Abstract
Purpose
Twelve-month safety and effectiveness results for canaloplasty combined with trabeculotomy using the OMNI surgical system in pseudophakic eyes or combined with cataract surgery in mild-to-moderate open-angle glaucoma (OAG).
Setting
Sixteen centers in 11 US states (AL, AR, CA, CT, KS, LA, MO, NY, SD, TN, TX).
Design
Retrospective, case series, open-label.
Methods
A total of 136 eligible patients from 20 surgeons: mild-to-moderate OAG (visual field mean deviation (MD) not worse than −12 dB), 12-month follow-up, preoperative medicated IOP >18 mmHg and ≤36 mmHg on ≤5 medications. One eye per patient enrolled. Endpoints included proportion with ≥20% reduction in IOP or IOP between 6 and 18 mmHg (inclusive) and on the same or fewer medications without secondary surgical intervention (SSI) (primary success), mean IOP, change in IOP, ocular hypotensive medication use, and proportion of patients with a 20% or greater reduction in IOP at 12 months. Safety: adverse events (AE) and best corrected visual acuity (BCVA).
Results
Primary success was met by 71%. Mean IOP was reduced (22.3–15.9 mmHg, p < 0.0001). Medications went from 1.9 ± 1.3 to 1.3 ± 1.2 (p < 0.001). AE were mild and as expected for angle surgery. The most frequent AE were BCVA loss (6%), mild inflammation (4%), IOP elevation (3%), and clinically significant hyphema (3%). There were 4 (3%) SSI.
Conclusion
The OMNI system provides effective IOP reduction, sustained IOP control, and meaningful medication reduction for up to 12 months postoperative. The present study confirms and extends the results from ROMEO.
Keywords: viscodilation, MIGS, open-angle glaucoma, glaucoma surgery, canaloplasty, trabeculotomy, OMNI
Introduction
The ROMEO study provided the first data from multiple surgeons and study sites demonstrating the effectiveness of circumferential canaloplasty and trabeculotomy (OSS; OMNI Surgical System, Sight Sciences, Inc, Menlo Park, CA) in mild-to-moderate open-angle glaucoma (OAG).1,2 The study was the basis of the FDA approved OSS indication to “reduce IOP in adult patients with open-angle glaucoma”. ROMEO included pseudophakic patients undergoing a standalone procedure as well as patients undergoing a procedure combined with phacoemulsification cataract surgery. Both cohorts were stratified by preoperative baseline intraocular pressure (IOP); >18 mmHg, and ≤18 mmHg. While ROMEO provided early clinical data useful in guiding treatment decisions, the sample size was relatively small due to the short period of time the OSS had been available prior to the start of ROMEO. The aim of ROMEO 2 was to expand on and confirm the results of the original ROMEO study with an increased number of investigative sites and surgeons contributing cases. The focus of this expansion was on the high baseline IOP cohort from ROMEO, “Group 1”, baseline IOP greater than 18 mmHg, for two reasons; first, the low, controlled IOP cohort had a reasonably large sample size (N = 81), while the high baseline IOP group was substantially smaller (N = 48, 24 combined with phacoemulsification cataract surgery and 24 standalone in pseudophakic eyes), and second, confirmation of the ROMEO results with a larger sample size would further support OSS as an effective IOP-lowering treatment option for this group of patients poorly controlled by medication.1,2
Methods
ROMEO 2 was a retrospective, multicenter study with 16 participating ophthalmic practices in 11 states (AL, AR, CA, CT, KS, LA, MO, NY, TN, TX, SD). Eligible cases included pseudophakic eyes where the OSS was used as a standalone procedure (“standalone”), and eyes undergoing an OSS procedure concomitant with phacoemulsification cataract surgery (“+cataract”). Eligibility criteria were unchanged from the original ROMEO study with the single exception that baseline pre-operative IOP must have been >18 mmHg. In brief, these were as follows: Underwent canaloplasty and trabeculotomy using the OSS with at least 12 months of follow-up (acceptable window 273–456 days) before the study start; at least 180° canaloplasty and at least 90° of trabeculotomy; cataract requiring surgery or pseudophakic at the time of OSS surgery; diagnosis of OAG; mild-to-moderate glaucoma (mean deviation −12 dB or better); cup:disc ≤0.9; IOP > 18 mmHg and on 0–5 topical IOP-lowering medications preoperatively; open angles (Shaffer grade ≥3). Exclusions included any of the following procedures within 6 weeks of OSS surgery: SLT or ALT, trabecular or supraciliary stent, ciliary ablation. Patients were excluded if they had a history of trabeculectomy or other bleb-forming procedure, or a previous canaloplasty or trabeculotomy. No other procedure could have been performed at the time of OSS aside from cataract extraction.
