Abstract
At the outset of the coronavirus disease 2019 (COVID-19) pandemic, it was clear that a vaccine would be crucial for global health efforts. The Pfizer and BioNTech teams came together in a race against the virus, working to design, test, manufacture, and distribute a safe and efficacious vaccine in record time for people around the world. Here, we provide backstory commentary from the pharmaceutical scientist perspective on the challenges and solutions encountered in the development of the Pfizer-BioNTech mRNA COVID-19 vaccine (BNT162b2; b2; Comirnaty®; tozinameran). We discuss the foundational science that led to the decision to use an mRNA-based approach. We also describe key challenges in the identification of an optimal vaccine candidate and testing in clinical trials, the continuous efforts to improve the vaccine formulation in response to changing global health priorities and facilitate vaccine accessibility, and how vast quantities of vaccine doses were manufactured and safely delivered to every corner of the globe, all without compromising quality, science, and safety. The key to successfully delivering a safe and efficacious vaccine within nine months was a result of extraordinary, real-time, parallel effort and across-the-board collaboration between stakeholders on a global scale.
Keywords: Global Health, Lipid Nanoparticles, mRNA, Vaccine, Vaccine delivery
Abbreviations: CDMO, contract development and manufacturing organization; CMA, Conditional Marketing Authorization; COVID-19, coronavirus disease 2019; CTA, clinical trial applications; EMA, European Medicines Agency; EUA, Emergency Use Authorization; GMP, Good Manufacturing Practice; IVT, in vitro transcription; mRNA, messenger RNA; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; FDA, Food and Drug Administration; WHO, World Health Organization
Introduction
Each stage of vaccine development, spanning pre-clinical and clinical research and testing, regulatory approvals, manufacturing, and successful distribution, has inherent challenges under the best of circumstances. These steps typically occur sequentially and usually take years to accomplish. During a pandemic, when time is life 1 and the need for an expedited and successful process is heightened, overcoming challenges in a safe and swift manner becomes a matter of great public health concern.
News broke of a novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) spreading globally in late 2019, and the gravity of the subsequent disease it caused (coronavirus disease 2019, COVID-19) was soon realized in the subsequent months. On March 11, 2020, the World Health Organization (WHO) declared a pandemic, and on March 13, 2020, Pfizer announced their partnership with BioNTech to co-develop a messenger RNA (mRNA)-based vaccine to prevent COVID-19.2 , 3 Both companies, which had an existing partnership to develop an mRNA-based influenza vaccine, signed a letter of intent on March 17, 2020 to join hands in the race against the virus and immediately commence the joint COVID-19 vaccine development program (Project Lightspeed).3 , 4
Here, from the pharmaceutical scientists’ perspective, we describe some of the challenges encountered during the development of the Pfizer-BioNTech mRNA COVID-19 vaccine (BNT162b2; b2; Comirnaty®; tozinameran) on a hyper-expedited timeline (Fig. 1 ), and the solutions utilized to successfully bring the first COVID-19 vaccine (and the first mRNA-based vaccine) to patients within nine months without compromising quality, science, and safety. This endeavor is the result of an unprecedented effort by thousands of individuals working collaboratively across Pfizer and BioNTech, who continue to respond to the evolving global status of COVID-19.
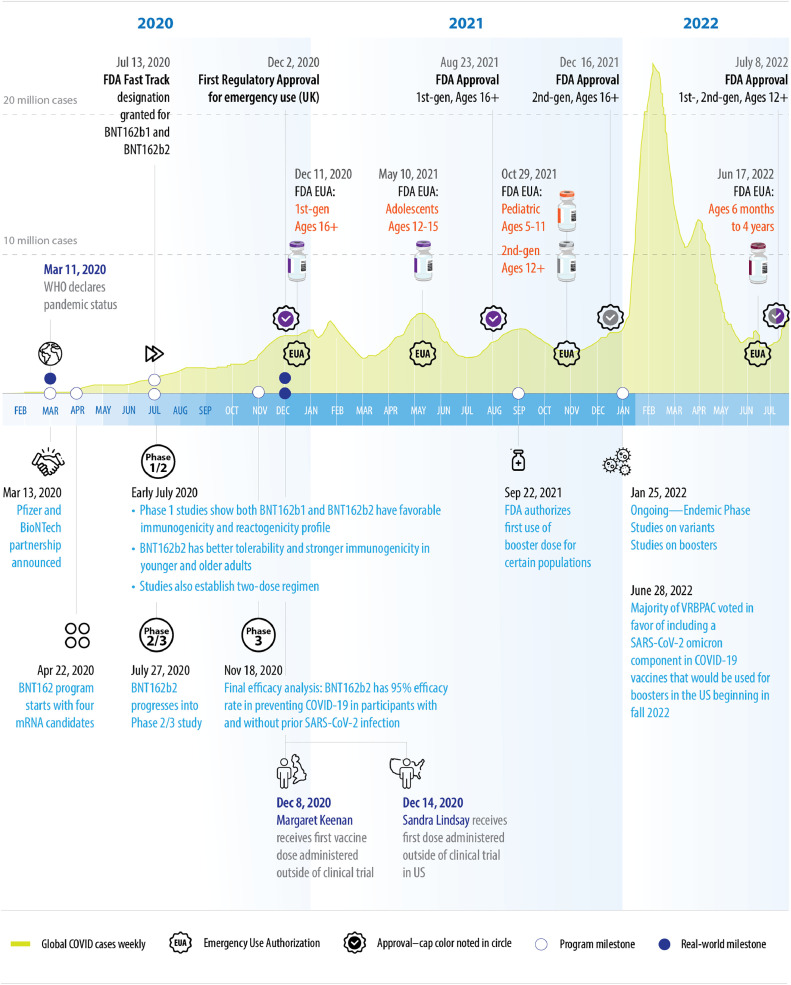
Fig. 1.
