO2 fraction of inspired oxygen; HR hazard ratio; CI confidence interval
Fig. 5.
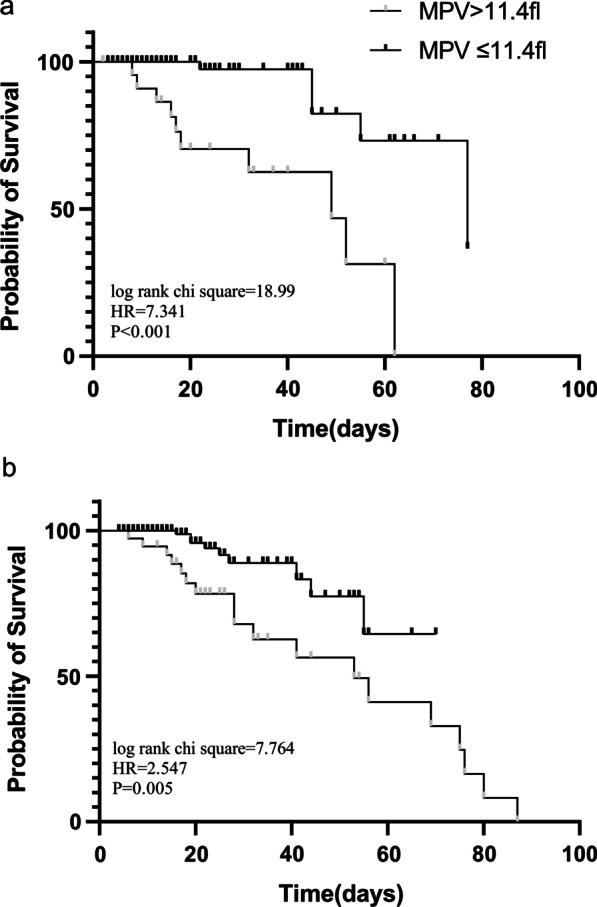
Kaplan–Meier survival curve according to the cutoff value of MPV for in-hospital mortality in female subgroup (a) and male subgroup (b). MPV, mean platelet volume
Discussion
In this research, we indicated MPV > 11.4 fl within 24 h after IMV was positively associated with in-hospital mortality among patients experiencing IMV. MPV > 11.4 fl with the AUC of ROC (0.848; 95%CI, 0.800–0.889) had a moderate predictive ability for in-hospital mortality and MPV > 11.4 fl was an independent predictor for in-hospital mortality. The HR of MPV > 11.4 fl for predicting mortality was 2.642 after adjusting for potential confounders, suggesting that the mortality risk of patients with MPV > 11.4 fl is much higher than patients with MPV ≤ 11.4 fl. The relationship between MPV > 11.4 fl and in-hospital mortality was stronger in female patients than in male patients.
Some studies had reported the similar results. A study suggested that MPV cutoff > 9,45fL at day 1, > 8,95fL at day 2 and > 8, 85fL at day 3 were independent predictor factors of mortality in sepsis [27]. Chen reported that MPV > 10.5 fl were associated with in-hospital mortality in severe pneumonia patients [28]. A retrospective study also found that the elevated MPV was related to poor outcome [29], however they did not find the association between the MPV at admission and in-hospital mortality in ICU patients. In our study, we focused on patients with IMV, and also find MPV > 11.4 fl was associated with in hospital mortality. The cutoff values were different, which may due to the difference in study population, severity of diseases, baseline characteristics, and even adjusted confounders.
The underly mechanisms of higher MPV with poor prognosis in patients with IMV remain hazy. The findings could be elaborated by several possible reasons as below.
Patients with mechanical ventilations are often critically ill and undergo a more serious status and stronger inflammatory response [30, 31]. Endotracheal intubation and mechanical ventilation can enhance this inflammatory reaction and stress. Moreover, nonsurvivors may experience more local and systemic inflammatory responses and stress. Hence, severe inflammatory response and stress were the possible mechanisms for higher MPV in nonsurvivors, which were induced by underlying disease, endotracheal intubation, and mechanical ventilation.