The study was reviewed by the WCG IRB (Puyallup, WA, USA). Because the study was retrospective and there were no study interventions, the IRB gave waiver of consent. No patient identifiable data were collected, and tenets of the Declaration of Helsinki were followed. This study is not listed on ClinicalTrials.gov as it is not an “Applicable Clinical Trial” (42 CFR 11.22(b)).
All available patients meeting the eligibility criteria were included for each participating investigative site to mitigate the risk of bias due to “cherry-picking”. A single eye from each patient could be included to ensure independence of data points, Where both eyes were eligible, only the right eye was included. No site enrolled >17% of the patients preventing overall study outcomes from undue influence by a single high enrolling site. Potential bias for favorable outcomes was mitigated by the retrospective design; all outcome measurements were completed prior to initiation of study data collection.
All data were extracted from patient medical records and included patient characteristics and history, IOP measurements, ocular hypotensive medication use, best corrected visual acuity (BCVA), and any complications (adverse events). Clock hours of canaloplasty and trabeculotomy were obtained from the operative notes. Purposely wide time windows for each of the study follow-up visits (Months 1, 6, 12) acknowledge that patients were followed as part of actual clinical practice rather than in the strict context of a prospective clinical trial. These time windows are aligned with World Glaucoma Association recommendations in Guidelines on Design and Reporting of Glaucoma Surgical Trials (2009).
A complete ophthalmic examination had been conducted for all patients prior to surgery. This included IOP (Goldmann applanation tonometry, mean of two repeated measures), gonioscopy, visual field assessment, slit-lamp and dilated fundus examinations. This exam was generally within 7 weeks of surgery (mean 25.2 days, median 20 days, 90th percentile 46 days).
Surgery with the OSS was standardized as all surgeons had received training in the device use provided by the manufacturer. In combined cases, the OSS procedure was performed following uncomplicated cataract surgery. In brief, viscoelastic was used to deepen the anterior chamber (AC). The OSS was introduced through a small (<2mm) clear corneal incision temporally into the AC (the same incision as for the cataract surgery in combined cases). The OSS was advanced across the AC, and the cannula tip was used to make a small goniotomy (<1mm). Schlemm’s canal was then accessed by insertion of the cannula, and the extension of the microcatheter for 180°. Retraction of the microcatheter dispensed a controlled amount of viscoelastic dilating the canal and collector channels. The microcatheter was again advanced into the canal for 180° to perform the trabeculotomy. Both hemispheres were dilated with viscoelastic in the majority of cases (65%) while a single hemisphere trabeculotomy was performed most frequently (82%). The extent of canaloplasty and trabeculotomy was according to surgeon preference and discretion and study eligibility required a minimum of 180° canaloplasty followed by a minimum 90° trabeculotomy. The procedure was completed with AC irrigation to remove viscoelastic, and pressurization of the AC with BSS. A standard regimen (with minor variation) of steroid and antibiotic drops was prescribed postoperatively.
The primary success endpoint was the percentage of patients at 12 months with an IOP between 6 and 18 mmHg (inclusive) OR with a 20% or more decrease from preoperative baseline IOP and no increase in number of ocular hypotensive medications and with no additional secondary surgical intervention for IOP control (SSI).
Other endpoints were as follows: proportion of patients with a 20% or greater reduction in IOP, percentage of patients between 6 mmHg and 18 mmHg, percent change in IOP, mean IOP, and mean IOP-lowering medications.
Ocular adverse events (AE) were recorded including ≥2 line decrease in BCVA (Snellen), IOP increase ≥10 mmHg above baseline one month or more post-surgery, any SSI for IOP control.
All patients meeting all inclusion criteria, and no exclusion criteria were included in the analysis. Pre-screening ensured that all included patients met eligibility criteria, therefore the analysis included all patients; there were no patients “lost to follow-up”. Where an SSI for IOP was performed during follow-up, the patient was considered a treatment failure for the purposes of binary endpoint analyses.
Demographics and baseline characteristics were analyzed with descriptive statistics (mean, maximum, minimum, standard deviation) and the two subgroups, +cataract and standalone were compared using t-tests, Chi-square, or Fisher’s exact test as appropriate. Primary and secondary binary outcomes are reported along with 95% confidence intervals. Mean IOP and mean medication number is reported at each time point. Follow-up timepoints were compared to baseline using t-tests or the non-parametric Wilcoxon signed rank test where non-normal. Statistical significance was set at 0.05 except where a Bonferroni adjustment was employed to account for multiple comparisons. No imputation was carried out for missing data where a procedure was not done (eg, BCVA) or where there was a missed visit. The sample for this study was a “convenience sample” that included all available and eligible cases at the participating centers.