Timeline of key events.1,2,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
Figure shows US FDA milestones; other country approvals not shown for simplicity.
mRNA as the Vaccine Technology Platform of Choice for the Pfizer-BioNTech COVID-19 Vaccine
There are many available vaccine technology platforms which the vaccine development team could have explored, including adenovirus, recombinant proteins, conjugation, and mRNA.1 mRNA vaccine technology quickly arose as the most viable path forward for COVID-19, as this approach has a more rapid manufacturing process and would enable a swifter response. While many think of mRNA-based vaccine technology as new, scientists have been studying this vaccine platform for decades prior to the breakthroughs brought about during the COVID-19 pandemic leading to the approvals of the first mRNA-based vaccines.16 mRNA was discovered in 1947-1961, and its ability to be transported to mouse/human cells in liposomes to induce protein expression was discovered in the late 1980s. The therapeutic potential of mRNA was subsequently realized in a range of applications, including viral vaccines in which mRNA is packaged in a viral vector.17 As mRNA is structurally unstable and can easily be degraded by ubiquitous RNAase enzymes, mRNA-based vaccine technology has been continually refined, for example, the backbone of the mRNA molecules can be modified to improve stability. Some modified mRNA formats include nucleoside-modified mRNA, uridine-containing mRNA, and self-amplifying mRNA.17 , 18
To achieve therapeutic effects, mRNA requires safe, effective, and stable delivery systems that protect it from degradation and allow for cellular uptake and release at the specific target cell. One such delivery system that has been successfully utilized are lipid nanoparticles.17 Previous studies had indicated that modified mRNA encapsulated in a lipid nanoparticle delivery system mobilizes a broader range of immune responses (both humoral and cellular) compared to traditional vaccine platforms.1
Other advantages of using synthetic mRNA in a lipid nanoparticle delivery system as the vaccine platform include that it is chemically defined, minimizes risk of anti-vector immunity, permits quick modifications for new variants and boosters, and enables rapid development and quick production scaling compared to traditional vaccine technologies.19 Part of this ability for rapid development/production was derived from (1) prior mRNA platform knowledge from an earlier collaboration between Pfizer and BioNTech for influenza, and (2) prior experience in manufacturing critical starting raw material (plasmid DNA) from Pfizer's Gene Therapy Program. With all advantages and challenges considered and fueled by the urgent need for a safe and effective COVID-19 vaccine in the face of increasing infection rates and death tolls, the Pfizer vaccine development team moved forward with an mRNA-based approach in partnership with BioNTech.
Challenges and Solutions in the Development of the COVID-19 Vaccine
Developing a vaccine for a novel virus involves many scientific challenges including scaling vaccine production for global distribution, overcoming complex regulatory interfaces, and logistical hurdles. For the development of Comirnaty®, the challenges were abundant, with key challenges including (1) the identification of an optimal vaccine candidate and testing in clinical trials on an expedited timeline, (2) continually updating the vaccine formulation to respond to changing global health priorities, (3) sourcing vast quantities of raw materials for manufacturing, and (4) ensuring quality control across all global sites. Throughout, effective collaborations with regulatory bodies were crucial for bringing the vaccine to patients quickly while prioritizing safety and regulatory compliance. Here, we describe the solutions utilized in collaboration with BioNTech and many key partners to develop, manufacture, and distribute Comirnaty®.
Identification of the Vaccine Candidate and Clinical Trial Challenges
To identify a single lead vaccine candidate more quickly, the team initially pursued a diverse set of four constructs in parallel, each representing different mRNA formats and target antigens.1 In late April 2020, approximately one month after the partnership between Pfizer and BioNTech began, the phase 1/2 clinical trials were initiated in Germany (NCT04380701)20 and the US (NCT04368728)21 with the following constructs:
-
•
BNT162b1 (b1): a nucleoside-modified mRNA targeting the smaller optimized receptor binding domain (RBD) from the spike protein of SARS-CoV-2.
-
•
BNT162b2 (b2): a nucleoside-modified mRNA targeting the P2-mutated full spike protein (Fig. 2 ).
-
•
BNT162a1: an uridine-containing mRNA targeting the RBD from the spike protein.
-
•
BNT162c2: a self-amplifying mRNA targeting the P2-mutated full spike protein.