Some studies suggested that serious inflammation could induce a systematic response with the release of thrombopoietin and lots of proinflammatory cytokines, mainly IL-6 and other substances that stimulate platelet activation and the massive production of young platelets in the blood circulation [27, 32, 33], but these large platelets function poorly competent, inducing thrombogenic activity and adverse clinical outcomes [32]. In the current study, patients with MPV > 11.4 fl had higher APACHE II score, longer ICU LOS, more complications including shock, AKI, acute liver dysfunction, gastrointestinal bleeding, and acute coronary syndrome, higher frequency treatment of vasopressor use and CRRT. Moreover, patients with MPV > 11.4 fl showed lower PaO2/FIO2, higher lactate, and higher CRP level, suggesting that those patients suffer more serious status and show more intense inflammatory response. In addition, a previous study had reported this platelet count was inversely related with MPV [34], in this study patients with MPV > 11.4 fl had a lower platelet count than that in patients with MPV ≤ 11.4 fl. MPV was also negatively associated with platelet count among these patients. We inferred that thrombocyte consumption caused by severe inflammation could result in higher MPV. Besides that, some studies reported that hypoxemia may enhance platelet consumption and promote bone marrow activation. Our study showed that nonsurvivors had much lower PaO2/FIO2, which could be another explanation for the increased MPV levels in nonsurvivors. In all, these factors could result in a higher MPV in nonsurvivors. According, we believe that MPV might be a more useful predicting factor for in-hospital mortality.
Besides that, we also found that the relationship between higher MPV and in-hospital mortality was much stronger in female patients than in male patients, which indicating that MPV was more likely to reflect in-hospital mortality in female patients. This was in line with previous study [28], reporting that MPV was associated with mortality among severe pneumonia female patients. Although it has been reported that there were differences in platelet function between male and female mice [35], the mechanism of the relationship was unclever. Female have higher platelet responsiveness [36], platelet counts [37], and had a higher propensity to activate integrin αIIbβ3, to degranulate and expose P-selectin, and to form platelet–leukocyte aggregates [38]. Women platelets have an intrinsic higher propensity to be activated by inflammatory stimuli compared to men [39]. The sexual dimorphism affects both the pro-aggregating and the immune-modulatory function of platelets [39]. Those may be potential mechanisms. With higher platelet responsiveness and platelet counts, the platelet may release more pro-inflammatory factors and immune regulatory factors, and interact more with granulocytes, leading to more serious inflammatory reactions in female patients. In addition, a study reported that a higher MPV might be a surrogate marker associated with metabolic disturbance only in women [40], which may be another possible mechanism to explain our results. More researches are needed to furtherly confirm this relationship.
Limitations
There were some limitations in this study. First, this is a single-center retrospective study with a small sample, so the results of this study ought to be generalized with caution. Second, we did not investigation nutritional status and antibiotics use which could affect the inflammatory response and in-hospital mortality. Third, there were about 20% postsurgical patients who tended to survive, this could cause lower in-hospital mortality compared to other studies [2, 5], and this possibly caused a bias in the interpretation of the results. Fourth, only the MPV within 24 h after intubation was studied, the change of MPV through ICU course were not evaluated due to lacking of data. Fifth, the AUC of 0.848 of MPV > 11.4 fl by ROC curve suggested that MPV alone is not enough to predict mortality. More predictive factors and models should be further studied in future. Sixth, the underlying mechanism of the sex difference between MPV and mortality was not clarified.
Conclusion
The ability of MPV for predicting in-hospital mortality among patients undergoing IMV has not been studied before. The study suggested that MPV is a more useful maker for predicting in-hospital mortality, and MPV > 11.4 fl was an independent predictor factor for in-hospital mortality. This relationship was much stronger in female patients. MPV is reported in routine blood counts with low cost and availability, making it a profitable marker with immediate clinical applicability, especially in limited resource sites.
Acknowledgements
Not applicable.
Abbreviations
- MPV
Mean platelet volume
- IMV
Invasive mechanical ventilation
- ICU
Intensive care unit
- CRP
C-reaction protein
- ROC
Receiver-operating characteristics
- COPD
Chronic obstructive pulmonary disease
- BMI
Body mass index
- LOS
Length of stay
- APACHE II
Acute physiology and chronic health evaluation II
- RR
Respiratory rate
- HR
Heart rate
- SBP
Systolic blood pressure
- SPO2
Peripheral oxygen saturation
- PaCO2
Arterial carbon dioxide tension
- PaO2
Arterial oxygen tension
- FiO2
Fraction of inspired oxygen
- MPV
Mean platelet volume
- PDW
Platelet distribution width
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase. AUC: area under the curve
- CI
Confidence interval
- HR
Hazard ratio
- CRRT
Continuous renal replacement therapy
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
Author contributions
YZ and ZL designed the research; ZC conducted the research; YZ and ZL collected and analyzed the data; all authors wrote the paper; and all authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data sets supporting the results of this article are included within the article.