Results
The dataset included 136 eyes, 60 standalone and 76 +cataract. Patients were majority White (108, 79%), female (76, 56%), and with a diagnosis of POAG (121, 89%). Baseline characteristics and demographics were generally not statistically different between the +cataract and standalone subgroups except that a higher proportion of the standalone group were POAG (57, 95% vs 64, 84%), and the non-White racial mix was somewhat different between the two groups (Table 1).
Table 1.
Demographic and Baseline Characteristics
| Parameter | ALL (N=136) | +Cataract (N=76) | Standalone (N=60) | P-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 73.8 (7.9) | 73.2 (8.1) | 74.4 (7.6) | 0.3695 |
| Gender, n (%) | ||||
| Female | 76 (56) | 36 (47) | 40 (67) | 0.3695 |
| Male | 60 (44) | 40 (53) | 20 (33) | |
| Race, n (%) | ||||
| White | 108 (79) | 60 (79) | 48 (80) | 0.0148 |
| Black/African-American | 9 (7) | 2 (2.6) | 7 (12) | |
| Asian | 5 (3.7) | 3 (3.9) | 2 (3.3) | |
| Other | 11 (8) | 10 (13) | 1 (1.7) | |
| Not reported | 3 (2.2) | 1 (1.3) | 2 (3.3) | |
| Glaucoma Diagnosis, n (%) | ||||
| Primary open-angle (POAG) | 121 (89) | 64 (84) | 57 (95) | 0.0375 |
| Pseudoexfoliation (PXG) | 11 (8) | 10 (13) | 1 (1.7) | |
| Pigmentary (PG) | 4 (2.9) | 2 (2.7) | 2 (3.3) | |
| Visual field mean deviation (dB), mean (SD) | −4.6 (3.6) | −4.4 (3.8) | −4.7 (3.4) | 0.6900 |
| Cup-to-disc ratio (SD) | 0.7 (0.2) | 0.7 (0.2) | 0.7 (0.2) | 0.9158 |
| Preoperative IOP (mmHg), mean (SD) | ||||
| ALL | 22.3 (3.7) | 22.0 (3.5) | 22.7 (4.0) | 0.3058 |
| POAG (n = 121) | 22.2 (3.5) | 21.8 (3.2) | 22.5 (3.9) | |
| PXG (n = 11) and PG (n = 4) | 23.7 (4.9) | 23.3 (4.8) | 25.5 (5.9) | |
| Preoperative Medications, mean (SD) | ||||
| ALL | 1.9 (1.3) | 1.9 (1.3) | 1.9 (1.4) | 0.7312 |
| POAG (n = 121) | 1.9 (1.3) | 1.8 (1.3) | 2.0 (1.4) | |
| PXG (n = 11) and PG (n = 4) | 1.6 (0.5) | 2.0 (1.4) | 1.7 (1.5) |
Notes: P-values from two-tailed t-test, Chi-square, or Fisher’s exact test as appropriate.
Abbreviations: dB, decibels; IOP, intraocular pressure; SD, standard deviation; POAG, primary open-angle glaucoma; PXG, pseudoexfoliation glaucoma; PG, pigmentary glaucoma; +Cataract, OMNI procedure in combination with cataract surgery.
Primary success, ie, the percentage of patients at 12 months with an IOP between 6 and 18 mmHg (inclusive) OR with a 20% or more decrease from preoperative baseline IOP and no increase in number of ocular hypotensive medications and with no additional SSI for IOP control, was met by 70.6% of the patients (96 of 136, 95% confidence interval [CI] 62.9–78.3%). A “qualified” primary success criteria, agnostic to medication use, was met by 82.4% of the patients (112 of 136, 95% CI 76–89%). A 20% or greater reduction in IOP was achieved by 65% (88 of 136, 95% CI 57–73%) of the patients and by 54% (74 of 136, 95% CI 46–62%) on the same or fewer medications as at baseline. At month 12, 79% had an IOP of 18 mmHg or less (108 of 136, 95% CI 73–86%), 69% (94 of 136, 95% CI 61–77%) on the same or fewer medications. The majority of patients had a decrease in medications or remained on the same number as preoperatively (117, 88.6%, 95% CI 83.3–93.9%) and the proportion of medication-free patients nearly doubled from 16.2% (n = 22) at baseline to 30.1% (n = 41) at month 12. Additional details are provided in Table 2.
Table 2.