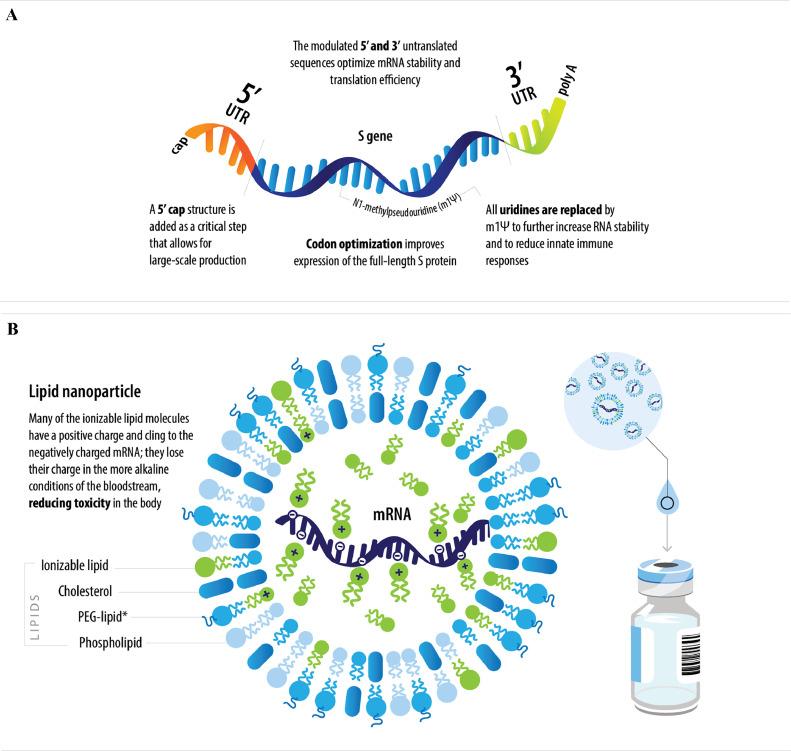
Fig. 2.
Schematic of the BNT162b2 (A) mRNA and (B) lipid nanoparticle.
*Lipid attached to polyethylene glycol.
PEG, polyethylene glycol; S, spike; UTR, untranslated region.
The RBD-based candidates contain the domain of the spike protein that facilitates cellular attachment and is thought to be most important for eliciting antibodies that can inactivate the virus. The P2-mutated candidates encode a full-length spike with two-point mutations (proline substitutions) within the central helix; this mutation stabilizes the spike protein in a prefusion conformation for optimal expression and immunogenicity.22 , 23 Each mRNA format is encapsulated in a lipid nanoparticle formulation (Fig. 2). In addition to these four diverse formats, different dose ranges were explored between 0.1 to 100 mcg depending on the type of construct.
Initial clinical data of the first vaccine candidate (b1) studied in the phase 1/2 trials were promising, and early predictions led to b1 being the anticipated candidate. However, emerging data on the second candidate b2 suggested it to have favorable reactogenicity in both younger and older participants in the phase 1 studies; earlier virus clearance and no evidence of virus in the lung in nonhuman primate challenge studies; and robust immunogenicity in both younger and older participants at the 30 mcg dose level.24 , 25 Based on these data, the team decided on July 27, 2020 to proceed with b2 at the dose of 30 mcg for the phase 2/3 study (NCT04368728).24 Narrowing down to the b2 construct and 30 mcg dose was a data-derived decision, expedited by the strategy of pursuing multiple diverse options in parallel in early stages rather than working through candidates sequentially.
As the clinical trials progressed, vaccine supply challenges arose during the phase 3 trial, with a very tight hand-to-arm supply of clinical trial material. For example, there was only one contract development and manufacturing organization (CDMO) making lipid nanoparticles at the initiation of the phase 2/3 trial that had planned to enroll ∼44,000 subjects. A typical expedited program development and manufacturing of phase 3 supplies can be 12 months or more depending on the molecule; for the COVID-19 vaccine, the timeline was ∼4-5 months. Several mitigation approaches were used to ensure timely clinical supply, including: sourcing of drug substance and using PCR-based template for clinical trial materials from BioNTech; use of multi-dose vials in the trial to conserve doses; scaling out the lipid nanoparticle manufacturing process to ensure higher throughput; CDMO relationships to ensure back-to-back manufacturing slots for continuous clinical supply; ensuring availability of starting materials, critical raw materials, custom lipids, glass vials, etc., by undertaking at-risk large order contracts with vendors; multiple fill nodes within the Pfizer network to work around fill capacity constraints; use of Pfizer private jets to deliver samples, drug substance, and supply to clinical trial sites; and rapid turnaround on multiple round-the-clock amendments to clinical trial applications (CTAs) from regulatory authorities/Boards of Health.