Declarations
Ethics approval and consent to participate
This study (NO.2020-KE-94) was approved by the Ethics Committee of the Beijing Chao-Yang Hospital, Capital Medical University. The requirement for informed consent was waived by the Ethics Committee of the Beijing Chao-Yang Hospital, Capital Medical University because of the retrospective nature of the study. All procedures were in accordance with Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rackley CR. Monitoring during mechanical ventilation. Respir Care. 2020;65(6):832–846. doi: 10.4187/respcare.07812. [DOI] [PubMed] [Google Scholar]
- 2.Vasilyev S, Schaap RN, Mortensen JD. Hospital survival rates of patients with acute respiratory failure in modern respiratory intensive care units. An international, multicenter, prospective survey. Chest. 1995;107(4):1083–1088. doi: 10.1378/chest.107.4.1083. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 4.Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest. 2000;118(4):1100–1105. doi: 10.1378/chest.118.4.1100. [DOI] [PubMed] [Google Scholar]
- 5.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 6.Urner M, Juni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8(9):905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi Y, Zhao Z, Frerichs I, Long Y, He H. Prevalence and prognosis of respiratory pendelluft phenomenon in mechanically ventilated ICU patients with acute respiratory failure: a retrospective cohort study. Ann Intensive Care. 2022;12(1):22. doi: 10.1186/s13613-022-00995-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y, Zhu B, Jiang L, Jiang Q, Wang M, Hua L, Xi X. A contemporary assessment of acute mechanical ventilation in Beijing: description, costs, and outcomes. Crit Care Med. 2017;45(7):1160–1167. doi: 10.1097/CCM.0000000000002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du B, An Y, Kang Y, Yu X, Zhao M, Ma X, Ai Y, Xu Y, Wang Y, Qian C, et al. Characteristics of critically ill patients in ICUs in mainland China. Crit Care Med. 2013;41(1):84–92. doi: 10.1097/CCM.0b013e31826a4082. [DOI] [PubMed] [Google Scholar]
- 10.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34(1):5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. doi: 10.1155/2019/9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sezgi C, Taylan M, Kaya H, Selimoglu Sen H, Abakay O, Demir M, Abakay A, Tanrikulu AC. Alterations in platelet count and mean platelet volume as predictors of patient outcome in the respiratory intensive care unit. Clin Respir J. 2015;9(4):403–408. doi: 10.1111/crj.12151. [DOI] [PubMed] [Google Scholar]
- 13.Zampieri FG, Ranzani OT, Sabatoski V, de Souza HP, Barbeiro H, da Neto LM, Park M, da Pinheiro SF. An increase in mean platelet volume after admission is associated with higher mortality in critically ill patients. Ann Intensive Care. 2014;4:20. doi: 10.1186/s13613-014-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim YL, Park KS, Ryu HJ, Park JT, Han SH, Yoo TH, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS ONE. 2015;10(3):e0119437. doi: 10.1371/journal.pone.0119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aydemir H, Piskin N, Akduman D, Kokturk F, Aktas E. Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets. 2015;26(4):331–335. doi: 10.3109/09537104.2012.701027. [DOI] [PubMed] [Google Scholar]
- 16.Reangvilaikul T, Udompongpaiboon P, Vattanavanit V. Predicting acute respiratory distress syndrome in influenza pneumonia patients using delta mean platelet volume. BMC Pulm Med. 2021;21(1):405. doi: 10.1186/s12890-021-01763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wang L, Jin L, Rong X, Tang X, Guo H, Liu X, Shi L, Tao G. Predictive value of MPV and plasma NT-ProBNP combined with the simplified geneva scale for the prognosis of acute pulmonary embolism. Evid-based Complement Altern Med: eCAM. 2021;2021:1292921. doi: 10.1155/2021/1292921. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Colakoglu SM, Genc Moralar D, Cekmecelioglu BT, Hergunsel GO. Relationship of mortality with neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume in patients undergoing acute abdominal surgery. Ulus Travma Acil Cerrahi Derg. 2020;26(5):735–741. doi: 10.14744/tjtes.2020.81783. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed MF, Ali A, Abbas A, Awad MS, Gouda M, Sediq AM. Mean platelet volume as a predictor of pulmonary hypertension in patients with stable COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1099–1108. doi: 10.2147/COPD.S176413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pafili K, Penlioglou T, Mikhailidis DP, Papanas N. Mean platelet volume and coronary artery disease. Curr Opin Cardiol. 2019;34(4):390–398. doi: 10.1097/HCO.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 21.Karim F, Akter QS, Khanom A, Haque S, Nahar S. Mean platelet volume in Type 2 Diabetes male. Mymensingh Med J: MMJ. 2020;29(3):659–663. [PubMed] [Google Scholar]