Intraocular Pressure (IOP) and Medication Outcomes: Baseline and Month 12
| Baseline | Month 12 | P value* | |
|---|---|---|---|
| Mean (SD) IOP, mmHg | |||
| ALL (N=136) | 22.3 (3.7) | 15.9 (4.4) | <0.00001 |
| POAG (n=121) | 22.2 (3.5) | 16.1 (4.5) | <0.00001 |
| SOAG (n=15) | 23.7 (4.9) | 15.0 (4.0) | <0.0001 |
| Eyes Meeting Primary Success (≥20% reduction in IOP) | NA | 95% CI | |
| OR with IOP between 6 and 18 mmHg (inclusive) on the same or fewer medications and no SSI), n (%) | |||
| ALL (N=136) | 96 (71) | 63–78% | |
| POAG (n=121) | 85 (70) | 62–78% | |
| SOAG (n=15) | 11 (73) | 50–96% | |
| Eyes with decrease in IOP ≥20%, n (%) | NA | 95% CI | |
| ALL (N=136) | 88 (65) | 57–73% | |
| POAG (n=121) | 76 (63) | 54–72% | |
| SOAG (n=15) | 12 (80) | 60–100% | |
| IOP ≥6, ≤18 mmHg, n (%) | |||
| ALL (N=136) | 1 (0.7) | 108 (79) | <0.001 |
| POAG (n=121) | 1 (0.8) | 96 (79) | <0.001 |
| SOAG (n=15) | 0 | 12 (80) | <0.001 |
| Mean (SD) Ocular Hypotensive Medications | |||
| ALL (N=136) | 1.9 (1.3) | 1.3 (1.2) | <0.001 |
| POAG (n=121) | 1.9 (1.3) | 1.3 (1.2) | <0.001 |
| SOAG (n=15) | 1.9 (1.3) | 1.1 (1.2) | 0.101 |
| Medication-Free, n (%) | |||
| ALL (N=136) | 22 (16) | 41 (30) | 0.014 |
| POAG (n=121) | 19 (16) | 34 (28) | 0.030 |
| SOAG (n=15) | 3 (20) | 7 (47) | 0.252 |
| Reduction in Medications ≥ 1, n (%) | NA | 95% CI | |
| ALL (N=136) | 64 (47) | 39–55% | |
| POAG (n=121) | 56 (46) | 37–55% | |
| SOAG (n=15) | 8 (53) | 28–78% |
Notes: *Comparison of month 12 and baseline. Two-tailed t-test (IOP and medication number), Chi-square or Fisher’s exact test (proportions). Baseline is the preoperative baseline.
Abbreviations: CI, confidence interval; SD, standard deviation; POAG, primary open-angle glaucoma; SOAG, secondary open-angle glaucoma including pseudoexfoliation and pigmentary.
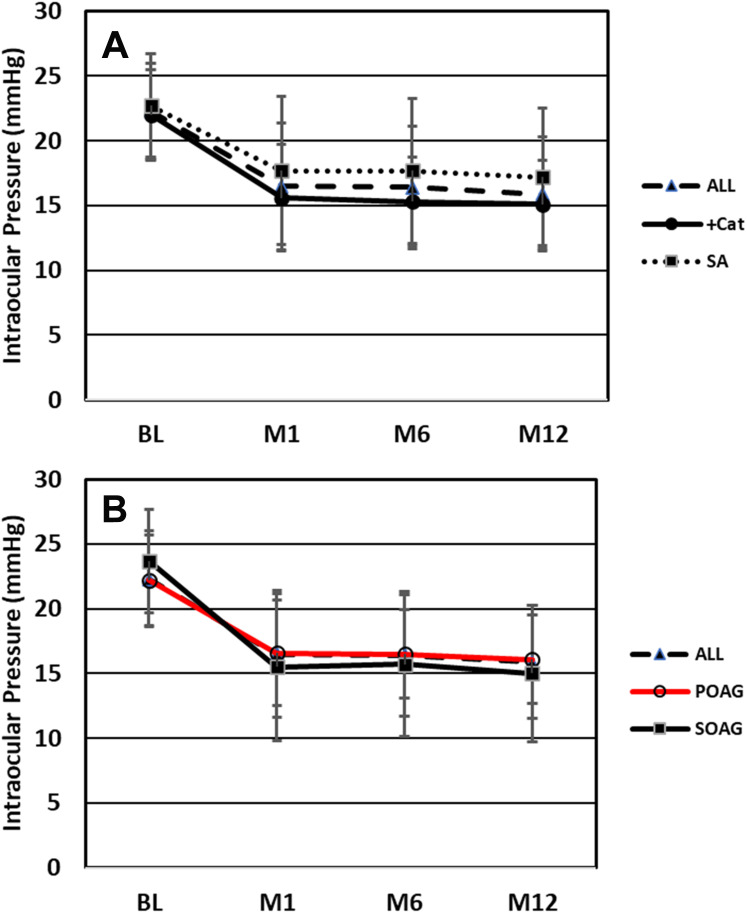
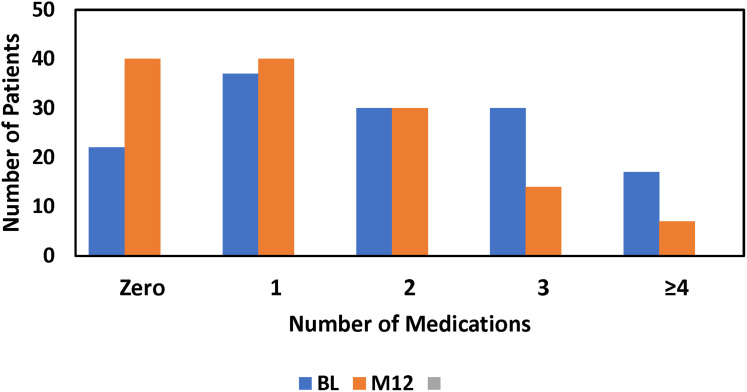
Medicated intraocular pressure was significantly decreased at all postoperative timepoints (Figure 1). Baseline mean IOP was 22.3 mmHg which decreased to 15.9 mmHg (−6.4 mmHg, −28.6%, P<0.00001) at month 12. Median percent change in IOP from baseline to month 12 was −30.0%. Preoperatively, patients were on an average of 1.9 medications with 22 (16%) on zero, 37 (27%) on one, 30 (22%) on two, 30 (22%) on three, and 17 (13%) on four or five. At month 12 (n=132 because medication data for 4 patients with an SSI was excluded), the mean number of medications was 1.3 (P<0.001 vs baseline) and these were shifted to generally fewer medications, 40 (31%), 40 (31%), 30 (23%), 14 (11%), and 7 (5%), respectively (Figures 2 and 3).
Figure 1.