Through use of these mitigation steps, coordinated collaboration, and prioritization of multiple stakeholders to ensure timely/adequate supplies, the clinical trials were successfully completed. On November 18, 2020, the results of the final efficacy analysis of the phase 3 study were announced: b2 met all primary efficacy endpoints with a favorable safety profile; the vaccine efficacy rate was 95% (p<0.0001) in participants without prior SARS-CoV-2 infection (first primary objective) and in participants with and without prior SARS-CoV-2 infection (second primary objective), measured from 7 days after the second dose.14 The vaccine was shown to elicit both neutralizing antibody and cellular immune responses to the spike antigen, which may contribute to protection against COVID-19.22 Based on these results, in December 2020, b2 became the first COVID-19 vaccine to be granted authorizations for emergency use. The first authorization was in the UK, followed shortly by the Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA) and the Conditional Marketing Authorization (CMA) by the European Medicines Agency (EMA) (Fig. 1).1 , 26 , 27
Adapting Formulations to Evolving Global Health Priorities and Regulatory Interactions to Bring the Vaccine to Patients
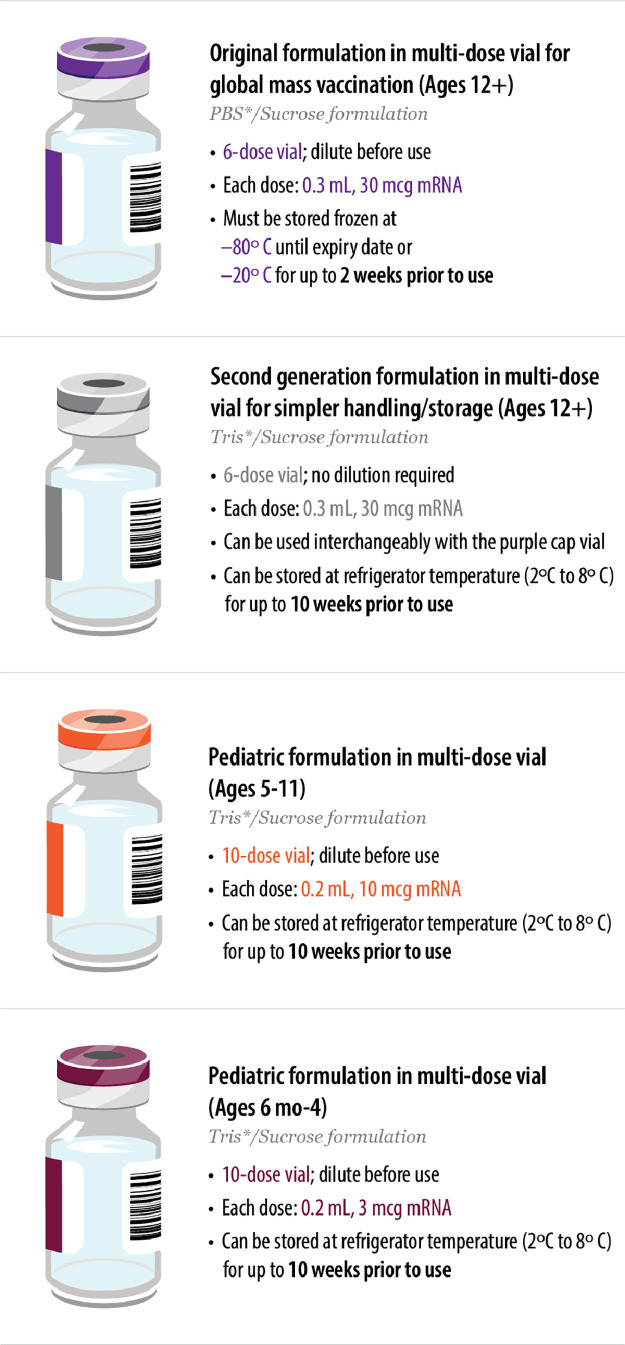
The b2 vaccine is a white to off-white suspension for intramuscular injection (pH: 6.9-7.9).7, 8, 9, 10 , 22 It is formulated in lipid nanoparticles for delivery into host cells; the lipid nanoparticles are composed of four different lipids (ALC-0159, ALC-0315, cholesterol, and DSPC).22 The vaccine formulations have evolved to address changing global health priorities and to simplify handling and storage to maximize vaccine availability and accessibility (Fig. 3 ). The cap colors for the multiple vaccine formulations now available were selected to help ensure vaccine administration sites differentiate between the formulations and intended patient group.
Fig. 3.
Vaccine formulations evolved to address changing global priorities and needs.7, 8, 9, 10
*PBS and Tris are buffering agents that help maintain the pH and stability of the product.
The initial vaccine formulation uses a PBS (phosphate-buffered saline)/sucrose buffer to help maintain the pH and stability of the product, and is provided in 2 mL clear type I glass vials with a purple flip-off plastic cap and a stopper (synthetic bromobutyl rubber) with aluminum seal.22 Prior to administration, the 0.45 mL vaccine concentrate requires dilution with sterile 0.9% sodium chloride injection, USP. Each 0.3 mL dose contains 30 mcg of a nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2.8 The vaccines are provided in multi-dose vials to maximize vaccine availability for mass global vaccinations.
The initial regulatory submissions took a conservative approach and indicated that each vial contained five doses of 0.3 mL.25 To minimize potential wasted vaccine, this was later updated to six doses of 0.3 mL following feedback from frontline healthcare providers with the use of low dead-volume syringe/needle combination (with dead volume of no more than 35 μL) to enable the extraction of six doses per vial (this may not be possible with standard needles/syringes).8 , 22
To ensure the quality and supply of the vaccines, a conservative approach was also taken for the initial global shipments following the initial authorizations in December 2020. Given the fragile nature of the mRNA vaccine, multi-dose vials were shipped in specially-designed thermal containers with real-time tracking during transport that can maintain ultra-low temperatures (-70°C ±10°C) for 10 days.1 Initial authorizations required storage of undiluted frozen vials at ultra-low temperatures for up to 30 days, undiluted thawed vials at standard refrigerator temperature (2⁰C to 8⁰C) for up to 5 days, and diluted thawed vials at refrigerator temperature or room temperature for use within 6 hours. In February 2021, based on continuous stability data in efforts to increase/improve vaccine accessibility, the FDA authorized undiluted frozen vials to be transported and stored at -25°C to -15°C for up to 2 weeks, in addition to the ultra-low temperatures.28 In May 2021, the FDA authorized longer storage of undiluted thawed vials at standard refrigerator temperatures, increasing from up to 5 days to up to 1 month.8 , 29 This would ensure the vaccine can be shipped and stored under increasingly flexible conditions and would also offer vaccine providers greater flexibility in how they manage their vaccine supply to remote areas.