- 22.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 23.Zheng Y, Luo Z, Cao Z. Mean platelet volume is useful for predicting weaning failure: a retrospective, observational study. BMC Anesthesiol. 2022;22(1):160. doi: 10.1186/s12871-022-01701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein DJ, Aaronson KD, Tatooles AJ, Silvestry SC, Jeevanandam V, Gordon R, Hathaway DR, Najarian KB, Slaughter MS, Investigators A. Gastrointestinal bleeding in recipients of the HeartWare ventricular assist system. JACC Heart Fail. 2015;3(4):303–313. doi: 10.1016/j.jchf.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Vélez-Páez JL, Legua P, Vélez-Páez P, Irigoyen E, Andrade H, Jara A, López F, Pérez-Galarza J, Baldeón L. Mean platelet volume and mean platelet volume to platelet count ratio as predictors of severity and mortality in sepsis. PLoS ONE. 2022;17(1):e0262356. doi: 10.1371/journal.pone.0262356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Li Y, Zeng Y, Tian Y, Wen Y, Wang Z. High mean platelet volume associates with in-hospital mortality in severe pneumonia patients. Mediators Inflamm. 2020;2020:8720535. doi: 10.1155/2020/8720535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Park M, Han S, Hwang JJ, Park SH, Park SY. An increase in mean platelet volume during admission can predict the prognoses of patients with pneumonia in the intensive care unit: a retrospective study. PLoS ONE. 2018;13(12):e0208715. doi: 10.1371/journal.pone.0208715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennus MP, van Vught AJ, Brabander M, Brus F, Jansen NJ, Bont LJ. Mechanical ventilation drives inflammation in severe viral bronchiolitis. PLoS ONE. 2013;8(12):e83035. doi: 10.1371/journal.pone.0083035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sellares J, Loureiro H, Ferrer M, Amaro R, Farre R, Torres A. The effect of spontaneous breathing on systemic interleukin-6 during ventilator weaning. Eur Respir J. 2012;39(3):654–660. doi: 10.1183/09031936.00037511. [DOI] [PubMed] [Google Scholar]
- 32.Machlus KR, Thon JN, Italiano JE., Jr Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165(2):227–236. doi: 10.1111/bjh.12758. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura S, Nagasaki M, Kunishima S, Sawaguchi A, Sakata A, Sakaguchi H, Ohmori T, Manabe I, Italiano JE, Jr, Ryu T, et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209(3):453–466. doi: 10.1083/jcb.201410052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butkiewicz AM, Kemona H, Dymicka-Piekarska V, Matowicka-Karna J, Radziwon P, Lipska A. Platelet count, mean platelet volume and thrombocytopoietic indices in healthy women and men. Thromb Res. 2006;118(2):199–204. doi: 10.1016/j.thromres.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. 1975;253(5490):355–357. doi: 10.1038/253355a0. [DOI] [PubMed] [Google Scholar]
- 36.Leng XH, Hong SY, Larrucea S, Zhang W, Li TT, López JA, Bray PF. Platelets of female mice are intrinsically more sensitive to agonists than are platelets of males. Arterioscler Thromb Vasc Biol. 2004;24(2):376–381. doi: 10.1161/01.ATV.0000110445.95304.91. [DOI] [PubMed] [Google Scholar]
- 37.Sloan A, Gona P, Johnson AD. Cardiovascular correlates of platelet count and volume in the Framingham Heart Study. Ann Epidemiol. 2015;25(7):492–498. doi: 10.1016/j.annepidem.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gremmel T, Kopp CW, Eichelberger B, Koppensteiner R, Panzer S. Sex differences of leukocyte-platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis. 2014;237(2):692–695. doi: 10.1016/j.atherosclerosis.2014.10.095. [DOI] [PubMed] [Google Scholar]
- 39.Sabetta A, Lombardi L, Stefanini L. Sex differences at the platelet-vascular interface. Intern Emerg Med. 2022;17(5):1267–1276. doi: 10.1007/s11739-022-02994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park BJ, Shim JY, Lee HR, Jung DH, Lee JH, Lee YJ. The relationship of platelet count, mean platelet volume with metabolic syndrome according to the criteria of the American Association of Clinical Endocrinologists: a focus on gender differences. Platelets. 2012;23(1):45–50. doi: 10.3109/09537104.2011.589014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets supporting the results of this article are included within the article.