(A) Mean intraocular pressure at the pre-surgical baseline and at each follow-up visit for All patients (N=136), Combined with phacoemulsification (+Cat) subgroup (N=76), and Standalone in pseudophakic eyes (SA) subgroup (N=60). (B) Mean intraocular pressure at the pre-surgical baseline and at each follow-up visit for All patients (N=136), primary open-angle glaucoma (POAG) subgroup (N=121), and secondary open-angle glaucoma (SOAG) subgroup (N=15). Error bars are ± 1 standard deviation. BL is presurgical baseline, M 1, M6, M12 = Months 1, 6, 12.
Figure 2.
Mean number of ocular hypotensive medications preoperatively and follow-up time points. Threshold of significance adjusted for multiple comparisons (Bonferroni) to P<0.0038.
Abbreviations: POAG, primary open-angle glaucoma; SOAG, secondary open-angle glaucoma; +Cat, procedure combined with phacoemulsification cataract surgery; SA, standalone procedure in pseudophakic eyes; ns, not significant; BL, presurgical baseline; M1, M6, M12, Months 1, 6, 12.
Figure 3.
Frequency distribution of patients on 0, 1, 2, 3, or ≥4 medications at presurgical baseline (BL), or month 12 (M12).
BCVA preoperatively was 20/40 or better for 103 patients, 20/32 or better for 81 patients, 20/25 or better for 58 patients and 20/20 or better for 27 patients. At month 12, there was a general improvement in BCVA with 118, 109, 85, and 51 patients in these respective categories. The improvement in BCVA was mainly seen, as expected, in patients undergoing OSS with cataract surgery, while BCVA for standalone OSS patients was generally similar at M12 and Baseline.
Intraoperative AE were infrequent (2, 1.5%) and included two incidents of iris touch. The most frequent AE were BCVA loss ≥2 lines Snellen, 8 (5.9%), two of which were related to posterior capsule opacification and subsequently resolved with YAG, one due to dry eye which was resolved with artificial tears, one secondary to correctopia and vitreous prolapse which resolved post-vitrectomy, one secondary to a subluxated IOL resolved after IOL exchange, and one resolving without intervention. For the two that had not resolved within the follow-up period, one went from 20/25 at baseline to 20/40 at month 6 and 12, and the other went from 20/20 at baseline and was 20/32 at months 1, 6 and 12. Other AE were mild anterior chamber inflammation, 6 (4.4%), all resolved with topical steroids; clinically significant hyphema ≥1 mm, 4 (2.9%), all resolved without intervention within 2 weeks; IOP increases ≥10 mmHg at or after 30 days postoperative, 4 (2.9%). Other AE with an incidence of 2% or less included mild corneal edema (2, 1.5%), mild central Descemet’s membrane change, corectopia, vitreous prolapse (all 1 occurrence, <1%). Adverse events are presented in Table 3. There were four SSI (2.9%) required; one in the combined with cataract surgery cohort (1.3%), a trabeculectomy with mitomycin C, and three in the standalone cohort (5.0%) including XEN gel stent (2) and micropulse laser (1).