To help simplify handling of the vaccine, a second-generation formulation of the vaccine was developed that does not require dilution prior to administration and allows for longer storage at standard refrigerator temperatures. The second-generation formulation (provided in multi-dose vials with gray caps) uses Tris (tromethamine) as the buffering agent, instead of PBS in the original purple cap formulation, and no longer contains sodium chloride and potassium chloride.30 All other aspects, including the antigen and the lipids of the vaccine, remain unchanged. The Tris/sucrose formulation allows for longer storage at standard refrigerator temperatures: frozen vials can be thawed and stored at 2°C to 8°C for up to 10 weeks,7 versus up to 1 month for the initial PBS/sucrose formulation.8 Vials can also be stored at room temperature for up to 12 hours, versus 6 hours for the original PBS/sucrose formulation. The Tris/sucrose formulation was shown to be analytically comparable to the original PBS/sucrose formulation.30 It received FDA authorization for emergency use in ages 12+ in October 2021 and received full FDA approval for ages 12+ in July 2022 (Fig. 1). The two formulations currently have the same FDA approvals and are approved to be used interchangeably for ages 12+.7 , 8
The two pediatric formulations are based on the second-generation formulation using the Tris/sucrose buffer and require dilution with sterile 0.9% sodium chloride injection, USP to adjust dosage prior to administration.9 , 10 The orange cap vial for individuals aged 5 through 11 years was authorized for emergency use by the FDA in October 2021; each 0.2 mL dose contains 10 mcg of a nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2.9 The maroon cap vial for individuals aged 6 months through 4 years was authorized for emergency use by the FDA in June 2022; each 0.2 mL dose contains 3 mcg of the nucleoside-modified mRNA.10 These pediatric vaccines are currently provided in multi-dose vials. Single-dose vials are being considered, so doses can be delivered on-demand at pediatrician offices.
Throughout the trials and development/refinement of the manufacturing process, a dialogue with various global regulatory agencies was crucial for ensuring vaccine deployment would not be stalled by regulatory roadblocks. Between April 2020 and April 2021 (the height of the pandemic), approximately 2100 queries were received from 24 markets. At the time of authoring this manuscript (July 2022), the number of queries have compounded to approximately 3255 from 49 markets. Innovative solutions were implemented to enable effective communication with FDA, EMA, and other health authorities, including proactive communications with health authorities, establishing rolling submission strategies with major health authorities, and aligning on a single, global EUA dossier applicable to most markets. In August 2021, Comirnaty® was approved in 70 global markets, and at the time of authoring this manuscript (July 2022) it has been launched in 186 markets globally.
Manufacturing, Distribution, and Quality Control on a Global Scale
It took approximately 3 months to transition from the first bench scale 10 mL in vitro transcription (IVT) reaction to the first production scale EUA drug product vial. In early 2021, following the initial authorizations, Pfizer had publicly committed to making 2.5 billion vaccine doses by the end of the year. For context, Pfizer's total vaccine production before COVID-19 was 200 million doses per year; the largest-volume vaccine before COVID-19 was the pneumococcal vaccine and it had taken a decade to reach the production levels at the time.1 Preparations for large-scale manufacturing were made in parallel with preclinical research and clinical trials, even before the vaccine candidate was identified, in an effort to roll out the vaccine as quickly as possible.1
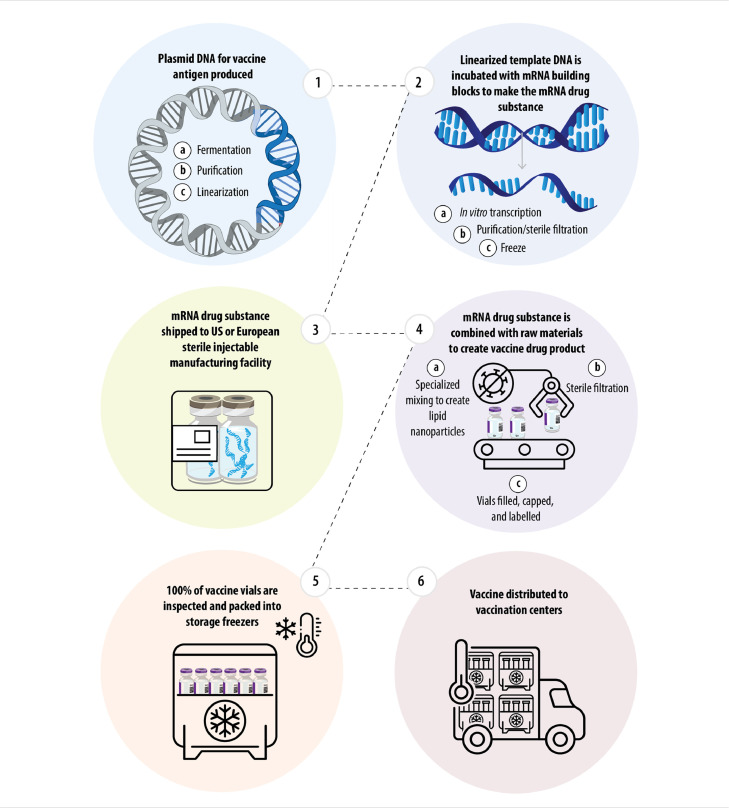
After careful assessment of complementary manufacturing capabilities and capacities across the network, the team devised a plan that would ensure safety and allow for optimal speed. Steps in the manufacturing of the mRNA drug substance and vaccine drug product are shown in Fig. 4 .1 The plasmid DNA that would serve as the template for the mRNA in the vaccine is generated in Chesterfield St. Louis, Missouri, USA. Following subsequent purification steps, the purified template DNA is linearized and sent to Andover, Massachusetts, USA for the manufacture of the mRNA drug substance. At the Andover facility, the linearized template DNA is incubated with mRNA building blocks to create the mRNA drug substance, which is then purified and shipped for formulation and further processing to two of the largest sterile injectable manufacturing sites in the US (Kalamazoo, Michigan) and Europe (Puurs, Belgium). The use of two separate sterile injectable manufacturing facilities in separate countries was intentional, to build redundancy into the system in the event of interruptions at one facility, and as a precaution in the event of export restrictions. At the sterile injectable manufacturing facilities, the mRNA drug substance undergoes specialized mixing with raw materials to form the lipid nanoparticles and thus creating the vaccine drug product, which would be sterilized and filled into vials for inspection and packing into storage freezers.1 In this multi-step commercial manufacturing process, the supply chain needed constant adjustment and recalibration to account for changing rate-limiting steps on a monthly basis. To meet continued demand, the mRNA and lipid nanoparticle manufacturing process was expanded to include multiple manufacturing sites within the Pfizer and BioNTech networks, and additional partnerships with contract manufacturing organizations were established.
Fig. 4.
Steps in the manufacturing of the mRNA drug substance and vaccine drug product.1
Ensuring the availability of the necessary quantity of raw materials/disposables needed for the mRNA, lipids, and other solutions for formulation at such a massive scale was a major concern. The four different lipids needed to synthesize the lipid nanoparticles (ALC-0159, ALC-0315, cholesterol, and DSPC) were a particular challenge to source, as two are proprietary and two are commodity. As these were new ingredients that were not used at scale in other vaccines, Pfizer began to produce the lipids internally to increase and ensure availability, in addition to production in close collaborations with the respective chemical companies. Obtaining vials and caps was also a challenge, with many vaccine suppliers vying for the same materials; leveraging existing relationships with suppliers was helpful in ensuring availability.1
In addition to the availability of raw materials at scale, focusing on Good Manufacturing Practice (GMP), the manufacturing team invented, designed, and ordered specialized formulation, storage, and transport tools, equipment, and software to prepare for the mass production/delivery of the first mRNA-based medical product.1 Instrumentation was another challenge, as both analytical labs and mRNA process development labs had to be re-designed with key instrumentation and operational workflows managed under accelerated timelines.
It was also crucial to demonstrate appropriate and comparable product quality and stability across the global manufacturing network. A series of comprehensive comparability evaluations were executed to demonstrate comparable processes and product quality for materials manufactured across the global network. For example, computational fluid dynamics was utilized to conduct mixing similarly across different facilities. Drug substance and drug products manufactured at new sites and/or increased scale were demonstrated to be comparable to historical product lots through lot release data and heightened characterization testing. The active substance specifications contain tests for appearance, pH, content (RNA Concentration), Identity of Encoded RNA Sequence, RNA Integrity, 5’- Cap, Poly(A) Tail, Residual DNA Template, dsRNA, Bacterial Endotoxin and Bioburden.31 The release and stability testing specifications for b2 finished product include tests for Appearance, Subvisible Particles, pH, Osmolality, LNP Size, LNP Polydispersity, RNA Encapsulation, RNA content, ALC-0315 content, ALC-0159 content, DSPC content, Cholesterol content, extractable volume, Lipid identities, Identity of encoded RNA sequence, Potency / in Vitro Expression, RNA Integrity, Bacterial Endotoxin, Sterility and Container Closure Integrity.31 Stability data from lots manufactured across the global network informed appropriate product storage guidance which could be followed at all global sites.
Storage and transport of the vaccine at ultra-low temperatures was another challenge that required innovative solutions. The team would design and implement a temperature-controlled thermal shipper which could hold up to 5,850 vaccine doses in the size of a carry-on suitcase and record location, temperature, and light exposure in real time.1 This logistical innovation could also serve as cold storage in settings without a suitable freezer. Pfizer also expanded ‘freezer farms’ at manufacturing plants around the world to house vaccine at appropriate temperatures;1 this incredible effort involved significant innovations outside the scope of this article.
By the end of 2021, Pfizer had successfully manufactured over 3 billion vaccine doses, and more than 2.6 billion doses were delivered to 166 countries/territories.32 At the time of authoring this manuscript (July 2022), 3.6 billion doses of the COVID-19 vaccine have been delivered to 180 countries and territories in every region of the world. Scaling up the manufacturing capabilities to hit these production targets was an enormous undertaking that required a dynamic and redundant supply chain, manufacturing precision, and unprecedented engineering and logistical efforts.