Table 3.
Adverse Events: Number and Frequency
| Adverse Event | Number (%) |
|---|---|
| Intraoperative | |
| Iris touch | 2 (1.5) |
| Postoperative | |
| BCVA loss of ≥2 lines Snellen at or after 3 months post-op | 8 (5.9) |
| Mild anterior chamber inflammation | 6 (4.4) |
| Clinically significant hyphema ≥ 1 mm | 4 (2.9) |
| IOP increase ≥10 mmHg at or after 30 days postoperative | 4 (2.9) |
| Mild corneal edema | 2 (1.5) |
| Central Descemet’s membrane change (mild) | 1 (<1) |
| Correctopia* | 1 (<1) |
| Vitreous prolapse* | 1 (<1) |
| TOTAL | 29 |
Note: *Correctopia and vitreous prolapse occurred in the same patient.
Discussion
ROMEO was the first multicenter study demonstrating the safety and effectiveness of OSS in mild-to-moderate OAG either with cataract surgery or as a standalone procedure.1,2 In ROMEO, patients with baseline IOP > 18 mmHg had a change in medicated IOP at 12 months of −6.8 mmHg and −0.9 medications for OSS combined with cataract surgery, and −6.2 mmHg and −0.5 medications for the standalone group. Taken together, there was a 6.5 mmHg decrease in IOP and a 0.7 decrease in medications. The results of ROMEO 2 are consistent and confirm the earlier results, the IOP reduction for all patients was 6.4 mmHg with a 0.6 decrease in medications. Like the ROMEO results, in ROMEO 2 IOP reduction was somewhat greater for the combined with cataract surgery cohort (−6.9 mmHg) than for the standalone cohort (−5.6 mmHg), which may reflect the contribution of cataract surgery to IOP reduction for the combined cohort,3 or the fact that more patients in the standalone group were on 2 or more medications at baseline (60% vs 54%) and had somewhat more advanced glaucoma (mean MD −4.7 dB vs −4.4 dB; proportion worse than −6 dB 30% vs 26%) suggesting IOP somewhat more refractory to treatment, or a combination of these factors. There was also a somewhat higher proportion of African-American patients in the standalone group (12% versus 2.6%) which could also have contributed to this finding although the proportion White was similar between the two groups (80% in standalone, 79% in the combined with cataract surgery cohort). Congruent with the notion that the standalone cohort was, on average, somewhat more refractory, the majority of the SSIs were in this group (3 vs 1) and there was a lower proportion with a 20% or better IOP reduction (53% vs 74%) or with IOP at or below 18 mmHg (80% vs 89.5%). While meaningful IOP and medication reduction was achieved for both subgroups, and both subgroups were closely matched in terms of baseline characteristics and demographics, the subtle differences mentioned above are in alignment with a slightly different threshold of disease for MIGS when performed with cataract surgery or as a standalone procedure. The need for cataract surgery presents an “open door” to add MIGS with little additional risk, whereas a standalone MIGS is only justified where warranted for control of glaucoma.
Most (89%) of patients in this study had a diagnosis of POAG. While the POAG results are nearly identical to those for all patients due to the preponderance of POAG in this study, it is worth noting that IOP and medication outcomes were as good or better in the small group (n=15) of SOAG eyes included in this study.
Since the publication of ROMEO, several other studies of OSS have been completed. These include the prospective, multicenter (US) GEMINI study which included a preoperative and terminal medication washout with 149 eyes undergoing OSS with phacoemulsification cataract surgery,4,5 a single-center, prospective 12-month Polish study of standalone and combination procedures,6 and a single-center German study with 24-month data for standalone cases that included both phakic and pseudophakic eyes.7 The GEMINI study showed an IOP decrease of 8.2 mmHg (from 23.8 to 15.6 mmHg, −35%) from preoperative to month 12 (both unmedicated following medication washout) and medications decreased from 1.8 to 0.4. Comparison of the GEMINI patient demographics and glaucoma characteristics to those of the present study show that, on average, the GEMINI patients were somewhat younger (68.3 years vs 72.9 years), had slightly less advanced glaucoma (visual field MD −3.7 dB vs −4.5 dB), and were more uniformly POAG (93% vs 84% for the +cataract cohort).5 However, the populations were racially similar (82% vs 81% White) and were under treatment with a similar number of medications preoperatively (1.8 vs 1.9). Despite the differences noted, IOP at month 12 was similar (15.6 vs 15.9 mmHg) albeit unmedicated for the GEMINI study and with an average of 1.3 medications in the present study.5 In a retrospective case series, Klabe reported IOP at 12 and 24 months of 14.7 and 14.9 mmHg at 12 and 24 months on 0.4 and 0.5 medications, while Grabska-Liberek reported, in a prospective case series, IOP of 12.7 mmHg on 0.64 medications at 12 months.6,7 IOP outcomes in patients treated with OSS show a remarkable consistency across studies, typically in the mid-teens.8 There is more apparent variability in medication reduction outcomes, but this can, at least in part, be attributed to the use of a baseline medication washout in some of the studies.5,7 Moreover, as the present study was retrospective and included 16 different centers, post-operative medication decisions were not standardized as is the case in prospective or single-center studies but were instead made according to individual surgeon discretion and standard of care.