Conclusion and Future Outlook
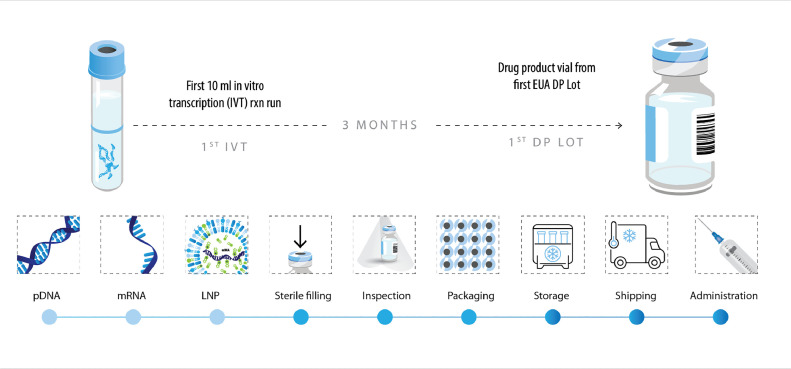
In the first year of Comirnaty® rollout, it is estimated that the vaccine contributed to averting almost 9 million symptomatic cases, 700,000 hospitalizations, and over 110,000 deaths.33 This was made possible by progressing our vaccine development program swiftly, going from the first 10 mL IVT reaction run to the first drug product lot with an EUA in 3 months (Fig. 5 ). Our team and collaborators did this while successfully ensuring the highest compliance and quality standards according to the normal vaccine development principles:19
1. The vaccine must be proven effective, meaning it can help prevent severe disease and hospitalizations in at least a majority of vaccinated people
2. The vaccine must be proven safe, with robust safety data generated from thousands of people
3. The vaccine must be consistently manufactured at the highest quality standards
Fig. 5.
The Comirnaty® development process was accelerated without compromising compliance or safety.
DP, drug product; EUA, Emergency Use Authorization; IVT, in vitro transcription; LNP, lipid nanoparticle; mRNA, messenger RNA; pDNA, plasmid DNA.
Throughout the process, a dialogue between the Pfizer-BioNTech development team, global regulatory agencies, and vaccine providers on-the-ground was crucial for raising practical challenges and addressing them appropriately. Such collaboration between scientific, medical, and policy stakeholders will remain essential as the pressing concerns of the COVID-19 pandemic continue to evolve. As SARS-CoV-2 transitions to an endemic virus, the healthcare system must adapt to a patient population with heterogeneous levels of prior immunity/exposure and the emergence of new viral variants. Ongoing studies aim to optimize booster vaccination strategies to continually offer all patients well-tolerated protection from severe COVID-19. As in the initial development of Comirnaty®, these ongoing/future studies will be built on a foundation of partnership across companies and countries, with a common goal of championing global health. We hope this collaborative spirit and transparent review of decision-making in the development of Comirnaty® proves useful to the broader scientific community for future vaccine development efforts.
Disclosures
Authors are employees of Pfizer.
Acknowledgements
The authors wish to thank the clinical trial participants and their families; sites, investigators and their dedicated staff; our clinical trial CRO and other partners; governments, the CDC, FDA, and other regulatory authorities worldwide; Operation Warp Speed; healthcare workers, first responders, essential workers, teachers, vendors, suppliers, other support agencies; and our fellow colleagues at BioNTech and Pfizer.
Medical writing support, including assisting authors with the development of the manuscript drafts and incorporation of comments, was provided by Grace Jeong, PhD and Meredith Whitaker, PhD of Alphabet Health (New York, NY), supported by Pfizer, according to Good Publication Practice guidelines (https://www.ismpp.org/gpp3).
References
- 1.Bourla A. 1st ed. HarperCollins Publishers; 2022. Moonshot. [Google Scholar]
- 2.World Health Organization . 2020. WHO Director-General's opening remarks at the media briefing on COVID-19.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at. [Google Scholar]
- 3.Pfizer . 2020. Pfizer Outlines Five-Point Plan to Battle COVID-19.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-outlines-five-point-plan-battle-covid-19 Available at. [Google Scholar]
- 4.Pfizer . 2020. Pfizer and BioNTech to Co-Develop Potential COVID-19 Vaccine.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-co-develop-potential-covid-19-vaccine Available at. [Google Scholar]
- 5.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2022. COVID-19 Dashboard.https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 Accessed on Aug 1. Available at. [Google Scholar]
- 6.US Food and Drug Administration . 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster Available at. [Google Scholar]
- 7.Pfizer . 2022. COMIRNATY® (COVID-19 Vaccine, mRNA) suspension for injection, for intramuscular use (gray cap)https://labeling.pfizer.com/ShowLabeling.aspx?id=16351&format=pdf Last Updated July Available at. Accessed Aug 1, 2022. [Google Scholar]
- 8.Pfizer . 2022. COMIRNATY® (COVID-19 Vaccine, mRNA) suspension for injection, for intramuscular use (purple cap)https://labeling.pfizer.com/ShowLabeling.aspx?id=15623&format=pdf Last Updated July Available at. Accessed Aug 1, 2022. [Google Scholar]
- 9.Pfizer . 2022. EUA Fact Sheet for Vaccination Providers (5 Through 11 Years of Age) - Orange Cap.https://labeling.pfizer.com/ShowLabeling.aspx?id=16073&format=pdf Last Updated June 17. Available at. Accessed Aug 1, 2022. [Google Scholar]
- 10.Pfizer . 2022. EUA Fact Sheet for Vaccination Providers (6 Months to 4 Years) - Maroon Cap.https://labeling.pfizer.com/ShowLabeling.aspx?id=17227&format=pdf Last Updated June 17. Available at. Accessed Aug 1, 2022. [Google Scholar]
- 11.US Food and Drug Administration . 2021. FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations.https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations Sep 22. Available at. [Google Scholar]
- 12.US Food and Drug Administration . 2021. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age.https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age Oct 29. Available at. [Google Scholar]
- 13.Pfizer . 2022. Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18 to 55 Years of Age.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based Jan 25 Available at. [Google Scholar]
- 14.Pfizer . 2020. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine November 18. Available at. [Google Scholar]
- 15.Pfizer . 2020. Pfizer and BioNTech Granted FDA Fast Track Designation for Two Investigational mRNA-based Vaccine Candidates Against SARS-CoV-2.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-granted-fda-fast-track-designation-two July 13 Available at. [Google Scholar]
- 16.Dolgin E. 2021. The tangled history of mRNA vaccines.https://www.nature.com/articles/d41586-021-02483-w Available at. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration . 2020. VRBPAC December 10, 2020 Presentation - BNT162b2 Vaccine Candidate Against COVID-19.https://www.fda.gov/media/144325/download December 10, 2020. Available at: Accessed April 1, 2022. [Google Scholar]
- 20.Pfizer . 2020. BioNTech and Pfizer Announce Regulatory Approval from German authority Paul-Ehrlich-Institut to Commence First Clinical Trial of COVID-19 Vaccine Candidates.https://www.pfizer.com/news/press-release/press-release-detail/biontech_and_pfizer_announce_regulatory_approval_from_german_authority_ paul_ehrlich_institut_to_commence_first_clinical_trial_of_covid_19_vaccine_ candidates April 22 Available at. [Google Scholar]
- 21.Pfizer . 2020. Pfizer and BioNTech Dose First Participants in the U.S. as Part of Global COVID-19 mRNA Vaccine Development Program.https://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_biontech_dose_ first_participants_in_the_u_s_as_part_of_global_covid_19_mrna_vaccine_development_ program May 05. Available at. [Google Scholar]
- 22.European Medicines Agency . 2022. Comirnaty - Summary of Product Characteristics.https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf Last Updated July 28 Available at. Accessed Aug 1, 2022. [Google Scholar]
- 23.Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci. 2017;114(35):E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfizer . 2020. Pfizer and BioNTech Choose Lead mRNA Vaccine Candidate Against COVID-19 and Commence Pivotal Phase 2/3 Global Study.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-choose-lead-mrna-vaccine-candidate July 27 Available at. [Google Scholar]
- 25.US Food and Drug Administration . 2020. VRBPAC December 10, 2020 Meeting Briefing Document- Sponsor.https://www.fda.gov/media/144246/download Available at. Accessed April 1, 2022. [Google Scholar]
- 26.Pfizer . 2020. Pfizer and BioNTech Celebrate Historic First Authorization in the U.S. of Vaccine to Prevent COVID-19.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-celebrate-historic-first-authorization December 11 Available at. [Google Scholar]
- 27.Pfizer . 2020. Pfizer and BioNTech Receive Authorization in the European Union for COVID-19 Vaccine.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-receive-authorization-european-union December 21 Available at. [Google Scholar]
- 28.US Food and Drug Administration . 2021. Coronavirus (COVID-19) Update: FDA Allows More Flexible Storage, Transportation Conditions for Pfizer-BioNTech COVID-19 Vaccine.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-more-flexible-storage-transportation-conditions-pfizer February 25 Available at. Accessed Aug 1, 2022. [Google Scholar]
- 29.US Food and Drug Administration . 2021. FDA In Brief: FDA Authorizes Longer Time for Refrigerator Storage of Thawed Pfizer-BioNTech COVID-19 Vaccine Prior to Dilution, Making Vaccine More Widely Available.https://www.fda.gov/news-events/press-announcements/fda-brief-fda-authorizes-longer-time-refrigerator-storage-thawed-pfizer-biontech-covid-19-vaccine May 19 Available at. [Google Scholar]
- 30.US Food and Drug Administration . 2021. Pfizer Decision Memorandum 10 29 2021.https://www.fda.gov/media/153947/download Available at. Accessed April 12, 2022. [Google Scholar]
- 31.European Medicines Agency . 2021. European Public Assessment Report (EPAR)https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf February 19 Available at. [Google Scholar]
- 32.Pfizer . 2021. Expanding COVID-19 Manufacturing Efforts to Increase Global Vaccine Access.https://www.pfizer.com/sites/default/files/investors/financial_reports/annual_reports/2021/story/expanding-covid-manufacturing-efforts/ Available at. [Google Scholar]
- 33.Di Fusco M, Marczell K, Deger KA, et al. Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. J Med Econ. 2022;25(1):605–617. doi: 10.1080/13696998.2022.2071427. [DOI] [PubMed] [Google Scholar]