Efforts to control IOP in mild-to-moderate glaucoma have historically been through pharmaceutical means; however, success is ultimately dependent on patient adherence. Sustained release drugs, laser trabeculoplasty and microinvasive glaucoma surgery (MIGS) are all therapeutic options that aim to provide IOP control that is not reliant on patient behavior.9 A further potential advantage of these modalities is a theoretically continuous and predictable effect on IOP, in contrast to topical drug treatment, where even when administered optimally, there is a peak and trough effect.10,11 Fluctuation in IOP has been shown to be a significant independent risk factor for progression of glaucoma.12 Recently, Pyfer et al showed that diurnal IOP fluctuations in IOP were significantly reduced (−35%) 1 year following OSS treatment.13 Analysis of 5-year visual field data from the HORIZON study has provided evidence supporting the hypothesis that surgical intervention in mild-to-moderate glaucoma with a MIGS procedure can slow glaucomatous progression.14
The safety outcomes in the present study were also consistent with ROMEO and other studies of OSS. Most notable was BCVA loss of ≥2 lines Snellen at or after 1 month post-operative. Most of these cases had causes unrelated to the procedure (eg, posterior capsular opacity, or malpositioned IOL) and all but two resolved with or without treatment. Hyphema (clinically significant ≥1 mm), mild anterior chamber inflammation, and IOP elevation ≥10 mmHg at or after 1 month were all ~3–4% and similar to ROMEO, and indeed other MIGS procedures,15,16 and all were transient. It is noteworthy that 97% of the eyes did not require an SSI over the course of the 12-month follow-up. This reproduces the OSS findings from GEMINI (no SSIs in 149 eyes) and the original ROMEO studies (9 SSIs in 129 patients, 7%), and is similar to what was observed for the first-generation iStent (4.3%).1,2,5,17
This report presents a large retrospective data set for the OSS collected from 16 investigational sites and 20 ophthalmic surgeons. There are some limitations to the study which should be discussed. First, eligibility required a preoperative IOP of >18 mmHg which was measured at one visit (mean of two repeated measurements). While this could create the risk of regression to the mean it is, in our view, an acceptable risk that would likely be relevant for only a small fraction of the study population. The alternative of using two or more preoperative visits to obtain a mean preoperative IOP would likely have been confounded with differences in number and class of medications used, given the generally poor IOP control preoperatively. Moreover, to be comparable to ROMEO 1, it was important to match eligibility criteria. Second, while there is overlap in investigational sites/surgeons between ROMEO 2 and ROMEO which could arguably account for the consistency of results, ROMEO 2 included five new centers with seven new surgeons who had not participated in the original ROMEO study. The new centers and surgeons would certainly have introduced some diversity and variability beyond what was measured. Nevertheless, the overall similarity in outcomes suggests generalizability and robustness. Third, the present study, is limited to 12 months of post-operative follow-up and additional follow-up beyond 12 months would allow better characterization of the durability of the treatment effect. We believe such data will be forthcoming with time. Similar to the original ROMEO study, ROMEO 2 was retrospective. Protocol defined measurement methods (eg. for IOP), strict surgical procedural guidelines, rules for medication introduction or discontinuation, and washout of IOP-lowering medication prior to treatment and at the endpoint are commonplace in prospective clinical trials but are not in a retrospective studies. While this lack of standardization could be viewed as a weakness, it is in fact a core strength of this study. No two ophthalmologists treat glaucoma in exactly the same way. Individual practitioners have different thresholds for surgical intervention, differing ideas about IOP targets and treatment escalation, and different ideas about when a medication can be discontinued. A prospective “one size fits all” clinical trial cannot capture this diversity of practice and therefore generalizability may be limited. However, in a study such as ROMEO or ROMEO 2, this diversity is embraced and the results may in some ways better inform other surgeons of the outcomes they might achieve in their own patients.
Funding Statement
Sight Sciences Inc. (SSI) provided financial support.
Data Sharing Statement
The authors do not intend to share participant-level data. Other queries or requests should be directed to the corresponding author (JD).
Author Contributions
ROMEO Study Group
Sebastian Heersink, MD, Jeremy Cotliar, MD, Gabriel Ferreira, MD, Steven D. Vold, MD, Louis Hirsch, MD, Blake Williamson, MD, Mark Gallardo, MD, Michael Hochman, MD, Deepan Selvadurai, MD, Ardalan Aminlari, MD, Anita Campbell, MD, Andrew Cho, MD, Jeffrey A Kammer, MD, Kenneth W. Olander, MD, Daniel Terveen, MD, Avneet K. Sodhi Gaur, MD, James T. Murphy, III, MD, J. Matthew Rouse, MD, Ryan H. Phan, MD, Adam R. Bleeker, MD, Michael McFarland, OD, Kavita Dhamdhere, MD, PhD, Jaime E. Dickerson, Jr, PhD.
Disclosure
JED Jr and KD are employees of SSI. JTM III is a consultant and speaker for SSI. DCT has received a research grant from SSI. AEA has nothing to disclose. The authors report no other conflicts of interest in this work.
References
- 1.Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47:907–915. doi: 10.1097/j.jcrs.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 2.Vold SD, Williamson BK, Hirsch L, et al. Canaloplasty and trabeculotomy with the OMNI System in pseudophakic patients with open-angle glaucoma: the ROMEO study. Ophthalmol Glaucoma. 2021;4:173–181. doi: 10.1016/j.ogla.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong JJ, Wasiuta T, Kiatos E, et al. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26(6):511–522. doi: 10.1097/IJG.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 4.Gallardo MJ, Sarkisian SR, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: interim results from the GEMINI Study. Clin Ophthalmol. 2021;15:481–489. doi: 10.2147/OPTH.S296740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallardo MJ Pyfer MF, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification for glaucoma. 12-month results of the GEMINI study. Clin Ophthalmol. 2022;16:1225–1234. doi: 10.2147/OPTH.S362932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabska-Liberek I, Duda P, Rogowska M, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm’s canal and collector channels and 360° trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol Feb. 2021;24:1120672121998234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klabe K, Kaymak H. Standalone trabeculotomy and viscodilation of Schlemm’s canal and collector channels in open-angle glaucoma using the OMNI surgical system: 24-month outcomes. Clin Ophthalmol. 2021;15:3121–3129. doi: 10.2147/OPTH.S325394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickerson JE, Dhamdhere K. Combined circumferential canaloplasty and trabeculotomy ab interno with the OMNI surgical system. Front Ophthalmol. 2021;1:106–114. doi: 10.36879/FrO.21.000106 [DOI] [Google Scholar]
- 9.Fingeret M, Dickerson JE. The role of minimally invasive glaucoma surgery devices in the management of glaucoma. Optom Vis Sci. 2018;95:155–162. doi: 10.1097/OPX.0000000000001173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishima S. Clinical pharmacokinetics of the eye. Proctor Lecture. Invest Ophthalmol Vis Sci. 1981;21:504–541. [PubMed] [Google Scholar]
- 11.Webers CA, Beckers HJ, Nuijts RM, Schouten JS. Pharmacological management of primary open-angle glaucoma: second-line options and beyond. Drugs Aging. 2008;25:729–759. doi: 10.2165/00002512-200825090-00002 [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Rabiolo A, Morales E, et al. Risk factors for fast visual field progression in glaucoma. Am J Ophthalmol. 2019;207:268–278. doi: 10.1016/j.ajo.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 13.Pyfer MF Gallardo M, Campbell A, et al. Suppression of diurnal. (9AM-4PM) IOP fluctuations with minimally invasive glaucoma surgery: an analysis of data from the prospective, multicenter, single-arm GEMINI study. Clin Ophthalmol. 2021;15:3931–3938. doi: 10.2147/OPTH.S335486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzard G. Visual Field Outcomes from the Randomized, Multicenter, Horizon Trial. Nashville, TN: American Glaucoma Society; 2022. [Google Scholar]
- 15.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126:811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed IIK, Fea A, Au L, et al. A prospective randomized trial comparing Hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology. 2020;127:52–61. doi: 10.1016/j.ophtha.2019.04.034 [DOI] [PubMed] [Google Scholar]
- 17.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi: 10.1016/j.jcrs.2012.03.025 [DOI] [PubMed] [Google Scholar]